Abstract
Background
Members of the predatory gastropod genus Conus use a venom comprised of a cocktail of peptide neurotoxins, termed conotoxins or conopeptides, to paralyze prey and conotoxin gene family members diversify via strong positive selection. Because Conus venoms are used primarily to subdue prey, the evolution of venoms is likely affected by predator-prey interactions.
Methodology/Principal Findings
To identify the selective forces that drive the differentiation of venoms within species of Conus, we examined the distribution of alleles of a polymorphic O-superfamily conotoxin locus of Conus ebraeus at Okinawa, Guam and Hawaii. Previous analyses of mitochondrial cytochrome oxidase I gene sequences suggest that populations of C. ebraeus, a worm-eating Conus, are not structured genetically in the western and central Pacific. Nonetheless, because the sample size from Guam was relatively low, we obtained additional data from this location and reexamined patterns of genetic variation at the mitochondrial gene at Okinawa, Guam and Hawaii. We also utilized a DNA-based approach to identify prey items of individuals of C. ebraeus from Guam and compared this information to published data on diets at Okinawa and Hawaii. Our results show that conotoxin allelic frequencies differ significantly among all three locations, with strongest differentiation at Hawaii. We also confirm previous inferences that C. ebraeus exhibits no genetic differentiation between Okinawa, Guam and Hawaii at the mitochondrial locus. Finally, DNA-based analyses show that eunicid polychaetes comprise the majority of the prey items of C. ebraeus at Guam; while this results compares well with observed diet of this species at Okinawa, C. ebraeus preys predominantly on nereid polychaetes at Hawaii.
Conclusions/Significance
These results imply that strong selection pressures affect conotoxin allelic frequencies. Based on the dietary information, the selection may derive from geographic variation in dietary specialization and local coevolutionary arms races between Conus and their prey.
Introduction
Members of the predatory marine gastropod genus Conus utilize a complex venom comprised of numerous neurotoxic peptides, termed conotoxins, to paralyze prey [1]. Conotoxins are encoded by members of large gene families and target a variety of different ion channels and cell receptors in prey [1]. Previous analyses of conotoxin gene family evolution reveal that conotoxins are subject to very strong positive selection [2]–[6]. Because venoms are used primarily to subdue prey, the selective forces responsible for the evolution of venom components are likely associated with predator-prey interactions. In particular, an arms race may occur between the conotoxins of Conus and the neuronal receptors and ion channels of prey, or Conus venoms may evolve to track changes in dietary specializations [4]. Although it is difficult to specifically test these hypotheses, information on the ecological and genetic correlates of variation in venom composition within species will enhance our understanding of the forces driving Conus venom evolution.
Snakes [7]–[10] and scorpions [11] show geographic variation in venom composition and these differences are potentially associated with differences in diets among populations of snakes, although this relationship is controversial [8], [9], [12], [13]. For example, Daltry et al. [9] reported that venom composition and diets of populations of a Malayan pitviper are correlated, but it is unclear what factors were responsible for the differences in venom composition among populations because venoms were characterized based on differences in isoelectric focusing patterns of whole venoms. Results from other venomous snakes suggest that coevolutionary arms races drive the evolution of venoms [14]. Nonetheless, very few studies have directly investigated intraspecific patterns of variation of genes involved with envenomation.
Recently, Duda and Lee [15] observed significant differences in allelic frequencies of two conotoxin loci of Conus miliaris at Easter Island, a population that has undergone ecological release at this isolated location and exhibits a broader dietary breadth than at other locations in the Indo-West Pacific [16]. These results suggest that strong selection pressures associated with dietary specialization affect venom composition of Conus and similar phenomena may affect the evolution of venoms of other taxa.
Conus ebraeus is one of the most widely distributed members of its genus and occurs in shallow water, tropical regions throughout the Indo-West and eastern Pacific, from the Red Sea to the shores of the Americas [17]. Duda and Palumbi [5] detected a polymorphic O-superfamily conotoxin locus of C. ebraeus (conotoxin locus E1) and identified two alleles at this locus. These alleles differ at nine nonsynonymous sites that are responsible for 7 amino acid substitutions within the 28 amino acids of the mature conotoxin peptides [5].
To identify the selective forces that drive the differentiation of venoms within species of Conus we examined intraspecific patterns of variation at the E1 locus of C. ebraeus at Okinawa, Guam and Hawaii. In particular, we genotyped individuals from Okinawa and Guam in the western Pacific and Hawaii in the central Pacific and compared allelic frequencies among locations. Previous phylogeographic analyses of mitochondrial cytochrome oxidase I (COI) sequences of populations of C. ebraeus in the Indo-West Pacific show no evidence of population genetic structure among samples from these regions [18], a pattern that is not necessarily unexpected given the potential for wide dispersal in this species due to a three to four week planktonic larval stage [19]. Nonetheless, because the sample size from Guam was relatively low (n = 10), we obtained COI sequences from additional individuals from Guam to further scrutinize the apparent lack of genetic differentiation of C. ebraeus at these locations. While diets of C. ebraeus from Hawaii and Okinawa have been described, information from Guam was not previously known. Thus to determine if differences in dietary specialization among samples of C. ebraeus from these three locations are associated with any observed differences in allelic frequencies at conotoxin locus E1, we described diets of individuals from Guam and compared these data to published information on diets of C. ebraeus from Okinawa [20] and Hawaii [21].
