Abstract
Acceleration of the wound healing process by using angiogenic peptides has been demonstrated previously. Here we used select laminin-111 peptides, A13 and C16, from the laminin α1 and γ1 chain, respectively, to test whether they are able to stimulate wound healing in a rat full thickness wound model. The 12-mer peptides C16 and A13 are highly angiogenic and bind to integrins αvβ3 and α5β1. We show that A13 increases wound reepithelialization as much as 17% over controls by day 4 and C16 increases coverage by 11%. Contraction of the treated wounds was increased as much as 11% for A13 and 8% for C16 at day 4. No differences were observed at day 7 with either peptide. The peptides also stimulated fibroblast migration in Boyden chamber assays. A13 increased cell migration as much as 2.4-fold on uncoated filters and as much as 16-fold on collagen type IV-coated filters over negative controls. Similarly, C16 also stimulated migration 1.8-fold on uncoated filters and as much as 12-fold on collagen-coated filters. A13 and C16 significantly decreased expression of the pro and active forms of matrix metalloproteinase 2 in foreskin fibroblasts indicating their role in collagen accumulation. We conclude that small bioactive angiogenic peptides can promote dermal wound healing and may offer a new class of stable and chemically manipulable therapeutics for wound healing.
Keywords: Laminin, basement membrane, wound healing, angiogenesis, peptides
Introduction
Wound healing involves a complex series of events, including an inflammatory phase, a proliferative phase, and a remodeling phase. These phases require several cell types, multiple signaling cascades, and various growth/ chemokine factors. Processes to enhance healing are of great interest because they not only increase the speed of the healing of “normal” wounds but they could also increase the healing of impaired wounds. One way to enhance healing is by accelerating the rate of angiogenesis, especially in chronic wounds that are characterized by significant tissue defects. Angiogenesis is necessary during the early wound healing process to remove debris, to supply essential nutrients and oxygen to the wound site, and to promote granulation tissue formation. Previous studies have shown that angiogenic factors are potent wound healing factors (Pierce et al., 1992, Malinda et al., 1997, Malinda et al., 1998, Obara et al., 2003, Zeng et al., 2007). Here we studied the effects of two previously identified angiogenic laminin-derived synthetic peptides on wound healing.
The laminins consist of a family at least 15 large trimeric basement membrane proteins. They are made up of a α, β, and γ chains. Laminin-111 is one of the best characterized laminins and is composed of the α1, β1, and γ1 chains (Aumailley et al., 2005, Ekblom et al., 2003, Malinda and Kleinman, 1996). It exists in a cruciform structure, is an important structural component of the basement membrane, and plays a role in tumor metastasis, cell spreading and attachment, and angiogenesis (Yamada, 1991, Kibbey et al., 1992, Nurcombe, 1992, Kleinman et al., 1993). Previous experiments have shown that the angiogenic properties of the protein can be further separated into discrete areas of the chains (Malinda et al., 1999, Ponce et al., 1999). Although several synthetic peptides showed strong angiogenic properties, we selected the two most potent ones for further study: A13 and C16. A13 on the α1 chain shares 66% conserved sequence homology with C16 on the γ1 chain and both use the same integrin receptors for cell attachment (Ponce and Kleinman, 2003). However, the two peptides have some differing levels of activity. Both peptides strongly promote cell adhesion, endothelial cell tube formation, and aortic vessel sprouting (Ponce et al., 1999, Malinda et al., 1999). While A13 stimulates endothelial cell migration at the 20 µg/ml concentration (Malinda et al., 1999), C16 peptide has much weaker migration activity (Ponce et al., 1999).
C16 and A13 peptides are highly conserved among the laminin chains. The γ1 chain is present in 10 of 15 laminins while the α1 chain is present in 2 of 15. A homologous, active form of A13 is also present on the α2, α4, and α5 chains. This means that all but two of the identified 15 laminins have either C16 or A13 sequences, and 8 laminin isoforms have both. The conservation of these peptide sequences across laminin isoforms suggests they are important in cell processes.
The role of the laminins during wound healing is not clear. Several studies have demonstrated that keratinocyte migration is necessary during human wound reepithelialization (Stenn and DePalma, 1988),(Olerud et al, 2000). Although laminin-322 is deposited as a part of a provisional matrix during wound healing, its role in keratinocyte migration and wound healing has been controversial (Sullivan et al, 2007). Using a diabetic wound healing animal model expressing reduced laminin-322 levels and poor keratinocyte migration, Sullivan et al. did not find any significant improvement in the migration of wound keratinocytes after topical laminin-322 treatment. Alternative evidence that laminins play a role in wound healing comes from work with matrikines, peptides liberated by partial proteolysis of extracellular matrix macromolecules. The epidermal growth factor-like repeats in laminin are examples of matrikines. At these sites, laminin binds to epidermal growth factor receptors where it acts to enhance fibroblast migration (Panayotou et al., 1989) (Tran et al., 2005). In the case of laminin-5, this activity appears to occur only following cleavage by matrix metalloproteinases (MMPs) (Giannelli et al., 1997). During wound healing, laminin-5 is expressed by keratinocytes at the leading edge of the dermal-epidermal junction (Amano et al., 2004). This correlates in time with keratinocyte migration and MMP-2 expression (Moses et al., 1996). This and other evidence suggest that the binding of laminin-5 to the epidermal growth factor receptor during wound healing provides pro-migratory tracks within the wound healing edges for migrating and proliferating keratinocytes (Tran and Wells, 2004). Since angiogenic endothelial cells, fibroblasts, and migrating wound keratinocytes express integrin molecules, α5β1 and αvβ3, that bind to our laminin-111 active peptides, we decided to investigate their role in wound healing.
Materials and Methods
Cell Culture
Foreskin fibroblasts were isolated from fresh human foreskins. Briefly, foreskins were washed 3x in PBS (Gibco, Invitrogen, Grand Island, NY) with gentamicin (Gibco, Invitrogen, Grand Island, NY) and fungizone (Gibco, Invitrogen, Grand Island, NY) then 3x with DMEM high glucose (Gibco, Invitrogen, Grand Island, NY) containing gentamicin and fungizone, and finally 3x in plain DMEM. The foreskin was then placed on a 100mm culture dish (BD Biosciences, Bedford, MA), excess tissue and fat were removed, and the foreskin was cut into 3mm square pieces. The pieces were transferred to a clean culture dish epidermal side up and placed in the CO2 incubator for approximately 1 hour without media. DMEM with 20% fetal bovine serum (BioWhittaker, Cambrex Bio Science Rockland, Inc., Baltimore, MD) was added in drops around each tissue so that a thin layer of media surrounded each tissue square and the dish was incubated for 3 days. The medium was partially changed and fresh medium was added after three days. This process was continued until fibroblasts had grown out from the tissues and were ready for subculture. Foreskin fibroblasts were cultured in DMEM with high glucose and 10% fetal bovine serum.
Migration assays
Foreskin fibroblast migration assays were conducted in Boyden chambers using either uncoated 12-mm pore PVP-free filters or filters coated with a 0.1 mg/ml solution of collagen IV (Trevigen, Gaithersburg, MD) as previously reported (Malinda et al., 1997). Each condition was assayed in triplicate wells at least three times unless otherwise indicated.
