Abstract
Previous studies have described that statins (inhibitors of cholesterol and isoprenoid biosynthesis) inhibit the output of amyloid-β (Aβ) in the animal model and thus decrease risk of Alzheimer's disease. However, their action mechanism(s) in APP processing and Aβ generation is not fully understood. Here we report that lovastatin treatment reduced Aβ output in cultured hippocampal neurons as a result of reduced Aβ precursor protein (APP) levels and β-secretase activities in low density Lubrol WX (non-ionic detergent) extractable lipid rafts (LDLR). Rather than altering cholesterol levels in lipid raft fractions and thus disrupting lipid raft structure, lovastatin decreased Aβ generation through down-regulating geranylgeranyl-pyrophosphate (GGPP) dependent endocytosis pathway. The inhibition of APP endocytosis by treatment with lovastatin and reduction of APP levels in LDLR fractions by treatment with phenylarsine oxide (a general endocytosis inhibitor) support the involvement of APP endocytosis in APP distribution in LDLR fractions and subsequent APP β-cleavage. Moreover, lovastatin-mediated down-regulation of endocytosis regulators, such as EEA1, dynamin-I and phosphatidylinositol-3 kinase activity, indicates that lovastatin modulates APP endocytosis possibly through its pleiotropic effects on endocytic regulators. Collectively, these data report that lovastatin mediates inhibition of LDLR distribution and β-cleavage of APP in a GGPP and endocytosis dependent manner.
Keywords: lovastatin, lipid rafts, geranylgeranylation, Alzheimer's disease, beta-Amyloid and endocytosis
Introduction
Accumulation of amyloid-β peptides (Aβ) is one of the “hallmarks” of Alzheimer’s disease (AD) (Halliday et al. 2000; Gupta and Pansari 2003; McGeer and McGeer 2003). Aβ is produced from Aβ precursor protein (APP) by sequential action of β-secretase and γ-secretase which aggregates to generate neurotoxic insoluble Aβ plaques (Halliday et al. 2000; Gupta and Pansari 2003; McGeer and McGeer 2003). On the other hand, cleavage of APP by α-secretase and γ-secretase generates soluble non-toxic products. Recent studies have demonstrated that the β-secretase- and α-secretase-mediated APP cleavages occur in separate membrane compartments; β-secretase in lipid rafts (a cholesterol and sphingomyelin rich microdomains) and α-secretase in non-lipid rafts membrane fractions (Riddell et al. 2001; Wolozin 2001). According to this model, a high level of cholesterol may favor Aβ production, whereas a reduced cholesterol level favors the non-amyloidogenic α-secretase pathway. Indeed, a number of studies have demonstrated that hypercholesterolemia and high cholesterol diet are risk factors of AD in human and animal models (Refolo et al. 2000; Fassbender et al. 2001; Buxbaum et al. 2002; Pappolla et al. 2003). Moreover, reduced incident of AD is reported in patients using statins (inhibitors of HMG-CoA reductase, a rate limiting enzyme in cholesterol biosynthesis) (Jick et al. 2000; Wolozin et al. 2000; Kojro et al. 2001; Chauhan 2003). Therefore, the ability of statins to reduce cholesterol synthesis has been suggested as a major mechanism for their anti-amyloidogenic property (Simons et al. 1998; Refolo et al. 2000; Fassbender et al. 2001; Kojro et al. 2001; Shie et al. 2002; Pappolla et al. 2003; Parvathy et al. 2004). However, the recent MIRAGE (Multi-Institutional Research in Alzheimer’s Genetic Epidemiology) trial demonstrated that AD is prevented by statins but not by non-statin cholesterol-lowering medications (Green et al. 2006), thus suggesting the occurrence of cholesterol-independent pathways in statin-mediated anti-AD effects. Indeed, statin (i.e. lovastatin, simvastatin and pravastatin) treatment has been shown to be ineffective on reduction of cholesterol levels in the brain of guinea pigs (Fassbender et al. 2001; Lutjohann et al. 2004) and in cultured hippocampal neurons up to 40 hrs treatment (Meske et al. 2003). The very long half life of brain cholesterol (4–6 months in rat) (Serougne-Gautheron and Chevallier 1973; Andersson et al. 1990) and observed inhibition of cholesterol release to peripheral blood as 24S-hydroxycholesterol (Lutjohann et al. 2004) by statin may contribute towards the observed unchanged cholesterol levels in statin-treated brain. Therefore, the exact mechanism for statin-mediated antiamyloidogenic activity is clear.
Recent reports have provided evidence that endosomes are major subcellular compartments for β-cleavage of APP because of their acidic pH and positive correlation between endocytic activity and β-cleavage of APP (Koo and Squazzo 1994; Refolo et al. 1995; Soriano et al. 1999; Walter et al. 2001; Bamberger et al. 2003; Ehehalt et al. 2003; Grbovic et al. 2003; Cataldo et al. 2004). Aβ generation is markedly reduced when endocytosis of APP is inhibited (Koo and Squazzo 1994; Soriano et al. 1999) but accelerated when endocytosis is stimulated (Grbovic et al. 2003). In neurons, APP endocytosis is known to be mediated by a clathrin-dependent pathway (Marquez-Sterling et al. 1997), and several geranylgeranylated small GTPases (i.e. RhoA and Rab5) are known to be involved in this event (Marquez-Sterling et al. 1997; Symons and Rusk 2003). Very recently, statins were demonstrated to have an inhibitory effect on endocytosis of cell surface molecules, where statins appear to regulate endocytosis via regulation of isoprenylation of small GTPases (Sidaway et al. 2004; Verhulst et al. 2004). Therefore, these studies suggest that statins may regulate APP endocytosis and APP β-cleavage via inhibition of protein isoprenylation.
This study describes a possible cholesterol-independent mechanism for statin-mediated anti-amyloidogenic process in primary cultured rat hippocampal neurons. We have observed that lovastatin reduces APP levels and β-secretase activity in low density lipid rafts (LDLR) as well as Aβ production in a GGPP dependent manner. The inhibition of APP endocytosis and reduction in early endosomal distribution of APP by lovastatin treatment and reduction of APP levels in LDLR fractions by phenylarsine oxide (PAO), an endocytosis inhibitor, suggest that lovastatin may modulate distribution of APP in LDLR by inhibiting APP endocytosis.
Materials and Methods
Cell culture and drug treatments
For hippocampal neuron cell culture, E17 Sprague Dawley rat hippocampi were used. Following treatment of hippocampi with 0.125% trypsin, the dissociated cells were plated on poly-D-lysine coated culture plates in Neurobasal medium (Invitrogen, San Diego, CA) with 2% B27 supplement (Invitrogen), L-glutamine (0.5 mM; Sigma-Aldrich Co., St. Louis, MO), L-glutamate (25 µM; Sigma-Aldrich Co.) and antibiotic/antimycotic agent (cocktail of penicillin, streptomycin and amphotericin B, Invitrogen). The media was completely exchanged with the same media just after cell attachment (about 3 hr). The cultures were maintained in 5% CO2 at 37°C for 7 days and exchanged with B27 free Neurobasal medium for drug treatment. Lovastatin (BioMol, Plymouth Meeting, PA), mevalonate (Sigma-Aldrich), phenylarsine oxide (Sigma-Aldrich), FTI-276 (Calbiochem, San Diego, CA) and GGTI-298 (Calbiochem) were prepared in dimethylsulfoxide (DMSO; Sigma). Geranylgeranyl-pyrophosphate (GGPP; Sigma) and farnesyl-pyrophosphate (FPP; Sigma) were prepared as liposomes as described previously (Sinha et al. 1999). Briefly, 200µl dipalmitoylphosphatidylcholine (25mM) and 200µl GGPP (200 µg) or 200µl FPP (200 µg) in methanol were mixed and dried under nitrogen gas flow. The dried lipid film was then dispersed in 0.5 ml of PBS at 50 °C and liposomes were made by sonication.
Analysis of α- and β-secretase activity and Aβ40/Aβ42 release
The activities of α- and β-secretases in post nuclear cell extract or lipid raft fractions were measured by using fluorogenic assay kits purchased from R&D Systems Inc. (Minneapolis, MN). The activities were measured by SPECTRAmax® Gemini XS® fluorimeter with SOFTmax PRO® software (Molecular Devices, Chicago, IL) with excitation at 345 nm and emission detection at 500 nm. For quantification of Aβ in media, culture media was centrifuged (1000xg for 10min at 4°C) and 100µl supernatant was used for colorimetric ELISA assay by using human Aβ (1–40) and (1–42) assay kits purchased from IBL. Co., Ltd. (Japan) or Wako chemicals (Japan) which are fully compatible with rat Aβ40 or Aβ42.
Western blot analysis and antibodies
Western blot analysis were performed as described previously (Won et al. 2003) by using antibody against N-terminal APP695 (22C11, Chemicon, Temecula, CA), C-terminal APP (Ab18813, Abcam, Cambridge, MA), BACE1 (Chemicon), ADAM10 (SantaCruz Biotech.), flotillin-1 (SantaCruz Biotech.), clathrin (SantaCruz Biotech.), PrP (SantaCruz Biotech.), CD71 (SantaCruz Biotech.), Rab5 (SantaCruz Biotech.), RhoA (SantaCruz Biotech.), EEA1 (Abcam), dynamin-1 (SantaCruz Biotech.) or phospho-Akt1 (Cell Signaling Tech. Inc. Panvers, MA).
Extraction of membrane microdomains
The cells in 150mm culture dish were washed in ice cold PBS twice and lysed in 0.4 ml MBS buffer [25 mM MES, pH 6.5, 150 mM NaCl, 1 mM Na3VO4 and protease inhibitor cocktail (Roche, Indianapolis, IN)] containing 0.5 % Triton X-100 (Sigma) or Lubrol WX (ICN Biochemicals, Cleveland, OH) for 30 min on ice with 10 strokes in a tightly fitted Dounce homogenizer. The homogenates were centrifuged at 1,000xg for 10min at 4°C and the resulting supernatants were analyzed for protein quantity. The post-nuclear lysates with the same protein quantities were mixed with the same volume of 80% Nycodenz (Nycomed Pharma, Roskilde, Denmark) in MBS buffer with appropriate detergent. The resulting 40% Nycodenz containing lysate mixtures were overlayed with two volumes of 30% and one volume of 5 % Nycodenz in MBS with the appropriate detergent. Following centrifugation for 2 hr at 80,000 xg in a TLV-100 rotor (Beckman, Fullerton, CA), 10 equal volumes of fractions were collected.