Methods
Specimen and fecal sample collections
We obtained specimens of C. ebraeus from Sesoko Island, Okinawa; Pago Bay, Guam; and Kahe Point, Oahu, Hawaii. These locations are similar in that they consist of shallow subtidal reef habitats. For specimens from Guam, we placed single snails in individual containers (3 ounce plastic cups) with approximately 100 ml of seawater. Containers were examined every 6–12 hours to determine if specimens had defecated. When feces were observed, they were removed with small plastic pipettes and placed in 2 ml centrifuge tubes. As much seawater as possible was then removed from samples and approximately 2 ml of 95% ethanol was added to each vial. Venom ducts of specimens from Guam and Hawaii were dissected from individual snails and stored in RNAlater (Ambion) as per manufacturer's recommendations. Other snail tissues were stored in 70–95% ethanol.
Conotoxin E1 allelic sequences and analyses
We prepared cDNA from venom duct mRNA as described previously [4] from 18 individuals of C. ebraeus from Hawaii, 29 individuals from Guam and 5 individuals from Okinawa. We extracted genomic DNA (gDNA) using the E.Z.N.A.™ Mollusc DNA Kit (Omega Bio-Tek, Doraville, Georgia, USA) from approximately 25 mg of foot tissue of an additional 13 individuals from Okinawa.
The primers TOX1 (CATCGTCAAGATGAAACTGACGTG) and TOX2 (CACAGGTATGGATGACTCAGG) of Duda and Palumbi [4] were used to amplify alleles of locus E1 from cDNA. Because O-superfamily conotoxin genes contain a large intron just upstream from the conotoxin coding region, we designed locus-specific primers to amplify locus E1 from gDNA (preE1: AAACTCCAAGTGGACCAGGGAATG; 3utrE1: GGAAATATCAGGCGCCCCACG).
Amplification products from all but 19 of the individuals from Guam were cloned using a TA cloning kit (Invitrogen). Inserts from at least five positive clones of each product were sequenced, giving greater than 93% probability that both alleles of heterozygotes were detected (i.e., P = 1−0.5(n−1), where P = the probability that both alleles are detected and n = the number of inserts sequenced), or until two distinct allelic sequences were identified. Because sequences of a second, apparently monomorphic locus were detected from two individuals from Okinawa and Guam (i.e., locus E2 from [22]), additional inserts were sequenced from these individuals until at least five E1 allele sequences were obtained. On average we sequenced 9 inserts per amplification product.
Sequences of E1 alleles were aligned in Sequencher 4.8 using the contig assembly tool with assembly parameters set to 100% sequence identity to identify sets of identical sequences from multiple individuals. All putative amplification artifacts were removed from subsequent analyses.
We directly sequenced amplification products from cDNA of 19 individuals from Guam as well as from cDNA and gDNA of the 18 individuals from Okinawa (amplifications of the individuals from Okinawa were also cloned as described above; the direct sequencing was conducted to verify results from cloning). The resultant chromatograms of these sequences were examined for the presence of double peaks and genotypes of individuals were determined based on confirmed allele sequences obtained previously from other individuals.
We examined the relationships of alleles and their geographic segregation by constructing a statistical parsimony network [23] with TCS 1.21 [24]. To determine if significant differences in allelic frequencies occur among samples from the three locations, we estimated pairwise F-statistics and conducted an analysis of molecular variance (AMOVA) [25] with Arlequin [26]. The program performed 10,010 permutations to assess the degree to which obtained estimates were different from those obtained from a random assignment of alleles to populations. We used Modeltest 3.7 [27] to determine the most appropriate model of nucleotide substitution and used this model in all relevant calculations in Arlequin.
COI sequences and analyses
We extracted DNA using the E.Z.N.A.™ Mollusc DNA Kit (Omega Bio-Tek, Doraville, Georgia, USA) from approximately 25 mg of foot tissue of 22 specimens of C. ebraeus from Guam. We amplified a region of approximately 644 bases of the mitochondrial COI gene with Folmer primers LCO1490 and HCO2198 [28]. We prepared the products for cycle sequencing by diluting 1∶5 in sterile water. Sequencing was performed in both directions at the University of Michigan DNA Sequencing Core. We analyzed chromatograms with Sequencher 4.8 (Gene Codes Corporation, Ann Arbor, Michigan, USA) and used a text editor to align sequences to published COI sequences of 18 individuals of C. ebraeus from Okinawa, 10 individuals from Guam and 17 from Hawaii (GenBank Accession numbers EF547559-EF547576, EF547612-EF547628 and EF547602-EF547611).
We constructed a statistical parsimony network [23] of COI haplotype sequences with TCS version 1.21 [24]. We calculated pairwise Φ ST values and conducted an AMOVA with Arlequin version 2.0 [26]. As above, 10,100 permutations of the data were performed to determine the degree to which obtained estimates were different than those obtained from a random assignment of haplotypes to populations. As in Duda and Lessios [18], we used Tamura-Nei distances [29] in all relevant calculations.
