Abstract
Human conception, indeed fertilization in general, takes place in a fluid, but what role does fluid dynamics have during the subsequent development of an organism? It is becoming increasingly clear that the number of genes in the genome of a typical organism is not sufficient to specify the minutiae of all features of its ontogeny. Instead, genetics often acts as a choreographer, guiding development but leaving some aspects to be controlled by physical and chemical means. Fluids are ubiquitous in biological systems, so it is not surprising that fluid dynamics should play an important role in the physical and chemical processes shaping ontogeny. However, only in a few cases have the strands been teased apart to see exactly how fluid forces operate to guide development. Here, we review instances in which the hand of fluid dynamics in developmental biology is acknowledged, both in human development and within a wider biological context, together with some in which fluid dynamics is notable but whose workings have yet to be understood, and we provide a fluid dynamicist’s perspective on possible avenues for future research.
This review is about fluid dynamics, that is to say fluid flow. All biology involves fluids; biochemistry takes place in aqueous solution, and the human body, for example, is more than half water. However, many biological processes occur in static fluids within which some substance diffuses, and the fluid merely provides the medium in which this may take place. Our interest here, on the other hand, is in instances in which biological development utilizes, or is affected by, the movement of fluid. This is so in the process of fertilization that initiates development, whose fluid dynamics has been investigated in depth with regard to human reproduction (Fauci and Dillon, 2006). But what of later on in development? Fluid dynamics—and we must interject that it should not be forgotten that both liquids and gases are fluids—has an enormous number of biological applications and is fundamental on many levels from cells to ecosystems; there is a huge literature on the interaction of organisms with their fluid environment. Hitherto, however, rather less attention has been paid to the role of fluid dynamics during the development of an organism.
While this role can often be subsumed under the heading of morphogenesis, we may classify examples into various categories according to their ultimate effect on the organism. In the first instance, there is the role of fluid dynamics in the growth of a single cell through the movement of fluid within a cell termed cytoplasmic streaming, or cyclosis. Fluid dynamics also has a hand in the formation of the body plan itself. This is laid down at the earliest stages of development, and fluid dynamics is fundamental in various forms in different organisms, both within the cell after fertilization when in some organisms flow in the cytoplasm is determinant in setting the anteroposterior or dorsoventral axes, and at a later stage when in many vertebrates a temporary structure, the node, is formed that uses fluid flow to set the left-right axis. The node may be considered an organ with a transient existence in the embryo. The next category into which we may sort examples of fluid dynamics is the development of organs that will go on to carry fluid in the adult. Here, we find the organogenesis of the heart, kidneys, brain, lungs, and so on. There is also the development of structures that will not carry fluid when complete, but utilize fluid flow in their construction. Here, we encounter a host of interesting cases, of the self-assembly of supramolecular structures such as fibrous composites and biominerals that have recourse to fluid dynamics during their assembly, and also of fluid flow used to remove an existing structure and then to rebuild, as in molting in arthropods and nematodes. Last, we may consider a further way in which fluid flow may influence development. The development of an organism may be altered by its external fluid environment. An organism may create an external flow which affects its development, or it may react to external flow which also alters its development. In the former category there are flows about embryonic mollusks, amphibians, and fish within their eggs, and about other larval fish once hatched; in the latter there is the effect of air or water velocity—of wind or current—during the development of organisms like plants, sponges, and corals.
Wherever we look in detail at developmental processes in which fluid is present we are beginning to find that fluid dynamics shapes ontogeny. In this review we present examples of developmental fluid dynamics and attempt from our perspective as fluid dynamicists to predict what is to come in this field of interdisciplinary research.
PHYSICAL PROCESSES AND DRIVING MECHANISMS
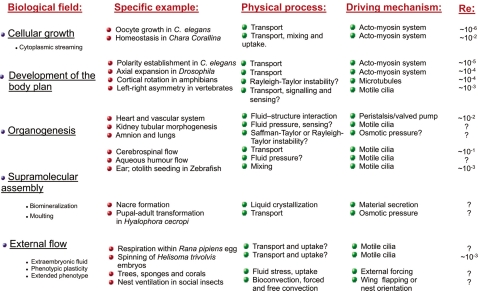
Fluid dynamics is involved in several different physical processes in development (see Table 1). A first is that fluid pressure is used to enlarge tubes and maintain them at a given size. Examples are the lung, heart, kidneys, etc.; we may note the related use of fluid pumped into developing structures to unroll and stiffen them in plants—turgor pressure—insects and cnidarians—the hydrostatic skeleton—and elsewhere. Next, there is the role of transporting material with the fluid, in the brain, the node, and so on. Then, there is the related idea of mixing material; the ear is an example. Liquid crystallization, involved in the supramolecular assembly of tissues such as molluskan nacre, involves a combination of transport and mixing to take material to the site and ensure it is in a state to crystallize.
Table 1.
Fluid dynamics in developmental biology.
 |
|---|
If we look at how fluid flow is induced in these processes, we find that in many instances motile cilia are employed to drive fluid motion. This is the case, for example, in the developing kidney, ear, and brain. However, cilia are not the only mechanism for moving fluids; the heart is the obvious example of a different approach: it initially uses peristalsis and subsequently develops into a chambered valved pump. The actomyosin system is another ubiquitous mechanism for the generation of fluid motion within the cell. The physical mechanism of cyclosis based on actomyosin is varied. In Chara what moves the fluid is drag produced by particles—small vesicles—transported by actin tracks, while in C. elegans, it seems to be the result of the contraction of the cellular cortex.
Osmotic pressure is a further general physical mechanism for setting fluids in motion, to which recent work on the aquaporin gene family which encodes proteins that function as membrane channels—the channels through which liquids are secreted and adsorbed by cells (Liu and Wintour, 2005)—provides a useful counterpoint. Buoyancy forces can also induce flow, as in cortical rotation in amphibians, and lead to the appearance of flow instabilities such as the Rayleigh–Taylor instability, where a heavier fluid underlies a lighter one, appearing in developmental biology. A further flow instability that may have implications in development is the Saffman–Taylor instability, in which a less viscous fluid pushes a more viscous one, and in doing so the front between the two fluids puckers into so-called viscous fingers; this may appear in the lung, as well as in structures found in fossil ammonoids.
The first quantity to calculate when one begins to investigate a fluid flow phenomenon is the Reynolds number. This dimensionless number measures the relative importance of viscous and inertial forces in the flow, and is important because flows with the same Reynolds number show the same types of behavior.
To calculate it one needs to estimate three quantities: a characteristic length scale L and velocity U, and the kinematic viscosity of the fluid, ν=μ/ρ, its viscosity divided by its density. The Reynolds number is then
We have been able to estimate the Reynolds number of a few of the flows we discuss here from data in the literature (see Table 1). In general, we can see that developmental flows have Reynolds number less than 1, which implies viscosity dominates inertia. The world of flow at Re<1, called creeping flow, is one in which we as humans have little direct experience, making it dangerous to attempt to apply intuition learnt in “our” world. It is the world of life at low Reynolds number (Purcell, 1977), in which turbulence does not exist, and flow ceases the moment driving stops, within which development generally occurs.
FLUID FLOW DURING CELLULAR GROWTH: CYTOPLASMIC STREAMING
When looking at the role fluid dynamics plays in shaping an organism, an obvious place to start should be the minimal constituent and building block of all living organisms: a single cell. Although cells differ greatly in their size, structure and function, all share a common feature, namely, a large proportion of their volume is a fluid: cytoplasm. Cytoplasmic streaming, also termed cyclosis, is a persistent circulation of the fluid contents of cells that occurs in organisms as diverse as amoebae, protozoa, and fungi. The fluid flow can take on many intriguing geometries, from fountain streaming, in which the motion near the central axis of the cell is opposite to that near the periphery, to spiral rotational streaming, with counter-propagating currents arranged like the colors on a barber pole. It is now well established that cytoplasmic streaming is crucial in some cases of cell locomotion; for instance in amoebae. However, only in few instances has its role during development and cellular growth been clearly established or understood.
Cytoplasmic streaming is involved during oocyte growth in Caenorhabditis elegans. The large single-cell oocyte, when fully grown, presents already the amount of messenger ribonucleic acid (mRNA) and protein it will need during embryogenesis to generate most of the cells of the adult organism. It has been shown that most of the oocyte materials originate in an anucleate region shared by all developing oocytes and named the gonad core (Wolke et al., 2007). The material originated there is then actively transported by actomyosin dependent cytoplasmic streaming as depicted in Fig. 1(i). However, the mechanism by which the streaming is generated is still unclear, the latest experimental results suggest that the cytoplasm is pulled into oocytes by forces generated very close to the enlarging oocytes at a rate of about 0.1 μm s−1, giving a Reynolds number of order 10−6.
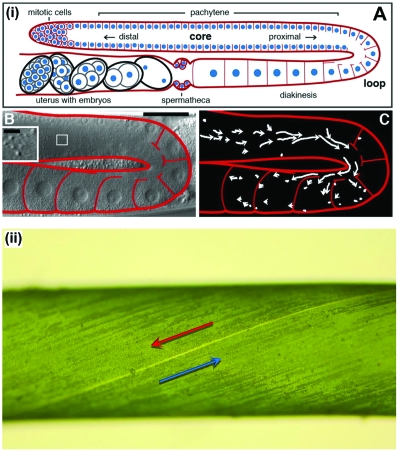
Figure 1. Role of cytoplasmic streaming in cellular growth.
(i) Proximal streaming in the wild-type C. elegans gonad and its role in oocyte growth. (A) Diagram of one arm of an adult hermaphrodite gonad. Plasma membranes, red; nuclei, blue. Somatic sheath cells enclose most of the gonad, but are not shown for simplicity. (B) Single image from time-lapse movie. DIC particles are shown at high magnification in inset. (C) 2-min particle tracks. (Wolke et al., 2007). (ii) Spiral rotational streaming in Chara corallina internodal cell. Regions of opposite flow direction are arranged like the colors on a barber pole, separated by two spiraling lines of missing chloroplasts termed indifferent zones. (i) is reproduced with the permission of the Company of Biologists.
Another instance in which cytoplasmic streaming is involved in cellular growth is in the green plants. While the fluid velocities found in streaming in the previous example are at most a few micrometers per minute, in larger cells cytoplasmic streaming can reach a fraction of a millimeter per second. For instance, in the alga Chara corallina, which historically has been among the organisms of choice for studies of streaming, fluid flows at speeds up to 100 μm s−1 in cells several centimeters long (Kamiya and Kuroda, 1956), giving a Reynolds number of order 10−2. While this is slow on a human scale, it greatly outpaces molecular diffusion on the scales of interest for single cells. For this reason, it has long been conjectured that streaming plays an important role in cell metabolism, helping to transport and mix the cell contents in a manner that enhances fitness. But how exactly does streaming effect metabolic rates, such as photosynthetic activity, and growth? Recent work in this direction has shown that internal mixing and the transient dynamical response to changing external conditions can indeed be enhanced by the spiral rotational streaming found in Chara (Goldstein et al., 2008); see Fig. 1(ii). The possibility that this may have developmental consequences is made clear by the coincidence of the exponential growth phase of these algae and the point of maximum enhancement of those processes.
FLUID FLOW IN THE DEVELOPMENT OF THE BODY PLAN
One of the most primitive developmental processes, yet one of the most evident morphologically, is the establishment of the axes that determine an organism’s body plan. In vertebrates, all three axes—dorsoventral, anteroposterior, and left-right—are laid down during the early stages of embryonic life by robust mechanisms involving external inputs and complex internal cascades of genetic expressions. The intrinsic chirality of the resulting three-dimensional structure is a beautiful and intriguing example of complete symmetry breaking. The external inputs to the genetic program are in some cases fluid flows.
