Abstract
The promoters of MEA (FIS1), FIS2, and FIE (FIS3), genes that repress seed development in the absence of pollination, were fused to β-glucuronidase (GUS) to study their activity pattern. The FIS2∷GUS product is found in the embryo sac, in each of the polar cell nuclei, and in the central cell nucleus. After pollination, the maternally derived FIS2∷GUS protein occurs in the nuclei of the cenocytic endosperm. Before cellularization of the endosperm, activity is terminated in the micropylar and central nuclei of the endosperm and subsequently in the nuclei of the chalazal cyst. MEA∷GUS has a pattern of activity similar to that of FIS2∷GUS, but FIE∷GUS protein is found in many tissues, including the prepollination embryo sac, and in embryo and endosperm postpollination. The similarity in mutant phenotypes; the activity of FIE, MEA, and FIS2 in the same cells in the embryo sac; and the fact that MEA and FIE proteins interact in a yeast two-hybrid system suggest that these proteins operate in the same system of control of seed development. Maternal and not paternal FIS2∷GUS, MEA∷GUS, and FIE∷GUS show activity in early endosperm, so these genes may be imprinted. When fis2, mea, and fie mutants are pollinated, seed development is arrested at the heart embryo stage. The seed arrest of mea and fis2 is avoided when they are fertilized by a low methylation parent. The wild-type alleles of MEA or FIS2 are not required. The parent-of-origin-determined differential activity of MEA, FIS2, and FIE is not dependent on DNA methylation, but methylation does control some gene(s) that have key roles in seed development.
Seed development in flowering plants involves an essential but complex set of developmental processes initiated by interactions of the male and female gametophytic cells. These interactions occur during the double fertilization process, which generates the embryo and the endosperm; the new sporophytic tissues are packaged in the sporophytic tissues of the previous generation, the integuments, and other maternal tissues of the ovule, forming a seed (reviewed in refs. 1 and 2). The egg cell and the central cells of the early embryo sac do not normally show any development in the absence of pollination and fertilization. However, in many families of plants, seed development can occur without fertilization of these cells (autonomous apomixis) or where only fertilization of the central cell occurs (pseudogamous apomixis). A range of pre- and postmeiotic developmental pathways have evolved that generate seed-derived progeny with a conserved maternal genotype (apomictic progeny) (3).
Three genes have now been identified that code for proteins that repress seed development and, in particular, endosperm development, in the absence of pollination and fertilization. Mutations in these genes, FIS1 or MEA (4–6), FIS2 (4, 5), and FIS3 or FIE (4, 5, 7), result in two significant phenotypes: initiation of seed development in the absence of pollination, and if pollination does occur, an arrest of seed development with the embryo at the heart stage, with the endosperm failing to cellularize.
MEA and FIE are related in sequence to two classes of Drosophila polycomb genes, Enhancer of zeste and Extra sex combs, respectively (8, 9). Similar genes have been identified in other organisms (10, 11). Sequence analysis shows that FIS2 is likely to be a DNA-binding transcriptional regulatory protein (12). Because mutation in each of these genes results in similar phenotypes, it is likely that all three genes participate in a polycomb-like control of genes involved in seed development, particularly endosperm development. In understanding the roles of these genes, it is crucial to know in which tissues and cells these loci are active and to determine whether all three genes are active in the same cell types and at the same developmental times.
There are some data describing tissue-specific expression of MEA and FIE (13, 14). In situ hybridization studies have shown that MEA mRNA occurs, before fertilization, in the eight-nuclei embryo sac, in the egg cell, and in the central cell (14). After fertilization, MEA mRNA was detected in all cells of the suspensor and the embryo until the heart and torpedo stage of embryo development, thereafter becoming weaker. FIE, on the other hand, is expressed in many sporophytic, as well as gametophytic, tissues, as shown by reverse transcriptase–PCR of RNA pools derived from various tissues (13).
MEA has been shown to be imprinted (14, 15); during early seed development only the maternal allele is expressed (14). The allele from the pollen as well as the maternal allele is expressed after the torpedo stage of embryo development (15). This difference in pattern of expression in early seed development of the paternally and maternally derived genes is similar to the control of imprinted genes reported in a number of cases in the animal kingdom (16). These expression data fit with earlier genetic observations in which reciprocal crosses involving wild-type and mutant alleles of the three FIS genes did not give identical results, indicating a parent-of-origin effect (4, 6, 7).
In this paper we report an analysis of the activity patterns of reporter gene constructs of all three genes and show differential parent-of-origin activity for each locus. We also present evidence, from yeast two-hybrid experiments, of physical interactions of MEA and FIE proteins, as would be expected in a polycomb-type system.
In mea, fis2, and fie mutants, pollination with wild-type pollen leads to arrested seed development; however, in the mea mutant, this phenotype can be rescued by the inclusion of a ddm1 mutant allele in the paternal plant (14). ddm1 is associated with reduced methylation levels and chromatin restructuring (17). Vielle-Calzada et al. (14) suggest that the rescue of the arrested-seed phenotype is caused by the reactivation of the paternally derived wild-type MEA allele in the developing seed, the reactivation being dependent on the reduced methylation level or on chromatin restructuring caused by the ddm1 allele.
