Abstract
The vegetative development of the maize shoot can be divided into juvenile and adult phases based on the types of leaves produced at different times in shoot development. Models for the regulation of phase change make explicit predictions about when the identity of these types of leaves is determined. To test these models, we examined the timing of leaf type determination in maize. Clones induced in transition leaf primordia demonstrated that the juvenile and adult regions of these leaves do not become clonally distinct until after the primordium is 700 μm in length, implying that these cell fates were undetermined at this stage of leaf development. Adult shoot apices were cultured in vitro to induce rejuvenation. We found that leaf primordia as large as 3 mm in length can be at least partially rejuvenated by this treatment, and the location of rejuvenated tissue is correlated with the maturation pattern of the leaf. The amount and distribution of juvenile tissue in rejuvenated leaves suggests that rejuvenation occurs nearly simultaneously in all leaf primordia. In vitro culture rejuvenated existing leaf primordia and the P0 primordium, but did not change the identity of subsequent primordia or the total number of leaves produced by the shoot. This result suggests that leaf identity can be regulated independently of the identity of the shoot apical meristem, and it implies that vegetative phase change is not initiated by a change in the identity of the shoot apical meristem.
Plants produce different types of leaves or leaf-like organs at different stages in their development (a phenomenon called heteroblasty or phase change) (1–4). Among these are cotyledons, the variety of leaf types produced during the vegetative growth of the shoot, inflorescence bracts, and the highly modified leaves found in flowers and other reproductive structures. Genetic and molecular analyses of floral morphogenesis have provided a detailed model (the ABC model) for the regulation of floral organ identity (5, 6). In contrast, the specification of vegetative organ identity remains very poorly understood (7).
One of the questions that is still unresolved is when the identity of a leaf is determined. Many investigators have reported that heteroblastic variation in leaf anatomy and morphology is discernable very early in leaf development (8–13), and have therefore concluded that leaf identity is specified during the earliest stages of leaf initiation. However, this conclusion has rarely been tested experimentally. The effect of exogenous factors on leaf morphology has been studied extensively (1, 4), but there is surprisingly little information about when phase-specific aspects of leaf anatomy and morphology are determined during leaf development. Sussex and Clutter (14) found that adult leaf primordia of the fern Osmunda cinnamomea develop as juvenile leaves when cultured on a medium containing low levels of sucrose, demonstrating that leaf identity is not specified until after leaf initiation in this species. In addition, experimental analyses of leaf determination in two aquatic species, Hippuris vulgaris (15) and Ranunculus flabellaris (16), have shown that some heteroblastic features of leaf anatomy can be modified by exogenous conditions up until a leaf has nearly fully expanded. Unfortunately, the relevance of these experimental results to the regulation of leaf identity in other species is difficult to predict. In contrast to most terrestrial species, aquatic plants undergo dramatic, reversible changes in leaf morphology in response to a wide range of environmental conditions. This plasticity may indicate that leaf development in aquatic species is regulated in a fundamentally different way than in species with more stable patterns of leaf phenology. Furthermore, in contrast to many strongly heteroblastic terrestrial species, terrestrial and aquatic leaf forms in aquatic plants are morphologically similar until quite late in leaf development (15). It is also unclear how closely leaf development in ferns resembles leaf development in more advanced species (17), given that fern leaves are compound (many flowering plants have simple leaves) and are almost indeterminate. Fern leaves not only grow for a very long time, but also can be transformed directly into shoots in culture (18, 19), a phenomenon which, to our knowledge, has never been documented in flowering plants.
Based on the pattern of juvenile and adult tissue in transition leaves, several investigators have speculated that the phase identity of the maize leaf is determined after leaf initiation (20, 21). This hypothesis has never been tested experimentally, however. One source of information about this issue is the phenotype of an unstable allele of Gl15, a putative transcription factor that is required for the production of some juvenile epidermal traits (22–24). Juvenile leaves of gl15-m1 plants have an adult epidermis with sectors of juvenile tissue resulting from the excision of the Spm element in gl15-m1. Based on the small size of these sectors, Moose and Sisco (23) concluded that Gl15 is required for the expression of juvenile traits late in leaf development. Whether this late temporal requirement for Gl15 corresponds to the time at which leaf identity is actually determined is unknown, however, because Gl15 regulates only a subset of phase-specific traits and is downstream of genes that may act much earlier in leaf development (22, 24).
