Abstract
Experimental infection with the gammaherpesvirus Bovine herpesvirus 4 (BoHV-4) rarely establishes disease, yet BoHV-4 is commonly associated with uterine disease in cattle. Uterine disease involves co-infection with bacteria such as Escherichia coli, which stimulate the production of prostaglandin E2 (PGE2) by endometrial cells. BoHV-4 replication depends on Immediate Early 2 (IE2) gene transactivation, and in the present study, PGE2, E. coli or its lipopolysaccharide (LPS), up-regulated the IE2 gene promoter in uterine cells. Bacterial co-infection is important for BoHV-4 uterine disease.
Introduction
Abortion may follow infection with a variety of α-, ß- and γ-herpesvirus, but viral causes of uterine disease are seldom investigated in cattle. Although postpartum metritis affects up to 40% of cattle, causing considerable infertility and economic loss, it has been assumed that most diseases are of bacterial origin and virus isolation or serology is rarely considered (Sheldon et al. 2004). The first isolation of bovine herpesvirus 4 (BoHV-4) from a case of bovine metritis was reported in U.S.A. (Park et al. 1973). Later, several other isolates were obtained from cows with reproductive disorders from different countries, including Italy (Castrucci et al. 1986). In Belgium, BoHV-4 seroprevalence was associated with postpartum metritis and chronic infertility of cattle (Czaplicki et al. 1998). Postpartum metritis has also been associated with BoHV-4 in the USA (Frazier et al. 2002), Spain (Monge et al. 2006) and Serbia (Nikolin et al. 2007). There is a lacuna in the knowledge about the direct correlation between viral infection and uterine pathology.
The BoHV-4 Immediate Early (IE) genes are expressed immediately during cell infection, do not require prior viral protein synthesis for their expression and their expression is mediated by the pool of transcription factors made by the cell, already present at the moment of infection and able to transactivate at the transcriptional level the IE promoters. BoHV-4 IE2 protein (replication and transcription activator homologous, Rta) encoded by open reading frame 50 (ORF 50) is well conserved among γ-herpesviruses (Zimmermann et al. 2001). Rta expression plays a primary role in initiating viral lytic replication, not only during reactivation of latently infected non-permissive cells but also during de novo infection of permissive cells (Donofrio et al. 2004, van Santen 1993). Viruses are restricted to using the metabolic and biosynthetic pathways of the cells that they infect. These pathways vary between cell types, lineage, stage of differentiation and with the state of cell activation. There are many examples of viruses that replicate in specific cells and at particular stages of cell growth, differentiation or activation. This includes the reactivation of cytomegalovirus when host cells differentiate into macrophages; initiation of papillomavirus replication by keratinocytes and replication of minute virus in testicular cells. The key mechanism mediating these effects is the regulation of viral gene expression at the transcriptional level by host cell factors. In a previous study (Donofrio et al. 2007) the interaction, tropism and outcome of BoHV-4 challenge of endometrial epithelial and stromal cells were investigated. In the present report to further investigate the mechanisms associated with endometrial tropism, a molecular switch involving the viral immediate early 2 (IE2) gene has been associated to extracellular stimuli belonging to the intrauterine microenvironment, as bacterial contamination.
Materials and methods
Reagents
Purified LPS (from E. coli, O55:B5 and O111:B4), prostaglandin E2 (PGE2), idomethacin and Polymyxin B were purchased from Sigma–Aldrich (St. Louis, http://www.sigmaaldrich.com). Heat-killed E. coli isolate 361 and 154, were isolated from a case of clinical bovine endometritis associated with pyrexia as described (Sheldon et al. 2002).
Endometrial cell isolation and primary cultures
Six bovine uteri from post-pubertal non-pregnant BoHV-4 serum negative animals with no evidence of genital disease were collected at a local abattoir immediately after slaughter and kept on ice until further processing in the laboratory. The physiological stage of the reproductive cycle for each genital tract was determined by observation of the ovarian morphology. Genital tracts with an ovarian Stage I corpus luteum were selected for endometrial tissue and cell culture, and only the horn ipsilateral to the corpus luteum was used.
