Abstract
We studied 21 COPD patients in stable clinical conditions to evaluate whether changes in lung function induced by cumulative doses of salbutamol alter diffusing capacity for carbon monoxide (DLCO), and whether this relates to the extent of emphysema as assessed by high resolution computed tomography (HRCT) quantitative analysis. Spirometry and DLCO were measured before and after cumulative doses of inhaled salbutamol (from 200 μg to 1000 μg). Salbutamol caused significant increments of forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and flows at 30% of control FVC taken from both partial and maximal forced expiratory maneuvers. Functional residual capacity and residual volume were reduced, while total lung capacity did not change significantly. DLCO increased progressively with the incremental doses of salbutamol, but this became significant only at the highest dose (1000 μg) and was independent of the extent of emphysema, as assessed by radiological parameters. No significant changes were observed in CO transfer factor (DLCO/VA) and alveolar volume (VA). The results suggest that changes in lung function induced by cumulative doses of inhaled salbutamol are associated with a slight but significant increase in DLCO irrespective of the presence and extent of emphysema.
Keywords: lung volumes, high resolution computed tomography, chronic obstructive bronchitis, emphysema
Introduction
In chronic obstructive pulmonary disease (COPD) carbon monoxide uptake in the lung (DLCO) is determined to assess the associated presence of emphysema (Pauwels et al 2001). Reduction of the parameter is deemed to be due to functional and/or anatomical reductions of the alveolar and capillary surfaces available for gas exchange (Krogh 1915; Cadigan et al 1961; Rose et al 1979). For bronchodilator therapy may improve lung volume recruitment as a result of a decrease in RV in both chronic obstructive bronchitis and emphysema (Cerveri et al 2000), and thus increase the amount of gas inhaled with a single inspiration, it would be reasonable to expect an increase in DLCO in COPD after bronchodilatation. However, the few studies conducted in this regard gave contrasting results. Two studies demonstrated that at least for diagnostic purposes, the test may be safely performed either before or after dilator agonists (Jones et al 1961; Chinn et al 1988), whereas a study showed a significant increase in DLCO with inhaled terbutaline 1.5 mg (Åkesson et al 2000). Part of these discrepancies could be explained by the different degree of bronchodilatation achieved with different pharmacological agents and/or the variable association of emphysema with chronic obstructive bronchitis in COPD, both possibly affecting the DLCO measurement as a result of different lung volume and pulmonary vascular bed recruitment.
Gaining information on the effects of the bronchodilator agents on the test is clinically relevant as DLCO is a functional marker of the progression of a disease treated with different therapeutic protocols and doses of beta-2 agonists. Indeed, the variable course of the disease and/or the chronic effects of bronchodilator therapy may lead to recruitment of alveolar volume within lung regions previously served by closed or near-closed airways and/or improvement of blood perfusion across the lung over time, thus potentially affecting the DLCO and its interpretation.
On this ground, we wondered whether DLCO varies with the degree of pharmacologically-induced bronchodilatation and/or extent of emphysema in COPD.
To test this hypothesis, we studied lung function and diffusing capacity before and after cumulative doses of inhaled salbutamol. The study was conducted in a group of 21 COPD patients well characterized for degree of emphysema, as assessed by radiological criteria.
Methods
Patients
Eighteen male and tree female patients affected by COPD, as defined by the international guidelines (Pauwels et al 2001) participated in the study (Table 1). Six of them were current smokers and fifteen former smokers, with an average smoking history of 51.6 ± 18.8 pack-years. Airflow obstruction ranged from moderately severe to very severe. All patients were required to abstain from short-acting bronchodilators for at least 12 h prior to each study session, to be in stable clinical conditions, and not to have suffered from respiratory exacerbation in the previous four weeks. The Ethics Committee approved the experimental protocol, and a written informed consent was obtained prior to the study from each subject.
Table 1.
Main anthropometric, functional, and imaging data
| Mean ± SD | Range | |
|---|---|---|
| Age, yr | 69 ± 8 | 54–84 |
| BMI, Kg/m2 | 27 ± 4 | 19–34 |
| FEV1, % pred | 45 ± 16 | 19–69 |
| VC, % pred | 76 ± 19 | 40–123 |
| FRC, % pred | 156 ± 32 | 104–223 |
| RV, % pred | 176 ± 49 | 120–288 |
| TLC, % pred | 112 ± 14 | 93–145 |
| DLCO, % pred | 57 ± 23 | 24–105 |
| DLCO/V A, % pred | 50 ± 19 | 21–108 |
| PaCO2, mmHg | 37 ± 4 | 27–44 |
| PaO2, mmHg | 66 ± 8 | 52–76 |
| Hb, g/dl | 14 ± 2 | 12–17 |
| RA950, % | 19 ± 7 | 6–31 |
Notes: Data are means ± SD.
