Abstract
Introduction
It is unclear whether the care of breast cancer patients complies with the German breast cancer guideline and achieves the appropriate standard of care. For this reason the guideline’s requirements were compared with the empirically determined health care provision in the German state of Schleswig-Holstein.
Methods
The OVIS-study is based on a postal questionnaire survey of medical care in 1927 breast cancer patients between 02/2003 to 02/2005. Statements such as „should be carried out“ and „mandatory procedure“ were cross-referenced with specific reference ranges.
Results
Locally invasive tumors were rare (pT 3/4: 8%). Preoperatively, 95% of patients received mammography, 78% breast ultrasound, 92% a chest x-ray, and 81% an abdominal ultrasound. 71% of all patients had breast conserving treatment, of whom 96% had irradiation of the remaining breast tissue. Axilla lymph node dissection was reported for 91%, axillary irradiation for 36%, and a systemic therapy for 95%.
Discussion
Local quality indicators comply in general with the German guideline. The high rate of axillary irradiation could be viewed as inadequate provision.
Keywords: breast cancer, diagnosis, treatment, quality assurance, guideline
With 55 100 new cases per year, breast cancer is the most common cancer in women in Germany (1). According to the age standardized rate based on the world standard population (WASR), 80 cases of breast cancer occur per 100 000 women (2). In the German state of Schleswig-Holstein, the annual incidence of breast cancer is slightly above the national average (absolute number 2468 women; WASR 92/100 000), but is at the same level as the incidence in Denmark, where an WASR of 89/100 000 has been described (3). In addition to almost complete registration of breast cancer cases and a heterogeneous distribution of risk factors across Germany, the higher incidence in Schleswig-Holstein is also likely to be due to initiatives in the early detection of breast cancer. A program for quality assured breast cancer diagnostics (QuaMaDi) was implemented in a sample region of 25% of the Schleswig-Holstein population in 2001, and since 2005 this program has covered the entire state (4, 5). This program is one of four pillars of the network "Betrifft Brust" ("Concerning: Breast") which is aiming to optimize the early detection of breast cancer.
Since 2003, a population-based project has studied the oncological care in Schleswig-Holstein (OVIS study) in women with breast cancer (n=2366) (6). In the context of this study, cancer patients were asked to record details of their medical care and quality of life in a self-filled questionnaire. For a part cohort of 1141 women, the treating doctors have also been consulted.
Data from the OVIS study have enabled us to conduct evaluative health services research and follow two fundamentally different intentions and questions. On the one hand, medical care can be documented and compared from patients’ as well as the doctors’ perspectives. Are the data from patients and doctors consistent? Can patients’ responses relating to their illness, diagnosis, and therapy be used as reliable data sources? On the other hand, can the collected data be compared with guidelines and/or data from the Bundesgeschäftsstelle Qualitätssicherung gGmbh (BQS; the federal office for quality assurance)? What is the situation with regard to medical care? Are there areas where care provision is insufficient? Is there a potential or a need for optimization?
It is known from the literature that the quality of medical care varies between states and within states, as well as between those administering treatment (7–11). Guidelines are set out and implemented; the objective is to achieve evidence based, appropriate, and economical patient care across areas – independently of individual persons or locations. This target can be met only when guideline recommendations are applied in clinical practice. Some studies have shown that medical care improves as a result of setting out and implementing guidelines (8, 9, 11, 12), whereas others did not find optimized care as a result of guidelines alone (7, 9, 10). Since mid-2004, Germany has had a national interdisciplinary S3 guideline for the diagnosis, therapy, and aftercare of breast cancer in women (13). The appendix to the guideline provides reference ranges/areas for selected indicators, whereas for other indicators the text uses phrases such as "mandatory procedure" or "individual decision." To level out the care situation, these phrases need to be transformed into reference ranges. Subsequently, the patient data collected in the OVIS study were used to check whether diagnosis and therapy of breast cancer in Schleswig-Holstein were performed according to the guideline and independently of hospital and treating physician.
