Abstract
Background
Rapid growth of the embryonic heart occurs by addition of progenitor cells of the second heart field to the poles of the elongating heart tube. Failure or perturbation of this process leads to congenital heart defects. In order to provide further insight into second heart field development we characterized the insertion site of a transgene expressed in the second heart field and outflow tract as the result of an integration site position effect.
Results
Here we show that the integration site of the A17-Myf5-nlacZ-T55 transgene lies upstream of Hes1, encoding a basic helix-loop-helix containing transcriptional repressor required for the maintenance of diverse progenitor cell populations during embryonic development. Transgene expression in a subset of Hes1 expression sites, including the CNS, pharyngeal epithelia, pericardium, limb bud and lung endoderm suggests that Hes1 is the endogenous target of regulatory elements trapped by the transgene. Hes1 is expressed in pharyngeal endoderm and mesoderm including the second heart field. Analysis of Hes1 mutant hearts at embryonic day 15.5 reveals outflow tract alignment defects including ventricular septal defects and overriding aorta. At earlier developmental stages, Hes1 mutant embryos display defects in second heart field proliferation, a reduction in cardiac neural crest cells and failure to completely extend the outflow tract.
Conclusions
Hes1 is expressed in cardiac progenitor cells in the early embryo and is required for development of the arterial pole of the heart.
Introduction
Cardiac progenitor cells of the second heart field (SHF) contribute to the rapid growth of the embryonic heart by adding cells to the poles of the heart tube [1]. At the arterial pole, SHF cells give rise to right ventricular and outflow tract (OFT) myocardium in addition to smooth muscle at the base of the great arteries [2]. The SHF originates in splanchnic mesoderm medial to the cardiac crescent from which the linear heart tube is derived and is characterized by the expression of Fgf10, Isl1 and Tbx1 [3]–[5]. The contribution of SHF derived cells to the heart is coordinated with that of cardiac neural crest cells, which are essential for dividing the embryonic OFT into the ascending aorta and pulmonary trunk [6]. Interactions between these two cell types in the pharyngeal region prior to their addition to the heart are critical for normal OFT development; perturbations in SHF or neural crest development results in failure to correctly elongate and divide the OFT, resulting in congenital heart defects including overriding aorta, double outlet right ventricle and common arterial trunk [6]–[8]. Despite recent advances in our understanding of OFT development, the molecular mechanisms maintaining SHF cells in a progenitor state and regulating their contribution to the elongating OFT remain poorly defined.
Notch intercellular signaling has been implicated in multiple aspects of heart morphogenesis including cushion development, trabecular growth, atrioventricular patterning, neural crest differentiation and arterial pole development [9]–[12] (reviewed in [13]). Mutations in the genes encoding the Notch ligand JAGGED1 or the NOTCH2 receptor have been identified in Alagille syndrome, associated with arterial pole defects including tetralogy of Fallot, and mutations in Notch1 are associated with aortic valve disease [14]–[16]. The Hey (Hairy and Enhancer of Split related) family of basic-helix-loop-helix transcription factors is thought to mediate Notch signaling in the developing cardiovascular system [13]. Hey2 mutant mice display arterial pole alignment and ventricular septal defects, associated with activation of the atrial program in ventricular myocardium [17]–[20]. Members of the related Hes (Hairy and Enhancer of Split) family of transcription factors are also activated by Notch signaling and play critical roles in development including maintaining progenitor cell populations, controlling binary cell fate decisions and regulating boundary formation [21]. Hes1, encoding a transcriptional repressor, plays central roles in cell proliferation and differentiation processes in multiple cell types and is required to maintain progenitor cells in an undifferentiated state [22]–[26].
Recently the A17-Myf5-nlacZ-T55 (T55) transgene has been shown to be expressed in the SHF and OFT of the developing mouse heart as a result of an integration site position effect [27]. We have localized the T55 transgene integration site upstream of Hes1 and show that the transgene is expressed in a subset of Hes1 expression sites, including pharyngeal mesoderm of the SHF. Although Hes1 has not been previously implicated in heart development, mice lacking Hes1 display arterial pole alignment anomalies, including overriding aorta and ventricular septal defects, common components of congenital heart disease in man. These defects are preceded by impaired deployment of cells of the SHF and cardiac neural crest revealing a requirement for Hes1 during OFT development.
Results
Characterization of the T55 transgene integration site
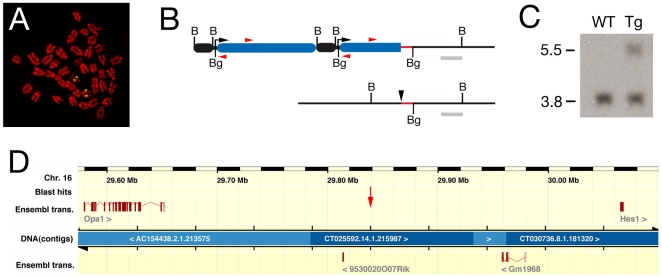
The Myf5 A17-nlacZ-T55 (T55) transgene is expressed in the SHF and OFT as a result of an integration site position effect [27]. In order to identify new genes expressed in the SHF, we characterized the transgene integration site. Using fluorescent in situ hybridization (FISH) the insertion site was mapped to chromosome 16 B2–B4 (Fig. 1A). An inverse PCR approach with primers in the 5′ region of the nlacZ reporter gene was used to isolate a transgene-integration site junction fragment containing 256 bp of flanking sequence (Fig. 1B). Southern blot and PCR analysis confirmed the presence of this junction specifically in DNA from mice carrying the T55 transgene (Fig. 1C, data not shown).
Figure 1. Molecular characterization of the T55 transgene integration site.
(A) Fluorescent in situ hybridization to metaphase chromosomes prepared from T55 splenocytes showing transgene localization to chromosome 16 B2–B4. (B) Map of the T55 integration site (top) and endogenous locus (bottom) showing the structure of the 3′ end of the transgene array (black box, A17 Myf5 enhancer; blue box, nlacZ reporter gene) and the position of the inverse PCR primers (red arrowheads); B, BamHI; Bg, BglII. Also shown are the flanking sequence isolated by inverse PCR (red) and the Southern blot probe (grey) used to identify the predicted 5.5 kb BamHI fragment in transgenic DNA in addition to a 3.8 kb wildtype fragment (C). (D) Ensembl map of the genomic region surrounding the integration site (red arrow) on chromosome 16, showing the position of the flanking genes Opa1 and Hes1 and intermediate gene predictions based on EST alignments.