In vivo wound assay
Six full thickness 8 mm punch biopsy wounds were made on the dorsal surface of each rat as previously described (Bhartiya et al., 1992, Sidhu et al., 1996). A13 and C16 (synthetic laminin peptides, produced by the CBER Facility for Biotechnology Resources FDA, Bethesda, MD containing the NH2-terminal amide) were each applied topically at the time of wounding. A range of 2.5 to 25 µg in 50 µl treatments was applied at the time of wounding and again after 24 h to each wound. Controls for topical treatment were three rats that received identical amounts of dH20 at the time of wounding and at 24 h. Separate rats were used for controls to prevent mixing of peptide and control through potential peptide transfer along facial planes, through any leakage or via the circulation. At days 4 and 7 post-wounding, each wound was collected and fixed in 10% buffered formalin. The samples were sectioned, and sections from the middle of each wound were stained with hematoxylin and eosin or Masson’s Trichrome (American Histolabs, Gaithersburg, MD). This experiment was performed three times using three rats for each test condition except C16 at 25 µg in 50 µl which was performed twice with three rats each and A13 at 25 µg in 50 µl which was performed once with three rats.
Morphometric measurements
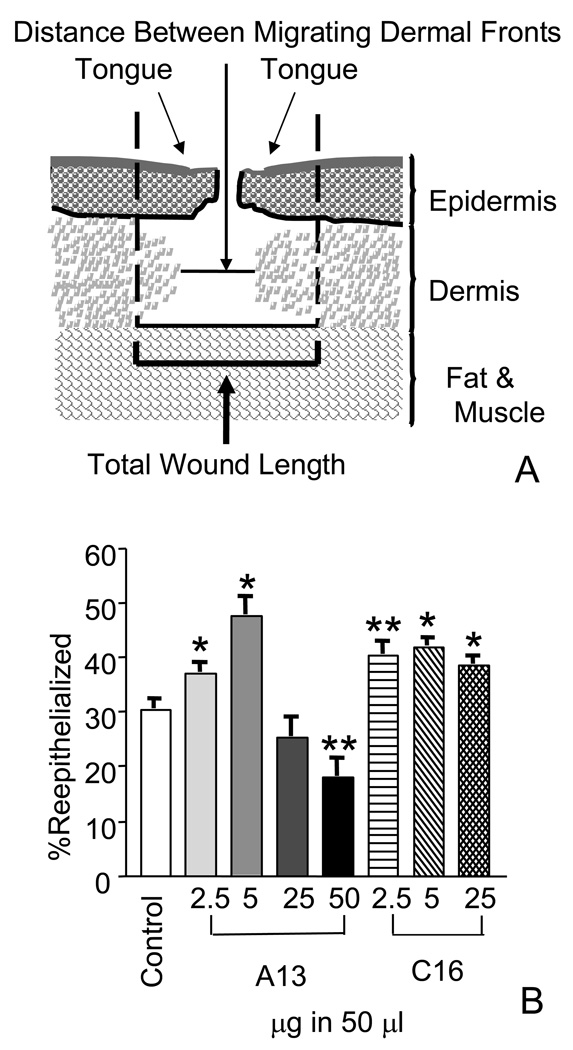
Histologic sections and IPLab imaging software (BD Biosciences, Bedford, MA) were used to measure the reepithelialization and the contraction of the wound. Epithelial migration was determined by measuring the lengths of the tongues of epithelium migrating from either side of the wound over the wound bed from the zone of proliferation at the margin of the uninjured and wounded skin (Fig 1A). This was compared to the length of the total wound to generate the “percent reepithelialized”. The equation used was Tongue + Tongue /Total Wound Length ×100 = % reepithelialized. Contraction as measured by the percent granulated was also determined. It was calculated as the total wound size as compared to the granulating dermal layer as visualized by Masson’s Trichome staining. The equation used was: 1-Distance Between Migrating Dermal Fronts/Total Wound Length ×100=% granulation.
Figure 1.
Laminin-derived peptides increased reepithelialization when applied topically. A) Diagram of a full thickness punch wound. The reepithelialization as measured by % coverage and contraction as measured by % granulation were used to calculate wound closure. % reepithelialized= Tongue + Tongue /Total Wound Length ×100. % granulated = 1- Distance Between Migrating Dermal Fronts/Total Wound Length ×100 B) Quantitation of wound reepithelialization in wounds harvested at day four. Wounds were treated with peptides ranging from 2.5 to 50 µg in 50 µl. Treatment with A13 at the 2.5 and 5 µg in 50 µl level, wounds showed a significant increase in the increase in epithelial coverage of the wound, 7% (±2.5) and 17% (±3.8), respectively. At the higher dose of 50 µg in 50 µl, A13 showed a statistically significant level of inhibition of reepitheilization at 12% (±3.8). C16 peptide treatment also increased reepithelialization in topical treatments. At all doses, a significant increase in the levels of reepithelialization was observed. At 2.5 µg, a 10% (±2.9) increase in coverage was observed. At 5 µg, coverage increased to 11% (±2.4) and at higher doses, 25 µg in 50 µl, an 8% (± 2.2) increase in coverage was observed. Measurements are expressed as the mean % increase ± SEM. (*p≤0.008, **p≤0.02).
Zymograms
Early passage HUVECs (p 4–5) were grown in complete media until 60–70% confluent. The cells were washed three times with PBS and incubated with increasing doses of peptide 0–200 µg/ml in endothelial cell serum-free media (Gibco, Invitrogen, Grand Island, NY) supplemented with MITO+ serum extender (BD Biosciences, Bedford, MA). After 6 hrs, conditioned media were collected, and the cells were lysed in mPER buffer (Pierce, Rockford, IL) containing Complete Protease Inhibitor Cocktail (Roche, Indianapolis, IN). Media were concentrated using Millipore UltraFree 100K concentrators. Concentrated samples were normalized based on protein determinations (Pierce microBCA protein assay, Pierce, Rockford, IL), and electrophoresed on 10% zymogram gels containing gelatin (BioRad, Hercules, CA). Gels were incubated with zymogram renaturation buffer for 30 min (BioRad, Hercules, CA) and developed with zymogram development buffer (BioRad, Hercules, CA) overnight at 37°C. The reaction was stopped by staining the gels with 0.5% Coomassie blue, 50% methanol, and 10% acetic acid for 1 hr. Gels were destained using 50% methanol and 10% acetic acid. Each experiment was repeated at least three times.
Note: Extending the incubation time of HUVECs with the peptides to test the possibility of altered MMP expression was not possible since HUVEC do not survive longer than four days under SFM conditions.
Zymograms performed using human foreskin fibroblasts (p 7–14) were similarly done, except that the cells were grown in DMEM, high glucose containing 10% fetal bovine serum (Gibco, Invitrogen, Grand Island, NY). Equal volumes of unconcentrated foreskin media samples were run on the zymogram gels. Quantitation of a representative gel was performed using Gel Quantitation Software by ImageJ (Public Domain Software, NIH). Briefly, the image of the gel was inverted, each band was identified, a peak was generated and the software was used to calculate the area under the peak.
Results
Reepithelialization and contraction are enhanced in peptide treated wounds
A full thickness wound healing model was used to assess the effects of two synthetic laminin peptides on wound healing. This model has been previously used to measure two parameters of wound healing: the reepithelialization and the contraction of the wound (Malinda et al., 1997, Malinda et al., 1998). We first studied the topical effect of increasing doses of peptides A13 and C16 on wound reepithelialization, and the results were compared to their vehicle controls. Wounds treated with A13 at 2.5 µg showed a 7% (±2.5) (p=0.0081, n=34) increase in the percent coverage of the wound as compared to its control (Fig. 1B). At dosage of 5 µg, a 17% (±3.8) (p≤0.0001, n=51) increase in coverage was observed. When levels of A13 were increased 10-fold, reepithelialization was retarded. This was apparent at 50 µg where a 12% (±3.8) (p=0.017, n=5) decrease in wound coverage compared to its control was observed.