We have investigated the role of DNA methylation in the control of gene activity, using an antisense construct of the DNA methyltransferase-1 gene (MET1; ref. 18). No pollen-derived MEA∷β-glucuronidase gene (GUS) or FIS2∷GUS activity resulted from any of the crosses, irrespective of the methylation status of the male gametophytic genome. In experiments where the pollen introduced either mea or fis2 mutant alleles or MEA or FIS2 wild-type alleles, the seed development results were equivalent, showing that the rescue of the arrested-seed phenotype is not dependent on the activity of pollen-derived MEA or FIS2 genes in the developing seed. These results implicate other genes in modulating downstream genes in the rescue of the maternal defect of these mutants.
Materials and Methods
MEA, FIS2, and FIE Promoter∷GUS Fusion Constructs.
The MEA∷GUS fusion contains 2,070 bp of nucleotide sequence upstream of the predicted translational start site, exons 1–15, the first 13 bp of exon 16, and introns 1–15 in the vector pBI101-3 (CLONTECH). MEA genomic fragments were excised from a bacterial artificial chromosome (BAC) clone (IGF 10O11). The FIS2∷GUS fusion contains 3,189 bp of nucleotide sequence upstream of the predicted translational start site; exons 1, 2, and 3; the first 39 bp of exon 4; and the first three introns in the vector pBI101-3. FIS2 genomic sequences were excised from the cosmid 18H1 (12). The FIE∷GUS fusion contains 6,970 bp of FIE promoter sequence upstream of the predicted start codon, the first eight exons and eight introns of genomic sequence, and 50 bp of the ninth exon in the vector pBI101-3 (CLONTECH). The FIE genomic DNA fragment was excised from the BAC IGF 10A3. The results with these reporter gene constructs were consistent with in situ and reverse transcriptase–PCR results reported elsewhere (6, 13).
All binary constructs were mobilized in Agrobacterium tumefaciens AGL1, and the T-DNA was introduced into Arabidopsis ecotypes WS or C24, using the “floral dip” transformation protocol (19).
Microscopy and GUS Staining.
After GUS staining (20), ovules were directly cleared with lactophenol and observed with differential interference contrast microscopy. The rescued seeds and control were sectioned (21).
MEA and FIE cDNA Cloning.
MEA and FIE fragments were amplified from genomic DNA with primers 5′-GATCCAAACAACACTATGTG-3′ and 5′-CCCATCCATGAACAT-3′ and 5′-TCCATCGACAGACATGGCTG-3′ and 5′-CTCGCAAATGTGCAGAGTCTTGTG-3′, respectively, and used to screen a silique cDNA library (12). A 2,328-bp MEA cDNA insert, identical to the published MEA sequence, and a 1,372-bp FIE cDNA spanning the entire coding region were isolated.
Yeast Strains, Transformation, and Two-Hybrid Constructs and Assays.
The lithium acetate procedure (22) was used to transform yeast strain PJ69-4A (MATa, trp1-901, leu2-3,112, Ura3-52, His3-200, gal4Δ, gal80Δ, GAL2-ADE2, LYS2∷GAL1-HIS3, met2∷GAL7-lacZ (23, 24).
The Matchmaker two-hybrid system (CLONTECH) was used with cDNAs inserted into either the DNA-binding domain (DBD) vector pGBT9 or the transcriptional activation domain (AD) vector pGAD424. A full-length MEA cDNA clone was inserted into both vectors to produce pGBT-MEA and pGAD-MEA. Each contains 15 amino acid residues between the end of the GAL4 fusion and the MEA translation start site. MEA deletion constructs were made by digesting the MEA cDNA clone with suitable restriction enzymes and cloning them into pGBT9. The construct pGBT-MEAΔN61 lacks the first 60 amino acid residues, pGBT-MEAΔ513C has the first 512 residues, pGBT-MEAΔ320C has the first 319 residues, and pGBT-MEAΔ61C has the first 61 residues. pGBT-FIS2 and pGAD-FIS2 each contain a full-length 2.4-kb FIS2 cDNA insert. Forty-three amino acid residues that originate from the FIS2 5′ noncoding region of the cDNA clone are present between the end of the GAL4 fusion and the predicted FIS2 protein start site. pGAD-FIE contains a 1.4-kb FIE cDNA insert and an additional 19 amino acid residues between the end of the GAL4 activation domain fusion and the predicted protein start site. pGBT-EZA1 contains the full-length 2.7-kb EZA1 cDNA (AF1001163) insert cloned into the EcoRI site of pGBT9, and the resulting fusion protein contains an additional 27 amino acid residues between the end of the GAL4 DBD and the putative EZA1 translational start. All fusion junctions between cDNA inserts and the two-hybrid vectors were confirmed by sequencing.
Results
Interaction of Seed Development Repressor Genes.
