Abstract
Arabinosyltransferases (AraTs) play a critical role in mycobacterial cell wall biosynthesis and are potential drug targets for the treatment of tuberculosis, especially multi-drug resistant forms of M. tuberculosis (MTB). Herein, we report the synthesis and acceptor/inhibitory activity of Araf α(1→5) Araf disaccharides possessing deoxygenation at the reducing sugar of the disaccharide. Deoxygenation at either the C-2 or C-3 position of Araf was achieved via a free radical procedure using xanthate derivatives of the hydroxyl group. The α(1→5)-linked disaccharides were produced by coupling n-octyl α-Araf 2-/3-deoxy, 2-fluoro glycosyl acceptors with an Araf thioglycosyl donor. The target disaccharides were tested in a cell free mycobacterial AraTs assay as well as an in vitro assay against MTB H37Ra and M. avium complex strains.
Introduction
Tuberculosis (TB) is one of the primary killers worldwide in spite of the availability of several potent anti-mycobacterial agents.1 Millions die annually from this disease, and the problem is amplified by the apparent synergism with HIV.2,3 Mycobacterial diseases have attracted renewed attention in recent years because of their increased incidence worldwide and the emergence of multi-drug resistant (MDR) and extensively drug resistant (XDR) strains.4 MDR-TB infections are significantly more difficult to treat with second-line therapies that are typically more expensive and have considerable side-effects. XDR-TB5 develops when these second-line drugs are also misused or mismanaged and therefore become ineffective. Because XDR-TB is resistant to first- and second-line drugs, treatment options are seriously limited.
During the last two decades, new programs have been initiated to elucidate the systems biology of the tubercle bacillus with a focus on new, valid targets for novel anti-tubercular drug discovery. Many unique metabolic processes occur during the biosynthesis of cell wall components, including arabinogalactan and mycolic acids.6 Among the front line drugs for treatment of TB, two drugs isoniazid (INH) and ethambutol (EMB) target the mycobacterial cell wall that is essential for the survival of pathogen.7 The structure of the cell wall has now been systematically elucidated in terms of its component complex polysaccharides, the specific chemical linkages therein, and the macromolecular structure of the mycolylarabinogalactan complex.8 The two major oligosaccharide portions, lipoarabinomannan (LAM) and arabinogalactan (AG), contain arabinofuranose (Araf) units.
Chemical analysis of the fragments of LAM and AG have revealed that both are composed of linear arabinan consisting of α (1→5) linked Araf units, and a branched Araf hexasaccharide at the terminus with α (1→3) and β(1→2) linked Araf units. The assembly of the arabinan portions of cell wall polysaccharides in mycobacteria involves a family of AraTs9 that promote the polymerization of Araf units using decaprenolphosphoarabinofuranose (DPA) as the sugar donor. Mycobacterial viability requires an intact arabinan, and thus compounds that inhibit these glycosyltransferases (GTs) are both useful biochemical tools as well as potential lead compounds for new selective anti-tubercular agents as Araf is not present in mammals.
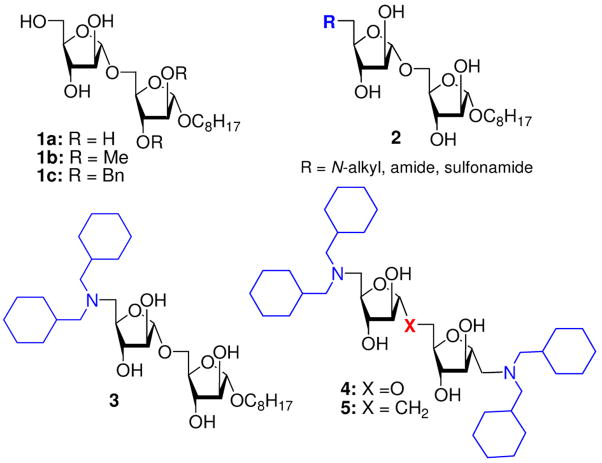
At the inception of the mycobacterial GTs program, our intention was to prepare prototype disaccharides that would be substrates for assay development and could probe the acceptor activity of the various cell wall GTs.10,11 Neoglycosides 1a, 1b and 1c (Chart 1) were previously synthesized and evaluated for their potential as acceptors/inhibitors.11 As those efforts advanced, our work turned to examining the various substitution patterns of the acceptor disaccharides to study the acceptor tolerance for various alterations, and the ability of these substitutions to affect inhibition relative to the standard acceptor disaccharides for each transferase. Based on the substrate activity of a control acceptor, n-octyl Araf α(1→5) Araf (1a), several analogs possessing N-alkyl, amide and sulphonamide functionalities at the C-5 position of the non-reducing sugar were prepared and screened as competitive inhibitors.12 Among a small set of disaccharides 2, N,N-dicyclohexylamino disaccharide analog 3 showed superior activity as an inhibitor.12
Chart 1.
Previously synthesized Araf α(1→5)Araf disaccharide analogs
Ideally, and with the growing body of SAR information, we could begin to move from what might be considered routine acceptor-like and non-drug-like disaccharides to compounds that would more closely fit drug-like molecules. Next, we prepared symmetrical C-linked and pseudo-symmetrical O-linked disaccharides 4 and 5 possessing N,N-dicyclohexylamino moieties. Both C-sugars and C-linked disaccharides may offer advantages as enzyme probes and inhibitors, and most importantly, this linkage may prevent glycosidase-mediated cleavage. However, both 4 and 5 showed only modest inhibitory activity in the cell free assay system as well as in vitro against MTB H37Ra and M. avium strains.13 In a parallel study, the Lowary group has also synthesized Araf di- and trisaccharide analogs possessing substitution at the C-5 position(s) of the non-reducing sugars; activity was not reported for these compounds.14
Our eventual goal was to move from prototype acceptor disaccharides to potent drug-like GTs inhibitors. In this work, our goal was to assess the requirement for typical saccharide-like OH substitutions (e.g. hydroxy to deoxy sugars), and some of these substitutions are reported herein. Secondly, the 2-deoxy-2-fluoro-Araf substitution is known to stabilize glycosidic linkages, and might improve drug-like properties of even more saccharide-like inhibitors.
Deoxy sugars as well as their fluoro counterparts are present in many natural products and are a medicinally useful group of compounds.15 Deoxy derivatives have been prepared as inhibitors of glycosidases,16 GTs,17 and also to establish which hydroxyl groups are involved in interaction with lectins.18 The preparation and biological activity of deoxy sugars and deoxy sugar oligosaccharides have been reviewed.19 Some of the general methods for the preparation of deoxy sugars are reductive methods, using such starting materials as epoxides, thio sugars, C-halosugars, carboxylate esters, sulfonates, or even by direct reduction of hydroxyl groups. Unsaturated sugars are also excellent sources for deoxygenated derivatives via electrophilic addition of hydrogen halides, and glycals through acid catalyzed addition of water or alcohols.20 Other more direct, approaches have also been developed recently21 and the syntheses of a small number of deoxy furanose derivatives, especially 2-deoxy-glycosides, have also been reported.22 Specifically, 2-deoxy Araf di- and trisaccharides were recently reported through reductive desulfonylation.23
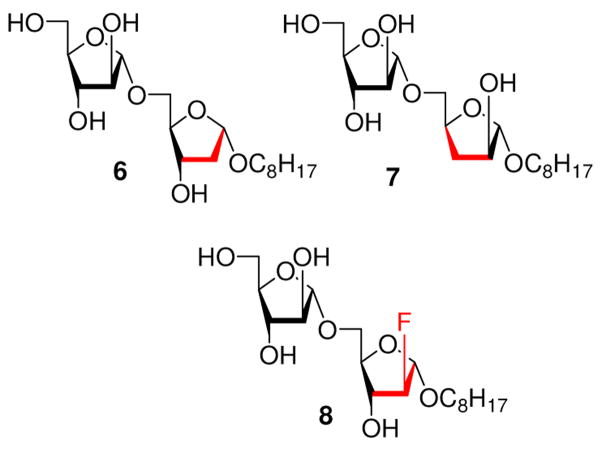
The most common preparative method used is radical chain chemistry for the transformation of a secondary alcohol to the corresponding deoxy derivative. Firstly, alcohols are converted to a thiocarbonyl derivative (thioxobenzoates, xanthates, or thiocarbonylimidazolides), and, on reduction with tributyltin hydride, these derivatives afford deoxy compounds in good yields.24 Utilizing this approach, we report the synthesis of 1-O-octyl-Araf α(1→5) Araf disaccharides 6 and 7 possessing deoxygenation at the 2-and 3-position of the reducing end respectively as shown in Figure 1. Also, disaccharide 8 was synthesized possessing 2-deoxy-2-fluoro at the reducing end of the disaccharide (Figure 1) starting from 2-fluoro-1,3,5-tri-O-benzoyl-α-D-arabinofuranose. These analogs were prepared in order to further define the growing SAR profile of the mycobacterial AraTs relative to the simple Araf α(1→5) Araf template acceptor.
Figure 1.
Target Araf α(1→5)Araf disaccharides
Result and Discussion
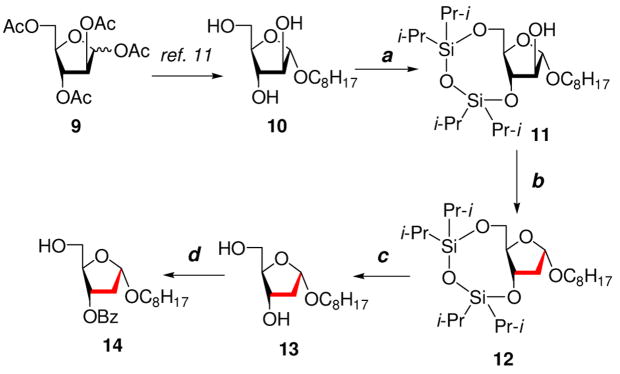
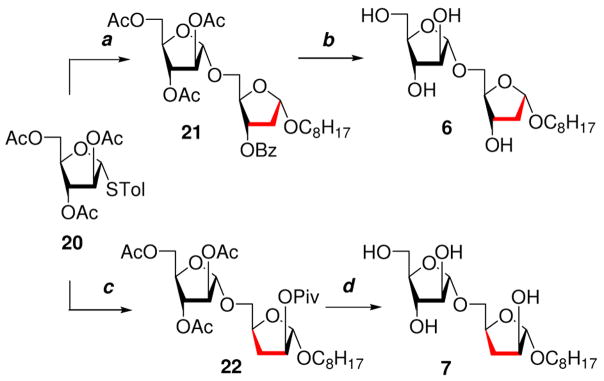
The synthesis of target 2-deoxy and 3-deoxy Araf disaccharides 6 and 7 were initiated from a common known Araf glycoside precursor 1125 (Schemes 1 and 2). The synthesis of precursor 11 began with commercially available D-arabinose that efficiently transformed to 1,2,3,5-tetra-O-acetyl-D-arabinofuranose 9.26 Glycoside 9 was subsequently converted to n-octyl α-D-arabinofuranoside (10) as reported earlier.11 Reaction of 10 with 1,3-dichloro-1,1,3,3-tetraisopropyl-disiloxane (TIPDSCl2) in pyridine and purification by column chromatography on SiO2 afforded the 3,5-silyl blocked glycoside 11 (Scheme 1).
Scheme 1.
Reagents and conditions: (a) TIPSCl2, pyridine, 0 °C, 4 h, 87%; (b) (i) CS2, NaH, MeI, DMF, 0 °C–rt, 30 min, (ii) Bu3SnH, AIBN, rt, 3 h, overall yield 86%; (c) Et4N+F−, THF, rt, 3 h, 89%; (d) (i) TrCl, DMAP, pyridine, 45 °C, overnight, (ii) BzCl, pyridine, 50 °C, overnight; (iii) 5% TFA/CHCl3, −20 °C, 4 h, overall yield 65%.
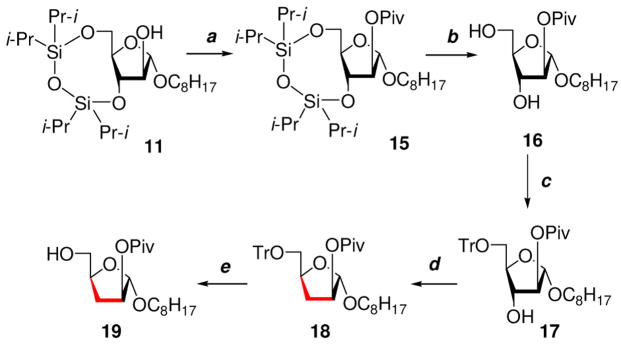
Scheme 2.
