Abstract
Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist developed as a smoking cessation aid, showed antidepressant-like activity in the forced swim test in two mouse strains. In addition, a low varenicline dose significantly enhanced the effects of moderately active doses of the selective serotonin reuptake inhibitor sertraline. These findings are consistent with the notion that reducing α4β2 nicotinic acetylcholine receptor activity either by antagonists or by partial agonists that can partially activate or desensitize acetylcholine receptors is associated with antidepressant-like properties. These data suggest that varenicline may have antidepressant potential and can augment antidepressant responses of selective serotonin reuptake inhibitors when combined.
Keywords: Forced Swim Test, Varenicline, Nicotinic Acetylcholine Receptor, Partial Agonist Antidepressant
1. Introduction
Neuronal nicotinic acetylcholine receptor antagonists and partial agonists may have utility in the treatment of depression, either alone or combined with antidepressants (Shytle et al, 2003; Picciotto et al, 2008). Preclinical studies in the forced swim test and the tail suspension test, established mouse models of antidepressant efficacy, have demonstrated antidepressant-like activity of the non-selective nicotinic acetylcholine receptor antagonist mecamylamine and the selective α4β2 nicotinic acetylcholine receptor partial agonists, cytisine (Caldarone et al., 2004; Rabenstein et al., 2006; Mineur et al., 2007) and ispronicline (Gatto et al., 2004). A recent study confirmed the antidepressant-like properties of mecamylamine, but found that a full agonist of the α4β2 subtype and an agonist or antagonist of the α7 subtype lacked efficacy in the forced swim test and the tail suspension test (Andreasen et al., 2008). Recent clinical studies (Dunbar et al, 2007; George et al., 2008) have demonstrated that mecamylamine has an antidepressant-enhancing effect upon co-administration with selective serotonin reuptake inhibitors (SSRIs). Taken together, these data suggest that selectively reducing the activity of α4β2 nicotinic acetylcholine receptors by the action of either antagonists or partial agonists is associated with antidepressant effects. Partial agonists can modulate ACh signaling not only via their decreased efficacy at the receptor, but also via inactivating nicotinic acetylcholine receptors by desensitization (Hogg and Bertrand, 2007; Picciotto et al., 2008).
This study used the mouse forced swim test to investigate the antidepressant potential of the selective α4β2 nicotinic acetylcholine receptor partial agonist varenicline (Coe et al., 2005), an efficacious smoking cessation aid (Cahill et al., 2007). Varenicline has at least 100-fold higher affinity for α4β2 than for other subtypes, is a 45% agonist as compared with nicotine and can potently desensitize α4β2 nicotinic acetylcholine receptors with an IC50 value of 6 nM for inhibiting nicotine-evoked currents (Rollema et al., 2007). The antidepressant-like effects of varenicline were assessed in two independent laboratories in two different mouse strains, C57BL/6J and CD-1, with each lab using a slightly different modification of the forced swim test. In CD-1 mice, varenicline’s antidepressant-like effects were also compared with the effects of two classical antidepressants, the SSRI sertraline and the tricyclic antidepressant amitriptyline, the latter of which is known to produce a consistent and robust response in the forced swim test (e.g. Caldarone et al., 2004). Finally, varenicline was also tested in CD-1 mice after co-administration with sertraline, to examine possible synergistic effects of combining this nicotinic acetylcholine receptor partial agonist with a classical antidepressant. Enhancement of antidepressant-like activity in the forced swim test by co-administration of mecamylamine and amitriptyline was previously described (Caldarone et al., 2004) and this augmentation strategy has been clinically demonstrated by adding mecamylamine to antidepressant treatments (Dunbar et al., 2007; George et al., 2008).