Increased wound coverage was also seen with peptide C16 at all concentrations tested. A significant coverage of 10% (±2.9) (p=0.0011, n=35) was observed when wounds were treated with 2.5 µg of C16 (Fig. 1B). When the dosage was doubled, an 11% (±2.4) (p≤0.0001, n=39) increase in wound coverage was observed. At the higher peptide dose, 25 µg, wound coverage remained elevated at 8% (± 2.2) (p=0.0009, n=18) over the control (Fig. 1B). Altogether, these results demonstrate the ability of both peptides to promote wound healing. A13 showed increased reepithelialization by as much as 17% above its control wounds, and C16 showed consistent reepithelialization ranging between 8–11% above controls at all doses of peptide tested.
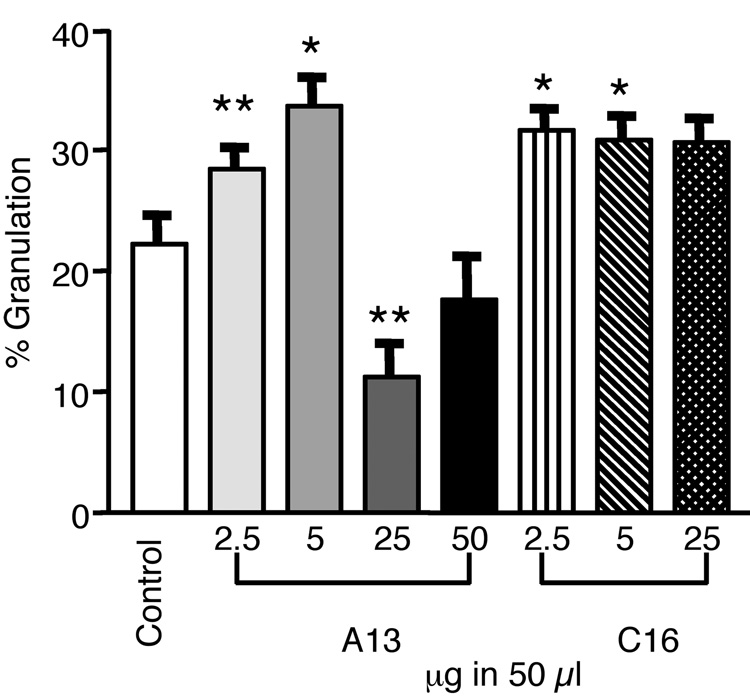
The effect of peptide treatment on wound contraction, as measured by the amount of granulation tissue within the wound, was also investigated. Our results demonstrated that at day 4, A13 promoted a 6% (± 2.6) (p=0.017, n=34) increase in granulation (Fig. 2) over control at the lowest peptide dose tested, 2.5 µg. The level of granulation paralled that observed with reepithelialization. When the A13 dose was doubled, the amount of granulation observed at the wound almost doubled to 11% (±3) (p=0.0002, n=51). However, at higher doses of A13, a different effect was observed. At 25 µg, an 11% (±3) (p=0.0098, n=5) decrease in granulation below the control level was detected. The highest dose tested (50 µg) also showed a slight decrease in granulation as compared to the control, but the amount was not found to be statistically significant (Fig. 2). The effect of C16 on wound granulation was also studied. We found that treatment with 2.5 µg of C16 peptide resulted in a 9% (±3) increase in granulation (p=0.0006, n=35) over the control (Fig. 2). This increase remained constant at higher peptide concentrations. Granulation remained at 9% (±3) (p=0.0022, n=39) at the 5 µg dose and showed a slightly lower increase of 8% (±3) (p=0.0024, n=18) after an initial 10-fold peptide increase (25 µg dose) (Fig. 2). These data demonstrate that A13 and C16 can also promote significant tissue granulation. A13 stimulated granulation levels ranging between 6 and 11% at the two lowest peptide amounts tested, and C16, again, showed a consistent response of 9% at all doses.
Figure 2.
Treatment with laminin-derived peptides enhanced granulation when applied topically. Wounds were harvested at day 4. Wounds were treated with 2.5 to 50 µg in 50 µl. A13 treatments showed significant increases in granulation. At 2.5 µg, A13 showed a 6% (± 2.6) increase. Granulation increased at the 5 µg dose to 11% (±3). At higher doses of A13, a decrease was observed. At 25 µg, an 11% (±3) decrease in granulation was observed. The effect at the 50 µg dose was not statistically significant. C16 treatments resulted in 9% (±3) increase in granulation at 2.5 µg and 5 µg doses. A slightly lower increase of to 8% (±3) occurred at the 25 µg dose. Measurements are expressed as the mean % increase ± SEM. (*p≤0.002, **p≤0.02).
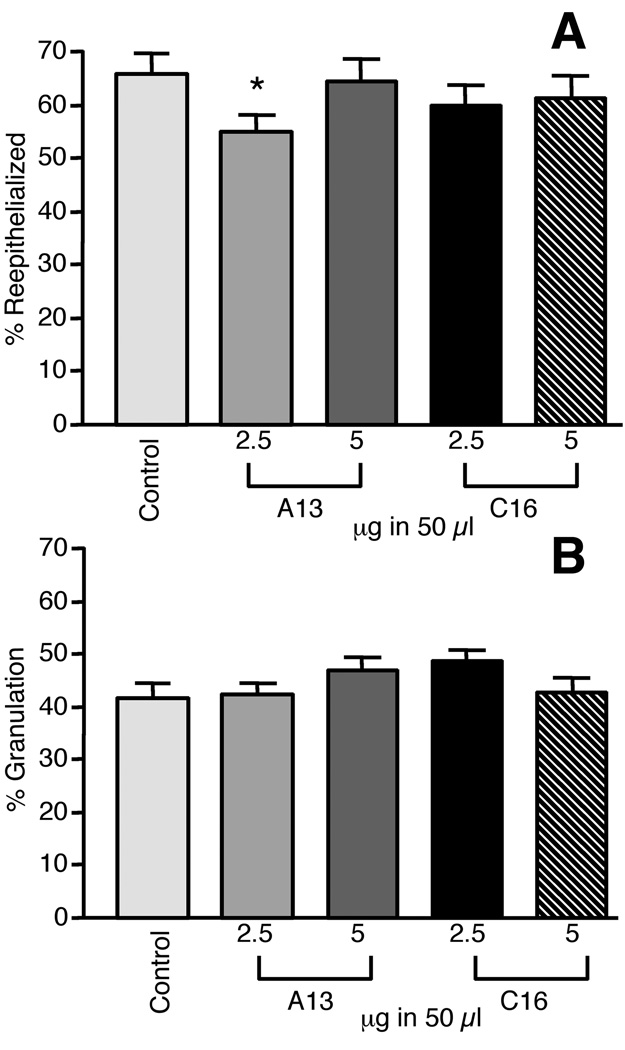
By day 7, there were no differences between the peptide-treated wounds and controls in terms of epithelialization and granulation tissue (Fig. 3A and B). Treatment of wounds with peptide C16 at the two lowest doses (2.5 and 5 µg) did not show significant deviation from baseline reepithelialization. A13 treated wounds showed what appeared to be an inhibition of reepithelialization, 10% (± 5) (p=0.05 n=17), at the 2.5 µg dosage. This result was not observed at the higher dose of 5 µg (Fig. 3A). When granulation was assessed at day 7, we did not find any significant differences as compared to their controls after treatment with either A13 or C16 at the 2.5 or 5 µg doses (Fig. 3B).