(a) (CH3)3CCOCl, pyridine, rt, overnight, 97%; (b) Et4N+F−, THF, rt, 3 h, 91%; (c) TrCl, DMAP, Pyridine, 45 °C, 3 h, 85%; (d) (i) CS2, NaH, MeI, DMF, 0 °C–rt, 30 min (ii) Bu3SnH, AIBN, rt, 3 h, overall yield 83%; (e) 5% TFA/CHCl3, −20 °C, 4 h, 83%.
The synthesis of n-octyl 2-deoxy glycosyl acceptor 14 was achieved in three steps starting from precursor glycoside 11. The deoxygenation at the 2-position in glycoside 11 was carried out in one pot by converting 11 to the xanthate intermediate through reaction with CS2, MeI in the presence of NaH followed by radical reduction with Bu3SnH and AIBN. After workup and purification, 2-deoxy glycoside 12 was obtained in 86% overall yield. Glycoside 12 was desilylated using Et4N+F− in THF to give 13 in 89% yield. The final step in the synthesis of glycosyl acceptor 14 was carried out without purification of the intermediates. The 5-OH group in 13 was selectively tritylated, and the product was subsequently benzoylated and detritylated to give 14 in 65% overall yield after purification. The 1H NMR spectrum of glycosyl acceptor 14 in CDCl3 showed the anomeric proton at δ 5.27 ppm as a doublet of doublets (J1,2eq = 0.9 Hz, J1,2ax = 5.2 Hz). The H-2 axial and equatorial protons in 14 were observed as ddd at δ 2.43 ppm and δ 2.21 ppm respectively. These assignments were made on the basis of coupling constant correlation and nOe experiments. The decoupled and DEPT 13C NMR spectra of 14 were also supportive of the structure, and showed the anomeric carbon and C-2 at δ 103.64 and δ 39.31 ppm respectively.
The synthesis of 3-deoxy acceptor glycoside 19 began from glycoside 11 (Scheme 2). The 2-hydroxyl group in 11 was protected by a pivaloyl group to give 15 that, on desilylation using Et4N+F− in THF, produced 16 in excellent yield. The 5-OH group of compound 16 was then selectively tritylated to produce glycoside 17. Compound 17 was deoxygenated via a free radical pathway in two steps as described for 12 to give the 3-deoxy glycoside 18 in 83% yield after purification. To access the desired 3- deoxy glycosyl acceptor 19, compound 18 was detritylated at −20 °C using TFA in CHCl3. The structure of 3-deoxy glycoside 19 was supported by the 1H NMR spectrum that showed the anomeric proton at δ 4.99 ppm as a singlet and signals from axial and equatorial protons at C-3 were observed as ddd at δ 1.72 and δ 2.47 ppm respectively. The 13C NMR spectra of 19 showed the anomeric carbon and C-3 at δ 105.97 and δ 31.51 ppm respectively.
Both 2-deoxy and 3-deoxy Araf glycosyl acceptors 14 and 19 were subjected to glycosylation using the known thioglycoside donor 2027 as represented in Scheme 3. Glycosyl acceptor 14 on reaction with thioglycoside 20 in the presence of activator NIS and Lewis acid Sn(OTf)2 gave disaccharide 21 in 85% yield after purification. Disaccharide 21 was fully characterized by the 1H NMR spectrum and anomeric proton signals were evident as a doublet at δ 5.28 ppm (H-1, J1,2ax = 4.7 Hz) and as a singlet at δ 5.13 ppm (H-1′), suggesting 1,2-trans glycosylation. The assigned structure was further supported by the 13C NMR spectrum in which the signals from the anomeric carbons C-1 and C-1′ were observed at δ 104.02 ppm and δ 105.68 ppm respectively. Disaccharide 21 was deprotected using 7N NH3/MeOH to produce the 2-deoxygenated disaccharide 6. The 1H NMR spectrum of 6 in CD3OD at 600 MHz showed the anomeric proton H-1 as a doublet of doublets at δ 5.11 ppm (J1,2eq = 1.9 Hz, J1,2ax = 5.5 Hz) and the H-1′ proton at δ 4.89 ppm as a doublet (J1′,2′= 1.2 Hz). The 13C NMR spectrum of 6 showed anomeric carbons C-1′ and C-1 at δ 109.68 and 105.24 ppm respectively.
Scheme 3.
Reagents and conditions. (a) 14, NIS, Sn(OTf)2, CH2Cl2, 0 °C, 30 min, 85%; (b) 7N NH3/MeOH, rt, overnight, 85%; (c) 19, NIS, Sn(OTf)2, CH2Cl2, 0 °C, 30 min, 87%; (d) 7N NH3/MeOH, rt, overnight, 87%; (e) NaOMe, MeOH, rt, overnight, 75%.
The synthesis of 3-deoxygenated disaccharide 22 was accomplished by the reaction of glycosyl acceptor 19 and glycosyl donor 20 similar to the synthesis of 21. The α-glycosylation was supported by the 1H NMR spectrum of 22 that showed the anomeric H-1′ signal at 5.09 ppm, but the H-1 signal was unresolved from the H-3′ proton signal. The 13C NMR spectrum of 22 showed anomeric carbons C-1 and C-1′ signals at 106.04 and 105.65 ppm respectively. Global de-protection of disaccharide 22 with 25% NaOMe in MeOH solution resulted in disaccharide 7. The 1H NMR spectrum of 7 at 600 MHz in CD3OD showed the anomeric protons H-1 and H-1′ at δ 4.87 ppm and δ 4.92 ppm as a singlet and doublet (J1′,2′ = 1.2 Hz) respectively. The 13C NMR spectrum of 7 showed anomeric carbons at δ 110.09 and δ 109.56 ppm assigned to C-1 and C-1′ respectively.
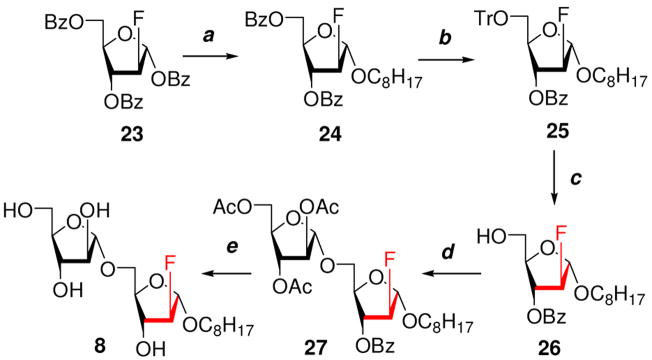
The synthesis of 2-fluoro disaccharide 8 was achieved starting from known glycoside 2-deoxy-2-fluoro-1,3,5-tri-O-benzoyl-α-D-arabinofuranose 2328 as presented in Scheme 4.
Scheme 4.
Reagents and conditions. (a) C8H17OH, SnCl4, CH3CN, rt, 30 min, 54%; (b) (i) 7N NH3/MeOH, rt, overnight, (ii) TrCl, DMAP, pyridine, 45 °C, 48 h, (iii) BzCl, pyridine, rt, overnight, overall yield 81%; (c) 5% TFA/CHCl3, −20 °C, 4 h, 81%; (d) 20, NIS, Sn(OTf)2, CH2Cl2, 0 °C, 30 min, 88%; (e) 7N NH3/MeOH, rt, overnight, 88%.
The 2-fluoro Araf 23 was treated with n-octanol in CH3CN in presence of Lewis acid SnCl4 similar to a reported glycosylation method29 and resulted in a mixture of α- and β-octyl glycosides. After chromatographic purification on SiO2, the desired α-octyl Araf glycoside 24 was obtained in 54% yield. The α-configuration in 24 was supported by the 1H NMR spectrum that showed the anomeric proton as a doublet at δ 5.30 ppm (J1,F = 10.3 Hz) and the H-2 proton at δ 5.10 as a doublet of doublets (J2,3 = 0.7 Hz, J2,F = 49.66 Hz). The 13C NMR decoupled spectrum of 24 showed C-1 and C-2 as doublets at δ 105.16 (J1,F = 34.8 Hz) and δ 98.25 ppm (J2,F = 181.9 Hz) respectively. The synthesis of glycoside 25 was achieved in an overall yield of 81% by a three step reaction sequence in one reaction vessel. In the first step, glycoside 24 was debenzoylated by NH3/MeOH. After completion of the reaction, it was concentrated under vacuum to syrup that was utilized without purification. In the second step, the 5-hydroxyl group was selectively tritylated using TrCl in pyridine at 50 °C. In the third step, the tritylated glycoside was benzoylated by adding BzCl in the same reaction vessel to give glycoside 25. Purified 25 was treated with 5% TFA in CHCl3 at −20 °C to remove the trityl group to give acceptor glycoside 26 in 81% yield. The structure of glycoside 26 was characterized by 1H NMR, 13C NMR and HR-ESIMS spectra.
Coupling of the glycosyl acceptor 26 and thioglycosyl donor 20 was carried out in the presence of activator NIS and the Lewis acid Sn(OTf)2 at 0 °C to yield disaccharide 27 in 88% yield. The 1,2-trans glycosylation was supported by the 1H NMR spectrum that displayed anomeric signals as a doublet at δ 5.25 ppm (J1,F = 10.4 Hz, H-1) and a signlet at δ 5.17 ppm (H-1′). The 13C NMR spectrum 27 showed anomeric carbons at δ 105.51 as a singlet and δ 105.01 ppm as a doublet (2J1,F = 34.8 Hz). Finally, removal of all acyl protecting groups in 27 by NH3/MeOH produced the final 2-deoxy-2-fluoro glycoside 8 in 88% yield. The 1H NMR spectrum in CD3OD of disaccharide 8 showed anomeric signals at δ 5.06 (d, J1,F = 12.2 Hz) and δ 4.94 (d, J1′,2′;= 1.2 Hz) whereas the 13C NMR spectrum showed anomeric carbons at δ 109.68 (C-1′) and δ 106.5 (d, 2JF,C-1 = 127.6 Hz).
All new compounds were characterized using 1H NMR, 13C NMR and HR-ESIMS. The NMR shifts of mono and disaccharides were compared with literature results.10,12,15,30 nOe, decoupling and D2O exchange experiments were performed to confirm NMR assignments as needed.
Biological activity
In vitro activity
The arabinofuranosyl disaccharides were screened against M. tuberculosis (MTB H37Ra) and M. avium (NJ 211) in vitro as reported.31 Disaccharides 6, 7 and 8 were active in the concentration range >12.8≤128 μg/mL for both strains.
Cell free assay
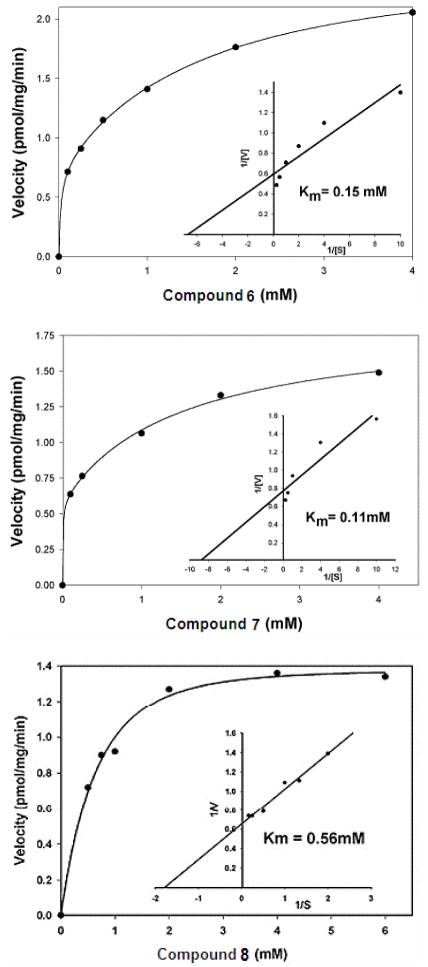
Based on the previous use of specific Araf-based neoglycolipid acceptors,32 compounds 6–8 were assessed as acceptors and competitive inhibitors. Assays were performed in the presence of membranes, resulting in [14C]Araf incorporation from the established enzymatic Araf donor DP-[14C]Araf by compounds 6, 7 and 8. TLC autoradiography (Figure 2) demonstrated the enzymatic conversion of 6, 7 and 8 to their corresponding homologated products. The enzymatic kinetic analysis for compounds 6, 7 and 8 (Figure 3) gave Km values of 0.15 mM, 0.11 mM and 0.56 mM respectively. Further competition-based, experiments established that 6, 7 and 8 were weak competitive inhibitors of their native acceptor 1a [Araf(1→5)Araf-O-C8H17] in the AraTs assay resulting in an IC50 values of 2.45 mM, 2.15 mM, 3.58 mM, respectively.