MEA and FIE are related to the polycomb genes Enhancer of zeste and Extra sex combs of Drosophila (6, 12, 13). In the fly these gene products interact as part of a protein complex that is associated with changes in chromatin architecture and repression of gene expression (25). We sought evidence of interaction between the Arabidopsis MEA and FIE proteins, using a yeast two-hybrid system.
Yeast cotransformed with the pGBT-MEA and either pGAD-MEA or pGAD-FIE constructs grew without added histidine or adenine, indicating that MEA polypeptides can autoassociate and that they can interact physically with the FIE protein (Fig. 1iA). Yeast cells cotransformed with constructs producing MEA and FIS2 or FIE and FIS2 were not able to grow in the absence of histidine or adenine, indicating that the FIS2 protein does not physically interact with either the MEA or FIE (Fig. 1iA) proteins.
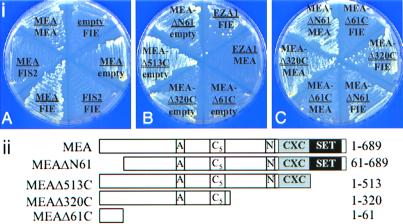
Figure 1.
(i) Interactions between the FIS2, MEA, FIE, and EZA1 proteins in the yeast two-hybrid system. The plates show growth of yeast containing different plasmid combinations on SC minus tryptophan, leucine, and histidine media containing 1 mM 3-aminotriazole. The label “empty” corresponds to either the bait vector pGBT9 or the prey vector pGAD424 without insert. (ii) Schematic drawing showing MEA proteins of different lengths used in the two-hybrid system.
We used deletion analysis of the MEA coding region to determine whether specific domains of the protein were involved in homo- and heterodimer formation. Four deletion constructs of the MEA coding region were tested for their interaction with either intact MEA or FIE polypeptides (Fig. 1ii). Removal of the MEA SET domain and fusion of the truncated protein to the GAL4 DBD in the pGBT9 vector (MEAΔ513C) enhanced growth (Fig. 1iB). Two other truncated GAL4 DBD fusions, MEAΔN61 and MEAΔ320C, produced slow growth of transformed yeast cells on the selective medium, suggesting a weak activation of the reporter genes, presumably through dimerization. A third deletion construct, MEAΔ61C, did not support growth on the selective medium. Deletion MEAΔN61, which removes 61 amino acids from the amino terminus, did not decrease MEA homodimer formation but did reduce MEA/FIE heterodimer formation (Fig. 1iC). The MEAΔ61C construct, which contains only the first 61 amino acid residues, fused to the GAL4 DBD sequence, failed to support either homo- or heterodimer formation, suggesting that the dimerization domain of MEA is downstream of amino acid residue 61. The third deletion, MEAΔ320C, which spans both the acidic and the cysteine-rich (C5) regions of the protein, was able to interact with both MEA and FIE proteins to support growth (Fig. 1iC). These results suggest that the domain between amino acid residues 61 and 320 is required both for MEA homodimerization and for MEA/FIE heterodimerization.
Another Arabidopsis protein of the MEA class, EZA1 (GenBank accession no. AF1001163), was also tested for possible protein–protein interactions with MEA, FIE, and FIS2. Cotransformation of EZA1 and FIE constructs into the yeast system resulted in weak growth of the yeast cells on both selection media. This result may reflect a degree of heterodimerization with FIE, but there was no evidence for interaction between EZA1 and MEA or EZA1 and FIS2 (Fig. 1iB); nor did EZA1 form homodimers.
We did not find any evidence for the interaction of FIS2 with either MEA or FIE (Fig. 1iA). This lack of evidence could mean that another protein may be needed in the complex to facilitate the interaction of FIS2 with MEA and FIE, or it may be that FIS2, a zinc finger protein, functions in a manner analogous to that of the Hunchback zinc finger protein of Drosophila (8, 26). The Hunchback protein does participate in the polycomb gene expression system but acts before the formation of the polycomb complex, and it does not show any physical interaction with the complex.
Patterns of Activity of the Seed Repressor Genes.
Because MEA, FIS2, and FIE are expressed at low levels (6, 12, 13), we used promoter∷GUS fusions to analyze their expression during ovule and seed development. The FIS2, FIE, and MEA∷GUS fusion proteins were all expressed in the female gametophyte before pollination and in the developing endosperm after pollination (Fig. 2). FIS2∷GUS activity (Fig. 2i A–H) was first evident in the unfused polar nuclei (Fig. 2iA) and subsequently in the nucleus of the central cell in the embryo sac (Fig. 2iB). After fertilization, FIS2∷GUS activity was associated with the primary endosperm nucleus and subsequently with the dividing endosperm nuclei (Fig. 2i C–F). FIS2∷GUS activity continued to be associated with the free endosperm nuclei until a cenocytic cyst formed in the chalazal region of the embryo sac (Fig. 2iG). FIS2∷GUS activity in the free endosperm nuclei ceased before the time of endosperm cellularization but continued in the nuclei of the chalazal cyst. FIS2∷GUS activity was occasionally observed in the synergids and the egg apparatus in a small proportion of the unfertilized embryo sacs (Fig. 2iH).