We have previously described two models for the regulation of leaf identity during shoot development (4). The meristem-patterning model proposes that leaf identity is specified by dividing the shoot apical meristem (SAM) into discrete identity domains, similar to the way that the floral meristem is divided into four domains early in flower development. According to this model, the identity of cells in the SAM determines the identity of the leaves produced by these cells; thus, juvenile leaves arise from a juvenile region of the meristem, adult leaves arise from an adult region of the meristem, and transition leaves arise from cells at the boundary between these domains. The apex- patterning model proposes that leaf identity is regulated by factors that originate outside the SAM, and which act simultaneously on the SAM and all existing undetermined organs (4, 20). This model assumes that changes in leaf identity can occur without a prior change in the identity of cells in SAM.
These models make explicit predictions about the timing of leaf determination. The meristem-patterning model predicts that leaf identity is established early in the development of a leaf primordium because identity is specified by the character of cells in the SAM. The apex-patterning model predicts that leaf identity is specified after the leaf has been initiated and can be modified independently of the identity of the SAM. To test these predictions, we examined the timing of leaf-type determination in maize. Using clonal analysis, we defined when juvenile and adult regions of transition leaf primordia become clonally distinct. We also took advantage of the observation that adult shoot apices can be rejuvenated in culture (25, 26) and examined the effect of this treatment on the fate of existing leaf primordia. The results presented here demonstrate that the phase identity of a leaf is specified after a leaf primordium is well established and support the predictions of the apex-patterning model of vegetative phase change.
Materials and Methods
Clonal Analysis.
The plants used in this study were the product of a cross between the inbred OH43 and a stock carrying the albino mutation wd in a W23-inbred background (OH43 × wd, Ring-Wd C-I, W23). Developmentally uniform seedlings (n = 350) were γ irradiated (700 R, 137Cs, 12 min) 8 days after being planted in the greenhouse. To determine the length of leaves six and seven in this population, 10 specimens were fixed in formalin/acetic acid/alcohol at the time of irradiation, dehydrated, cleared in benzyl benzoate-four-and-a-half solution (27), and examined with a differential interference contrast microscope. At maturity, leaves with sectors that spanned juvenile and adult domains were harvested, the boundary between these domains was outlined with an indelible pen, and these leaves were then photocopied.
Apex Culture.
The plants used for apex culture were the same genotype as those used for clonal analysis. These plants were grown in the greenhouse with supplemental illumination in Metromix 200 (Scotts) and cultured 3 weeks after planting. Control plants grown under the same conditions were subsequently transplanted to larger pots and allowed to mature.
After removing the root system and expanded leaf blades, seedlings were surface sterilized and seven or eight leaves were removed to produce specimens containing six adult leaf primordia. Specimens representing the range of developmental stages in the population were fixed in formalin/acetic acid/alcohol on the same day shoot apices were placed in culture, embedded in paraffin, sectioned, and stained with periodic acid/Schiff reagent-hematoxylin, and the lengths of all of the visible leaf primordia on these apices was then determined. The length of the enclosed leaf primordia on each cultured apex was determined by measuring the length of the outermost leaf with an ocular micrometer and using this information to find comparable specimens among those that had been analyzed histologically.
Shoot tips were initially grown in a growth chamber (22°C; 18:6 photoperiod) in 24-well tissue culture dishes on a medium consisting of Murashige and Skoog salts (GIBCO/BRL)/Gamborg's vitamins (Sigma)/40 μM glycine (Sigma)/30 g/liter sucrose (Sigma)/100 mg/liter myo-inositol (Sigma)/0.10 μM kinetin (Sigma)/6 g/liter agar, pH 5.8. After ≈8 days, apices were transferred into Magenta boxes onto a medium identical to the one described above except that kinetin was replaced with 0.04 mg/liter 2,4-dichlorophenoxyacetic acid (Sigma). Specimens developed roots in 2–3 weeks on this medium. After about 4 weeks, specimens were transplanted in Metromix 200 (Scotts) and grown to maturity in the greenhouse.
Fully expanded leaves were removed as the plants developed and were examined under a dissecting microscope for the presence of epicuticular wax and epidermal hairs. Regions with epicuticular wax were outlined on the leaf in ink, and the leaf was then photocopied. Epidermal peels from apical, middle, and basal parts of representative leaves were obtained by enzymatic digestion and were stained with toluidine blue as described (28).
Results
Clonal Analysis.
In maize, it is possible to distinguish two types of leaves, juvenile and adult, based on differences in a large number of epidermal traits and the expression of the dominant mutation, Ragged leaf (21, 24, 29, 30). In this study, juvenile identity was defined as the production of epicuticular wax, lack of epidermal hairs and bulliform cells, weakly crenulated epidermal cell walls, and purple cell wall staining with toluidine blue. Adult cell identity was defined as the absence of epicuticular wax, the production of epidermal hairs and bulliform cells, highly crenulated epidermal cell walls, and aqua cell wall staining with toluidine blue. The first five leaves of the plants used in these experiments were uniformly juvenile. Leaves 6 and 7 were transition leaves and possessed juvenile tissue at the apex and margins of the leaf blade and adult tissue at the center and base of the blade, respectively. Leaves 8 and above were uniformly adult.