2. Materials and Methods
Forced swim test experiments were performed in C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) at Yale University and in CD-1 mice (Charles River Laboratories, Wilmington, MA, USA) at Pfizer Inc. In both sets of experiments male mice were housed under standard conditions (temperature 21 ± 2 °C, 12:12 h light/dark cycle, lights on at 07:00 h, food and water ad lib) for at least 2 weeks and experiments were performed when mice were 14–16 weeks old and weighed 30–35 grams. Varenicline tartrate (Pfizer, Groton, CT, USA) was dissolved in water and administered i.p., sertraline HCl (Pfizer, Groton, CT, USA) was dissolved in 1% acetic acid and administered s.c., and amitriptyline HCl (Sigma, St. Louis, MO, USA) was dissolved in water and administered s.c. All test compounds were administered to C57BL/6J and CD-1 mice at a volume of 10 ml/kg with pretreatment times of 30–60 min, based on the half life of varenicline in mice of 1.4 h (Obach et al., 2006). C57BL/6J mice were placed in beakers filled with 15 cm deep water (~25 °C), 30 min after drug or vehicle administration and the time spent immobile was continuously recorded over a 15 min period (Mineur et al., 2007). Data were expressed as the mean (± S.E.M.) time in seconds spent immobile (n = 10 for each treatment) and were analyzed by ANOVA with Treatment as the main factor, using Statview 5 (SAS institute, Cary, NC, USA). For the forced swim test in CD-1 mice, animals were placed in beakers filled with 10 cm deep water (23–25 °C), 60 min after dosing with test compound or vehicle, and behavior was scored every 30 seconds over a 5 minute period as either immobile (score = 1), or active (score = 0), giving a total score between 0 (fully active for 5 min) and 10 (continuously immobile for 5 min). Data for CD-1 mice were expressed as the mean swim score ± S.E.M. (n = 10–30 for each treatment) and analyzed with the non-parametric Kruskal–Wallis test followed by Bonferroni-corrected Mann-Whitney U tests. Since drug-induced changes in locomotor activity can confound the interpretation of forced swim test data, the test compounds were also tested for effects on spontaneous locomotor activity in locomotor activity chambers. All animal procedures were approved by the Institutional Animal Care and Use Committees at Yale University and Pfizer Global Research and Development.
3. Results
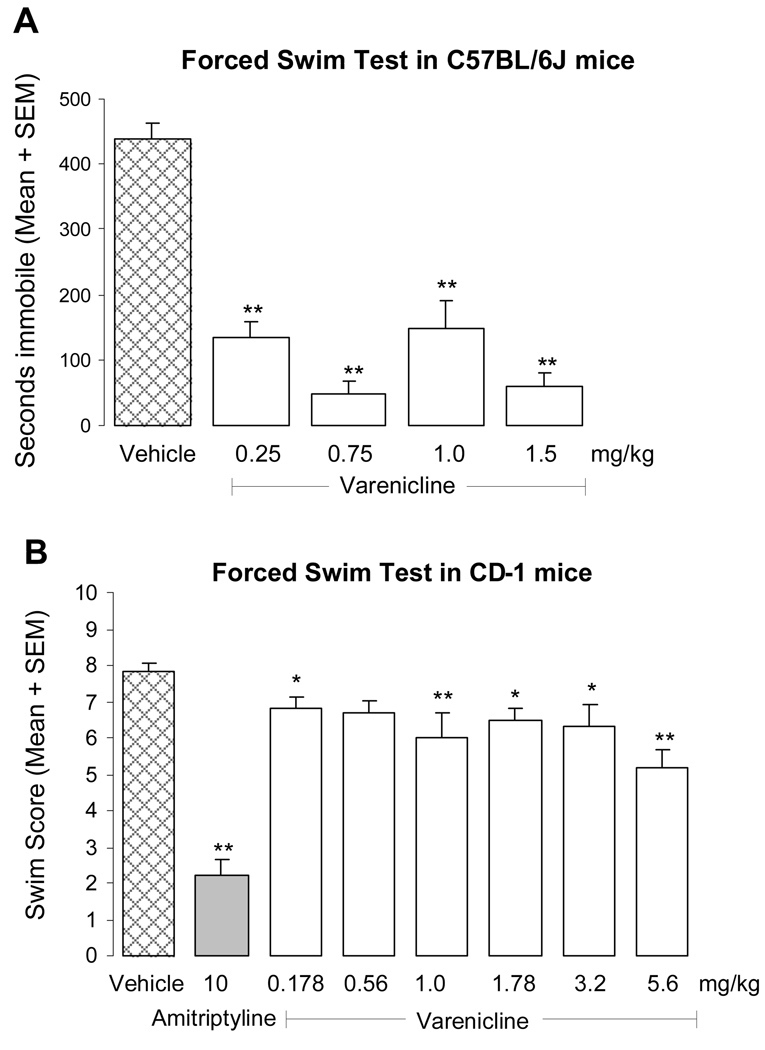
Varenicline significantly reduced immobility in both mouse strains at most doses tested (Fig. 1). The effect size in C57BL/6J mice (0.25–1.5 mg/kg i.p., Fig. 1A) was greater than in CD-1 mice (0.178 – 5.6 mg/kg i.p., Fig. 1B), with the response in the latter strain being comparable to that of sertraline in terms of effect size and dose-response relationship (sertraline data not shown). Strain differences could account for variations in the degree of efficacy in these forced swim test studies and are well-documented for several mouse behavioral responses (e.g. Mineur et al., 2006). In addition, differences in monitoring times and scoring methods could also contribute to the observed effect sizes in each method. The fact that low doses of varenicline (i.e. 0.25 mg/kg in C57BL/6J mice and 0.178 mg/kg in CD-1 mice) have significant effects in both experiments is consistent with varenicline’s high in vitro binding affinity (Ki = 0.15 nM) and inactivating potency (IC50 = 6 nM) at α4β2 nicotinic acetylcholine receptors, as well as with its potent activity in in vivo models, such as increasing mesolimbic dopamine release with an ED50 of 0.03 mg/kg after oral administration (Rollema et al., 2007). Since inactive doses were not obtained in either of the studies and a clear dose-response relationship was not demonstrated, further studies will be necessary to define the responses at the low end of the dose range. A lack of dose-dependency of the forced swim test effects may suggest that once a dose of the partial agonist is administered that is sufficient to reduce endogenous ACh signaling or that achieves maximum receptor occupancy, the response will remain the same over a wide dose range, resulting in a plateauing of the effect. The forced swim test data for varenicline generated in each laboratory in different mouse strains are thus in good agreement, despite variations in the protocols. Finally, at the doses used for the forced swim test, none of the test compounds increased locomotor activity, indicating that the results are not affected by stimulant effects.