Polarity establishment in the nematode Caenorhabditis elegans is controlled during oogenesis by cytoplasmic streaming. Once the oocyte is formed, but prior to the first cellular division, cytoplasmic streaming proves essential for the correct establishment and maintenance of the cell anterior-posterior polarity. An active cortical contraction initiated near the point of fertilization generates fountain streaming consisting of a cortical motion flowing towards the anterior of the cell accompanied with a central cytoplasmic flow directed towards the posterior end [Fig. 2(i)] with a Reynolds number of order 10−5. The established flow acts to distribute asymmetrically PAR proteins, key components in the establishment of cell polarity (Hird, 1993; Munro et al., 2004). Similar fountain streaming is also known to produce nuclear migration along the anterior-posterior axis—a motion termed axial expansion—in the early syncytial embryo of the fruit fly Drosophila (von Dassow and Schubiger, 1994), where the Reynolds number of order 10−4.
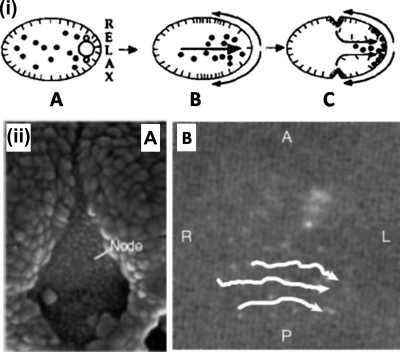
Figure 2. Fluid flow in the development of the body plan.
(i) Polarity establishment in C. elegans. Anterior is at the left. Some component of the asters of the sperm pronucleus induces relaxation of cortical tension at the posterior end of the egg (A). This induces a movement of contractile elements from this end [curved arrows in (B)], and a simultaneous flow of cytoplasm towards this [straight arrow in (B)]. (C) P granules (black circles) are segregated to the posterior end of the egg by the cytoplasmic flow. (ii) Left-right development in vertebrates. Node of the mouse embryo (a) and leftward transport of microbeads by the cilia induced nodal flow (b). (i) © Hird and White, 1993. Originally published in (Hird, 1993). (ii) (A) (from Vogan and Tabin, 1999) and (B) (from Nonaka et al., 2002) are reprinted by permission of Macmillan Publishers, Ltd.
In amphibians, an interesting observation is that when a fertilized egg is inverted before the first cell division, the embryo either develops with the pigmentation of dorsal and ventral skin reversed, or it does not develop past gastrulation at all (Chung and Malacinski, 1982, 1983; Wakahara et al., 1984; Neff et al., 1984). This has led to a series of experiments on this zygote being undertaken in the microgravity of space, whereon the embryos produced normal larvae (Neff et al., 1986; Black, 1996). Moreover, there is abundant experimental evidence that links the alignment of cortical microtubules—those just below the cell membrane—with the spontaneous cortical rotation that happens before the first cell division (Elinson and Rowning, 1988; Gerhart et al., 1989). This cortical rotation is known to correlate precisely with the later developing dorsoventral and left-right axes of the embryo (Elinson and Holowacz, 1995; Chang et al., 1999). Some experiments also show that forced cortical rotation—rotating the cortex using an external apparatus—works as well in dorsal-ventral axis determination (Phillips et al., 1996). All these observations sustain the idea that a physical mechanism is operating at this stage of development. The Reynolds number of the flow is of order 10−4. An interesting hypothesis is that the denser yolk in one hemisphere of the cell and the lighter cytoplasm in the other suffer a Rayleigh–Taylor instability (Faber, 1995)—the heavier fluid overlies the lighter one—on inversion of the cell. Asymmetric sloshing of the yolk causes the cortical microtubules to align in one direction and its effects are thus similar to that of a normal cortical rotation, while axisymmetric flow, on the other hand, prevents normal pattern formation for microtubules and stops the normal development of the embryo as if the cortical rotation had not occurred (Nouri et al., 2008). In all these instances, therefore, cytoplasmic streaming is fundamental for the correct development of the organism.
Nodal flow: left-right development
In the early 1990s, Brown and Wolpert (1990) proposed a conceptual model for the de novo generation of left-right asymmetry in mammalian embryos in which the initial break in symmetry involved a chiral molecule. But it seems that nature, in mice at least, prefers to use not a chiral molecule, but another chiral structure—a molecular motor—to elicit advective transport in a well-defined direction and thence to trigger lateralization. The realization that, in many vertebrates, a transient fluid dynamical phenomenon is the trigger for the development of lateralization has been a vital step forward in our understanding of morphogenesis.
In a series of elegant experimental observations in embryonic mice, work by Nonaka et al. (1998, 2002) was the first to establish categorically the existence of a link between fluid movement and lateralization in vertebrates. First, they reported the existence of a directional flow of extraembryonic fluid from the right to the left of the ventral node of wild-type animals and its absence in iv mutants which present lateralization defects. Second, this so-called nodal flow was identified, at least in mice, as preceding all known left-right asymmetric gene expression. Finally, they showed that an exogenous reversal of the fluid flow direction leads to a complete reversal of the morphological asymmetry—situs inversus—in developing embryos. The detailed understanding of this fluid dynamical link has been the subject of extensive research in the last few years. It is now well established that a distinct type of motile 9+0 monocilium is responsible for setting the nodal fluid in motion. The node floor is lined with tens of such cilia, all of which rotate clockwise when seen from the ventral side. The way by which this rotation drives a leftward nodal flow—whose Reynolds number is of order 10−3—can be explained based solely on simple abstract symmetry arguments and fundamental hydrodynamics. The only chirally symmetric way to produce a chirally asymmetric flow is to use the tridimensional and pseudovectorial character of rotations. This leads to the conclusion that the clockwise-rotating cilia should be tilted towards the posterior end of the node. When the direction of the cilia rotation is coupled by means of this tilt with the previously established anteroposterior and dorsoventral axes of the embryo, a uniquely determined handedness emerges (Cartwright et al., 2004).
The subsequent steps in the process are still obscure. The information on the symmetry broken by the nodal flow must be transmitted to the rest of the embryo, but the specific mechanisms involved are unclear. The structure of the nodal flow gives support to a possible robust mechanism of gradient formation for a morphogen concentration: a signaling molecule—probably a protein—is released into the flow, distributed within the node by the combination of three processes—advection or the hydrodynamical transport of a given substance, molecular, or another type of diffusion, and a chemical deactivation reaction—and its concentration is detected by chemoreceptors within the node. If the balance between these three elements is correct, a gradient in the morphogen concentration can build up in the appropriate direction to trigger the differentiation between the left and the right sides of the developing embryo. Moreover, alterations of this balance originated in the debilitation of the right-left flow outside of a given range can be shown to produce the opposite gradient (Cartwright et al., 2004), which may explain the origin of the large proportions of situs inversus in some genetically originated situations, i.e., that of the inv mutant (Okada et al., 1999). The most recent experimental evidence shows that the morphogen is released in a discrete fashion: morphogen parcels wrapped in a lipid vesicle known as nodal vesicular parcels, are transported by the nodal flow, and break in proximity with the cilia and the walls of the node to deliver there the morphogens they contain (Tanaka et al., 2005). This process can be modeled by a variant of the morphogen model (Cartwright et al., 2007, 2008).
FLUID FLOW IN ORGANOGENESIS: DEVELOPMENT OF ORGANS THAT WILL CONTAIN FLUIDS IN THE ADULT
Organisms utilize a multiplicity of fluids that are fundamental to different physiological systems. In adult humans, for example, there is found in one or both sexes air, amniotic fluid, aqueous humour, bile, blood, cerebrospinal fluid, cerumen, Cowper’s fluid, chyle, chyme, endolymph, female ejaculate, flatus, interstitial fluid, intracellular fluid, lymph, menses, breast milk, mucus, pancreatic juice, perilymph, pleural fluid, saliva, sebum, semen, sweat, synovial fluid, tears, urine, and vaginal fluid; and this list is likely not complete. Tubes transporting fluid and containers in which to store it are a vital part of the organs making up these systems, and in many cases we are beginning to understand that their morphogenesis is controlled by the fluid flow itself (Lubarsky and Krasnow, 2003). Without fluid dynamics, that is to say without the movement of fluid, organs such as the heart [Figs. 3I, 3J], the brain 3C, 3D, the ear 3E, 3F, the eyes 3A, 3B, the kidneys 3G, 3H, and the lungs 3K, 3L, do not develop correctly; fluid flow is an intrinsic part of the development of these systems and shapes their growth.
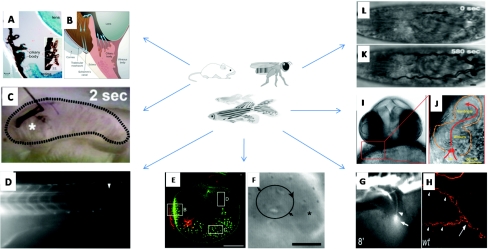
Figure 3. Fluid flow implicated in organogenesis in mouse (A)–(C), zebrafish (D)–(J) and fruit fly (K)–(L) embryos.
(A)–(B) Flow of aqueous humour: first produced in the ciliary body of the eye, it circulates throughout the anterior part of the eye, and then exits primarily through the trabecular meshwork (Calera et al., 2006; Alward, 2003). (C) Flow of exogenously microinjected India ink in the mouse brain showing the direction of the cerebrospinal flow and the parallel neuroblast migration (Sawamoto et al., 2006). (D) Fluid flow in the central canal of the spinal cord in a zebrafish embryo (Kramer-Zucker et al., 2005). (E)–(F) Beating cilia and kinocilia in zebrafish ear during otolith seeding (Hammond and Whitfield, 2006; Riley et al., 1997). (G)–(H) Cilia in the pronephric ducts (arrows indicate the point were the pronephric ducts merge at the cloaca) and the associated flow through the pronephric kidney observed by injected dye into the circulation (Kramer-Zucker et al., 2005). (I)–(J) Valveless atrio-ventricular junction in the embryonic zebrafish heart indicating mean flow direction (red arrows) and streak-like imprints left by moving blood cells (Hove et al., 2003). (K)–(L) Liquid clearance and gas filling of a Drosophila embryo. The initiation of the gas filling (first gas bubble) and its completion, when all tracheal branches are gas filled, are shown (Tsarouhas et al., 2007). (A), (D), (E), (G) and (H) are reproduced with the permission of the Company of Biologists. (B) from Alward, 2003 and (C) from Sawamoto et al., 2006 are reprinted with permission of AAAS. (F) from Riley et al., 1997, (K) and (L) from Tsarouhas et al., 2007 are reprinted with permission of Elsevier. (I) and (J) are reprinted by permission of Macmillan Publishers, Ltd. (Hove et al., 2003).
Blood flow: the heart and vascular system
Blood flow, or haemodynamics, is the area in which many early applications of fluid dynamics to biology were worked out. For many reasons the blood-flow problem is at the heart of the novel field of biofluid mechanics (Skalak et al., 1989). Haemodynamics has been a source of a complex variety of fluid-dynamical problems at many different scales. For example, modeling the circulatory system implies understanding the coupling between elasticity and fluid motion in networks of flexible and collapsible elastic tubes that connect the large arteries to the capillary ones (Skalak et al., 1989). Interesting phenomena like the appearance of self-excited oscillations induced by this coupling and other types of instabilities seem to play an important role in the circulatory system and have also been analyzed as a diagnostic tool (Grotberg and Jensen, 2004). On the other hand, the properties of the transport of cells composing the blood and the consequent effects on the blood rheology pose fascinating fluid-dynamical challenges.
