Abstract
An enantioselective total synthesis of (+)-cassiol is reported. The complex derived from Pd2(pmdba)3 and enantiopure t-BuPHOX ligand catalyzes enantioconvergent decarboxylative alkylation to generate the quaternary carbon stereocenter at an early stage. The overall synthetic strategy involves a convergent late-stage coupling of two fragments. The synthesis features a longest linear sequence of eight steps.
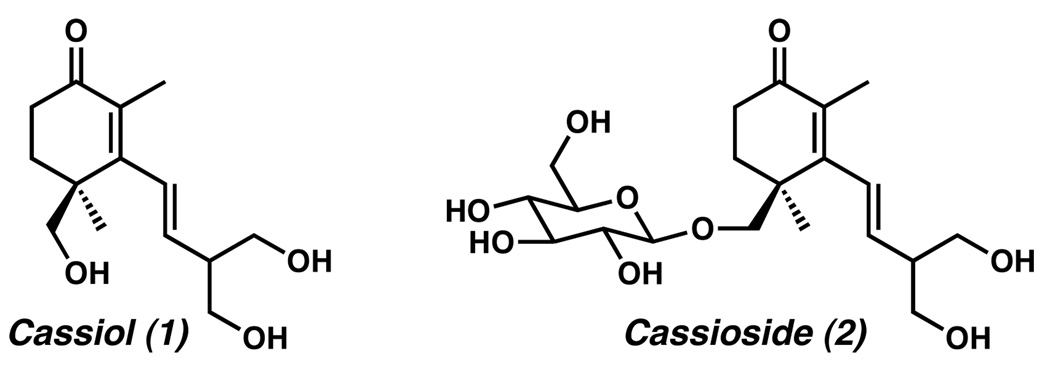
In 1988, Fukaya reported the isolation of (−)-cassioside (2) (Figure 1) from the stem bark of Cinnamonum cassia Blume.1 This glycosylated sesquiterpenoid exhibited potent antiulcerogenic activity in rats. The aglycon of (−)-cassioside, (+)-cassiol (1), demonstrated even stronger antiulcerogenic activity than observed with the glycosylated precursor. Given this useful biological property, (+)-cassiol (1) has attracted a great deal of attention from synthetic laboratories.2 Herein, we report an expedient enantioselective synthesis of (+)-cassiol with a longest linear sequence of eight steps.
Figure 1.
(+)-Cassiol (1) and (−)-cassioside (2).
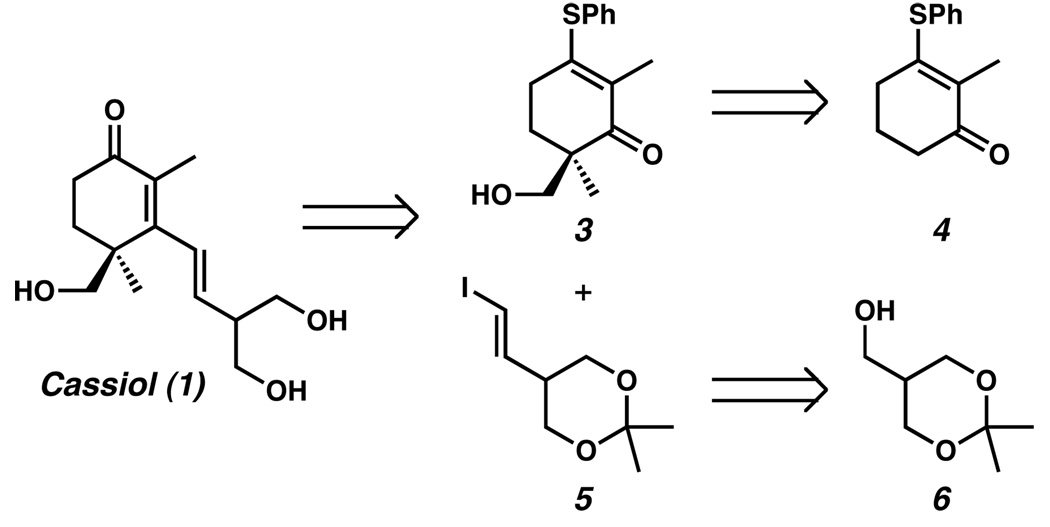
A principal challenge to the synthesis of (+)-cassiol (1) is the presence of an all-carbon quaternary stereocenter.3 Several total syntheses of cassiol have been reported; however, most have relied on chiral pool starting materials, or chiral auxiliaries.4,5 Few of these syntheses addressed the challenge of catalytic enantioselective quaternary carbon stereocenter generation. For example, successful catalytic enantioselective approaches have utilized Diels–Alder,6 intramolecular alkylidine insertion,7 and enzymatic8 reactions to form the quaternary carbon. We envisioned a different strategy9 wherein the key quaternary stereocenter would be installed through an enantioselective Pd-catalyzed allylic alkylation method recently developed in our laboratories.10 Our plan consisted of coupling two complex pieces (3 and 5, Scheme 1) in the final step of the synthesis through a Stork–Danheiser-type addition/rearrangement reaction.11 Precursors 3 and 5 would in turn be available from known vinylogous thioester 412 and primary alcohol 6,13 respectively.
