Abstract
In this paper, we report direct measurement of an influx of extracellular Ca2+ induced by gamete fusion in flowering plants. This result was obtained during maize in vitro fertilization with the use of an extracellular Ca2+-selective vibrating probe. Ca2+ influx recorded at the surface of isolated egg cells, with or without adhesion of a male sperm cell, was close to zero and stable over time. Gamete fusion, however, triggered a Ca2+ influx in the vicinity of the sperm entry site with a delay of 1.8 ± 0.6 sec. The Ca2+ influx spread subsequently through the whole egg cell plasma membrane as a wavefront, progressing at an estimated rate of 1.13 μm⋅sec−1. Once established, Ca2+ influx intensities were sustained, monotonic and homogeneous over the whole egg cell, with an average peak influx of 14.92 pmol⋅cm−2⋅sec−1 and an average duration of 24.4 min. The wavefront spread of channel activation correlates well with the cytological modifications induced by fertilization, such as egg cell contraction, and with the cytosolic Ca2+ (c[Ca2+]) elevation previously reported. Calcium influx was inhibited effectively by gadolinium, possibly implicating mechanosensitive channels. Furthermore, artificial influxes created by incubation with Ca2+ ionophores mimicked some aspects of egg activation. Taken together, these results suggest that, during fertilization in higher plants, gamete membrane fusion starts the first embryonic events by channel opening and Ca2+ influx. In turn, c[Ca2+] may work as a trigger and possibly a space and time coordinator of many aspects of egg activation.
Keywords: maize, channels, vibrating ion probe
Fertilization in higher plants is an extremely complex process, involving the interaction of several cells and tissues and a double fusion inside the embryo sac. In maize, in vitro fertilization (IVF) procedures allow the in vitro manipulation and study of the two cytological fusions that lead to a full seed: the sperm–egg cell fusion that initiates the development of the embryo (1–3) and the sperm–central cell fusion that leads to the formation of the endosperm (4). Attention has been paid to the changes triggered by sperm–egg fusion at the cellular and molecular levels (reviewed in refs. 5 and 6). In particular, a transient elevation of cytosolic calcium concentration (c[Ca2+]) has been observed to be triggered after sperm–egg fusion in a medium containing 5mM CaCl2 (7). Because increasing or decreasing external Ca2+ changed significantly the rate of sperm–egg fusion (3), the extracellular Ca2+ dependence of this calcium rise was not investigated in this study.
One question not yet addressed concerns the origin of this calcium rise. Data from animal systems indicate that calcium can be mobilized from internal stores and/or arise from an influx of extracellular Ca2+ during fertilization, depending on the species investigated (8). In brown algae, a calcium influx is believed to be induced by gamete fusion and seems to play a direct role in egg activation (9, 10). We do not know yet whether this is the case for flowering plants.
The Ca2+ selective vibrating probe (for review, see refs. 11 and 12) developed a decade ago by Kühtreiber and Jaffe (13) is a powerful and strictly noninvasive method for measuring minute extracellular calcium gradients with spatial and temporal resolutions of a few microns/seconds. The calcium-vibrating electrode records externally the net flux of calcium across the plasma membrane of a single cell by measuring potential differences between two points perpendicular to the plasma membrane and by subsequently converting the microvolt signal to flux data and current densities. Application of this technique to pollen tubes (14–16) and roots (17–20) indicates the potential of the vibrating probe for recording and analyzing calcium fluxes in the vicinity of plant cells and for elucidation of the role of extracellular calcium in developmental processes.
In this study, we have used the Ca2+-selective vibrating probe to measure calcium fluxes during different steps of the IVF process in maize. We demonstrate that an influx of calcium, which can be inhibited by gadolinium, is induced after sperm–egg fusion and propagates from the fusion site as a wavefront. Moreover, artificial induction of calcium influxes triggers a number of postfusion events. Temporal and spatial patterns of the calcium influx and its possible role in egg activation are discussed.
Materials and Methods
Plant Material.
Maize plants (Zea mays L.) of inbred line A188 and hybrid DH5×DH7 were grown in the Lisbon Botanical Garden or in the Gulbenkian Institute for Science (Oeiras, Portugal) nursery fields. Ears were bagged to prevent pollination and were collected at receptivity stage, when emerging silks reached 8–13 cm in length.
Isolation of Female and Male Gametes.