Identification of prey
In most cases, feces of C. ebraeus contain small pieces of undigested prey and Conus individuals typically consume a single prey item every other night [21]. Feces of specimens of C. ebraeus from Guam were examined with a compound microscope. Based on characteristics of setae, acicula and jaw parts present in feces we tentatively identified prey as eunicid (Order Eunicida, Family Eunicidae) or nereid (Order Phyllodocida, Family Nereididae) polychaetes (i.e., the presumed prey of C. ebraeus [21], [30]–[33]). We extracted DNA from single pieces of feces with the E.Z.N.A.™ Mollusc DNA Kit (Omega Bio-Tek, Doraville, Georgia, USA) as per manufacturer's recommendations. We amplified a region of the mitochondrial 16S gene with annelid-specific primers 16SANNF2 (GCGGTATCCTGACCGTGCWAAGGTA) or 16SANNF3 (GTATCCTGACCGTGCWAAGGTAGC) and 16Spr1 (CTCTAAGCCAACATCGAGGTG); the two downstream primers, 16SANNF2 and 16SANNF3, were modified from the annelid-specific primer 16SANNF (GCGGTATCCTGACCGTRCWAAGGTA) designed by Sjölin et al. [34]. In some cases, two rounds of PCR were needed to produce sufficient quantities of template for sequencing. Amplification products were diluted 1∶5 in water and used directly as sequencing templates. Sequencing was performed in both directions at the University of Michigan DNA Sequencing Core facilities. Chromatograms were examined with Sequencher 4.8 (Gene Codes Corporation, Ann Arbor, Michigan, USA). Sequences were aligned using Se-Al v2.0a11 [35] to sequences of polychaetes from GenBank. Phylograms were constructed using neighbor-joining based on Kimura 2-parameter distances with PAUP* [36].
Comparisons of diets among populations
We compared published data on diets of C. ebraeus from Okinawa (reference) and Hawaii [21] to data we recovered from individuals from Guam. We used data from individuals with shell lengths ≥13 mm (i.e., adult snails) from Okinawa and combined information of prey items recovered from the alimentary tracts of adult snails from multiple locations from Hawaii. We calculated proportional similarity indices (PSI) [37] to estimate the levels of dietary differences at Guam and Hawaii; PSI values are independent of sample size [38]. We also used G-test of independence and Fisher's exact test [39] for comparisons in which expected frequencies were <5 to test if diets differ significantly among locations.
Results
Conotoxin E1 alleles
We obtained E1 genotypes for 18 individuals of C. ebraeus from Hawaii, 29 individuals from Guam and 18 individuals from Okinawa (Table 1). The alleles inferred from direct sequencing of amplification products from individuals from Okinawa were identical to those inferred from cloning. Genotype information for two additional individuals from Hawaii were obtained from data reported in Duda and Palumbi [5]. While the E1a allele reported here is the same as that reported by Duda and Palumbi [5], their E1b allele corresponds to our E1d allele.
Table 1. Sample size, numbers of alleles and diversity statistics of Conus ebraeus conotoxin locus E1.
| Location | Number of individuals | Number of alleles (unique1) | Gene diversity (SE2) | Nucleotide diversity (SE2) |
| Okinawa | 18 | 9 (4) | 0.837 (0.039) | 0.125 (0.065) |
| Guam | 29 | 6 (2) | 0.682 (0.041) | 0.092 (0.048) |
| Hawaii | 20 | 4 (1) | 0.553 (0.070) | 0.088 (0.046) |
number of unique alleles observed.
standard error.
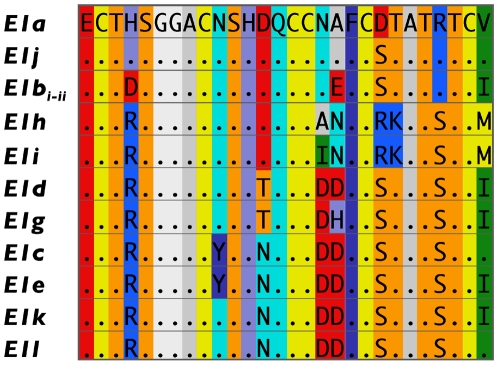
We identified 12 alleles from the 67 individuals examined (GenBank accession numbers FJ804530-FJ804536 and FJ834433-FJ834437) (Table 1). These alleles exhibit 16 polymorphic sites and differ at 1 to 11 nonsynonymous sites within the toxin coding region of the sequences that are responsible for 1 to 8 amino acid substitutions (Figs. 1, 2). Allele E1bii differs uniquely from all other alleles at a nonsynonymous site in the prepro region of the transcript, the only substitution observed outside of the toxin coding region. Although all nucleotide substitutions are associated with amino acid substitutions, a few occur in codons that exhibit two or more changes and in these cases one of the possible substitutional pathways involves a synonymous substitution. But otherwise no synonymous substitutions were observed among alleles.
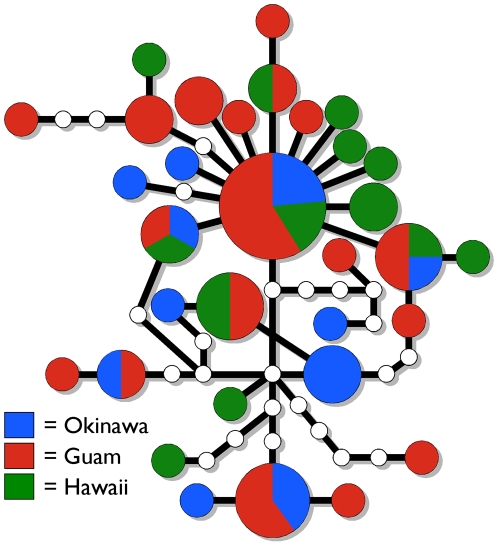
Figure 1. Haplotype network of alleles of conotoxin locus E1 of Conus ebraeus at Guam and Hawaii.