Quantification of cholesterol levels and analysis of cholesterol stability
Cholesterol levels in cellular or each membrane microdomain fraction were measured by using Amplex® Red Cholesterol Assay Kit (Invitrogen) as described in manufacturer’s instruction. For the analysis of cholesterol half life, the cells were grown for a week and then labeled with [4-14C] cholesterol (1 µCi, 50–60 mCi/mmol from American radio labeled Chemicals, Inc) overnight. After washing three times in B27 free media, the cells were treated with statins for 36 hrs under B27 free condition (exogenous cholesterol free condition) and total lipids were extracted from cell homogenates using the Folch method as described earlier (Khan et al. 2000). Non-polar lipids were resolved on high performance thin layer chromatography in a solvent system consists of hexane:ether:acetic acid (75:25:01). Cholesterol was visualized by iodine and autoradiography and scraped for radioactive counts using LS 6500 multi-purpose scintillation counter from Beckman Coultier.
Quantification of APP and transferrin endocytosis
Three sets of neuron cell culture on 6 well plates were washed with ice-cold Neurobasal media and incubated for 20 min at 4°C with human transferrin conjugated with Alexa Fluor® 594 (10 µg/ml; Invitrogen) or anti-APP antibody (22C11; 5 µg/ml) labeled with Alexa Fluor® 594 Fab fragments (Invitrogen) in B27 free Neurobasal media. After another wash with ice-cold Neurobasal media, the first set of cell cultures were lysed in 200µl of PNT buffer (PBS, 0.2% NP-40, and 0.2% Triton X-100) and the cell lysates were designated as “total surface binding (TSB)”. The second set of cell cultures were washed with ice-cold acetate buffer (0.5 M acetate and 0.5 M NaCl) or acidic PBS (pH2.5) to remove surface bound transferrin or 22C11 antibody, respectively. Following lysis with PNT buffer, the cell lysates were designated as “acid stable binding (ASB)”. The third set of cell cultures were incubated for 30 min at 37°C to allow internalization of fluorescent transferrin or 22C11. The fluorescent transferrin or 22C11 that remained on the surface was removed by washing with ice-cold acetate buffer or acidic PBS, respectively. Following lysis with PNT buffer, the cell lysates were designated as “internalized transferrin or APP (INT)”. The fluorescence in each lysate was measured by SPECTRAmax® Gemini XS® fluorimeter with SOFTmax PRO® software with excitation at 590 nm and emission detection at 617 nm with 610nm cut off. The “specific endocytic activity (SEA)” of transferrin or APP was calculated by “SEA=(INT-ASB)/(TSB-ASB)”.
For immunocytochemical analysis of cellular APP distribution and internalization, the neuron cells plated on poly-D-lysine coated two chamber slides (Becton, Dickinson and Company, Franklin Lakes, NJ) were treated with lovastatin for 36 hrs and then incubated with 22C11 anti-APP antibody for 1hr at 4°C. Following washing with PBS, the cells were fixed with 4% formaldehyde containing PBS for staining of cell surface APP, or were further incubated in Neurobasal media at 37°C for 2hrs to allow internalization and then washed with acid PBS (pH2.5) to remove cell surface 22C11 prior to fixing with formaldehyde containing PBS for staining of internalized APP. The cell surface or internalized 22C11 antibody was visualized by using Alexa Fluo 488 conjugated Goat anti-mouse antibody (Invitrogen) and fluorescent microscope and DP70 image system with 40X objective (Olympus, Japan).
Subcellular fraction
The cells in 150mm culture dish were washed in ice cold PBS twice and lysed in sucrose buffer (20 mM Hepes-NaOH, pH 7.4, 1 mM EDTA, 0.25 M sucrose, 1 mM Na3VO4 and protease inhibitor cocktail) with 20 strokes in a tight fitted Dounce homogenizer. The homogenates were centrifuged at 1,000xg for 10min at 4°C and the resulting post nuclear fractions designated as S1 were further centrifuged at 15,000xg for 20 min at 4°C to pellet mitochondria and broken membrane fragments (P15). The resulting post mitochondrial fractions (S15) were further centrifuged at 100,000 xg for 40 min at 4°C to separate cytosolic fractions (S100) and microsomal fractions (P100). For immunoprecipitation of Rab5 positive microsomal vesicles, P100 were dissolved in the sucrose buffer and incubated with anti-Rab5 polyclonal antibody for 2hr at 4°C. The Rab5 positive vesicles were pulled-down by using protein-G conjugated with Sepharose (Santa Cruz Biotech.). The APP levels in Rab5 positive P100 vesicles as well as in S1, P15, S15, P100 and S100 were quantified by Western immunoblot using 22C11 APP antibody. For subcellular organelle fractionation, the cell lysates were centrifuged at 3,000xg for 20 min to remove unbroken cells, nuclei and heavy mitochondrial fractions. The resulting 250µl of supernatant were loaded on the top of Optiprep® (Axis-Shield, Oslo, Norway) step density gradient (0, 1.25, 2.5, 5, 10 and 20%) in 20 mM Hepes-NaOH, pH 7.4, 1 mM EDTA, 0.25 M sucrose. Following centrifugation for 30min at 80,000 xg in a TLV-100 rotor (Beckman), 10 equal volumes of fractions were collected.
RESULTS
Lovastatin reduced Aβ generation but increased cellular APP levels in primary hippocampal neurons
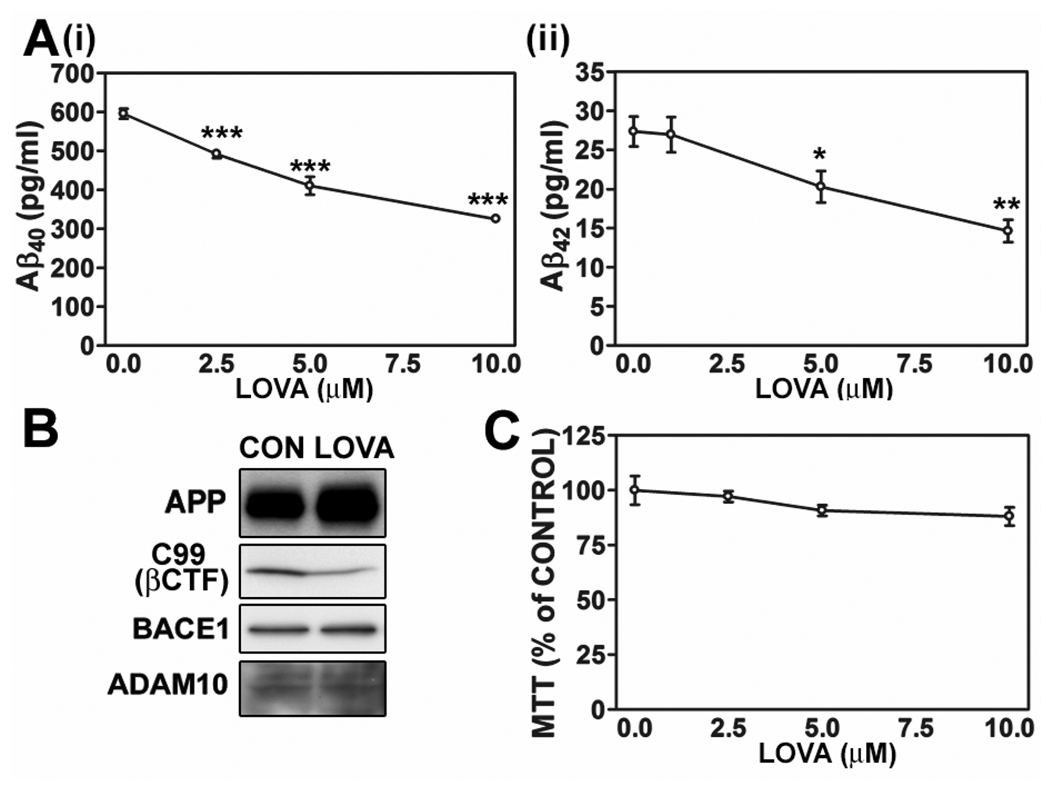
To examine the effect of statin on Aβ output in neurons, primary hippocampal neurons in culture were treated with different concentrations of lovastatin (1, 5 and 10 µM) for 36 hrs. As reported previously (Wolozin et al. 2000; Fassbender et al. 2001; Kojro et al. 2001), the lovastatin treatment reduced Aβ40 and Aβ42 output (Fig. 1A-i and -ii) and cellular levels of C99 (APP C-terminal fragment produced by action of β-secretase) (Fig. 1B) without altering protein levels of BACE1 (one of β-secretases) and ADAM10 (one of α-secretases) (Fig. 1B) and cell viability (Fig. 1C). However, lovastatin treatment increased cellular APP levels (Fig. 1B). These results indicate that lovastatin-mediated reduction of Aβ generation may be mediated by reduced β-secretase-mediated APP cleavage rather than reduction of protein levels of APP and β-secretase.
Fig. 1.
Lovastatin reduces Aβ generation but increases cellular APP levels in primary hippocampal neurons. The primary rat hippocampal neurons were treated with lovastatin (LOVA) for 36hrs, and secreted Aβ40 and Aβ42 levels (A), cell viability (B) and protein levels of APP695, C99 (β-secretase processed C-terminal fragment of APP; βCTF), BACE1 and ADAM10 (C) were measured. All experiments were performed at least three times and showed the same tendency. The vertical bar on each group indicates the standard error of mean (* P < 0.05, ** P < 0.01, *** p < 0.001 compared to control group).