Not surprisingly, blood flow has been also intensively studied in connection with the development of the cardiovascular system, but the importance of its role at this stage has only recently begun to be properly assessed. It is now increasingly accepted that haemodynamics is instrumental in many stages of the formation and maturation of the developing circulatory system in the embryo. Once again, flow influences spread across many scales. For example, changes in the cytoskeletal structure and gene expression patterns induced by flow shear have been observed in cultured cardiac endothelial cells (Topper and Gimbrone, Jr., 1999; Davies, 1995). Moreover, recent in vivo measurements in zebrafish embryos show that the flow-induced forces in the developing heart—where the Reynolds number is of order 10−2—are stronger than the mechanical sensitivity of the cells at very early stages of the heart development (Hove et al., 2003). This seems to imply that nature might be using the flow forces to control genetic expression and cellular differentiation. Furthermore, a perturbation of the flow by interposing an obstacle results in an abnormally developed heart with extra chambers, diminished looping, defective valves, etc. However, the hypothesis of the regulatory role of flow induced endothelial share stress has been put in perspective after experiments by Bartman et al. (2004) suggesting that the failure of endothelial cushion formation could also be directly linked to genetically induced suppression of myocardial function. Since the absence of myocardial function is concomitant with the absence of blood flow and therefore stresses, this issue is still unresolved and properly designed experiments to isolate mutually the two effects are needed (Mironov et al., 2005).
Microbiofluidic experiments in which blood flow is sustained by purely hydrodynamical means at the same time as myocardial function is suppressed by genetic, biochemical, and/or pharmacological actions may now be feasible and would provide a clear-cut resolution to the flow versus function issue. Mironov et al. (2005) consider also some alternatives for the role of blood flow in heart tissue morphogenesis. For example, flow may be necessary for the transport of endothelial progenitor stem cells, whose role in cushion tissue morphogenesis and valvulogenesis cannot be neglected; if proven, this possibility would provide an unexpected conceptual connection between the role of flow in the development of the heart and the cerebrospinal system (vide infra).
The full understanding of the morphogenesis of the heart still presents a number of conceptual difficulties. The processes by which the original serial tubular peristaltic pump of the mammalian embryo (Forouhar et al., 2006)—which in the chordates remains as such even in adulthood (Kriebel, 1970; Wenning and Meyer, 2007)—develops into a four-chambered parallel pump is still a matter of debate even at the fundamental level (Moorman and Christoffels, 2003; Moorman et al., 2004). Development here might be thought of in both an embryonic and an evolutionary sense. In a way, this is not surprising since the whole transformation involves parallel changes in several physical and biological processes in mutual interaction, such as the fluid-mechanical forces and the structural actions and reactions, the mechanoelectrical excitable behavior of the developing tissues, the biochemistry of development, etc.
It has been suggested, for example, that the early embryonic heart does not beat to pump blood for the purpose of transport (Burggren, 2004) but that its primary role is rather to regulate heart morphogenesis. Indeed, the standard hypothesis about the function of beating in the early development of the embryo, so-called convective synchronotropy, which states that the heart begins to beat just at the moment at which diffusive transport ceases to be efficient throughout the embryo, seems to be contradicted by the fact that severe disruptions of the flow, including ablation of the whole heart, allow the embryo to live for a relatively long period of time afterwards. On the contrary, the competing idea of prosynchronotropy, substantiated by accurate estimations of the transport needs in the embryo, proposes that beating starts much before diffusion becomes inefficient. The role of such early beating is then sought in the necessity of shear from flow to trigger and control several events in cardiac morphogenesis, such as dextral bending or looping of the heart (Taber, 2006, and others), although the precise influence of hydraulic forces is still not well understood. The other alternative hypothesis is that beating might be required simply for vasculogenesis and angiogenesis.
Fluid flow is now appreciated to play a key role in the development of the vascular system in general (Patterson, 2005). Fluid stress, for instance, has been shown to induce differentiation of stem cells into vascular endothelial cells in in vitro experiments (Yamamoto et al., 2004; Wang et al., 2005). There is mounting evidence that flow as a nongenetic or environmental factor is as important as genetic prespecification for the correct development of the vascular system (Jones et al., 2006). For example, during the development of the chick embryo, flow has been shown to be an important factor in the regulation of arterial-venous differentiation in the yolk sac, so that alterations of the flow pattern may lead to a large disruption of the differentiation process (Noble et al., 2003). It is obvious that the comprehension of the impact of flow stresses at the cellular level is not only important in development but also for the understanding of processes occurring in adult organisms such as neovascularization and vascular remodeling during tissue healing, arterioesclerosis, etc. (Resnick et al., 2003).
Several molecules have been identified as forming part of mechanosensors that inform the endothelium of the characteristics of the flow within either developing or adult vessels. These include cell-matrix and cell-cell junction molecules such as integrins and adherens. For example, the molecule responsible for platelet endothelial cell adhesion, known as PECAM-1, functions via phosphorylation and ulterior binding; it has been found through several means, including the mechanical pulling of individual molecules, that this phosphorylation is highly sensitive to mechanical stresses (Resnick et al., 2003). Analogously, other molecules controlling membranal structures at the level of ion channels, tyrosine kinase receptors, caveolae, etc., as well as those mentioned above influencing the cytoskeleton structure, have been identified as mechanically sensitive. All this suggests not only that a knowledge of the flow patterns is important for the understanding of vascular development, but also that an appropriate treatment of the interrelation of the flow pattern with the chemical reactions involved in the communication of this information to the cell is necessary. We foresee an important role for reaction-advection-diffusion modeling in studies of this developmental process.
Urine and blood flow: the kidneys
As the kidneys are organs fundamentally concerned with filtering and cleaning blood it is not surprising that, like in the heart, fluid forces should play a role in their development. The development of the kidney necessitates the assembly of a network of tubes (Lubarsky and Krasnow, 2003) in which each tube should grow to a given size. The whole process is an example of branching morphogenesis (Lubkin, 2007). How does the size-sensing step work? The details are not yet known, but the tube walls possess mechanosensory cilia sensitive to deflection (Praetorius and Spring, 2001). It may be that these send a signal to stop growth once their deflection is in a given range. The deflection will vary with the flow velocity in the tube. At the low Reynolds numbers of the flow in the kidney, the flow will be laminar, corresponding to Hagen–Poiseuille flow in a pipe (Tritton, 1988). The flow velocity at distance r from the edge of a pipe of radius a is Vr=G/(4μ)(a2−r2), where G is the pressure gradient and μ the viscosity. Hence, if the latter two quantities and the length r of the cilium are fixed, the flow velocity and thence the deflection will increase as the square of the tube radius. This argument supposes the pressure gradient is constant; i.e., a fixed “head”; another alternative is that the flow rate is constant, when the velocity and deflection would decrease with the tube size, and yet another possibility is that the deflection-dependent signal from the cilia regulates growth by altering the volume of fluid being produced; the pressure is given by the fluid volume produced, and the tubes distend under pressure. In the kidney of the adult mouse epithelial microvilli regulate flow volume in proximal tubules in this way (Du et al., 2004). Another factor that may be implicated in tubular morphogenesis in the kidney is the alignment of the cells in the tube walls (Simons and Walz, 2006).
Most work has been carried out on the embryonic kidney in zebrafish. As with the heart, blood flow is required for glomerular assembly in zebrafish, apparently acting via a stretch-responsive signaling system in the vessel wall (Serluca et al., 2002). Moreover, motile cilia are found to drive urine flow in the zebrafish larval kidney and disruption of cilia structure or motility results in pronephric cyst formation Figs. 3G, 3H; such motile cilia may possibly be present in the human embryonic kidney too (Kramer-Zucker et al., 2005).
Polycystic kidney disease (PKD) is a human disease directly related to a failure of sensing in the tube-size-determining step of the fluid dynamical developmental system in the kidney. Defective mechanosensing cilia in the kidney lead to tubules growing beyond their normal size into expanding cysts that eventually disrupt the topology of the network and cause a progressive loss of renal function (Lubarsky and Krasnow, 2003; Ibañez-Tallon et al., 2003; Yokoyama, 2004; Simons and Walz, 2006; Yoder, 2007;Fliegauf et al., 2007).
The fetal metanephric kidney produces a large volume of dilute urine: in the most common animal model—sheep—used for the study of fetal renal function it has been shown that the rate of urine production per body weight is more than ten times what it is in the adult. This urine contributes to maintaining the flow of amniotic fluid (Liu and Wintour, 2005).
Amniotic fluid, lung fluid, urine, and air flow: the amnion and lungs
Amniotic fluid is no longer thought to be a “stagnant pool” and there are now recognized to be active mechanisms for its production and removal, but the role of this fluid dynamics in the development of the fetus is not fully understood (Hooper and Harding, 1995; Mann et al., 1996; Modena and Fieni, 2004; Olver et al., 2004; Beall et al., 2007). Stem cells have been found in human amniotic fluid, whose role in development is also unknown (De Coppi et al., 2007).
Together with fetal urine from the kidneys, the most important inflow to the amniotic fluid is from the lung (Modena and Fieni, 2004; Olver et al., 2004; Beall et al., 2007). The fluid dynamics of the adult human lung has been studied for decades (Pedley, 1977), but much less is understood of its development. Like the kidney, lung development involves the assembly of a network of tubes (Lubarsky and Krasnow, 2003) and is an instance of branching morphogenesis (Lubkin, 2007). While the genetic basis of the process is now beginning to be understood (Warburton, 2008; Metzger et al., 2008), this has yet to be linked to the fluid dynamics. The developing lung is filled with lung fluid, which is of slightly different composition than the amniotic fluid which, together with urine, it goes on to form once expelled from the lung. Expansion of the mammalian lung is apparently controlled by the secretion of fluid into the lumina (Bryant and Mostov, 2007). This secretion may be controlled by osmotic pressure (Lubarsky and Krasnow, 2003; Olver et al., 2004; Bryant and Mostov, 2007). The vocal chords act as a valve that gives the lungs an overpressure compared to the amniotic fluid outside, leading to their expansion (Olver et al., 2004). In addition to this fluid secretion, it has been proposed that growth is controlled by forces exerted by foetal breathing movements and peristaltic contractions (Liu et al., 1992; Xu et al., 1998; Schittny et al., 2000; Del Riccio et al., 2004).
The process of lung expansion in fruit flies has been investigated in detail and involves a sequence of three events (Tsarouhas et al., 2007). Some 10.5 h after eggs are laid, epithelial cells lining the tubes begin to secrete large amounts of proteins into the tube lumen. This must provide a large osmotic pressure differential to inflate the tubes, through which, over the following 30 min, the diameter of the tubes increases 2.5- to 3-fold. Some 7.5 h later, much of the liquid in the tube lumen is removed by the same epithelial cells. Shortly before the embryo is ready to hatch, the liquid remaining in the lung is exchanged for gas, as shown in Figs. 3K, 3L. In mammals a similar series of processes take place during gestation and at birth. Lung liquid is secreted across the pulmonary epithelium into the lung lumen under an osmotic pressure gradient established by the movement of chloride ions in the same direction. It is not known exactly when in gestation lung liquid secretion begins, but fluid is present by midgestation in fetal sheep and continues to be produced until term. Fetal lung liquid leaves the lungs via the trachea, whereat approximately half is swallowed, and the remainder, together with fetal urine, goes on to make up the amniotic fluid volume. If the fetal trachea is obstructed, preventing the outward flow of lung liquid, the fetal lung expands with accumulated liquid. At birth, the lung liquid must be replaced by air as breathing begins so that respiratory gas exchange can take place. It is clear that this implies the absorption of the liquid, and this is thought to take place by the inversion of the osmotic pressure gradient that drives liquid secretion across the pulmonary epithelium during fetal life (Liu and Wintour, 2005). At the same time air penetrates deeper into the lungs with each breath, as the interface between lung fluid and air is driven forward into the lungs by the incoming air (Hooper et al., 2007). This situation, of a less viscous fluid—air—pushing a more viscous one—lung liquid—has much fluid-dynamical interest and, depending on the geometry, can lead to the Saffman–Taylor or Rayleigh–Taylor flow instabilities of a less viscous fluid pushing a more viscous one, or a heavier fluid above a lighter one (Faber, 1995).