Haplotypes are illustrated as circles; hypothetical haplotypes that were not observed are illustrated as small, empty circles. Areas of circles are proportional to frequencies of alleles; pie diagrams illustrate the allelic frequencies at each location.
Figure 2. Predicted amino acid sequences of the toxin coding region of alleles of conotoxin locus E1 of Conus ebraeus.
Amino acids are provided as single letter codes; dots are used to indicate identity to the amino acid of the first sequence given. To illustrate radical amino acid substitutions, amino acids with similar chemical properties are provided in similar background colors (coloring based on the ‘amino’ scheme utilized in Jmol, a Java viewer for chemical structures (http://www.jmol.org/)). Amino acid sequences of E1bi and E1bii were combined because they exhibit no nonsynonymous substitutions with the toxin coding region.
Three alleles are common and comprise 80.6% of the alleles observed in combined samples from Okinawa, Guam and Hawaii: E1a (45.5%), E1bi (21.6%) and E1c (13.4%). Two of these common alleles (E1a and E1c) occurred at all three locations (Fig. 1), but one (E1bi) was absent from Hawaii even though it was relatively frequent at both Guam (31.0%) and Okinawa (30.6%) and was either the most common allele (Okinawa) or second most common allele (Guam) at these locations. Otherwise, Hawaii has only one other allele in common with Okinawa (E1d), and Guam and Okinawa share just one other allele (E1h). The other seven alleles were unique to single locations (Table 1, Fig. 1), and these alleles were relatively rare with frequencies less than or equal to 10.0% at each location. Okinawa exhibits the greatest diversity of alleles and Hawaii exhibits the lowest (Table 1, Fig. 1). Pairwise Φ ST values calculated from these data were large and significant for all comparisons with the pairwise comparisons between Hawaii and the other two locations yielding the largest values of Φ ST (Table 2). Results from AMOVA showed that while most of the genetic variance was partitioned within populations (89.8%), a significant proportion of the variance was partitioned among populations (10.2%) and gave a highly significant Φ ST value (0.102, P<0.00001).
Table 2. Pairwise Φ ST values among populations of Conus ebraeus estimated from analysis of sequences of conotoxin locus E1 (below diagonal) and mitochondrial COI sequence data (above diagonal).
| Okinawa | Guam | Hawaii | |
| Okinawa | −0.007NS | 0.022NS | |
| Guam | 0.069P = 0.010 | −0.004NS | |
| Hawaii | 0.137P = 0.002 | 0.111P = 0.0011 |
Probabilities that observed Φ ST values deviate from a null hypothesis of no difference between populations were determined from the proportion of 10,100 permutations of haplotypes between populations that gave Φ ST values greater than or equal to the observed Φ ST (NS = not significant).
COI haplotype data
We obtained COI sequences from 22 individuals of C. ebraeus from Guam (GenBank accession numbers FJ804508-FJ804529) and combined these with published sequences from 10, 18 and 17 individuals from Guam, Okinawa and Hawaii respectively [18] (GenBank accession numbers provided in Methods). No obvious structure is apparent in the network constructed from the haplotype sequences (Fig. 3). Moreover, in contrast to results from analysis of conotoxin E1 alleles, the pairwise Φ ST values are small and not significantly different from zero (Table 2). Also, results from AMOVA showed that only a small proportion of the variance was partitioned among populations (0.03%) and gave a small and insignificant Φ ST value (0.0003, P = 0.430).
Figure 3. Haplotype network of COI sequences from 67 individuals of Conus ebraeus at Okinawa, Guam and Hawaii.
Haplotypes are illustrated as circles, with hypothetical haplotypes that were not observed illustrated as small, empty circles. Areas of circles are proportional to the haplotype frequencies; pie diagrams illustrate frequencies of haplotypes at each location.
Dietary analyses
We examined 52 fecal samples of 50 individuals of C. ebraeus from Guam. We recovered putative 16S sequences from 50 of the 52 samples, but 5 of these sequences did not align well with 16S sequences of annelids. Four of these sequences were similar to published sequences of ribosomal RNA genes of bacteria, including Bacillus cereus (n = 3) and Frankia (n = 1). Based on results from NCBI BLASTn searches, the other sequence showed similarity to rRNA sequences of various protists and bacteria, but exhibited poor matches to any particular sequences in GenBank. Because fecal samples of these five samples contained obvious hard parts of eunicid or nereid polychaetes, we suspect that DNAs from prey were highly degraded and our primers amplified templates of other species that were present in the feces or collected along with the fecal materials. The other 45 sequences (GenBank accession numbers FJ804537-FJ804572 and FJ907334-FJ907342) aligned well with either eunicid or nereid polychaete 16S sequences, including 37 sequences that exactly matched or were very similar to published sequences of Palola sp. [40] (Fig. 4). Most sequences (n = 19) were identical to sequences of Schulze's [40] Palola clade A3 that were detected by Schulze at several locations in the western Pacific (e.g., Guam, Pohnpei, Yap) (Fig. 4). The second most common set of 16S haplotypes (n = 10) group with sequences of Palola (clades A4 and A5) but they did not match any Palola sequences reported by Schulze [40] (Fig. 4). Seven other sequences group with Schulze's [40] sequences of Palola clade A9 from Micronesia (Kosrae and Pohnpei) (Fig. 4). Seven of the eight sequences that did not cluster with sequences of Palola in phylogenetic reconstructions exhibited few (0–3) nucleotide substitutions amongst each other and clustered with sequences of various nereids; the eighth sequence grouped with eunicids but was clearly not Palola (Fig. 4). Of the two individuals that defecated twice, one apparently consumed both a nereid and an unidentified eunicid (CebG121, see Fig. 4). Sequences from the other were identical to sequences from Palola clade A3 reported by Schulze [40]; either the individual consumed two Palola worms or feces from the same prey item were defecated twice. Based on microscopic examination, 43 fecal samples contained structures that resembled features of eunicid polychaetes; 9 others contained structures characteristic of nereids. These identifications based on polychaete hard part anatomy were entirely consistent with the DNA-based identifications.