Lovastatin reduced APP levels in low-density Lubrol WX extractable lipid raft fractions
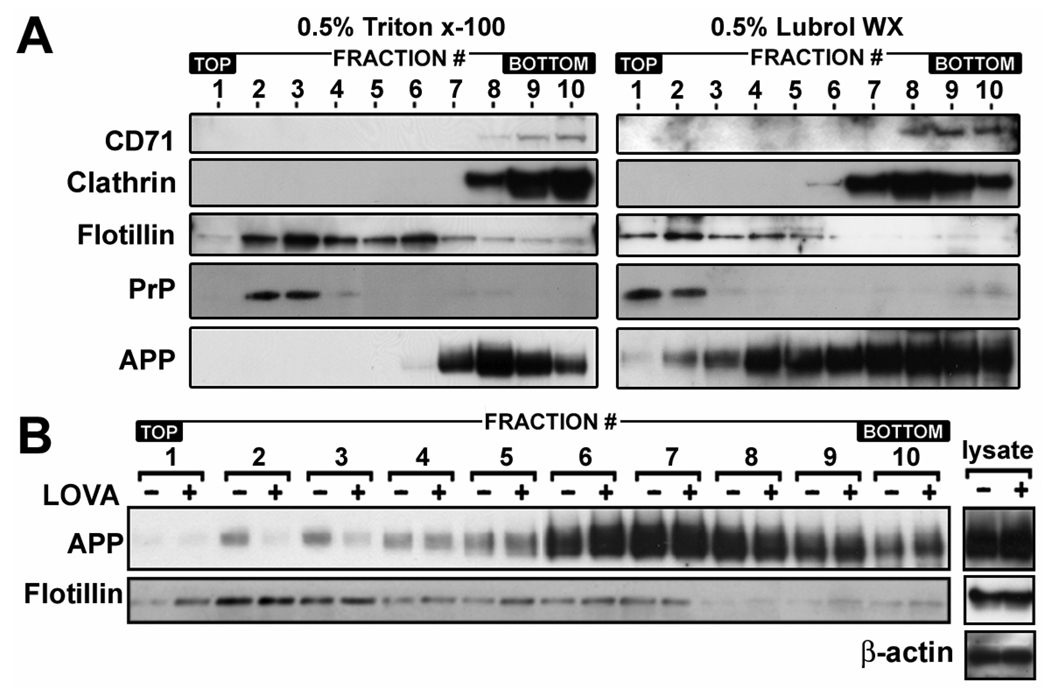
The amyloidogenic process of APP is known to be associated with cholesterol and sphingolipid rich membrane microdomains called “lipid rafts” (Riddell et al. 2001; Wolozin 2001). Since subsets of rafts and their chemical and physical properties are dependent on types of detergent used (Madore et al. 1999; Roper et al. 2000; Simons and Toomre 2000; Riddell et al. 2001; Rouvinski et al. 2003), we studied APP distribution in membrane microdomains prepared with two types of non-ionic detergents; Triton X-100 and Lubrol WX (Fig. 2A). In 0.5% Triton X-100, the majority of APP was associated with detergent soluble fractions (CD71 and clathrin positive fractions; # 8~10) and only a small amount of APP (less than 1%) was associated with the flotillin positive fractions (fraction # 6) (Fig 2A). On the other hand, in 0.5% Lubrol WX, APP was broadly distributed in the entire gradient. About 40.1% of APP were distributed in flotillin positive fractions (#1~6) and 59.9% was in non-lipid raft membrane fractions (#7~10). These data indicate that significant amounts of APP molecules on the neuronal membranes may be associated with specific membrane microdomains which are insoluble in Lubrol WX but soluble in Triton X-100.
Fig. 2.
Lovastatin reduces APP levels in low-density lipid raft fractions. A. To determine the distribution of APP in membrane microdomains, the lipid raft fractions from rat hippocampal neurons were extracted by using 0.5% Triton X-100 or 0.5% Lubrol WX and the distribution of APP in the lipid raft fractions was determined by comparing protein levels of APP and non-lipid raft markers (CD71 and clathrin), caveolae marker (flotillin-1) or marker for glycosylphosphatidylinositol-anchored protein containing lipid rafts (prion protein, PrP) by Western immunoblot analysis. B. To examine the effect of lovastatin on APP levels in lipid rafts, the neuron cells were cultured for 36 hrs in the presence or absence of lovastatin (5 µM). Following the extraction of lipid raft fractions in 0.5% Lubrol WX, the protein levels of APP in each fraction were analyzed by Western immunoblot analysis. As loading control of density gradient centrifugation, β-actin levels were measured from post nuclear lysates (lysate). In addition, the lack of correlation between protein levels of APP and flotillin-1 in low density lipid raft fractions (fraction# 2 and 3) in lovastatin treated and untreated control gradient fractions indicate that the observed alterations in APP levels are not due to the differences of protein amounts in each group of fractions. All experiments were performed at least three times and showed the same tendency.
Since lipid rafts are the site for β-secretase-mediated APP cleavage (Riddell et al. 2001), we examined the possible role of lovastatin in the regulation of APP distribution in Lubrol WX insoluble lipid raft fractions. As shown in the Fig. 2B, we observed that lovastatin treatment caused selective reduction of APP levels only in low density fractions of Lubrol WX insoluble lipid rafts (LDLR) (#1~3) which contained higher amounts of cholesterol than other flotillin positive fractions (Fig. 5B), and were enriched with PrP, a glycosyl phosphatidyl inositol-anchored protein (GPI-AP) (Fig. 2A). However, APP levels in other lipid raft fractions (# 4~6) and detergent soluble fractions (#7~10) were not reduced by lovastatin treatment (Fig. 2B). In contrast to APP, protein levels of flotillin, a caveolae protein, in fractions # 2 and 3 were not altered by lovastatin treatment under similar conditions (Fig. 2B). Secondly, the same amount of control and lovastatin treated cell lysates were charged on the gradients as represented by equal amounts of β-actin levels in Fig. 2B (denoted by lysate), and equal volumes of fractions were collected from the respective gradients. Therefore, lovastatin mediated reduction in APP levels in LDLR fractions is not due to differences in protein loading or general reduction in membrane associated protein levels. The same experimental procedures for lipid raft preparation were also performed for data in Fig. 3B, Fig. 4A, Fig. 6A, 6E, Fig. 7G and Fig. 8E (data not shown), thus, it indicates that the observed changes in APP and BACE1 protein levels in those figures are not due to differences in protein loading or general reduction in membrane associated protein levels.
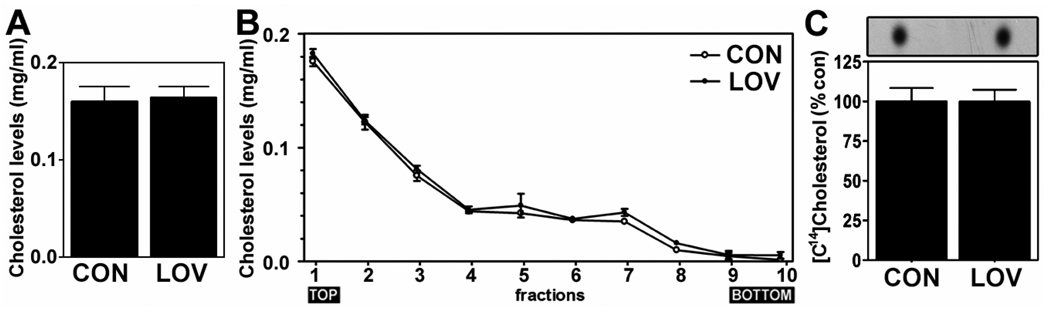
Fig. 5.
Cellular cholesterol levels were not altered by lovastatin treatment. To examine the involvement of cholesterol in lovastatin-mediated anti-amyloidogenic effect, the effect of lovastatin (5µM/36hrs) on the levels of cholesterol in post nuclear whole cell extracts (A) and in lipid raft fractions (B) were measured. To analyze half life of cholesterol under lovastatin treatment, the neurons were labeled with [14C]-cholesterol and treated with lovastatin (5µM). Thirty six hr after, the levels of [14C]-cholesterol were measured as described in Experimental Procedure (C).
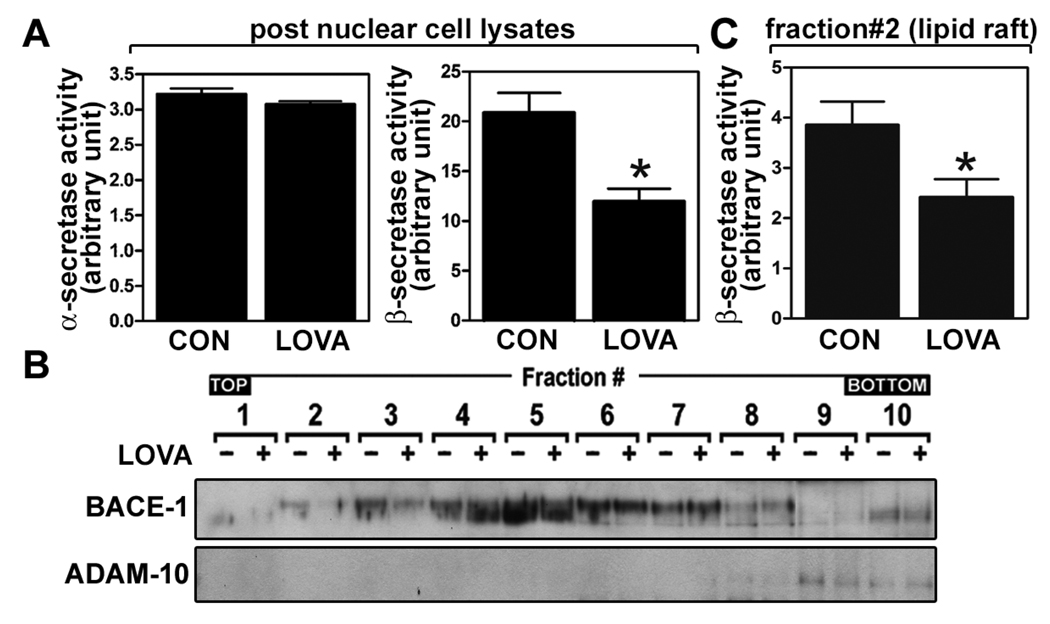
Fig. 3.
Lovastatin reduces β-secretase activity without altering α-secretase activity. To examine the effect of lovastatin on α- and β-secretases, the α- and β-secretase activities were measured from primary cultured hippocampal neuron cells after treatment with lovastatin (5µM/36hrs) (A). For the lipid raft distribution of β-secretase, the lipid raft fractions were extracted from post nuclear fractions by using 0.5% Lubrol WX and enzyme levels of α- and β-secretases (B) and β-secretase enzyme activity in low density lipid raft fraction (fraction # 2) (C) were measured. All experiments were done at least three times and showed same tendency. The vertical bar indicates the standard error of mean (* P < 0.05 compared to control group).
Fig. 4.