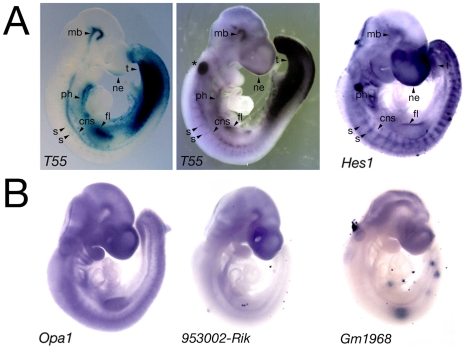
A nucleotide BLAST search using the 256 bp flanking sequence revealed that this sequence mapped to chromosome 16 B2, consistent with the FISH localization (Fig. 1D). The insertion site junction lies between two genes, Optic Atrophy 1 (Opa1), encoding a mitochondrial dynamin-related GTPase, and Hairy/Enhancer of Split 1 (Hes1), encoding a basic-helix-loop-helix containing transcriptional repressor, situated 258 kb and 224 kb from the isolated sequence respectively. Opa1 and Hes1 are part of a 4 gene synteny block centered on Hes1 that is conserved in zebrafish. The expression profiles of Hes1 and Opa1, together with those of 4 EST sequences and 2 predicted genes mapping between Hes1 and Opa1, were evaluated by wholemount in situ hybridization at E9.5 (Fig. 2). Hes1 transcripts showed a regionalized expression profile overlapping in distribution with the T55 transgene (Fig. 2A, Fig. 3), including pharyngeal, forelimb, tail, intersomitic, neural tube, midbrain and nasal ectoderm expression sites (Fig. 2A). In contrast, Opa1 transcripts were broadly expressed in the embryo and riboprobes detecting EST sequences between Opa1 and Hes1, including the predicted genes 9530020007RIK and GM1968, revealed either low-level expression in the anterior region of the embryo or no expression (Fig. 2B). Together, these results suggest that Hes1 may be the endogenous target of the cis-regulatory sequences trapped by the T55 transgene.
Figure 2. Comparison of transgene and integration site gene expression profiles.
(A) E9.5 embryos after X-gal staining (T55 embryo, left) or in situ hybridization with a lacZ (T55 embryo, middle) or Hes1 riboprobe (right). Overlapping expression sites include the ventral pharyngeal region (ph), forelimb (fl), ventral neural tube (cns), segmental intersomitic region (s), midbrain (mb), tail region (t) and nasal ectoderm (ne). Asterisk indicates lacZ riboprobe trapping in the otic vesicle. (B) Expression profiles of Opa1 and two predicted genes mapping to the intergenic region at E9.5.
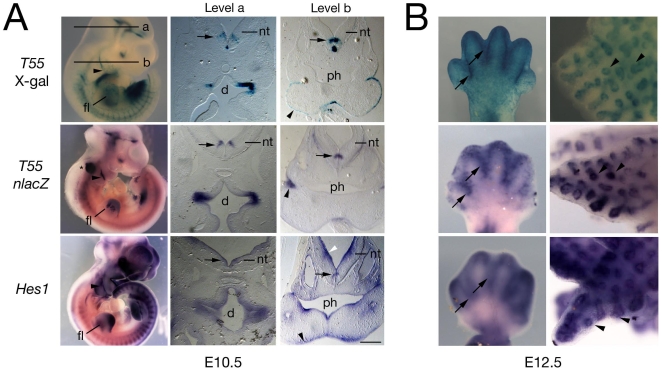
Figure 3. Comparison of T55 and Hes1 expression during embryonic development.
(A) E10.5 wholemount embryos (left) and cryosections after X-gal staining (top) or in situ hybridization with a lacZ (T55 embryo, middle) or Hes1 riboprobe (bottom). The transgene is expressed at a subset of sites of Hes1 expression including the forelimb (fl), pharyngeal region (including ectoderm, arrowheads), neural tube (nt), including a specific population of neurons in the ventral neural tube (arrows) and diencephalon (d); ph, pharynx. Asterisk indicates probe trapping in the otic vesicle. (B) E12.5 forelimb (left) and lung (right) after X-gal staining (top) and nlacZ (middle) or Hes1 (bottom) in situ hybridization, showing similar transgene and Hes1 expression in the interdigital region (arrows) and pulmonary epithelium (arrowheads). Scale bar: 200 µm.
Additional gene expression studies were carried out to test this hypothesis. The distribution of Hes1 transcripts was compared with that of nlacZ, together with β-galactosidase expression, in T55 embryos at E10.5 and E12.5. At E10.5, T55 and Hes1 expression overlapped in pharyngeal epithelia, the pericardial region and the forelimb (Figs 3A, 4A). T55 transcripts accumulated in a subset of Hes1 expression sites in the central nervous system, including specific populations of neurons in the brain and ventral neural tube (Fig. 3A). At E12.5, expression of the transgene and Hes1 was observed in the interdigital region of the developing limb-buds and pulmonary endoderm (Fig. 3B). These experiments revealed that the T55 transgene and Hes1 are co-expressed in a subset of Hes1 expression sites.
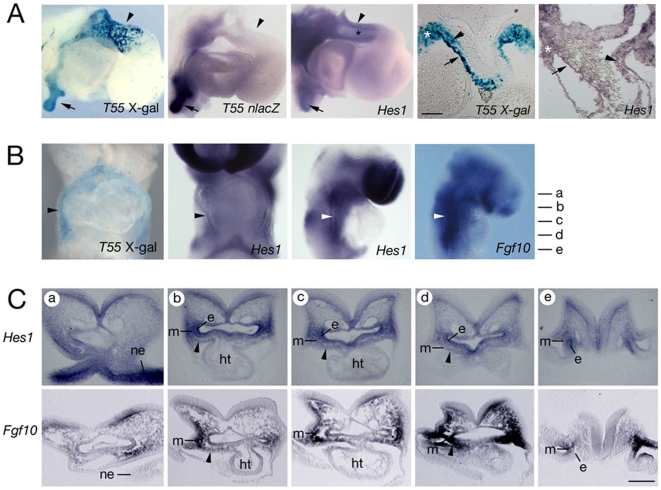
Figure 4. Hes1 is expressed in the second heart field.