Fig. 1.
Effects of varenicline and amitriptyline in the mouse forced swim test. (A): Effects of vehicle and varenicline (0.25–1.5 mg/kg i.p.) in C57BL/6J mice. Immobility time was measured in seconds for 15 min and expressed as mean ± S.E.M. (n=10, ** p<0.01 vs vehicle).
(B): Effects of vehicle, amitriptyline (10 mg/kg) and varenicline (0.178–5.6 mg/kg) in CD-1 mice. Immobility (score 1) and activity (score 0) were recorded every 30 sec for 5 min and expressed as mean swim score ± S.E.M. (n=10–30, * p<0.05, ** p<0.01 vs vehicle).
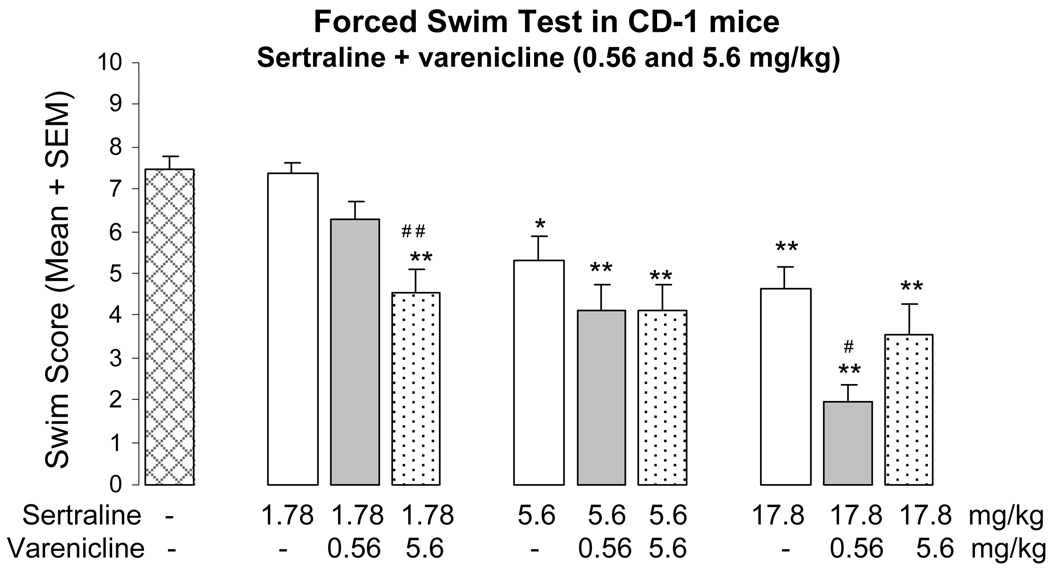
A comparison with the effects of the classical antidepressants amitriptyline and sertraline in CD-1 mice shows that varenicline administration results in comparable swim scores as the SSRI sertraline at doses of 5.6 mg/kg and above (Fig. 2). At 10 mg/kg, the tricyclic antidepressant amitriptyline, which consistently yields pronounced dose-dependency and near maximal efficacy in dose-response studies (data not shown; see e.g. Caldarone et al, 2004), reduced immobility scores with greater efficacy than varenicline or sertraline when given alone (Fig. 1B). Co-administration of varenicline and sertraline resulted in lower swim scores than after each sertraline dose alone. Combining 0.56 and 5.6 mg/kg of varenicline with the lowest (1.78 mg/kg) and highest (17.8 mg/kg) sertraline dose, respectively, reduced the swim score significantly more than the corresponding sertraline doses alone. Of particular interest is the finding that co-administration of the lowest varenicline dose of 0.56 mg/kg with 17.8 mg/kg sertraline had the same pronounced effect on the swim score as 10 mg/kg amitriptyline (Fig. 1B, Fig. 2).