This article describes empirically collected care data from women with breast cancer and compares these data with the S3 guidelines. These data have thus far not been available at the population level.
Methods
The OVIS study
In Schleswig-Holstein, all doctors are legally obliged to report patients with tumor disease to the state’s cancer registry. It is up to the patients to decide whether they are in principle willing to participate in research studies. Those patients who agree can be identified via the cancer registry if the need arises and can be contacted by the study team (14). The participants in the OVIS study had to meet the following criteria:
A primary tumor diagnosis meeting ICD-10 diagnosis C50 criteria (Breast cancer)
Age at diagnosis of 18 to <85 years
Place of residence in Schleswig-Holstein at the time of diagnosis
Diagnosis made between January 2002 and June 2004
Willingness in principle to participate in research projects.
From February 2003, potential study participants were contacted by letter and received information about the study, and were asked to provide written consent and to participate. Where necessary, a reminder was sent after 4 and 8 weeks. The study questionnaire (15,16) was used to collect data on medical care (diagnosis, therapy, aftercare, rehabilitation) and quality of life (EORTC QLQ-C30) (17). The study protocol was approved by the ethics committee at Lübeck University. All patients gave written consent.
Transformation of study phrases into reference areas
Guidelines aim to provide appropriate, scientifically founded, current, and economical diagnosis, therapy, aftercare, and rehabilitation, while considering the individual patient’s disease stage (13). For female breast cancer, a national interdisciplinary S3 guideline has existed since 2004, which was valid until December 2007 (13). Beforehand, a draft S1 guideline and different guidelines and evidence based recommendations had been available in Germany since 2001. These included, for example, "Evidence based recommendations for the primary treatment of breast cancers," "Evidence based recommendations for the treatment of locoregionally recurrent breast cancers and breast cancer with distant metastases" from the AGO-Organkommission Mamma (AGO, Arbeitsgemeinschaft Gynäkologische Onkologie – the working group for gynecological oncology in the breast organ committee), "Principles of modern radio-therapy (radio-oncology from the Arbeitsgemeinschaft Radiologische Onkologie (ARO) der Deutschen Krebsgesellschaft (the working group for radio-oncology of the German cancer society), and international guidelines such as "Management of breast cancer in women" from the Scottish Intercollegiate Guidelines Network. The available evidence was put together in the national S3 guideline.
The S3 guideline gives specific targets for some quality indicators that in some cases should be achieved in more than 95% of cases. For other areas, phrases such as "mandatory procedure," "the treatment aims to," "should be carried out," and "complementary/additional treatment if required," or "individual decision" were chosen (table 1). As a first step it became necessary to transform these phrases into suitable reference ranges. For the phrase "mandatory procedure," it was assumed that in an ideal scenario, 100% of patients should receive this treatment. To do justice to individual circumstances or individual patients’ wishes, however, the phrase was assigned a reference range of >95%. The phrases "should be carried out" and "the treatment aims to" were interpreted as follows: if possible, these treatments should be given (in the ideal scenario, this means >95%), but for these recommendations, more than for the preceding ones, individual factors such as age, general condition, size of breast, tumor size, and the patients’ wishes should be considered. The reference rage is therefore wide, at 50 to 95%. X-radiography of the thorax on two levels is indicated in every case, to exclude lung metastases; but if the patient expresses a wish or if current radiograms from another source are available, this is not obligatory. The phrases "complementary/additional treatment if required" and "individual decision" were interpreted as follows: these measures are optional measures, which can be administered because of medical particularities or because of patients’ wishes. The range is therefore <10%.