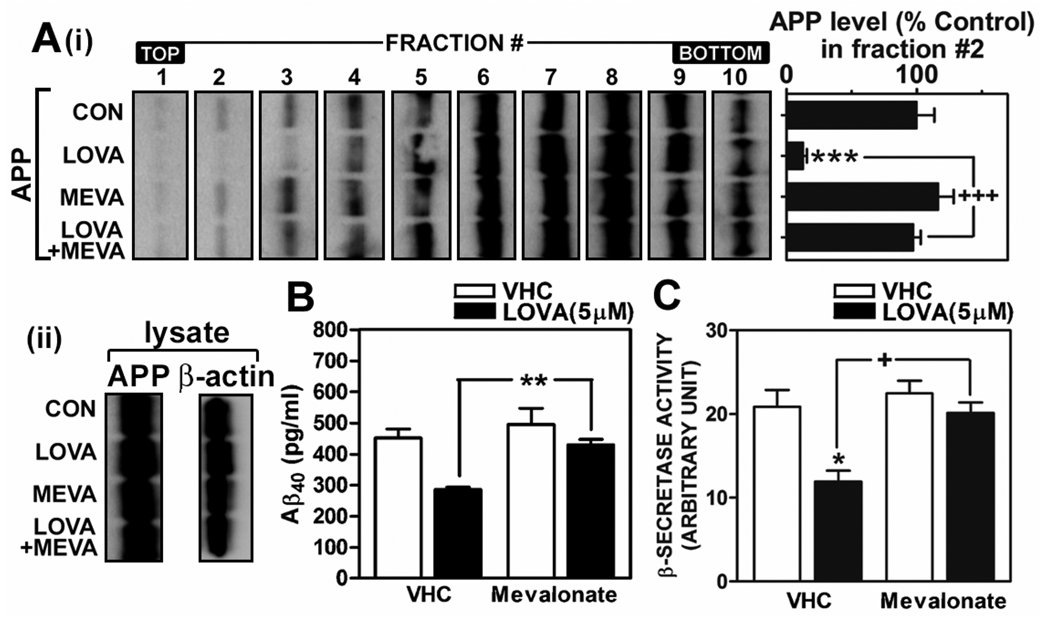
Mevalonate reverses lovastatin-mediated reduction of APP levels in low density lipid raft fractions, β-secretase activity and Aβ generation. To define the role of metabolites of the mevalonate pathway in lovastatin-mediated anti-amyloidogenic effects, primary cultured hippocampal neuron cells were treated with lovastatin (5µM) in the presence or absence of mevalonate (250µM) for 36 hrs and then APP levels in lipid rafts (A), Aβ40 levels in media (B) and β-secretase activity in post nuclear cell lysate (C) were measured as described in materials and methods. For the lipid raft distribution of APP, the lipid raft fractions were extracted from post nuclear fractions by using 0.5% Lubrol WX and APP levels in lipid raft fraction were measured (A). As loading control of density gradient centrifugation, β-actin levels were measured from post nuclear lysates (lysate) by Western immunoblot analysis (A-ii). All experiments were performed at least three times. VHC (vehicle) represents dimethylsulfoxide treatment as control. The vertical bar indicates the standard error of mean (* P < 0.05, ** P < 0.01, *** p < 0.001 compared to control group; + P < 0.05, +++ p < 0.001 compared to lovastatin treated group).
Fig. 7.
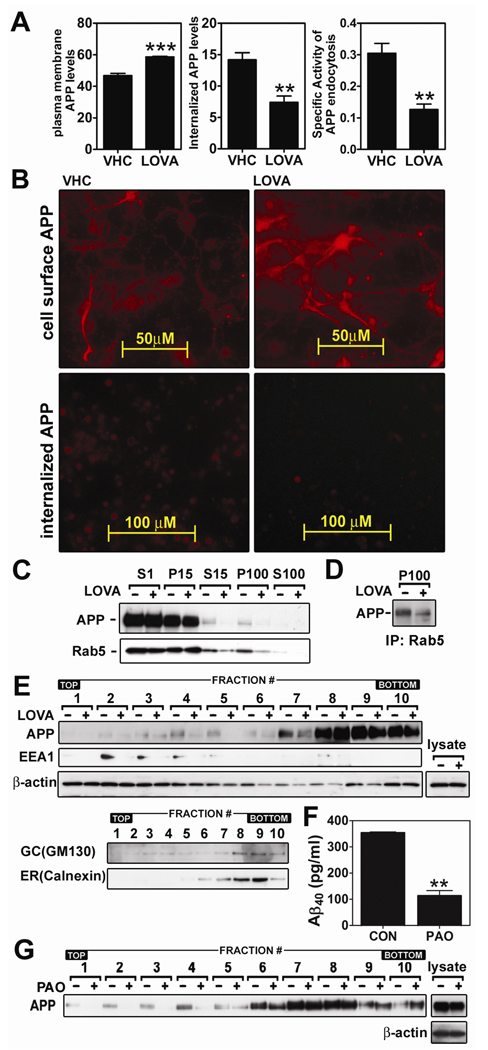
Anti-amyloidogenic activity of lovastatin is mediated via inhibition of APP endocytosis. To examine the effect of lovastatin on APP endocytosis, hippocampal neuronal cells were treated with lovastatin (LOVA; 5µM/36hrs) and cell surface and internalized APP levels were measured by fluorometric (A) or fluoromicroscopic (B) methods. For this the cells were incubated with APP antibody (22C11) at 4°C and fixed for staining of cell surface APP or further incubated at 37°C and washed with acid PBS and fixed for staining of internalized APP. To examine the effect of lovastatin on endosomal APP levels, APP and Rab5 levels in post nuclear (S1), light mitochondrial (P15), post-light mitochondrial (S15), microsomal (P100) and post microsomal (S100) fractions were measured by Western immunoblot analysis (C). For analysis of APP association with Rab5 in the microsomes, the P100 fractions were immunoprecipitated (IP) by using anti-Rab5 polyclonal antibody and co-precipitated APP levels were quantified by Western immunoblot using 22C11 APP antibody (D). For analysis of subcellular distribution of APP, subcellular organelles were purified by Optiprep® density gradient centrifugation (E). As loading control of density gradient centrifugation, β-actin levels were measured from post nuclear lysates (lysate) by Western immunoblot analysis. In addition, no correlation between protein levels of APP or EEA1 and β-actin in each subcellular fraction indicated that the observed alteration in APP and EEA1 levels may not due to protein amounts in each fractions. To examine the involvement of APP endocytosis in the regulation of Aβ40 generation (F) and APP distribution in lipid raft fractions (G), the cells were treated with phenylarsine oxide (PAO; 200nM/18hrs). For confirmation of equal amount protein loading in the process of lipid raft extraction, β-actin levels were measured from post nuclear lysates (lysate) by Western immunoblot analysis. All experiments were performed at least three times. The vertical bar indicates the standard error of mean (** P < 0.01, *** p < 0.001 compared to control group; CON).
Fig. 8.
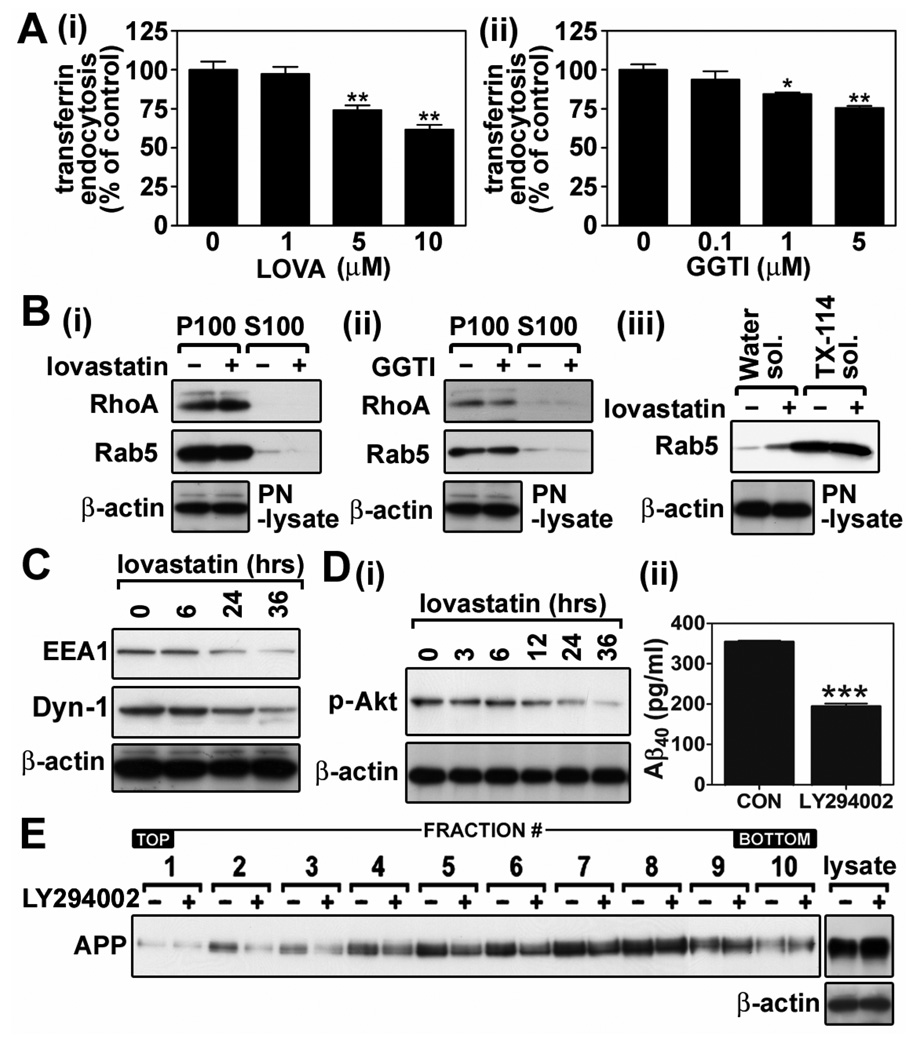
Pleiotropic roles of lovastatin in the down-regulation of endocytosis may be involved in the reduction of APP endocytosis. A. To examine the effect of lovastatin on clathrin-mediated transferrin endocytosis, hippocampal neuron cells were treated with lovastatin (5µM/36hrs) (i) or GGTI-298 (GGTI; 1µM/36hrs) (ii) and incubated with fluorescent transferrin. The endocytosis of fluorescent transferrin was measured as described in materials and methods. B. To characterize the possible involvement of geranylgeranylation small GTPases in lovastatin-mediated antiamyloidogenesis, hippocampal neuron cells were treated with lovastatin (i) or GGTI-298 (GGTI) (ii), and the levels of membrane (P100) or cytoplasm (S100) associated RhoA and Rab5 were measured. For analysis of geranylgeranylated Rab5 protein levels, post-nuclear cell lysates were extracted by using Triton X-114 (iii). Rab5 level in water soluble (Water sol.) or Triton X-114 soluble (TX-114 sol.) represent ungeranylgeranylated or geranylgeranylated Rab5, respectively. The β-actin levels in post-nuclear cell lysates (PN-lysate) were analyzed for loading controls of differential centrifugation and TX-114 extraction. C. To characterize the possible involvement of EEA1 (an early endosomal antigen 1) and dynamin-I in lovastatin-mediated anti-amyloidogenesis, the levels of these proteins from post-nuclear fractions were measured after treatment with lovastatin (5µM/36 hrs). To characterize the possible involvement of phosphatidylinositol 3-kinase (PI3-K)/Akt pathway in lovastatin-mediated anti-amyloidogenesis, the effect of lovastatin on Akt phosphorylation (D-i) and the effect of LY294002 (a PI3-K inhibitor; 20µM/24hr) on Aβ40 levels in culture media (D-ii) or distribution of APP in lipid raft fractions (E) were analyzed. For confirmation of equal amount protein loading in the process of lipid raft extraction, β-actin levels were measured from post nuclear lysates (lysate) by Western immunoblot analysis. All experiments were done at least three times and showed the same tendency. The vertical bar indicates the standard error of mean (* P < 0.05, ** P < 0.01, *** p < 0.001 compared to control group).