Scheme 1.
Retrosynthetic analysis of (+)-cassiol (1).
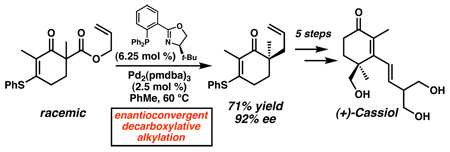
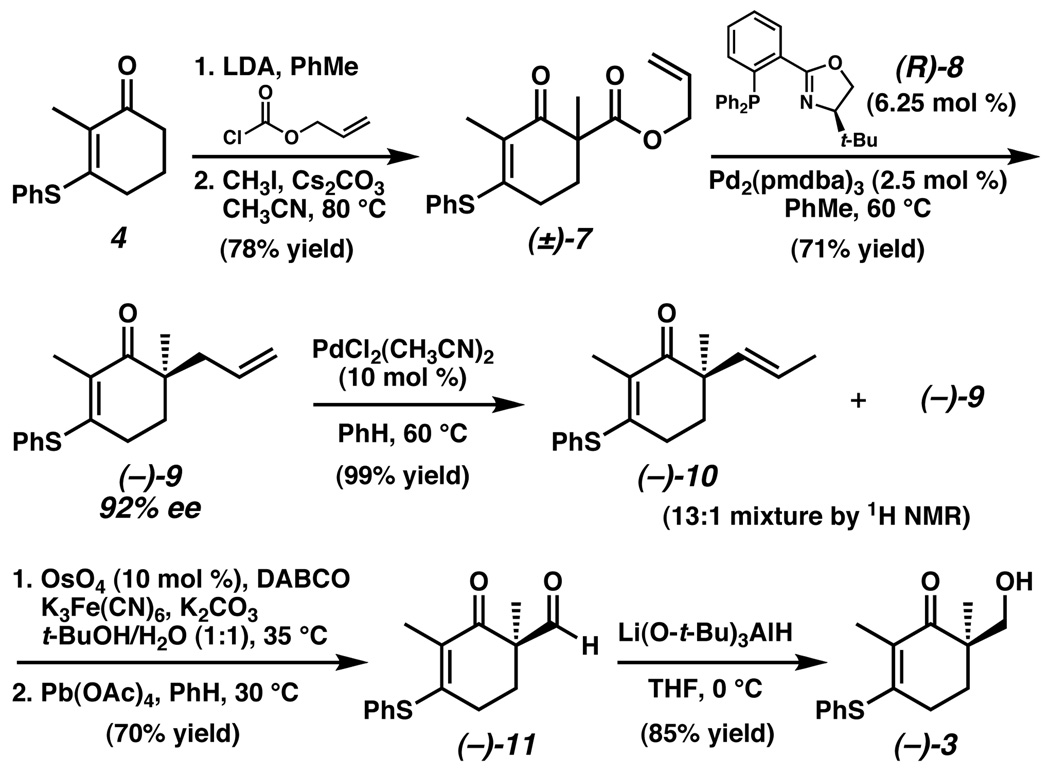
Our synthesis commenced with the deprotonation of vinylogous thioester 4 with LDA and acylation of the resulting enolate with allyl chloroformate (Scheme 2). Subsequent position-selective alkylation with iodomethane provided racemic β-ketoester 7 in 78% overall yield from 4. In the presence of the catalyst complex derived from Pd2(pmdba)3 and (R)-t-BuPHOX (8),14 β-ketoester (±)-7 was readily transformed into allyl ketone (−)-9 in good yield and excellent enantiomeric excess.12a Isomerization of the terminal alkene occurred upon exposure to catalytic PdCl2(CH3CN)2 in hot benzene, resulting in quantitative recovery of an inseparable 13:1 mixture of E-alkene 10 and starting material 9.
Scheme 2.