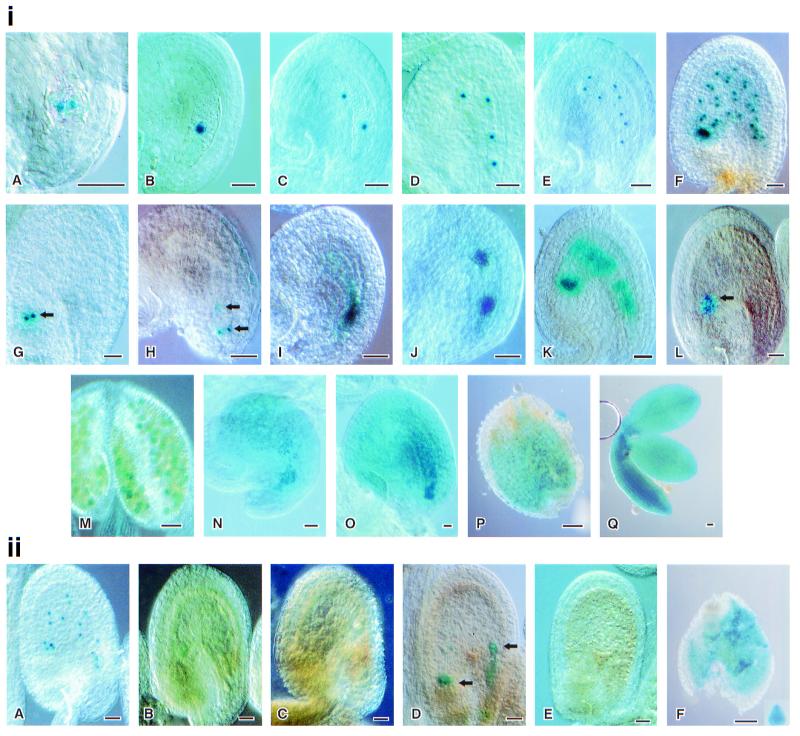
Figure 2.
FIS2, MEA, and FIE∷GUS fusion activity and imprinting. (i) FIS2, MEA, and FIE∷GUS fusion activity. (A–H) FIS2∷GUS activity. (A and B) Unpollinated ovules showing GUS activity in the central cell nucleus. (C–F) Free endosperm nuclei divide from 2 to 32. (G) Nuclei in cyst region retaining GUS activity. (H) Synergids and possible egg nuclei showing GUS activity. (I–L) MEA∷GUS activity. (I) Unpollinated ovule showing GUS activity in the central cell nucleus. (J and K) MEA∷GUS activity in dividing endosperm nuclei. (L) Nuclei in cyst region retaining GUS activity. (M–Q) FIE∷GUS activity. (M) Microspores showing transient GUS activity. (N) Unpollinated ovule showing GUS activity. The central cell region is dense. (O) Ovule 24 h after pollination, showing diffuse GUS activity. (P and Q) Seven-day-old seed dissected to stain embryo and rest of the seed separately, showing the GUS activity in embryo and inside the seed coat. (ii) FIS2, MEA, and FIE imprinting. (A) Twenty-four-hour-old ovule with maternally derived FIS2∷GUS, showing GUS activity. (B) Twenty-four-hour-old ovule pollinated with a FIS2∷GUS plant, showing no GUS activity. (C) Twenty-four-hour-old ovule pollinated with a MEA∷GUS plant, showing no GUS activity. (D) Forty-eight-hour-old ovule showing GUS activity in cyst and possibly in embryo after pollination with MEA∷GUS. (E) No GUS activity seen in an Landsberg erecta (L.er) ovule pollinated with FIE∷GUS 48 h after pollination. (F) Endosperm and heart stage embryo showing GUS activity in an ovule pollinated by a FIE∷GUS plant 4 days after pollination. (Bars = 0.05 mm.)
The activity pattern of MEA∷GUS was similar to that of FIS2∷GUS, but the localization around the nuclei was more diffuse (Fig. 2i I–L). MEA∷GUS protein, before pollination, occurred in the fused polar cells (Fig. 2iI). After fertilization, MEA∷GUS activity was associated with dividing endosperm nuclei (Fig. 2i J–K), and the final pattern of activity was similar to that of FIS2∷GUS plants, with GUS activity maintained in the chalazal cyst but ceasing in the other nuclei before endosperm cellularization (Fig. 2iL). In a small number of embryo sacs GUS staining was observed in some nuclei at the micropylar end of the embryo sac at the time of endosperm cellularization (data not shown). Vielle-Calzada et al. observed MEA expression in both embryo and endosperm after pollination (14).
FIE∷GUS protein was diffuse throughout the embryo sac, and before anthesis was observed in the developing pollen microspores (Fig. 2i M and N); this activity was transient and was not evident in mature pollen. The diffuse distribution of product of the FIE∷GUS construct in the embryo sac persisted after fertilization and was observed in both the cellularized endosperm and embryo (Fig. 2i O–Q). GUS activity was observed in some sporophytic tissues: leaves, silique wall, and stamen filaments.