Egg cells were isolated manually by using a protocol from Kranz et al. (1) and Faure et al. (3). They were individually rinsed two to three times and stored in microdroplets of a solution containing 500 mM mannitol and 0.0001% bovine albumin, fraction V (Sigma), pH 5.7, under liquid paraffin (Merck, Darmstadt, Germany). Male gametes were released from freshly collected tricellular pollen grains by a pH/osmotic shock in 500 mM mannitol (1), 2–3 min before IVF.
IVF.
IVF was performed according to Faure et al. (3) but by using a modified fusion medium composed of 500 mM mannitol, 2 mM MgCl2, 0.01 to 5.0 mM CaCl2, 0.0001% BSA, fraction V, pH 5.7.
For each IVF experiment, several male gametes were first transferred to the bottom center of a 35-mm Petri dish containing 5 ml of fusion medium. When the male gametes stopped moving, an egg cell was introduced in their vicinity. A Ca2+-selective electrode was positioned 5 mm from the cells. The medium was allowed to settle for 10 min to avoid stirring artifacts. One male gamete was moved manually into contact with the egg cell by use of a tungsten microneedle previously washed in 10 mM EGTA. The medium was again allowed to settle for 1–2 min. During this period, the electrode was moved gently toward the adhering male and female gametes. A background reference was taken for 10–30 sec before Ca2+ flux measurement proceeded on the plasma membrane of the egg cell during the adhesion and fusion steps. Fusion usually took place 2–35 min after adhesion. Experiments were performed on the stage of an inverted microscope (Nikon Eclipse TE-300). Images were collected by using a CCD camera (Panasonic WV-BP332) and recorded on VHS video (Thomson VPH 6990, Chroma ProII) synchronized with the electrophysiological recording.
Extracellular Flux Measurements.
We used a self-referencing Ca2+-selective vibrating electrode (12, 21, 22), modified for direct DC coupling (11). Signals were measured by a purpose-built electrometer (Applicable Electronics, West Yarmouth, MA). The electrodes were calibrated by using a standard series of CaCl2 solutions (from 0.1 to 10.0 mM) before use. The average response of the electrodes was about 28–30 mV/pCa. Electrodes with a response of less than 28 mV/pCa were considered non-Nernstian and were discarded.
Electrode vibration and positioning were achieved with a three-dimensional positioner (Applicable Electronics). Data acquisition, preliminary processing, and control of the stepper motor-driven positioner were driven by computer through specific control boards (Applicable Electronics) and integrated by using the software package aset (Automated Scanning Electrode Techniques, ScienceWares, Falmouth, MA). The self-referencing vibrating probe was vibrated perpendicular to the egg cell surface and positioned such that it was approximately 1 μm away at its nearest vibration point (see Fig. 1). The vibrating probe oscillated with an excursion distance of 10 μm. A cycle of recording was composed of four consecutive events. In each of the two extreme positions (proximal and distal), there is lag period in which no recording takes place, allowing the probe to settle after each movement. This is followed by a measuring period, in which the signal is averaged by the software. The total length of the cycle is set to avoid hysteresis and to allow sufficient stabilization of the probe response. In our specific measuring conditions, each data point was generated every 2.89 sec. The two settled averages were then subtracted from each other (this subtraction represented the self-referencing feature of the probe). The cycle was repeated over time.
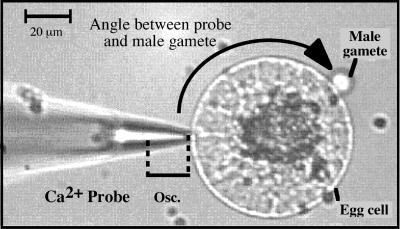
Figure 1.
Micrograph from a typical Ca2+ flux measurement during maize IVF. The Ca2+-selective vibrating probe was positioned approximately 1 μm from the surface of the egg cell at its nearest vibration point. The probe oscillated with an excursion of 10 μm away from the cell (Osc.). The angle between the probe and the adhering male gamete is indicated by the black arrow.
Cell measurements were alternated with reference measurements taken by vibrating the electrode in the medium at more than 100 μm from any gamete or contaminating amyloplasts. These reference values generally fluctuated around 0 ± 15 μV (noise level).
Ion fluxes were calculated by assuming cylindrical diffusion by using Fick's first law of diffusion (12, 21). Negative values for the voltage output corresponded to inward Ca2+ flux toward the cell and positive values to efflux. All results are expressed as average ± standard error.
Inhibition with Gadolinium.
GdCl3 treatments were performed after fusion, when the Ca2+ influx had started. Ca2+ flux measurement was stopped before 50 μl of a 1 mM GdCl3 (Sigma) stock aqueous solution was added to the fusion medium contained in the Petri dish to obtain a final concentration of 10 μM. Five to ten minutes later, a background reference was taken, and membrane fluxes were recorded.