Enantioselective synthesis of alcohol (−)-3.a
apmdba = bis(4-methoxybenzylidene)acetone; DABCO = 1,4-diazabicyclo[2.2.2]octane.
In order to convert the propenyl sidechain of 10 into a hydroxymethylene unit, we sought to carry out an oxidative olefin cleavage reaction. This transformation, however, proved challenging. Competing oxidative reactions of the thioester moiety appeared to occur rapidly under ozonolysis conditions. Modified Lemieux–Johnson conditions (OsO4, NaIO4, 2,6-lutidine, dioxane/water)15 were also investigated, but led to a complex mixture of products lacking the desired compound. Both Upjohn dihydroxylation (OsO4, NMO, acetone)16 and Sharpless asymmetric dihydroxylation conditions (AD-mix-α or AD-mix-β, t-BuOH/H2O)17 resulted in slow and only partial conversion to the desired diol product.
Confronted by these difficulties, we considered possible opportumities to improve reactivity for our system. We wished to take advantage of the well-precedented rate-acceleration of amine additives in osmium-catalyzed dihydroxylation reactions,18 but we were reasoned that the bulky chiral ligands employed in the Sharpless protocol might hamper reactivity toward our sterically encumbered, enantiomerically enriched olefin. Warren, Wyatt, and coworkers had found DABCO to be a convenient achiral ligand for non-enantioselective dihydroxylations,19 and we reasoned that the smaller steric size of this ligand might improve the rate of oxidation in our system. This proved to be the case, and dihydroxylation proceeded smoothly with no evidence of undesired vinylogous thioester oxidation.20 Immediate exposure of the crude diol to Pb(OAc)4 furnished pure aldehyde (−)-11 in 70% overall yield for the two oxidative transformations.
Treatment of aldehyde (−)-11 with NaBH4 or NaBH(OAc)3 resulted in rapid reduction of both carbonyl groups present in the substrate. Fortunately, use of the bulkier reducing agent Li(O-t-Bu)3AlH circumvented the problem of overreduction and provided the desired alcohol (−)-3 in good yield. Chiral HPLC analysis of (−)-3 indicated that no significant erosion of enantiomeric purity had occured over the course of these steps.
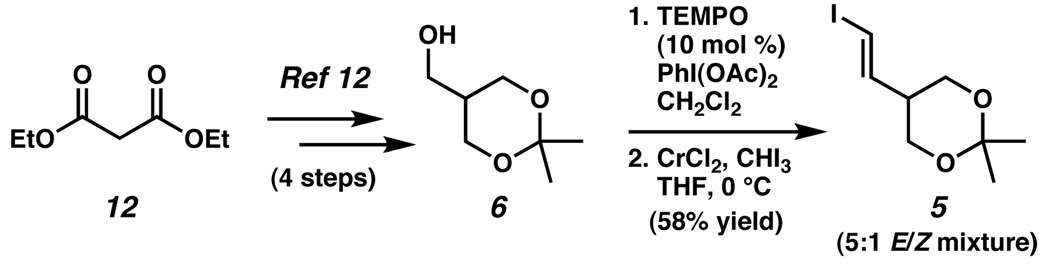
Turning our attention to the synthesis of vinyl iodide 5, we prepared alcohol 6 in four known steps from diethyl malonate.13 Catalytic oxidation of alcohol 6 to the corresponding aldehyde was accomplished with TEMPO and PhI(OAc)2 as the stoichiometric co-oxidant (Scheme 3).21 Takai olefination22 of the crude aldehyde to stereoselectively introduce the alkene functionality yielded vinyl iodide 5 in a 5:1 E/Z ratio.
Scheme 3.
Synthesis of vinyl iodide 5.a
aTEMPO = 2,2,6,6-Tetramethyl-piperidin-1-oxyl
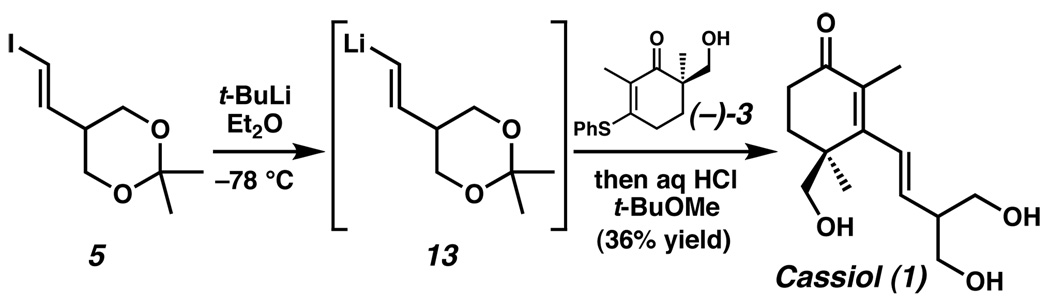
In the final step of the synthesis, lithium-halogen exchange of vinyl iodide 5 (2 equiv) through exposure to t-BuLi (4.25 equiv) in diethyl ether furnished vinyllithium 13. Addition of a solution of alcohol (−)-3 (1 equiv) to the solution of organolithium 13 and subsequent acid-catalyzed rearrangement and hydrolysis yielded (+)-cassiol (1).7 The spectroscopic data (1H NMR, 13C NMR, IR, HRMS, optical rotation) were identical to the reported data for natural 1.