The endometrium was cut into strips and placed into serum-free RPMI-1640 (Sigma) supplemented with 50 IU/ml of penicillin, 50 μg/ml of streptomycin and 2.5 μg/ml of Amphotericin B (Sigma), working under sterile conditions. The strips were then chopped into 1 mm3 pieces and placed into HBSS (Sigma) and used as previously described (Fortier et al. 1988) with the following modifications. Briefly, tissue was digested in 25 ml sterile filtered digestive solution, which was made by dissolving 50 mg trypsin III (Roche), 50 mg collagenase II (Sigma), 100 mg BSA (Sigma) and 10 mg DNase I (Sigma) in 100ml phenol-red free HBSS. Following a 1.5 h incubation in a shaking water bath at 37°C, the cell suspension was filtered through a 40 μm mesh (Fisher Scientific) to remove undigested material and the filtrate was resuspended in phenol-red free HBSS containing 10% FBS (Sigma) and 3 μg/ml trypsin inhibitor (Sigma) (Washing medium). The suspension was centrifuged at 100 × g for 10 min and following two further washes in washing medium the cells were resuspended in RPMI-1640 containing 10% FBS, 50 IU/ml of penicillin, 50 μg/ml of streptomycin and 2.5 μg/ml of Amphotericin B. The cells were plated at a density of 1 × 105 cells in 2 ml per well using 24-well plates (Nunc). To obtain separate stromal and epithelial cell populations, the cell suspension was removed 18 h after plating, which allowed selective attachment of stromal cells (Fortier et al. 1988). The absence of immune cells in the uterine cell cultures was confirmed by RT-PCR for the CD45 pan-leukocyte marker as previously described (Herath et al. 2006). The culture media was changed every 48 h until the cells reached confluence. All cultures were maintained at 37°C, 5% CO2 in air, in a humidified incubator.
Plasmids
The IE2 promoter region of the BoHV-4 genome (nucleotide 61,391 to 62,534; GenBank accession number AF318571; (Zimmermann et al. 2001) was generated by PCR, using total DNA isolated from BoHV-4-infected MDBK cells as template and a pair of IE2 promoter primers (sense: 5′-aaacccggtaccgccagtgccaagctttttaag-3′; antisense: 5′-gggaactagctagcctgttgttctgctccctttta-3′) containing an artificial KpnI site on the 5′ end and a NheI site on the 3′ end respectively.
One microgram sample DNA was amplified over 35 cycles, each cycle consisting of denaturation at 94 °C for 1 min, primer annealing at 55 °C for 1 min, and chain elongation with High Fidelity PCR Enzyme Mix (Fermentas) at 72°C for 2 min. PCR amplification was carried out in a final volume of 50 μl, containing 0.2 mM deoxinucleoside triphosphate and 0.25 μM of each primer. In the first cycle, the samples were denatured at 94°C for 5 min, and in the last cycle the extension step was increased to 7 min. The amplicon was column purified, KpnI, NheI digested and subcloned into pGL3 Basic vector (Promega, UK) to generate pIE2prom-Luc. pTK-Renilla were obtained from Promega, UK. All constructs were sequenced to guarantee the fidelity of the PCR products.
Transient Transfection
Confluent BES cells in twenty four-well plates were co-transfected with 0.5 μg of pIE2prom-Luc or 0.5 μg pGL3 empty vector, as a negative control and 0.05 μg of pRK-Renilla to normalize the efficiency of transfection, using LTX transfection reagent (Invitrogen, UK) as suggested by manufacturer. Transfection mixture was prepared in DMED without serum and antibiotics and left on the cells for 6 h at 37°C, 5% CO2 in air, in a humidified incubator. After 6 h, the transfection mixture was replaced with complete medium (RPMI-1640, 10% FBS, 50 IU/ml of penicillin, 50 μg/ml of streptomycin and 2.5 μg/ml of Amphotericin B) and left to recover for 18 h at 37°C, 5% CO2 in air, in a humidified incubator. 24 h post transfection, cells were treated with different reagents for period.
Luciferase reporter assay
Luciferase reporter assay was performed with a Dual Luciferase Reporter Assay System kit (Promega) with minor modifications. Following treatments, cells were washed with PBS, lysed with 100 μl of lysis passive buffer by freeze-thawing at −80 °C. 10 μl of the cell lysete was added to 50 μl of LAR and Luciferase activity were determined with a PerkinElmer Victor3 Multilabel Counter, according to the manufacturer's specifications. Individual assays were normalized for Renilla Luciferase activity with a second reading, adding 50 μl of Stop & Glo substrate.