Cerebrospinal fluid flow: the brain
The importance of the cerebrospinal fluid in connection with abnormal brain functioning has been considered since ancient times; Hippocrates described the condition of enlargement of the brain ventricles, or hydrocephalus. It is, however, quite recently that increasing and more general evidence has been gathered that shows that this fluid plays a vital role in the correct development of the brain, as well as in its correct functioning once developed (Nico et al., 2003; Miyan et al., 2003). Currently, a precise knowledge of the effects of alterations of the flow pattern during development is far from being only an academic matter but is also an evolving subject in neonatology of the utmost practical importance at a clinical level (Oi and Rocco, 2006).
The conceptual similarity of the mechanisms at work in cerebrospinal fluid flow and in other biofluidic systems in the body is quite suggestive. For example, there is good evidence indicating that the flow of cerebrospinal fluid in the ventricles of the adult mammalian brain can assist the migration of new neurons towards their destination in the olfactory bulb (Clarke, 2006). This flow—with Reynolds number of order 10−1—is directed by cilia that line the ventricular surface (Sawamoto et al., 2006) just as happens in several other instances in the organism. Indeed, ependymal cells possess motile 9+2 cilia of the same type of those in the respiratory tract, although significantly longer and beating at twice the frequency: about 40 Hz in rats. Although the complete role of these cilia and the relevance of the fluid transport that they induce is not fully elucidated, it is probable from the physical point of view that cilium-induced flow is dominant at least for the cerebrospinal fluid transport along the narrowest channels of the system, complementing the effects of changing blood pressure on the brain vessels as a circulatory agent (Ibañez-Tallon et al., 2003; Fliegauf et al., 2007).
If this is true in the adult, there is a good possibility that cilium-induced flow is a mechanism active in development, too. Although that has not yet been shown, the pressure of cerebrospinal fluid is known to be important for the physical expansion of the embryonic brain (Desmond and Jacobson, 1977). As is the case with the kidneys, a failure of this process to function normally may lead to developmental diseases. Correlations of human congenital hydrocephalus with cilia-related genetic diseases have been observed, for example, in patients with primary ciliary diskynesia or Kartagener’s syndrome (Ibañez-Tallon et al., 2003). As for animal models, both in rats (Fliegauf et al., 2007) and in zebrafish (Kramer-Zucker et al., 2005) it has been demonstrated quite convincingly that a lack of ciliary motility leads to the development of hydrocephalus.
Aqueous humour flow: the eye
The front of the eye contains a transparent liquid, the aqueous humour, that flows into the eye from the ciliary body where it is produced, and flows out through the trabecular meshwork into Schlemm’s canal and finally joins the venous circulation (Bill, 1975; Brubaker, 1982). The balance between aqueous humour production and outflow determines the pressure within the eye; an elevated pressure can lead to problems such as glaucoma.
Our knowledge of the development of the eye is as yet incomplete, but there are indications that the flow of aqueous humour flow during development may be of considerable importance (Wulle, 1972; Mizokami et al., 1994). The commencement of aqueous humour flow occurs at the same time as intercellular spaces, openings, or vacuoles in the trabecular meshwork, are formed (Smelser and Ozanics, 1971; Johnstone and Grant, 1973).
From such observations alone one cannot conclude whether aqueous humour flow is a cause, or an effect, of the development of the eye, but more recent work in mice demonstrates that mutants whose aqueous humour production is reduced, resulting in a loss of intraocular pressure, smaller eyes, and lack of normal eye protrusion. Examination shows that the eye is flaccid with a loss of vitreal space, together with adhesion of the retina to the posterior surface of the lens, changes in the layering of the retina, disorganization of the bilayered structure of the ciliary epithelium and evidence of lens fibre fusion and distortion of the lenticular structure (Calera et al., 2006). All this suggests that the flow is indeed vital for correct development. A better understanding of these developmental processes may lead to novel treatments for glaucoma (Alward, 2003).
Endolymph flow: the ear
The adult ear of vertebrates contains two fluids, endolymph and perilymph. In fish, at least, flow of endolymph is important during ear development. Fish have an auditory system that differs from the human one. Instead of a cochlea in the inner ear, fish possess three mineral masses termed otoliths in each ear. These are implicated in both balance and hearing (Popper et al., 2005; Cartwright and Khokhlov, 2008). In the main fish model, zebrafish, otolith growth is initiated at 18–18.5 h with localized accretion of free-moving precursor particles. This process, referred to as otolith seeding, is regulated by two classes of cilia: First, kinocilia of precociously forming hair cells termed tether cells bind seeding particles, thereby localizing otolith formation. Tether cells usually occur in pairs at the anterior and posterior ends of the ear. Second, beating cilia distributed throughout the ear agitate the seeding particles, thereby inhibiting premature agglutination. Constraining particles with laser tweezers causes them to fuse into large untethered masses and bringing such masses into contact with tethered otoliths causes them to fuse, greatly enhancing otolith growth. Seeding particles and beating cilia disappear soon after 24 h, and the rate of otolith growth decreases by almost 90% (Riley et al., 1997). The available data show particle velocities of order 20 μm s−1 within the otic vesicle of some 100 μm length, so if the viscosity of the endolymph is close to water, the Reynolds number is of order 10−3.
It is not known whether a similar process occurs in the human ear, in which, rather than otoliths, is found a mass of small otoconia involved in balance.
Other systems in which fluid flow may influence organogenesis: the gut, pancreas, lymphatic system, bones, etc.
Besides the examples we have presented above, there are indications that fluid flow may influence the development of many other organs. In the developing digestive system, as with the mammalian lung, expansion of the intestinal lumen in zebrafish is apparently controlled by the movement of fluid into the lumen (Bagnat et al., 2007). In a seemingly similar fashion to the kidney, cilia are involved in pancreatic development and ciliary abnormalities are associated with cyst formation in pancreatic ducts (Nielsen et al., 2008).
The development of the lymphatic system has recently been shown to be very plastic (Drayton et al., 2006); and may also be guided by fluid flow. The lymph is fed by flow from the capillary vessels through the tissues of the body to the lymph system, and a primary function of the lymphatic system is to maintain interstitial fluid balance (Boardman and Swartz, 2003). Interstitial flow may be implicated in guiding the growth and organization of the developing lymphatic capillary network (Boardman and Swartz, 2003; Lee, 2003; Helm et al., 2005; Rutkowski and Swartz, 2007). Furthermore, interstitial fluid flow through bone is believed to be involved in the development of bone architecture (Hillsley and Frangos, 1994; Owan et al., 1997; Rutkowski and Swartz, 2007; Shapiro, 2008). Considering the long, yet probably incomplete, list of fluids in the human body we gave in the introduction to this section, undoubtedly more examples of organogenesis influenced by fluid flow will come to light.
FLUID FLOW IN THE DEVELOPMENT OF SUPRAMOLECULAR STRUCTURES
How does an organism assemble itself? After the cells make the molecules that are to form part of the structure, how do they form into tissue; flesh and bone? This is the problem of supramolecular assembly in biology, which is as yet in general unresolved. One prediction we may make is that fluid flow will in many instances turn out to be involved, both in transporting materials and in their self-assembly.
Fibrous composites and biominerals
One hint that this is so is in the large number of tissues that have a structure of fibers set in a matrix in a similar fashion to artificial fibrous composites like fiberglass or plywood. To name just a selection, bone and cornea, arthropod cuticle and eggshell, and plant cell wall are all examples of such natural fibrous composites. The morpologies of these composites strongly suggest a link to liquid crystals: although these materials are solids, the hypothesis is that they must have self-assembled during an earlier mobile phase before solidification to produce this liquid-crystalline organization (Neville, 1993). It is clear that fluid flow must be involved in these liquid crystallization processes, although as yet the means of both the initial liquid-crystal formation and of its subsequent solidification are in most cases unknown (Cowin, 2004).
One such structure that has been researched in more detail than most is nacre, or mother of pearl. Many species of mollusk secrete a mineral shell about themselves. Fluid flow in the liquid filled extrapallial space between the mantle and the shell of mollusks is of a peculiar nature. Liquid containing the components of the shell is continually excreted from the cells of the mantle and self-organizes into the shell structure. In the case of those species that produce a nacreous coating of the interior surface of the shell, at least, this process of self organization, gelification and solidification into nacre involves an intermediate state involving a cholesteric liquid-crystal structure of chitin crystallites that is subsequently coated with protein and mineralized (Cartwright and Checa, 2007; Cartwright et al., 2008). This may be the first instance in which we are able to understand the process of liquid crystallization and solidification of a fibrous composite.
Nacre is of interest because it is in the intersection of fibrous composites with a second group of supramolecular biological structures: it is also a biomineral. Biominerals like bone and teeth, shell and carapace have long been studied (Bouligand, 2004). In those instances, like that of nacre formation, in which the biomineralization process has been examined in detail, fluid flow has been found to be involved. Such is the case with two biominerals we have already mentioned: we discussed above how fluid flow is implicated in bone and in otolith formation, and it is likely that further examples will come to light as our knowledge of the so-called “soft chemistry” involved in biological mineral deposition improves.
A related instance in which fluid flow may have had a critical role in the formation of hard tissue is in the ammonoids, fossil cephalopod mollusks whose shell was partitioned by septa. The septa dividing the chambers have long been noted to have an extremely complex fractal morphology of folds at their margins, and one hypothesis to explain this is that the folds are frozen viscous fingers, formed when the soft body of the organism, displaced under the pressure of the cameral fluid filling the chamber, suffered a Saffman–Taylor instability (Checa and García-Ruiz, 1996). The idea competes with a rival hypothesis that the folds were directly under genetic control; it is clearly rather more complicated to resolve this question with an extinct organism than with one in which it is possible to experiment directly with live examples.
Molting and metamorphosis
There are undoubtedly a great many more body structures whose development depends on moving fluids, but as yet there has been very little work on understanding the fluid dynamics involved. Let us mention just one instance: a fascinating example of developmental fluid dynamics in a context far from human development occurs during molting in ecdysozoans. Molting is a complex developmental process associated both with exoskeleton renewal in arthropods and nematodes in general and also with the even more complicated process of metamorphosis in insects. Here, fluid flow is utilized not only to build as in the previous case, but first to breakdown; to enable the chemical substances necessary to dissolve the old exoskeleton to arrive where they are required. Something is now known about the molecular control of molting (Ewer, 2005), but how is the process carried out ? Whether a molt is a simple or a metamorphic one, molting fluid is involved in dissolving the old cuticle and forming the new one.
In the silkmoth, Hyalophora cecropi, during the pupal-adult transformation molting fluid is secreted in the exuvial space as soon as the epidermis retracts from the old pupal cuticle. This fluid persists for the first 19 days of the 21 days of pharate adult life. For the first two-thirds of this period the molting fluid is gel-like and has no obvious effect on the overlying pupal cuticle. However, on about the 14th day of pharate adult development, the molting gel of the pharate adult liquifies and begins to hydrolyze the proteins and chitin of the pupal endocuticle. By the 20th day the endocuticle has disappeared, leaving a thin crisp exocuticle and epicuticle. On the 19th day, molting fluid begins to be absorbed and, by the 20th day, most of it is gone from the exuvial space. On the 21st day, the insect emerges (Lensky et al., 1970). It is thought that the molting fluid is secreted and reabsorbed by osmotic processes (Jungreis and Harvey, 1975).
EXTERNAL FLUID FLOW
As well as fluids internal to an organism, its external fluid environment may also affect development. Practically all organisms exist within a fluid: either air, water, or some aqueous solution. One variable is clearly the temperature of the fluid, but another is the velocity of the air or water: the wind or current in which an organism develops. The phenotype of an organism can vary depending on properties of its external fluid medium during development; sessile organisms exposed to wind or current provide a clear example of such phenotypical plasticity. Motile organisms may take advantage of external flow, and larval stages of many of the organisms composing zooplankton are found to alter their behavior according to the flow they find themselves in. Such organisms are also of interest because they straddle the boundary between the world of viscous flow at low Reynolds number and that of inertial flow at higher Reynolds number.