Figure 3.
A: Comparison of laminin-derived peptides and control on wound healing at day seven. Dosages of 2.5 and 5 µg in 50 µl of A13 and C16 were tested. Only A13 at the 2.5 µg dose showed a 10% (± 5) inhibition of reepithelialization. Other treatment had no significant effect. B. Treatment with peptides at the 2.5 and 5 µg in 50 µl doses had no significant effect on granulation. Treatment with A13 and C16 had no statistically significant difference in the granulation of the wounds. Measurements are expressed as the mean % increase ± SEM. (*p=0.048)
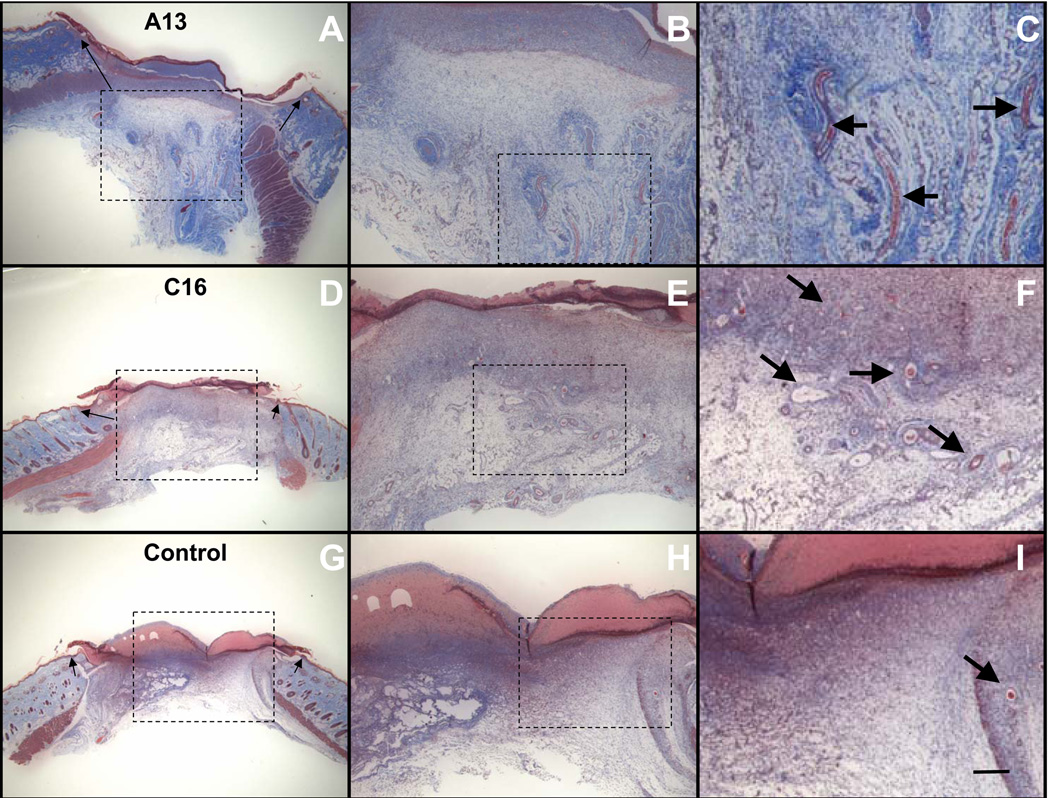
Since previous experiments had demonstrated the angiogenic abilities of A13 and C16 (Malinda et al., 1999, Ponce et al., 1999), we also examined if their presence increased angiogenesis in the wound area. As shown in figure 4 (B, C, E, F), we observed an increase in capillary ingrowth in the A13- and C16-treated wounds as compared to the control at day 4 (Fig. 4 H and I). These are examples of wounds treated at the 5 µg doses for A13 and C16. Treated wounds contained more and larger vessels than the controls. This trend was also observed in the A13- and C16-treated wounds at the 2.5 µg dose (data not shown). Vessels were large and filled with red blood cells while the controls had smaller, less numerous vessels if they were present at all (H, I). Peptide-treated wounds also appeared to show an increase in collagen deposition as compared to the control at day 4 and an increase in fibroblasts. Collagen deposition was visually assessed by Masson Trichrome staining of the wound tissue. An increase in blue staining fibers was observed in the A13- and C16-treated wounds as compared to the control. This was observed at the 5 µg and 2.5 µg doses. Angiogenesis and granulation were not assessed at the 25 µg dose.
Figure 4.
Laminin peptides enhanced wound healing in rats. Masson’s Trichrome stained sections of 4-day punch wounds show collagen in blue and endothelial cells in red: Arrows on Panels A, D and G indicate the edge of the original wound. Boxed areas of the wound show areas magnified on the following image. A) Wound treated with laminin-derived peptide A13 (5 µg/50 µl). Epithelial tongues are visible migrating over the dermis as reepithelialization proceeds. B–C) Granulation tissue is infiltrated with cells and a moderate number of vessels (see arrows). Enhanced collagen production was present in the treated sections (dark blue staining fibers). D) Wound treated with laminin peptide C16 (5 µg/50 µl). E–F) Here areas of granulation show neovascularization (see arrows) and enhanced numbers of fibroblasts. Collagen levels also appear elevated in the treated wounds (blue staining fibers). G) Vehicle control. H–I) Control shows few cells and little vascularization (see arrow). Magnification: A,D,G: (0.96x), B,E,H: 2.4x, C,F,I: 4.8x bar size: 1 mm
Laminin peptides promote cell migration
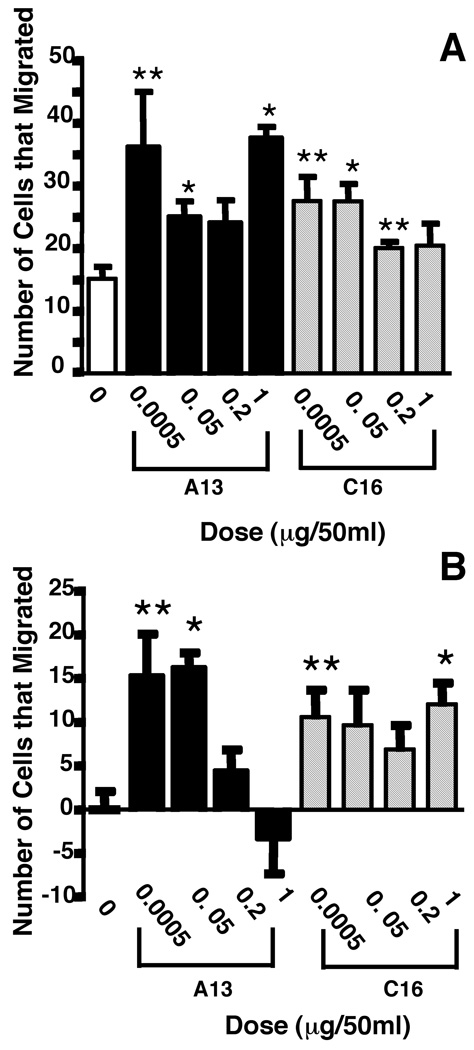
Cell migration is important in wound healing. To determine if laminin-derived peptides can influence the migration of fibroblasts which are known to be involved in wound healing, we performed cell migration experiments in Boyden chambers with human foreskin fibroblasts. Previous data had shown that A13 significantly stimulated the migration of endothelial cells on collagen type IV-coated filters (Yamada, 1991). In this study, we investigated the effect of A13 and C16 on the migration of fibroblasts on plain filters and on filters coated with collagen type IV. Our results indicate that A13 stimulated the migration of fibroblasts 2.4-fold over background levels in the presence of only 0.0005 µg/50 µl (p=0.04) of peptide on plain, uncoated filters (Fig. 5A). A 1.6-fold increase in cell migration was also observed with 0.05 µg/50 µl of A13 (p=0.0086). At higher doses, 0.2 µg/50 µl, we also observed a 1.6-fold migration increase over controls. The results tend towards significance with the Welch’s test (p=0.0698) but it is not significant unless the Unpaired t-test is used (p=0.0477). At much higher doses, 1 µg/50 µl, a 2.5-fold increase in migration was again observed (p=0.0002). When the experiment was performed on coated filters (Fig. 5B), the number of cells that migrated as compared to the negative control increased approximately 15-fold with 0.0005 µg/50 µl (p=0.0146) of A13, and the increase was 16-fold for the 0.05 µg/50 µl dose (p< 0.0001). At higher concentrations, we did not observe any significant migration as compared to their controls at 0.2 µg/50 µl, and the migration appeared to be slightly inhibited at 1 µg/50 µl (p=0.5048).