Fig. 2.
Effects of vehicle, sertraline alone (1.78, 5.6 and 17.8 mg/kg i.p.) and of co-administration of each dose of sertraline with varenicline (0.56 and 5.6 mg/kg s.c.) in the forced swim test in CD-1 mice. Immobility (score 1) and activity (score 0) were recorded every 30 sec for 5 min and expressed as mean swim score ± S.E.M. (n=10–30). * p<0.05 vs vehicle, ** p<0.01 vs vehicle, # p<0.05, # # p<0.01 vs corresponding dose of sertraline alone (1.78 and 17.8 mg/kg)
4. Conclusion
The present results are in agreement with previous reports that both nicotinic acetylcholine receptor antagonists and partial agonists show antidepressant-like efficacy in animal depression models and can also increase antidepressant activity when co-administered with classic antidepressants such as SSRIs or tricyclic antidepressants (Caldarone et al., 2004; Rabenstein et al., 2006; Mineur et al., 2007; Andreasen et al., 2008; Dunbar et al., 2007; George et al., 2008). Reduced ACh signaling via α4β2 nicotinic acetylcholine receptors resulting in antidepressant-like activity can thus be achieved with antagonists by blocking α4β2 nicotinic acetylcholine receptors, and with low doses of partial agonists by attenuating α4β2 nicotinic acetylcholine receptor function, either by their decreased efficacy at nicotinic acetylcholine receptors or by inactivation of nicotinic acetylcholine receptors via desensitization (Hogg and Bertrand, 2005; Picciotto et al., 2008). With regard to the studies on the effect of co-administration of an SSRI with a partial agonist, it is noteworthy that the combination of the SRRI sertraline with the lowest test dose of the potent and selective α4β2 nicotinic acetylcholine receptor partial agonist varenicline produced maximal enhancement of antidepressant-like activity, resulting in an amitriptyline-like reduction of the swim score. This is consistent with the notion that further decreased ACh signaling, in addition to the small effect that SSRIs have on AChR activity via their weak nicotinic acetylcholine receptor antagonist properties (Shytle et al, 2002), can significantly enhance the antidepressant effects of SSRIs.
This study provides evidence that varenicline, the first nicotinic acetylcholine receptor ligand approved for pharmacotherapeutic use (as Chantix or Champix), has activity in an animal model that has good predictive validity for antidepressant activity.
These preclinical data therefore suggest that varenicline may have potential as an antidepressant, either alone or, given its ability to amplify the SSRI response, as an augmentation strategy for depression in combination with classical antidepressants. Future clinical trials will be needed to confirm these preclinical data and to assess whether the predictive validity of the forced swim test as an animal model of antidepressant response extends beyond monoamine-based classical antidepressants.
Acknowledgements
Supported by a collaborative grant from Pfizer and by NIH grants MH077681 and DA00436 to MRP.
References
- Andreasen JT, Olsen GM, Wiborg O, Redrobe JP. Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J. Psychopharmacology. 2008 doi: 10.1177/0269881108091587. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev. 2007;1:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol. Psychiatry. 2004;56:657–664. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, DW Schulz DW, Tingley FD, O’Neill BT. Varenicline: an α4β2 nicotinic receptor partial agonist for smoking cessation. J. Med. Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Dunbar GC, Kuchibhatla R, Krishnan R. Mecamylamine for the treatment for depressed patients who were poor responders to citalopram first line therapy; a double blind placebo controlled study; 46th Ann. ACNP meeting; Dec 9–13, 2007; Boca Raton, FL. 2007. [Google Scholar]
- Gatto GJ, Bohme GA, Caldwell WS, Letchworth SR, Traina1 VM, Obinu MC, Laville M, Reibaud M, Pradier L, Dunbar G, Bencherif M. TC-1734: An Orally Active Neuronal Nicotinic Acetylcholine Receptor Modulator with Antidepressant, Neuroprotective and Long-Lasting Cognitive Effects. CNS Drug Reviews. 2004;10:147–166. doi: 10.1111/j.1527-3458.2004.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: A preliminary study. J. Clin. Psychopharmacol. 2008;28:340–344. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Bertrand D. Partial agonists as therapeutic agents at neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 2007;73:459–468. doi: 10.1016/j.bcp.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52:1256–1262. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O’Connell TN, Zandi KS, Miller S, Coe JW. Metabolism and disposition of varenicline, a selective α4β2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab. Dispos. 2006;34:121–130. doi: 10.1124/dmd.105.006767. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, DH. Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Progress in Neurobiology. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not α2- or α7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology. 2006;189:395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, III, Willams KE. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7:525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]