Table 1. Health care indicators, reported, and deducted reference values and observed rates in the OVIS study.
| Health care indicator | Evidence based recommendations for primary treatment of breast cancer (organ committee working group, consensus 2001) | Phrase or reported values from S3 guideline breast cancer (version 2004) | Deducted reference value (percent) | Observed rate in OVIS study in % (95% confidence interval) |
| Basic diagnostics*1 | ||||
| Mammography | Recommended | Mandatory procedure | >95 | 94.8 [94; 96] |
| Ultrasonography | Recommended | Mandatory procedure | >95 | 78.2 [76; 80] |
| Magnetic resonance imaging | Insufficient data to warrant general recommendation | To be added if required | <10 | 4.8 [4; 6] |
| Pretherapeutic staging*2 | ||||
| Abdominal ultrasonography | Preoperative diagnostics include ... | Should be carried out | 50-95 | 81.2 [79; 83] |
| Chest x-ray on two levels | Preoperative diagnostics include ... | Should be carried out | 50-95 | 92.2 [91; 93] |
| Skeletal scintigraphy | If required | Should be carried out | 50-95 | 87.1 [86; 89] |
| Therapy | ||||
| Breast conserving therapy | All patients should be informed about (. . .) breast conserving therapy, list of indications | Aim (. . .) is*3In pT1 and pT2-Ca > 60%*4 | 50-95 | Total: 70.9 [69; 73] T1: 83.9 [82; 86] T2: 62.7 [59; 66] |
| Radiotherapy after breast conserving therapy | Postoperative radiotherapy of remaining breast tissue is (. . .) standard | > 95%*5 | T3/4: 19.6 [14; 27] 96.3 [95; 97] | |
| Axillary surgery | Obligatory component of both operative methods (breast conserving therapy and mastectomy) | > 95%*6 | 90.6 [89; 92] | |
| Axillary radiotherapy | Radiotherapy of (. . .) axillary lymph drainage pathways is of slight importance (. . .) If 4 or (more lymph nodes are positive (. . .) principally recommended (. . .). | Individual decision*7 | <10 | 35.8 [34; 38] |
| Systemic therapy | Standard treatment | > 90%*8 | 95.1 [94; 96] | |
Found in S3 guideline: *1 statement 1; *2 statement 3; *3 statement 8; *4 appendix 7A4.2; *5 appendix 7 A6.2; *6 appendix 7 A4.6; *7 statement 27; *8 appendix 7 A7.
Statistical analysis
It has been reported that women receive different medical care depending on their age (10, 18, 19). The OVIS cohort was therefore subdivided into four age groups: (≤50, >50 to 60, >60 to70, and >70 years of age).
The results are described using relative frequencies, means, and standard deviations (SD). Differences between the age groups with regard to quantitative variables were tested for significance using the chi square test (two sided). Twelve tests altogether were done and the significance level was adjusted using the Bonferroni method (probability of error 0.05/12=0.004). Confidence intervals for observed rates were calculated using the traditional Altman procedure (20).
Results
For the time period in question, 5248 breast cancer patients were included in the cancer registry. These women are the total study population. Of these, 2366 had agreed to be contacted vis-à-vis study participation (eligible patients). Of these, 1927 responded to the initial letter. The participation rate was 81% with regard to the eligible patients; relative to the total study population it was 37%. A comparison of basic characteristics shows that women with a more favorable tumor, node, metastasis (TNM) grade and women with urban residences tended to be more likely to be eligible and also participated more often (table 2). Table 1 shows the rates based on patient data for the basic diagnostic tests, staging, and therapy as listed in the S3 guideline. Deviations from the reference ranges were found for the indicators ultrasonography, axillary surgery, and radiotherapy after axillary dissection.
Table 2. Characteristic data from different study cohorts.