Figure 5.
Foreskin fibroblast migration was stimulated in the presence of laminin peptides. A) A13 in the lower wells of a Boyden chamber stimulated migration above background levels on uncoated filters. There was a 1.6- to 2.5-fold increase in migration above baseline depending on the concentration of peptide tested. C16 peptide tested in the Boyden chamber also stimulated migration above baseline levels. A 1.3- to 1.8-fold increase was seen in migration on uncoated filters. (*p≤0.009, **p≤0.04), B) Migration of foreskin fibroblasts on collagen-coated filters was enhanced. A13 added to the bottom well of the Boyden chamber caused a 15- to 16-fold increase in migration at the lower concentrations, however, tests at higher concentrations resulted in lower fold increases or at the highest a decrease in migration. C16 treatment of the media in the lower well caused an approximate 7- to 12-fold increase in migration. For this experiment all background levels of migration were subtracted. (*p≤0.004, **p≤0.015).
The effect of the angiogenic peptide C16 on the migration of human foreskin fibroblasts was also tested on uncoated and collagen type IV-coated filters. Similar to the results observed with peptide A13 on uncoated filters, C16 showed a 1.8-fold increase in migration at the 0.0005 µg/50 µl (p= 0.01) and 0.05 µg/50 µl (p=0.0028) peptide doses (Fig. 5A). The two highest amounts of peptide (0.2 and 1 µg/50 µl) also showed a similar increase in cell migration over their control (1.3 and 1.7 fold-increase, respectively), but due to differences in their variability, the results were not statistically significant. In contrast, fibroblast cell migration increased approximately 10-fold, as compared to its control, with 0.0005 µl/50 µl of C16 on collagen type IV-coated filters (p=0.0127) (Fig. 5B). We obtained a similar 10-fold increase in the migration of fibroblasts with a 0.05 µg/50 µl dose (p=0.056). At higher C16 concentrations, we also observed a 7 and 12-fold increase in the number of migrating fibroblasts with peptide doses of 0.2 and 1 µg/50 µl, respectively. However, due to variation between samples, the numbers approached but did not quite reached statistical significance, except at the 1 µg/50 µl dose where migration was 12-fold (p=0.0035). The migratory response of fibroblasts to A13 and C16 suggest that the peptides can help mobilize fibroblasts, the primary collagen secreting cell within the wound, a requirement necessary for wound healing. Furthermore, they also indicate that the effects on wound granulation could be due in part to fibroblast migration.
Matrix metalloproteinase processing and production are altered by peptide treatment
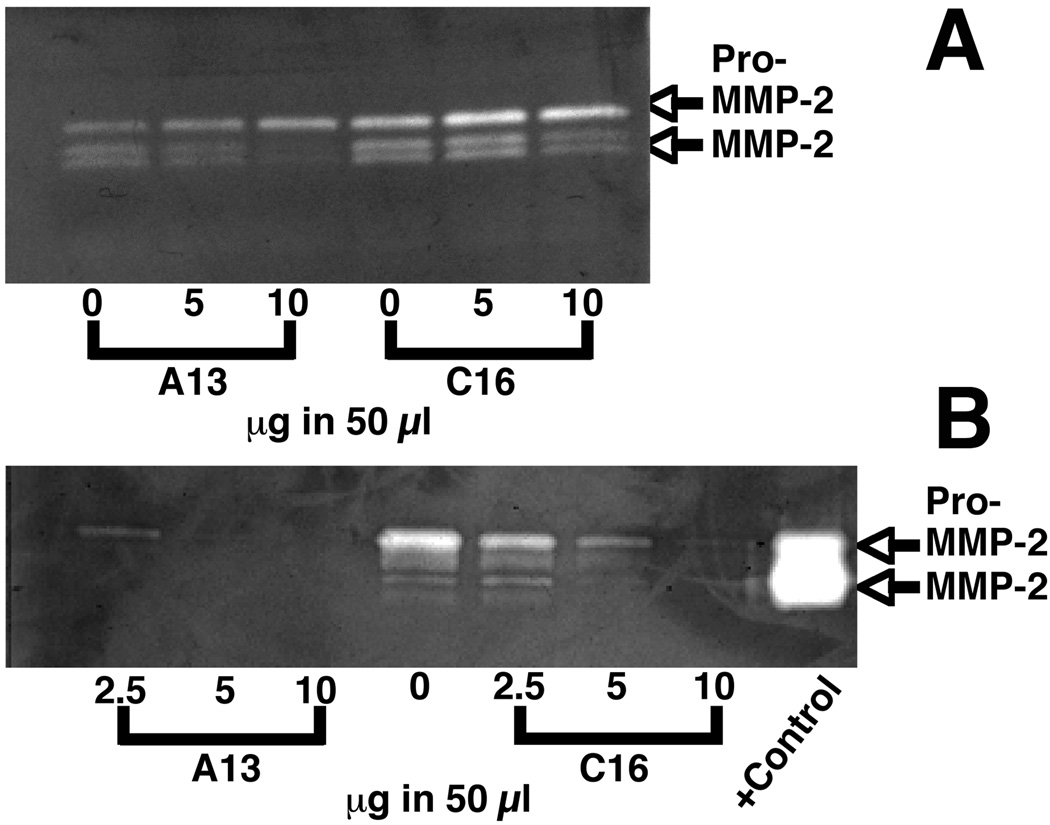
Matrix metalloproteinases are involved in blood vessel formation via degradation of the underlying basement membrane and in wound healing with the remodeling of the collagen and other matrix proteins present in the wound. We determined the effects of peptide treatment on two of the cell types active in angiogenesis and in wound healing: endothelial cells and fibroblasts. HUVECs were treated with either A13 or C16 at several doses. A13 decreased the processing of pro-MMP-2 to active MMP-2 at the 10 µg/50 µl dose (Fig 6A). This was not observed at the lower 5 µg/50 µl dose. C16 peptide showed a similar activity. Pro-MMP and MMP-2 activity was at the control level at the 5 µg/50 µl dose and MMP-2 activity was decreased at the 10 µg/50 µl dose. Quantitation studies support that levels of MMP expression are not changing rather that levels of pro-MMP-2 to MMP-2 processing are decreased at the highest peptide doses. This was observed in two of three zymograms.