Figure 4. Neighbor-joining tree constructed from Kimura 2-parameter distances [48] of sequences of a region of the mitochondrial 16S gene.
Bootstrap values from 1000 replicates are presented on branches; branches with bootstrap values less than 50% were collapsed. The tree is rooted to a sequence from Arenicola marina (Order Capitellida, Family Arenicolidae). Sequences obtained from feces are indicated in bold; names include the specimen code and fecal sample number. Names of sequences of polychaetes from GenBank are given with their GenBank accession numbers. Sequences from fecal materials of CebG121 and CebG412, the two individuals that defecated twice over a 24-hour period, are indicated with asterisks.
C. ebraeus from Hawaii shows differences in the proportions of eunicids and nereids that are consumed in comparison to individuals from Okinawa and Guam (Table 3) and respective similarity indices are low (Hawaii-Guam: PSI = 0.572; Hawaii-Okinawa: PSI = 0.483). These differences are highly significant for both comparisons G>17 and P<0.0001. On the other hand, eunicids (i.e., Palola sp.) and nereids comprise a nearly equivalent proportion of the diets of snails at Guam and Okinawa (PSI = 0.911) and diets are not significantly different (G = 0.873, P = 0.35; Fisher's exact test: P = 0.67). We conducted all tests based on the frequencies of combined eunicid and nereid polychaetes consumed at each location and did not include frequencies of other polychaete families.
Table 3. Prey recovered from gut contents or feces of Conus ebraeus from locations in the Indo-West Pacific.
Discussion
Our analyses reveal significant differences in allelic frequencies of conotoxin locus E1 of C. ebraeus from Okinawa, Guam and Hawaii (Table 2). On the contrary, results from examination of additional COI sequences from Guam corroborate inferences by Duda and Lessios [18] that C. ebraeus exhibits no genetic differentiation among locations in the western and central Pacific (Table 2). Analysis of fecal samples shows that C. ebraeus at Guam predominantly preys on eunicid polychaetes (Palola sp.) (Table 3; Fig. 4). This latter result is consistent with observations of diets of C. ebraeus at Okinawa [20] as well as at the western Indian Ocean, the eastern Indian Ocean, the Great Barrier Reef and Papua New Guinea in the Indo-West Pacific [30]–[33], but contrasts with data from Hawaii [21] as well as the Seychelles [41] where nereid polychaetes comprise a larger proportion of prey items (see Table 3).
Geographic variation of conotoxin E1 allelic frequencies
The significant differences in allelic frequencies of an expressed conotoxin locus at Okinawa, Guam and Hawaii contrasts strongly with the lack of genetic differentiation observed at COI sequences from these locations (Table 2; Figs. 1, 3). Mitochondrial gene sequences tend to coalesce four times faster than nuclear genes due to the uniparental inheritance and haploid condition of the mitochondrial genome [42]. Thus the significant differences in allelic frequencies at conotoxin locus E1 are unlikely to be solely due to neutral mechanisms (i.e., drift) and so localized selection pressures may have contributed to the genetic differentiation of this locus at these locations. As implied by the strong segregation of E1 alleles in light of the apparent lack of genetic structure observed at mitochondrial gene sequences, selection may be strong enough to counteract the homogenizing effect of gene flow. Alternatively, C. ebraeus may have experienced a recent selective sweep of mitochondrial genomes, but this seems unlikely given the diversity of haplotypes observed (Fig. 3).
We have not examined the functions of the predicted peptide sequences of the E1 alleles, but conotoxin peptides that differ at single amino acid positions show differences in function [43], [44]. Moreover, many of the amino acid substitutions result in charge differences among the translated peptides (Fig. 2) and all nucleotide substitutions among alleles are associated with amino acid substitutions. Thus, the sequence variation exhibited by the E1 alleles is presumably manifested as functional variation in the gene products of these alleles. Aside from alleles E1h and E1i, most of the amino acid variation is exhibited by the three most commonly observed alleles (E1a, E1bi and E1c) and these show six to ten nucleotide substitutions between them that are responsible for four to seven amino acid differences in the translated peptides. One of the common alleles (E1bi) is absent at Hawaii. On the other hand, most of the rare alleles (E1bii, E1d, E1e, E1g, E1j, E1k and E1l) are just a few nucleotide substitutions removed from the three common alleles. Thus, much of the presumed functional diversity of the gene products of the alleles of locus E1 is unequally distributed among these locations. As a whole, Okinawa apparently exhibits the greatest functional diversity at this locus.