Figure 2.
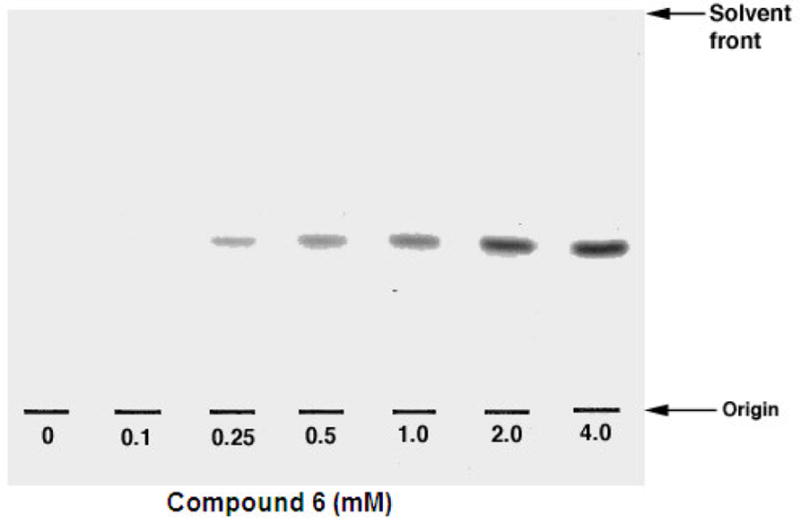
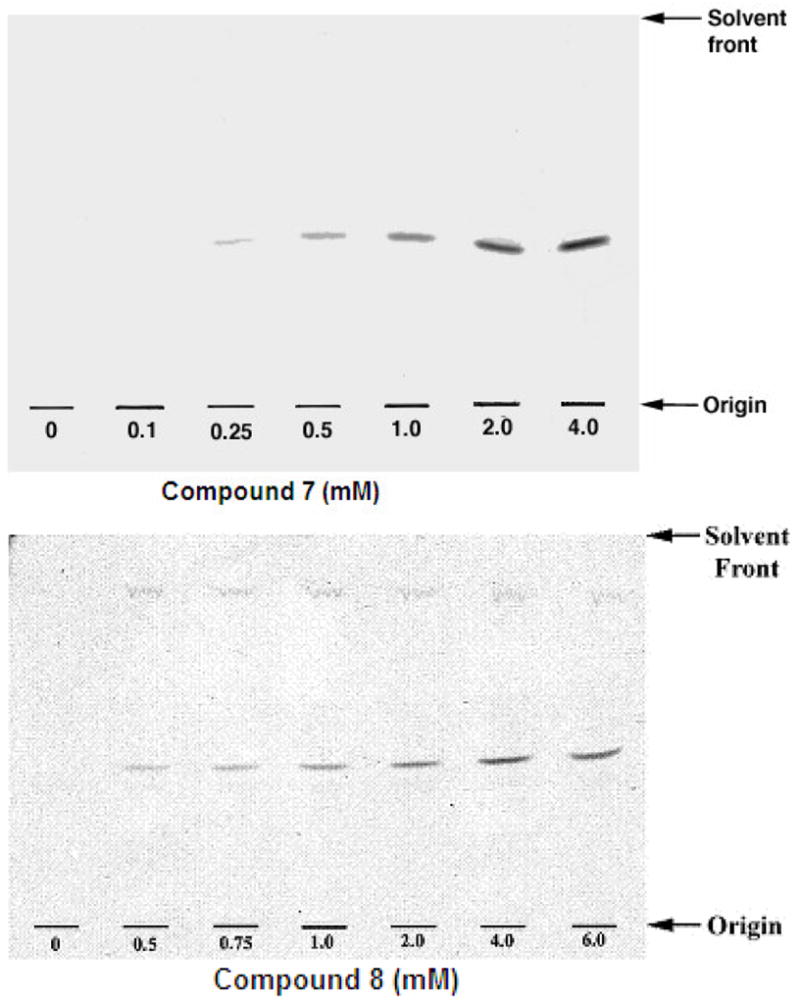
TLC autoradiograms of reaction products produced through the inclusion of 6, 7 and 8, mycobacterial membranes and DP[14C]A. The TLC/autoradiography performed using CHCl3:MeOH:NH4OH:H2O (65:25:0.4:3.6) and products revealed through exposure to Kodak X-Omat film at −70 °C for 3 days.
Figure 3.
Kinetic analysis of acceptors 6, 7 and 8. The inset illustrates the double reciprocal plot for substrates for the mycobacterial AraTs.
Conclusion
In conclusion, we have efficiently prepared three new α (1→5)–linked Araf disaccharides 6, 7 and 8 possessing deoxygenation at the 2- or 3-position of the reducing sugar as biochemical probes to study substrate specificity of AraTs in MTB. Deoxygenation removes a potential metabolic site, but, additionally, the added hydrophobicity may play an important role for inhibiting AraTs as has been noted in reports by us and others.11–14 The activity of these disaccharides as acceptors and competitive inhibitors was evaluated, and only moderate activities were seen against the AraTs in a cell free assay system as well as in the invitro antibacterial assay against MTB H37Ra and MAC strain NJ211. Clearly, the deoxygenated disaccharides can act as modest acceptors, and can affect bacterial growth. In a previous study, compounds 1b and 1c (possessing methyl and benzyl protecting groups at the reducing end of an Araf disaccharide), the reported IC50 values were 3.70 and 1.12 mM respectively.11 The IC50 values found for three new deoxy disaccharides 6, 7 and 8 were comparable to values obtained for compounds 1b and 1c. Deoxygenation, i.e. alteration in hydrophilicity, at the reducing end in the disaccharides 6, 7, and 8 did not significantly alter AraTs substrate or competitive inhibitory activity when compared to other disaccharides bearing substitution at the reducing and non-reducing ends.11–14 Currently, bis(cyclohexylmethyl)amino substituted Araf disaccharide 3 is the only reported disaccharide that has shown both enzyme inhibition and prevention of mycobacterial growth.12 The results from this investigation also clearly indicate that the reducing end sugar can be altered to other similar pentose ring systems to effectively bind in the catalytic region of AraTs enzymes.
Experimental
Synthesis
All reactions were performed under a dry argon atmosphere and reaction temperatures were measured externally. All chemicals and anhydrous solvents from Aldrich were used as is in the reactions. Whenever necessary, compounds and starting materials were dried by azeotropic removal of water with toluene under reduced pressure. Reactions were monitored by thin-layer chromatography (TLC) on precoated E. Merck silica gel (60F254) plates (0.25 mm) and visualized using UV light (254 nm) and/or heating after spray with (NH4)2SO4 solution (150 g ammonium sulfate, 30 mL H2SO4, 750 mL H2O). All solvents used for work-up and chromatography were reagent grade from Fisher Scientific. Flash chromatography was carried out on Fischer silica gel 60 (230–400 Mesh). The 1H and 13C NMR spectra were recorded on Nicolet NT 300NB instrument at 300 MHz and 75 MHz respectively. Coupling constants (J) values are reported in Hz and chemical shifts are in ppm (δ) relative to residual solvent peak or internal standard (TMS). The peak assignments in NMR were done on the basis of coupling constant values and whenever needed nOe experiments were performed. The HR-ESIMS were recorded on a BioTof-2 time-of-flight mass spectrometer.
Octyl 3,5-O-1,1,3,3-tetra-iso-propyldisilyl-α-D-arabinofuranoside (11)25
Octyl α-D-arabinofuranoside 1011 (3.00 g, 11.5 mmol) was dissolved in dry pyridine (100 mL) under argon atmosphere and cooled to 0 °C. TIPSCl2 (4.0 mL, 12.6 mmol) was added and the reaction mixture was stirred at 0 °C for 4 hr. The solvent was evaporated to dryness and the remaining oil was dissolved in 150 mL CHCl3. The CHCl3 solution was washed with deionized water (2×50 mL) and dried over Na2SO4. It was concentrated to a syrup that on purification by column chromatography over silica gel using CHCl3–MeOH (95:5) gave compound 11 (4.44 g, 77% yield). HR-ESIMS: m/z Found 527.3193 [M+Na]+, calcd 527.3200 for C25H52O6Si2Na. 1H NMR (CDCl3): δ 4.87 (1H, dd, J1,2 = 0.7 Hz, J1,2-OH = 2.5 Hz, H-1), 4.18 (1H, dd, J3,4 = 3.0 Hz, J2,3 = 6.3 Hz H-3), 4.14 (1H, ddd, J1,2 = 0.7 Hz, J2,2-OH = 2.5 Hz, J2,3 = 6.3 Hz, H-2), 3.99 (1H, dd, J4,5a = 3.1 Hz, J5a,5b = 12.5 Hz, H-5a), 3.93 (1H, dd, J4,5b = 3.7 Hz, J5a,5b = 12.5 Hz, H-5b), 3.88 (1H, ddd, J3,4 = 3.0 Hz, J4,5a = 3.1 Hz, J4,5b = 3.7 Hz, H-4), 3.70 (1H, m, OCH2), 3.41 (1H, m, OCH2), 2.00 (1H, d, J1,2-OH = J2,2-OH = 2.5 Hz, 2-OH), 1.58 (2H, m, CH2), 1.29 (10H, m, 5xCH2), 1.05 (28H, m, 4xCH, 8xCH3), 0.88 (3H, m, CH3). 13C NMR (CDCl3): δ 106.75 (C-1), 82.64 (C-4), 80.71 (C-2), 76.94 (C-3), 68.36 (OCH2), 61.55 (C-5), 31.82, 29.63, 29.36, 29.24, 26.07, 22.65 (CH2), 17.46, 17.33, 17.26, 17.19, 17.14, 17.09, 17.05, 16.99, 14.09 (CH3), 13.50, 13.15, 12.81, 12.56 (CH).
Octyl 2-deoxy-3,5-di-O-1,1,3,3-tetra-iso-propyldisilyl-α-D-arabinofuranoside (12)
A solution of compound 11 (1.60 g, 3.17 mmol) was dissolved in dry DMF (25 mL) under argon atmosphere and cooled to 0 °C. CS2 (1.1 mL, 18.38 mmol) was added and the reaction mixture was stirred at 0 °C for 15 minutes followed by addition of NaH (380 mg, 9.51 mmol). The reaction mixture was further stirred for 30 minutes and MeI (2.0 mL, 31.70 mmol) was added dropwise. The solution was stirred for 20 minutes at 0 °C and allowed to warm to room temperature over 30 minutes. Deionized water (50 mL) was added to the reaction mixture followed by extraction with EtOAc (3×100 mL). The combined EtOAc extract was washed with deionized water (50 mL), brine solution (50 mL), dried over Na2SO4 and concentrated to yellow oil. The oil was dried under vacuum at room temperature for 1 hr and was used further without purification. The crude thiocarbonyl intermediate product was dissolved in dry toluene (30 mL) and Bu3SnH (3.2 mL, 12.01 mmol) followed by AIBN (113 mg, 0.69 mmol) were added. The reaction mixture was refluxed for 3 hrs, cooled to room temperature and concentrated under vacuum. The crude product was purified over silica gel column using cyclohexane–EtOAc (9:1) as the eluent to produce compound 12 (1.33 g, yield 86%) with slight impurities of tributyltin salts and was used as such for next step. ESIMS: m/z Found 511.3249 [M+Na]+, calcd 511.3251 for C25H52O5Si2Na. 1H NMR (CDCl3): δ 5.04 (1H, dd, J1,2eq = 3.5 Hz, J1,2ax = 5.7 Hz, H-1), 4.29 (1H, m, J3,4 = 6.8 Hz, J2eq,3 = 7.0 Hz, J2ax,3 = 8.5 Hz, H-3), 4.02 (1H, m, H-5a), 3.85 (1H, m, H-4, H-5b), 3.70 (1H, m, OCH2), 3.39 (1H, m, OCH2), 2.45 (1H, ddd, J1,2ax = 5.7 Hz, J2ax,3 = 8.5 Hz, J2ax,2eq = 13.3 Hz, H-2ax), 1.94 (1H, ddd, J1,2eq = 3.5 Hz, J2eq,3 = 7.0 Hz, J2ax,2eq = 13.3 Hz, H-2eq), 1.55 (2H, m, CH2), 1.27 (10H, m, 5xCH2), 1.05 (28H, m, 4xCH, 8xCH3), 0.88 (3H, m, CH3).
