Abstract
Objective
We hypothesized that low presenting systolic blood pressure (SBP) predicted cardioembolic stroke aetiology.
Design
Active and passive surveillance were used to identify all ischaemic strokes as part of the Brain Attack Surveillance in Corpus Christi (BASIC) population-based study. Multinomial logistic regression was used to examine the association between stroke subtype and first documented SBP in the medical record.
Setting
Nueces County, Texas, USA (313,645 residents in 2000). The community is urban with the majority of the population residing in the city of Corpus Christi. The area is served by seven adult acute care hospitals.
Patients
Three hundred eight cases with completed ischaemic stroke and determined subtype aetiology between January 2000 and December 2002.
Results
Lower presenting SBP was associated with stroke subtype (p=0.001). This association remained significant in the final model adjusted for age and history of coronary artery disease (CAD). The odds of cardioembolic versus small vessel occlusion increased by 20% (OR=1.20, 95% CI: 1.07-1.35) for every 10 mm Hg decrease in presenting SBP. Other covariates including race/ethnicity, gender, history of hypertension, and diabetes were neither significant predictors of stroke subtype, nor did they confound the association of SBP and stroke subtype. A 5 year increase in age increased the odds of cardioembolic subtype by 25% (OR 1.25, 95% CI: 1.07-1.47).
Conclusions
Lower initial SBP and older age at ischaemic stroke presentation were associated with cardioembolic stroke. Suspicion of cardioembolic stroke should be increased in those presenting with low SBP.
Introduction
Identifying the ischaemic stroke subtype is crucial for providing directed secondary stroke prophylaxis. Internists are commonly the primary in-hospital care providers for acute stroke patients when evaluation of stroke subtype occurs. Early clues to ischaemic stroke subtype may facilitate an expeditious, thorough evaluation for stroke mechanism and allow the clinician to institute proper preventive therapy. Patients with acute ischaemic stroke present to the hospital with a wide range of blood pressures. Worse neurological outcome is associated with either low or markedly elevated blood pressures on presentation.[1, 2] To date we are aware of no studies in which the presenting blood pressure has been used to attempt to predict the stroke subtype. Prior work has focused on trends in blood pressure during the first 24-48 hours after the onset of stroke.[3-7] An association between lower blood pressure and the cardioembolic subtype is suggested by these studies; a possible mechanism is pre-existing heart disease. [7]
We assessed the ability of presenting blood pressure to predict modified TOAST subtype classification among ischaemic stroke patients in a population-based study.[8-10] We hypothesized that lower presenting systolic blood pressure (SBP) would be an independent predictor of cardioembolic stroke subtype.
Methods
Study Setting
The Brain Attack Surveillance in Corpus Christi (BASIC) Project is a population-based stroke surveillance study in Nueces County, Texas, USA, which had 313,645 residents in 2000. The community is urban with the majority of the population residing in the city of Corpus Christi. The area is served by seven adult acute care hospitals. The methods utilized to collect patient data and determine stroke subtype as part of the BASIC project have previously been described.[10, 11]
Study Cohort
Briefly, both active and passive surveillance were used to identify all strokes among those >44 years old presenting to any emergency department or hospital. Completed ischaemic strokes, between January 2000 and December 2002, were identified by trained abstractors and validated by board-certified neurologists utilizing source documentation. The first ischaemic stroke event per individual captured by BASIC was utilized.
Data Sources
A random sample of validated stroke cases were selected for extended chart review, in which detailed information was collected including medical test results, laboratory results, and the discharge summary. First recorded systolic blood pressures after presentation to the emergency department (or hospital for direct admissions) were abstracted from the medical record.
Stroke subtype classification
Ischaemic stroke cases were classified by two fellowship-trained stroke neurologists according to a previously published [10], modified version of the TOAST criteria. The stroke subtype categories were large-artery atherosclerosis, cardioembolic, small-vessel occlusion, stroke of other determined aetiology, stroke of undetermined aetiology, and an additional category of nonlacunar stroke of unknown aetiology (NLUE). The NLUE category comprised large artery territory strokes that had insufficient evidence for categorization into large-artery atherosclerosis versus cardioembolic; this categorisation has been utilised previously.[10, 12] The neurologists were blinded to age and were unaware of the study question of the relationship of blood pressure to ischaemic stroke subtype. The inter-rater agreement for stroke subtype was high (kappa = 0.80, p < 0.001).[10]
Statistical Methods
Frequencies, means, and other descriptive statistics were calculated for the demographic and clinical characteristics of the patients in the study, both for the overall sample and by stroke subtype. Chi-squared tests, ANOVA F-tests, and the Kruskal-Wallis test were used to assess differences in demographic and clinical characteristics between stroke subtype groups and between patients with a classified stroke subtype and those with an undetermined aetiology. Stroke of other determined aetiology was excluded from further analysis due to low numbers (n=5).