Statistics
Experiments were performed with 4 replicates at each time point and each experiment repeated three times. Statistical differences were tested by ANOVA.
Results
Activation of BoHV-4 promoter in BES cells
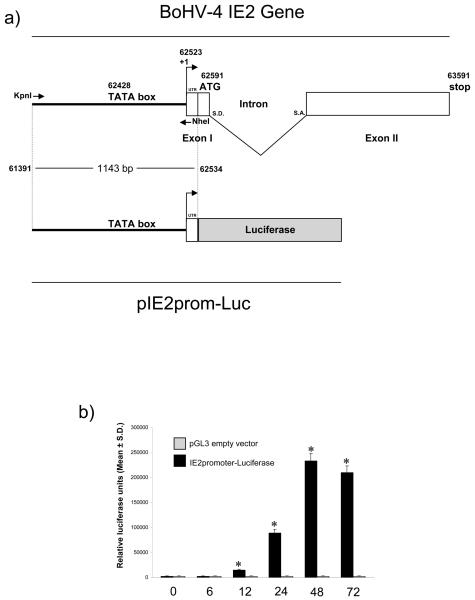
To be able to quantify transactivation of the IE2 promoter in BES cells, a luciferase reporter construct was developed by sub-cloning the BoHV-4 IE2 promoter in front of the pGL3 Luciferase reporter vector (Promega) open reading frame to generate pIE2prom-Luc (Fig. 1a). BES cells were co-transfected with pIE2prom-Luc or pGL3 empty vector as a negative control, and pRK-Renilla (Promega, UK) to normalize the efficiency of transfection. Cells were harvested at different times after transfection and Luciferase activity measured. The IE2 promoter was activated within 12 h of transfection and reached the maximum level by 48 h (Fig. 1b). The observation that 24 h was in the middle of the linear range of the promoter activation was used to select 24 h after transfection as the optimal time to analyze the IE2 promoter activity in subsequent experiments.
Fig. 1.
a) Diagram showing the genomic region containing BoHV-4 IE2 gene (not on scale), where the exon II (whit box) contains most of the IE2 ORF, except the translation initiation codon contained in the exon I (whit box) together with the 5′ UTR. The splicing donor (S. D.) and splicing acceptor (S. A.) sites are indicated. During splicing, the intron is removed and the two exons (I and II) are joined together to generate the IE2 ORF. Sense and antisense primers used for the PCR amplification of the promoter region sequence (1,143 bp) are indicated by arrows, spanning the full promoter containing the TATA box and the first 15 non coding nucleotides (UTR) of the first exon. The KpnI and NheI restriction sites have been introduced into the 5′ end of the primers, for sub-cloning the amplicon in front of the Luciferase ORF (grey box) contained into the pGL3 vector. Numbers indicates nucleotide position in the BoHV-4 full genome published sequence (GeneBank accession number: AF318573) (Zimmermann et al. 2001). b) IE2 promoter activity in BES cells at different time post transfection with pIE2prom-Luc or pGL3 empty vector. The data represents the mean relative Luciferase units after normalizing with the cotransfected Renilla activity. Each reaction was done in quadruplicate, and each point represents the average ± standard deviation from three experiments (*P<0.001).