The mosaic character of leaves 6 and 7 provides a way to study when phase-specific aspects of leaf identity become determined. We reasoned that if the identity of these transition leaves is determined during the early stages of leaf initiation (during the formation of the leaf buttress, for example), then the cell lineage of juvenile and adult parts of the leaf should become distinct before the emergence of the leaf primordium, or shortly afterward. On the other hand, if juvenile and adult cell identities are specified later in leaf development, then juvenile and adult parts of the leaf should share the same lineage for some time after leaf initiation.
We tested this prediction by generating marked clones in transition leaf primordia. Seedlings heterozygous for the albino mutation wd were irradiated 8 days after planting, when leaf 6 was ≈700 μm and leaf 7 was ≈300 μm in length (Fig. 1). When these leaves were fully expanded, they were examined for sectors that spanned juvenile and adult regions of the leaf blade (as defined by the presence or absence of epicuticular wax and epidermal hairs). Twelve such sectors were identified out of a total of ≈165 sectors; six of these were in leaf 6, and six were in leaf 7 (Fig. 2). This result demonstrates that single cells in transition leaf primordia are capable of giving rise to both juvenile and adult tissue. If these domains had been specified at the time of irradiation, sectors would have been confined to either juvenile or adult parts of the leaf blade. We conclude that the phase identity of a transition leaf is specified after it is 700 μm in length.
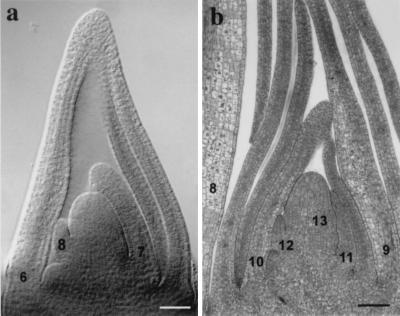
Figure 1.
Shoot apices of juvenile and adult maize shoots. (a) Cleared specimen of the shoot apex of an 8-day-old maize seedling with two transition leaf primordia (leaves 6 and 7) and an adult leaf primordium (leaf 8). Plants at this developmental stage were used for clonal analysis. (b) Longitudinal section through the shoot apex of a plant with six adult leaf primordia (leaves 8–13). Plants at this developmental stage were used for apex culture. (Bar = 100 μm.)
Figure 2.
Somatic clones spanning the boundary between juvenile (gray) and adult (black) regions of leaves 6 and 7. Only 6 of 12 leaves with such sectors are illustrated here. Leaf 6 was 700 μm and leaf 7 was 300 μm at the time of irradiation (see Fig. 1).
It should be emphasized that all of the sectors we observed were located in mesophyll tissue, whereas phase identity was defined by epidermal traits. This is because albino sectors are invisible to the naked eye in the epidermis, but are quite obvious in mesophyll tissue. The conclusion that these sectors span the boundary between juvenile and adult parts of the leaf therefore depends on the assumption that cells in the epidermis and mesophyll do not become displaced relative to one another during leaf expansion; in other words, that the cell lineage of the mesophyll reflects the cell lineage of the epidermis. We do not believe that this is a serious concern because all of the sectors we observed extended for three or more centimeters on either side of the boundary (Fig. 2). If cells in the epidermis and the mesophyll are displaced, it seems unlikely that this would involve such a great distance. It is also significant that the shape and orientation of clonal sectors did not resemble the shape and orientation of the boundary between juvenile and adult tissue. Clonal sectors were typically long, thin, and regular in shape, whereas the boundary between different cell types was quite irregular and usually did not run parallel to the adjacent sector (Fig. 2). If cell identity was established early in leaf development and was propagated by cell division during the subsequent growth of the leaf, the orientation and shape of the juvenile–adult boundary should resemble the orientation and shape of clonal sectors in the same region of the leaf. The irregular shape of the juvenile–adult boundary suggests that these domains were specified relatively late in leaf development, the irregularity being caused by variation in the cell division patterns of many different cell lineages.
Apex Culture.
To explore the relationship between the maturation state of a leaf primordium and the specification of phase-specific leaf identity, we took advantage of the observation that adult shoot apices can be rejuvenated by growing the shoot apex in vitro (26). This study also reported that existing leaf primordia are rejuvenated in culture, but did not provide any information about the nature of this reversion and the size of the affected primordia. These features were characterized more extensively in the following experiment.