Octyl 2-deoxy-α-D-arabinofuranoside (13)
Slightly impure compound 12 (1.20 g, 2.66 mmol) was dissolved in a mixture of dry THF (20 mL) and CH3CN (20 mL) under argon atmosphere, and Et4N+F− (834 mg, 5.59 mmol) was added. The reaction mixture was stirred at room temperature for 3 hrs and concentrated to syrup. Purification over silica gel column using CHCl3–MeOH (9:1) gave pure compound 13 (538 mg, 89%). HR-ESIMS: m/z Found 269.1727 [M+Na]+, calcd 269.1729 for C13H26O4Na. 1H NMR (DMSO-d6): δ 4.98 (1H, dd, J1,2eq = 2.6 Hz, J1,2ax = 5.8 Hz, H-1), 4.81 (1H, d, J3,3-OH = 5.3 Hz, 3-OH), 4.62 (1H, t, J5a,5-OH = J5b,5-OH = 5.7 Hz, 5-OH), 3.90 (1H, dddd, J2eq,3 = 4.9 Hz, J3,4 = 5.2 Hz, J3,3-OH = 5.3 Hz, J2ax,3 = 8.4 Hz, H-3), 3.67 (1H, ddd, J4,5a = 3.5 Hz, J3,4 = 5.2 Hz, J4,5b = 5.7 Hz, H-4), 3.57 (1H, m, OCH2), 3.49 (1H, ddd, J4,5a = 3.5 Hz, J5a,5-OH = 11.4 Hz, H-5a), 3.35 (1H, dd, J4,5b = J5,5-OH = 5.7 Hz, J5a,5b = 11.4 Hz, H-5b), 3.27 (1H, m, OCH2), 2.27 (1H, ddd, J1,2ax = 5.8 Hz, J2ax,3 = 8.4 Hz, J2ax,2eq = 13.4 Hz, H-2ax), 1.62 (1H, dddd, J1,2eq = 2.6 Hz, J2eq,3 = 4.9 Hz, J = 7.6 Hz, J2ax,2eq = 13.4 Hz, H-2eq), 1.47 (2H, m, CH2), 1.27 (10H, m, 5xCH2), 0.87 (3H, m, CH3).
Octyl 3-O-benzoyl-2-deoxy-α-D-arabinofuranoside (14)
To a dry pyridine (10 mL) solution of compound 13 (497 mg, 2.01 mmol) were added TrCl (672 mg, 2.40 mmol) and DMAP (25 mg, 0.20 mmol) at room temperature. The reaction mixture was heated at 45 °C overnight and TLC confirmed the completion of the reaction. It was cooled to room temperature and BzCl (0.36 mL, 3.07 mmol) was added dropwise. The reaction mixture was heated at 50 °C overnight, cooled to room temperature and concentrated under vacuum to give a syrup. The syrup was dissolved in CHCl3 (30 mL) and cooled to −20 °C, and 5% TFA in CHCl3 (11 mL) was added dropwise. The reaction mixture was stirred for 4 hrs at the same temperature, co-evaporated with ethanol (2×20 mL), and concentrated to syrup. Column chromatography using cyclohexane–EtOAc (1:1) on the oil gave glycoside 14 (396 mg, overall yield in three steps 65%) as a colorless syrup. HR-ESIMS: m/z Found 373.1996 [M+Na]+, calcd 373.1985 for C20H30O5Na. 1H NMR (CDCl3): δ 8.06 (2H, m, Ar), 7.57 (1H, m, Ar), 5.33 (1H, ddd, J2eq,3 = 2.2 Hz, J3,4 = 3.8 Hz, J2ax,3 = 5.9 Hz, H-3), 5.27 (1H, dd, J1,2eq = 0.9 Hz, J1,2ax = 5.2 Hz, H-1), 4.25 (1H, dd, J3.4 = J4,5a = 3.8 Hz, J4,5b = 4.0 Hz, H-4), 3.88 (1H, dd, J4,5a = 3.8 Hz, J5a,5b = 11.8 Hz, H-5a), 3.83 (1H, dd, J4,5b = 4.0 Hz, J5a,5b = 11.8 Hz, H-5b), 3.72 (1H, m, OCH2), 3.43 (1H, m, OCH2), 2.43 (1H, ddd, J1,2ax = 5.2 Hz, J2ax,3 = 5.9 Hz, J2ax,2eq = 13.4 Hz, H-2ax), 2.21 (1H, ddd, J1,2eq = 0.9 Hz, J2eq,3 = 2.2 Hz, J2ax,2eq = 13.4 Hz, H-2eq), 1.59 2H, (m, CH2), 1.30 (10H, m, 5xCH2), 0.85 (3H, m, CH3). 13C NMR (CDCl3): δ 166.69 (C=O), 133.15 (CH, Ar), 129.86 (C, Ar), 129.70, 128.32 (each CH, Ar), 103.64 (C-1), 83.55 (C-4), 74.86 (C-3), 67.46 (OCH2), 62.74 (C-5), 39.31 (C-2), 31.79, 29.34, 29.39, 29.25, 26.24, 22.62 (each CH2), 14.04 (CH3).
Octyl 2-O-pivaloyl-3,5-O-1,1,3,3-tetra-iso-propyldisilyl- α-D-arabinofuranoside (15)
Compound 11 (2 g, 3.96 mmol) was dissolved in dry pyridine (10 mL) and pivaloyl chloride (0.6 mL, 4.75 mmol) and DMAP (48 mg, 0.40 mmol) were added. The reaction mixture was stirred overnight at room temperature and TLC showed completion of the reaction. It was concentrated to a syrup, and purification by column chromatography over silica gel using cyclohexane–EtOAc (95:5) gave compound 15 (2.26 g) in 97% yield. HR-ESIMS: m/z Found 611.3760 [M+Na]+, calcd 611.3775 for C30H60O7Si2Na. 1H NMR (CDCl3): δ 5.10 (1H, dd, J1,2 = 1.1 Hz, J2,3 = 4.4 Hz, H-2), 4.78 (1H, d, J1,2 = 1.1 Hz, H-1), 4.32 (1H, dd, J2,3 = 4.4 Hz, J3,4 = 7.0 Hz, H-3), 3.98 (3H, m, H-4, H2-5), 3.64 (1H, m, OCH2), 3.41 (1H, m, OCH2), 1.58 (2H, m, CH2), 1.27 (10H, m, 5xCH2), 1.20 (9H, s, 3xCH3), 1.05 (28H, m, 4xCH, 8xCH3), 0.87 (3H, m, CH3). 13C NMR (CDCl3): δ 177.42 (C=O), 105.48 (C-1), 84.16 (C-2), 81.43 (C-4), 76.55 (C-3), 67.79 (OCH2), 62.20 (C-5), 38.61 (C), 31.83, 29.50, 29.34, 29.24 (CH2), 27.03 (CH3), 26.04, 22.65 (CH2), 17.42, 17.32, 17.29, 17.13, 17.05, 16.98, 16.94, 14.06 (CH3), 13.58, 13.22, 12.78, 12.53 (CH2).
Octyl 2-O-pivaloyl-α-D-arabinofuranoside (16)
Compound 15 (2.20 g, 3.74 mmol) was treated with Et4N+F− (1.00 g, 6.73 mmol) as described in the synthesis of compound 13. Purification over silica gel column using CHCl3–MeOH (95:5) gave pure compound 16 (1.18 g, 91%). HR-ESIMS: m/z Found 369.2249 [M+Na]+, calcd 369.2253 for C18H34O6Na. 1H NMR (CDCl3): δ 5.06 (1H, s, H-1), 4.81 (1H, dd, J1,2 = 0.9 Hz, J2,3 = 2.5 Hz, H-2), 4.13 (1H, ddd, J4,5b = 3.3 Hz, J4,5a = 4.6 Hz, J3,4 = 5.8 Hz, H-4), 3.96 (1H, ddd, J2,3 = 2.5 Hz, J3,4 = J3,3-OH = 5.8 Hz, H-3), 3.89 (1H, ddd, J4,5a = 4.6 Hz, J5a,5-OH = 7.9 Hz, J5a,5b = 11.9 Hz, H-5a), 3.73 (2H, m, H-5b, OCH2), 3.44 (1H, m, OCH2), 3.16 (1H, d, J3,3-OH = 5.8 Hz, 3-OH), 1.82 (1H, dd, J5b,5-OH = 5.2 Hz, J5a,5-OH = 7.9 Hz, 5-OH), 1.59 (2H, m, CH2), 1.27 (10H, m, 5xCH2), 1.22 (9H, s, 3xCH3), 0.88 (3H, m, CH3). 13C NMR (CDCl3): δ 178.91 (C=O), 105.19 (C-1), 85.32 (C-2), 84.15 (C-4), 76.44 (C-3), 67.90 (OCH2), 62.02 (C-5), 38.71 (C), 31.79, 29.45, 29.31, 29.21 (CH2), 27.04 (CH3), 26.06, 22.63 (CH2), 14.07 (CH3).
Octyl 2-O-pivaloyl-5-O-trityl-α-D-arabinofuranoside (17)
To a dry pyridine (20 mL) solution of compound 16 (1.18 g, 3.41 mmol) were added trityl chloride (1.16 g, 4.16 mmol) and DMAP (42 mg, 0.34 mmol), and the reaction mixture was stirred at 50 °C overnight. Co-evaporation with toluene (2×50 mL) resulted in an oil that was dissolved in CHCl3 (100 mL), washed with water (2×20 mL), dried over Na2SO4 and concentrated. Column chromatography (cyclohexane–EtOAc 95:5) gave compound 17 as an oil (1.70 g, 85%). HR-ESIMS: m/z Found 611.3339 [M+Na]+, calcd 611.3349 for C37H48O6Na. 1H NMR (CDCl3): δ 7.46 (6H, m, Ar), 7.37 (9H, m, Ar), 5.05 (1H, s, H-1), 4.81 (1H, dd, J1,2 = 0.7 Hz, J2,3 = 2.2 Hz, H-2), 4.28 (1H, dd, J3,4 = 4.7 Hz, J4,5b = 5.3 Hz, J4,5a = 5.8 Hz, H-4), 3.96 (1H, ddd, J2,3 = 2.2 Hz, J3,4 = 4.7 Hz, J3,3-OH = 7.1 Hz, H-3), 3.77 (1H, m, OCH2), 3.29 (1H, dd, J4,5a = 5.8 Hz, J5a,5b = 9.7 Hz, H-5a), 3.19 (1H, dd, J4,5b = 5.3 Hz, J5a,5b = 9.7 Hz, H-5b), 3.04 (1H, d, J3,3-OH = 7.1 Hz, 3-OH), 1.60 (2H, m, CH2), 1.31 (10H, m, 5xCH2), 1.08 (9H, s, 3xCH3), 0.88 (3H, m, CH3). 13C NMR (CDCl3): δ 178.43 (C=O), 143.81 (C), 128.66, 127.79, 126.98 (CH), 105.10 (C-1), 86.58 (C), 83.95 (C-2), 83.86 (C-4), 77.22 (C-3), 67.80 (OCH2), 63.82 (C-5), 38.53 (C), 31.78, 29.51, 29.31, 29.20 (CH2), 26.94 (CH3), 26.08, 22.61 (CH2), 14.06 (CH3).