In summary, we report a brief and convergent total synthesis of the antiulcerogenic natural product (+)-cassiol. Our route requires eight linear steps from vinylogous ester 4 and proceeds in 12% overall yield. Employing our recently developed enolate alkylation technology, the key quaternary carbon stereocenter was generated at an early stage. The versatile reactivity of the allyl group enabled installation of the hydroxymethylene unit present in the natural product through chemoselective oxidation and reduction reactions. Late-stage installation of the diol sidechain via Stork–Danheiser-type addition/rearrangement completed the synthesis. Other synthetic efforts featuring enantioselective enolate functionalization reactions are underway.23
Scheme 4.
Preparation of (+)-cassiol (1).
Acknowledgement
We thank Samantha R. Levine and Michael R. Krout (Caltech) for helpful discussions and experimental assistance. We thank the NIH-NIGMS (R01 GM080269-01), Caltech Amgen Scholars Program (undergraduate fellowship to KVP), Eli Lilly (predoctoral fellowship to JTM), Amgen, Bristol-Myers Squibb, Merck Research Laboratories, Abbott Laboratories, Boehringer-Ingelheim, and Caltech for financial support.
Footnotes
Supporting Information Available
Experimental details and NMR spectra of all intermediates. These materials are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Shiraga Y, Okano K, Akira T, Fukaya C, Yokoyama K, Tanaka S, Fukui H, Tabata M. Tetrahedron. 1988;44:4703–4711. [Google Scholar]
- 2.For an overview of previous syntheses of (+)-cassiol, see: Colombo MI, Rúveda EA. J. Braz. Chem. Soc. 1998;9:303–312.
- 3.For recent reviews of the synthesis of quaternary stereocenters, see: Cozzi PG, Hilgraf R, Zimmermann N. Eur. J. Org. Chem. 2007;36:5969–5994. Trost BM, Jiang C. Synthesis. 2006:369–369. Christoffers J, Baro A, editors. Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis. Weinheim: Wiley; 2005. Douglas CJ, Overman LE. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5363–5367. doi: 10.1073/pnas.0307113101. Denissova I, Barriault L. Tetrahedron. 2003;59:10105–10146.
- 4.For syntheses of (+)-cassiol using chiral auxiliaries, see: Takemoto T, Fukaya C, Yokoyama K. Tetrahedron Lett. 1989;30:723–724. Irie O, Fujiwara Y, Neomoto H, Shishido K. Tetrahedron Lett. 1996;37:9229–9232. Maiti S, Achari B, Banerjee AK. Synlett. 1998:129–130. Momose T, Toyooka N, Nishio M, Shinoda H, Fujii H, Yanagino H. Heterocycles. 1999;51:1321–1343. Colombo MI, Zinczuk J, Mischne MP, Rúveda EA. Tetrahedron: Asymmetry. 2001;12:1251–1253. Shen X, Wu Y-K, Zhang F. Chin. J. Chem. 2002;20:1439–1444. Colombo MI, Zinczuk J, Bohn ML, Rúveda EA. Tetrahedron: Asymmetry. 2003;14:717–725.
- 5.One total synthesis of (−)-cassioside (2) using a chiral auxiliary has also been reported, see: Boeckman RK, Jr, Liu Y. J. Org. Chem. 1996;61:7984–7985. doi: 10.1021/jo961613q.