Shoot apices with six immature adult leaf primordia were dissected from plants that had initiated a total of 13 leaves, and were cultured on the medium used by Irish and Karlen (26). The length of the outermost leaf primordium was measured before the apex was placed in culture; the lengths of younger leaf primordia were estimated from a histological examination of specimens at the same developmental stage. The developmental identity of each leaf was determined after it was fully expanded by examining it under a dissecting microscope for the presence of epicuticular wax and epidermal hairs. It was sometimes difficult to determine the fate of the oldest leaf on each apex because it expanded very little and usually died before it was feasible to remove it for analysis. However, younger leaves grew to a larger size and were quite manageable.
Table 1 illustrates the fate of successive leaves on 19 cultured shoots. All of the leaf primordia that were present on these apices when they were placed in culture (leaves 8–13) were either partially or completely rejuvenated in culture. Partially rejuvenated leaves had adult tissue at the tip of the leaf blade and juvenile tissue at the base of the leaf blade, and the relative amount of these tissues was proportional to the age of leaf at the time the apex was placed in culture (Figs. 3 and 4). The largest leaf primordia on these shoots (leaf 8) ranged in size from 1.7 to 3.2 mm at the time of culture (Table 1) and gave rise to leaves that had a very small amount of juvenile tissue at the base of the leaf blade. Leaves 9 and 10 and the majority of leaves at node 11 were also partially rejuvenated (Fig. 4). Leaves 12 and 13 were less than 200 μm at the time of culture and in most cases developed as completely juvenile leaves. However, several leaves at these nodes resembled normal transition leaves in that they had juvenile tissue at the tip of the leaf blade and adult tissue at the base of the blade; we refer to these leaves as “second transition leaves.” In two cases, the primordia of leaf 13 developed as adult leaves. Most P0 leaves developed as second transition leaves, or as adult leaves (Table 1). The vast majority of P-1 leaves and all subsequent leaves were adult. In summary, leaf primordia between 400 μm and 3.2 mm in length were partially rejuvenated, primordia less that 200–300 μm in length were completely rejuvenated, and uninitiated leaves developed as either second transition leaves or adult leaves.
Table 1.
Developmental fate of leaves produced by shoot apices cultured in vitro
| Leaf no. | Length,* mm | No. of leaves†
|
||||
|---|---|---|---|---|---|---|
| Rejuv. | Juvenile | 2nd trans. | Adult | Tassel | ||
| 8 | 1.7–3.2 | 10 | ||||
| 9 | 1.2–1.7 | 18 | ||||
| 10 | 0.5–1 | 19 | ||||
| 11 | 0.3–0.4 | 11 | 8 | |||
| 12 | 0.2 | 3 | 14 | 2 | ||
| 13 | Buttress | 12 | 5 | 2 | ||
| 14 | P0 | 1 | 12 | 6 | ||
| 15 | P-1 | 2 | 17 | |||
| 16 | P-2 | 1 | 18 | |||
| 17 | P-3 | 11 | 8 | |||
| 18 | P-4 | 2 | 9 | |||
| 19 | P-5 | 2 | ||||
Length of the leaf primordium when the apex was placed in culture; the P0 to P-6 primordia were invisible at this time.
Number of leaves with the indicated identity. n = 19, except in the case of leaves 8 and 9, where some leaves became necrotic before they could be scored. Rejuv., rejuvenated adult leaf, with juvenile tissue at the base of the leaf blade and adult tissue at the tip of the blade; Juvenile, completely juvenile leaf; 2nd trans., juvenile tissue at the tip of the leaf blade, adult tissue at the base; Adult, completely adult leaf; Tassel, tassel produced at the indicated position.
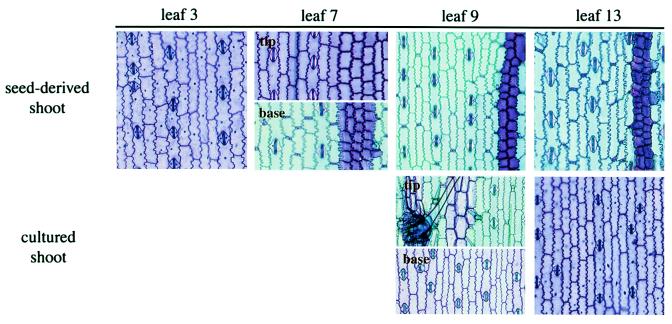
Figure 3.
Epidermal peels from leaves of control shoots and rejuvenated shoots stained with toluidine blue. Note that leaf 9 of the rejuvenated shoot has juvenile and adult tissue in a reversed orientation relative to leaf 7, which is a normal transition leaf.