| Study cohort at baseline*1 (n = 5248) | Potential participants*2 (n = 2366) | OVIS participants (n = 1927) | |
| Age at diagnosis in years (Mean ± SD) | 60.7 ± 12.1 | 59.6 ± 11.7 | 58.8 ± 11.3 |
| Dwelling category | |||
| City | 47.7% | 51.1% | 51.4% |
| Country | 51.9% | 48.9% | 48.6% |
| Tumor, node, metastasis T (tumor) | |||
| T1 | 48.5% | 51.3% | 54.6% |
| T2 | 34.4% | 35.8% | 35.4% |
| T3 | 4.8% | 4.6% | 4.8% |
| T4 | 6.9% | 4.4% | 3.6% |
| Unknown | 5.4% | 3.9% | 1.4% |
| Tumor, node, metastasis N (regional lymph nodes) | |||
| N0 | 53.8% | 58.8% | 62.0% |
| N1 | 30.4% | 30.5% | 30.7% |
| N2 | 3.7% | 3.5% | 3.5% |
| N3 | 0.4% | 0.1% | – |
| Unknown | 11.8% | 7.0% | 3.9% |
| Tumor, node, metastasis M (distant metastases) | |||
| M0/Unknown | 95.2% | 97.1% | 97.5% |
| M1 | 4.8% | 2.9% | 2.5% |
*1Inclusion criteria apply, reported by name or anonymously;*2 Inclusion criteria apply, reported by name, corresponds to eligible patients, SD; standard deviation.
The latter indicator depends on the cancer stage. Radiotherapy was given to 26% of N0 patients (no lymph nodes affected), 54% of N1 patients (1 to 3 lymph nodes affected), and 63% of N2 patients (4 to 9 lymph nodes) (<0.001). No relation to year of diagnosis or year of therapy was found.
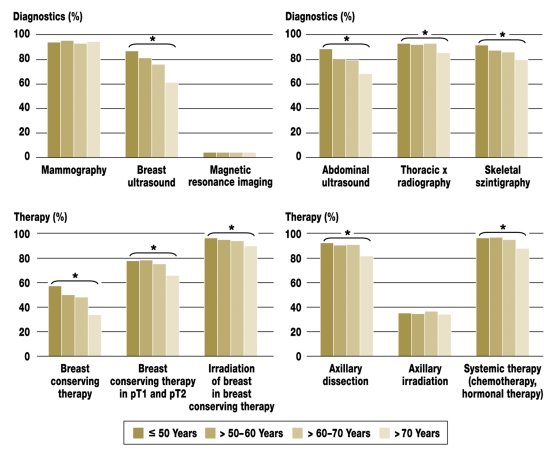
With regard to preoperative diagnostic measures and staging, differences between the age groups reached significance (all p<0.001) with respect to the frequency of breast ultrasonography, thoracic x-radiography, skeletal scintigraphy, and abdominal ultrasonography. With regard to therapy, differences reach significance (all p<0.001) between age groups with respect to breast conserving therapy, radiotherapy of the remaining breast tissue, axillary dissection, and systemic therapy. Diagnostic and therapeutic procedures were used more rarely with increasing age (figure).
Figure.
Age dependence of preoperative diagnostics, staging, and therapy; *p=0.001, chi-square test, comparison of four age groups with respect to procedures listed
Discussion
Guidelines as an instrument of medical care should provide appropriate, scientifically founded, current, and economical diagnostics, therapy, and rehabilitation, while considering the disease stage. Guidelines should be the basis for medical decision processes that materially affect medical actions. The supraordinate aim of the cited S3 guidelines is the area-wide implementation of a multidisciplinary, quality-assured, and cross-sectoral therapy for breast cancer, to reduce mortality in, and improve the quality of life of, women with breast cancer in the medium and long term (13).
In Schleswig-Holstein, attempts have been made for several years in the areas of detection of and delivering medical care for breast cancer. These have recently been combined under the umbrella "Concerning: Breast". This includes BreastLife, a training program for breast self examination aimed especially at young women. The program aims to raise awareness of the topic of breast cancer and regular participation in early detection screening programs. QuaMaDI is a second project, which aims to achieve curative, quality assured breast cancer diagnostics for women of all age groups. The mammographies are administered according to national and European guidelines, the mammograms are analyzed by two independent reviewers, and the diagnostic tests are conducted in reference centers (13, 21). The mammography screening consists of nation-wide coverage, quality assured, radiography screening of asymptomatic women aged 50-69 according to the European Commission guideline (21). The disease management program (DMP) breast cancer aims to implement multidisciplinary, quality assured, and cross sectoral therapy. In spite of these efforts it is thus far unknown at the population level whether the diagnosis and therapy of breast cancer in Schleswig-Holstein comply with the guidelines.