Multinomial logistic regression was used to study the association between stroke subtypes and presenting systolic SBP. Multinomial logistic regression generalizes binary logistic regression by allowing the outcome of interest to be categorized into more than two possible outcomes, in this case, multiple stroke subtypes. In binary logistic regression, the odds of the event versus no event are calculated; in multinomial logistic regression, the odds of one type of event (e.g., cardioembolic) versus a reference type of outcome are computed. We used small vessel occlusion as the referent stroke subtype, as past work has demonstrated this subtype to generally present with the highest systolic blood pressure.[4, 6, 7] The results from multinomial logistic regression were adjusted for other predictor and confounding variables and were used to calculate adjusted probabilities of each of the possible stroke subtypes. Age, gender, ethnicity, and history of hypertension, diabetes, and coronary artery disease (CAD) were considered as covariates. Atrial fibrillation was not considered as a covariate because it was used to classify stroke as cardioembolic. Variables were kept in the model if they were confounders (assessed by the ten-percent rule of confounding) or independent predictors (p<0.05) of stroke subtype. Deciles of SBP were used to construct Pigeon and Heyse’s goodness of fit statistic to test for lack of fit of the multinomial regression models.[13] For the models fitted, the test statistic had a chi-square distribution with 27 degrees of freedom. We decided a priori to exclude undetermined cases from the primary analysis as this is a clinically heterogeneous subgroup. However, to be sure that this decision did not affect our results, the multinomial logistic regression was repeated including the undetermined cases.
The estimated models were used to predict the probability of each stroke subtype for a continuous range of values of SBP. The predicted probabilities were then plotted against SBP. In practical situations, categories of SBP may be more useful than continuous changes in SBP. Thus, the models were re-estimated using quartiles of SBP. Bar graphs depicting the odds ratios (OR) for each subtype were constructed based on the model with SBP quartiles. For all calculations, the highest SBP quartile was the referent. The odds of cardioembolic, NLUE, and large vessel versus small vessel occlusion (referent stroke subtype) were calculated to depict the association between subtype and quartiles of SBP. Additionally, the odds of small vessel occlusion versus all other stroke subtypes combined were computed to depict the association between small vessel occlusion and increasing SBP.
A sensitivity analysis was conducted to assess the possible effects of misclassification of stroke subtype. Specifically, the NLUE stroke category contained both cardioembolic and large artery stroke and therefore a potential source of misclassification bias if just the cardioembolic stroke group was considered in the analysis. For the sensitivity analysis, stroke subtypes were dichotomized into two broader categories. One category included cardioembolic and the NLUE stroke groups, while the second included large artery and small vessel occlusions. To analyze the dichotomized stroke subtypes binary logistic regression was used.
This project was approved by the University of Michigan Institutional Review Board, the University of Texas at Houston Committee for the Protection of Human Subjects, and the Institutional Review Boards of all involved hospitals. The data were analyzed using SAS Version 9.1 (SAS Corporation, Cary, NC).
Results
Study Enrolment and Demographics
Abstractors screened 13,291 possible cerebrovascular events. Of these, 2,378 were subsequently validated as stroke. A random sample of 711 patients was selected for extended data abstraction. Two hundred and ninety three of these patients with other stroke types were excluded from the analysis (199 transient ischaemic attacks (TIA), 80 intracerebral haemorrhages, 11 subarachnoid haemorrhages, and one event in which it was unclear if it was ischaemic versus hemorrhagic stroke). Of the 418 remaining ischaemic stroke patients with extended abstraction data, five events for patients with more than one stroke during the study period and 14 patients with no subtype data were excluded from the analysis. Finally, 5 patients with ischaemic stroke of other determined aetiology were excluded from the analysis. The analytic sample consisted of 308 patients with determined stroke aetiology and 86 patients with ischemic stroke subtype of undetermined aetiology.