Geographic variation in diets of Conus ebraeus
While the focal prey of C. ebraeus are eunicid polychaetes (i.e., members of the genus Palola) at most locations in the Indo-West Pacific (e.g., the Maldives, eastern Indian Ocean, Great Barrier Reef, Okinawa and Guam) [30]–[33], at Hawaii and the Seychelles this species predominantly preys on nereid polychaetes [21], [41]. These results suggest that C. ebraeus exhibits geographic variation in dietary specialization and the diet of this species is distinct at Hawaii and the Seychelles compared to at other locations. The distinctiveness of the diets at Hawaii and the Seychelles could though be explained by seasonal variation or ontogenetic shifts in diets of C. ebraeus, and microhabitat differences in prey availability. Diets of Conus populations are relatively fixed and no major differences in diets of populations have been observed over short time scales, during different times of the year or across short microgeographic scales (A.J. Kohn, University of Washington, personal communication). Thus, we suspect that seasonal changes in diet are unlikely to explain the unique diet observed at Hawaii and Seychelles, especially given the large number of specimens that were examined and extent of the sampling at Hawaii [21]. At Okinawa, C. ebraeus shows definite changes in diet during development: juvenile and subadult individuals (shell lengths <13 mm) prey solely on syllid polychaetes (Order Phyllodocida, Family Syllidae) and adults (shell lengths ≥13 mm) predominantly eat eunicid polychaetes [20]. Nonetheless, we restricted our comparisons to diets of adult snails and so this factor seems unlikely to be responsible for the observed unique diets of C. ebraeus at Hawaii and the Seychelles. Kohn [21] studied diets of C. ebraeus from a variety of locations around the island of Oahu, Hawaii. These locations included several marine bench habitats and several subtidal reef habitats, the latter of which are probably similar in characteristics to the locations where we studied C. ebraeus from Guam and where Duda et al. [20] studied it at Okinawa. Kohn [21] observed that C. ebraeus diets differ among these habitats, presumably reflecting differences in prey availability, with nereid polychaetes comprising a greater proportion of prey items at marine benches than at subtidal reef habitats. Nereids may be more common within the algal mats of reef benches than in subtidal reef habitats (A.J. Kohn, University of Washington, personal communication). But at the Seychelles, C. ebraeus occurred predominantly in microhabitats with few algae and so the preference for nereids at this location cannot be explained by differences in the availability of prey associated with particular microhabitats. Therefore, although we cannot necessarily exclude the possibility that the observed differences in diet of C. ebraeus stem from variation in prey abundance among different microhabitats where this species occurs (especially at Hawaii), it seems unlikely given the data from the Seychelles.
Sources of selection
Because Conus venoms are used primarily to subdue prey, selection at E1 may be associated with geographic differences in dietary specialization, possibly at Hawaii given the greater proportion of nereid polychaetes consumed by C. ebraeus here (but see above). Thompson's [45] coevolutionary alternation with escalation hypothesis posits that predators' specializations for prey alternate and these changes could result in the rapid evolution of characters related to predation in predators. Accordingly, the evolution of Conus venoms may be strongly affected by dietary shifts such that changes in venom composition are driven by changes in specializations for particular prey, a process that presumably occurred in C. miliaris at Easter Island [15]. In particular, differences in the availability of prey between locations may lead to location-specific dietary specializations and localized selection pressures on venoms. But this proposition cannot explain the differences in allelic frequencies at Guam and Okinawa where diets of C. ebraeus are nearly identical (Table 3). Instead, C. ebraeus may exhibit geographic variation in the intensity or direction of selection pressures that emanate from predator-prey interactions such that coevolution of C. ebraeus and its prey exhibit a mosaic pattern of coevolutionary hotspots and coldspots sensu Thompson's (2005) geographic mosaic theory of coevolution. Clearly results from additional investigations are needed to test these hypotheses.
DNA-based dietary analyses of Conus
Although DNA-based methods for identifying diets of various taxa were developed some time ago [46], [47], to our knowledge this is the first time this approach has been utilized for examining diets of Conus. Our current success rate is reasonably high (i.e., roughly 87% of the fecal samples yielded polychaete sequences); improved extraction techniques or different amplification methods could improve this rate. More importantly, the level of resolution in identifying diets of Conus offered from recovery of prey 16S sequences is clearly superior to that provided by traditional morphological-based identification of Conus prey. For example, while DNA-based methods show that prey of C. ebraeus at Guam are members of four divergent clades of Palola sp. (Fig. 4), morphological-based methods would not be able to determine this because the members of these clades exhibit no morphological synapomorphies [40].
Conclusions
We found that C. ebraeus exhibits significant differences in conotoxin allelic frequencies at Okinawa, Guam and Hawaii despite results from analysis of mitochondrial COI sequences that suggest that no population genetic structure occurs among these locations. These patterns imply that selection is responsible for the differences in allelic frequencies of the conotoxin locus and that neutral mechanisms alone cannot explain these observations. Although presumed differences in diet at Hawaii could be associated with differences in allelic frequencies at this location, C. ebraeus at Okinawa and Guam exhibit nearly identical diets and yet allelic frequencies differ significantly between these locations as well. Thus, divergence in allelic frequencies may result from differences in selection pressures not associated with differences in dietary specialization, such as variation in the intensity of predator-prey arms races at different locations. Presumably similar factors generate intraspecific variation in venom composition and the geographic segregation of this variation in other venomous organisms, but additional studies of geographic variation in venom composition and its functional significance are clearly needed to more thoroughly evaluate these assertions.