Cell Wall Detection.
Egg cells and zygotes were stained for 5 min in the dark in fusion medium containing 0.001% calcofluor white M2R (Sigma) under liquid paraffin and then washed twice with fusion medium before observation of cellulose staining (see ref. 23) by using an epifluorescence inverted microscope (Nikon Eclipse TE-300).
Ca2+ Ionophore Treatments.
Isolated egg cells were incubated for 40 min in a medium composed of 500 mM mannitol, 5 mM CaCl2, 0.0001% BSA, fraction V, to which 20 μM A23187 or 5 μM ionomycin was added. Egg cells were then washed twice in 500 mM mannitol containing 0.0001% BSA, fraction V, before observation of cellulose staining by using calcofluor white (see above).
Results
Ca2+ Fluxes Before Gamete Fusion.
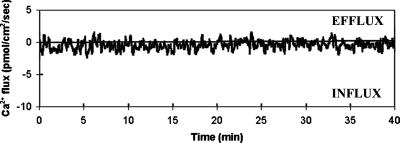
The time course of Ca2+ fluxes was determined for egg cells before and during adhesion with a male gamete. The Ca2+ fluxes of isolated egg cells (n = 10) were recorded mostly in a fusion medium containing 50 μM CaCl2, the condition used for fertilization measurements. Small and stable Ca2+ fluxes were measured, ranging between an efflux of 1.29 and an influx of 6.02 pmol⋅cm−2⋅sec−1. No significant variations of this pattern were observed during more than 60 min. Adhesion of one male gamete to the egg cell did not change this situation significantly (Fig. 2). In all adhering male and female gametes examined (n = 133), Ca2+ fluxes measured on the egg cell plasma membrane were comparable to those of isolated egg cells (influx of 0.46 ± 0.04 pmol⋅cm−2⋅sec−1 for the cell of Fig. 2). Ca2+ fluxes remained stable for more than 2 h (n = 31). Thus, before fertilization, isolated egg cells and egg cells adhered with a male gamete are in a near-resting state of calcium equilibrium, with no prevalent net movement of this ion taking place. This is shown clearly in some samples for which the currents measured are indistinguishable from the noise level.
Figure 2.
Typical Ca2+ flux measurements on an egg cell adhered with a male gamete before fusion took place (n = 112). Only very small and stable influxes or effluxes were detected, which are close to the noise level independently assessed as 0.60 ± 0.08 pmol⋅cm−2⋅sec−1.
A Ca2+ Influx Is Triggered by Gamete Fusion.
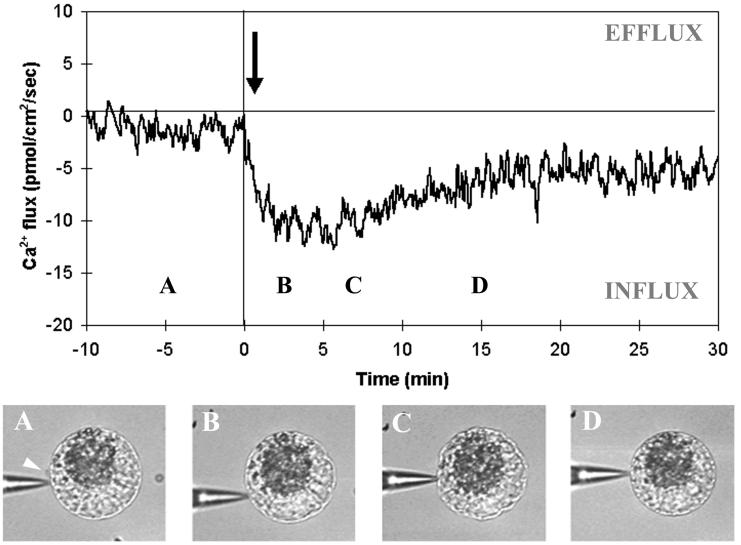
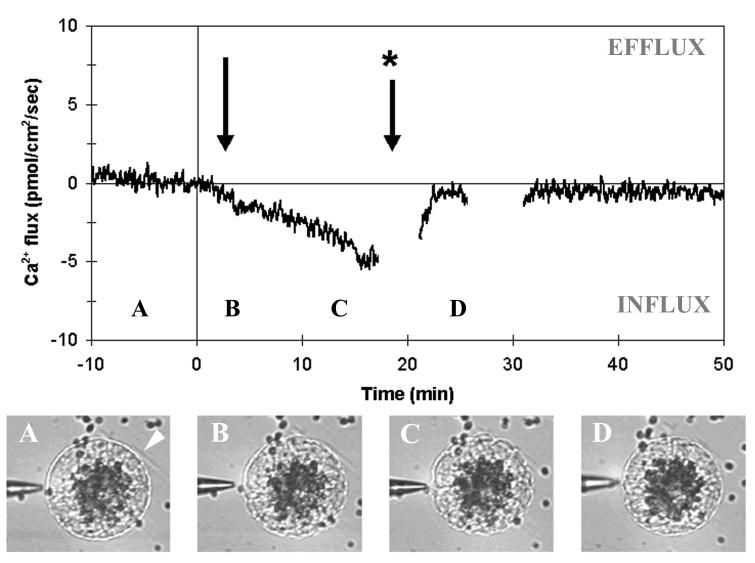
The nominal 50 μM Ca2+ was found to be the best compromise between a good rate of fusogenicity (55%) and recording of biologically significant Ca2+ fluxes. All measurements were therefore made in 50 μM CaCl2 solution. In this medium, the real Ca2+ concentration measured with the calcium-specific electrode in the vicinity of the cells varied between 50 and 200 μM Ca2+. Under these conditions, male gamete fusion with the egg cell was systematically followed a few seconds later by the onset of a long-lasting Ca2+ influx (n = 61, Fig. 3). Peak intensity of Ca2+ influxes ranged between 0.11 and 63.19 pmol⋅cm−2⋅sec−1, with a peak average of 14.9 ± 1.83 pmol⋅cm−2⋅sec−1 and an average duration of 24.4 ± 3.13 min, spanning from 2.8 min to more than 2 h. Despite the fact that there is a wide range of influx net magnitudes, there is always a postfusion influx in all measured cells, irrespective of the flux direction before fusion. This postfusion influx was significantly greater than baseline noise in all fertilized egg cells studied. Furthermore, more than 50% of the measured cells display an influx peaking at more than 10 pmol⋅cm−2⋅sec−1.
Figure 3.
Ca2+ flux measurements during maize IVF. A typical recording is shown (n = 61) illustrating the onset of a Ca2+ influx after fusion. Time 0 is chosen arbitrarily as the time of gametic fusion, as asserted by direct microscopic observation. The arrow shows the detectable onset of a Ca2+ influx after fusion. A clear Ca2+ influx was always detected in the egg membrane, with a delay to fusion dependent on the relative position of the probe and fusion site (black arrow in Fig. 1). A, B, C, and D refer to the time when the pictures (Bottom) were taken. The following events are depicted: (A) egg cell before fusion (male gamete position is shown by a white arrow); (B) egg cell after fusion, just when contraction has started; (C) strong egg cell contraction; and (D) egg cell reshaping.
Two hours after fusion, we could measure fluxes on the zygote surface (n = 11) for various positions of the vibrating probe. There were no significant differences in flux intensity when recordings were made at the two opposites poles of the zygote (as an example, for one zygote, influx intensities measured ranged between 4.02 and 4.06 pmol⋅cm−2⋅sec−1). These data suggest that, once triggered, the Ca2+ influx was homogeneous over the surface of the zygote, indicating that after the postfusion alterations of Ca2+ influx, the previous apolar condition was reset.
Ca2+ Influx Onset Is Propagated as a Wavefront from the Gamete Fusion Site.
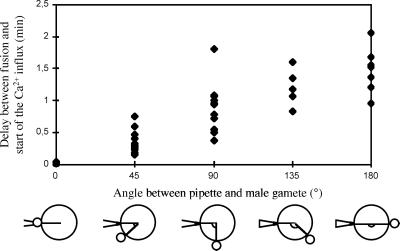
We tested whether the position of the probe on the female plasma membrane respective to that of the adhering male gamete could lead to any differences in the measurement of the Ca2+ influx. The delay between gamete fusion and the measured influx of Ca2+ increased with the distance between the probe and fusion site (Fig. 4; n = 37). The influx started immediately after fusion (average delay was 1.8 ± 0.6 sec; n = 4) when the probe was positioned on the egg cell as close as possible to the adhering male gamete. A significant delay between fusion and onset of influx was observed (average delay was 1.47 ± 0.13 min; n = 7) when the probe was positioned on the egg cell at the opposite side of the adhering male gamete. This result suggests that the influx spreads from the fusion site to the opposite pole of the egg cell.
Figure 4.