Lovastatin treatment reduced β-secretase activity but had no effect on α-secretase activity
Statin treatment or low cholesterol conditions are known to stimulate α-secretase mediated production of soluble APP ectodomain (Kojro et al. 2001; Parvathy et al. 2004; Pedrini et al. 2005). In addition, depletion of cellular cholesterol by cholesterol sequester (i.e. β-methyl cyclodextrin) is known to translocate BACE1 from lipid rafts to non-lipid raft fractions and thus to reduce its activity (Riddell et al. 2001). To examine the involvement of α- or β-secretase in anti-amyloidogenic activity of lovastatin, we examined the effect of lovastatin treatment on protein levels of BACE1 and ADAM10 and associated enzyme activities. We observed that lovastatin treatment did not alter α-secretase activity significantly (Fig. 3A). However, lovastatin treatment significantly reduced β-secretase activities in cell lysates (Fig. 3A) and BACE1 protein levels in LDLR fractions (fraction# 1~3 in Fig. 3B). In addition, the observed reduction in β-secretase activity in LDLR fraction (#2) (Fig 3C) was well correlated with reduced protein levels of BACE1 in LDLR fraction (Fig. 3B). Therefore, these data document that the decrease in Aβ output by lovastatin treatment may be due to the reduction of protein levels and enzymatic activity of BACE1 in LDLR fractions along with reduction in LDLR APP levels.
Lovastatin exerted its anti-amyloidogenic role through inhibition of the mevalonate pathway
Statins are a family of inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and thus down-regulate synthesis of isoprenoids and cholesterol through inhibition of mevalonate formation. To examine if lovastatin exerts its anti-amyloidogenic activity through metabolites of the mevalonate pathway, mevalonate was supplemented to lovastatin treatment. As shown in Fig. 4A-i, mevalonate fully reversed the inhibitory actions of lovastatin on APP distribution in LDLR fractions (#2~3). Moreover, mevalonate supplementation also reversed lovastatin-mediated reduction in Aβ output (Fig. 4B) and a β-secretase activity in post nuclear extracts (Fig. 4C). These results indicate that lovastatin exerts its anti-amyloidogenic role through inhibition of the mevalonate pathway (HMG-CoA reductase).
Since cholesterol is one of the end products of the mevalonate pathway and an integral component of lipid rafts mediating APP β-cleavage (Fassbender et al. 2001; Riddell et al. 2001), lovastatin could exert its anti-amyloidogenic actions through depletion of cholesterol in neurons. However, we observed that lovastatin did not alter cholesterol levels in the post-nuclear cell lysates (Fig. 5A). To test if lovastatin exerts its role through selective reduction of cholesterol levels in specific lipid raft fractions, cholesterol levels in lipid raft fractions were also examined (Fig. 5B). Similar to its effect in post-nuclear extracts, lovastatin treatment did not alter cholesterol levels in LDLR fractions (# 1~3). Previous reports describe that cholesterol depletion reduces distribution of caveolae proteins including flotillins and caveolins in lipid raft fractions (Garcia-Marcos et al. 2005; Gaus et al. 2005; Chintagari et al. 2006). Therefore, the observed lack of differences in LDLR-associated flotillin levels between control and lovastatin treated cells (Fig. 2B) suggests no alteration of lipid raft integrity by lovastatin treatment. Taken together, these data indicate that lovastatin treatment under our experimental conditions alters neither cholesterol content in lipid raft nor the integrity of lipid raft. Cholesterol is one of the stable lipids in the CNS (Serougne-Gautheron and Chevallier 1973; Andersson et al. 1990). In Fig. 5C, we observed that the inhibition of HMG-CoA reductase by lovastatin treatment (5µM/36hr) did not alter pre-labeled cholesterol levels in neuronal cells under cholesterol free media conditions, thus suggesting that cholesterol in in vitro culture of neurons was also stable up to 36 hrs of lovastatin treatment.
Lovastatin exerted its anti-amyloidogenic effect in a geranylgeranyl-pyrophosphate dependent manner
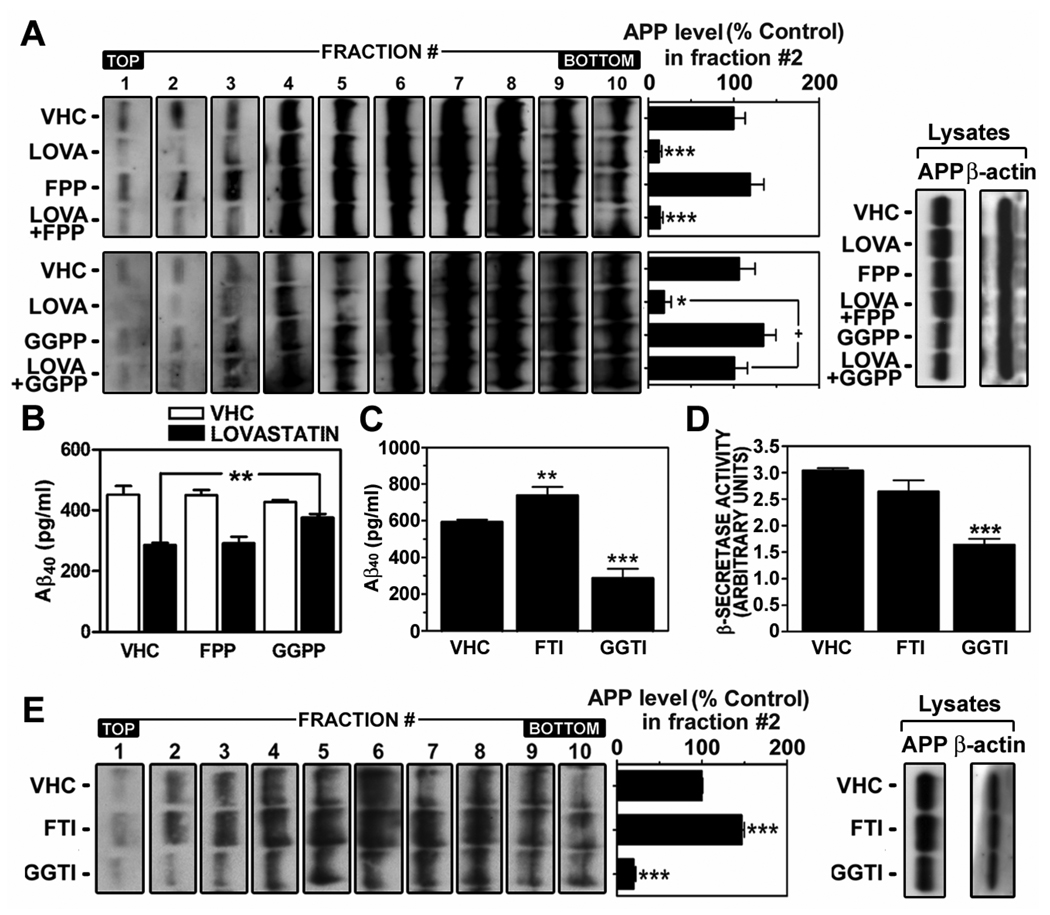
Metabolites of the mevalonate pathway such as farnesyl-pyrophosphate (FPP) and geranylgeranyl-pyrophosphate (GGPP) are utilized by farnesyl-transferase and geranylgeranyl-transferase, respectively, for isoprenylation of proteins. The role of protein isoprenylation in statin-mediated anti-amyloidogenesis was recently reported (Pedrini et al. 2005; Ostrowski et al. 2007), where inhibition of geranylgeranylation by statin treatment induced α-secretase mediated APP ectodomain shedding and reduced Aβ generation. Similar to this study, we observed that GGPP supplementation, but not FPP, reversed the lovastatin-mediated reduction of LDLR distribution of APP (Fig. 6A) and Aβ output to media (Fig. 6B). The role of GGPP in lovastatin-mediated anti-amyloidogenic activity was further supported by geranylgeranyl-transferase inhibitor (GGTI-298)-mediated reductions of Aβ40 output to media (Fig. 6C), β-secretase activity (Fig. 6D) and APP levels in LDLR fractions (Fig. 6E). On the other hand, farnesyl-transferase inhibitor (FTI-276) failed to reduce Aβ40 output to media (Fig. 6C), β-secretase activity (Fig. 6D) and APP levels in LDLR fractions (#1~3) (Fig. 6E). These results document that inhibition of GGPP synthesis and subsequent inhibition of protein geranylgeranylation (i.e. small GTPases) are involved in lovastatin-mediated reduction of APP levels in LDLR fractions and Aβ generation.
Fig. 6.