The suitability of retrospective patients’ self reported data for the purposes of health services research was shown in the context of the OVIS study by analyzing reliability (15) and validity (16). The most important result of our study is the fact that patients’ self reported data reflect their medical care accurately as long as they are not asked for medical details (for example, the type of chemotherapy regimen). Responses to questions whether a woman has received chemotherapy or radiotherapy to the breast tissue, for example, showed a high degree of consistency between patients’ reports and doctors’ reports. By comparing the care situation with a valid standard a guideline in this case areas of routine care can be classified as adequately provided, overprovided, underprovided, or inappropriately provided (22). Even if these classifications are rather general, they represent an opportunity to evaluate care in a fundamental way that requires little effort. If patients’ self reported data from the OVIS study are compared with the care situation as per the S3 guideline, it transpires that medical care is adequate with regard to basic diagnostics and pretherapeutic staging. Only ultrasonography misses the guidance standard of >95%, reaching 78%. The proportion of women who received breast conserving therapy is in the desirable range of >60%. It would be desirable, however, for the axillary lymph nodes to be operated on in more cases and for radiotherapy after axillary dissection to be administered in fewer cases. Especially the latter indicator may be interpreted as a sign of inappropriate care provision – whether the S3 guideline or the evidence based recommendations of the AGO organ committee are consulted. Since the recommendation for radiotherapy was taken out of the S3 guideline, and an "individual decision" is the applicable phrase used instead, this indicator will require further monitoring.
Nagel et al, in a population-based study from eastern Thuringia, which included cohorts from 1996 and 1997, showed that 46% of patients received breast conserving therapy. After breast conserving therapy, 91% received adjuvant radiotherapy (23). These results may possible be explained with the known phenomenon of a temporal delay between the publication of an innovation and its nation-wide coverage implementation. In the case of equivalence of breast conserving therapy plus radiotherapy of the remaining breast tissue and mastectomy, with regard to recurrence free survival of patients with breast cancer, Engel et al documented a comparable development. Five years after the first publication in 1981, the proportion of women who had breast conserving treatment for breast cancer was 10%; 5 years later it had risen to 20%. Another 5 years later it was 50% (22). The current BQS quality report (24) gives a proportion of breast conserving therapy of 83% for 2006, with a reference range of 60-80%, for breast cancer patients at stage pT1 or pT2.
In the context of the study reported in this article, almost all care indicators are age dependent – fewer treatments are administered the older the patient. The S3 guideline, however, says only in statement 43 that older patients should not be excluded from systemic therapy because of their age alone. Age dependency for breast ultrasonography may seem plausible because the density of the breast tissue decreases with increasing age and therefore mammograms can be interpreted more easily. However, with regard to pretherapeutic staging, it is not easy to understand why in older women, no investigations are made to exclude lung, liver, and skeletal metastases. The literature provides reports of sinking adherence to guidelines and a lower quality of care with increasing age of patients (18, 19, 23, 25).
A limitation of our study is the fact that we could not capture the reasons behind the deviation from guideline recommendations. Deviations from guidelines as a rule are caused by 3 factors: Lacking knowledge of the guideline, subjective reasons that are due to doctors’ or patients’ decisions, or structural causes. Further, unknown reasons for the deviation from the standard will have to be considered. Another possible limitation of our study is the population-based participation rate of 40%, which means that selection bias of the possible study participants with regard to guideline compatible therapy cannot be excluded. This effect, however, can be assumed to be smaller than in studies in which patients were not recruited in a population-based manner-for example, where recruitment took place in a hospital. The high participation rate of 81% of contacted patients who were treated in a multitude of hospitals makes possible selection bias and response bias with regard to guideline compliance unlikely. Further, the invitation letter did not make any mention of a comparison between the care situation and treatment guidelines. It is not inconceivable, however, that the care given to study participants and non-study participants may be systematically different, because of hospital data such as the UICC stage (UICC, Union internationale contre le cancer) and origin, differentiated by city and state. Explorative multivariate analyses, however, have not shown a relevant influence of these variables on guideline compliant care.