Influence of the position of the probe, respective to that of the adhering male gamete, on the measurement of the Ca2+ influx induced by fusion. Lag time between fusion and onset of Ca2+ influx is plotted against the angle between the male gamete and the Ca2+-selective electrode. The drawing (Lower) illustrates the different positions estimated visually during the adhesion procedure. Only differences of at least 45° have been taken into account.
For the 37 samples, we calculated the membrane rates of propagation of the Ca2+ influx, which was an average of 1.13 ± 0.01 μm⋅sec−1, assuming a linear surface propagation algorithm.
Ca2+ Influx and Cytological Events During IVF.
The visible cytological events characteristic of IVF were recorded in parallel to the flux measurements and consist of the following successive stages: adhesion, fusion, cell contraction in most of the samples (58 of 62), and restoration of spherical shape around 40 min after fusion. The intensity and duration of the contraction, as well as the degree of cytoplasmic reorganization after fusion, were variable. We could determine no meaningful correlation between these characteristics and the intensity and duration of the Ca2+ influx. The same lack of correlation applies to the various real Ca2+ concentrations in the bathing medium (ranging from 50 to 200 μM). Thus, the total amount of Ca2+ entering the fusion product was not directly correlated with visible cytological manifestations.
Calcium Influx Is a Sufficient Condition for Cell Wall Deposition.
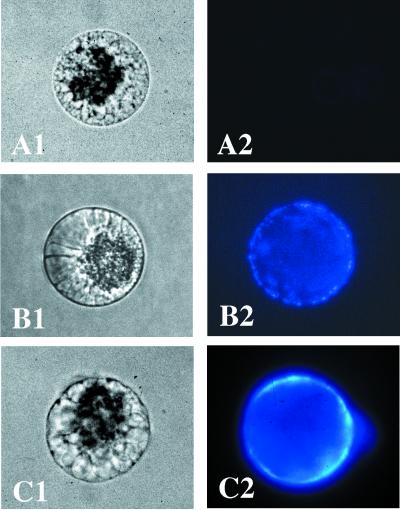
We first verified that a cellulosic cell wall was still established after recording of a Ca2+ influx at 50 μM CaCl2 (n = 4; Fig. 5B), as is the case around fusion products obtained in the presence of 5 mM CaCl2 (n = 11).
Figure 5.
Cell wall establishment around egg cells after fertilization or Ca2+ ionophore treatment. Transmission micrograph (A1) and fluorescent micrograph (A2) of an isolated egg cell. Transmission micrograph (B1) and fluorescent micrograph (B2) of a 2-h zygote. Transmission micrograph (C1) and fluorescent micrograph (C2) of egg cells 2 h after incubation for 40 min with A23187. (Bar = 20 μm.)
To test whether calcium influx was sufficient to trigger other cellular events, we investigated whether an artificial Ca2+ influx could induce the establishment of a cell wall. We treated unfertilized egg cells with two Ca2+-ionophores in the presence of 5 mM CaCl2. A cell wall could be detected 40 min after treatment with either 20 μM A23187 (Fig. 5C) or 5 μM ionomycin (n = 29 and 4, respectively). These results suggest that Ca2+ influx is a sufficient condition to induce establishment of a cellulosic cell wall on the unfertilized egg cell of maize. Furthermore, to our knowledge, these are the first experiments showing that Ca2+ ionophore treatment induces cell wall deposition around eggs of flowering plants.
Ca2+ Influx Is Mediated by Gadolinium-Sensitive Channels.
Gd3+ provided an extremely reproducible and effective action. Addition of 10 μM GdCl3 (final concentration) to the fusion medium had no significant effect on the Ca2+ fluxes measured on unfertilized egg cells (n = 3) or on egg cells with adhered male gametes (n = 3). However, addition of the same quantity at various times after fusion (ranging from 8.35 min to 85 min) systematically inhibited the Ca2+ influx set by gamete fusion (n = 17; Fig. 6). On average, inhibition of the Ca2+ influx reached 95% of the net influx set by gamete fusion.
Figure 6.
Effect of GdCl3 on the Ca2+ influx set after gametic fusion. Ca2+ fluxes were first recorded during IVF before addition of GdCl3 to the fusion medium. As in Fig. 3, time 0 is chosen arbitrarily as the time of gametic fusion. The single arrow indicates the onset of a Ca2+ influx. GdCl3 (10 μM final concentration) was then added (arrow with asterisk). A typical recording is shown (n = 17). GdCl3 inhibits 93.07% of the Ca2+ influx induced by fusion. A, B, C, and D refer to the time when the pictures (Lower) were taken. The following events are depicted: (A) egg cell before fusion (male gamete position is shown by white arrow); (B) egg cell after fusion, just when contraction has started; and (C) strong egg cell contraction, which was abolished immediately (D) by GdCl3 addition.