Lovastatin may exert its anti-amyloidogenic effect through inhibition of geranylgeranyl-pyrophosphate synthesis. To examine the involvement of isoprenoids in lovastatin-mediated anti-amyloidogenic activity, hippocampal neuron cells were treated with farnesyl-pyrophosphate (FPP) or geranylgeranyl-pyrophosphate (GGPP) in the presence or absence of lovastatin (LOVA; 5µM/36hrs), followed by measurement of level of APP in membrane microdomain fractions (A) and Aβ40 levels in culture media (B) as described in materials and methods. To examine the involvement of farnesylation and geranylgeranylation in lovastatin-mediated anti-amyloidogenic activity, the cells were treated with FTI-276 (FTI; a farnesyl-transferase inhibitor; 5 µM/36hrs) or GGTI-298 (GGTI; a geranylgeranyl-transferase inhibitor; 1 µM), followed by measurement of Aβ40 levels in culture media (C) and β-secretase activity (D) and APP levels in lipid raft fractions (E). For confirmation of equal amount protein loading in the process of lipid raft extraction, β-actin levels were measured from post nuclear lysates (lysate) by Western immunoblot analysis (A and E). All experiments were done at least three times and showed the same tendency. VHC (vehicle) represents dimethylsulfoxide treatment as control. The vertical bar indicates the standard error of mean (* P < 0.05, ** P < 0.01, *** p < 0.001 compared to control group).
Lovastatin-mediated reduction of APP levels in LDLR fractions may be mediated by down-regulation of APP endocytosis
Since processes of endocytosis and early endosomal targeting of APP are crucial for its β-cleavage (Roheim et al. 1979; Walter et al. 2001; Bamberger et al. 2003; Ehehalt et al. 2003; Grbovic et al. 2003; Cataldo et al. 2004), we examined the effect of lovastatin (5µM/36hr) on cell surface APP endocytosis by fluorometric and fluoromicroscopic analysis. As shown in the figure 7A, lovastatin induced accumulation of cell surface APP levels but decreased intracellular APP levels, which reflect 60% decrease in APP endocytosis activity. Similar results were also observed in microscopic analysis (Fig. 7B), where we observed that lovastatin treatment for 36hrs increased the intensity of cell surface APP staining but decreased internalized APP levels.
To examine whether the lovastatin-mediated inhibition of APP endocytosis alters subcellular APP distribution, post-nuclear cell lysates were fractionated by differential centrifugation and APP levels in those fractions were analyzed. We observed that lovastatin treatment decreased APP levels in post-mitochondrial (S15) and microsomal (P100) fractions which contain small vesicles including endosomes, endocytic and other membrane trafficking vesicles (Fig. 7C). However, lovastatin did not reduce APP levels in post nuclear (S1) and mitochondrial (P15) fractions which mainly contain large subcellular organelles (i.e. mitochondria, E.R., peroxisomes and Golgi) and broken plasma membrane. These data indicate that lovastatin selectively reduces APP levels associated with intracellular small membranous vesicles (microsomal fraction). To examine if the reduction in APP levels in microsomal fractions results from reduction in APP levels in endosomes and/or endocytic vesicles, the protein levels of Rab5 in these fractions and colocalization of Rab5 and APP in microsomal fractions were examined (Fig. 7C and D). Rab5 is a regulator of endosomal targeting of endocytic vesicles and is normally present in plasma membrane fractions (S1 and P15) as well as in endosomes and endocytic vesicles (S15 and P100) (Woodman 2000). Interestingly, we observed that lovastatin selectively reduced Rab5 protein levels in microsome containing fractions (S15 and P15) (Fig. 7C). Since Rab5 positive vesicles contained APP as shown in Fig 7D, these results suggest that the reduced APP levels in microsomal fractions may be associated with reduction of Rab5 levels and/or Rab5-associated membrane vesicles in those fractions.
To examine whether lovastatin-mediated reduction in APP levels in P100 fractions are associated with reduction of APP levels in early endosomal fractions, equal amounts of post heavy mitochondrial fractions (S3), as represented by equal amounts of β-actin levels (Fig. 7E, denoted by lysate), were subjected to gradient centrifugation. The gradients were fractionated as equal volume of fractions and the levels of APP and EEA1 (early endosomal protein) in each fraction were examined by Western immunoblot analysis. As shown in Fig. 7E, lovastatin treatment reduced APP levels in the early endosomal fractions (#2~4), indicating that lovastatin-inhibited APP endocytosis causes reduction of APP levels in early endosomes. In addition, lovastatin treatment also reduced EEA1 levels in early endosomal fractions (Fig. 7E). Since EEA1 and Rab5 are key regulators in endocytosis process (Mills et al. 1999), the reduction of these protein levels in endosomes and/or endocytic vesicles may be related to reduced endocytosis activity and thus reduced APP levels in early endosomes.
To examine whether endocytosis of APP affect its distribution in LDLR fractions, we examined the effect of endocytosis inhibitor on APP distribution in LDLR fractions. For this, cellular endocytic activity was inhibited by phenylarsine oxide treatment (PAO; 200nM/18hrs). PAO is an inhibitor of tyrosine phosphatases and is known to increase β-secretase-mediated APP shedding by inhibition of APP endocytosis and thus induction of cell surface APP accumulation (Daukas and Zigmond 1985; Tong et al. 2000; Zeng et al. 2003; Pedrini et al. 2005). As shown in Fig. 7F, PAO treatment resulted in reduction of Aβ output. Moreover, PAO also reduced APP levels in LDLR fractions (#1~4) (Fig. 7G). Taken together, this data indicates that APP endocytosis may be associated with APP distribution in LDLR fractions and subsequent Aβ output.
Pleiotropic role of lovastatin in down-regulation of endocytosis regulators
Since lovastatin treatment reduced protein levels of microsomal Rab5 and early endosomal EEA1 (Fig. 7C and E), lovastatin-mediated reduction in APP endocytosis may be due to non-selective down-regulation of cellular endocytic activity. Therefore, we analyzed the effect of lovastatin on transferrin endocytosis activity which is mediated by a clathrin-dependent pathway (Marquez-Sterling et al. 1997; Bonifacino and Traub 2003). As depicted in Fig. 8A-i, lovastatin treatment dose dependently inhibited transferrin endocytosis activity. Moreover, similar inhibition in transferrin endocytosis was also observed by GGTI treatment, an inhibitor of geranylgeranyl-transferase (Fig. 8A-ii). Therefore, these data suggest that lovastatin may reduce APP endocytosis through inhibition of the clathrin-dependent pathway in a GGPP dependent manner.
Activation of Rab5, a geranylgeranylated small GTPase, through GTP loading and subsequent interaction with effecter proteins including Rabaptin-5, phosphatidylinositol 3-kinases (PI3-K), EEA1 and Rabenosyn-5, are known as rate limiting steps of clathrin-dependent and -independent endocytosis (Rybin et al. 1996; Zerial and McBride 2001; Pelkmans et al. 2004). Therefore, inhibition of geranylgeranylation of Rab5 could be one of mechanisms for lovastatin and GGTI mediated inhibition of clathrin-dependent endocytosis pathway as reported in N2a neuroblastoma cells recently (Ostrowski et al. 2007). In hippocampal neuron cells, however, neither lovastatin nor GGTI treatment altered the levels of membrane associated (P100) Rab5 and other geranylgeranylated small GTPases, such as RhoA (Fig. 8B-i and ii). Further, lovastatin treatment did not alter the isoprenylated Rab5 levels as depicted by unaltered Rab5 levels in Triton X-114 fractions (Fig. 8B-iii), although there was a small increase in Rab5 protein levels in water soluble fractions (unisoprenylated Rab5). These data suggest that the geranylgeranyl moiety of Rab5 is stable under our experimental conditions and that the observed lovastatin-mediated inhibitory role in APP endocytosis may not be through down-regulation of Rab5 or RhoA geranylgeranylation.
In Fig. 7E, we observed that lovastatin treatment reduced EEA1 protein levels in endosomal fractions. EEA1 and dynamin are crucial in endocytic vesicle fusion and fission processes (Mills et al. 1999; Antonny 2004). In Fig. 8C, we observed that lovastatin treatment decreased protein levels of EEA1 and dynamin in a time dependent manner (Fig. 8C). Moreover, lovastatin treatment also inhibited the PI3-K pathway, another key regulator of endocytosis (Zerial and McBride 2001). The observed reduction in phosphorylated Akt (PKB) levels by lovastatin treatment (Fig. 8D-i) and reductions of Aβ output (Fig. 8D-ii) and LDLR APP levels (Fig. 8E) by LY294002 treatment (a PI3-K inhibitor; 20µM/24hr) indicate that lovastatin-induced inhibition of APP endocytosis and subsequent reduction of Aβ output may be mediated by lovastatininduced down-regulation of EEA1 and dynamin and inhibition of PI3-K pathway.
Discussion
Differential solubilization of membrane microdomains in Triton X-100 at 4 °C has been widely used for study of lipid raft structure and function (Brown and Rose 1992). However, there are still many open questions, including the question whether more than one kind of raft domain exist on the plasma membrane. In support of the existence of different types of rafts, Röper et al. (Roper et al. 2000) previously identified a cholesterol-rich microdomain that is distinct from the classical lipid rafts based on its solubility in Triton X100 but its resistance to another non-ionic detergent, Lubrol WX. Moreover, Vetrivel et al. showed that γ-secretase as well as APP are placed in distinct membrane microdomains which are characterized by Lubrol WX insolubility (Vetrivel et al. 2004; Vetrivel et al. 2005). In hippocampal neurons, we observed that almost half of APP is associated with Lubrol WX insoluble microdomains which were characterized by colocalization of flotillin-1, a caveolin family protein (Fig. 2A). These microdomains are distinct from classical Triton X100 insoluble microdomains because of almost complete absence of APP in Triton X100 insoluble fractions (Fig. 2A). Although the physical and biochemical differences of Triton X100 and Lubrol WX extractable lipid rafts are not understood at present, the data described in this study indicate that almost half of cellular APP is associated with specific lipid boundaries which are distinct from α-secretase containing Lubrol WX soluble fractions (Fig. 3B) and Triton X100 insoluble lipid raft fractions (Fig. 2A).