(A) Comparison of transgene and Hes1 expression in right lateral views of hearts after X-gal staining and in situ hybridization at E10.5 (left three panels). X-gal positive cells are observed in the distal OFT wall (arrowhead) and pulmonary endoderm (arrow); nlacZ transcripts are observed in pulmonary endoderm but not the OFT. Low-level Hes1 transcript accumulation is observed in the distal OFT (black asterisk). Transverse sections (right two panels) show β-galactosidase and low-level Hes1 transcript accumulation in the pericardial region (white asterisks), superior OFT wall (arrow) and mesenchymal cells in the OFT (arrowhead). (B) At E8.5 X-gal and Hes1 positive cells are observed in ventral and right views in the pericardial wall (black arrowhead) and pharyngeal mesoderm (white arrowheads) where Fgf10 transcripts also accumulate. (C) E8.5 sections at the levels indicated in (B), showing Hes1 transcripts in neuroepithelium (ne), endoderm (e) and mesoderm (m) lateral and ventral to the pharynx, including the SHF (arrowheads), compared with Fgf10 expression. Note that caudally (level e) Hes1 and Fgf10 transcripts are observed in endoderm and mesoderm respectively. ht, heart tube. Scale bars: (A, C) 100 µm.
Hes1 is expressed in the second heart field
In T55 embryos, β-galactosidase activity is detected in the superior wall of the OFT and contiguous pericardial mesoderm at E10.5 [27]; nlacZ transcripts in T55 positive embryos accumulate in pericardial mesoderm but were not observed in the myocardial OFT wall. β-galactosidase activity in the OFT wall is thus the result of either extremely low-level transcription or β-galactosidase perdurance from prior transcription in OFT progenitor cells (Fig. 4A). At this timepoint Hes1 transcripts accumulate in pericardial mesoderm; after extensive staining, Hes1 transcript accumulation was detected in cells in the superior wall of the OFT (Fig. 4A). Hes1 transcripts also accumulate at low-level in cells likely to correspond to cardiac neural crest cells in the distal OFT; T55 transgene expression is also observed in these cells (Fig. 4A). Analysis of earlier developmental timepoints revealed that the T55 transgene is expressed in splanchnic mesoderm dorsal to the heart tube at E8.5 (Fig. 4B) [27]. Endogenous Hes1 transcripts were observed in the dorsal pericardial wall and in mesoderm lateral to the pharynx, in addition to pharyngeal epithelia and neuroectoderm. The expression of Hes1 in pharyngeal mesoderm overlaps with that of Fgf10; however, the mesodermal expression domain of Fgf10 extends further, both in a posterior direction and into cranial mesoderm, than that of Hes1 (Fig. 4C). Hes1 is therefore coexpressed with Fgf10 in pharyngeal mesoderm dorsal to the early heart tube, including the region of the SHF.
Heart defects in Hes1 mutant mice
Hes1 mutant mice die during late fetal development with defects affecting neural, eye, pancreas, pituitary and lung development, among other organs [22]–[26]. This phenotype reflects the critical role of Hes1 in maintaining progenitor cell populations and regulating differentiation during organogenesis [22]. In order to investigate whether Hes1 is required for SHF development, we analyzed cardiac development in Hes1 mutant mice. SHF defects affect OFT formation and arterial pole alignment [1], [4], [7] and embryos were scored at E15.5 for abnormalities in ventriculoarterial alignment. During normal development, the ascending aorta obtains an independent connection with the left ventricle and the pulmonary trunk with the right ventricle. 21/127 Hes1 −/− embryos were recovered (expected 32/127; p>0.05)) and 8/21 (38%) mutant embryos displayed an externally visible dextraposed ascending aorta; this phenotype was not observed in Hes1 +/− or Hes1 +/+ embryos (Fig. 5A). Histological sections of selected embryos revealed that the aorta failed to obtain a dorsal position with respect to the pulmonary trunk resulting in a ventricular septal defect and overriding aorta (observed in 3 out of 4 Hes1 −/− hearts with a dextraposed aorta that were sectioned). The extent to which the aorta overrides the right ventricle was <50% in the hearts scored (Fig. 5). Overriding aorta and ventricular septal defects were not observed in 1 of the 4 sectioned hearts with a dextraposed aorta or in 6 additional Hes1 −/− hearts without an externally visible dextraposed aorta. No defects were observed on histological analysis of 5 Hes1 +/− and 5 Hes1 +/+ hearts. Loss of Hes1 therefore results in defects in normal alignment of the aorta and left ventricle during cardiac remodeling in a significant fraction of mutant embryos.
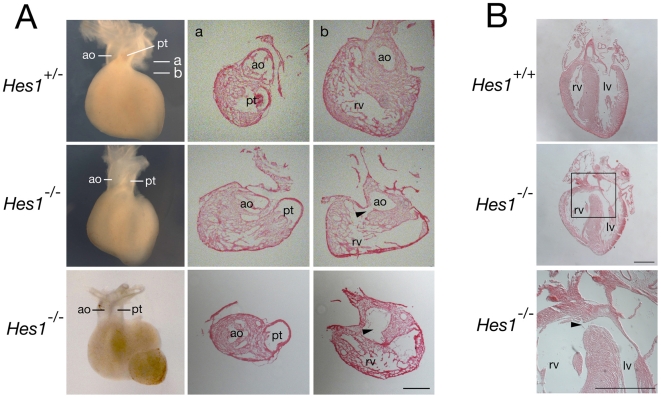
Figure 5. Congenital heart defects in Hes1 mutant mice.
(A) E15.5 hearts in ventral wholemount views (left) and cryostat sections at the levels indicated showing a normal configuration of the ascending aorta (ao) and pulmonary trunk (pt) in Hes1 +/− hearts (top) and a dextraposed aorta overriding a ventricular septal defect (arrowhead) in Hes1 −/− hearts (middle (mild) and bottom (severe)). Note that the aorta in the bottom heart symmetrically overrides the ventricular septal defect. (B) Paraffin sections of E18.5 hearts in a frontal plane showing a ventricular septal defect (arrowhead) in a Hes1 −/− (middle and bottom) but not Hes1 +/+ heart (top). lv, left ventricle; rv, right ventricle. Scale bar: (A) 200 µm; (B) 500 µm.