The concept of internal versus external flow depends on what we consider as the organism. In fact the amniotic fluid we discussed earlier is just such an external fluid environment in developing placental mammals, strictly controlled by being within the mother. A further example of such control is the manipulation by an organism of oxygen levels in the egg by regulating flow in its extraembryonic fluid: the embryos of some mollusks, amphibians, and fish move within the egg to stir the extraembryonic fluid. Developing organisms outside a womb or an egg do not have the luxury of such regulation, and must manipulate their external flow environment directly. Adults may also care for the young by inducing flow to regulate heat and oxygen levels; this is seen in nest ventilation and brood aeration in both terrestrial and marine organisms.
Phenotypical response of sessile organisms
One facet of fluid flow external to an organism is the phenotypical response of organisms to flow. The effect of fluid flow on development is notable in sessile organisms. Both trees exposed to continual wind (de Langre, 2008), sponges (Warburton, 1960), and corals (Kaandorp et al., 1996) in a continual current grow in a more compact or “stunted” habit than the same species in a sheltered position (Fig. 4). In the case of trees, individual branches have been found to be thicker and the root system to be both denser and asymmetric in trees growing in a windy site (de Langre, 2008). Plants show a host of engineering solutions to the structural stresses to which they are subjected, such as ribbing on leaves, reinforcing collars on the stems of grasses, and prestressed wood in the trunks of trees (Gordon, 1981), and they actively control such factors during development: they tune their height and stem diameter as they grow in response to the wind (Moulia et al., 2006). The response of plant growth and development to mechanical stimulation has been termed thigmomorphogenesis (Jaffe, 1973). But wind affects plant growth not only by mechanical flexion, but also through air flow itself, which decreases boundary layer thickness and so increases rates of evaporation and carbon dioxide uptake. A study that separated these two effects showed that while the mechanical action of wind produced shorter, stronger stems of the sunflower Helianthus annuus, air flow alone led to increased plant height and reduced stem strength (Smith and Ennos, 2003). Clearly, the response of plants to wind is complex and multifactored and understanding this phenotypical plasticity requires examining the fluid dynamics alongside the cellular and molecular mechanisms that produce these developmental changes.
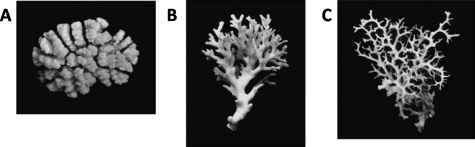
Figure 4. Phenotypical response of a sessile organism to flow: Growth forms of the stony coral.
Form (A) originates from an exposed site, (B) from a semiprotected site, and (C) from a site sheltered from water movement. Reprinted by permission of the American Physical Society (Kaandorp et al., 1996).
Plankton dynamics and larval swimming
Organisms, both sessile and motile, may utilize flow in their environment to their advantage. Plankton dynamics is an example where fluid dynamics is pivotal at the level of an ecosystem; there is great interest at present in understanding the problem of plankton patchiness (Abraham, 1998). When we consider zooplankton that are the larval stages of many organisms, we can see that this is an instance of fluid dynamics interacting with development. A great many larvae adapt their swimming response to the flow environment they find themselves in: some larvae swim less in rapid currents, some orient themselves with respect to shear, and some chose to which surface attach to themselves depending on the velocity of the flow (reviewed in Koehl and Reidenbach (2007)). Haeckel originally made a division between drifting organisms—plankton—and swimming organisms—nekton. That distinction becomes blurred as we see that even weak swimmers like zooplankton are not merely being passively advected by the flow, but rather may actively utilize the fluid flow in their environment.
An adjunct to this argument is that during development many of these larval stages increase in size and in doing so pass from the world of low Reynolds number, Re<1, where viscous forces rule and inertia is not important, to the higher Reynolds number world at Re>1 in which inertia is now dominant. As brine shrimp larvae grow through this changeover, even though the flapping motion of their appendages does not change, their propulsion mechanism alters from drag-based rowing to inertial swimming (Williams, 1994). Larval fish switch from drag-based swimming at low Re to inertial propulsion when they grow larger, and intermittent swimming becomes more energetically advantageous as the importance of viscous force declines at higher Re. Another example is provided by scallops: mollusks which swim by jet propulsion by squirting water out of the mantle cavity while clapping their shells together. Small juvenile scallops cannot use this inertial mode of locomotion effectively and are sedentary; larger scallops can jet (reviewed in Koehl (1996)).
External flow to regulate oxygen and heat levels
It is a classical observation that motile cilia occur on the skin of amphibian embryos (Assheton, 1896) and larval skin of the toad Xenopus is now used as a model system for understanding the developmental mechanisms that polarize cilia (Mitchell et al., 2007). In the frog Rana pipiens it is found that ciliated cells appear in the future epidermis during the neural plate stage and that the number of ciliated cells increases greatly during development of the neural folds, so that motile cilia become widely distributed over the entire embryonic surface. Posthatching the cilia begin to regress in larvae, apparently by resorption. It is thought that the motile cilia function to facilitate respiration within the egg and are also important in the movement of mucus across the embryo (Kessel et al., 1974). The embryonic axolotl, Ambystoma mexicanum, too has been found to have similar motile cilia (Billett and Courtenay, 1973).
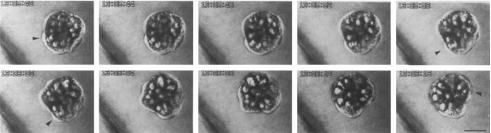
Mollusks share with amphibians the characteristic of laying gelatinous egg clutches, and we find the same behavior in molluskan embryos. In the pond snail, Helisoma trivolvis, embryos are seen to rotate or spin within the egg (Diefenbach et al., 1991) (Fig. 5). The behavior noted in this gastropod mollusk is seen to be driven by cilia on the embryo surface. It is found that on lowering oxygen levels the cilia beat faster, so is thought that hypoxia stimulates this response. Oxygen must reach the embryo by crossing the extraembryonic liquid surrounding it, and in the absence of flow the oxygen will diffuse across, but if the diffusive flux is insufficient, the ciliary movement will stir the liquid, which will increase the oxygen transport to the embryo. The rotation of the embryo as a whole is then a consequence of this behavior coupled with the alignment of ciliary beat directions on the embryo surface (Hunter and Vogel, 1986; Goldberg et al., 2008); we can estimate the Reynolds number to be of order 10−3. A similar phenomenon is observed in the cephalopod mollusk Loligo pealei (Arnold and Williams-Arnold, 1980); as this ciliary flow in the embryo is found in both snails and squid, it presumably has a certain generality in mollusks. In fish, embryo movements may serve a similar need for increasing oxygen levels; embryonic salmon Salmo salar are found to flutter their fins (Peterson et al., 1991).
Figure 5. Flow regulation in the extraembryonic fluid: time-lapse video micrographs of the pond snail Helisoma trivolvis embryo showing embryo spinning within the egg.
Arrowheads in the first, fifth, sixth, and tenth frames of the image series reference the angular change in position of the foot primordium. The diagonal band in the lower left corner of each frame is the egg capsule wall (Diefenbach et al., 1991). Reprinted by permission of Wiley & Sons, Ltd.
Other organisms perform similar movements in their developmental stages, but are not constrained by being within an egg. For example, after hatching, there is present a ciliary current over the skin of the lungfish, Neoceratodu forsteri, for some weeks during its larval phase (Whiting and Bone, 1980). Just as for embryos within the egg, this movement in the free larva is thought to serve for respiration; a further hypothesis is that the induced current serves to keep the unprotected skin of larvae free of debris, parasites, and predatory protozoa.
Not only may the developing organisms themselves produce flow, but also the adults may produce such flows as part of care for the young. In marine organisms brood aeration or oxygenation is found in many groups, including mollusks, crustaceans, and fish. A similar phenomenon in terrestrial organisms is nest ventilation. In social insects this is vital for maintaining the temperature within the range for the correct development of the brood. This may be accomplished either by forced convection—adults fanning with their wings, in bees, for example—or by the very architecture of the nest itself, which in some termite species is constructed so as to provide free convection (Turner, 2002; Jones and Oldroyd, 2007). Such structures may be seen as examples applied to developmental fluid dynamics of what Dawkins (1989) dubbed the extended phenotype.
The importance of flow as a regulator of heat and oxygen levels even reaches as far as humans. A statistical study has shown that the risk of sudden infant death syndrome, or cot death, in human infants, is reduced when a baby sleeps with a fan on; whether the benefit is caused by the fan regulating temperature or oxygen levels is unclear (Coleman-Phox et al., 2008).
DISCUSSION
The research of fluid flow in biology has a long history. At the level of the single organism there have been studied the fluid dynamics of swimming bacteria and fish, and flying birds and insects (Childress, 1981; Happel and Brenner, 1983; Vogel, 1994; Dudley, 2000; Wang, 2005), and even of creatures that walk on water (Bush and Hu, 2006). A large amount of work too has been performed on fluid dynamics in physiology, in particular on pulmonary fluid dynamics (Pedley, 1977), and on the functioning of the vascular systems of animals (Skalak et al., 1989; Vogel, 1992; Grotberg and Jensen, 2004) and of plants (Canny, 1977; Rand, 1983). Yet the appreciable number of examples we have assembled here, in which biological development has recourse to fluid dynamics, both in humans, and throughout the biological world as a whole, has not been viewed as a coherent ensemble; each individual case of developmental fluid dynamics has been seen in isolation. By collecting them together we have found some patterns begin to emerge (see Table 1): fluid flow is seen to shape the development of the cell itself, as well as of many of the organs that will carry fluid in the adult, to be involved in the self-organization of supramolecular structures, and to be vital, in some organisms at least, in setting the body plan itself. Moreover, organisms are affected by and in turn manipulate fluid flow in their external environment. These external flows can be viewed as an extension to the outside world of flows created within the organism to alter development; there is a conceptual continuity from flow within a developing organ, to flow in the amniotic fluid about a mammalian fetus, to flow about the embryo in an amphibian, molluskan, or piscine egg, to flow about a larva now unprotected by its egg. Phenotypic plasticity can be seen in some organisms in their response to external fluid flows; plants, sponges, and corals exhibit polymorphism depending on air or water velocity—on wind or current—during their development. Some organisms even manipulate fluid flow in their external environment by constructing structures, such as the nests of bees and termites, that provide airflow to produce the optimum temperature for brood development either with or without the active intervention of the adults; this is the concept of the extended phenotype involved in developmental fluid dynamics.
We presented above a list of fluids in the adult human that is probably not exhaustive, and the development of the organs that produce and store most of these fluids is not understood. It seems probable, given what we have seen of the development of organs where something is known of the fluid dynamics, that fluid flow will be involved in many more cases. Moreover, work aimed directly or indirectly at understanding human development has naturally been given priority by researchers, while there is a considerable amount of research to do regarding how fluid flow influences development in the wider biological world beyond. In fact, one might hazard that examples will be found in any organism whose development is examined in detail.
A corollary to the importance of fluid flow in development is that when the process goes wrong, fluid dynamics is intimately implicated in the resulting developmental disease, and to understand and eventually to correct such problems we need to comprehend the fluid dynamics along with the genetics. PKD (Yoder, 2007), and hydrocephaly (Oi and Rocco, 2006; Sawamoto et al., 2006) are instances of such fluid-dynamical diseases in humans. Both of these diseases are related to genetic errors in the make up of cilia involved in sensing and producing flow (Ibañez-Tallon et al., 2003; Fliegauf et al., 2007). To understand and overcome these diseases, we need a better comprehension of the fluid dynamics.