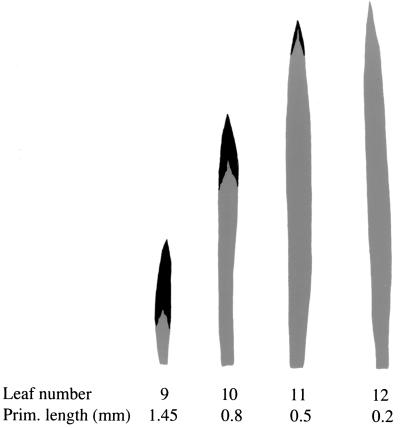
Figure 4.
Distribution of juvenile (gray) and adult (black) tissue in a series of rejuvenated leaves from a single cultured shoot. The length of these leaves at the time the apex was placed in culture is indicated.
Cultured apices produced approximately the same total number of leaves as seed-derived plants. The total number of leaves produced by cultured shoot tips before and after culture was 16.8 ± 0.2, whereas as seed-derived plants produced a total of 15.8 ± 0.1 leaves (Fig. 5). This result is consistent with the observations of Irish and Jegla (31), who found that apices cultured with six leaf primordia produced only as many leaves as seed-derived plants, whereas apices with two to three leaf primordia are completely reset in culture.
Figure 5.
Mature plants derived from seed (a) and a cultured shoot apex (b).
Root primordia were first visible 2–3 weeks after apices were placed in culture, by which time most rejuvenated leaves were at least half their final length. This is well beyond the point at which cell division in the leaf ceases (32), suggesting that roots played little or no role in rejuvenation.
Discussion
The Phase Identity of a Leaf Is Determined After Leaf Initiation.
The results of this study demonstrate that phase-specific epidermal traits in maize are determined after leaf initiation in a gradual fashion that mirrors the basipetal pattern of maturation typical of the maize leaf. These results are consistent with the results of studies of leaf development in ferns (14) and aquatic plants (15, 16), as well as experiments on the determination of vegetative/floral organ identity in Impatiens balsamina (33) and Arabidopsis thaliana (34) and demonstrate that heteroblastic aspects of leaf identity are not specified until after the leaf primordium is well established. By using clonal analysis, we found that single cells in an existing transition leaf primordium are capable of giving rise to both juvenile and adult tissue. These cellular identities must therefore be determined after the stage at which these leaves were irradiated, i.e., at a length greater than 700 μm. Because sectors induced at later stages of development are quite small (32), it is difficult to use clonal analysis to determine when juvenile and adult regions of a transition leaf finally become distinct. Consequently, to obtain more accurate information about the timing and spatial patterning of leaf determination, we examined the sensitivity of adult leaf primordia to conditions that induce rejuvenation. The results of this latter experiment suggest that the phase identity of a leaf is not completely determined until the primordium is greater than 3 mm in length because primordia as large as 3 mm produced a small amount of juvenile tissue at the base of the leaf blade. Because we were unable to identify a stage at which leaf primordia were insensitive to rejuvenation, it is possible that leaf primordia remain undetermined until they are significantly larger than 3 mm. Given the basipetal pattern of leaf maturation, it is likely that the leaf sheath remains undetermined for a much longer time than the rest of the leaf.
Rejuvenation Occurs Simultaneously Throughout the Shoot Apex.
We found that the juvenile tissue in rejuvenated adult leaves was confined to the base of the leaf blade, and the extent of this rejuvenated region was correlated with the age of a leaf at the time it was placed in culture. Specifically, younger leaves had a greater amount of juvenile tissue than the next older leaf on the apex. This pattern is explained by the basipetal maturation pattern of the maize leaf. In maize, as in many other species, cellular differentiation begins at the tip of the leaf, and then gradually progresses toward the leaf base (20, 32, 35); therefore, in an immature leaf, cells at the base of a leaf are likely to be less highly determined than cells at the tip. It follows that because leaves are initiated sequentially, successively older leaf primordia on a shoot apex have proportionately larger regions of determined tissue at their tip. If all of the leaf primordia on the apex underwent rejuvenation more-or-less simultaneously, the distribution of adult and juvenile tissue in a series of rejuvenated leaves should reflect the determination state of these leaves at a single time point in shoot development. That is, older leaves should have less juvenile tissue than younger leaves, and the rejuvenated region should increase acropetally in successively younger leaves. Because this is the pattern we observed, we conclude that all of the leaf primordia on a cultured apex are rejuvenated more-or-less simultaneously.