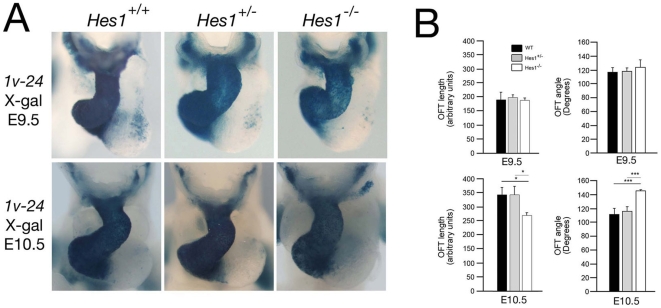
The etiology of this defect was analyzed by examining Hes1 −/− embryos at earlier stages of heart development. In order to visualize SHF progenitor cells and OFT development in mutant embryos, we crossed Hes1 +/− mice with the Mlc1v-nlacZ-24 transgenic line, in which transgene integration upstream of the Fgf10 locus results in expression in pharyngeal mesoderm, including cells of the SHF, OFT and right ventricle (Fig. 6A) [3]. We analyzed OFT morphology at two different days of development (E9.5 and E10.5) and measured OFT length and the angle between the proximal and distal regions of the OFT (Fig. 6B). OFT length and angle in Hes1 −/− embryos was indistinguishable from that of control littermates at E9.5. In contrast, at E10.5, we observed a significantly increased angle between the proximal and distal regions of the OFT compared to somite-matched control littermates; moreover, OFT length was concomitantly decreased (Fig. 6B). These results demonstrate that the Hes1 −/− OFT is shorter and straighter than that of control littermates at midgestation.
Figure 6. Outflow tract morphogenesis in Hes1 −/− embryos.
(A) Ventral whole mount views of X-Gal stained hearts from E9.5 and E10.5 Hes1 +/+, Hes1 +/− and Hes1 −/− embryos carrying the Mlc1v-nlacZ-24 transgene. (B) Histograms showing OFT length measurements and the angle between the proximal and distal region of the OFT from Hes1 +/+, Hes1 +/− and Hes1 −/− hearts at E9.5 and E10.5. At E10.5, but not E9.5, Hes1 −/− embryos display a shorter, straighter OFT (*, p<0.05; ***, p<0.001, Student's t-test).
Loss of Hes1 impairs SHF proliferation without altering differentiation
The decrease in OFT length in Hes1 −/− embryos is suggestive of a defect in SHF development. Hes1 is known to maintain progenitor cell populations in the developing embryo through the regulation of differentiation and proliferation [22]–[26]. We therefore analyzed SHF differentiation at E9.5; no precocious accumulation of sarcomeric myosin heavy chain or α-actinin was observed in splanchnic mesodermal SHF cells in the dorsal pericardial wall of Hes1 −/− embryos at this stage (Fig. 7A). The SHF contributes to both myocardium and smooth muscle at the arterial pole of the heart [2]. Normal myocardial and smooth muscle differentiation at the base of the great arteries were observed in mutant hearts at E12.5 using SM22α and α-actinin immunochemistry (data not shown).
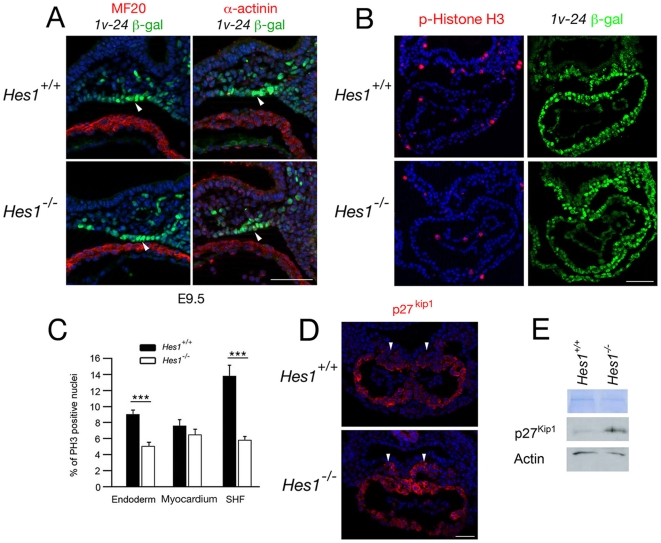
Figure 7. Impact of loss of Hes1 on differentiation and proliferation in the second heart field.
(A) Immunochemistry with anti-MHC (left), anti-α-actinin (right) and anti-β-galactosidase antibodies in transverse sections through the caudal pharyngeal region of an E9.5 embryo carrying the Mlc1v-nlacZ-24 transgene. Note the β-galactosidase positive α-actinin and MF20 negative cells in the dorsal pericardial wall (arrowheads). Nuclei are labeled with Hoechst (blue). (B) Immunochemistry with anti-phospho-Histone H3 and anti-β-galactosidase antibodies in paraffin sections of E8.5 Hes1 +/+ and Hes1 −/− embryos carrying the Mlc1v-nlacZ-24 transgene. (C) Histogram comparing the percentage of phospho-Histone H3 positive nuclei in pharyngeal endoderm, myocardium and SHF of Hes1 +/+ and Hes1 −/− embryos. A decrease in phospho-Histone H3 positive nuclei is observed in Hes1 −/− SHF and endoderm (p<0.001, Student's t-test). (D) Immunochemistry with an anti-p27kip1 antibody in paraffin sections at E8.5. Note that p27kip1 is expanded in the SHF of Hes1 −/− hearts (arrowheads). Nuclei are labeled with Hoechst (blue). (E) Western blot of microdissected heart and ventral pharyngeal regions of Hes1 +/+ and Hes1 −/− embryos showing elevated p27kip1 protein levels in the absence of Hes1. Scale bars (A): 50 µm; (B): 100 µm; (D) 50 µm.