The strengths of the study include the large, population-based, representative sample and the additional interviews with doctors with regard to diagnostics, therapy, and tumor data, in 1141 patients. When the data from the doctors are included, the quality of care is similar. The only exception is preoperative breast ultrasonography, which according to the doctors was given to merely 54% of patients.
Conclusion
The current care situation for women with breast cancer in Schleswig-Holstein can be assumed to be adequate. The nation-wide coverage implementation of the S3 guideline seems to have been successful. But the indicator "radiotherapy after axillary dissection" should be checked; inappropriate care provision with regard to this indicator cannot be excluded. Particular attention should go to the care of older women, because almost all diagnostic and therapeutic measures are reported more rarely in older patients.
This study was supported by Deutsche Krebshilfe (German Cancer Aid) (70-2901-BU I).
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (ed.) in Zusammenarbeit mit dem Robert Koch-Institut. Krebs in Deutschland. Häufigkeiten und Trends. 5. überarbeitete, aktualisierte Ausgabe. Saarbrücken. Riegelsberg: RoBo-Print; 2006. [Google Scholar]
- 2.International Agency for Research on Cancer. GLOBOCAN www-dep.iarc.fr/GLOBOCAN/table2.asp?cancer=132®ion;=99&sex;=2&sort;=1&submit;=Exec. 2002.
- 3.Institut für Krebsepidemiologie e.V. (Hrsg.) Krebs in Schleswig-Holstein. Band 4: Inzidenz und Mortalität im Jahr 2004. Lübeck: Schmidt-Römhild; 2006. [Google Scholar]
- 4.Katalinic A, Bartel C, Raspe H-H, Schreer I. Beyond mammography screening: quality assurance in breast cancer diagnosis (The QuaMaDi Project) Br J Cancer. 2007;96:157–161. doi: 10.1038/sj.bjc.6603506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katalinic A, Waldmann A Schriftenreihe des IKE - Heft 3 Evaluation des Modellvorhabens Qualitätsgesicherte Mammadiagnostik (QuaMaDi) www.krebsregister-sh.de/berichte/heft3.pdf. Lübeck: Schmidt-Röhmhild; 2006. Abschlussbericht - Berichtszeitraum 2001 bis 2005. Lübeck. [Google Scholar]
- 6.Pritzkuleit R, Waldmann A, Raspe H, Katalinic A. The population-based oncological health care study OVIS - Recruitment of the patients and analysis of the non-participants. Eur J Cancer Care. 2007 doi: 10.1186/1471-2407-8-311. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latosinsky S, Fradette K, Lix L, Hildebrand K, Turner D. Canadian breast cancer guidelines: have they made a difference? CMAJ. 2007;176:771–776. doi: 10.1503/cmaj.060854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ottevanger PB, De Mulder PH, Grol RP, van Lier H, Beex LV. Adherence to the guidelines of the CCCE in the treatment of node-positive breast cancer patients. Eur J Cancer. 2004;40:198–204. doi: 10.1016/s0959-8049(03)00660-9. [DOI] [PubMed] [Google Scholar]
- 9.Ray-Coquard I, Philip T, de Laroche G, et al. A controlled „before-after“ study: impact of a clinical guidelines programme and regional cancer network organization on medical practice. Br J Cancer. 2002;86:313–321. doi: 10.1038/sj.bjc.6600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaapveld M, de Vries EG, Otter R, de Vries J, Dolsma WV, Willemse PH. Guideline adherence for early breast cancer before and after introduction of the sentinel node biopsy. Br J Cancer. 2005;93:520–528. doi: 10.1038/sj.bjc.6602747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White V, Pruden M, Giles G, et al. The management of early breast carcinoma before and after the introduction of clinical practice guidelines. Cancer. 2004;101:476–485. doi: 10.1002/cncr.20401. [DOI] [PubMed] [Google Scholar]