Thus in accordance with their mutant phenotypes, MEA, FIS2, and FIE are expressed in the female gametophyte before pollination. However, in contrast to MEA and FIS2, the FIE product is found not only in the endosperm but also in the embryo and in other sporophytic tissues, indicating that FIE might have functions other than those in endosperm development. Our inability to make a fie/fie homozygote also indicates a critical requirement for this gene in the zygote.
Activity of the Repressor Genes Depends on the Parent of Origin.
We and others have shown that each of these genes shows a parent-of-origin effect; paternally derived wild-type genes are not able to rescue the maternally derived lesion in the corresponding gene (4, 6, 7). One interpretation of this observation is that the genes are imprinted and are not expressed when derived paternally (14, 15). To examine the pattern of activity of MEA and to investigate the possibility that FIS2 and FIE are also imprinted, we have investigated the activity of these genes in transgenic GUS protein fusion lines.
In contrast to the endosperm-limited activity of the maternally derived FIS2∷GUS transgene (Fig. 2iiA), no product was observed when FIS2∷GUS was introduced by pollen (Fig. 2iiB). Similarly, there was no GUS protein during the early stages of endosperm formation when MEA∷GUS was introduced by pollen (Fig. 2iiC). However, 48 h after pollination (early globular stage), paternal MEA∷GUS expression was observed in the chalazal cyst and sometimes in the embryo itself (Fig. 2iiD). Paternally derived FIE∷GUS expression was not observed in either embryo or endosperm until 3 days after pollination (Fig. 2iiE). At 4 days after pollination, FIE∷GUS expression was observed in early heart-stage embryos and in the cellularized endosperm (Fig. 2iiF). There was no expression of paternally derived FIS2∷GUS at any developmental stage in either ovules or seeds.
Our data show that MEA is imprinted during early endosperm development and that later in endosperm development the imprinting breaks down, with MEA∷GUS being expressed in the chalazal cyst. We also observed embryo expression of paternally derived MEA∷GUS genes at low frequency at 48 h after pollination. Earlier, Kinoshita et al. (12) showed imprinting only in endosperm expression, and Vielle-Calzada et al. (11) showed imprinting in both embryo and endosperm, using reverse transcriptase–PCR analyses. Our method, using the sensitive GUS reporter gene, indicates that the imprinting of MEA shows both a spatial and a temporal pattern. We have further shown that all three FIS genes show maternal expression in the developing seed, and each has a unique time of cessation of activity as seed development proceeds.
Arrested Seed Development in the Repressor Mutants Is Rescued by Pollen from a Hypomethylated Plant.
When the homozygous mea and fis2 mutants are pollinated with wild-type pollen, double fertilization occurs and both embryo and endosperm development are initiated. The developing endosperm fails to cellularize, and the syncytial nuclei continue to divide, expanding the endosperm mass. The embryo develops normally but arrests at the heart/torpedo stage. Viable seeds are not formed. A similar result was reported (7) for the fie ovules in a FIE/fie heterozygote. The aborted seeds of mea were rescued when pollinated by a line carrying the ddm1 mutation (14). We tested the role of DNA methylation in the rescue of mea, fis2, and fie embryos. We pollinated homozygous mea and fis2 plants with pollen from wild-type C24 plants and from C24 plants homozygous for the antisense MET1 construct, in which DNA methylation levels were approximately 15% of the wild-type level. When mea homozygotes were pollinated with the anti-MET1 pollen, 98.8% of the seeds were viable (Fig. 3A). Endosperm and embryo development were normal (Fig. 3P) compared with 24% viable seed when mea plants were pollinated with wild-type C24 pollen (Fig. 3B). When homozygous fis2 plants were pollinated with C24 anti-MET1 pollen, 99% of seeds were viable (Fig. 3 C and M), whereas wild-type C24 pollen rescued only 1.9% of the affected seed (Fig. 3 D and L). We were unable to produce a fie homozygote, but when a FIE/fie heterozygote was pollinated with C24 pollen, arrested and normal seeds were produced in a ratio of 1:1 (109:111) (Fig. 3F). In contrast, when pollen was taken from a C24 anti-MET1 plant, all seeds were fully developed and viable but were in two distinct size classes in a 1:1 ratio (105:112) (Fig. 3E).