E. Coli stimulates the BoHV-4 IE2 gene promoter
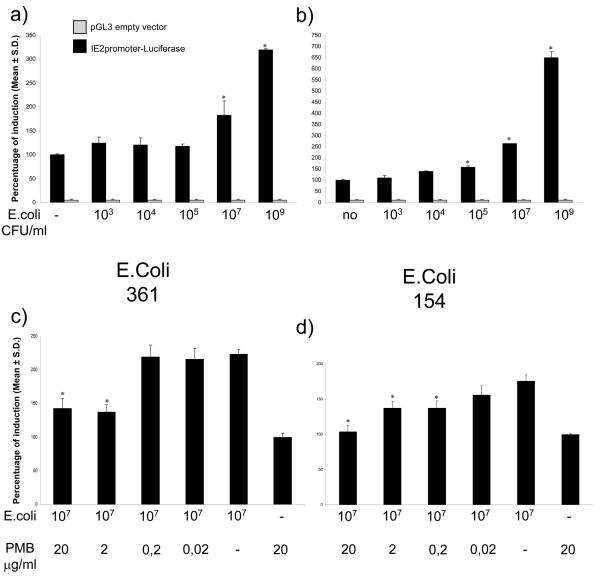
The capability of bacteria to stimulate the BoHV-4 IE2 gene promoter was investigated using E. coli because they are the most frequently bacterial isolates from the uterus of postpartum cattle with uterine disease (Olson et al. 1984, Sheldon et al. 2004). Two strains of E. coli isolated from the uterus of postpartum cattle were used: E. coli 154, is a pathogenic strain associated with severe metritis and E. coli 361 was from a clinically normal animal. The bacteria were heat-killed as described previously (Herath et al. 2006) and used at a range of concentrations spanning those present in the uterus of postpartum cattle (Dohmen et al. 2000, Williams et al. 2007). BES cells were challenged for 6h with the bacteria, starting 24 h after transfection with pIE2prom-Luc or pGL3 empty vector and pTK-Renilla. Both strains of E. coli increased IE2 promoter activity in a concentration dependent manner (Fig. 2a and b). Much of the host's immune response and pathology associated with E. coli is attributable to the bacterial endotoxin, lipopolysaccharide (LPS). LPS exerts its effect by binding to the TLR4/CD14/MD2 receptor complex, present in many cell types, including endometrial cells (Herath et al. 2006). To test if LPS contributed to activation of the IE2 promoter by E. coli, BES cells were treated for 6 h with E. coli, in the presence of different concentration of the LPS inhibitor Polymyxin B (PMB; SIGMA, UK), 24 h after transfection with pIE2prom-Luc or pGL3 empty vector and pTK-Renilla. PMB reduced the stimulatory effect of LPS (Fig. 2c and d).
Fig. 2.
Transiently transfected BES cells with pIE2prom-Luc (black bars) or pGL3 (gray bars) empty vector for 24 h. The cells were then stimulated for 6 h with 103, 104, 105, 107, or 109 CFU/ml E. coli isolate 361 (a) or isolate 154 (b). The results are expressed as percentage of induction, where 100% is the untreated control (−). Inhibition of the effect of E. coli isolate 361 (c) or isolate 154 (d) by increasing concentration of the LPS antagonist, polymixin B (PMB). The data represents the mean relative Luciferase units after normalizing with the cotransfected Renilla activity. Each reaction was done in quadruplicate, and each point represents the average ± standard deviation from three experiments. Values differ significantly from E. coli untreated control (in a and b, *P<0.001) and PMB untreated control (in c and d, *P<0.001).
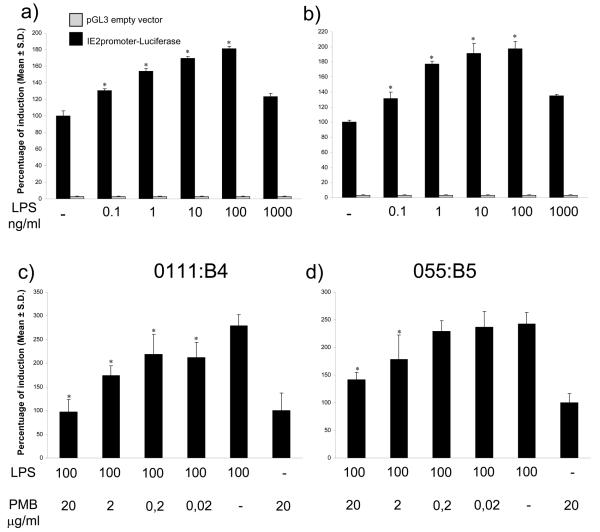
Specific stimulation of the BoHV-4 IE2 gene promoter by LPS
To further determine the role of LPS in IE2 activation, the experiment was repeated with pure (O55:B5, Sigma) and ultrapure LPS (O111:B4, Invivogen) from different E.coli serotypes, at concentrations reflecting the range measured in the uterine lumen of postpartum cows (Williams et al. 2007). Both forms of LPS stimulated the IE2 promoter in a concentration dependent manner (Fig. 3a and b). A GFP reporter construct for IE2 was used to confirm these observations. Both forms of LPS increased BES fluorescence indicating activation of the IE2 promotor (see supplemental data). To determine if the response was associated with LPS and not contamination of the compounds, BES cells were treated with PMB before challenge with LPS. The IE2 response to LPS was reduced by PMB (Fig. 3c and d), with the greater reduction for O111:B4 probably reflecting the greater purity of this preparation compared with O55:B5.