The distribution pattern of juvenile and adult tissue in rejuvenated leaves is similar to the pattern of juvenile and adult tissue in normal transition leaves, albeit in a reversed orientation. In a series of transition leaves, juvenile tissue is confined to a progressively smaller region of the leaf apex in the same way that adult tissue is confined to a progressively smaller region of the leaf apex in rejuvenated leaves. This similarity suggests that the normal juvenile-to-adult transition may involve both a change in the fate of the shoot apex and a simultaneous change in the fate of existing leaf primordia (4, 20). This conclusion is supported by the observation that the number of transition and rejuvenated leaves is correlated with the number of immature leaf primordia at the apex of juvenile and adult shoots. At the time a seedling undergoes the transition to adult development, it has approximately three leaves less than 1 mm in length; in contrast, the 3-week-old adult apices used in our rejuvenation experiments had five leaves in this size range (Fig. 1). If the process of shoot maturation is similar to the process of shoot rejuvenation, one would expect the number of transition leaves produced by a seedling to be less than the number of rejuvenated leaves produced by an adult apex because at any point in time there are fewer immature leaves in a juvenile shoot than in an adult shoot. Consistent with this expectation, the plants used in this study had two transition leaves and usually produced four partially rejuvenated leaves in culture (Table 1).
The hypothesis that phase change involves a rapid, global transformation in the identity of all of the organs at the shoot apex (the apex patterning model of phase change) has also been proposed for the transition from vegetative to reproductive growth (33, 34, 36, 37). In response to a floral-inductive stimulus, some plants produce intermediate organs with both vegetative and floral cell types (37). In I. balsamina (33) and A. thaliana (34), these intermediate structures have been shown to arise from leaf and bud primordia that were present on the shoot apex at the time of the photoinductive stimulus. Because these intermediate vegetative/floral organs are not regularly produced during the transition from vegetative to floral development, the question arises as to whether this phenomenon is a normal feature of the floral transition. The morphology of the leaves produced during the early stages of the floral transition suggests that it is. In Arabidopsis, adult rosette leaves and bracts (leaves produced in the inflorescence) have distinctive patterns of trichome distribution (38). These distinct patterns occur in discrete proximal-distal domains of the intermediate organs produced during the early stages of inflorescence development, in the same way that juvenile and adult cell types are located in proximal-distal domains of transition leaves or rejuvenated leaves. This feature, and the fact that the relative size and location of these leaf- and bract-like domains fit in the expected pattern, suggest that these transition leaves are produced by primordia that are present before the floral transition and are transformed by this process.
Phase Change Is Not Initiated by a Change in the Identity of the Shoot Apical Meristem.
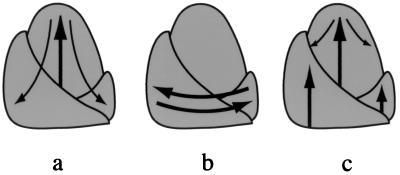
The vegetative-to-floral transition is known to be regulated by factors produced by leaves and translocated to the shoot apex (39, 40). In some species, leaves also play a critical role in maintaining the floral identity of the shoot (41, 42). In contrast, the source of the factors regulating vegetative phase change is very poorly understood (43). Several possible mechanisms are illustrated in Fig. 6. Some investigators believe that the developmental phase of the shoot is regulated autonomously by the developmental state of cells in the SAM (44). This mechanism predicts that any change in leaf identity (whether initiated by factors originating within or outside the SAM) is preceded by a change in the identity of cells in the SAM (Fig. 6a). A second possibility has been suggested by Irish et al. (26, 31). These investigators have suggested that the SAM is “neutral with respect to phase” and propose that the adult phase is initiated and maintained by signals originating from preexisting leaves, with no direct involvement of the SAM (26) (Fig. 6b). This model implies that juvenile identity is a default condition that is transformed into adult identity by factors produced by juvenile leaves. We favor a third possibility that combines features of these two models. Specifically, we proposed that phase change is regulated by factors that originate outside the SAM and which act independently on leaf primordia and the SAM (Fig. 6c). In this scenario, the SAM does not regulate the initial change in leaf identity, but functions to maintain the subsequent developmental state of the shoot once the identity of the SAM has been established.
Figure 6.
Models for the regulation of phase change. ➞, Primary signals; →, secondary signals. (a) The meristem-autonomous model postulates that vegetative identity is regulated entirely by the SAM. Thus, the primary event in phase change is a change in the identity of cells in the SAM. (b) This diagram illustrates the hypothesis (26) that adult leaf identity is regulated entirely by interactions between preexisting leaves and newly formed leaf primordia. (c) The meristem-patterning model proposes that vegetative identity is regulated by factors that act independently on existing leaf primordia and the SAM. The maintenance of developmental phase is regulated in part by changes in the character of the SAM.