Ehehalt et al. reported previously that clustering of β-secretase and APP containing lipid rafts is crucial for Aβ generation (Ehehalt et al. 2003), thus indicating that simple distribution of APP or β-secretase (i.e. BACE1) in lipid raft fractions may not be sufficient to mediate APP β-cleavage. Individual rafts may contain less than 10~30 protein molecules because of their limited size (~50 nm) (Pralle et al. 2000), thus, two different species of raft proteins would rarely be expected to be in the same raft. This means not only localization of APP or BACE1 in the lipid raft fractions but also interaction between APP and BACE1 containing lipid rafts should be required for APP β-cleavage. In this study, we observed that lovastatin selectively reduced the APP distribution in LDLR fractions (fraction# 1~3 in Fig. 2B, Fig. 4A and Fig. 6A). However, it is not clear whether these LDLR fractions serve as platforms for APP and BACE1 interaction. Nevertheless, the observed lovastatin-mediated decrease in protein levels and enzymatic activities of BACE1/β-secretase (Fig. 3B and C) only in LDLR fractions and their reflection to β-secretase activity in whole cell lysates (Fig. 3A) suggest that lovastatin-mediated regulation of APP and β-secretase distribution in LDLR fractions may be essential for amyloidogenic cleavage of APP.
Cholesterol is an essential component for maintaining lipid raft integrity and has been regarded as a crucial regulatory factor for amyloidogenesis (Simons et al. 2001; Ehehalt et al. 2003). Therefore, ability of statin to reduce cholesterol synthesis has been suggested as a major mechanism for their anti-amyloidogenic activity (Fassbender et al. 2001). However, in cultured neurons, lovastatin did not alter cholesterol levels in post nuclear cell lysates and lipid rafts fractions (Fig. 5A and B). Therefore, lovastatin-mediated reductions of LDLR APP levels and subsequent Aβ generation might be mediated by pathway(s) other than cholesterol depletion-mediated disruption of lipid raft structure. The observed lack of effects of lovastatin on flotillin levels in LDLR fractions (Fig. 2B) also supports this hypothesis because distribution of flotillin as well as caveolin in lipid raft fractions is dependent on membrane cholesterol levels (Garcia-Marcos et al. 2005; Gaus et al. 2005; Chintagari et al. 2006). Rather than cholesterol depletion, our data indicates that lovastatin may exert it inhibitory role in APP distribution in LDLR and Aβ generation through depletion of GGPP (Fig. 6). This data is consistent with a recent study (Ostrowski et al. 2007) where lovastatin and simvastatin reduced Aβ generation in a geranylgeranylation dependent manner. However, these studies differ regarding the role of statins in RhoA/Rab5 membrane association and cellular APP accumulation. Ostrowski et al. have reported that lovastatin or simvastatin treatment reduced membrane association of Rab5 and RhoA in N2a neuroblastoma cells (Ostrowski et al. 2007). However, in our study using the rat hippocampal neurons, there were no changes in membrane association and geranylgeranylation of Rab5 and RhoA by lovastatin treatment, thus suggesting that lovastatin may exert it anti-amyloidogenic activity through modulating geranylgeranylation of other protein(s). Although the explanation for the difference in Rab5/RhoA geranylgeranylation between these experiments is not well understood at present, use of different cell types (N2a neuroblastoma vs. primary rat hippocampal neurons vs. mouse cortical neurons) or different experimental conditions (24hrs vs. 36hr treatment with simvastatin vs. lovastatin) may account for these differences. Rather than modulation of RhoA and Rab5 geranylgeranylation, we observed that lovastatin treatment modulated protein levels of EEA1 and dynamin-1 and activity of PI3-kinase, proteins responsible for clathrin-dependent/-independent endocytosis pathway (Nichols 2003; Pelkmans et al. 2004; Echarri and Del Pozo 2006). These findings indicate that lovastatin treatment is expected to modulate a broad range of cellular endocytosis processes rather than selective inhibition of APP endocytosis. Indeed, lovastatin is reported to inhibit endocytosis of GPI-anchored ecto-5′-nucleotidase, a lipid raft associated protein which is internalized via a clathrin-independent pathway (Ledoux et al. 2002). Moreover, we observed that lovastatin inhibited endocytosis of transferrin, a non-lipid raft associated protein which is internalized via a clathrin-dependent pathway (Fig. 8A). Therefore, lovastatin may exert its inhibitory role in APP endocytosis through non-specific and global inhibition of cellular endocytosis activity via down-regulating geranylgeranylation of certain protein(s), rather than Rab5 and RhoA.
In neurons, the clathrin-dependent pathway is considered as a major pathway for APP endocytosis (Marquez-Sterling et al. 1997; Bonifacino and Traub 2003). However, APP internalized in non-lipid raft fractions via clathrin-dependent pathway may not be a favorable substrate for β-secretase which is associated with lipid raft fractions. Therefore, the internalized APP would have to relocate to BACE containing lipid raft fractions (i.e. LDLR) for its BACE mediated processing in endosomes. In figure 7G, we observed that the treatment of neuronal cells with PAO, a general endocytosis inhibitor, reduced APP levels in LDLR fractions with inhibiting Aβ generation. Therefore, this data suggests that endocytosis regulates APP distribution in LDLR fractions. However, whether the APP in LDLR fractions originates from early endosomes is not clear at present. Further, PAO inhibits protein tyrosine kinases, thus, expected to affect various cellular physiological functions. Therefore, the exact role of endocytosis on endosomal APP distribution in LRLR fractions needs further investigation.
At present, the mechanism of APP endocytosis via the clathrin-independent pathway is not well understood. However, the reported role of cell surface GPI-anchored proteins in APP β-processing (Sambamurti et al. 1999) and age-dependent increase in neuronal caveolin expression and its regulatory role in APP β-processing (Kang et al. 1987) suggests a possible role of cell surface lipid rafts in APP β-processing. Since lovastatin reduces endocytosis of GPI-anchored protein as reported previously (Ledoux et al. 2002), there is a possibility that lovastatin also down-regulates APP endocytosis via inhibition of clathrin-independent lipid raft endocytosis. Even though APP is internalized through a clathrin-independent pathway as a part of lipid raft and/or caveolae containing vesicles, APP may not be readily accessible to BACE due to their localization in separate lipid raft moieties. Ehehalt et al. previously suggested that endocytosis may induce clustering of APP and BACE containing lipid rafts (Ehehalt et al. 2003). Under this scenario, if the clustering process lowers the density of lipid rafts through endosomal metabolism of lipid raft components or through other unknown pathways, inhibition of APP endocytosis is expected to reduce APP levels in LDLR fractions as observed in figure 2B.
This study documents a pleiotropic inhibitory role of lovastatin in APP endocytosis by down-regulation of EEA1, dynamin and PI3-kinase. No alteration in cholesterol content in neurons by lovastatin treatment (Fig. 5A) and ability of GGPP to reverse the effects of lovastatin on reduction of Aβ generation and β-secretase activity (Fig. 6A, B and C) support the conclusion that the anti-amyloidogenic activity of lovastatin is mediated by GGPP, a lipid required for membrane association of certain signaling proteins such as small GTPases. The observed lack of difference in membrane association of RhoA or Rab5 following lovastatin treatment suggests the involvement of other proteins in geranylgeranylation dependent reduction of EEA1, dynamin and PI3-kinase (proteins associated with endocytosis process) and reduction of Aβ production. Since a large number of proteins are known to be isoprenylated, different proteins may have different isoprenylation rates and/or different half lives. The observed inhibitions of APP endocytosis and its specific distribution in LDLR fractions and reduced Aβ generation by lovastatin suggest the participation of lovastatin-sensitive APP containing lipid rafts (LDLR) in neuronal amyloidogenic activity.
Acknowledgement
* We thank to Ms. Joyce Bryan and Ms. Michaela Rose for laboratory assistance and secretarial assistance, respectively. This study was supported in part by grants from National Institute of Health (NS-22576, NS-34741, NS-37766, AG-25307, RR018823 and RR015455).