Proliferation within the pharyngeal region of Hes1 −/− embryos was evaluated by scoring for the proliferation markers phospho-Histone H3 and Ki67, revealing M-phase and all proliferating cells respectively. SHF progenitors were identified in the dorsal pericardial wall and pharyngeal mesenchyme at E8.5 using Isl1 or β-galactosidase immunochemistry in Mlc1v-nlacZ-24 embryos. A reduction in the number of phospho-Histone H3 positive (mitotic) Mlc1v-nlacZ-24 positive cells was observed, from 13.7% in wildtype embryos to 5.7% in Hes1 −/− embryos (p<0.001; Fig. 7B, 7C). Moreover, 11.7% of Mlc1v-nlacZ-24 positive cells were Ki67-negative (non-proliferating) in mutant embryos compared to 5.7% in wildtype embryos (p<0.001; data not shown). A reduction in proliferating cell numbers was also observed in pharyngeal endoderm from 9% in wildtype embryos to 5% in mutant embryos (p<0.001). These results suggest that cell cycle progression in the SHF and pharyngeal endoderm is impaired in mutant embryos. In contrast, no differences in the percentage of phospho-Histone H3 or Ki67 positive cells were observed in the linear heart tube of Hes1 −/− embryos at the same E8.5 timepoint. Equivalent low numbers of Caspase 3-positive cells were found in the pharyngeal region of wildtype and mutant embryos, suggesting that apoptosis levels are unaltered in the absence of Hes1 (data not shown).
Hes1 has been shown to control proliferation through the transcriptional repression of cyclin-dependent kinase inhibitors including p27kip1 and p57kip2 [28], [29]. We investigated p27kip1 distribution in the SHF of Hes1 −/− embryos and observed an expansion of p27kip1 positive cells in SHF cells in the dorsal pericardial wall proximal to the dorsal mesocardium in 3/4 Hes1 −/− hearts (Fig. 7D). Immunoblot analysis revealed a 2.15 fold increase in p27kip1 levels in microdissected ventral pharyngeal regions including the SHF in the absence of Hes1 (Fig. 7E). Together, these results suggest that loss of Hes1 perturbs proliferation, but not differentiation, of SHF progenitor cells, associated with elevated p27kip1levels.
Cardiac neural crest cell defects in Hes1 mutant embryos
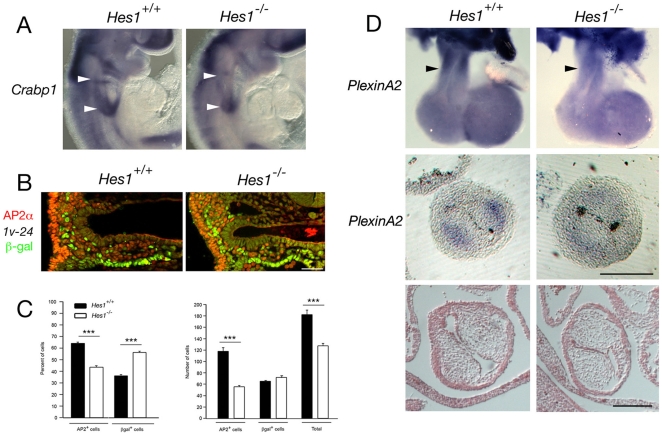
Cardiac neural crest cells are critically required for OFT septation and normal SHF deployment [6]. In addition to the above defects in SHF development we observed a reduction in Crabp1 expression in the region of the 3rd and 4th–6th pharyngeal arches in Hes1 −/− embryos at E9.5 (Fig. 8A). Transverse sections of the caudal pharynx at E9.5 revealed a reduction in AP-2α positive neural crest cells underlying the pharynx (Fig. 8B). Quantification of neural crest (AP-2α positive) and SHF (Mlc1v-nlacZ-24 β-galactosidase positive) nuclei revealed a significant decrease in neural crest cell numbers in mutant embryos compared to wildtype littermates (Fig. 8B, 8C). Subsequently, reduced PlexinA2 transcript accumulation was observed in the distal OFT of Hes1 −/− embryos at E11.5 (Fig. 8D). Analysis of OFT cushion morphology at E11.5 revealed normal cushion development in the distal region of the OFT although slight hypocellularity was noted in proximal OFT cushions (Figs 8D, data not shown). Loss of Hes1 therefore affects two critical cell populations entering the arterial pole of the heart, the SHF and cardiac neural crest cells, leading to impaired heart tube elongation and arterial pole alignment defects at later stages of development.
Figure 8. Loss of Hes1 impairs neural crest development.
(A) In situ hybridization at E9.5 showing decreased Crabp1 transcript accumulation in the caudal pharyngeal region of Hes1 −/− compared to Hes1 +/+ embryos (arrowheads). (B) Immunochemistry with anti-AP-2α (red) and anti-β-galactosidase (green) antibodies in a transverse section through the caudal pharynx of an E9.5 embryo carrying the Mlc1v-nlacZ-24 transgene. (C) Histograms showing the percentage of β-galactosidase positive nuclei and the percentage of AP-2α positive nuclei in the pharyngeal region of Hes1 +/+ and Hes1 −/− embryos (left) and the numbers of total, β-galactosidase positive and AP-2α positive nuclei (right). Note the decrease in the number of AP-2α positive cells in Hes1 −/− embryos (p<0.001, Student's t-test). (D) In situ hybridization at E11.5 showing decreased PlexinA2 transcript accumulation in the OFT of Hes1 −/− compared to Hes1 +/+ hearts (black arrowheads) in wholemount (top) and transverse sections (middle). Histological analysis reveals normal OFT cushion morphology in Hes1 −/− embryos at E11.5 (bottom). Scale bars (B): 50 µm; (D): 200 µm.
Discussion
Here we identify Hes1 as a transcription factor encoding gene expressed in the SHF and show that Hes1 is required for normal OFT development. Characterization of the insertion site of the T55 transgene reveals that Hes1 is the likely endogenous target of regulatory sequences trapped by the transgene. The integration site lies within a region of conserved synteny between mouse and zebrafish that may correspond to a genomic regulatory block, such as have been proposed to contain conserved non-coding elements targeting developmental regulatory genes [30]. Our data suggest that regulatory elements within this block control the spatiotemporal expression pattern of Hes1. Comparison of T55 and Hes1 expression profiles suggest that the transgene has trapped only a subset of the cis-regulatory elements mediating Hes1 activation. In contrast, sites of β-galactosidase accumulation but not Hes1 expression may result from inherent differences in transcript versus β-galactosidase protein stability. Further differences may arise from the fact that Hes1 is highly regulated both transcriptionally and post-transcriptionally; oscillations in Hes1 protein levels play a critical role in measuring developmental time in unsegmented paraxial mesoderm [21]. β-galactosidase activity in a subregion of OFT myocardium may thus in part provide a readout of prior heterogeneity in Hes1 expression in SHF progenitor cells.