Octyl 3-deoxy-2-O-pivaloyl-5-O-trityl-α-D-arabinofuranoside (18)
A solution of compound 17 (1.70 g, 2.89 mmol) was dissolved in dry DMF (25 mL) under argon atmosphere and cooled to 0 °C. CS2 (1.01 mL, 16.75 mmol) was added and reaction mixture was stirred at 0 °C for 15 minutes followed by addition of NaH (347 mg, 8.67 mmol). Reaction mixture was further stirred for 30 min, MeI (1.8 mL, 28.9 mmol) was added dropwise. The reaction was stirred for 20 min at 0 °C and allowed to warm to room temperature in 30 min. Deionized water (50 mL) was added to reaction mixture and extracted with EtOAc (3×100 mL). The combined EtOAc extract was washed with deionized water (50 mL), aq. satd. NaCl solution (50 mL), dried over Na2SO4 and concentrated to a yellow oil. It was dried under vacuum at room temperature for 1 hr and was used further without purification. The crude intermediate product was dissolved in dry toluene (30 mL) and Bu3SnH (2.0 mL, 7.35 mmol) followed by AIBN (69 mg, 0.42 mmol) were added. The reaction mixture was refluxed for 3 hrs, cooled to room temperature and concentrated under vacuum. The crude product was purified over silica gel column using cyclohexane–EtOAc (98:2) as the eluent to produce compound 18 (1.37 g, yield 83%). HR-ESIMS: m/z Found 595.3405 [M+Na]+, calcd 595.3394 for C37H48O5Na. 1H NMR (CDCl3): δ 7.45 (6H, m, Ar), 7.26 (9H, m, Ar), 4.98 (1H, dd, J2,3ax = 1.3 Hz, J2,3eq = 6.2 Hz, H-2), 4.96 (1H, s, H-1), 4.51 (1H, dddd, J3ax,4 = 4.3 Hz, J4,5b = 5.8 Hz, J4,5a = 6.4 Hz, J3eq,4 = 8.5 Hz, H-4), 3.68 (1H, m, OCH2), 3.41 (1H, m, OCH2), 3.31 (1H, dd, J4,5a = 6.4 Hz, J5a,5b = 9.2 Hz, H-5a), 3.00 (1H, dd, J4,5b = 5.8 Hz, J5a,5b = 9.2 Hz, H-5b), 2.48 (1H, ddd, J2,3eq = 6.2 Hz, J3eq,4 = 8.5 Hz, J3ax,3eq = 14.2 Hz, H-3eq), 1.70 (1H, ddd, J2,3ax = 1.3 Hz, J3ax,4 = 4.3 Hz, J3ax,3eq = 14.2 Hz, H-3ax), 1.54 (2H, m, CH2), 1.30 (10H, m, 5xCH2), 1.02 (9H, s, 3xCH3), 0.88 (3H, m, CH3).
Octyl 3-deoxy-2-O-pivaloyl-α-D-arabinofuranoside (19)
Compound 18 (1.00 g, 1.75 mmol) was dissolved in CHCl3 (30 mL) and cooled to −20 °C, and 5% TFA in CHCl3 (11 mL) was added dropwise. The reaction mixture was stirred for 4 hr, co-evaporated with ethanol (2×20 mL), and concentrated to syrup. Column chromatography (cyclohexane–EtOAc, 3:1) yielded compound 19 (479 mg, yield 83%) as a colorless syrup. HR-ESIMS: m/z Found 353.2296 [M+Na]+, calcd 353.2304 for C18H34O5Na. 1H NMR (CDCl3): δ 5.05 (1H, dd, J2,3ax = 1.3 Hz, J2,3eq = 6.4 Hz, H-2), 4.99 (1H, s, H-1), 4.31 (1H, m, H-4), 3.77 (1H, ddd, J4,5a = 3.1 Hz, J5a,OH = 5.7 Hz, J5a,5b = 11.8 Hz, H-5a), 3.66 (1H, m, OCH2), 3.60 (1H, ddd, J4,5b = 5.5 Hz, J5b,OH = 6.7 Hz, J5a,5b = 11.8 Hz, H-5b), 3.41 (1H, m, OCH2), 2.47 (1H, ddd, J2,3eq = 6.4 Hz, J3eq,4 = 8.8 Hz, J3ax,3eq = 14.4 Hz, H-3eq), 1.87 (1H, dd, J5a,OH = 5.7 Hz, J5b,OH = 6.7 Hz, 5- OH), 1.72 (1H, ddd, J2,3ax = 1.3 Hz, J3ax,5a = 5.7 Hz, J3ax,5b = 6.7 Hz, H-3ax), 1.58 (2H, m, CH2), 1.28 (10H, m, 5xCH2), 1.19 (9H, s, 3xCH3), 0.88 (3H, m, CH3). 13C NMR (CDCl3): δ 177.60 (C=O), 105.97 (C-1), 78.34 (C-4), 77.44 (C-2), 67.39, (OCH2), 64.76 (C-5), 38.57 (C), 31.80 (CH2), 31.51 (C-3), 29.51, 29.33, 29.21 (CH2), 27.03 (CH3), 26.07, 22.62 (CH2), 14.07 (CH3).
Octyl 2,3,5-tri-O-acetyl-α-D-arabinofuranosyl-(1→5)-2- deoxy-3-O-benzoyl-α-D-arbinofuranoside (21)
Donor glycoside 20 (695 mg, 1.82 mmol), acceptor glycoside 14 (425 mg, 1.21 mmol) and activated, powdered 4Å molecular sieves (200 mg) in dry CH2Cl2 (15 mL) were cooled at 0 °C under argon atmosphere. The mixture was stirred for 15 min, and NIS (327 mg, 1.45 mmol) followed by Sn(OTf)2 (51 mg, 0.12 mmol) was added to initiate coupling. It was stirred for 30 min at rt, and the reaction was quenched by addition of Et3N (1 mL). The reaction mixture was diluted with CH2Cl2 (20 mL) and filtered through a celite pad. The filtrate was washed with 10% Na2S2O3 (20 mL), followed by washing with saturated aqueous NaHCO3 (20 mL). The organic layer was dried over Na2SO4, the solvent was removed under vacuum, and the residue was purified by column chromatography (cyclohexane–EtOAc, 3:1) to give pure disaccharide 21 as a colorless oil (627 mg, 85%). HR-ESIMS: m/z Found 631.2741 [M+Na]+, calcd 631.2725 for C31H44O12Na. 1H NMR (CDCl3): δ 8.05 (2H, m, Ar), 7.57 (1H, m, Ar), 7.43 (2H, m, Ar), 5.43 (1H, ddd, J3,4 = 3.0 Hz, J2eq,3 = 4.6 Hz, J2ax,3 = 7.7 Hz, H-3), 5.28 (1H, d, J1,2ax = 4.7 Hz, H-1), 5.13 (1H, s, H-1′), 5.11 (1H, d, J2′,3′, = 1.3 Hz, H-2′), 4.97 (1H, dd, J2′,,3′, = 1.3 Hz, J3′,,4′, = 4.6 Hz, H-3′), 4.43 (1H, dd, J4′,5a′, = 3.0 Hz, J5a′,,5b′, = 11.3 Hz, H-5a′), 4.36 (1H, dd, J3,4 = J4,5a = 3.0 Hz, J4,5b = 5.7 Hz, H-4), 4.27 (1H, ddd, J4′,5a′, = 3.0 Hz, J3′,,4′, = 4.6 Hz, J4′,5b′, = 5.7 Hz, H-4′), 4.21 (1H, dd, J4′,5b′, = 5.7 Hz, J5a′,5b′, = 11.3 Hz, H-5b′), 4.00 (1H, dd, J4.5a = 3.0 Hz, J5a5b = 11.1 Hz, H-5a), 3.71 (2H, m, H-5b, OCH2), 3.42 (1H, m, OCH2), 2.46 (1H, ddd, J1,2ax = 4.7 Hz, J2ax,3 = 7.7 Hz, J2ax,2eq = 13.1 Hz, H-2ax), 2.10 (1H, m, H-2eq), 2.09, 2.10, 2.11 (each 3H, s, 3xCH3), 1.58 (2H, m, CH2), 1.31 (10H, m, 5xCH2), 0.86 (3H, m, CH3). 13C NMR (CDCl3): δ 170.52, 170.07, 169.42, 166.36 (4xC=O), 133.04 (CH, Ar), 130.00 (C, Ar), 129.63, 128.28 (each CH2, Ar), 105.68 (C-1′), 104.02 (C-1), 82.50 (C-4), 80.90 (C-4′), 80.85 (C-2′), 77.06 (C-3′), 74.83 (C-3), 67.42 (OCH2), 66.67 (C-5), 63.26 (C-5′), 39.25 (C-2), 31.77, 29.76, 29.37, 29.23, 26.23, 22.58 (6xCH2), 20.71, 20.68, 20.64, 14.02 (4xCH3).
Octyl 2,3,5-tri-O-acetyl-α-D-arabinofuranosyl-(1→5)-3- deoxy-2-O-pivaloyl-α-D-arbinofuranoside (22)
Donor glycoside 20 (503 mg, 1.32 mmol), acceptor glycoside 19 (290 mg, 0.90 mmol) were coupled as described for the synthesis of disaccharide 21. The crude disaccharide was purified by column chromatography (cyclohexane–EtOAc, 3:1) to give pure disaccharide 22 (449 mg, 87%) as a colorless oil. HR-ESIMS: m/z Found 611.3038 [M+Na]+, calcd 611.3001 for C29H48O12Na. 1H NMR (CDCl3): δ 5.13 (1H, d, J = 1.8 Hz, H-2′), 5.09 (1H, s, H-1′), 5.03 (1H, dd, J2,3ax = 1.7 Hz, J2,3eq = 6.4 Hz, H-2), 4.98 (2H, m, H-1, H-3′), 4.42 (1H, dd, J4′5a′= 2.1 Hz, J5a′,5b′= 10.6 Hz, H-5a′), 4.36 (1H, m, H-4), 4.24 (2H, m, H-4′, H-5b′), 3.82 (1H, dd, J4,5a = 6.2 Hz, J5a,5b = 10.3 Hz, H-5a), 3.65 (1H, m, OCH2), 3.51 (1H, dd, J4,5b = 5.5 Hz, J5a,5b = 10.3 Hz, H-5b), 3.40 (1H, m, OCH2), 2.47 (1H, ddd, J2,3eq = 6.4 Hz, J3eq,4 = 8.2 Hz, J3ax,3eq = 14.2 Hz, H-3eq), 2.10 (6H, s, 2xCH3), 2.09 (3H, s, CH3), 1.71 (1H, ddd, J2,3ax = 1.7 Hz, J = 5.0 Hz, J3ax,3eq = 14.2 Hz, H-3ax), 1.55 (2H, m, CH2), 1.28 (10H, m, 5xCH2), 1.19 (9H, s, 3xCH3), 0.88 (3H, m, CH3). 13C NMR (CDCl3): δ 177.69, 170.56, 170.12, 169.54 (C=O), 106.04 (C-1), 105.65 (C-1′), 81.19 (C-2′), 80.22 (C-4′), 77.32 (C-2), 77.10 (C-3′), 76.12 (C-4), 69.66 (OCH2), 67.41 (C-5), 63.18 (C-5′), 38.53 (C), 32.51 (C-3), 31.79, 29.50, 29.33, 29.21 (CH2), 27.02 (CH3), 26.05 (CH2), 22.61 (CH2), 20.76, 20.72 (CH3), 14.06 (CH3).
Octyl 2-deoxy-2-fluoro-3,5-O-dibenzoyl-α-D-arabinofuranoside (24)
Compound 2328 (900 mg, 1.94 mmol) was dissolved in dry CH3CN (200 mL) was added SnCl4 (0.27 mL, 2.33 mmol) at room temperature and the mixture was stirred for 15 min. To this solution, n-octanol (0.37 mL, 2.33 mmol) was added dropwise over a period of 30 min. It was again stirred for 1 hr at room temperature. Celite (2.0 g) was added cooled in ice-water bath and a saturated aqueous NaHCO3 solution was added dropwise to precipitate tin salts. After complete precipitation, the mixture was filtered through celite and washed with chloroform (2×10 mL), concentrated to a syrup, and re-dissolved in CHCl3 (100 mL). The solution was next washed with water (2×25 mL) and brine (2×25 mL), dried over Na2SO4 and concentrated under vacuum to give a crude oil. Column chromatography (cyclohexane–EtOAc, 7:1) gave the desired α-isomer 24 as a colorless oil (403 mg, 54% yield). HR-ESIMS: m/z Found 495.2153 [M+Na]+, calcd 495.2154 for C27H33FO6Na. 1H NMR (CDCl3): δ 8.05 (4H, m, Ar), 7.57 (2H, m, Ar), 7.43 (4H, m, Ar), 5.48 (1H, ddd, J2,3 = 0.7 Hz, J3,4 = 4.7 Hz, J3,F = 22.4 Hz, H-3), 5.30 (1H, d, J1,2 = 0 Hz, J1,F = 10.3 Hz, H-1), 5.10 (1H, dd, J1,2 = 0.0 Hz, J2,3 = 0.7 Hz, J2,F = 49.66 Hz, H-2), 4.74 (1H, dd, J4,5a = 3.5 Hz, J5a,5b = 11.9 Hz, H5a), 4.62 (1H, dd, J4,5b = 4.6 Hz, J5a,5b = 11.9 Hz, H5b), 4.50 (1H, dd, J4,5a = 3.5 Hz, J4,5b = 4.6 Hz, H-4), 3.76 (1H, m, OCH2), 3.49 (1H, m, OCH2), 1.59 (2H, m, CH2), 1.30 (10H, m, 5xCH2), 0.86 (3H, m, CH3). 13C NMR (CDCl3): δ 166.37, 165.77 (2xC=O), 133.68, 133.18, 129.97, 129.90, 128.59, 128.44 (Ar), 105.16 (J1,F = 34.8 Hz, C-1), 98.25 (J2,F = 181.9 Hz, C-2), 81.00 (J4,F = 1.8 Hz, C-4), 77.63 (J3,F = 30.5 Hz, C-3), 67.61 (OCH2), 63.83 (C-5), 31.90, 29.58, 29.45, 29.35, 26.23, 22.73 (CH2), 14.16 (CH3).