Acknowledgments
We thank Barry Smith, Alex Kerr, Jason Biggs and others at the University of Guam Marine Lab for coordinating fieldwork at Guam. University of Guam students Marielle Terbio, Chris Rosario, Cabrini Rivera and Jon Lim provided tremendous assistance at Guam. We thank Mike Hadfield and members of the Kewalo Marine Lab for logistical support in collecting at Oahu. We thank Laura Geyer and Alan Kohn for specimens from Okinawa. Alan Kohn, Christian Voolstra and one anonymous reviewer provided valuable comments on earlier drafts that considerably improved this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the United States National Science Foundation (IOS 0718370). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Olivera BM, Walker C, Cartier GE, Hooper D, Santos AD, et al. Speciation of cone snails and interspecific hyperdivergence of their venom peptides potential - Evolutionary significance of introns. Molecular Strategies in Biological Evolution. 1999;870:223–237. doi: 10.1111/j.1749-6632.1999.tb08883.x. [DOI] [PubMed] [Google Scholar]
- 2.Conticello SG, Gilad Y, Avidan N, Ben-Asher E, Levy Z, et al. Mechanisms for evolving hypervariability: The case of conopeptides. Molecular Biology and Evolution. 2001;18:120–131. doi: 10.1093/oxfordjournals.molbev.a003786. [DOI] [PubMed] [Google Scholar]
- 3.Duda TF., Jr Differentiation of venoms of predatory marine gastropods: Divergence of orthologous toxin genes of closely related Conus species with different dietary specializations. Journal of Molecular Evolution. 2008;67:315–321. doi: 10.1007/s00239-008-9155-8. [DOI] [PubMed] [Google Scholar]
- 4.Duda TF, Jr, Palumbi SR. Molecular genetics of ecological diversification: Duplication and rapid evolution of toxin genes of the venomous gastropod Conus. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:6820–6823. doi: 10.1073/pnas.96.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duda TF, Jr, Palumbi SR. Evolutionary diversification of multigene families: Allelic selection of toxins in predatory cone snails. Molecular Biology and Evolution. 2000;17:1286–1293. doi: 10.1093/oxfordjournals.molbev.a026412. [DOI] [PubMed] [Google Scholar]
- 6.Duda TF, Jr, Palumbi SR. Gene expression and feeding ecology: evolution of piscivory in the venomous gastropod genus Conus. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:1165–1174. doi: 10.1098/rspb.2004.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chippaux JP, Williams V, White J. Snake venom variability - methods of study, results and interpretation. Toxicon. 1991;29:1279–1303. doi: 10.1016/0041-0101(91)90116-9. [DOI] [PubMed] [Google Scholar]
- 8.Creer S, Malhotra A, Thorpe RS, Stocklin R, Favreau P, et al. Genetic and ecological correlates of intraspecific variation in pitviper venom composition detected using matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS) and isoelectric focusing. Journal of Molecular Evolution. 2003;56:317–329. doi: 10.1007/s00239-002-2403-4. [DOI] [PubMed] [Google Scholar]
- 9.Daltry JC, Wuster W, Thorpe RS. Diet and snake venom evolution. Nature. 1996;379:537–540. doi: 10.1038/379537a0. [DOI] [PubMed] [Google Scholar]
- 10.Fry BG, Wickramaratna JC, Jones A, Alewood PF, Hodgson WC. Species and regional variations in the effectiveness of antivenom against the in Vitro neurotoxicity of death adder (Acanthophis) venoms. Toxicology and Applied Pharmacology. 2001;175:140–148. doi: 10.1006/taap.2001.9233. [DOI] [PubMed] [Google Scholar]
- 11.Martin MF, Rochat H, Marchot P, Bougis PE. Use of high-performance liquid-chromatography to demonstrate quantitative variation in components of venom from the scorpion Androctonus australis Hector. Toxicon. 1987;25:569–573. doi: 10.1016/0041-0101(87)90293-5. [DOI] [PubMed] [Google Scholar]
- 12.Sasa M. Diet and snake venom evolution: Can local selection alone explain intraspecific venom variation? Toxicon. 1999;37:249–252. doi: 10.1016/s0041-0101(98)00121-4. [DOI] [PubMed] [Google Scholar]
- 13.Wüster W, Daltry JC, Thorpe RS. Can diet explain intraspecific venom variation? Reply. Toxicon. 1999;37:253–258. [Google Scholar]
- 14.Mackessy SP, Sixberry NA, Heyborne WH, Fritts T. Venom of the brown treesnake, Boiga irregularis: ontogenetic shifts and taxa-specific toxicity. Toxicon. 2006;47:537–548. doi: 10.1016/j.toxicon.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Duda TF, Jr, Lee T. Ecological release and venom evolution of a predatory marine snail at Easter Island. PLoS ONE. 2009;4(5):e5558. doi: 10.1371/journal.pone.0005558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohn AJ. Ecological shift and release in an isolated population - Conus miliaris at Easter Island. Ecological Monographs. 1978;48:323–336. [Google Scholar]
- 17. Röckel D, Korn W, Kohn AJ. 1995. Manual of the living Conidae. Wiesbaden, Germany: Verlag Christa Hemmen; 3 v [Google Scholar]
- 18.Duda TF, Jr, Lessios HA. Connectivity of populations within and between major biogeographic regions of the tropical Pacific in a widespread marine gastropod. Coral Reefs (in press) 2009 [Google Scholar]
- 19. Kohn AJ, Perron FE. 1994. Life history and biogeography: Patterns in Conus. Oxford: Clarendon Press; vi, 106 [Google Scholar]
- 20.Duda TF, Jr, Kohn AJ, Matheny AM. in review. Genetic, morphological, and ecological differentiation in a widespread tropical marine gastropod: Conus ebraeus and its cryptic sister.