Hes1 is known to be a downstream component of the Notch signaling pathway and a critical regulator of multiple stem cell populations in the developing embryo [22]. Accelerated differentiation in Hes1 mutant embryos results in depletion of progenitor cell populations and a failure of late differentiating cell types [22]–[26]. Here we show that Hes1 is required for normal deployment of SHF progenitor cells. However, we did not observe accelerated differentiation in the SHF of Hes1 −/− embryos. A possible explanation lies in the observation that Hey1 and Hey2 transcripts are also detectable in pharyngeal mesoderm (data not shown). Hes1 can heterodimerize with Hey1 to effect strong transcriptional repression and Hes1, Hes3 and Hes5 are known to have overlapping functions in the developing nervous system, uncovered by analysis of compound null embryos [31], [32]. Hes1 may therefore overlap in function with other members of the Hes and Hey gene families to regulate SHF development. In support of this argument, overriding aorta has been observed in embryos lacking Hey2 [17]–[20].
Hes1 also maintains progenitor cell populations in the developing embryo by regulating proliferation. In particular, Hes1 represses transcription of the CDK inhibitors p27kip1 and p57kip2 [28], [29]. We observed that levels of the mitotic marker phospho-Histone H3 are reduced in the SHF of Hes1 mutant embryos at E8.5, whereas the number of Ki67-negative quiescent cells is increased. p27kip1 protein distribution at this stage reflects the low proliferative nature of differentiated cells in the linear heart tube. We observed that p27kip1 was expanded and upregulated in the dorsal pericardial wall of Hes1 mutant embryos, consistent with release from Hes1 mediated repression and providing new mechanistic insights into the regulation of SHF proliferation.
In addition to reduced proliferation in the SHF, the Hes1 cardiac phenotype is likely to be compounded by the observation that less neural crest cells are found in the ventral pharyngeal region and distal OFT of mutant embryos. Hes1 −/− embryos have defects in neural development that may affect the number of migrating neural crest cells [23] and low-level Hes1 transcript accumulation is observed in neural crest cells in the distal OFT. Alternatively, loss of endodermal or SHF signals may indirectly impact on neural crest cell numbers. Neural crest ablation in avian embryos results in impaired SHF deployment and a shortened OFT [6], [8]; reduction of neural crest in the caudal pharynx could thus in turn influence SHF development in Hes1 −/− embryos. OFT alignment defects in Hes1 mutant embryos have been independently observed by another group who, in addition, noted aortic arch artery abnormalities associated with a defective smooth muscle contribution at E11.5 (P. Scambler, personal communication). Neural crest cell anomalies may therefore also contribute directly to the arterial pole phenotype of Hes1 mutant embryos. While we did not observe defects in great artery smooth muscle development, slight hypocellularity of OFT cushions could result from reduced neural crest cell numbers. Alternatively, epithelial-mesenchymal transition of OFT endothelial cells, known to be regulated by Notch signalling [9], may be defective in Hes1 −/− embryos, possibly due to a later role of Hes1 in the OFT itself. Dissecting the requirement for Hes1 in different cell-types by conditional mutagenesis will determine the relative importance of different sites of Hes1 expression (including pharyngeal mesoderm, endoderm, ectoderm, neural crest and endothelial cells) during OFT morphogenesis.
The early defect in OFT elongation is likely to underlie the subsequent ventriculoarterial alignment defects observed in fetal Hes1 −/− hearts. Intriguingly, a dextraposed aorta and ventricular septal defect are observed in less than half of mutant embryos whereas a shorter OFT was observed in all mutant embryos scored at E10.5. Given that there is no loss of Hes1 −/− embryos between E10.5 and E15.5, this suggests that there is a degree of phenotypic recovery. The embryonic OFT obtains its maximal length at E10.5 and subsequently becomes incorporated into the ventricular outlets and non-myocardial base of the great arteries; OFT length in Hes1 −/− embryos may be on the borderline of that required to achieve normal alignment.
Overriding aorta and ventricular septal defects are common components of human congenital heart defects. Defects in OFT development in human patients carrying JAGGED1 or NOTCH2 mutations or in mice carrying mutations in Notch ligands, receptors or target genes demonstrate the importance of this intercellular signaling pathway in arterial pole development [13]–[20]. Notch signaling has also been implicated in neural crest and valve development [9], [12], [16]. The regulation of SHF progenitor cell deployment during heart tube extension may be an additional mechanism by which Notch intercellular signaling controls heart development. Future investigation of the upstream control of Hes1 expression in the SHF will test this hypothesis.
Materials and Methods
Mice
A17-Myf5-nlacZ-T55 (T55) and Mlc1v-nlacZ-24 transgenic mice have been previously described [3], [27]. Hes1+/− mice were kindly provided by François Guillemot (NIMR, UK) and maintained on a CD1 outbred background [23]. DNA isolated from tail-tips or yolk sacs was genotyped for Hes1 alleles as described [33]. Mlc1v-nlacZ-24 and A17-Myf5-nlacZ-T55 transgenic mice were genotyped by X-gal staining or by PCR [3], [27]. Mouse care and procedures were in accordance with guidelines approved by the Departmental Direction of Veterinary Services of the French Ministry of Agriculture.
Isolation of a transgene integration site junction fragment
Inverse PCR was performed using SphI and BglII digested T55 hemizygous tail-tip DNA, as described [3]. Primers A and B were used for inverse PCR using the Expand PCR system (Roche). A 2.6 kb PCR product containing 256 bp of flanking sequence was subcloned using the pGemT-Easy vector (Promega). DNA sequences were aligned using NCBI BLAST, DNA Strider (Release 1.4) and the Ensembl mouse genome browser. Southern blot mapping was carried out with a 770 bp probe generated by PCR using primers C and D that detects a 3.8 kb wildtype fragment and a 5.5 kb fragment subsequent to transgene integration in BamHI digested genomic DNA. PCR validation was carried out using primers E, F and G. Primers E and F generate a 500 bp product from the wildtype locus and F and G a 374 bp product after transgene integration.