Octyl 2-deoxy-2-fluoro-3-O-benzoyl-5-O-trityl-α-D-arabinofuranoside (25)
To a solution of saccharide 24 (260 mg, 0.55 mmol) in dry methanol (5 mL) was added 7N NH3/MeOH (10 mL) and the reaction mixture was stirred overnight at room temperature. TLC showed no starting material and it was concentration under vacuum to an oil. The crude product was dissolved in dry pyridine (4 mL) and trityl chloride (108 mg, 0.39 mmol) and DMAP (4 mg, 0.03 mmol) were added. The reaction mixture was stirred at 50 °C overnight, cooled to room temperature and BzCl (0.05 mL, 0.44 mmol) was added. The reaction mixture was heated overnight at 50 °C. Co-evaporation with toluene (2×25 mL) resulted in an oil that was dissolved in CHCl3 (100 mL), washed with water (2×20 mL), dried over Na2SO4 and concentrated. Column chromatography (cyclohexane– EtOAc, 95:5) gave compound 25 as oil (272 mg, overall yield 81%). HR-ESIMS: m/z Found 633.2997 [M+Na]+, calcd 633.2987 for C39H43FO5Na. 1H NMR (CDCl3): δ 8.03 (2H, m, Ar), 7.51 (9H, m, Ar), 7.20 (9H, m, Ar), 5.41 (1H, ddd, J2,3 = 1.0 Hz, J3,4 = 5.1 Hz, J3,F = 22.6 Hz, H-3), 5.25 (1H, d, J1,F = 10.6 Hz, H-1), 4.99 (1H, dd, J2,3 = 1.0 Hz, J2,F = 49.7 Hz, H-2), 4.32 (1H, dd, J4,5a = 2.5 Hz, J = 9.5 Hz, H-5a), 3.75 (1H, ddd, J4,5b = 6.6 Hz, J = 9.5 Hz, H-5b), 3.49 (1H, dd, J4,5a = 2.5 Hz, J4,5b = 6.6 Hz, H-4), 3.41 (2H, m, OCH2), 1.59 (2H, m, CH2), 1.30 (10H, m, CH2), 0.86 (3H, m, CH3).
Octyl 2-deoxy-2-fluoro-3-O-benzoyl-α-D-arabinofuranoside (26)
Compound 25 (80 mg, 0.13 mmol) was dissolved in CHCl3 (3 mL) and cooled to −20 °C, and 5% TFA in CHCl3 (1.3 mL) was added dropwise. The reaction mixture was stirred for 1 hr and cold aqueous satd. NaHCO3 solution (10 mL) was added. It was extracted with CHCl3 (2×15 mL), washed with deionized water (2×10 mL) and dried over Na2SO4. After concentration and column chromatography (cyclohexane–EtOAc, 5:1) compound 26 (38 mg, yield 81%) was obtained. HR-ESIMS: m/z Found 391.1891 [M+Na]+, calcd 391.1891 for C20H29FO5Na. 1H NMR (CDCl3): δ 8.04 (2H, m, Ar), 7.60 (1H, m, Ar), 7.46 (2H, m, Ar), 5.38 (1H, ddd, J1,3 = 0.9 Hz, J3,4 = 4.8 Hz, J3,F = 23.0 Hz, H-3), 5.25 (1H, d, J1,2 = 0 Hz, J1,F = 10.4 Hz, H-1), 5.10 (1H, dd, J1,4 = 1.1 Hz, J2,F = 49.7 Hz, H-2), 4.24 (1H, dd, J = 4.3 Hz, 8.4 Hz, H-4), 3.93 (2H, m, H2-5), 3.74 (1H, m, OCH2), 3.48 (1H, m, OCH2), 2.15 (1H, dd, J = 5.3, 7.9 Hz, 5-OH), 1.60 (2H, m, CH2), 1.29 (10H, m, 5xCH2), 0.86 (3H, m, CH3). 13C NMR (CDCl3): δ 166.01 (C=O), 133.63, 129.89 (CH), 129.81 (C), 128.52 (CH), 104.84 (d, 2J1,F = 34.8 Hz, C-1), 98.43 (d, 1J2,F = 181.9 Hz, C-2), 83.29 (d, 3J4,F = 1.8 Hz, C-4), 77.30 (d, 2J3,F = 29.0 Hz, C-3), 67.44 (OCH2), 62.32 (C-5), 31.81, 29.49, 29.37, 29.26 (CH2), 26.14 (CH2), 22.65 (CH2), 14.07 (CH3).
Octyl 2,3,5-tri-O-acetyl-α-D-arabinofuranosyl-(1→5)-2- deoxy-2-fluoro-3-O-benzoyl-α-D-arbinofuranoside (27)
Glycosyl donor 20 (47 mg, 0.12 mmol), glycosyl acceptor 26 (30 mg, 0.08 mmol) were coupled as described for the synthesis of disaccharide 21. The crude disaccharide was purified by column chromatography (cyclohexane–EtOAc, 3:1) to give pure disaccharide 27 (45 mg, 88%) as a colorless oil. HR-ESIMS: m/z Found 649.2620 [M+Na]+, calcd 649.2631 for C31H43FO12Na. 1H NMR (CDCl3): δ 8.03 (2H, m, Ar), 7.59 (1H, m, Ar), 7.45 (2H, m, Ar), 5.51 (1H, ddd, J2,3 = 0.9 Hz, J3,4 = 5.1 Hz, J3,F = 22.3 Hz, H-3), 5.25 (1H, d, J1,F = 10.6 Hz, H-1), 5.18 (1H, d, J2′,,3′, = 1.3 Hz, H-2′), 5.17 (1H, s, H-1′), 5.04 (1H, dd, J2,3 = 0.9 Hz, J2,F = 43.8 Hz, H-2), 4.95 (1H, m, H-3′), 4.44 (1H, dd, J4′,,5′,a = 3.4 Hz, J5′,a,5′,b = 11.6 Hz, H-5′a), 4.33 (2H, m, H-4, H-4′), 4.21 (1H, dd, J4′,,5′,b = 5.6 Hz, J5′,a,5′,b = 11.6 Hz, H-5′b), 4.03 (1H, dd, J4,5a = 3.8 Hz, J5a,5b = 11.3 Hz, H-5a), 3.83 (1H, dd, J4,5b = 3.1 Hz, J5a,5b = 11.3 Hz, H-5b), 3.74 (1H, m, OCH2), 3.47 (1H, m, OCH2), 2.09 (6H, s, 2xOCH3), 2.08 (3H, s, OCH3), 1.58 (2H, m, CH2), 1.28 (10H, m, 5xCH2), 0.86 (3H, m, CH3). 13C NMR (CDCl3): δ 170.62, 170.43, 169.38 (COCH3), 165.58 (COC6H5), 133.53, 129.82 (CH), 129.17 (C), 128.49 (CH), 105.51 (C-1′), 105.01 (d, 2J1,F = 34.8 Hz, C-1), 98.70 (d, 2J2,F = 182.5 Hz, C-2), 81.38 (d, 3J4,F = 1.2 Hz, C-4), 81.04 (C-4′), 80.74 (C-2′), 77.00 (C-3′), 76.61 (d, 2J3,F = 18.9 Hz, C-3), 67.47 (OCH2), 65.49 (C-5), 63.38 (C-5′), 31.80, 29.46, 29.36, 29.26, 26.13, 22.63 (CH2), 20.78, 20.74, 20.62 (COCH3), 14.06 (CH3).
Octyl α-D-arabinofuranosyl-(1→5)-2-deoxy-α-D-arbinofuranoside (6)
To a solution of disaccharide 21 (200 mg, 0.33 mmol) in dry methanol (5 mL) was added 7N NH3/MeOH (5 mL) and the reaction mixture was stirred overnight at room temperature. Concentration in vacuum, and column chromatography (CHCl3–MeOH, 9:1) gave the de-blocked disaccharide containing a minor impurity. For the final purification, an aqueous solution (5 mL) of the disaccharide was passed through a small column packed with Bio- Beads™ SM-4 (20–50 mesh) and lyophilized to afford 6 as a hygroscopic solid (105 mg, 85%). HR-ESIMS: m/z Found 401.2156 [M+Na]+ calcd 401.2164 for C18H34O8Na. 1H NMR (CD3OD): δ 5.11 (1H, dd, J1,2eq = 1.9 Hz, J1,2ax = 5.5 Hz, H-1), 4.89 (1H, d, J1′,,2′, = 1.2 Hz, H-1′), 4.15 (1H, ddd, J2eq,3 = 5.6 Hz, J2ax,3 = 7.9 Hz, J3,4 = 8.6 Hz, H-3), 4.00 (1H, ddd, J4,5a = J4,5b = 4.6 Hz, J3,4 = 8.6 Hz, H-4), 3.95 (1H, dd, J1′,,2′, = 1.2 Hz, J2′,,3′, = 3.3 Hz, H-2′), 3.94 (1H, ddd, J4′,,5′,a = 3.3 Hz, J4′,,5′,b = 5.6 Hz, J3′,,4′, = 6.3 Hz, H-4′), 3.81 (1H, dd, J2′,,3′, = 3.3 Hz, J3′,,4′, = 6.3 Hz, H-3′), 3.78 (1H, dd, J4,5a = 4.6 Hz, J5a,5b = 11.8 Hz, H-5a), 3.73 (1H, dd, J4′,,5′,a = 3.3 Hz, J5′,a,5′,b = 11.8 Hz, H-5a′), 3.68 (1H, m, OCH2), 3.62 (1H, dd, J4′,,5′,b = 5.6 Hz, J5′,a,5′,b = 11.8 Hz, H-5b′), 3.54 (1H, dd, J4,5b = 4.6 Hz, J5a,5b = 11.8 Hz, H-5b), 3.38 (1H, m, OCH2), 2.35 (1H, ddd, J1,2ax = 5.5 Hz, J2ax,3 = 7.9 Hz, J2ax,2eq = 13.6 Hz, H-2ax), 1.82 (1H, dddd, J1,2eq = 1.9 Hz, J = 3.7 Hz, J2eq,3 = 5.6 Hz, J2ax,2eq = 13.6 Hz, H-2eq), 1.55 (2H, m, CH2), 1.32 (10H, m, 5xCH2), 0.89 (3H, m, CH3). 13C NMR (CD3OD): δ 109.68 (C-1′), 105.24 (C-1), 85.79 (C-4′), 84.89 (C-4), 83.31 (C-2′), 78.84 (C-3′), 72.82 (C-3), 68.88 (OCH2), 68.31 (C-5), 63.08 (C-5′), 42.10 (C-2), 33.00, 30.75, 30.49, 30.41, 27.31, 23.70 (6xCH2), 14.42 (CH3).