- 21.Kohn AJ. The ecology of Conus in Hawaii. Ecological Monographs. 1959;29:47–90. [Google Scholar]
- 22.Duda TF, Jr, Remigio EA. Variation and evolution of toxin gene expression patterns of six closely related venomous marine snails. Molecular Ecology. 2008;17:3018–3032. doi: 10.1111/j.1365-294X.2008.03804.x. [DOI] [PubMed] [Google Scholar]
- 23.Templeton AR, Crandall KA, Sing CF. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA-sequence data. 3. Cladogram estimation. Genetics. 1992;132:619–633. doi: 10.1093/genetics/132.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clement M, Posada D, Crandall KA. TCS: a computer program to estimate gene genealogies. Molecular Ecology. 2000;9:1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- 25.Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes - application to human mitochondrial-DNA restriction data. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider S, Roessli D, Excoffier L. Geneva: Genetics and Biometry Laboratory, University of Geneva; 2000. Arlequin ver 2.0. A software for population genetics data analysis. [Google Scholar]
- 27.Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 28.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology. 1994;3:294–299. [PubMed] [Google Scholar]
- 29.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial-DNA in humans and chimpanzees. Molecular Biology and Evolution. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 30.Kohn AJ. Maximal species richness in Conus: diversity, diet and habitat on reefs of northeast Papua New Guinea. Coral Reefs. 2001;20:25–38. [Google Scholar]
- 31.Kohn AJ, Nybakken JW. Ecology of Conus on eastern Indian-Ocean fringing reefs - diversity of species and resource utilization. Marine Biology. 1975;29:211–234. [Google Scholar]
- 32.Marsh H. Observations on the food and feeding of some vermivorous Conus on the Great Barrier Reef. Veliger. 1971;14:45–53. [Google Scholar]
- 33.Reichelt RE, Kohn AJ. Feeding and distribution of predatory gastropods on some Great Barrier Reef platforms. Proceedings of the Fifth International Coral Reef Congress. 1985;5:191–196. [Google Scholar]
- 34.Sjölin E, Erséus C, Källersjö M. Phylogeny of Tubificidae (Annelida, Clitellata) based on mitochondrial and nuclear sequence data. Molecular Phylogenetics and Evolution. 2005;35:431–441. doi: 10.1016/j.ympev.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Rambaut A. Oxford: University of Oxford; 1996. Se-Al: Sequence alignment editor. [Google Scholar]
- 36.Swofford DL. Sunderland, MA: Sinauer & Associates; 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. [Google Scholar]
- 37.Whittaker RH. A study of summer foliage insect communities in the Great Smoky Mountains. Ecological Monographs. 1952;22:1–44. [Google Scholar]
- 38.Kohn AJ, Riggs AC. Sample-size dependence in measures of proportional similarity. Marine Ecology-Progress Series. 1982;9:147–151. [Google Scholar]
- 39.Sokal RR, Rohlf FJ. New York: Freeman; 1995. Biometry: the principles and practice of statistics in biological research.887 [Google Scholar]
- 40.Schulze A. Phylogeny and genetic diversity of palolo worms (Palola, Eunicidae) from the tropical North Pacific and the Caribbean. Biological Bulletin. 2006;210:25–37. doi: 10.2307/4134534. [DOI] [PubMed] [Google Scholar]
- 41.Duda TF, Jr, Kohn AJ, Matheny AM. in preparation. Genetic, morphological, and ecological differentiation in a widespread tropical marine gastropod: Conus ebraeus and its cryptic sister.
- 42.Birky CW, Maruyama T, Fuerst P. An approach to population and evolutionary genetic theory for genes in mitochondria and chloroplasts, and some results. Genetics. 1983;103:513–527. doi: 10.1093/genetics/103.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams DJ, Smith AB, Schroeder CI, Yasuda T, Lewis RJ. omega-conotoxin CVID inhibits a pharmacologically distinct voltage sensitive calcium channel associated with transmitter release from preganglionic nerve terminals. Journal of Biological Chemistry. 2003;278:4057–4062. doi: 10.1074/jbc.M209969200. [DOI] [PubMed] [Google Scholar]
- 44.Lewis RJ, Nielsen KJ, Craik DJ, Loughnan ML, Adams DA, et al. Novel omega-conotoxins from Conus catus discriminate among neuronal calcium channel subtypes. Journal of Biological Chemistry. 2000;275:35335–35344. doi: 10.1074/jbc.M002252200. [DOI] [PubMed] [Google Scholar]
- 45.Thompson JN. Chicago: University of Chicago Press; 2005. The geographic mosaic of coevolution.456 [Google Scholar]
- 46.Höss M, Kohn M, Pääbo S, Knauer F, Schroder W. Excrement analysis by PCR. Nature. 1992;359:199–199. doi: 10.1038/359199a0. [DOI] [PubMed] [Google Scholar]
- 47.Kohn M, Knauer F, Stoffella A, Schroder W, Pääbo S. Conservation genetics of the European brown bear - a study using excremental PCR of nuclear and mitochondrial sequences. Molecular Ecology. 1995;4:95–103. doi: 10.1111/j.1365-294x.1995.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 48.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]