This model is based on the pattern of rejuvenation we observed in cultured shoot apices. We found that only existing leaf primordia and the P0 leaf were rejuvenated in culture. Leaves initiated two or more plastochrons after the apex was placed in culture (P-1 to P-5) were completely adult. Furthermore, most P0 leaves developed as second transition leaves and were therefore partially adult. These observations suggest that the vegetative phase of the SAM was not affected by our culture conditions; i.e., the SAM remained in an adult phase. The reproductive phase of the shoot was also unaffected by our culture conditions, as all cultured shoots produced only as many additional leaves as control, seed-derived meristems. Previous studies (25, 26) have shown that both the vegetative and reproductive identity of the SAM can be completely reset by removing all but two or three leaf primordia. Thus, the SAM is capable of undergoing complete rejuvenation under the appropriate conditions, but did not do so under our experimental conditions. These results suggest that rejuvenation was not initiated by a change in the SAM and support the hypothesis (4, 20) that vegetative phase change is initiated by factors that originate outside the SAM and which act independently on the SAM and on undetermined organs at the shoot apex.
If the SAM does not initiate vegetative phase change, then how is this process regulated? It is easy to imagine a role for the root system in this process. For example, phase change might take place once the shoot apex was a certain distance from the juvenilizing effect of the root system. Alternatively, the root system might be the source of maturation factors that increase in amount as the root system develops. But whereas there is evidence that the root system functions in the regulation of flowering (45–47), at present there is little indication that it plays a role in vegetative phase change. In particular, we observed no obvious correlation between root production and the production of rejuvenated leaves in this study. Several studies of phase change in woody plants have suggested that the root system may promote juvenile development (45, 48) but the evidence for this conclusion is quite weak.
Another possibility is that, as in the case of reproductive phase change, vegetative phase change is regulated by factors produced by leaves or leaf primordia. As noted above, based on the observation that adult apices completely revert to the juvenile phase when they are cultured with only two leaf primordia, Irish and Karlen (26) have proposed that shoot maturation depends on factors produced by preexisting leaf primordia. These investigators also noted that an alternative interpretation of their results is that leaf primordia stabilize the developmental state of the shoot rather than specifying this state. Future studies should make it possible to distinguish between these alternatives.
Acknowledgments
We thank R. Kerstetter for helpful comments on this manuscript. This work was funded by National Science Foundation IBN-97–28733. Sabbatical support to J.A.J.O. from Villanova University is gratefully acknowledged.
Abbreviation
- SAM
shoot apical meristem
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180301597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180301597
References
- 1.Allsopp A. Adv Morphol. 1967;6:127–171. doi: 10.1016/b978-1-4831-9953-5.50008-1. [DOI] [PubMed] [Google Scholar]
- 2.Goebel K. Organography of Plants. Part I. General Organography (English translation by Balfour, I. B.) Oxford: Clarendon; 1900. [Google Scholar]
- 3.Jones C S. Int J Plant Sci. 1999;160:S105–S111. doi: 10.1086/314215. [DOI] [PubMed] [Google Scholar]
- 4.Kerstetter R A, Poethig R S. Annu Rev Cell Dev Biol. 1998;14:373–398. doi: 10.1146/annurev.cellbio.14.1.373. [DOI] [PubMed] [Google Scholar]
- 5.Coen E S, Meyerowitz E M. Nature (London) 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- 6.Weigel D. Annu Rev Genet. 1995;29:19–39. doi: 10.1146/annurev.ge.29.120195.000315. [DOI] [PubMed] [Google Scholar]
- 7.Sylvester A W, Smith L, Freeling M. Annu Rev Cell Dev Biol. 1996;12:257–304. doi: 10.1146/annurev.cellbio.12.1.257. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan D R. Cellule. 1980;73:137–203. [Google Scholar]