Figure 6.
Peptide treatment decreases MMP-2 activity. A) Representative zymogram of media collected from HUVECs cultured with for 18 hrs with 5 and 10 µg/ in 50 µl of A13 or C16. Pro-MMP-2 levels were slightly decreased over control levels at the 5 and 10 µg/50 µl doses of C16 and at the dose 10 µg/50 µl of A13. Processed MMP-2 was decreased as compared to controls at the 5 and 10 µg/50 µl doses of C16 and steadily decreased as the concentration of the peptide A13 was increase. This occurred in two of three cases. B) Representative zymogram of media collected from foreskin fibroblasts cultured for 18 hrs with 2.5, 5, and 10 µg/ in 50 µl of A13 or C16. Treatment with A13 decreased MMP-2 levels below background even at the lowest concentration tested. C16 treatment showed an incremental decrease of MMP levels with trace amounts of MMP detectable at the highest concentration. This result was repeated 3 times.
We also tested the effects of peptide treatments on MMPs produced by human foreskin fibroblasts. With C16 we observed that there was a significant decrease in the levels of pro and active MMP-2 produced. 2.5, 5 and 10 µg/50 µl doses all decreased MMP2 significantly below baseline levels (Fig 6B). The largest decrease in MMP levels was observed at the 5 and 10 µg/50 µl dose, with MMP expression at the 10 µg/50 µl being only faintly visible. Bands were observed at the 72 kDa level for pro-MMP-2, and faint bands at lower molecular weights representing the activated forms of MMP-2. Quantitation showed a 51% decrease in pro-MMP-2 levels at the 2.5 µg/50 µl dose as compared to the baseline levels. There was a 87% decrease at the 5 µg/50 µl dose and a 98% decrease at the 10 µg/50 µl dose. Processed MMP-2 showed similar decreases. Cells cultured in the presence of A13 also showed highly significant decreases in activity over cells cultured in unsupplemented media. Only a faint band of pro-MMP-2 was visible even at the 2.5 µg/50 µl dose. Quantitation showed a 91% decrease in pro-MMP-2 levels at the 2.5 µg/50 µl dose and a 99% or greater decrease at the 5 and 10 µg/50 µl doses. Quantitation of processed MMP-2 also showed a similar decrease. These observations demonstrate that overnight treatment with peptide decreases production of pro and processed MMP-2. MMP-9 was secreted from either the HUVECs or fibroblasts. Longer incubation times with fibroblasts showed no differences on the zymograms indicting that maximum MMP expression had been achieved at the timepoints we chose.
Discussion
Angiogenic molecules enhance wound healing. In this paper, we assessed the ability of A13 and C16 to stimulate wound healing in an in vivo full thickness wound healing assay. Studies with A13 showed that the peptide significantly stimulated wound closure at 4 days post wounding with the maximum level of stimulation observed at 5 µg. Both granulation and repithelialization were stimulated most at this concentration. However, wound healing was inhibited at higher doses (5–10X larger). A similar effect was observed in the fibroblast migration assays on collagen. Migration on collagen was significantly stimulated at the 0.0005 and 0.05 µg /50 µl dose, but at the higher dose of 0.2 µg/50 µl migration was no longer statistically significantly stimulated over background. At 1 µg/50 µl migration was inhibited. A different effect was observed on plain filters. Here fibroblast migration was stimulated above background levels for 0.0005 µg/50 µl through 1 µg/50 µl with migration levels increasing at the higher dose. The ability of fibroblasts to migrate on plain filters demonstrates that migration is not stimulated by contaminates. Differences in migration between collagen and uncoated filters may be due to increased interactions of the fibroblasts with the basement membrane.
These observations are consistent with those reported earlier (Malinda et al., 1999). Angiogenic activity was observed at the 5–10 µg/50 µl in tube forming assays, at 5–20 µg/50 µl in aortic sprouting, and at 1 µg/50 µl for HUVEC migration. Tube formation and aortic sprouting were stimulated at all concentrations tested while migration showed a bell shaped dose response curve – higher and lower doses exhibited less significant activity. It is “normal” in migration assays for active compounds to become inactive at higher doses-perhaps due to the loss of the attractive gradient. This bell shaped activity is similar to what is observed with the in vivo experiments. Higher doses appear to lead to an inhibition of wound healing. These results suggest that the migratory activity of the cells is more readily affected at higher peptide concentrations. The exact reason for this effect is not clearly understood, but we cannot rule out peptide aggregation. We have observed that the peptides can gel under certain conditions that may lead to the inability to access active sites or to compete with native active sites. Differences observed between plain and collagen-coated filters suggest that the peptides increase the fibroblasts’ ability to migrate over a matrix. This could also be the reason why we also observed increased wound coverage and granulation tissue formation. Despite the differences observed between the lowest and highest peptide amounts used, both peptides can promote reepithelialization of the wounds at 4 days.
Wound healing with the C16 laminin peptide occurred in a somewhat different manner. There was no inhibitory activity at high peptide concentrations. Reepithelialization and granulation tissue formation occurred at virtually the same levels at all doses tested at day 4. Foreskin fibroblast migration on plain filters showed a slight decrease at higher concentrations (0.2–1 µg/50 µl) while migration on collagen-coated filters did not show much change between the lowest and highest dose tested.
Wound healing was not significantly accelerated at the seven day time point. This most likely was due to the treatment schedule used. Wounds were treated twice: the day of injury and the following day. Once scab formation occurred (48 hours), topical treatment ceased since the peptide was hard to deliver to the wound. Future experimentation with an alternative application method that could allow the peptide to reach the wound could yield enhanced healing at day seven for C16 and A13. In addition, in an earlier study (Wysocki et al., 1999) on acute and chronic wounds, we showed that in acute wounds there is a decrease in MMP-9 expression on day 4 to about half that seen at day 1 and that at day 4 there is an increase in the expression of plasminogen activator inhibitor-1. Thus, the presence of inhibitors at day 4 begins to play a role in the dramatic changes that may have been seen earlier and quickly reaches a plateau phase in acute wounds. This type of inhibitor effect could also be influencing the wound healing we observed in this study.
A13 and C16 treatments of HUVECS, depending on dose, had minimal effects or inhibited MMP-2 activity. Since MMPs are necessary for the remodeling that occurs during angiogenesis and wound healing, this could help explain the differences we observed with peptide treatment when higher levels of peptide are used. These observations are consistent with wound healing being stimulated the most at the 2.5 and 5 µg/50 µl dose for A13 while at higher peptide concentrations, healing is delayed. Studies by Okada et al. (Okada et al., 1997) in a rat incisional model show that MMP-2 and 9 increase the first day after wound healing. Pro-MMP-2 levels reach a maximum on day 5 with MMP-2 also appearing elevated. MMP-2 levels tapered off through day 14. Similar trends were observed in the excisonal rat (Soo et al., 2000) and mouse (Madlener et al., 1998) models. The timing of maximal MMP-2 expression would correspond to an enhanced level of MMP-2 at day 4 when we took our first time point in our wound healing model. Okada et al. also found that levels of MMP-2 were highest in the granulation tissue. We did not see a reproducible elevation in MMP-2 in fibroblasts with peptide treatment. The decrease we observed clearly is independent of the ability of the fibroblasts ability to migrate into the wound. Migration was enhanced by peptide treatment in vitro and in vivo and granulation tissue formation and remodeling was increased in treated wounds. The lack of pro and processed MMP-2 observed could potentially be due to the in vitro nature of the cells, in vitro nature of the experiment or the source of cells. Further experiments are necessary to address this issue.