Some differences existed between cases with determined and those with undetermined aetiology. Specifically, those with undetermined aetiology were more likely to have a history of stroke or TIA (45.9% versus 32.9%, p=0.02), were less likely to have a history of coronary artery disease (25.9% versus 38.6%, p=0.04), and had a lower National Institutes of Health Stroke Scale (NIHSS) score (median 2 versus 3, p=0.02) as compared with the determined aetiology group.
The demographic and clinical data for the patients are reported in Table 1. Of those with known aetiology, nearly 30% were NLUE, 27% cardioembolic, 24% small vessel occlusion, and 19% large-artery atherosclerosis.
Table 1.
Demographics, risk factors, and clinical characteristics of patients with determined versus undetermined subtype
| Stroke Subtype | Overall Determined N=308 |
Cardioembolic N=84 (27.3%) |
NLUE* N=91 (29.5%) |
Large Artery N=56(18.2%) |
Small Vessel N=77(25.0%) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||||
| Gender (%Female) | 179 | 58% | 185 | 60% | 169 | 55% | 188 | 61% | 179 | 58% |
| Race/Ethnicity (%MA) | 151 | 49% | 183 | 60% | 132 | 43% | 154 | 50% | 136 | 44% |
| Age, mean (SD) | 73 | (11) | 78 | (11) | 72 | (12) | 70 | (11) | 72 | (10) |
| Smoking Status | ||||||||||
| Never | 217 | 71% | 238 | 77% | 232 | 75% | 201 | 65% | 189 | 61% |
| Former | 48 | 16% | 31 | 10% | 47 | 15% | 47 | 15% | 66 | 21% |
| Current | 43 | 14% | 39 | 13% | 29 | 9% | 59 | 19% | 53 | 17% |
| Health Insurance | 293 | 95% | 301 | 98% | 284 | 92% | 302 | 98% | 288 | 94% |
| Clinical Characteristics | ||||||||||
| History of Hypertension | 233 | 76% | 235 | 76% | 217 | 70% | 246 | 80% | 240 | 78% |
| Diabetes | 139 | 45% | 125 | 41% | 136 | 44% | 162 | 53% | 140 | 46% |
| History of Stroke or TIA | 101 | 33% | 121 | 39% | 81 | 26% | 118 | 38% | 92 | 30% |
| Coronary Artery Disease | 119 | 39% | 183 | 60% | 106 | 34% | 90 | 29% | 84 | 27% |
| High Cholesterol | 62 | 20% | 33 | 11% | 68 | 22% | 95 | 31% | 64 | 21% |
| Atrial Fibrillation | 46 | 15% | 143 | 46% | 14 | 4% | 11 | 4% | 4 | 1% |
| Excessive ETOH | 15 | 5% | 11 | 4% | 17 | 6% | 17 | 6% | 16 | 5% |
| NIHSS, median (IQR) | 3 | (2,7) | 6 | (1,10) | 3 | (1,7) | 3 | (1,8) | 3 | (2,4) |
| Presented within 3 hours | 88 | 29% | 34 | 41% | 16 | 18% | 15 | 27% | 24 | 32% |
| Systolic BP (SD) | 161 | (30) | 152 | (32) | 160 | (30) | 164 | (28) | 168 | (28) |
| Diastolic BP (SD) | 84 | (18) | 84 | (19) | 85 | (19) | 82 | (15) | 87 | (16) |
| Mean Arterial Pressure (SD) | 110 | (19) | 107 | (21) | 110 | (20) | 109 | (17) | 114 | (17) |
| Diagnostic Testing | ||||||||||
| Any neuroimaging | 305 | 99% | 304 | 99% | 305 | 99% | 308 | 100% | 304 | 99% |
| Magnetic resonance imaging | 129 | 42% | 77 | 25% | 135 | 44% | 187 | 61% | 152 | 49% |
| Transthoracic echocardiogram | 149 | 48% | 172 | 56% | 146 | 47% | 176 | 57% | 132 | 43% |
| Transesophageal echocardiogram |
9 | 3% | 4 | 1% | 14 | 4% | 0 | 0% | 24 | 8% |
| Electrocardiogram | 268 | 87% | 282 | 92% | 268 | 87% | 275 | 89% | 268 | 87% |
| Conventional angiography | 16 | 5% | 4 | 1% | 7 | 2% | 49 | 16% | 10 | 3% |
| Carotid duplex sonography | 197 | 64% | 165 | 54% | 196 | 64% | 214 | 70% | 224 | 73% |
Means or medians reported with standard deviation or interquartile range as appropriate in parentheses.