Oligonucleotide primer sequences
A 5′-AAAACGACGGGATCATCGCGAGCCATGC-3′
B 5′-ACTGGAGGCTGAAGTTCAGATGTGCGGC-3′
C 5′-GGTGGCAGCAGTAATCCATGATCTTG-3′
D 5′-TGTCAAATCAGATCTCTCCAGATGCC-3′
E 5′-CCTGCTGCTTTGGACAATGC-3′
F 5′-AAGTCGTTGGAGACCTTGTC-3′
G 5′-ACCGCTGGATAACGACATTG-3′
Wholemount analysis, histology and immunochemistry
Embryos were dated taking the day of the plug as embryonic day (E) 0.5. Hearts were dissected at E15.5 and analyzed using a Zeiss Lumar stereo dissecting microscope; selected hearts were subsequently analyzed after cryostat or paraffin embedding and sectioning using standard techniques. Sections were stained with haemotoxilin and eosin or treated for 15 min with antigen unmasking solution (Vector) prior to immunohistochemistry using standard protocols. Primary antibodies used were: β-galactosidase (1/300, Cappel), MF20 (1/20, DSHB), AP-2α (1/25, DSHB clone 3B5), α-actinin (1/200, DSHB), p27kip1 (1/200, Cell Signaling), phospho-Histone H3 (1/400, Upstate), Ki67 (1/25, Dako), Isl1 (1/100, DSHB clones 402D6 and 394D5) and Caspase 3 (1/100, Cell Signaling). Secondary antibodies used were: anti-mouse Alexa 488 (Interchim), anti-mouse Cy3 (Jackson), anti-rat biotin (Jackson), anti-rabbit Alexa 488 (Interchim) and anti-rabbit Cy3 (Jackson). All secondary antibodies were used at a 1/200 dilution. The anti-rat biotinylated secondary was amplified using the Renaissance TSA Fluorescence System (Perkin Elmer), according to the manufacturer's instructions. Slides were counterstained with Hoechst and observed using an ApoTome microscope (Zeiss). Mesenchymal Ap-2α positive nuclei were scored at E9.5 in 5 or more 7 µm sections, 14 µm apart, from 3 mutant and 3 control embryos. X-gal staining was carried out as described [3]; embryos were collected and fixed for 10 min in 4% paraformaldehyde, extensively washed in 1× PBS and stained for 5 hours at 37°C in a solution containing 4 mg/ml of X-gal. After staining, the samples were washed in PBS, post-fixed and observed under a Zeiss Lumar stereomicroscope. For each experiment a minimum of 3 embryos of each genotype was scored. OFT length and angle were measured from stereotyped ventral views of X-gal stained Mlc1v-nlacZ-24 embryos at E9.5 (24–26 somites) and E10.5 (30–35 somites) using Metamorph software.
Immunoblotting
Protein lysates were obtained from microdissected E8.5 hearts and ventral pharyngeal regions of control and mutant embryos. Proteins were separated by SDS-PAGE and transferred to Hybond-C extra membranes (Amersham Biosciences) prior to blocking with 5% dry milk for 2 hours. Membranes were incubated overnight at 4°C with primary antibodies (anti-actin, diluted 1/2000, Sigma; anti-p27kip1, diluted 1/1000, Cell Signaling Technology) followed by chemiluminescent detection (Western Lightening, Enhanced Luminol reagent, Perkin Elmer). Quantification of immunoblots was carried out using Image Quant TL software (Amersham Biosciences).
In situ hybridization
Whole mount in-situ hybridization was performed as previously described [3]. The Hes1 riboprobe was synthesised by T7 RNA polymerase from a 553 bp template amplified by PCR using primers Hes1F and Hes1R:
Hes1F 5′-GAGAGGCTGCCAAGGTTTTT-3′
Hes1R 5′-GTAATACGACTCACTATAGGGTCAAATAAACTTCCCCAAAGGA-3′
The following riboprobes were prepared from plasmid templates: Crabp1 [34], Fgf10 [3], Isl1 [35], PlexinA2 [36], lacZ [3]. Riboprobes for Opa1, 953002-Rik, Gm1968, Hey1 and Hey2 were generated using T7 RNA polymerase from PCR amplified templates. Primer details are avaliable on request. For each experiment a minimum of two embryos per genotype were scored.
Fluorescent in situ hybridization
Fluorescent in situ hybridization was carried out as described [37]. Briefly, metaphase spreads were prepared from male mice carrying the T55 transgene. Concanavalin A-stimulated splenic cells were cultured for 72 hours with 5-BrdU added for the final 6 hours of culture (60 mg/ml of medium) to ensure chromosomal R-banding. The biotinylated lacZ probe was mixed with hybridization solution at a final concentration of 50 mg/ml and used at 200 ng per slide after competition of aspecific repetitive sequences. The hybridized probe was detected by fluorescent isothiocyanate-conjugated avidin (Vector laboratories). Chromosomes were counterstained with propidium iodide. A total of 50 metaphase cells were analyzed.
Cell proliferation and apoptosis analysis
Proliferation was evaluated by immunochemical detection of phospho-Histone H3 and Ki67 at E8.5. Data were obtained from at least four sections per embryo for three embryos of each genotype. Equivalent total cell numbers were scored in wildtype and homozygous null embryos. For phospho-Histone H3 mean total cell numbers counted per section were 56.1 (endoderm), 59.6 (heart) and 49.4 (Mlc1v-nlacZ-24 transgene positive SHF cells) for wildtype embryos and 59.2 (endoderm), 52.3 (heart) and 42.5 (Mlc1v-nlacZ-24 transgene positive SHF cells) for Hes1 −/− embryos. For Ki67 mean total Mlc1v-nlacZ-24 transgene positive SHF cell numbers counted per section were 62 for wildtype embryos and 64 for Hes1 −/− embryos. Statistical analysis was carried out using Student's t-test. Cell death was evaluated at E9.5 using a Caspase 3 antibody and scored as above.