Previous studies have shown that different laminin isoforms can play a role in wound healing (Nguyen et al., 2000, Blinova et al., 1997). Laminins-1, 5, 6 and 10 (laminin 111, 332, 311 and 511), are expressed in the skin basement membrane zone at the dermal/epidermal junction of normal skin (Chan, 1997, Fleischmajer et al., 1998, Li et al., 2007). During wound healing, precursor laminin-5 is expressed in the provisional basement membrane at the keratinocyte leading edge of the wound,(Goldfinger et al., 1998, Lampe et al., 1998) and it can promote keratinocyte migration (Nguyen et al., 2000). Laminin-10 is involved in the dermal microvascular blood vessels (Li et al., 2007) and along with laminin-6 shares the gamma 1 chain containing the C16 peptide. Furthermore, previous studies have also shown that laminins derived from placenta, laminin 2 and 4 (laminins 211 and 221), can promote wound healing (Blinova et al., 1997). Both laminins contain the laminin gamma 1 chain which has the C16 sequence. In addition, these laminin isoforms share the same alpha 2 chain which contains a homologous A13 sequence which is 75% identical to A13. These data indicate that the A13 and C16 sequences are present 4 times within these laminins, and that the sequences could play an important physiological role during wound healing. In addition, the integrin α5β1 molecule, which binds to both A13 and C16, has increased expression in migrating keratinocytes (Grinnell, 1992) and fibroblasts (Gailit et al., 1996) adjacent to the wound and in the granulation tissue during wound healing, further suggesting the importance of these peptides.
The use of growth factors and other stimulatory agents to augment and improve the healing of wounds, especially chronic wounds, is important in the clinical arena. The 20% improvement seen in our studies that have targeted effects on epithelization and granulation tissue formation are of potential therapeutic value and are similar to the early preclinical (LeGrand, 1998) and clinical studies reported for PDGF-BB in wound healing for diabetic foot ulcers (Steed, 1995, Smiell et al., 1999) and pressure ulcers (Robson et al., 1992, Rees et al., 1999, Kallianinen et al., 2000) where only modest effects on the improvement in ulcer depth or volume were around 15 to 20%, respectively (Robson et al., 1992). Subsequent analysis of four randomized studies (Smiell et al., 1999) found a 39% increase in complete healing compared to the placebo group, but the actual difference in complete healing was 50% versus 36% or a 14% improvement. Thus, while our effects may seem modest, they may be clinically significant for some individuals who have chronic wounds that have not healed or are considered treatment failures with standard of care. Early preclinical studies with PDGF-BB demonstrated mixed results (LeGrand, 1998). One problem with these studies is the lack of a good animal model for human chronic wounds, often requiring a “leap of faith” (LeGrand, 1998) to further develop potentially viable chronic wound treatments alone or as an adjuvant to standard of care or surgery. The dose of any new drug requires determination of the best dose to maximize response as was done with the PDGF-BB studies (Smiell et al., 1999),(Robson et al., 1992),(Rees et al., 1999).
Our data demonstrate that exogenous laminin peptides play a role in wound healing. Fibroblast migration, wound contraction, reepithelialization, and angiogenesis all were enhanced in the peptide-treated wounds. Laminins containing the peptide sequences exist in the basement membrane zone at the dermal/epidermal junction. After injury, proteolytic processing could free these peptides from the intact laminins, and they then could aid in the repair process. It is possible that the peptides play an initial role in wound healing and as healing progresses they then turn to a more regulatory role in the wound maturation process. It should be noted that these peptides were tested in an animal model of wound healing and that the complex wound environments in diabetic and elderly patients may have a different response to these peptides. We conclude that these peptides have the potential to be developed into new therapeutic agents for wound healing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano S, Akutsu N, Ogura Y, Nishiyama T. Increase of laminin 5 synthesis in human keratinocytes by acute wound fluid, inflammatory cytokines and growth factors, and lysophospholipids. Br J Dermatol. 2004;151:961–970. doi: 10.1111/j.1365-2133.2004.06175.x. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Bhartiya D, Sklarsh JW, Maheshwari RK. Enhanced wound healing in animal models by interferon and an interferon inducer. J Cell Physiol. 1992;150:312–319. doi: 10.1002/jcp.1041500214. [DOI] [PubMed] [Google Scholar]
- Blinova MI, Paramonov BA, Kukhareva LV, Gorelik Iu V, Nikitina Iu M, Voronkina IV. [Effect of fibroblasts, collagen, and laminin on the healing of wounds formed after dissection of split skin flaps in rats] Biull Eksp Biol Med. 1997;124:229–232. [PubMed] [Google Scholar]
- Chan LS. Human skin basement membrane in health and in autoimmune diseases. Front Biosci. 1997;2:d343–d352. doi: 10.2741/a196. [DOI] [PubMed] [Google Scholar]
- Ekblom P, Lonai P, Talts JF. Expression and biological role of laminin-1. Matrix Biol. 2003;22:35–47. doi: 10.1016/s0945-053x(03)00015-5. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R, Utani A, MacDonald ED, Perlish JS, Pan TC, Chu ML, et al. Initiation of skin basement membrane formation at the epidermo-dermal interface involves assembly of laminins through binding to cell membrane receptors. J Cell Sci. 1998;111(Pt 14):1929–1940. doi: 10.1242/jcs.111.14.1929. [DOI] [PubMed] [Google Scholar]
- Gailit J, Xu J, Bueller H, Clark RA. Platelet-derived growth factor and inflammatory cytokines have differential effects on the expression of integrins alpha 1 beta 1 and alpha 5 beta 1 by human dermal fibroblasts in vitro. J Cell Physiol. 1996;169:281–289. doi: 10.1002/(SICI)1097-4652(199611)169:2<281::AID-JCP7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JC. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J Cell Sci. 1992;101(Pt 1):1–5. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- Kallianinen LK, Hirshberg J, Marchant B, Rees RS. Role of platelet-derived growth factor as an adjunct to surgery in the management of pressure ulcers. Plast Reconstr Surg. 2000;106:1243–1248. doi: 10.1097/00006534-200011000-00001. [DOI] [PubMed] [Google Scholar]