An aspect that has been notable in writing this review is the number of instances in which we have had to have recourse to earlier works to find comments about the involvements of fluids in many systems; the latest works and reviews sometimes concentrate on the molecular biology, to the exclusion of all else. There are few quantitative data on almost any of the developmental flows we have been discussing. Clearly it is vital to understand the molecular biology of a system. Yet in focusing exclusively on this aspect, an important part of the question—how genes produce structures—is being ignored. To borrow a useful simile, if one is asked how an aeroplane flies, the answer that comes to mind is to do with lift; only after establishing the physical principles of flight might one discuss which controls the pilot manipulates, or the program the autopilot uses, to fly the aeroplane (Lubkin, 2007). Yet biology today concentrates almost exclusively on the pilot—the genes—and ignores how structures are produced in physical terms. A correction then needs to be made to bring our understanding of these physical processes of morphogenesis in line with our growing knowledge of the molecular biology. Progress will come from biologists and fluid dynamicists together: biologists need to provide more quantitative data, and fluid dynamicists need to apply themselves to these important biological problems.
It is worth recalling that a century ago the case for the importance of osmotic pressure as a physical driving mechanism for biological morphogenesis was put by Leduc (1911) with his ideas of plasmogeny; at the same time Herrera (1903), who called the field synthetic biology, was convinced that buoyancy forces were of greater importance. Both, together with others working in the area at that time, did a great deal of work attempting to mimic biological morphogenesis with physicochemical systems such as chemical gardens (Cartwright et al., 2002), while Haeckel (1917) highlighted the appearance of liquid crystals in biology. Today, with the benefit of a hundred years of hindsight, we know that these ideas were completely lacking the directing input that genetics provides to biological systems, but at the same time we can acknowledge that they were on the right track in the sense that biology does make use of physics; and we now see that osmotic and buoyancy forces, together with liquid crystallization and other physical mechanisms, have, as they suspected, a role in morphogenesis. We hope to have shown here that, as their contemporary D’Arcy Thompson (1917) put it, “a certain mathematical aspect of morphology, to which as yet the morphologist gives little heed, is interwoven with his problems, complementary to his descriptive task, and helpful, nay essential, to his proper study and comprehension of growth and form.”
References
- Abraham, E R (1998). “The generation of plankton patchiness by turbulent stirring.” Nature (London) 10.1038/35361 391, 577–580. [DOI] [Google Scholar]
- Alward, W LM (2003). “A new angle on ocular development.” Science 299, 1578–1581. [DOI] [PubMed] [Google Scholar]
- Arnold, J M, and Williams-Arnold, L D (1980). “Development of the ciliature pattern on the embryo of the squid Loligo pealei: a scanning electron microscope study.” Biol. Bull. 159, 102–116. [Google Scholar]
- Assheton, R (1896). “Notes on the ciliation of the ectoderm of the amphibian embryo.” J. Cell. Sci. 38, 465–484. [Google Scholar]
- Bagnat, M, Cheung, I D, Mostov, K E, and Stainier, D YR (2007). “Genetic control of single lumen formation in the zebrafish gut.” Nat. Cell Biol. 9, 954–960. [DOI] [PubMed] [Google Scholar]
- Bartman, T, Walsh, E C, Wen, K-K, Mckane, M, Ren, J, Alexander, J, Rubenstein, P A, and Stainier, D YR (2004). “Early myocardial function affects endocardial cushion development in zebrafish.” PLoS Biol. 2, e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall, M H, van den Wijngaard, J PHM, van Gemert, M JC, and Ross, M G (2007). “Amniotic fluid water dynamics.” Placenta 28, 816–823. [DOI] [PubMed] [Google Scholar]
- Bill, A (1975). “Blood circulation and fluid dynamics in the eye.” Physiol. Rev. 55, 383–417. [DOI] [PubMed] [Google Scholar]
- Billett, F, and Courtenay, T (1973). “A stereoscan study of the origin of ciliated cells in the embryonic epidermis of Ambystoma mexicanum.” J. Embryol. Exp. Morphol. 29, 549–558. [PubMed] [Google Scholar]
- Black, S (1996). “Regulative development of Xenopus laevis in microgravity.” Adv. Space Res. 17, 209–217. [DOI] [PubMed] [Google Scholar]
- Boardman, K C, and Swartz, M A (2003). “Interstitial flow as a guide for lymphangiogenesis.” Circ. Res. 92, 801–808. [DOI] [PubMed] [Google Scholar]
- Bouligand, Y (2004). “The renewal of ideas about biomineralisations.” Comptes Rendus Palevol 3, 617–628. [Google Scholar]
- Brown, N A, and Wolpert, L (1990). “The development of handedness in left/right asymmetry.” Development 109, 1–9. [DOI] [PubMed] [Google Scholar]
- Brubaker, R F (1982). “The flow of aqueous humor in the human eye.” Trans. Am. Ophthalmol. Soc. 80, 391–474. [PMC free article] [PubMed] [Google Scholar]
- Bryant, D, and Mostov, K (2007). “Development: inflationary pressures.” Nature (London) 449, 549–550. [DOI] [PubMed] [Google Scholar]
- Burggren, W W (2004). “What is the purpose of the embryonic heart beat? or how facts can ultimately prevail over physiological dogma.” Physiol. and Biochem. Zoology 77, 333–345. [DOI] [PubMed] [Google Scholar]
- Bush, J WM, and Hu, D L (2006). “Walking on water: biolocomotion at the interface.” Annu. Rev. Fluid Mech. 10.1146/annurev.fluid.38.050304.092157 38, 339–369. [DOI] [Google Scholar]
- Calera, M R, Topley, H L, Liao, Y, Duling, B R, Paul, D L, and Goodenough, D A (2006). “Connexin43 is required for production of the aqueous humor in the murine eye.” J. Cell. Sci. 119, 4510–4519. [DOI] [PubMed] [Google Scholar]
- Canny, M J (1977). “Flow and transport in plants.” Annu. Rev. Fluid Mech. 10.1146/annurev.fl.09.010177.001423 9, 275–296. [DOI] [Google Scholar]
- Cartwright, J HE, and Checa, A G (2007). “The dynamics of nacre self-assembly.” J. R. Soc., Interface 4, 491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright, J HE, Checa, A G, Escribano, B, and Sainz-Diaz, C I (2008). “Spiral and target patterns in nacre: layer growth of a liquid crystal.” preprint. [DOI] [PMC free article] [PubMed]
- Cartwright, J HE, García-Ruiz, J M, Novella, M L, and Otálora, F (2002). “Formation of chemical gardens.” J. Colloid Interface Sci. 10.1006/jcis.2002.8620 256, 351–359. [DOI] [Google Scholar]
- Cartwright, J HE, and Khokhlov, S (2008). “How do fish hear? The physics of the otolith.” preprint.
- Cartwright, J HE, Piro, O, and Tuval, I (2004). “Fluid-dynamical basis of the embryonic development of left-right asymmetry in vertebrates.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0402001101 101, 7234–7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright, J HE, Piro, N, Piro, O, and Tuval, I (2007). “Embryonic nodal flow and the dynamics of nodal vesicular parcels.” J. R. Soc., Interface 4, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright, J HE, Piro, N, Piro, O, and Tuval, I (2008). “Fluid dynamics of nodal flow and left-right patterning in development.” Dev. Dyn. 10.1002/dvdy.21672237, 3477–3490. [DOI] [PubMed] [Google Scholar]
- Chang, P, Perez-Mongiovi, D, and Houliston, E (1999). “Organisation of Xenopus oocyte and egg cortices.” Microsc. Res. Tech. 44, 415–429. [DOI] [PubMed] [Google Scholar]
- Checa, A G, and García-Ruiz, J M (1996). “Morphogenesis of the septum in ammonoids.” In Ammonoid Paleobiology, Landman, N H, Tanabe, K, and Davis, R A, eds., Plenum, New York. [Google Scholar]
- Childress, S (1981). Mechanics of Swimming and Flying, Cambridge University Press, Cambridge. [Google Scholar]
- Chung, H, and Malacinski, G (1982). “Pattern formation during early amphibian development: embryogenesis in inverted anuran and urodele.” Dev. Biol. 93, 444–452. [DOI] [PubMed] [Google Scholar]
- Chung, H, and Malacinski, G (1983). “Reversal of developmental competence in inverted amphibian eggs.” J. Embryol. Exp. Morphol. 73, 207–220. [PubMed] [Google Scholar]
- Clarke, J (2006). “Cell migration: neurons go with the flow.” Curr. Biol. 16, R337–R339. [DOI] [PubMed] [Google Scholar]
- Coleman-Phox, K, Odouli, R, and Li, D-K (2008). “Use of a fan during sleep and the risk of sudden infant death syndrome.” Arch. Pediatr. Adolesc. Med. 162, 963–968. [DOI] [PubMed] [Google Scholar]
- Cowin, S (2004). “Do liquid crystal-like flow processes occur in the supramolecular assembly of biological tissues?” J. Non-Newtonian Fluid Mech. 10.1016/j.jnnfm.2004.01.012 119, 155–162. [DOI] [Google Scholar]
- Davies, P F (1995). “Flow mediated endothelial mechanotransduction.” Physiol. Rev. 75, 519–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins, R (1989). The Extended Phenotype: The Long Reach of the Gene, Oxford University Press, Oxford. [Google Scholar]
- De Coppi, P, et al. (2007). “Isolation of amniotic stem cell lines with potential for therapy.” Nat. Biotechnol. 10.1038/nbt1274 25, 100–106. [DOI] [PubMed] [Google Scholar]
- de Langre, E (2008). “Effects of wind on plants.” Annu. Rev. Fluid Mech. 10.1146/annurev.fluid.40.111406.102135 40, 141–168. [DOI] [Google Scholar]
- Del Riccio, V, Van Tuyl, M, and Post, M (2004). “A poptosis in lung development and neonatal lung injury.” Pediatr. Res. 55, 183–189. [DOI] [PubMed] [Google Scholar]
- Desmond, M E, and Jacobson, A G (1977). “Embryonic brain enlargement requires cerebrospinal fluid pressure.” Dev. Biol. 57, 188–98. [DOI] [PubMed] [Google Scholar]
- Diefenbach, T J, Koehncke, N K, and Goldberg, J I (1991). “Characterization and development of rotational behavior in Helisoma embryos: role of endogenous serotonin.” J. Neurobiol. 22, 922–934. [DOI] [PubMed] [Google Scholar]
- Drayton, D L, Liao, S, Mounzer, R H, and Ruddle, N H (2006). “Lymphoid organ development: from ontogeny to neogenesis.” Nat. Immun. 7, 344–353. [DOI] [PubMed] [Google Scholar]
- Du, Z, Duan, Y, Yan, Q S, Weinstein, A M, Weinbaum, S, and Wang, T (2004). “Mechanosensory function of microvilli of the kidney proximal tubule.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0405179101 101, 13068–13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, R (2000). The Biomechanics of Insect Flight: Form, Function, Evolution, Princeton University Press, Princeton. [Google Scholar]