Acknowledgments
We are grateful to François Guillemot for Hes1 +/− mice, Ted Chang and Margaret Buckingham for Myf5 A17-nlacZ-T55 mice and Peter Scambler and Kelly Lammerts van Bueren for discussion and sharing unpublished data.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Inserm Avenir program, the Fondation de France, the European Community's Sixth Framework Program contract (Heart Repair) LSHM-CT-2005-018630, the Agence National pour la Recherche and the 2008 ISHR-ES/Servier Research Fellowship (FR). FR is an Inserm postdoctoral fellow. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 2.Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, et al. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol. 2005;281:78–90. doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 4.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H, Morishima M, Wylie JN, Schwartz RJ, Bruneau BG, et al. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development. 2004;131:3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- 6.Hutson MR, Kirby ML. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol. 2007;18:101–110. doi: 10.1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward C, Stadt H, Hutson M, Kirby ML. Ablation of the secondary heart field leads to tetralogy of Fallot and pulmonary atresia. Dev Biol. 2005;284:72–83. doi: 10.1016/j.ydbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Waldo KL, Hutson MR, Stadt HA, Zdanowicz M, Zdanowicz J, et al. Cardiac neural crest is necessary for normal addition of the myocardium to the arterial pole from the secondary heart field. Dev Biol. 2005;281:66–77. doi: 10.1016/j.ydbio.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, et al. Notch signaling is essential for ventricular chamber development. Dev Cell. 2007;12:415–429. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutenberg JB, Fischer A, Jia H, Gessler M, Zhong TP, et al. Developmental patterning of the cardiac atrioventricular canal by Notch and Hairy-related transcription factors. Development. 2006;133:4381–4390. doi: 10.1242/dev.02607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.High FA, Zhang M, Proweller A, Tu L, Parmacek MS, et al. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J Clin Invest. 2007;117:353–363. doi: 10.1172/JCI30070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niessen K, Karsan A. Notch signaling in cardiac development. Circ Res. 2008;102:1169–1181. doi: 10.1161/CIRCRESAHA.108.174318. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Krantz ID, Deng Y, Genin A, Banta AB, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 15.McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet. 2006;79:169–173. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 17.Gessler M, Knobeloch KP, Helisch A, Amann K, Schumacher K, Rhode E, Fischer A, Leimeister C. Mouse gridlock: no aortic coarctation or deficiency, but fatal cardiac defects in Hey2 −/− mice. Curr Biol. 2002;12:1601–4. doi: 10.1016/s0960-9822(02)01150-8. [DOI] [PubMed] [Google Scholar]
- 18.Donovan J, Kordylewska A, Jan YN, Utset MF. Tetralogy of fallot and other congenital heart defects in Hey2 mutant mice. Curr Biol. 2002;12:1605–1610. doi: 10.1016/s0960-9822(02)01149-1. [DOI] [PubMed] [Google Scholar]
- 19.Xin M, Small EM, van Rooij E, Qi X, Richardson JA, et al. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc Natl Acad Sci U S A. 2007;104:7975–7980. doi: 10.1073/pnas.0702447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokubo H, Miyagawa-Tomita S, Johnson RL. Hesr, a mediator of the Notch signaling, functions in heart and vessel development. Trends Cardiovasc Med. 2005;15:190–194. doi: 10.1016/j.tcm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Kageyama R, Ohtsuka T, Kobayashi T. The Hes gene family: repressors and oscillators that orchestrate embryogenesis. Development. 2007;134:1243–1251. doi: 10.1242/dev.000786. [DOI] [PubMed] [Google Scholar]
- 22.Kageyama R, Ohtsuka T, Tomita K. The bHLH gene Hes1 regulates differentiation of multiple cell types. Mol Cells. 2000;10:1–7. doi: 10.1007/s10059-000-0001-0. [DOI] [PubMed] [Google Scholar]
- 23.Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, et al. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- 24.Tomita K, Ishibashi M, Nakahara K, Ang SL, Nakanishi S, et al. Mammalian hairy and Enhancer of split homolog 1 regulates differentiation of retinal neurons and is essential for eye morphogenesis. Neuron. 1996;16:723–734. doi: 10.1016/s0896-6273(00)80093-8. [DOI] [PubMed] [Google Scholar]
- 25.Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 27.Bajolle F, Zaffran S, Meilhac SM, Dandonneau M, Chang T, et al. Myocardium at the base of the aorta and pulmonary trunk is prefigured in the outflow tract of the heart and in subdomains of the second heart field. Dev Biol. 2008;313:25–34. doi: 10.1016/j.ydbio.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 28.Murata K, Hattori M, Hirai N, Shinozuka Y, Hirata H, et al. Hes1 directly controls cell proliferation through the transcriptional repression of p27Kip1. Mol Cell Biol. 2005;25:4262–4271. doi: 10.1128/MCB.25.10.4262-4271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Georgia S, Soliz R, Li M, Zhang P, Bhushan A. p57 and Hes1 coordinate cell cycle exit with self-renewal of pancreatic progenitors. Dev Biol. 2006;298:22–31. doi: 10.1016/j.ydbio.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 30.Kikuta H, Laplante M, Navratilova P, Komisarczuk AZ, Engstrom PG, et al. Genomic regulatory blocks encompass multiple neighboring genes and maintain conserved synteny in vertebrates. Genome Res. 2007;17:545–555. doi: 10.1101/gr.6086307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iso T, Chung G, Hamamori Y, Kedes L. HERP1 is a cell type-specific primary target of Notch. J Biol Chem. 2002;277:6598–6607. doi: 10.1074/jbc.M110495200. [DOI] [PubMed] [Google Scholar]
- 32.Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, et al. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004;131:5539–5550. doi: 10.1242/dev.01436. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, et al. Ectopic pancreas formation in Hes1 -knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J Clin Invest. 2006;116:1484–1493. doi: 10.1172/JCI27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrelson Z, Kelly RG, Goldin SN, Gibson-Brown JJ, Bollag RJ, et al. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development. 2004;131:5041–5052. doi: 10.1242/dev.01378. [DOI] [PubMed] [Google Scholar]
- 35.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- 36.Mesbah K, Camus A, Babinet C, Barra J. Mutation in the Trapalpha/Ssr1 gene, encoding translocon-associated protein alpha, results in outflow tract morphogenetic defects. Mol Cell Biol. 2006;26:7760–7771. doi: 10.1128/MCB.00913-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theveniau-Ruissy M, Dandonneau M, Mesbah K, Ghez O, Mattei MG, et al. The del22q11.2 candidate gene Tbx1 controls regional outflow tract identity and coronary artery patterning. Circ Res. 2008;103:142–148. doi: 10.1161/CIRCRESAHA.108.172189. [DOI] [PubMed] [Google Scholar]