Non-lacunar stroke of either possible cardioembolic or possible large-artery aetiology
Results of subtype prediction modelling
In the unadjusted model, presenting SBP was strongly associated with stroke subtype (p=0.001). The model did not show a significant lack of fit (χ2 = 27.2, p=0.44). The odds of cardioembolic stroke versus small vessel occlusion increased by 20% (OR=1.20, 95% confidence interval [CI]: 1.08-1.34) for every 10 mmHg decrease in presenting SBP. The odds ratios for other stroke subtypes versus small vessel occlusion are given in Table 2. The NLUE aetiology had an intermediate risk between that of cardioembolic and large artery atherosclerosis.
Table 2.
Odds of stroke subtype for a 10 unit decrease in SBP — unadjusted model.
| Stroke Subtype | OR | 95%CI | |
|---|---|---|---|
| Cardioembolism | 1.20 | 1.08 | 1.34 |
| NLUE* | 1.10 | 0.99 | 1.22 |
| Large Artery | 1.04 | 0.93 | 1.17 |
| Small Vessel | 1.00 | Referent | |
(NLUE: non-lacunar stroke of either possible cardioembolic or possible large-artery aetiology)
In the adjusted multinomial logistic regression model (lack of fit χ2= 24.2, p=0.62), the only other variables included as predictive of subtype were age and history of CAD. Other covariates including race/ethnicity, gender, history of hypertension, and diabetes, were neither significant predictors of stroke subtype, nor did they confound the association of SBP and stroke subtype. The adjusted association between SBP and cardioembolic aetiology did not change and remained significant (Table 3). That is, the odds of cardioembolic versus small vessel occlusion increased by 20% (OR=1.20, 95% CI: 1.07-1.35) for every 10 mm Hg decrease in presenting SBP. The odds of cardioembolic aetiology versus small vessel occlusion increased by 25% for a 5 year increase in age (OR=1.25, 95% CI: 1.07-1.47), but were not significantly changed versus the other subtypes. Similarly, the odds of cardioembolic versus small vessel occlusion more than tripled for those with CAD compared to those without (OR= 3.12, 95% CI: 1.56- 6.24). The odds of any other subtype versus small vessel occlusion did not differ between those with and without CAD. Repeating the multinomial logistic regression including the undetermined cases did not significantly alter the relationship between stroke subtype and presenting blood pressure.
Table 3.
Odds of stroke subtype - adjusted model
| Stoke Subtype |
OR** | 95% CI | ||
|---|---|---|---|---|
| SBP | ||||
| (10mmHg) | Cardioembolic | 1.20 | 1.07 | 1.35 |
| (p=0.01) | NLUE* | 1.12 | 1.11 | 1.25 |
| Large Artery | 1.04 | 1.03 | 1.18 | |
| Small Vessel | 1.00 | Referent | ||
| Age (5yr) | Cardioembolic | 1.26 | 1.07 | 1.48 |
| (p<0.01) | NLUE* | 1.01 | 0.88 | 1.16 |
| Large Artery | 0.93 | 0.80 | 1.09 | |
| Small Vessel | 1.00 | Referent | ||
| CAD | Cardioembolic | 3.12 | 1.56 | 6.24 |
| (p<0.01) | NLUE* | 1.33 | 0.67 | 2.64 |
| Large Artery | 1.15 | 0.52 | 2.54 | |
| Small Vessel | 1.00 | Referent | ||
(NLUE: non-lacunar stroke of either possible cardioembolic or possible large-artery aetiology)
Referent: Small Vessel
Probabilities of stroke subtype over range of SBP
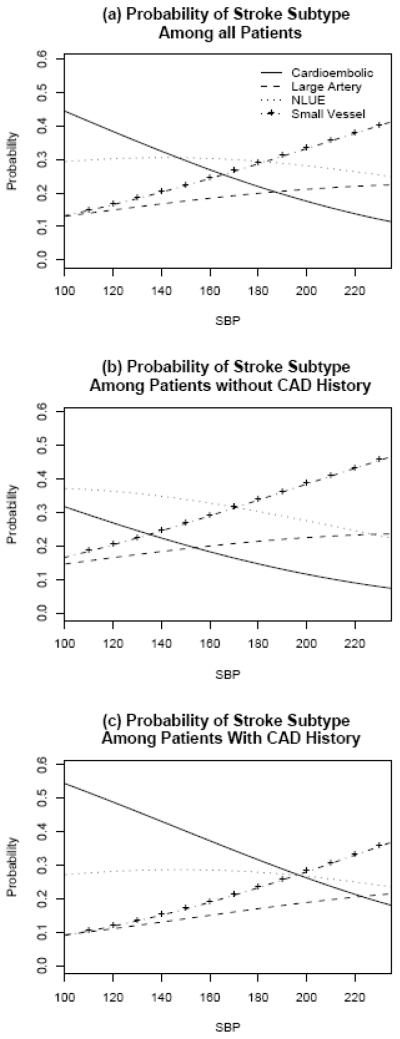
The age-adjusted probabilities of stroke subtype over a range of presenting SBP were calculated for patients (a) overall, and those (b) with and (c) without history of CAD, and are depicted graphically in Figure 1. As an example, a 73 year old patient (average age in the sample) with a presenting SBP of 100mmHg, had approximately a 44% probability of cardioembolic stroke.(Figure 1a) If the patient had a history of CAD, this probability increased to 55%.(Figure 1c)
Figure 1.