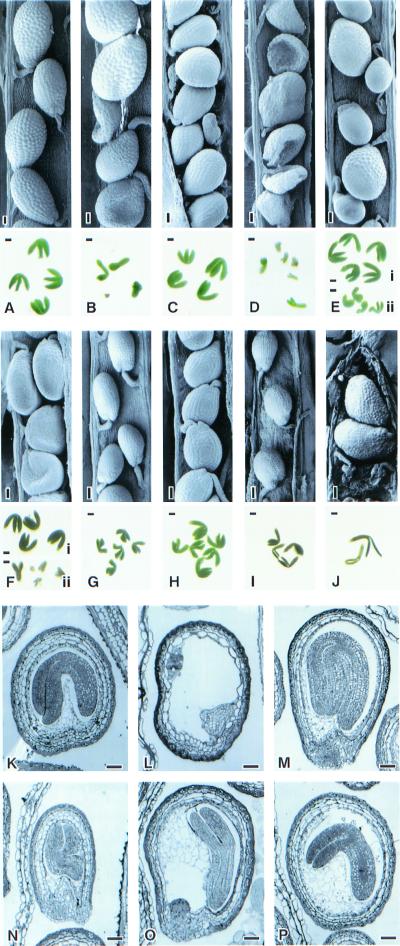
Figure 3.
mea, fis2, and fie seed rescued by C24 anti-MET1 pollen. (A) mea/mea seeds and embryos pollinated by C24 anti-MET1. (B) mea/mea pollinated with C24 pollen. Some seeds collapsed. Dissected embryos show arrest. (C) fis2/fis2 seeds and embryos rescued with C24 anti-MET1 pollen (D) fis2/fis2 pollinated with C24 pollen. Embryos are arrested. (E) FIE/fie pollinated with C24 anti-MET1 pollen, showing two types of seeds: fully developed big seeds with big embryos and fully developed small seeds with small embryos. (F) FIE/fie pollinated with C24 pollen. Half of the seeds are fully developed and others are aborted. (G) L.er pollinated with C24 anti-MET1 pollen. Seeds and embryos are smaller than those in H. (H) L.er selfed seeds and embryos. (I) C24 anti-MET1 selfed seeds smaller than the seeds in J. (J) C24 anti-MET1 pollinated with L.er. Seeds become bigger than these in I. (K) Section of a fully developed wild-type embryo. (L) Section of fis2 arrested embryo. (M) Section of fis2 rescued embryo by C24 anti-MET1. (N) Section of embryo from L.er × C24 anti-MET1. (O) Section of fie developing embryo with cellularized endosperm rescued by C24 anti-MET1. (P) mea developing embryo with cellularized endosperm rescued by C24 anti-MET1. (Bars = 0.05 mm.)
These results, where pollen from hypomethylated plants produced normal seed development in mea, fis2, and fie ovules, suggest that under these conditions some gene(s) in the male genome overcome(s) the abnormalities generated in the ovules carrying mutant alleles of any of the three loci. The results could mean that the male-derived alleles of MEA, FIS2, and FIE normally do not function in the early development of the seed, but if they are derived from a hypomethylated male genomic environment they function to remove the deficiencies in the mutant ovules. Alternatively, the seed rescue may not require functional MEA, FIS2, and FIE genes but may result from the action of other genes, the normal pattern of activity of which is changed if DNA methylation levels are low in the pollen parent.
Rescue of Female mea and fis2 by Hypomethylated Pollen Does Not Require a Functional Paternally Derived MEA or FIS2.
A wild-type MEA allele, derived from a male with reduced DNA methylation, was reported to be needed for production of viable seeds in the mutant ovules (14). This observation was interpreted to mean that the male MEA allele is normally inactive in the developing seed and that DNA methylation is involved in the imprinting of this gene. Our results do not confirm these conclusions.
When mea and fis2 homozygotes were pollinated by mea/mea anti-MET1 or fis2/fis2 anti-MET1 pollen, seed rescue occurred. When we crossed mea homozygotes with a MEA/mea anti-MET1 pollen parent plant heterozygous for the anti-MET1 construct 248/251, seeds were rescued, in comparison to 12/130 seeds rescued in a cross with wild-type (L.er/C24) pollen. Similarly, fis2 plants crossed with pollen from a FIS2/fis2 anti-MET1 plant rescued 278/298 seeds, in contrast to 1/150 rescued seeds with the wild-type (L.er/C24) cross.
These results indicate that the reactivation of paternally derived MEA and FIS2 genes cannot be responsible for the seed rescue we have observed in the mea and fis2 ovules. Thus hypomethylation must alter the activity of other, as-yet-unidentified, genes derived from the pollen that have a role in the early developing seed.
In contrast to these unknown genes that respond to the low methylation levels, we found that, although MEA and FIS2 are pollen-inactive, they are not reactivated by low methylation levels. This conclusion was based on experiments where we used the same MEA∷GUS and FIS2∷GUS reporter gene constructs in a hemizygous state in either wild-type or anti-MET1 plants as pollen parents on wild-type L.er plants. No GUS expression was observed in the early development of endosperm or embryo for either reporter gene construct (Fig. 2ii A–E). Identical results were obtained from the wild-type and anti-MET1 pollen parents. Thus these two genes are regulated so that the male-derived allele is not active in the early development of the ovule. This control is not dependent on methylation. Even though we estimated the level of methylation in the pollen parent to be 25% of wild-type levels, the methylation level was reduced sufficiently to give seed rescue.
Seed Size.