- 6.Corey EJ, Guzman-Perez A, Loh T-P. J. Am. Chem. Soc. 1994;116:3611–3612. [Google Scholar]
- 7.Taber DF, Meagley RP, Doren DJ. J. Org. Chem. 1996;61:5723–5728. and references therein. [Google Scholar]
- 8.(a) Uno T, Watanabe H, Mori K. Tetrahedron. 1990;46:5563–5566. [Google Scholar]; (b) Trost BM, Li Y. J. Am. Chem. Soc. 1996;118:6625–6633. [Google Scholar]; (c) Miyaoka H, Kajiwara Y, Hara M, Suma A, Yamada Y. Tetrahedron: Asymmetry. 1999;10:3189–3196. [Google Scholar]
- 9.For a review of enantioselective methodologies inspired by target-directed synthesis, see: Mohr JT, Krout MR, Stoltz BM. Nature. 2008;455:323–332. doi: 10.1038/nature07370.
- 10. Behenna DC, Stoltz BM. J. Am. Chem. Soc. 2004;126:15044–15055. doi: 10.1021/ja044812x. Mohr JT, Behenna DC, Harned AM, Stoltz BM, editors. Angew. Chem., Int. Ed. 2005;44:6924–6927. doi: 10.1002/anie.200502018. Seto M, Roizen JL, Stoltz BM, editors. Angew. Chem., Int. Ed. 2008;47:6873–6876. doi: 10.1002/anie.200801424. (d) For a review of the development of the enantioselective Tsuji allylation in our lab and others, see: Mohr JT, Stoltz BM. Chem.-Asian J. 2007;2:1476–1491. doi: 10.1002/asia.200700183.
- 11.Stork G, Danheiser RL. J. Org. Chem. 1973;38:1775–1776. [Google Scholar]
- 12.(a) The use of a vinylogous thioester is important to the success of the subsequent enantioselective alkylation reaction, see: Levine SR, Krout MR, Stoltz BM. Org. Lett. doi: 10.1021/ol802409h. in press. (b) For the use of vinylogous thioesters in a related system and the preparation of 4, see: Trost BM, Bream RN, Xu J, editors. Angew. Chem., Int. Ed. 2006;45:3109–3112. doi: 10.1002/anie.200504421.
- 13.Bates HA, Farina J, Tong M. J. Org. Chem. 1986;51:2637–2641. [Google Scholar]
- 14.For the development of phosphinooxazoline (PHOX) ligands, see: Helmchen G, Pfaltz A. Acc. Chem. Res. 2000;33:336–345. doi: 10.1021/ar9900865. Williams JMJ. Synlett. 1996:705–710.
- 15.Yu W, Mei Y, Kang Y, Hua Z, Jin Z. Org. Lett. 2004;6:3217–3219. doi: 10.1021/ol0400342. [DOI] [PubMed] [Google Scholar]
- 16.VanRheenen V, Kelly RC, Cha DY. Tetrahedron Lett. 1976;17:1973–1976. [Google Scholar]
- 17.Kolb HC, Van Nieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483–2547. [Google Scholar]
- 18.Criegee R, Marchand B, Wannowius H. Justus Liebigs Ann. Chem. 1942;550:99–133. [Google Scholar]
- 19.Eames J, Mitchell HJ, Nelson A, O’Brien P, Warren S, Wyatt P. J. Chem. Soc., Perkin Trans. 1. 1999:1095–1103. [Google Scholar]
- 20.Other amine additives (DMAP, quinuclidine) were not as effective as DABCO
- 21.De Mico A, Margarita R, Parlanti L, Vescovi A, Piancatelli G. J. Org. Chem. 1997;62:6974–6977. [Google Scholar]
- 22.Takai K, Nitta K, Utimoto K. J. Am. Chem. Soc. 1986;108:7408–7410. doi: 10.1021/ja00279a068. [DOI] [PubMed] [Google Scholar]
- 23.For other synthetic applications of this reaction from our laboratory, see: McFadden RM, Stoltz BM. J. Am. Chem. Soc. 2006;128:7738–7739. doi: 10.1021/ja061853f. Mohr JT, Nishimata T, Behenna DC, Stoltz BM. J. Am. Chem. Soc. 2006;128:11348–11349. doi: 10.1021/ja063335a. Behenna DC, Stockdill JL, Stoltz BM, editors. Angew. Chem., Int. Ed. 2007;46:4077–4080. doi: 10.1002/anie.200700430. White DE, Stewart IC, Grubbs RH, Stoltz BM. J. Am. Chem. Soc. 2008;130:810–811. doi: 10.1021/ja710294k. Marinescu SC, Nishimata T, Mohr JT, Stoltz BM. Org. Lett. 2008;10:1039–1042. doi: 10.1021/ol702821j. Enquist JA, Jr, Stoltz BM. Nature. 2008;453:1228–1231. doi: 10.1038/nature07046.