Probability of stroke subtype The probability of stroke subtype based on presenting blood pressure for unadjusted (a) and adjusted (b) and (c) models. NLUE: non-lacunar stroke of either possible cardioembolic or possible large-artery aetiology.
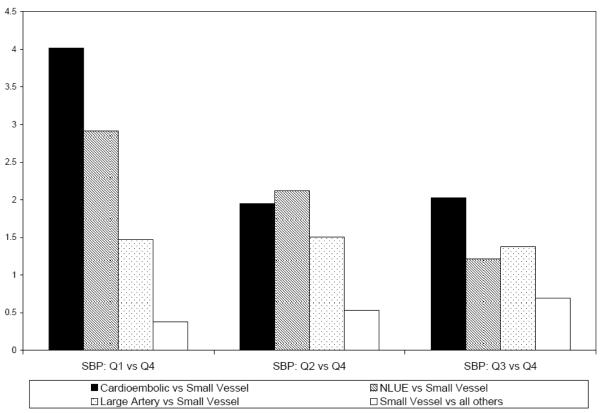
The odds ratios of the analyzed subtypes were also calculated for each quartile of presenting SBP, and are presented in Figure 2. For example, within the first quartile of presenting SBP (<140mmHg), the odds of cardioembolic stroke were four times larger than the odds of small vessel occlusion.
Figure 2.
Adjusted odds ratios of stroke subtype versus small vessel occlusion by quartiles of systolic blood pressure Systolic blood pressure quartiles: Q1 90-139 mm Hg, Q2 140-159 mm Hg , Q3 160-181 mm Hg, Q4 182-260 mm Hg. For all odds ratios, Q4 was the referent. NLUE: non-lacunar stroke of either possible cardioembolic or possible large-artery aetiology.
Sensitivity Analysis
The results from the sensitivity analysis yielded similar relationships between stroke subtype and SBP. Specifically, a 10 mmHg decrease in SBP was associated with a 14% increased odds of the cardioembolic combined with the NLUE subtype versus the other two stroke subtypes combined (95% CI: 5%-24%). A five year increase in age was associated with a 14% increased odds of cardioembolic combined with the NLUE subtype versus other subtypes combined (95% CI: 4.5%-27%). Finally, the odds of cardioembolic combined with the NLUE versus other stroke types combined were 1.87 times higher among those with a history of coronary artery disease versus those without (95% CI: 1.13-3.10).
Discussion
We found that lower presenting systolic blood pressure was associated with cardioembolic ischaemic stroke subtype. If future work confirms this association, clinicians could consider a more thorough evaluation for cardiac sources of emboli in individuals who present with a lower blood pressure. Such a strategy may help maximize the number of patients who are initiated on appropriate secondary prevention strategies to reduce the burden of recurrent stroke. Current guidelines recommend anti-coagulation to prevent recurrence in most cases of cardioembolic stroke, though acute anticoagulation is not recommended.[14, 15] Additional data would be needed, including cost considerations, before widespread changes in practice could be recommended.