- 9.Kaplan D R. Cellule. 1973;69:253–290. [Google Scholar]
- 10.Merrill E K. Can J Bot. 1986;64:2645–2649. [Google Scholar]
- 11.Merrill E K. Can J Bot. 1986;64:2650–2661. [Google Scholar]
- 12.Richards J. Bot Gaz. 1983;144:247–259. [Google Scholar]
- 13.Bruck D K, Kaplan D R. Am J Bot. 1980;67:337–346. [Google Scholar]
- 14.Sussex I M, Clutter M E. Phytomorphology. 1960;10:87–99. [Google Scholar]
- 15.Goliber T E, Feldman L J. Am J Bot. 1990;77:399–412. [Google Scholar]
- 16.Bruni N, Young J P, Dengler N G. Can J Bot. 1995;74:823–837. [Google Scholar]
- 17.Goliber T, Kessler S, Chen J J, Bharathan G, Sinha N. Curr Top Dev Biol. 1999;43:259–290. doi: 10.1016/s0070-2153(08)60384-1. [DOI] [PubMed] [Google Scholar]
- 18.Steeves T A. In: Trends in Plant Morphogenesis. Cutter E G, editor. New York: Wiley; 1966. pp. 200–219. [Google Scholar]
- 19.Caponetti J D, Steeves T A. Can J Bot. 1963;41:545–556. [Google Scholar]
- 20.Sylvester A W, Cande W Z, Freeling M. Development (Cambridge, UK) 1990;110:985–1000. doi: 10.1242/dev.110.3.985. [DOI] [PubMed] [Google Scholar]
- 21.Bongard-Pierce D K, Evans M M S, Poethig R S. Int J Plant Sci. 1996;157:331–340. [Google Scholar]
- 22.Moose S P, Sisco P H. Genes Dev. 1996;10:3018–3027. doi: 10.1101/gad.10.23.3018. [DOI] [PubMed] [Google Scholar]
- 23.Moose S P, Sisco P H. Plant Cell. 1994;6:1343–1355. doi: 10.1105/tpc.6.10.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans M M, Passas H J, Poethig R S. Development (Cambridge, UK) 1994;120:1971–1981. doi: 10.1242/dev.120.7.1971. [DOI] [PubMed] [Google Scholar]
- 25.Irish E E, Nelson T M. Planta. 1988;175:9–12. doi: 10.1007/BF00402876. [DOI] [PubMed] [Google Scholar]
- 26.Irish E E, Karlen S. Int J Plant Sci. 1998;159:695–701. [Google Scholar]
- 27.Herr J M., Jr Stain Technol. 1982;57:161–169. doi: 10.3109/10520298209066609. [DOI] [PubMed] [Google Scholar]
- 28.Dudley M, Poethig R S. Genetics. 1993;133:389–399. doi: 10.1093/genetics/133.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poethig R S. Genetics. 1988;119:959–973. doi: 10.1093/genetics/119.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeling M, Lane B. In: The Maize Handbook. Freeling M, Walbot V, editors. New York: Springer; 1994. pp. 17–28. [Google Scholar]
- 31.Irish E, Jegla D. Plant J. 1996;11:63–71. [Google Scholar]
- 32.Poethig R S, Szymkowiak E J. Mayclica. 1995;40:67–76. [Google Scholar]
- 33.Battey N H, Lyndon R F. Ann Bot (London) 1988;61:9–16. [Google Scholar]
- 34.Hempel F D, Zambryski P C, Feldman L J. Plant Cell. 1998;10:1663–1676. doi: 10.1105/tpc.10.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharman B C. Ann Bot (London) 1942;6:245–284. [Google Scholar]
- 36.Hempel F D, Feldman L J. Plant J. 1995;8:725–731. doi: 10.1046/j.1365-313x.1995.08050725.x. [DOI] [PubMed] [Google Scholar]
- 37.Battey N H, Lyndon R F. Bot Rev. 1990;56:162–189. [Google Scholar]
- 38.Telfer A, Bollman K M, Poethig R S. Development (Cambridge, UK) 1997;124:645–654. doi: 10.1242/dev.124.3.645. [DOI] [PubMed] [Google Scholar]
- 39.Bernier G. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:175–219. [Google Scholar]
- 40.Colasanti J, Yuan Z, Sundaresan V. Cell. 1998;93:593–603. doi: 10.1016/s0092-8674(00)81188-5. [DOI] [PubMed] [Google Scholar]
- 41.Pouteau S, Tooke F, Battey N. Plant Physiol. 1998;118:1191–1201. doi: 10.1104/pp.118.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tooke F, Pouteau S, Battey N. J Exp Bot. 1998;49:1681–1688. [Google Scholar]
- 43.Hackett W P, Murray J R. In: Biotechnology of Ornamental Plants. Geneve R L, Preece J E, Merkle S A, editors. New York: CAB International; 1997. pp. 73–86. [Google Scholar]
- 44.Wareing P F. In: Manipulation of Flowering. Atherton J G, editor. Boston: Butterworth; 1987. pp. 83–92. [Google Scholar]
- 45.Robinson L W, Wareing P F. New Phytol. 1969;69:67–78. [Google Scholar]
- 46.Schwabe W W, Al-Doori A H. J Exp Bot. 1973;24:969–981. [Google Scholar]
- 47.McDaniel C N. Planta. 1980;148:462–467. doi: 10.1007/BF00552661. [DOI] [PubMed] [Google Scholar]
- 48.Doorenbos J. Proc Koninkl Nederl Akademie van Wetenschappen Ser C. 1954;57:99–102. [Google Scholar]