- Elinson, R, and Holowacz, T (1995). “Specifying the dorsoanterior axis in frogs: 70 years since Spemann and Mangold.” Curr. Top Dev. Biol. 30, 253–285. [DOI] [PubMed] [Google Scholar]
- Elinson, R, and Rowning, B (1988). “A transient array of parallel microtubules in frog eggs: potential tracks for a cytoplasmic rotation.” Dev. Biol. 10.1016/0012-1606(88)90281-3 128, 185–197. [DOI] [PubMed] [Google Scholar]
- Ewer, J (2005). “How the ecdysozoan changed its coat.” PLoS Biol. 3, e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber, T E (1995). Fluid Dynamics for Physicists, Cambridge University Press, Cambridge. [Google Scholar]
- Fauci, L J, and Dillon, R (2006). “Biofluidmechanics of reproduction.” Annu. Rev. Fluid Mech. 10.1146/annurev.fluid.37.061903.175725 38, 371–394. [DOI] [Google Scholar]
- Fliegauf, M, Benzing, T, and Omran, H (2007). “When cilia go bad: cilia defects and ciliopathies.” Nat. Rev. Mol. Cell Biol. 8, 880–893. [DOI] [PubMed] [Google Scholar]
- Forouhar, A S, Liebling, M, Hickerson, A, Nasiraei-Moghaddam, A, Tsai, H-J, Hove, J R, Fraser, S E, Dickinson, M E, and Gharib, M (2006). “The embryonic vertebrate heart tube is a dynamic suction pump.” Science 10.1126/science.1123775 312, 751–753. [DOI] [PubMed] [Google Scholar]
- Gerhart, J, Danilchik, M, Doniach, T, and Roberts, S (1989). “Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development.” Development 107, 37–51. [DOI] [PubMed] [Google Scholar]
- Goldberg, J, Doran, S, Shartau, R, Pon, J, Ali, D, Tam, R, and Kuang, S (2008). “Integrative biology of an embryonic respiratory behaviour in pond snails: the ‘embryo stir-bar hypothesis’.” J. Exp. Biol. 211, 1729–1736. [DOI] [PubMed] [Google Scholar]
- Goldstein, R E, Tuval, I, and van de Meent, J W (2008). “Microfluidics of cytoplasmic streaming and its implications for intracellular transport.” Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0707223105 105, 3663–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, J E (1981). Structures, or, Why Things Don’t Fall Down, Penguin, London. [Google Scholar]
- Grotberg, J B, and Jensen, O E (2004). “Biofluid mechanics in flexible tubes.” Annu. Rev. Fluid Mech. 10.1146/annurev.fluid.36.050802.121918 36, 121–147. [DOI] [Google Scholar]
- Haeckel, E (1917). Kristallseelen: Studien über das anorganische Leben, Alfred Kroner, Leipzig; translated and annotated in Mackay, A L, (2007), Ernst Haeckel and Biological Form, Lulu.com. [Google Scholar]
- Hammond, K L, and Whitfield, T T (2006). “The developing lamprey ear closely resembles the zebrafish otic vesicle: otx 1 expression can account for all major patterning differences.” Development 133, 1347–1357. [DOI] [PubMed] [Google Scholar]
- Happel, J, and Brenner, H (1983). Low Reynolds Number Hydrodynamics, Prentice Hall, Englewood Cliffs, NJ. [Google Scholar]
- Helm, C L, Fleury, M, Zisch, A, Boschetti, F, and Swartz, M (2005). “Synergy between interstitial flow and vegf directs capillary morphogenesis in vitro through a gradient amplification mechanism.” Proc. Natl. Acad. Sci. U.S.A. 102, 15779–15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera, A L (1903). Nociones de Biología, Imprenta de la Secretaria de Fomento, Mexcio, facsimile ed., Universidad Autónoma de Puebla, Mexico, 1992 (in Spanish). [Google Scholar]
- Hillsley, M V, and Frangos, J A (1994). “Bone tissue engineering: the role of interstitial fluid flow.” Biotechnol. Bioeng. 10.1002/bit.260430706 43, 573–581. [DOI] [PubMed] [Google Scholar]
- Hird, S N, and White, J G (1993). “Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans.” J. Cell. Sci. 121, 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper, S B, et al. (2007). “Imaging lung aeration and lung liquid clearance at birth.” FASEB J. 21, 3329–3337. [DOI] [PubMed] [Google Scholar]
- Hooper, S B, and Harding, R (1995). “Fetal lung liquid: a major determinant of the growth and functional development of the fetal lung.” Clin. Exp. Pharmacol. Physiol. 22, 235–241. [DOI] [PubMed] [Google Scholar]
- Hove, J R, Koster, R W, Forouhar, A S, Acevedo-Bolton, G, Fraser, S E, and Gharib, M (2003). “Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis.” Nature (London) 10.1038/nature01282 421, 172–177. [DOI] [PubMed] [Google Scholar]
- Hunter, T, and Vogel, S (1986). “Spinning embryos enhance diffusion through gelatinous egg masses.” J. Exp. Mar. Biol. Ecol. 96, 303–308. [Google Scholar]
- Ibañez-Tallon, I, Heintz, N, and Omran, H (2003). “To beat or not to beat: roles of cilia in development and disease.” Hum. Mol. Genet. 12, 27R–35R. [DOI] [PubMed] [Google Scholar]
- Jaffe, M (1973). “Thigmomorphogenesis: the response of plant growth and development to mechanical stimulation.” Planta 114, 143–157. [DOI] [PubMed] [Google Scholar]
- Johnstone, M, and Grant, W (1973). “Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes.” Am. J. Ophthalmol. 75, 365–383. [DOI] [PubMed] [Google Scholar]
- Jones, E AV, Le Noble, F, and Eichmann, A (2006). “What determines blood vessel structure? Genetic prespecification vs. hemodynamics.” J. Physiol. (London) 21, 388–395. [DOI] [PubMed] [Google Scholar]
- Jones, J C, and Oldroyd, B P (2007). “Nest thermoregulation in social insects.” J. Insect Physiol. 33, 153–191. [Google Scholar]
- Jungreis, A M, and Harvey, W R (1975). “Role of active potassium transport by integumentary epithelium in secretion of larval-pupal moulting fluid during silkmoth development.” J. Exp. Biol. 62, 357–366. [DOI] [PubMed] [Google Scholar]
- Kaandorp, J A, Lowe, C P, Frenkel, D, and Sloot, P M A (1996). “Effect of nutrient diffusion and flow on coral morphology.” Phys. Rev. Lett. 10.1103/PhysRevLett.77.2328 77, 2328–2331. [DOI] [PubMed] [Google Scholar]
- Kamiya, N, and Kuroda, K (1956). “Velocity distribution of the protoplasmic streaming in Nitella cells.” Bot. Mag. Tokyo 69, 544–554. [Google Scholar]
- Kessel, R, Beams, H, and Shih, C (1974). “The origin, distribution and disappearance of surface cilia during embryonic development of Rana pipiens as revealed by scanning electron microscopy.” Am. J. Anat. 141, 341–360. [DOI] [PubMed] [Google Scholar]
- Koehl, M AR (1996). “When does morphology matter?” Annu. Rev. Ecol. Syst. 10.1146/annurev.ecolsys.27.1.501 27, 501–542. [DOI] [Google Scholar]
- Koehl, M AR, and Reidenbach, M A (2007). “Swimming by microscopic organisms in ambient water flow.” Exp. Fluids 43, 755–768. [Google Scholar]
- Kramer-Zucker, A G, Olale, F, Haycraft, C J, Yoder, B K, Schierand, A F, and Drummond, I A (2005). “Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis.” Development 132, 1907–1921. [DOI] [PubMed] [Google Scholar]
- Kriebel, M E (1970). “Wave front analyses of impulses in tunicate heart.” Am. J. Physiol. 218, 1194–1200. [DOI] [PubMed] [Google Scholar]
- Le Noble, F, Moyon, D, Pardanaud, L, Yuan, L, Djonov, V, Matthijsen, R, Bréant, C, Fleury, V, and Eichmann, A (2003). “Flow regulates arterial-venous differentiation in the chick embryo yolk sac.” Development 131, 361–375. [DOI] [PubMed] [Google Scholar]
- Leduc, S (1911). The Mechanism of Life, Rebman, London. [Google Scholar]
- Lee, R (2003). “Lessons from lymph: flow-guided vessel formation.” Circ. Res. 92, 701–703. [DOI] [PubMed] [Google Scholar]
- Lensky, Y, Cohen, C, and Schneiderman, H A (1970). “The origin, distribution and fate of the molting fluid proteins of the Cecropia silkworm.” Biol. Bull. 139, 277–295. [DOI] [PubMed] [Google Scholar]
- Liu, H, and Wintour, E M (2005). “Aquaporins in development—a review.” Reprod. Biol. Endocrinol 3, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M, Skinner, S J, Xu, J, Han, R N, Tanswell, A K, and Post, M (1992). “Stimulation of fetal rat lung cell proliferation in vitro by mechanical stretch.” Am. J. Physiol. Lung Cell. Mol. Physiol. 263, 376–383. [DOI] [PubMed] [Google Scholar]
- Lubarsky, B, and Krasnow, M A (2003). “Tube morphogenesis: making and shaping biological tubes.” Cell 10.1016/S0092-8674(02)01283-7 112, 19–28. [DOI] [PubMed] [Google Scholar]
- Lubkin, S R (2007). “Developmental biology: branching morphogenesis.” In Complex Systems Science in Biomedicine, Deisboeck, T S, and Kresh, J Y, eds., Springer, pp. 357–374. [Google Scholar]
- Mann, S E, Nijland, M J M, and Ross, M G (1996). “Mathematic modeling of human amniotic fluid dynamics.” Am. J. Obstet. Gynecol. 175, 937–944. [DOI] [PubMed] [Google Scholar]
- Metzger, R J, Klein, O D, Martin, G R, and Krasnow, M A (2008). “The branching programme of mouse lung development.” Nature (London) 453, 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov, V, Visconti, R P, and Markwald, R R (2005). “On the role of shear stress in cardiogenesis.” Endothelium 12, 259–261. [DOI] [PubMed] [Google Scholar]
- Mitchell, B, Jacobs, R, Li, J, Chien, S, and Kintner, C (2007). “A positive feedback mechanism governs the polarity and motion of motile cilia.” Nature (London) 447, 97–101. [DOI] [PubMed] [Google Scholar]
- Miyan, J A, Nabiyouni, M, and Zendah, M (2003). “Development of the brain: a vital role for cerebrospinal fluid.” Can. J. Physiol. Pharmacol. 81, 317–328. [DOI] [PubMed] [Google Scholar]
- Mizokami, K, Sugiura, T, and San Juan, R G (1994). “The development of human trabecular meshwork.” Med Electron Microsc. 27, 275–281. [Google Scholar]
- Modena, A B, and Fieni, S (2004). “Amniotic fluid dynamics.” Ateneo Parmense, Acta Bio-Med. 75, 11–13. [PubMed] [Google Scholar]
- Moorman, A FM, and Christoffels, V M (2003). “Cardiac chamber formation: development, genes, and evolution.” Physiol. Rev. 83, 1223–1267. [DOI] [PubMed] [Google Scholar]
- Moorman, A FM, Soufan, A T, Hagoort, J, Boer, P, and Christoffels, V M (2004). “Development of the building plan of the heart.” Ann. N.Y. Acad. Sci. 1015, 171–181. [DOI] [PubMed] [Google Scholar]
- Moulia, B, Coutand, C, and Lenner, C (2006). “Posture control and skeletal mechanical acclimation in terrestrial plants: implications for mechanical modeling of plant architecture.” Am. J. Bot. 93, 1477–1489. [DOI] [PubMed] [Google Scholar]
- Munro, E, Nance, J, and Priess, J R (2004). “Cortical flows powered by asymmetrical contraction transport par proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo.” Dev. Cell 10.1016/j.devcel.2004.08.001 7, 413–424. [DOI] [PubMed] [Google Scholar]