Prior studies examining the relationship between blood pressure during acute hospitalization and stroke subtype have demonstrated similar trends, with small vessel occlusion having higher, and cardioembolic stroke having lower, systolic blood pressures throughout the hospitalization.[3, 6, 7, 16] Our findings are congruent with the findings of these studies, and importantly expand this observation to a community setting without an academic medical centre. In addition, the current report provides estimates of risk of cardioembolic stroke given initial, presenting blood pressure rather than trends throughout acute hospitalization. This may allow for a higher suspicion of cardioembolic stroke at the time of presentation. The mechanism underlying the association of presenting blood pressure and ischaemic stroke subtype is uncertain. Possible factors include prior hypertension and pre-existing cardiac disease along with treatment for these conditions, as well as size and cerebral location of the stroke. Further evaluation of these mechanisms is warranted.
The distribution of stroke subtype in this community is similar to the overall distribution of stroke subtype reported in the TOAST trial, a meta-analysis of population based stroke incidence studies, and a population-based stroke study from Germany.[8, 17, 18] A recent population based study published from France did show a higher incidence of large artery atherosclerosis as the etiologic subtype (35.8%), however the investigators reported a similar proportion of cardioembolic stroke to the current study and the other studies.[19] About 20% of the patients in the current investigation had an “undetermined” classification. Prior studies using the TOAST classification system have characterized about one-third to one-half of subjects as “undetermined aetiology.”[8, 17, 20] This “undetermined” group was different in our sample from the determined group, notably their strokes were minimally less severe, and they more frequently had a history of prior stroke or TIA. The likely explanation for our study having a smaller “undetermined” group is the inclusion of the NLUE category which allowed for the classification of patients in whom insufficient information existed to distinguish between large artery and cardioembolic aetiology. In the current report, the point estimate of the odds ratio for the association between NLUE and presenting systolic blood pressure was intermediate between the definite cardioembolic and large artery groups. This is consistent with the presumed mix of both (cardioembolic and large artery) subtypes in the NLUE subtype.
This investigation has several important limitations. First, a significant portion of patients were classified as either NLUE or “undetermined.” It is likely that these groups contained missed cases of cardioembolic stroke. Perhaps less diagnostic testing was performed in patients with minor stroke, which would diminish the ability to assign a classification, and tend to increase the likelihood that patients with minor stroke would be classified as either NLUE or “undetermined.” Another limitation is that the time interval between stroke onset and blood pressure measurement was not collected in the BASIC study; blood pressure has been previously shown to decrease after stroke onset.[21, 22] In addition, the primary aims of BASIC did not include this sub-study and the findings should be used for hypothesis generation rather than direct application to widespread clinical practice. It is possible that clinicians were more thorough in examining for cardiac sources of emboli in patients with low systolic blood pressures. In addition, the BASIC study did not collect information on history of congestive heart failure. This and other cardiac parameters not included in our analysis may be helpful in predicting cardioembolic subtype in patients presenting with ischaemic stroke. Despite these limitations, this study was conceived without prior examination of the data, and the BASIC study abstractors were blinded to this study question, as were the study neurologists who assigned the subtypes.
In summary, this work demonstrates that lower presenting systolic blood pressure, along with increasing age and history of CAD are associated with classification into cardioembolic aetiology of ischaemic stroke in a representative bi-ethnic community. Further prospective exploration and confirmation of this relationship is needed, including further study of possible mechanisms for this observed association.
Acknowledgements
Financial disclosure The authors have no relevant financial interests related to the material in the manuscript.
Role of funding source This study was funded by the National Institutes of Health (NINDS R01 NS38916). The funding source had no involvement in study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Previous Presentation This study was presented as an abstract at the International Stroke Conference; February 20, 2008; New Orleans, Louisiana.