Octyl α-D-arabinofuranosyl-(1→5)-3-deoxy-α-D-arbinofuranoside (7)
To a solution of disaccharide 22 (160 mg, 0.35 mmol) in dry methanol (6 mL) was added 25% w/v NaOMe/MeOH (0.15 mL), and the reaction mixture was stirred overnight at room temperature. Concentration under vacuum and column chromatography (CHCl3–MeOH, 9:1) gave the deblocked disaccharide. For the final purification, an aqueous solution (5 mL) of the disaccharide was passed through a small column packed with Bio-Beads™ SM-4 (20–50 mesh), and the eluted fractions were lyophilized to afford 7 as a hygroscopic solid (77 mg, 75%). HR-ESIMS: m/z Found 401.2108 [M+Na]+, calcd 401.2146 for C18H34O8Na. 1H NMR (CD3OD): δ 4.92 (1H, d, J1′,,2′, = 1.2 Hz, H-1′), 4.87 (1H, s, H-1), 4.29 (1H, m, H-4), 4.08 (1H, dd, J2,3ax = 1.8 Hz, J2,3eq = 3.7 Hz, H-2), 3.96 (1H, m, H-4′), 3.97 (1H, dd, J1′,,2′, = 1.2 Hz, J2′,,3′, = 3.3 Hz, H-2′), 3.82 (1H, dd, J2′,,3′, = 3.3 Hz, J3′,,4′, = 5.8 Hz, H-3′), 3.80 (1H, dd, J4,5a = 5.3 Hz, J5a,5b = 10.5 Hz, H-5a), 3.73 (1H, dd, J4′,,5′,a = 3.4 Hz, J5′,a,5′,b = 11.8 Hz, H-5′a), 3.67 (1H, m, OCH2), 3.62 (1H, dd, J4′,,5′,b = 5.3 Hz, J5′,a,5′,b = 11.8 Hz, H-5′b), 3.55 (1H, dd, J4,5b = 6.5 Hz, J5a,5b = 10.5 Hz, H-5b), 3.37 (1H, m, OCH2), 2.34 (1H, ddd, J2,3eq = 3.7 Hz, J3eq,4 = 8.7 Hz, J3ax,3eq = 13.5 Hz, H-3eq), 1.67 (1H, ddd, J2,3ax = 1.8 Hz, J3ax,4 = 4.7 Hz, J = 13.5 Hz, H-3ax), 1.52 (2H, m, CH2), 1.30 (10H, m, 5xCH2), 0.89 (3H, m, CH3). 13C NMR (CD3OD): δ 110.09 (C-1), 109.56 (C-1′), 86.06 (C-4′), 83.08 (C-2′), 78.72 (C-3′), 78.29 (C-4), 76.11 (C-2), 70.66 (C-5), 68.21 (OCH2), 63.07 (C-5), 35.61 (C-3), 33.01, 30.70, 30.41, 27.28, 23.71 (CH2), 14.43 (CH3).
Octyl α-D-arabinofuranosyl-(1→5)-2-deoxy-2-fluoro-α- D-arbinofuranoside (8)
To a solution of disaccharide 27 (40 mg, 0.06 mmol) in dry methanol (3 mL) was added 7N NH3/MeOH (5 mL), and the reaction mixture was stirred overnight at room temperature. Concentration under vacuum and column chromatography (CHCl3–MeOH 9:1) gave disaccharide 8 as an oil (22 mg, 88%). HR-ESIMS: m/z Found 419.2051 [M+Na]+, calcd 419.2055 for C18H33FO8Na. 1H NMR (300 MHz, CD3OD): 5.06 (1H, d, 3J1,F = 12.2 Hz, H-1), 4.94 (1H, d, J1′,,2′, = 1.2 Hz, H-1′), 4.75 (1H, dd, J2,3 = 2.0 Hz, 2J2,F = 52.2 Hz, H-2), 4.14 (1H, ddd, J2,3 = 2.5 Hz, J3,4 = 6.7 Hz, 3J3,F = 26.3 Hz, H-3), 4.03 (1H, ddd, J3,4 = 6.7 Hz, J4,5b = 3.4 Hz, J4,5a = 5.9 Hz, H-4), 4.00 (1H, dd, J1′,,2′, = 3.3 Hz, J2′,,3′, = 3.3 Hz, H-2′), 3.97 (1H, ddd, J4′,,5a′, = 3.4 Hz, J4′,,5b′, = 4.9 Hz, J3′,,4′, = 6.1 Hz, H-4′), 3.86 (1H, dd, J4′,5′,a = 4.9 Hz, J5′,a5′,b = 11.3 Hz, H-5′a), 3.83 (1H, dd, J2′,,3′, = 3.3 Hz, J3′,,4′, = 6.1 Hz, H-3′), 3.75 (1H, dd, J4,5a = 3.4 Hz, J5a,5b = 11.9 Hz, H-5a), 3.70 (1H, m, OCH2), 3.67 (1H, dd, J4′,5b′= 3.4 Hz, J5a ′,5b ′= 11.3 Hz, H-5b′), 3.64 (1H, dd, J4,5b = 5.9 Hz, J5a,5b = 11.9 Hz, H-5b), 3.43 (1H, m, OCH2), 1.58 (2H, m, CH2), 1.34 (10H, m, 5xCH2), 0.90 (3H, m, CH3). 13C NMR (CD3OD): δ 109.68 (C-1′), 106.5 (d, 2JF,C-1 = 127.6 Hz, C-1), 103.37 (d, 1JF,C-2 = 181.3 Hz, C-2), 85.80 (C-4′), 83.25 (d, 3JF,C-4 = 4.9 Hz, C-4), 83.24 (C-2′), 78.84 (C-3′), 77.21 (d, 2JF,C-3 = 26.3 Hz, C-3), 68.69 (OCH2), 67.48 (C-5), 63.06 (C-5′), 33.00, 30.56, 30.46, 30.40, 27.21, 23.70 (6xCH2), 14.43 (CH3).
Biological
In vitro assay
In-vitro inhibition assays31 of the Araf disaccharides 6, 7 and 8 were performed on Mycobacterium tuberculosis (MTB H37Ra, ATCC 25177) and Mycobacterium avium (NJ 211).
Arabinosyltransferase assay32
Compounds 6, 7 and 8 at a range of concentrations from 0.1 to 6.0 mM which were stored as 100 mM ethanol stocks) and DP[14C]A [20,000 cpm, 9 mM, 10 μL (stored in chloroform/methanol, 2:1)], were dried under a stream of argon in a microcentrifuge tube (1.5 mL) and placed in a vacuum desiccator for 15 min to remove any residual solvent. The dried constituents of the assay were then resuspended in 8 μL of a 1% aqueous solution of Igepal. The remaining constituents of the AraTs assay containing 50 mM MOPS (adjusted to pH 8.0 with KOH), 5 mM β- mercaptoethanol, 10 mM MgCl2, 1 mM ATP, membranes (250 μg) were added to a final reaction volume of 80 μL. The reaction mixtures were then incubated at 37°C for 1 h. A CHCl3:CH3OH (1:1, 533 μL) solution was then added to the incubation tubes and the entire contents centrifuged at 18,000×g. The supernatant was recovered and dried under a stream of argon and re-suspended in C2H5OH:H2O (1:1, 1 mL) and loaded onto a pre-equilibrated [C2H5OH:H2O (1:1)] 1 mL Whatmann strong anion exchange (SAX) cartridge which was washed with 3 ml of ethanol. The eluate was dried and the resulting products partitioned between the two phases arising from a mixture of n-butanol (3 mL) and H2O (3 mL). The resulting organic phase was recovered following centrifugation at 3,500 × g and the aqueous phase was again extracted twice with 3 mL of n-butanol saturated water, the pooled extracts were back-washed twice with water saturated with n-butanol (3 mL). The n-butanol-saturated water fraction was dried and resuspended in 200 μL of n-butanol. The total cpm of radiolabeled material extractable into the n-butanol phase was measured by scintillation counting using 10% of the labelled material and 10 mL of EcoScintA (National Diagnostics, Atlanta). The incorporation of [14C]Araf was determined by subtracting counts present in control assays (incubation of the reaction components in the absence of the compounds). Another 10% of the labelled material was subjected to thin-layer chromatography (TLC) in CHCl3:CH3OH:NH4OH:H2O (65:25:0.5:3.6) on aluminium backed Silica Gel 60 F254 plates (E. Merck, Darmstadt, Germany). Autoradiograms were obtained by exposing TLCs to X-ray film (Kodak X-Omat) for 3 days. IC50 values for 6, 7 and 8 were evaluated by competition-based experiments performed by mixing either 6, 7 or 8 at various concentrations 0.5, 0.75, 1.0, 2.0, 4.0 and 6.0 mM with Araf α(1→5)Araf-O-C8H17 at 0.4 mM concentration followed by thin-layer chromatography/autoradiography (TLC not shown) as described earlier, and the extent of product formation was determined. The Rf on TLC of product formed by native acceptor 1a was found to be 0.33 and readily distinguishable from other product bands; the Rf values of products formed by 6, 7 and 8 were 0.40, 0.42 and 0.45 respectively.
Acknowledgments
This work was supported by NIH/NIAID grant R01AI45317. We acknowledge Dr. Kamal Tiwari for providing precursor compound 23. GSB acknowledges support in the form of a Personal Research Chair from Mr. James Bardrick, a Royal Society Wolfson Research Merit Award, as a former Lister Institute-Jenner Research Fellow, the Medical Research Council (G9901077 and G0500590) and The Welcome Trust (081569/2/06/2). The M. avium strain (NJ211) was kindly provided by Dr. Leonid Heifets, National Jewish Center for Immunology and Respiratory Diseases, Denver, CO, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) World Health Organization. http://www.who.int/mediacentre/factsheets/fs104/en/index.html.; (b) Palmer MV. Curr Top Microbiol Immunol. 2007;315:195. doi: 10.1007/978-3-540-70962-6_9. [DOI] [PubMed] [Google Scholar]; (c) Onyebujoh PC, Ribeiro I, Whalen CC. J Infect Dis. 2007;196:S35. doi: 10.1086/518657. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Churchyard GJ, Scano F, Grant AD, Chaisson RE. J Infect Dis. 2007;196:S52. doi: 10.1086/518662. [DOI] [PubMed] [Google Scholar]; (e) Clark T, Fitzgerald L. J Midwifery Womens Health. 2007;52:e33. doi: 10.1016/j.jmwh.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 2.(a) Onyebujoh PC, Ribeiro I, Whalen CC. J Infect Dis. 2007;196:S35. doi: 10.1086/518657. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Charles M, Pape JW. Curr HIV/AIDS Rep. 2006;3:139. doi: 10.1007/BF02696658. [DOI] [PubMed] [Google Scholar]; (b) Harries AD, Dye C. Ann Trop Med Parasitol. 2006;100:415. doi: 10.1179/136485906X91477. [DOI] [PubMed] [Google Scholar]; (c) Nelson M, Lipman M. Int J Clin Pract. 2006;60:976. doi: 10.1111/j.1742-1241.2006.01058.x. [DOI] [PubMed] [Google Scholar]; (d) Nahid P, Daley CL. Curr Opin Infect Dis. 2006;19:189. doi: 10.1097/01.qco.0000216631.36316.62. [DOI] [PubMed] [Google Scholar]; (e) Sharma SK, Mohan A, Kadhiravan T. Indian J Med Res. 2005;121:550. [PubMed] [Google Scholar]; (f) Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, Castro KG, Weyer K. J Infect Dis. 2007;196:S86. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 3.(a) Turenne CY, Wallace R, Jr, Behr MA. Clin Microbiol Rev. 2007;20:205. doi: 10.1128/CMR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Biet F, Boschiroli ML, Thorel MF, Guilloteau LA. Vet Res. 2005;36:411. doi: 10.1051/vetres:2005001. [DOI] [PubMed] [Google Scholar]; (c) Chatterjee D, Khoo KH. Cell Mol Life Sci. 2001;58:2018. doi: 10.1007/PL00000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Vier H, Schaberg T, Gillissen A. Pneumologie. 2007;61:606. doi: 10.1055/s-2007-980092. [DOI] [PubMed] [Google Scholar]; (b) Holtz TH, Riekstina V, Zarovska E, Laserson KF, Wells CD, Leimane V. Int J Tuberc Lung Dis. 2005;9:S258. [PubMed] [Google Scholar]; (c) CDC MMWR 20065530116557213 [Google Scholar]; (d) WHO. http://www.who.int/tb/xdr/en/index.html.