In an earlier section we noted that FIE/fie pollinated with FIE/FIE anti-MET1 gave rise to seeds of two size classes. To determine which of the seed sizes was derived from fie ovules and which from FIE ovules, we pollinated wild-type L.er (FIE/FIE) with anti-MET1 pollen. The seeds (Fig. 3 G and N) were significantly smaller than those from selfed L.er plants (Fig. 3 H and K) or from the reciprocal cross C24 anti-MET1 pollinated with L.er pollen (Fig. 3J). Thus the smaller seeds obtained when FIE/fie is crossed with anti-MET1 pollen are likely to be derived from the maternal carriers of the FIE allele, and the larger seeds, from the maternal carriers of the fie allele. Selfed C24 anti-MET1 plants also yielded small seeds (Fig. 3I). These results show that pollen from demethylated plants reduces seed size and that this reduction is countered by the mutation in mea, fis2, or fie in the maternal genes. The smaller seeds give rise to small seedlings that later grow into wild-type-size plants.
Scott et al. (27), on the basis of interploidy crosses, showed that pollen with a higher ploidy increases embryo size, whereas in the reciprocal cross, when there is a maternal gamete with a higher ploidy, the embryo size is decreased. Recently Adams et al. (28) have demonstrated that pollen from a demethylated plant reduces embryo size. Our results are in agreement with these data but also show that a maternally carried mea, fis2, or fie allele produces larger seeds and embryos than do wild-type alleles (after pollination by a demethylated male plant).
Discussion
The genes MEA, FIS2, and FIE repress seed development until the double fertilization event that follows pollination provides the signals for embryo and endosperm development. The experiments, in which we coupled the promoters of the three genes to the GUS reporter gene, have shown that after fertilization, MEA, FIS2, and FIE activities can be detected in the endosperm tissue and that FIE activity also occurs in some other sporophytic tissues. Before pollination the genes are expressed in the female gametophyte: MEA and FIS2 products are found in the two polar cells, in the central cell, and in all of the dividing cells of the endosperm. The FIE∷GUS fusion product also occurs in the central cell before pollination as well as in the developing endosperm. The expression of these genes in the polar nuclei and in the central cell could be necessary for the repression of endosperm development and of other processes in seed development; it is likely, given the sequence affinities of these genes, that repression is mediated by a polycomb complex involving MEA and FIE directly, with the FIS2 protein functioning as a related transcription factor. Our two-hybrid data, showing interaction of MEA and FIE proteins, support this model.
The activity patterns of all three genes have demonstrated that there are differentiated regions of the endosperm. MEA and FIS2 activity continues in the cenocytic cyst in the chalazal end of the endosperm after the activity of these genes ceases in the central and micropylar regions of the embryo sac. The cenocytic condition of the chalazal cyst continues beyond the initiation of cellularization of the remainder of the endosperm, which may also be a consequence of the presence or absence of the MEA and FIS2 gene products.
Our experiments have provided strong evidence that all three FIS genes are regulated such that the gene copies from pollen are not expressed in the early development of the seed. We have also shown that this control is not related to the level of genome methylation in the male, and therefore it is not likely that the regulation of these genes is dependent on DNA methyltransferase activity.
On the other hand, the data from the antisense MET1 pollination shows that some gene(s) in the male genome has altered expression in the early stages of seed development when the level of DNA methylation is reduced. If there is a null mutation in one of the two seed repression genes, MEA or FIS2, seed development is arrested, even if normal alleles of the genes are provided by the pollen, but the seed arrest phenotype is avoided if pollen is from a low-methylation-level plant, irrespective of whether a functional or mutant allele of MEA or FIS2 is present. Apparently other genes from the male genome are necessary for the restoration of normal seed development. The differential activities of these other genes could reflect activation as a result of the low methylation level in the male genome, or alternatively, they could be a consequence of reduced activity of the genes, which are normally active in the zygote.
Our data have provided some knowledge of the steps that may be needed to achieve normal seed development in the absence of pollination (autonomous apomixis). We have found that MEA, FIS2, or FIE must be inactivated to permit endosperm development in the absence of pollination. In mea and fis2 ovules the endosperm development extends to cellularization, although cellularization has not been observed in the fie background.
Perhaps one additional mutant gene could trigger embryo development; it is possible that if embryo development occurs it could remove the block to further endosperm development. On the other hand, a knockout mutation may be needed in another gene that normally represses the cellularization of the endosperm. We have the opportunity now to screen for such mutations.
If we are to succeed in producing an apomictic system in a mutagenesis program, then we need to achieve the balance of gene action normally applying to male- and female-derived genes in sexual seed development.
Acknowledgments
We thank Dr. Jean Finnegan for providing us with the C24 anti-MET1 plants, Ms. Celia Miller for excellent technical support, and Mr. Carl Davies for figure preparation. This work was funded by the Rockefeller Foundation and the Australian Centre for International Agricultural Research.