References
- 1.Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension. 2004;43:18–24. doi: 10.1161/01.HYP.0000105052.65787.35. [DOI] [PubMed] [Google Scholar]
- 2.Stead LG, Gilmore RM, Decker WW, Weaver AL, Brown RD., Jr. Initial emergency department blood pressure as predictor of survival after acute ischemic stroke. Neurology. 2005;65:1179–83. doi: 10.1212/01.wnl.0000180939.24845.22. [DOI] [PubMed] [Google Scholar]
- 3.Marcheselli S, Cavallini A, Tosi P, Quaglini S, Micieli G. Impaired blood pressure increase in acute cardioembolic stroke. J Hypertens. 2006;24:1849–56. doi: 10.1097/01.hjh.0000242410.42912.2d. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Garcia JL, Botia E, de La Sierra A, Villanueva MA, Gonzalez-Spinola J. Significance of elevated blood pressure and its management on the short-term outcome of patients with acute ischemic stroke. Am J Hypertens. 2005;18:379–84. doi: 10.1016/j.amjhyper.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Tanizaki Y, Kiyohara Y, Kato I, et al. Incidence and risk factors for subtypes of cerebral infarction in a general population: the Hisayama study. Stroke. 2000;31:2616–22. doi: 10.1161/01.str.31.11.2616. [DOI] [PubMed] [Google Scholar]
- 6.Vemmos KN, Spengos K, Tsivgoulis G, et al. Factors influencing acute blood pressure values in stroke subtypes. J Hum Hypertens. 2004;18:253–9. doi: 10.1038/sj.jhh.1001662. [DOI] [PubMed] [Google Scholar]
- 7.Vemmos KN, Tsivgoulis G, Spengos K, et al. Blood pressure course in acute ischaemic stroke in relation to stroke subtype. Blood pressure monitoring. 2004;9:107–14. doi: 10.1097/01.mbp.0000132424.48133.27. [DOI] [PubMed] [Google Scholar]
- 8.The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators Low Molecular Weight Heparinoid, ORG 10172 (Danaparoid), and Outcome After Acute Ischemic Stroke: A Randomized Controlled Trial. JAMA. 1998;279:1265–72. [PubMed] [Google Scholar]
- 9.Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Uchino K, Risser JM, Smith MA, Moye LA, Morgenstern LB. Ischemic stroke subtypes among Mexican Americans and non-Hispanic whites: the BASIC Project. Neurology. 2004;63:574–6. doi: 10.1212/01.wnl.0000133212.99040.07. [DOI] [PubMed] [Google Scholar]
- 11.Morgenstern LB, Smith MA, Lisabeth LD, et al. Excess stroke in Mexican Americans compared with non-Hispanic Whites: the Brain Attack Surveillance in Corpus Christi Project. Am J Epidemiol. 2004;160:376–83. doi: 10.1093/aje/kwh225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Q, Cole JW, O’Connell JR, et al. Phosphodiesterase 4D polymorphisms and the risk of cerebral infarction in a biracial population: the Stroke Prevention in Young Women Study. Human molecular genetics. 2006;15:2468–78. doi: 10.1093/hmg/ddl169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pigeon JG, Heyse JF. An Improved Goodness of Fit Statistic for Probability Prediction Models. Biometrical Journal. 1999;41:71–82. [Google Scholar]
- 14.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 15.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 16.Toyoda K, Okada Y, Fujimoto S, et al. Blood pressure changes during the initial week after different subtypes of ischemic stroke. Stroke. 2006;37:2637–9. doi: 10.1161/01.STR.0000242781.80832.cc. [DOI] [PubMed] [Google Scholar]
- 17.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of Ischemic Stroke Subtypes According to TOAST Criteria: Incidence, Recurrence, and Long-Term Survival in Ischemic Stroke Subtypes: A Population-Based Study. Stroke. 2001;32:2735–40. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 18.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–73. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 19.Bejot Y, Caillier M, Salem D Ben, et al. Ischemic stroke subtypes and associated risk factors: a French population-based study. Journal of neurology, neurosurgery, and psychiatry. 2008 doi: 10.1136/jnnp.2008.150318. [DOI] [PubMed] [Google Scholar]
- 20.Schneider AT, Kissela B, Woo D, et al. Ischemic Stroke Subtypes: A Population-Based Study of Incidence Rates Among Blacks and Whites. Stroke. 2004;35:1552–6. doi: 10.1161/01.STR.0000129335.28301.f5. [DOI] [PubMed] [Google Scholar]
- 21.Broderick J, Brott T, Barsan W, et al. Blood pressure during the first minutes of focal cerebral ischemia. Annals of emergency medicine. 1993;22:1438–43. doi: 10.1016/s0196-0644(05)81993-6. [DOI] [PubMed] [Google Scholar]
- 22.Majersik JJ, Smith MA, Zahuranec DB, Sanchez BN, Morgenstern LB. Population-based analysis of the impact of expanding the time window for acute stroke treatment. Stroke. 2007;38:3213–7. doi: 10.1161/STROKEAHA.107.491852. [DOI] [PubMed] [Google Scholar]