- Kibbey MC, Grant DS, Kleinman HK. Role of the SIKVAV site of laminin in promotion of angiogenesis and tumor growth: an in vivo Matrigel model. J Natl Cancer Inst. 1992;84:1633–1638. doi: 10.1093/jnci/84.21.1633. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Weeks BS, Schnaper HW, Kibbey MC, Yamamura K, Grant DS. The laminins: a family of basement membrane glycoproteins important in cell differentiation and tumor metastases. Vitam Horm. 1993;47:161–186. doi: 10.1016/s0083-6729(08)60446-x. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Nguyen BP, Gil S, Usui M, Olerud J, Takada Y, et al. Cellular interaction of integrin alpha3beta1 with laminin 5 promotes gap junctional communication. J Cell Biol. 1998;143:1735–1747. doi: 10.1083/jcb.143.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGrand EK. Preclinical promise of becaplermin (rhPDGF-BB) in wound healing. Am J Surg. 1998;176:48S–54S. doi: 10.1016/s0002-9610(98)00177-9. [DOI] [PubMed] [Google Scholar]
- Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9–18. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Madlener M, Parks WC, Werner S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res. 1998;242:201–210. doi: 10.1006/excr.1998.4049. [DOI] [PubMed] [Google Scholar]
- Malinda KM, Goldstein AL, Kleinman HK. Thymosin beta 4 stimulates directional migration of human umbilical vein endothelial cells. Faseb J. 1997;11:474–481. doi: 10.1096/fasebj.11.6.9194528. [DOI] [PubMed] [Google Scholar]
- Malinda KM, Kleinman HK. The laminins. Int J Biochem Cell Biol. 1996;28:957–959. doi: 10.1016/1357-2725(96)00042-8. [DOI] [PubMed] [Google Scholar]
- Malinda KM, Nomizu M, Chung M, Delgado M, Kuratomi Y, Yamada Y, et al. Identification of laminin alpha1 and beta1 chain peptides active for endothelial cell adhesion, tube formation, and aortic sprouting. Faseb J. 1999;13:53–62. [PubMed] [Google Scholar]
- Malinda KM, Sidhu GS, Banaudha KK, Gaddipati JP, Maheshwari RK, Goldstein AL, et al. Thymosin alpha 1 stimulates endothelial cell migration, angiogenesis, and wound healing. J Immunol. 1998;160:1001–1006. [PubMed] [Google Scholar]
- Moses MA, Marikovsky M, Harper JW, Vogt P, Eriksson E, Klagsbrun M, et al. Temporal study of the activity of matrix metalloproteinases and their endogenous inhibitors during wound healing. Journal of cellular biochemistry. 1996;60:379–386. doi: 10.1002/(SICI)1097-4644(19960301)60:3%3C379::AID-JCB9%3E3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Nguyen BP, Ryan MC, Gil SG, Carter WG. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr Opin Cell Biol. 2000;12:554–562. doi: 10.1016/s0955-0674(00)00131-9. [DOI] [PubMed] [Google Scholar]
- Nurcombe V. Laminin in neural development. Pharmacol Ther. 1992;56:247–264. doi: 10.1016/0163-7258(92)90019-v. [DOI] [PubMed] [Google Scholar]
- Obara K, Ishihara M, Ishizuka T, Fujita M, Ozeki Y, Maehara T, Saito Y, Yura H, Matsui T, Hattori H, Kikuchi M, Kurita A. Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates woundhealing in healing-impaired db/db mice. Biomaterials. 2003;24:3437–3444. doi: 10.1016/s0142-9612(03)00220-5. [DOI] [PubMed] [Google Scholar]
- Okada A, Tomasetto C, Lutz Y, Bellocq JP, Rio MC, Basset P. Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of pro-gelatinase A. J Cell Biol. 1997;137:67–77. doi: 10.1083/jcb.137.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olerud JE, Usui ML, Gibran NS, Muffley LA, Mansbridge JN, Carter WG. The epithelial cells in wound margins of diabetic ulcers are highly proliferative. J Invest Dermatol. 2000;114:857. [Google Scholar]
- Panayotou G, End P, Aumailley M, Timpl R, Engel J. Domains of laminin with growth-factor activity. Cell. 1989;56:93–101. doi: 10.1016/0092-8674(89)90987-2. [DOI] [PubMed] [Google Scholar]
- Pierce GF, Tarpley JE, Yanagihara D, Mustoe TA, Fox GM, Thomason A. Platelet-derived growth factor (BB homodimer), transforming growth factor-beta 1, and basic fibroblast growth factor in dermal wound healing. Neovessel and matrix formation and cessation of repair. Am J Pathol. 1992;140:1375–1388. [PMC free article] [PubMed] [Google Scholar]
- Ponce ML, Kleinman HK. Identification of redundant angiogenic sites in laminin alpha1 and gamma1 chains. Exp Cell Res. 2003;285:189–195. doi: 10.1016/s0014-4827(03)00056-9. [DOI] [PubMed] [Google Scholar]
- Ponce ML, Nomizu M, Delgado MC, Kuratomi Y, Hoffman MP, Powell S, et al. Identification of endothelial cell binding sites on the laminin gamma 1 chain. Circ Res. 1999;84:688–694. doi: 10.1161/01.res.84.6.688. [DOI] [PubMed] [Google Scholar]
- Rees RS, Robson MC, Smiell JM, Perry BH. the Pressure Ulcer Study Group Becaplermin gel in the treatment of pressure ulcers: A phase II randomized, double-blind, placebo-controlled study. Wound Rep Reg. 1999;7:141–147. doi: 10.1046/j.1524-475x.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- Robson MC, Phillips LG, Thomason A, Altrock BW, Pence PC, Heggers JP, et al. Recombinant human platelet-derived growth factor-BB for the treatment of chronic pressure ulcers. Ann Plast Surg. 1992;29:193–201. doi: 10.1097/00000637-199209000-00001. [DOI] [PubMed] [Google Scholar]
- Sidhu GS, Thaloor D, Singh AK, Raghunath PN, Maheshwari RK. Enhanced biosynthesis of extracellular matrix proteins and TGF-beta 1 by polyinosinic-polycytidylic acid during cutaneous wound healing in vivo. J Cell Physiol. 1996;169:108–114. doi: 10.1002/(SICI)1097-4652(199610)169:1<108::AID-JCP11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: A combined analysis of four randomized studies. Wound Repair Regen. 1999;7:335–346. doi: 10.1046/j.1524-475x.1999.00335.x. [DOI] [PubMed] [Google Scholar]
- Soo C, Shaw WW, Zhang X, Longaker MT, Howard EW, Ting K. Differential expression of matrix metalloproteinases and their tissue-derived inhibitors in cutaneous wound repair. Plast Reconstr Surg. 2000;105:638–647. doi: 10.1097/00006534-200002000-00024. [DOI] [PubMed] [Google Scholar]
- Steed D. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg. 1995;21:71–78. doi: 10.1016/s0741-5214(95)70245-8. [DOI] [PubMed] [Google Scholar]
- Stenn KS, dePalma L. Re-epithelialization. New York: Plenum Press; 1988. [Google Scholar]
- Sullivan SR, Underwood RA, Sigle RO, Fukano Y, Muffley LA, Usui ML, Gibran NS, Antezana MA, Carter WG, Olerud JE. Topical application of laminin-332 to diabetic mouse wounds. J Dermatol Sci. 2007;48:177–188. doi: 10.1016/j.jdermsci.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KTGL, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004;12:262–268. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- Tran KT, Lamb P, Deng JS. Matrikines and matricryptins: Implications for cutaneous cancers and skin repair. Journal of dermatological science. 2005;40:11–20. doi: 10.1016/j.jdermsci.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wysocki AB, Kusakabe AO, Chang S, Tuan T-L. 1999;7:154–165. doi: 10.1046/j.1524-475x.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- Yamada KM. Adhesive recognition sequences. J Biol Chem. 1991;266:12809–12812. [PubMed] [Google Scholar]
- Zheng Y, Watanabe M, Kuraishi T, Hattori S, Kai C, Shibuya M. Chimeric VEGF-ENZ7/PIGF specifically binding to VEGFR-2 accelerates skin wound healing via enhancement of neovascularization. Arterioscler Thromb Vasc Biol. 2007;27:503–511. doi: 10.1161/01.ATV.0000256459.06671.3c. [DOI] [PubMed] [Google Scholar]