Abbreviations
- FIS
fertilization-independent seed
- MEA
Medea
- FIE
fertilization-independent endosperm
- GUS
β-glucuronidase
- MET1
methyltransferase-1
- DBD
DNA-binding domain
- AD
activation domain
- L.er
Landsberg erecta
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.170292997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.170292997
References
- 1.Chaudhury A M, Craig S, Dennis E S, Peacock W J. Curr Opin Plant Biol. 1998;1:26–31. doi: 10.1016/s1369-5266(98)80123-4. [DOI] [PubMed] [Google Scholar]
- 2.Berger F. Curr Opin Plant Biol. 1999;2:28–32. doi: 10.1016/s1369-5266(99)80006-5. [DOI] [PubMed] [Google Scholar]
- 3.Koltunow A. Plant Cell. 1993;5:1425–1437. doi: 10.1105/tpc.5.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhury A M, Ming L, Miller C, Craig S, Dennis E S, Peacock W J. Proc Natl Acad Sci USA. 1997;94:4223–4228. doi: 10.1073/pnas.94.8.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peacock J, Ming L, Craig S, Dennis E S, Chaudhury A. Induced Mutations and Molecular Techniques for Crop Improvement. Vienna: International Atomic Energy Agency; 1995. pp. 117–125. [Google Scholar]
- 6.Grossniklaus U, Vielle-Calzada J-P, Hoeppner M A, Gagliano W B. Science. 1998;280:446–449. doi: 10.1126/science.280.5362.446. [DOI] [PubMed] [Google Scholar]
- 7.Ohad N, Margossian L, Hsu Y C, Williams C, Repeth P, Fischer R L. Proc Natl Acad Sci USA. 1996;93:5319–5324. doi: 10.1073/pnas.93.11.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutjahr T, Frei E, Spicer C, Baumgartner S, White R, Noll M. EMBO J. 1995;14:4296–4306. doi: 10.1002/j.1460-2075.1995.tb00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones R S, Gelbart W M. Mol Cell Biol. 1993;13:6357–6366. doi: 10.1128/mcb.13.10.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobert O, Sures I, Ciossek T, Fuchs M, Ullrich A. Mech Dev. 1996;55:171–184. doi: 10.1016/0925-4773(96)00499-6. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher A, Lichtarge O, Schwartz S, Magnuson T. Genomics. 1998;54:78–88. doi: 10.1006/geno.1998.5509. [DOI] [PubMed] [Google Scholar]
- 12.Luo M, Bilodeau P, Koltunow A, Dennis E S, Peacock W J, Chaudhury A M. Proc Natl Acad Sci USA. 1999;96:296–301. doi: 10.1073/pnas.96.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohad N, Yadegari R, Margossian L, Hannon M, Michael D, Harada J J, Goldberg R B, Fischer R L. Plant Cell. 1999;11:407–415. doi: 10.1105/tpc.11.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vielle-Calzada J, Thomas J, Spillane C, Coluccio A, Hoeppner M, Grossniklaus U. Genes Dev. 1999;13:2971–2982. doi: 10.1101/gad.13.22.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinoshita T, Yadegari R, Harada J J, Goldberg R B, Fischer R. Plant Cell. 1999;11:1945–1952. doi: 10.1105/tpc.11.10.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wroe S F, Kelsey G, Skinner J A, Bodle D, Ball S T, Beechey C V, Peters J, Williamson C M. Proc Natl Acad Sci USA. 2000;97:3342–3346. doi: 10.1073/pnas.050015397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeddeloh J A, Stokes T L, Richards E J. Nat Genet. 1999;22:94–97. doi: 10.1038/8803. [DOI] [PubMed] [Google Scholar]
- 18.Finnegan E J, Peacock W J, Dennis E S. Proc Natl Acad Sci USA. 1996;93:8449–8454. doi: 10.1073/pnas.93.16.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clough S J, Bent A F. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 20.De Block M, De Brower D. Plant J. 1992;2:261–266. [Google Scholar]
- 21.Craig S, Beaton C D. J Microsc (Oxford) 1996;182:102–105. [Google Scholar]
- 22.James P, Halladay J, Craig E A. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose M D, Windston F, Hieter P. Methods in Yeast Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. [Google Scholar]
- 24.Gietz R D, Woods R A. Molecular Genetics of Yeast: Practical Approaches. London: Oxford Univ. Press; 1994. pp. 121–134. [Google Scholar]
- 25.Jones C A, Ng J, Peterson A J, Morgan K, Simon J, Jones R S. Mol Cell Biol. 1998;18:2825–2834. doi: 10.1128/mcb.18.5.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C C, Bienz M. Proc Natl Acad Sci USA. 1992;89:7511–7515. doi: 10.1073/pnas.89.16.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott R J, Spielman M, Bailey J, Dickinson H G. Development (Cambridge, UK) 1998;125:3329–3341. doi: 10.1242/dev.125.17.3329. [DOI] [PubMed] [Google Scholar]
- 28.Adams S, Vinkenoog R, Spielman M, Dickinson H G, Scott R J. Development (Cambridge, UK) 2000;127:2493–2502. doi: 10.1242/dev.127.11.2493. [DOI] [PubMed] [Google Scholar]