- Neff, A, Smith, R, and Malacinski, G (1986). “Amphibian egg cytoplasm response to altered g-forces and gravity orientation.” Adv. Space Res. 6, 21–28. [DOI] [PubMed] [Google Scholar]
- Neff, A, Wakahara, M, Jurand, A, and Malacinski, G (1984). “Experimental analyses of cytoplasmic rearrangements which follow fertilization and accompany symmetrization of inverted Xenopus eggs.” J. Embryol. Exp. Morphol. 80, 197–224. [PubMed] [Google Scholar]
- Neville, A C (1993). Biology of Fibrous Composites: Development Beyond the Cell Membrane, Cambridge University Press, Cambridge. [Google Scholar]
- Nico, B, et al. (2003). “Severe alterations of endothelial and glial cells in the blood-brain barrier of dystrophic mdx mice.” Glia 42, 235–251. [DOI] [PubMed] [Google Scholar]
- Nielsen, S K, Møllgård, K, Clement, C A, Veland, I R, Awan, A, Yoder, B K, Novak, I, and Christensen, S T (2008). “Characterization of primary cilia and hedgehog signaling during development of the human pancreas and in human pancreatic duct cancer cell lines.” Dev. Dyn. 237, 2039–2052. [DOI] [PubMed] [Google Scholar]
- Nonaka, S, Shiratori, H, Saijoh, Y, and Hamada, H (2002). “Determination of left-right patterning of the mouse embryo by artificial nodal flow.” Nature (London) 10.1038/nature00849 418, 96–99. [DOI] [PubMed] [Google Scholar]
- Nonaka, S, Tanaka, Y, Okada, Y, Takeda, S, Harada, A, Kanai, Y, Kido, M, and Hirokawa, N (1998). “Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking kif3b motor protein.” Cell 10.1016/S0092-8674(00)81705-5 95, 829–837. [DOI] [PubMed] [Google Scholar]
- Nouri, C, Luppes, R, Veldman, A, Tuszynski, J, and Gordon, R (2008). “Rayleigh instability of the inverted one-cell amphibian embryo.” Phys. Biol. 10.1088/1478-3975/5/1/015006 5, 015006. [DOI] [PubMed] [Google Scholar]
- Oi, S, and Rocco, C (2006). “Proposal of ‘evolution theory in cerebrospinal fluid dynamics’ and minor pathway hydrocephalus in developing immature brain.” Childs Nerv. Syst. 22, 662–669. [DOI] [PubMed] [Google Scholar]
- Okada, Y, Nonaka, S, Tanaka, Y, Saijoh, Y, Hamada, H, and Hirokawa, N (1999). “Abnormal nodal flow precedes situs inversus in iv and inv mice.” Mol. Cell 4, 59–468. [DOI] [PubMed] [Google Scholar]
- Olver, R E, Walters, D V, and Wilson, S M (2004). “Developmental regulation of lung liquid transport.” Annu. Rev. Physiol. 10.1146/annurev.physiol.66.071702.145229 66, 77–101. [DOI] [PubMed] [Google Scholar]
- Owan, I, Burr, D B, Turner, C H, Qiu, J, Tu, Y, Onyia, J E, and Duncan, R L (1997). “Mechanotransduction in bone: osteoblasts are more responsive to fluid forces than mechanical strain.” Am. J. Physiol.: Cell Physiol. 273, 810–815. [DOI] [PubMed] [Google Scholar]
- Patterson, C (2005). “Even flow: shear cues vascular development.” Arterioscler., Thromb., Vasc. Biol. 25, 1761–1762. [DOI] [PubMed] [Google Scholar]
- Pedley, T J (1977). “Pulmonary fluid dynamics.” Annu. Rev. Fluid Mech. 10.1146/annurev.fl.09.010177.001305 9, 229–274. [DOI] [Google Scholar]
- Peterson, R, Power, J, and Martin-Robichaud, D (1991). “Morphological basis of the pectoral fin flutter of embryonic Atlantic salmon (Salmo salar).” Can. J. Fish. Aquat. Sci. 48, 2223–2227. [Google Scholar]
- Phillips, C, Whalon, B, Moore, J, and Danilchik, M (1996). “Gravitational effects on the rearrangement of cytoplasmic components during axial formation in amphibian development.” Adv. Space Res. 17, 225–235. [DOI] [PubMed] [Google Scholar]
- Popper, A N, Ramcharitar, J, and Campana, S E (2005). “Why otoliths? Insights from inner ear physiology and fisheries biology.” Mar. Freshwater Res. 56, 497–504. [Google Scholar]
- Praetorius, H A, and Spring, K R (2001). “Bending the mdck cell primary cilium increases intracellular calcium.” J. Membr. Biol. 10.1007/s00232-001-0075-4 184, 71–79. [DOI] [PubMed] [Google Scholar]
- Purcell, E M (1977). “Life at low Reynolds number.” Am. J. Phys. 10.1119/1.10903 45, 3–11. [DOI] [Google Scholar]
- Rand, R H (1983). “Fluid mechanics of green plants.” Annu. Rev. Fluid Mech. 15, 29–45. [Google Scholar]
- Resnick, N, Yahav, H, Shay-Salit, A, Shushy, M, Schubert, S, Chen, L, Zilberman, M, and Wofovitz, E, (2003). “Fluid shear stress and the vascular endothelium: for better and for worse.” Prog. Biophys. Mol. Biol. 10.1016/S0079-6107(02)00052-4 81, 177–199. [DOI] [PubMed] [Google Scholar]
- Riley, B B, Zhu, C, Janetopoulos, C, and Aufderheide, K J (1997). “A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish.” Dev. Biol. 10.1006/dbio.1997.8736 191, 191–201. [DOI] [PubMed] [Google Scholar]
- Rutkowski, J M, and Swartz, M A (2007). “A driving force for change: interstitial flow as a morphoregulator.” Trends Cell Biol. 17, 44–50. [DOI] [PubMed] [Google Scholar]
- Sawamoto, K, et al. (2006). “New neurons follow the flow of cerebrospinal fluid in the adult brain.” Science 311, 629–632. [DOI] [PubMed] [Google Scholar]
- Schittny, J C, Miserocchi, G, and Sparrow, M P (2000). “Spontaneous peristaltic airway contractions propel lung liquid through the bronchial tree of intact and fetal lung explants.” Am. J. Respir. Cell Mol. Biol. 23, 11–18. [DOI] [PubMed] [Google Scholar]
- Serluca, F C, Drummond, I A, and Fishman, M C (2002). “Endothelial signaling in kidney morphogenesis: a role for hemodynamic forces.” Curr. Biol. 12, 492–497. [DOI] [PubMed] [Google Scholar]
- Shapiro, F (2008). “Bone development and its relation to fracture repair the role of mesenchymal osteoblasts and surface osteoblasts.” Eur. Cells Mater 15, 53–76. [DOI] [PubMed] [Google Scholar]
- Simons, M, and Walz, G (2006). “Polycystic kidney disease: cell division without a c(l)ue?.” Kidney Int. 70, 854–864. [DOI] [PubMed] [Google Scholar]
- Skalak, R, Ozkaya, N, and Skalak, T C (1989). “Biofluid mechanics.” Annu. Rev. Fluid Mech. 10.1146/annurev.fluid.21.1.167 21, 167–200. [DOI] [Google Scholar]
- Smelser, G, and Ozanics, V (1971). “The development of the trabecular meshwork in primate eyes.” Am. J. Ophthalmol. 71, 366–385. [DOI] [PubMed] [Google Scholar]
- Smith, V, and Ennos, A (2003). “The effects of air flow and stem flexure on the mechanical and hydraulic properties of the stems of sunflowers Helianthus annuus L.” J. Exp. Bot. 54, 845–849. [DOI] [PubMed] [Google Scholar]
- Taber, L A (2006). “Biophysical mechanisms of cardiac looping.” Int. J. Dev. Biol. 50, 323–332. [DOI] [PubMed] [Google Scholar]
- Tanaka, Y, Okada, Y, and Hirokawa, N (2005). “Fgf-induced vesicular release of sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination.” Nature (London) 435, 172–177. [DOI] [PubMed] [Google Scholar]
- Thompson, D W (1917). On Growth and Form, Cambridge University Press, Cambridge. [Google Scholar]
- Topper, J N, and Gimbrone, Jr, M A (1999). “Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype.” Mol. Med. Today 5, 40–46. [DOI] [PubMed] [Google Scholar]
- Tritton, D J (1988). Physical Fluid Dynamics, Oxford University Press, Oxford. [Google Scholar]
- Tsarouhas, V, Senti, K, Jayaram, S, Tiklova, K, Hemphala, J, Adler, J, and Samakovlis, C (2007). “Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila.” Dev. Cell 13, 214–225. [DOI] [PubMed] [Google Scholar]
- Turner, J S (2002). The Extended Organism: the Physiology of Animal-Built Structures, Harvard University Press, Cambridge, MA. [Google Scholar]
- Vogan, K J, and Tabin, C J (1999). “A new spin on handed asymmetry.” Nature (London) 397, 295–298. [DOI] [PubMed] [Google Scholar]
- Vogel, S (1992). Vital Circuits: on Pumps, Pipes, and the Workings of Circulatory Systems, Oxford University Press, Oxford. [Google Scholar]
- Vogel, S (1994). Life in Moving Fluids: the Physical Biology of Flow, Princeton University Press, Princeton. [Google Scholar]
- von Dassow, G, and Schubiger, G (1994). “How an actin network might cause fountain streaming and nuclear migration in the syncytial Drosophila embryo.” J. Cell Biol. 127, 1637–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakahara, M, Neff, A, and Malacinski, G (1984). “Topology of the germ plasm and development of primordial germ cells in inverted amphibian eggs.” Differentiation 26, 203–210. [DOI] [PubMed] [Google Scholar]
- Wang, H, Riha, G M, Yan, S, Li, M, Chai, H, Yang, H, Yao, Q, and Chen, C (2005). “Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line.” Arterioscler., Thromb., Vasc. Biol. 25, 1817–1823. [DOI] [PubMed] [Google Scholar]
- Wang, Z J (2005). “Dissecting insect flight.” Annu. Rev. Fluid Mech. 37, 83–210. [Google Scholar]
- Warburton, D (2008). “Order in the lung.” Nature (London) 453, 733–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton, F (1960). “Influence of currents on form of sponges.” Science 10.1126/science.132.3419.89 132, 89.13804209 [DOI] [Google Scholar]
- Wenning, A, and Meyer, E P (2007). “Hemodynamics in the leech: blood flow in two hearts switching between two constriction patterns.” J. Exp. Biol. 210, 2627–2636. [DOI] [PubMed] [Google Scholar]
- Whitting, H P, and Bone, Q (1980). “Ciliary cells in the epidermis of the larval Australian dipnoan. Neoceratodus.” Zoological J. of the Linnean Society 68, 125–137. [Google Scholar]
- Williams, T A (1994). “A model of rowing propulsion and the ontogeny of locomotion in Artemia larvae.” Biol. Bull. 187, 164–173. [DOI] [PubMed] [Google Scholar]
- Wolke, U, Jezuit, E A, and Priess, J R (2007). “Actin-dependent cytoplasmic streaming in C. elegans oogenesis.” Development 134, 2227–2236. [DOI] [PubMed] [Google Scholar]
- Wulle, K G (1972). “The development of the productive and draining system of the aqueous humor in the human eye.” Adv. Ophthalmol. 26, 296–355. [Google Scholar]
- Xu, J, Liu, M, Tanswell, A K, and Post, M (1998). “Mesenchymal determination of mechanical strain-induced fetal lung cell proliferation.” Am. J. Physiol. Lung Cell. Mol. Physiol. 275, 545–550. [DOI] [PubMed] [Google Scholar]
- Yamamoto, K, et al. (2004). “Fluid shear stress induces differentiation of flk-1-positive embryonic stem cells into vascular endothelial cells in vitro.” Am. J. Physiol. Heart Circ. Physiol. 288, H1915–H1924. [DOI] [PubMed] [Google Scholar]
- Yoder, B K (2007). “Role of primary cilia in the pathogenesis of polycystic kidney disease.” J. Am. Soc. Nephrol. 18, 1381–1388. [DOI] [PubMed] [Google Scholar]
- Yokoyama, T (2004). “Motor or sensor: a new aspect of primary cilia function.” Anatomical Science International 79, 47–54. [DOI] [PubMed] [Google Scholar]