- 5.(a) Goldman RC, Plumley KV, Laughon BE. Infect Disord Drug Targets. 2007;7:73–91. doi: 10.2174/187152607781001844. [DOI] [PubMed] [Google Scholar]; (b) Kim HR, Hwang SS, Kim HJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. Clin Infect Dis. 2007;45:1290. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]; (c) Matteelli A, Migliori GB, Cirillo D, Centis R, Girard E, Raviglion M. Expert Rev Anti Infect Ther. 2007;5:857. doi: 10.1586/14787210.5.5.857. [DOI] [PubMed] [Google Scholar]; (d) Dukes Hamilton C, Sterling TR, Blumberg HM, Leonard M, McAuley J, Schlossberg D, Stout J, Huitt G. Clin Infect Dis. 2007;45:338. doi: 10.1086/519292. [DOI] [PubMed] [Google Scholar]; (e) Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martín-Casabona N, Drobniewski F, Gilpin C, Havelková M, Lepe R, Lumb R, Metchock B, Portaels F, Rodrigues MF, Rüsch-Gerdes S, Van Deun A, Vincent V, Laserson K, Wells C, Cegielski JP. Emerg Infect Dis. 2007;13:380. doi: 10.3201/eid1303.061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Kremer LS, Besra GS. Expert Opin Investig Drugs. 2002;11:1033. doi: 10.1517/13543784.11.8.1033. [DOI] [PubMed] [Google Scholar]; (b) Alderwick LJ, Birch HL, Mishra AK, Eggeling L, Besra GS. Biochem Soc Trans. 2007;35:1325. doi: 10.1042/BST0351325. [DOI] [PubMed] [Google Scholar]; (c) Brennan P, Crick DC. Curr Top Med Chem. 2007;7:475. doi: 10.2174/156802607780059763. [DOI] [PubMed] [Google Scholar]; (d) Brennan PJ. Tuberculosis. 2003;83:9. [Google Scholar]; (e) Berg S, Kaur D, Jackson M, Brennan PJ. Glycobiology. 2007;17:35R. doi: 10.1093/glycob/cwm010. [DOI] [PubMed] [Google Scholar]; (f) Lowary TL. Mini Rev Med Chem. 2003;3:689–702. doi: 10.2174/1389557033487683. [DOI] [PubMed] [Google Scholar]
- 7.(a) Blanchard JS. Annu Rev Biochem. 1996;65:215. doi: 10.1146/annurev.bi.65.070196.001243. [DOI] [PubMed] [Google Scholar]; (b) Lowary TL. Mycobacterial Cell Wall Components. In: Fraser-Reid B, Tatsuta K, Thiem J, editors. Glycoscience: Chemistry and Chemical Biology. Springer; Berlin: 2001. pp. 2005–2080. [Google Scholar]
- 8.(a) Abou-Zeid C, Voiland A, Michel G, Cocito C. Eur J Biochem. 1982;128:363. doi: 10.1111/j.1432-1033.1982.tb06973.x. [DOI] [PubMed] [Google Scholar]; (b) Daffe M, Brennan PJ, McNeil M. J Biol Chem. 1990;265:6734. [PubMed] [Google Scholar]; (c) Brennen PJ, Nikaido H. Annu Rev Biochem. 1995;64:29. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]; (d) Besra GS, Khoo KH, McNeil MR, Dell A, Morris HR, Brennan P. Biochemistry. 1995;34:4257. doi: 10.1021/bi00013a015. [DOI] [PubMed] [Google Scholar]; (e) Lee RE, Brennan PJ, Besra GS. Curr Top Microbiol Immunol. 1996;215:1. doi: 10.1007/978-3-642-80166-2_1. [DOI] [PubMed] [Google Scholar]; (f) Mikusova K, Yagi T, Stern R, McNeil MR, Besra GS, Crick DC, Brennan PJ. J Biol Chem. 2000;275:33890. doi: 10.1074/jbc.M006875200. [DOI] [PubMed] [Google Scholar]
- 9.(a) Chatterjee D. Curr Opin Chem Biol. 1997;1:579. doi: 10.1016/s1367-5931(97)80055-5. [DOI] [PubMed] [Google Scholar]; (b) Daffe M, Draper P. Adv Microb Physiol. 1998;39:131. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]; (c) Alderwick LJ, Seidel M, Sahm H, Besra GS, Eggeling L. J Biol Chem. 2006;281:15653. doi: 10.1074/jbc.M600045200. [DOI] [PubMed] [Google Scholar]; (d) Seidel M, Alderwick LJ, Birch HL, Eggeling L, Besra GS. J Biol Chem. 2007;282:14729. doi: 10.1074/jbc.M700271200. [DOI] [PubMed] [Google Scholar]
- 10.(a) Pathak AK, Besra GS, Crick D, Maddry JA, Morehouse CB, Suling WJ, Reynolds RC. Bioorg Med Chem. 1999;7:2407. doi: 10.1016/s0968-0896(99)00199-6. [DOI] [PubMed] [Google Scholar]; (b) Pathak AK, Pathak V, Seitz L, Maddry JA, Gurcha SS, Besra GS, Suling WJ, Reynolds RC. Biorg Med Chem. 2001;9:3129. doi: 10.1016/s0968-0896(01)00179-1. [DOI] [PubMed] [Google Scholar]; (c) Pathak AK, Pathak V, Suling WJ, Gurcha SS, Morehouse CB, Besra GS, Maddry JA, Reynolds RC. Bioorg Med Chem. 2002;10:923. doi: 10.1016/s0968-0896(01)00343-1. [DOI] [PubMed] [Google Scholar]; (d) Pathak AK, Pathak V, Gurcha SS, Besra GS, Reynolds RC. Bioorg Med Chem Lett. 2002;12:2749. doi: 10.1016/s0960-894x(02)00536-x. [DOI] [PubMed] [Google Scholar]; (e) Pathak AK, Pathak V, Seitz L, Gurcha SS, Besra GS, Riordan JM, Reynolds RC. Biorg Med Chem. 2007;15:5629. doi: 10.1016/j.bmc.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathak AK, Pathak V, Maddry JA, Suling WJ, Gurcha SS, Besra GS, Reynolds RC. Bioorg Med Chem. 2001;9:3145. doi: 10.1016/s0968-0896(01)00180-8. [DOI] [PubMed] [Google Scholar]
- 12.Pathak AK, Pathak V, Kulshrestha M, Kinnaird D, Suling WJ, Gurcha SS, Besra GS, Reynolds RC. Tetrahedron. 2003;59:10239. [Google Scholar]; (g) Kremer L, Dover LG, Gurcha SS, Pathak AK, Reynolds RC, Besra GS. Carbohydr Bioengineering. 2002:178. [Google Scholar]
- 13.Pathak AK, Pathak V, Riordan JM, Suling WJ, Gurcha SS, Besra GS, Reynolds RC. Bioorg Med Chem Lett. 2007;17:4527. doi: 10.1016/j.bmcl.2007.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Cociorva OM, Lowary TL. Carbohydr Res. 2004;339:853. doi: 10.1016/j.carres.2003.12.015. [DOI] [PubMed] [Google Scholar]; (b) Ayers JD, Lowary Tl, Morehouse Cb, Besra GS. Bioorg Med Chem Lett. 1998;8:437. doi: 10.1016/s0960-894x(98)00049-3. [DOI] [PubMed] [Google Scholar]
- 15.(a) Hayashi T, Iwaoka T, Takeda N, Ohki E. Chem Pharm Bull. 1978:1786. doi: 10.1248/cpb.26.1786. [DOI] [PubMed] [Google Scholar]; (b) Barton DHR, Bringmann G, Lamotte G, Motherwell WB, Hay-Motherwell RS, Porter AEA. J Chem Soc Perkin Trans 1. 1980:2657. [Google Scholar]; (c) Thiem J, Klaffke W. Synthesis of deoxy oligosaccharides. In: Thiem J, editor. Topics in Current Chemistry, 154, Carbohydrate Chemistry. Spinger-Verlag; Berlin: 1990. p. 285. [Google Scholar]; (d) He XM, Liu HW. Annu Rev Biochem. 2002;71:701. doi: 10.1146/annurev.biochem.71.110601.135339. [DOI] [PubMed] [Google Scholar]; (d) Lindhorst TK. Antitumor and Antimicrobial Glycoconjugates. In: Fraser-Reid B, Tatsuta K, Thiem J, editors. Glycoscience: Chemistry and Chemical Biology. Springer-Verlag; Berlin: 2001. p. 2393. [Google Scholar]; (e) Witczak Zbigniew J, Whistler Roy L. Carbohydr Res. 1982;110:326. [Google Scholar]; (f) Oda K, Yoshida T, Uryu T. Macromolecules. 1994;27:315. [Google Scholar]
- 16.(a) Bock K, Adelhorst K. Carbohydr Res. 1990;202:131. doi: 10.1016/0008-6215(90)84076-7. [DOI] [PubMed] [Google Scholar]; (b) Hakamata W, Nishio T, Oku T. Carbohydr Res. 2000;324:107. doi: 10.1016/s0008-6215(99)00281-5. [DOI] [PubMed] [Google Scholar]; (c) Chiocconi A, Marino C, Otal E, de Lederkremer RM. Carbohydr Res. 2000;337:2119. doi: 10.1016/s0008-6215(02)00118-0. [DOI] [PubMed] [Google Scholar]
- 17.Westerlind U, Hagback P, Tidback B, Wiik L, Blixt O, Razi N, Norberg T. Carbohydr Res. 2005;340:221. doi: 10.1016/j.carres.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Lee T, Lee YC. Carbohydr Res. 1994;251:69. doi: 10.1016/0008-6215(94)84276-0. [DOI] [PubMed] [Google Scholar]
- 19.(a) Hanessian S. Adv Carbohydr Chem. 1996;21:143. doi: 10.1016/s0096-5332(08)60317-3. [DOI] [PubMed] [Google Scholar]; (b) Marzabadi C, Franck RW. Tetrahedron. 2000;56:8385. [Google Scholar]; (c) De Lederkremer RM, Marino C. Adv Carbohydr Chem. 2008;61:143. doi: 10.1016/S0065-2318(07)61004-X. [DOI] [PubMed] [Google Scholar]
- 20.(a) Das I, Pathak T. Org Lett. 2006;8:1303. doi: 10.1021/ol053082a. [DOI] [PubMed] [Google Scholar]; (b) Ravindran B, Sakthivel K, Suresh CG, Pathak T. J Org Chem. 2000;65:2637. doi: 10.1021/jo991380d. [DOI] [PubMed] [Google Scholar]
- 21.(a) Zhou M, O’Doherty GA. Org Lett. 2006;8:4339. doi: 10.1021/ol061683b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhou M, Doherty GA. J Org Chem. 2007;72:2485. doi: 10.1021/jo062534+. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tanaka H, Yoshizawa A, Takahashi T. Angew Chem, Int Ed. 2007;46:2505. doi: 10.1002/anie.200604031. [DOI] [PubMed] [Google Scholar]
- 22.(a) Mereyala HB, Kulkarni VR, Ravi D, Sharma GVM, Rao B, Reddy GB. Tetrahedron. 1992;48:545. [Google Scholar]; (b) Timmers CM, Verheijen JC, van der Marcel GA, Van Boom JH. Synlett. 1997:851. [Google Scholar]
- 23.Hou D, Lowary TL. Org Lett. 2007;9:4487. doi: 10.1021/ol7019108. [DOI] [PubMed] [Google Scholar]
- 24.Barton DHR, Ferreria JA, Jaszberenyi JC. In: In Preparative Carbohydrate Chemistry. Hanessian S, editor. Marcel Dekker, Inc; New York: 1997. pp. 151–205. [Google Scholar]
- 25.Callam CS, Lowary TL. J Org Chem. 2001;66:8961. doi: 10.1021/jo010827r. [DOI] [PubMed] [Google Scholar]
- 26.(a) Guthrie RD, Smith SC. Chem Ind (London) 1968:547. [Google Scholar]; (b) Kam BL, Barascut JL. Carbohydr Res. 1979;69:135. [Google Scholar]
- 27.D’Souza FW, Cheshev PE, Ayers JD, Lowary TL. J Org Chem. 1998;63:9037. [Google Scholar]
- 28.(a) Shortnacy-Fowler AT, Tiwari KN, Montgomery JA, Buckheit RW, Sheela F, Secrist JA., III Helv Chim Acta. 1999;82:2240. [Google Scholar]; (b)(a) Shortnacy-Fowler AT, Tiwari KN, Montgomery JA, Secrist JA., III Nucleosides Nucleotides Nucleic Acids. 2001;20:1583. doi: 10.1081/NCN-100105249. [DOI] [PubMed] [Google Scholar]
- 29.Pathak AK, El-Kattan YA, Bansal N, Maddry JA, Reynolds RC. Tetrahedron Lett. 1998;39:1497. [Google Scholar]
- 30.Mizutani K, Kasai R, Nakamura M, Tanaka O, Matsuura H. Carbohydr Res. 1989;185:27–38. [Google Scholar]
- 31.Suling WJ, Reynolds RC, Barrow EW, Wilson LN, Piper JR, Barrow WW. J Antimicrob Chemother. 1998;42:811. doi: 10.1093/jac/42.6.811. [DOI] [PubMed] [Google Scholar]
- 32.Lee RE, Brennan PJ, Besra GS. Glycobiology. 1997;7:1121. doi: 10.1093/glycob/7.8.1121. [DOI] [PubMed] [Google Scholar]