Fig. 3.
Transiently transfected BES cells with pIE2prom-Luc (black bars) or pGL3 (gray bars) empty vector for 24 h. The cells were than stimulated for 6 h with increasing concentrations of LPS O111:B4 (a) or O55:B5 (b). The results are expressed as percentage of induction, where 100% is the untreated control (−). Inhibition of 100 ng/ml O111:B4 (c) or O55:B5 LPS (d) activity by increasing concentration of PMB. The data represents the mean relative Luciferase units after normalizing with the cotransfected Renilla activity. Each reaction was done in quadruplicate, and each point represents the average ± standard deviation from three experiments. Values differ significantly from LPS untreated control (in a and b, *P<0.001) and PMB untreated control (in c and d, *P<0.001).
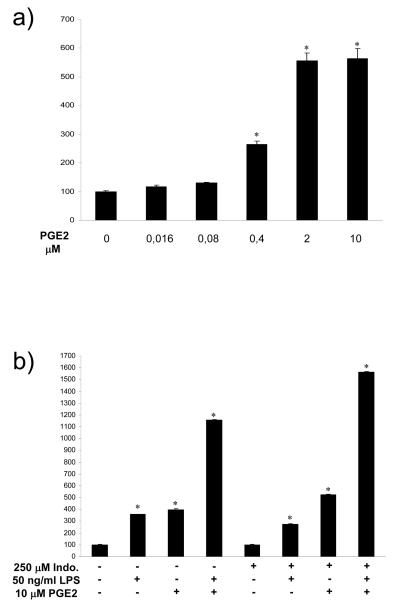
PGE2 stimulates the BoHV-4 IE2 gene promoter
PGE2 may also be involved in activation of the IE2 promoter when there is co-infection of the uterus with BoHV-4 and E. coli. E. coli stimulates BES cell secretion of PGE2 and 6 μM of PGE2 reactivated BoHV-4 replication in persistently infected bovine macrophages (Donofrio et al. 2005, Herath et al. 2006). So, BES cells were treated for 6 h with different concentrations of PGE2 (Sigma), 24 h after transfection with pIE2prom-Luc and pTK-Renilla. The IE2 promoter was stimulated by 0.4 μM PGE2, with maximal stimulation by 2 to 10 μM PGE2 (Fig. 5).
Independent and co-stimulatory effect of LPS and PGE2 on the BoHV-4 IE2 gene promoter
To explore if IE2 activation by LPS and PGE2 are independent, BES cells were challenged with LPS, PGE2, or their combination, 24 h after transfection with pIE2prom-Luc and pTK-Renilla, and 12 h after treatment with a pan-COX inhibitor (250 μM indomethacin; Sigma) or vehicle. LPS and PGE2 independently stimulated IE2 expression as expected. However, the presence of Indomethacin did not block the effect of LPS (Fig. 4b) so LPS may activate IE2 independent of PGE2. The combination of LPS and PGE2 appeared to exhibit a co-stimulatory effect of IE2.
Fig. 4.
Transiently transfected BES cells with pIE2prom-Luc (black bars) for 24 h. (a) The cells were then stimulated with increasing concentrations of PGE2 for 6 h. The results are expressed as percentage of induction, where 100% is the untreated control (−). (b) In a parallel experiment BES cells were untreated or pre treated with 250 μM of Indomethacin and than stimulated with PGE2, LPS or a combination of LPS and PGE2. The data represents the mean relative Luciferase units after normalizing with the cotransfected Renilla activity. Each reaction was done in quadruplicate, and each point represents the average ± standard deviation from three experiments (*, P<0.001).