In most cases (n = 15), the fusion product was still contracted before Gd3+ was added but recovered a spherical shape as soon as the Ca2+ influx was inhibited (Fig. 6). Cytoplasmic streaming was very active, and a cell wall was established (n = 3).
Discussion
This study provides description of a Ca2+ influx induced by gamete fusion in flowering plants. We have shown that the increase in Ca2+ flux initiated just after sperm–egg fusion takes place in the vicinity of the sperm entry site and subsequently spreads uniformly to the whole zygote plasma membrane. We were able to demonstrate that this influx represents a long-lasting inward-directed flux through the egg plasma membrane. The noninvasive nature of the ion-specific vibrating probe was essential to this approach, because other methods that interact physically with membrane integrity (e.g., patch clamp) have proved much less reproducible (A.F.A., C. Ojeda, C. Dumas, M. Rougier, and O. Rougier, unpublished work).
Characteristics of Calcium Influx.
Our results demonstrate that the small and steady fluxes of Ca2+ characteristic of unfertilized eggs are unmodified during sperm–egg adhesion. This result is consistent with our previous data showing that adhesion triggers no change in intracellular calcium concentration during IVF in maize (7). It cannot be excluded that a proportion of the measured extracellular calcium gradient is caused by apoplasmic exchange (16) rather than by transmembrane transport. Egg cells are true protoplasts, and no cell wall can be detected around them (ref. 23; see Fig. 5A). Newly formed cell wall material was detectable only in fusion products, and this material spreads unevenly on the cell surface several minutes after gamete fusion, well after the start of the Ca2+ influx. Thus we have good reason to consider that the changes in calcium fluxes measured immediately after fusion represent true transmembrane fluxes. To reinforce this view, we used gadolinium, a drug presumed to act on calcium-carrying channels. Although its use has to be considered with caution because of several limitations, including imperfect selectivity (24), gadolinium has proved an efficient inhibitor of calcium transport in plant (25, 26). The effect of gadolinium on fluxes measured at fertilization reflects the strong inhibition of calcium channels sensitive to this drug and located at egg plasma membrane. These channels remain to be identified. They could possibly be Ca2+ stretch-activated channels, because gadolinium has been reported often to inhibit this class of channels for the transport of Ca2+ in both plants (27) and animals (28, 29). Our results also show that once Ca2+ influx is established, this occurs homogeneously all over the zygote surface, suggesting that the Ca2+ channels mediating this influx are evenly distributed in the plasma membrane.
A Wave-Like Propagation?
Our data showing the propagation of a Ca2+ influx were obtained readily, because we knew both the site and time of sperm–egg fusion precisely. By contrast, during fertilization in animals, studies have allowed the recording only of membrane currents and not that of the ions carrying them (30, 31). Furthermore, most of these studies were performed with artificially activated rather than sperm-activated eggs (32). Sea urchin is the only system where current propagation was analyzed in detail during fertilization. In this species, establishment of the electrical continuity between the gametes precedes the fertilization current in the vicinity of sperm (33). Fertilization current spreads subsequently to the rest of the egg plasma membrane and propagates through a wave of channel opening that is probably caused by a cytoplasmic mechanism (30). A Ca2+ wave could be a good candidate for this mechanism, because it progresses simultaneously across fertilized sea urchin egg (34).
In maize IVF, propagation of the Ca2+ influx is carried by a mechanism of a yet unknown nature. Propagation is in the range of 1 μm⋅sec−1. Thus propagation through voltage-gated channels is highly improbable. Calculations of the rate of propagation suggest a linear surface propagation mode. This result is supported by the cytology of the fusion product: the plasma membrane is close to the vacuole that partially isolates this thin layer from the rest of the cytoplasm. This thin layer may behave as a semiautonomous entity. The estimated surface propagation rate is compatible with the values expected (35, 36) or reported (37) for slow-propagating calcium waves. These waves have been hypothesized as being the outcome of a mechanically driven process, most likely underpinned by stretch-activated channels either in the plasma membrane or in the endoplasmic reticulum. Our present data showing Gd3+ inhibition of the calcium influx, the obvious contraction of the egg cell after the onset of calcium influx and the immediate relaxation of the egg cell after Gd3+ addition, fit this model. In our present view, the following mechanistic sequence may occur: (i) membrane fusion, by the above-discussed mechanisms, would start a calcium influx close to the fusion site; (ii) a wave of contraction, presumably of actomyosin nature, would be triggered; (iii) this would then, in turn, trigger the opening of more stretch-activated calcium channels.