References
- Andersson M, Elmberger PG, Edlund C, Kristensson K, Dallner G. Rates of cholesterol, ubiquinone, dolichol and dolichyl-P biosynthesis in rat brain slices. FEBS Lett. 1990;269:15–18. doi: 10.1016/0014-5793(90)81107-y. [DOI] [PubMed] [Google Scholar]
- Antonny B. SNARE filtering by dynamin. Cell. 2004;119:581–582. doi: 10.1016/j.cell.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Cullen EI, Friedhoff LT. Pharmacological concentrations of the HMG-CoA reductase inhibitor lovastatin decrease the formation of the Alzheimer beta-amyloid peptide in vitro and in patients. Front Biosci. 2002;7:a50–a59. doi: 10.2741/A739. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA. Abeta localization in abnormal endosomes: association with earliest Abeta elevations in AD and Down syndrome. Neurobiol Aging. 2004;25:1263–1272. doi: 10.1016/j.neurobiolaging.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Chauhan NB. Membrane dynamics, cholesterol homeostasis, and Alzheimer's disease. J Lipid Res. 2003;44:2019–2029. doi: 10.1194/jlr.R300010-JLR200. [DOI] [PubMed] [Google Scholar]
- Chintagari NR, Jin N, Wang P, Narasaraju TA, Chen J, Liu L. Effect of cholesterol depletion on exocytosis of alveolar type II cells. Am J Respir Cell Mol Biol. 2006;34:677–687. doi: 10.1165/rcmb.2005-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daukas G, Zigmond SH. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J Cell Biol. 1985;101:1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echarri A, Del Pozo MA. Caveolae internalization regulates integrin-dependent signaling pathways. Cell Cycle. 2006;5:2179–2182. doi: 10.4161/cc.5.19.3264. [DOI] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, Keller P, Runz H, Kuhl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T. Simvastatin strongly reduces levels of Alzheimer's disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marcos M, Tandel S, Pochet S, Genin J, De Lorenzi M, Gomez F, Kumps A, Marino A, Dehaye JP. Cholesterol depletion perturbs calcium handling by rat submandibular glands. J Cell Physiol. 2005;203:429–438. doi: 10.1002/jcp.20241. [DOI] [PubMed] [Google Scholar]
- Gaus K, Rodriguez M, Ruberu KR, Gelissen I, Sloane TM, Kritharides L, Jessup W. Domain-specific lipid distribution in macrophage plasma membranes. J Lipid Res. 2005;46:1526–1538. doi: 10.1194/jlr.M500103-JLR200. [DOI] [PubMed] [Google Scholar]
- Grbovic OM, Mathews PM, Jiang Y, Schmidt SD, Dinakar R, Summers-Terio NB, Ceresa BP, Nixon RA, Cataldo AM. Rab5-stimulated up-regulation of the endocytic pathway increases intracellular beta-cleaved amyloid precursor protein carboxyl-terminal fragment levels and Abeta production. J Biol Chem. 2003;278:31261–31268. doi: 10.1074/jbc.M304122200. [DOI] [PubMed] [Google Scholar]
- Green RC, McNagny SE, Jayakumar P, Cupples LA, Farrer LA. Statin use and the risk of Alzheimer's disease: The MIRAGE Study. Alzheimer's & Dimentia. 2006;2:96–103. doi: 10.1016/j.jalz.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Gupta A, Pansari K. Inflammation and Alzheimer's disease. Int J Clin Pract. 2003;57:36–39. [PubMed] [Google Scholar]
- Halliday G, Robinson SR, Shepherd C, Kril J. Alzheimer's disease and inflammation: a review of cellular and therapeutic mechanisms. Clin Exp Pharmacol Physiol. 2000;27:1–8. doi: 10.1046/j.1440-1681.2000.03200.x. [DOI] [PubMed] [Google Scholar]
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Khan M, Contreras M, Singh I. Endotoxin-induced alterations of lipid and fatty acid compositions in rat liver peroxisomes. J Endotoxin Res. 2000;6:41–50. doi: 10.1177/09680519000060010601. [DOI] [PubMed] [Google Scholar]
- Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha -secretase ADAM 10. Proc Natl Acad Sci U S A. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- Ledoux S, Laouari D, Essig M, Runembert I, Trugnan G, Michel JB, Friedlander G. Lovastatin enhances ecto-5'-nucleotidase activity and cell surface expression in endothelial cells: implication of rho-family GTPases. Circ Res. 2002;90:420–427. doi: 10.1161/hh0402.105668. [DOI] [PubMed] [Google Scholar]
- Lutjohann D, Stroick M, Bertsch T, Kuhl S, Lindenthal B, Thelen K, Andersson U, Bjorkhem I, Bergmann Kv K, Fassbender K. High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids. 2004;69:431–438. doi: 10.1016/j.steroids.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Madore N, Smith KL, Graham CH, Jen A, Brady K, Hall S, Morris R. Functionally different GPI proteins are organized in different domains on the neuronal surface. Embo J. 1999;18:6917–6926. doi: 10.1093/emboj/18.24.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Sterling NR, Lo AC, Sisodia SS, Koo EH. Trafficking of cell-surface beta-amyloid precursor protein: evidence that a sorting intermediate participates in synaptic vesicle recycling. J Neurosci. 1997;17:140–151. doi: 10.1523/JNEUROSCI.17-01-00140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Meske V, Albert F, Richter D, Schwarze J, Ohm TG. Blockade of HMG-CoA reductase activity causes changes in microtubule-stabilizing protein tau via suppression of geranylgeranylpyrophosphate formation: implications for Alzheimer's disease. Eur J Neurosci. 2003;17:93–102. doi: 10.1046/j.1460-9568.2003.02433.x. [DOI] [PubMed] [Google Scholar]
- Mills IG, Jones AT, Clague MJ. Regulation of endosome fusion. Mol Membr Biol. 1999;16:73–79. doi: 10.1080/096876899294788. [DOI] [PubMed] [Google Scholar]
- Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003;116:4707–4714. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- Ostrowski SM, Wilkinson BL, Golde TE, Landreth G. Statins reduce amyloid-beta production through inhibition of protein isoprenylation. J Biol Chem. 2007 doi: 10.1074/jbc.M702640200. electronical publication ahead of print, manuscript# M702640200. [DOI] [PubMed] [Google Scholar]
- Pappolla MA, Bryant-Thomas TK, Herbert D, Pacheco J, Fabra Garcia M, Manjon M, Girones X, Henry TL, Matsubara E, Zambon D, Wolozin B, Sano M, Cruz-Sanchez FF, Thal LJ, Petanceska SS, Refolo LM. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology. 2003;61:199–205. doi: 10.1212/01.wnl.0000070182.02537.84. [DOI] [PubMed] [Google Scholar]
- Parvathy S, Ehrlich M, Pedrini S, Diaz N, Refolo L, Buxbaum JD, Bogush A, Petanceska S, Gandy S. Atorvastatin-induced activation of Alzheimer's alpha secretase is resistant to standard inhibitors of protein phosphorylation-regulated ectodomain shedding. J Neurochem. 2004;90:1005–1010. doi: 10.1111/j.1471-4159.2004.02521.x. [DOI] [PubMed] [Google Scholar]
- Pedrini S, Carter TL, Prendergast G, Petanceska S, Ehrlich ME, Gandy S. Modulation of statin-activated shedding of Alzheimer APP ectodomain by ROCK. PLoS Med. 2005;2:e18. doi: 10.1371/journal.pmed.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refolo LM, Sambamurti K, Efthimiopoulos S, Pappolla MA, Robakis NK. Evidence that secretase cleavage of cell surface Alzheimer amyloid precursor occurs after normal endocytic internalization. J Neurosci Res. 1995;40:694–706. doi: 10.1002/jnr.490400515. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Riddell DR, Christie G, Hussain I, Dingwall C. Compartmentalization of beta-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr Biol. 2001;11:1288–1293. doi: 10.1016/s0960-9822(01)00394-3. [DOI] [PubMed] [Google Scholar]
- Roheim PS, Carey M, Forte T, Vega GL. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci U S A. 1979;76:4646–4649. doi: 10.1073/pnas.76.9.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol. 2000;2:582–592. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- Rouvinski A, Gahali-Sass I, Stav I, Metzer E, Atlan H, Taraboulos A. Both raft- and non-raft proteins associate with CHAPS-insoluble complexes: some APP in large complexes. Biochem Biophys Res Commun. 2003;308:750–758. doi: 10.1016/s0006-291x(03)01470-0. [DOI] [PubMed] [Google Scholar]
- Rybin V, Ullrich O, Rubino M, Alexandrov K, Simon I, Seabra MC, Goody R, Zerial M. GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature. 1996;383:266–269. doi: 10.1038/383266a0. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Sevlever D, Koothan T, Refolo LM, Pinnix I, Gandhi S, Onstead L, Younkin L, Prada CM, Yager D, Ohyagi Y, Eckman CB, Rosenberry TL, Younkin SG. Glycosylphosphatidylinositol-anchored proteins play an important role in the biogenesis of the Alzheimer's amyloid beta-protein. J Biol Chem. 1999;274:26810–26814. doi: 10.1074/jbc.274.38.26810. [DOI] [PubMed] [Google Scholar]
- Serougne-Gautheron C, Chevallier F. Time course of biosynthetic cholesterol in the adult rat brain. Biochim Biophys Acta. 1973;316:244–250. doi: 10.1016/0005-2760(73)90014-3. [DOI] [PubMed] [Google Scholar]
- Shie FS, Jin LW, Cook DG, Leverenz JB, LeBoeuf RC. Diet-induced hypercholesterolemia enhances brain A beta accumulation in transgenic mice. Neuroreport. 2002;13:455–459. doi: 10.1097/00001756-200203250-00019. [DOI] [PubMed] [Google Scholar]
- Sidaway JE, Davidson RG, McTaggart F, Orton TC, Scott RC, Smith GJ, Brunskill NJ. Inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase reduce receptor-mediated endocytosis in opossum kidney cells. J Am Soc Nephrol. 2004;15:2258–2265. doi: 10.1097/01.ASN.0000138236.82706.EE. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Simons M, Keller P, Dichgans J, Schulz JB. Cholesterol and Alzheimer's disease: is there a link? Neurology. 2001;57:1089–1093. doi: 10.1212/wnl.57.6.1089. [DOI] [PubMed] [Google Scholar]
- Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Soriano S, Chyung AS, Chen X, Stokin GB, Lee VM, Koo EH. Expression of beta-amyloid precursor protein-CD3gamma chimeras to demonstrate the selective generation of amyloid beta(1–40) and amyloid beta(1–42) peptides within secretory and endocytic compartments. J Biol Chem. 1999;274:32295–32300. doi: 10.1074/jbc.274.45.32295. [DOI] [PubMed] [Google Scholar]
- Symons M, Rusk N. Control of vesicular trafficking by Rho GTPases. Curr Biol. 2003;13:R409–R418. doi: 10.1016/s0960-9822(03)00324-5. [DOI] [PubMed] [Google Scholar]
- Tong XK, Hussain NK, Adams AG, O'Bryan JP, McPherson PS. Intersectin can regulate the Ras/MAP kinase pathway independent of its role in endocytosis. J Biol Chem. 2000;275:29894–29899. doi: 10.1074/jbc.M004096200. [DOI] [PubMed] [Google Scholar]
- Verhulst A, D'Haese PC, De Broe ME. Inhibitors of HMG-CoA reductase reduce receptor-mediated endocytosis in human kidney proximal tubular cells. J Am Soc Nephrol. 2004;15:2249–2257. doi: 10.1097/01.ASN.0000136778.32499.05. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Kim SH, Chen Y, Barnes NY, Parent AT, Sisodia SS, Thinakaran G. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. J Biol Chem. 2005;280:25892–25900. doi: 10.1074/jbc.M503570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Fluhrer R, Hartung B, Willem M, Kaether C, Capell A, Lammich S, Multhaup G, Haass C. Phosphorylation regulates intracellular trafficking of beta-secretase. J Biol Chem. 2001;276:14634–14641. doi: 10.1074/jbc.M011116200. [DOI] [PubMed] [Google Scholar]
- Wolozin B. A fluid connection: cholesterol and Abeta. Proc Natl Acad Sci U S A. 2001;98:5371–5373. doi: 10.1073/pnas.101123198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- Won JS, Im YB, Key L, Singh I, Singh AK. The involvement of glucose metabolism in the regulation of inducible nitric oxide synthase gene expression in glial cells: possible role of glucose-6-phosphate dehydrogenase and CCAAT/enhancing binding protein. J Neurosci. 2003;23:7470–7478. doi: 10.1523/JNEUROSCI.23-20-07470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman PG. Biogenesis of the sorting endosome: the role of Rab5. Traffic. 2000;1:695–701. doi: 10.1034/j.1600-0854.2000.010902.x. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Tao N, Chung KN, Heuser JE, Lublin DM. Endocytosis of oxidized low density lipoprotein through scavenger receptor CD36 utilizes a lipid raft pathway that does not require caveolin-1. J Biol Chem. 2003;278:45931–45936. doi: 10.1074/jbc.M307722200. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]