Discussion
Bos taurus is particularly prone to uterine infection and metritis after parturition, (Sheldon et al. 2004). The most commonly recognised uterine pathogen is Escherichia coli (Dohmen et al. 2000, Olson et al. 1984, Sheldon et al. 2002, Sheldon et al. 2004, Williams et al. 2007). However, BoHV-4 is also consistently associated with metritis. Bacteria may stimulate BoHV-4 replication of the virus following the recruitment from the bloodstream to the site of inflammation of macrophages persistently infected with BoHV-4. This theory may explain how BoHV-4 can be isolated from healthy animals in the absence of inflammation.
Assuming IE2 is the molecular master switch for BoHV-4 replication, the capability of endometrial cells to transactivate the IE2 promoter was previously investigated by transient transfection of an EGFP-labelled construct containing the IE2 gene promoter and electroporated into endometrial epithelial and stromal cells, BT, BEL, MDBK and BEK cells. EGFP started to accumulate robustly as soon as 24 h after electroporation in the cytoplasm of stromal cells, in contrast to the other cell types where weak visible green cells appeared not before than 3 days post-electroporation (Donofrio et al. 2007). In conclusion, the present study provides evidence that bacterial co-infection is important for activation of the IE2 gene promoter in BES cells, necessary to activate the BoHV-4 lytic replication associated with uterine disease. Bacterial contamination of the uterine lumen is ubiquitous in postpartum cattle (Sheldon et al. 2004); and, BoHV-4 metritis is consistently associated with bacterial co-infection (Castrucci et al. 1986, Czaplicki et al. 1998, Frazier et al. 2002, Mehrotra et al. 1986, Monge et al. 2006, Nikolin et al. 2007, Park et al. 1973). Bacterial infection stimulates COX2 expression and production of PGE2 in bovine endometrial cells (Herath et al. 2006). The role of PGE2 on IE2 activation is particularly important in the uterus, as E. coli or LPS induce COX-2 expression and PGE2 production by BES cells (Herath et al. 2006). Indeed, LPS stimulates sufficient PGE2 secretion (~0.3 μM) to activate IE2 expression. Furthermore, BoHV-4 also induces COX-2 protein expression and PGE2 secretion in BES cells (Donofrio et al. 2007).
Here, we show that E. coli, LPS and PGE2 activate the BoHV-4 IE2 gene promoter, and this probably involves PGE2 dependent and independent pathways. These observations provide a plausible mechanism underlying the rapid activation of viral replication in the bovine endometrium associated with uterine disease. Indeed there could be a positive feedback loop between PGE2 production and viral replication, initiated by the bacterial co-infection. This mechanism could be important in other tissues other than the uterus, where bacterial and viral infections coexisting. Identification and exploration of the underlying mechanisms of viral-bacterial synergism will provide targets for prevention and treatment using drugs and vaccines.
Supplementary Material
Acknowledgements
We would like to thank The Royal Society, Italian Ministry of University and Scientific Research (Italian National Grant MIUR, PRIN 2005, 2005078885), the Fondazione Cariparma (Cassa di Risparmio di Parma, Italy). Sheldon is a BBSRC Research Development Fellow (Grant No. BB/D02028X/1).
References
- Castrucci G, Frigeri F, Cilli V, Donelli G, Ferrari M, Chicchini U, Bordoni E. A study of a herpesvirus isolated from dairy cattle with a history of reproductive disorders. Comp Immunol Microbiol Infect Dis. 1986;9:13–21. doi: 10.1016/0147-9571(86)90070-6. [DOI] [PubMed] [Google Scholar]
- Czaplicki G, Thiry E. An association exists between bovine herpesvirus-4 seropositivity and abortion in cows. Prev Vet Med. 1998;33:235–240. doi: 10.1016/s0167-5877(97)00036-6. [DOI] [PubMed] [Google Scholar]
- Dohmen MJW, Joop K, Sturk A, Bols PEJ, Lohuis JACM. Relationship between intra-uterine bacterial contamination, endotoxin levels and the development of endometritis in postpartum cows with dystocia or retained placenta. Theriogenology. 2000;54:1019–1032. doi: 10.1016/s0093-691x(00)00410-6. [DOI] [PubMed] [Google Scholar]