The Ca2+ Influx May Trigger Egg Activation.
We have shown that maize gamete fusion induces a long-lasting Ca2+ influx that overlaps the c[Ca2+] elevation observed independently by Ca2+ imaging (7). However, direct correlation between these two events was not established, and we still do not know whether the Ca2+ influx precedes the c[Ca2+] elevation. In animals and algae, egg activation is linked to a c[Ca2+] elevation in every system studied (8, 9). In nemertians (38), echiurians (39), and brown algae (9), this elevation seems to be sustained only by Ca2+ entry from the extracellular medium. In maize, the Ca2+ influx could be sufficient to sustain the long-lasting c[Ca2+] elevation because, in vivo, the egg cell probably has an important extracellular Ca2+ store at its disposal, resulting essentially from the degeneration of a synergid (40). Furthermore, the linear propagation of the influx along the membrane seems to indicate direct participation of the plasma membrane on the physiology of the process, pointing out the possible relevance of extracellular Ca2+. However, future investigations will be focused on the elucidation of the relative contribution of extra- and intracellular Ca2+ in the c[Ca2+] elevation.
Another pending question is the respective role of these two events in egg activation. A major egg activation event analyzed in relation to the Ca2+ influx is cell wall establishment. We observed a cell wall only after fertilization and measurement of a sperm-induced Ca2+ influx or after an ionophore-induced Ca2+ influx. Similar ionophore experiments performed in Fucus showed identical results (9, 41). Thus, in maize, as in Fucus, an artificial Ca2+ influx seems sufficient to induce the formation of a cell wall.
Future investigations will be required to establish the precise characterization of the sequence of egg activation events after IVF. In particular, a better understanding of spatiotemporal relationships between calcium influx and the c[Ca2+] elevation triggered by egg–sperm fusion is essential to elucidate their respective contribution to other postfertilization events and to early zygote development in flowering plants.
Acknowledgments
S.C. and J.A.F. acknowledge Fundação para Ciência e Tecnologia for financial support (PRAXIS/C/BIA/11034/1998) and Fundação Luso-Americana para o Desenvolvimento for partially financing the vibrating probe setup. The present collaboration between the Ecole Normale Supérieure and Instituto Gulbenkian de Ciência was funded by an Instituto de Cooperação Cientifica e Tecnológica International/Centre National de la Recherche Scientifique (CNRS) protocol (423/CNRS). We also thank Frédérique Rozier for technical help and Drs. Laura Zonia, Richard Parton, and Charlie Scutt for critical reading of the manuscript.
Abbreviations
- IVF
in vitro fertilization
- c[Ca2+]
cytosolic Ca2+ concentration
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180243697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180243697
References
- 1.Kranz E, Bautor J, Lörz H. Sex Plant Reprod. 1991;4:12–16. [Google Scholar]
- 2.Kranz E, Lörz H. Zygote. 1994;2:125–128. doi: 10.1017/s0967199400001878. [DOI] [PubMed] [Google Scholar]
- 3.Faure J-E, Digonnet C, Dumas C. Science. 1994;263:1598–1600. doi: 10.1126/science.263.5153.1598. [DOI] [PubMed] [Google Scholar]
- 4.Kranz E, Von Wiegen P, Quader P, Lörz H. Plant Cell. 1998;10:511–524. doi: 10.1105/tpc.10.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kranz E, Dresselhaus T. Trends Plant Sci. 1996;1:82–87. [Google Scholar]
- 6.Rougier M, Antoine A F, Aldon D, Dumas C. Sex Plant Reprod. 1996;9:324–329. [Google Scholar]
- 7.Digonnet C, Aldon D, Leduc N, Dumas C, Rougier M. Development (Cambridge, UK) 1997;124:2867–2874. doi: 10.1242/dev.124.15.2867. [DOI] [PubMed] [Google Scholar]
- 8.Stricker S A. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 9.Roberts S, Brownlee C. Zygote. 1995;3:191–197. doi: 10.1017/s0967199400002586. [DOI] [PubMed] [Google Scholar]