- 12.Tataru D, Robinson D, Moller H, Davies E. Trends in the treatment of breast cancer in Southeast England following the introduction of national guidelines. J Public Health (Oxf) 2006;28:215–217. doi: 10.1093/pubmed/fdl011. [DOI] [PubMed] [Google Scholar]
- 13.Nationale, interdisziplinäre S3-Leitlinie für die Diagnostik, Therapie und Nachsorge des Mammakarzinoms der Frau. AWMF-Registernummer 032/045. 2004 Juni; http://leitlinien.net. [Google Scholar]
- 14.Ministerpräsidentin des Landes Schleswig-Holstein. Landesraumordnungsplan Schleswig-Holstein. www.umwelt.schleswig-holstein.de/servlet/is/31643/LROPlan-1998-Textteil.pdf?command=downloadContent&filename;=LROPlan-1998-Textteil.pdf. 1998 [Google Scholar]
- 15.Dreckschmidt J Reliabilität eines Patientenfragebogens zur Evaluation der onkologischen Versorgung im Rahmen der OVIS-Studie. Dissertation. Universität zu Lübeck: Institut für Krebsepidemiologie e.V.; 2006. [Google Scholar]
- 16.Ritterhoff N. Vergleich von Angaben von Patienten und Ärzten in der OVIS-Studie. Dissertation. Universität zu Lübeck: Institut für Krebsepidemiologie e.V.; 2008. [Google Scholar]
- 17.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 scoring manual. (3rd ed) Brussels: European Organization for Research and Treatment of Cancer; 2001. [Google Scholar]
- 18.DeMichele A, Putt M, Zhang Y, Glick JH, Norman S. Older age predicts a decline in adjuvant chemotherapy recommendations for patients with breast carcinoma: evidence from a tertiary care cohort of chemotherapy-eligible patients. Cancer. 2003;97:2150–2159. doi: 10.1002/cncr.11338. [DOI] [PubMed] [Google Scholar]
- 19.Kosiak B, Sangl J, Correa-de-Araujo R. Quality of health care for older women: what do we know? Womens Health Issues. 2006;16:89–99. doi: 10.1016/j.whi.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Altman D, Machin D, Bryant TN, Gardner MJ. Statistics with confidence: confidence intervals and statistical guidelines. London: BMJ books; 2003. [Google Scholar]
- 21.Klassen AC, Curriero F, Kulldorff M, Alberg AJ, Platz EA, Neloms ST. Missing stage and grade in Maryland prostate cancer surveillance data, 1992-1997. Am J Prev Med. 2006;30:77–87. doi: 10.1016/j.amepre.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Engel J, Schubert-Fritschle G, Sauer H, Hölzel D. Disease-Management und Qualitätssicherung beim Mammakarzinom. Gynäkologe. 2002;35:1094–1104. [Google Scholar]
- 23.Nagel G, Röhrig B, Hoyer H, Füller J, Katenkamp D. Bevölkerungsbezogene Studie über die Anwendung adjuvanter Strahlentherapie bei Patientinnen mit Mammakarzinom. Strahlenther Onkol. 2002;178:589–596. doi: 10.1007/s00066-002-0985-9. [DOI] [PubMed] [Google Scholar]
- 24.Gemeinsamer Bundesausschuss. Der aktuelle BQS-Qualitätsreport www.bqs-qualitaetsreport.de.
- 25.Eisinger F, Ronda I, Puig B, Camerlo J, Giovannini MH, Bardou VJ. Breast cancer guidelines - Physicians’ intentions and behaviors. Int J Cancer. 2007;120:1136–1140. doi: 10.1002/ijc.22444. [DOI] [PubMed] [Google Scholar]