- Donofrio G, Cavirani S, Taddei S, Flammini CF. Activation of bovine herpesvirus 4 lytic replication in a non-permissive cell line by overexpression of BoHV-4 immediate early (IE) 2 gene. J Virol Methods. 2004;116:203–207. doi: 10.1016/j.jviromet.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Donofrio G, Herath S, Sartori C, Cavirani S, Flammini CF, Sheldon IM. Bovine herpesvirus 4 is tropic for bovine endometrial cells and modulates endocrine function. Reproduction. 2007a;134:183–197. doi: 10.1530/REP-07-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donofrio G, Martignani E, Cavirani S, Flammini CF. Exploiting persistent infection for selection of bovine herpesvirus 4 recombinants. J Virol Methods. 2005;128:6–13. doi: 10.1016/j.jviromet.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Donofrio G, Martignani E, Sartori C, Vanderplasschen A, Cavirani S, Flammini CF, Gillet L. Generation of a transposon insertion mutant library for bovine herpesvirus 4 cloned as a bacterial artificial chromosome by in vitro MuA based DNA transposition system. J Virol Methods. 2007b;141:63–70. doi: 10.1016/j.jviromet.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Fortier MA, Guilbault LA, Grasso F. Specific properties of epithelial and stromal cells from the endometrium of cows. J Reprod Fertil. 1988;83:239–248. doi: 10.1530/jrf.0.0830239. [DOI] [PubMed] [Google Scholar]
- Frazier KS, Baldwin CA, Pence M, West J, Bernard J, Liggett A, Miller D, Hines ME. Seroprevalence and comparison of isolates of endometriotropic bovine herpesvirus-4. J Vet Diagn Invest. 2002;14:457–462. doi: 10.1177/104063870201400602. [DOI] [PubMed] [Google Scholar]
- Herath S, Fischer DP, Werling D, Williams EJ, Lilly ST, Dobson H, Bryant CE, Sheldon IM. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology. 2006;147:562–570. doi: 10.1210/en.2005-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra ML, Shucla DC, Srivastava NC. Isolation of a new herpesvirus from cases of reproductive disorders in cow. Indian Journal of Animal Science. 1986;56:1196–1199. [Google Scholar]
- Monge A, Elvira L, Gonzalez JV, Astiz S, Wellenberg GJ. Bovine herpesvirus 4-associated postpartum metritis in a Spanish dairy herd. Research in Veterinary Science. 2006;80:120–125. doi: 10.1016/j.rvsc.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Nikolin VM, Donofrio G, Milosevic B, Taddei S, Radosavljevic V, Milicevic V. First Serbian isolates of bovine herpesvirus 4 (BoHV-4) from a herd with a history of postpartum metritis. New Microbiol. 2007;30:53–57. [PubMed] [Google Scholar]
- Olson JD, Ball L, Mortimer RG, Farin PW, Adney WS, Huffman EM. Aspects of bacteriology and endocrinology of cows with pyometra and retained fetal membranes. Am J Vet Res. 1984;45:2251–2255. [PubMed] [Google Scholar]
- Park JB, Kendrick JW. The isolation and partila characterization of a herpesvirus from a case of bovine metritis. Arch Gesamte Virusforsch. 1973;41:211–215. doi: 10.1007/BF01252768. [DOI] [PubMed] [Google Scholar]
- Sheldon I, Noakes D, Rycroft A, Pfeiffer D, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle 10.1530/rep.0.1230837. Reproduction. 2002;123:837–845. [PubMed] [Google Scholar]
- Sheldon IM, Dobson H. Postpartum uterine health in cattle. Anim Reprod Sci. 2004;82-83:295–306. doi: 10.1016/j.anireprosci.2004.04.006. [DOI] [PubMed] [Google Scholar]
- van Santen VL. Characterization of a bovine herpesvirus 4 immediate-early RNA encoding a homolog of the Epstein-Barr virus R transactivator. J. Virol. 1993;67:773–784. doi: 10.1128/jvi.67.2.773-784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, Fischer DP, Noakes DE, England GCW, Rycroft A, Dobson H, Sheldon IM. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology. 2007;68:549–559. doi: 10.1016/j.theriogenology.2007.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W, Broll H, Ehlers B, Buhk HJ, Rosenthal A, Goltz M. Genome sequence of bovine herpesvirus 4, a bovine Rhadinovirus, and identification of an origin of DNA replication. J Virol. 2001;75:1186–1194. doi: 10.1128/JVI.75.3.1186-1194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.