- 10.Robinson K, Wozniak M, Pu R, Messerli M. Curr Top Dev Biol. 1999;44:101–125. doi: 10.1016/s0070-2153(08)60468-8. [DOI] [PubMed] [Google Scholar]
- 11.Shipley A, Feijò J. In: Fertilization in Higher Plants. Cresti M, Cai G, Moscatelli A, editors. Berlin: Springer; 1999. pp. 235–252. [Google Scholar]
- 12.Smith P J S, Sanger R H, Jaffe L F. Methods Cell Biol. 1994;40:115–134. doi: 10.1016/s0091-679x(08)61112-7. [DOI] [PubMed] [Google Scholar]
- 13.Kühtreiber W M, Jaffe L F. J Cell Biol. 1990;110:1565–1573. doi: 10.1083/jcb.110.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierson E, Miller D, Callaham D, Shipley A, Rivers B, Cresti M, Hepler P. Plant Cell. 1994;6:1815–1828. doi: 10.1105/tpc.6.12.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierson E, Miller D, Callaham D, van Aken J, Hackett G, Hepler P. Dev Biol. 1996;174:160–173. doi: 10.1006/dbio.1996.0060. [DOI] [PubMed] [Google Scholar]
- 16.Holdaway-Clarke T, Feijò J, Hackett G, Kunkel J, Hepler P. Plant Cell. 1997;9:1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kochian L, Shaff J, Kühtreiber W, Jaffe L, Lucas W. Planta. 1992;188:601–610. doi: 10.1007/BF00197055. [DOI] [PubMed] [Google Scholar]
- 18.Schiefelbein J, Shipley A, Rowse P. Planta. 1992;187:455–459. doi: 10.1007/BF00199963. [DOI] [PubMed] [Google Scholar]
- 19.Hermann A, Felle H H. New Phytol. 1995;129:523–533. [Google Scholar]
- 20.Felle H, Hepler P. Plant Physiol. 1997;114:39–45. doi: 10.1104/pp.114.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kühtreiber W M, Jaffe L F. J Cell Biol. 1990;110:1565–1573. doi: 10.1083/jcb.110.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi A, Camacho P, Lechleitern J, Herman B. Physiol Rev. 1999;79:1089–1125. doi: 10.1152/physrev.1999.79.4.1089. [DOI] [PubMed] [Google Scholar]
- 23.Kranz E, von Wiegen P, Lörz H. Plant J. 1995;8:9–23. doi: 10.1105/tpc.10.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caldwell R A, Clemo H F, Baumgarten C M. Am J Physiol. 1998;275:C619–C621. doi: 10.1152/ajpcell.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- 25.Marshall J, Corzo A, Leigh R A, Sanders D. Plant J. 1994;5:683–694. [Google Scholar]
- 26.Geitmann A, Cresti M. J Plant Physiol. 1998;152:439–447. [Google Scholar]
- 27.Ding J P, Pickard B. Plant J. 1993;3:83–110. doi: 10.1111/j.1365-313x.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 28.Yang X-C. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. Nature (London) 1999;400:382–385. doi: 10.1038/22578. [DOI] [PubMed] [Google Scholar]
- 30.McCulloh D H, Chambers E L. J Gen Physiol. 1991;97:579–604. doi: 10.1085/jgp.97.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaffe L F, Nuccitelli R. J Cell Biol. 1974;63:614–628. doi: 10.1083/jcb.63.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaffe L A, Kado R T, Muncy L. J Physiol. 1985;368:227–242. doi: 10.1113/jphysiol.1985.sp015855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCulloh D H, Chambers E L. J Gen Physiol. 1992;99:137–175. doi: 10.1085/jgp.99.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen S S, Buck W R. Dev Biol. 1993;157:157–169. doi: 10.1006/dbio.1993.1120. [DOI] [PubMed] [Google Scholar]
- 35.Jaffe L F. Cell Calcium. 1993;14:736–745. doi: 10.1016/0143-4160(93)90099-r. [DOI] [PubMed] [Google Scholar]
- 36.Jaffe L F. BioEssays. 1999;21:657–667. doi: 10.1002/(SICI)1521-1878(199908)21:8<657::AID-BIES5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Fluck R A, Miller A L, Jaffe L F. J Cell Biol. 1991;115:1259–1265. doi: 10.1083/jcb.115.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stricker S A. Dev Biol. 1996;176:243–263. doi: 10.1006/dbio.1996.0131. [DOI] [PubMed] [Google Scholar]
- 39.Stephano J L, Gould M C. Dev Biol. 1997;191:53–68. doi: 10.1006/dbio.1997.8709. [DOI] [PubMed] [Google Scholar]
- 40.Russell S D. Int Rev Cytol. 1992;140:357–388. [Google Scholar]
- 41.Brawley S H, Quatrano R S, Wetherbee R. J Cell Sci. 1976;20:233–254. doi: 10.1242/jcs.20.2.233. [DOI] [PubMed] [Google Scholar]