Abbreviations: BMI, body mass index; DLCO, carbon monoxide diffusing capacity; FEV1, forced expiratory volume in one second; FRC, functional residual capacity; RV, residual volume; SD, standard deviation; TLC, total lung capacity; VC, vital capacity; VA, alveolar volume; PaCO2, arterial carbon dioxide tension; PaO2, arterial oxygen tension; Hb, hemoglobin; RA950, lung area with attenuation values lower than −950 HU.
Study design
The patients attended the laboratory in the morning of two separate days. On the first day, absolute lung volumes, DLCO, and partial (PEFV) and maximal (MEFV) flow-volume curves were measured at baseline (Step 1). The same measurements were repeated in the same order 15 min after each of the following sequential doses of salbutamol given through a spacer by metered dose inhaler: 200 μg (Step 2), 400 μg (Step 3), and 400μg (Step 4). The doses were chosen to cover at least part of the broad range of bronchodilator responses reported in COPD (Anthonisen et al 1986; Calverley et al 2003). On the second day, a high resolution computed tomography (HRCT) of the lung was obtained.
Lung function measurements
Inspired and expired volumes were obtained by numerical integration of mouth flow measured by a mass flow sensor (Sensor Medics, Yorba Linda, CA). After at least 8 regular tidal breaths, the patients were asked to expire forcefully from about 60% of vital capacity (VC) to residual volume (RV) (PEFV maneuver), to rapidly inspire to total lung capacity (TLC), and forcefully expire to RV (MEFV maneuver) without breath hold. The latter allowed the forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) to be computed. At least three technically acceptable curves were recorded. Flows at 30% of control FVC were taken on MEFV (Vmax30) and PEFV (Vpart30) curves.
Lung volumes were measured with the patients sitting in a body plethysmograph (Jaeger Masterlab, Jaeger, Würzburg, Germany) and panting against a closed shutter at a frequency slightly <1 Hz, cheeks supported by hands. TLC was obtained as the sum of thoracic gas volume (TGV) and the linked inspiratory capacity. Functional residual capacity (FRC) was obtained from TGV corrected for any difference between the volume at which the shutter was closed and the average end-expiratory volume of the four preceding regular tidal breaths. RV was the difference between TLC and VC. Predicted values for spirometry and lung volume are from Quanjer and colleagues (1979).
DLCO was measured according to the American Thoracic Society (ATS) recommendations (Crapo et al 1995) with a Baires System (Biomedin, Padua, Italy), using a gas mixture of 0.3% CO, 10% helium, and balance air. Breath holding time was measured by the method of Jones-Meade (Jones and Meade 1961). At each step of the study, two measurements of DLCO were made at a 4-min interval and were accepted if their inspired volumes were within 0.2 L between each other and 10% of the VC measured by spirometry. Adjustment for carboxyhemoglobin (HbCO) to account for the effect of increasing CO concentrations, and adjustment for Hb concentrations with repeated DLCO measurements were done according to the ATS statement (ATS 1987) in 10 patients who had their arterial blood drawn at the four steps of the study. In the other 11 patients, DLCO was corrected for the average slope of the linear regression analysis of DLCO adjusted versus nonadjusted for HbCO in the 10 patients. Reference values for DLCO are from Cotes (1979).
Imaging
Lung HRCT was performed with a third-generation, continuous-rotation-computerized tomograph with single scan acquisition (Somaton Plus, Siemens, Erlangen, Germany). Technical parameters were the following: 1mm collimation, 137 kVp, 195 mAs, and <1s scanning time. The lungs were scanned from the apex through the base at 1cm intervals and reconstructed with a lung algorithm.
All scan images were processed off-line using a semiautomated image-processing program, which extracts boundaries of the lungs, calculates lung cross sectional areas and histograms of attenuation values (CT numbers) of individual highlighted sections, and summarizes data to obtain the frequency distribution of attenuation values for both lungs. From the frequency distribution of CT numbers, mean CT number in Hounsfield units (HU) were derived. Percent of the whole lung area with attenuation values lower than −950 HU (RA950) was taken as an index of extent of emphysema (Gevenois et al 1995, 1996).
Statistical analysis
Data are expressed as mean ± standard deviation. Relationships between variables were tested by linear regression analysis. Analysis of variance (ANOVA) for repeated measures was used to compare the effects of increasing doses of salbutamol at the different steps. When F values were significant, Tukey’s post hoc test was used for multiple comparisons. P < 0.05 was considered statistically significant.
Results
The severity of airway obstruction, based on spirometric data, ranged from moderate to very severe (Table 1). Emphysema was variably present, as suggested by the wide ranges of RA950, lung hyperinflation and DLCO (Table 1).
Seventeen patients completed the four steps of study, and four asked to interrupt the test at step 3 after a cumulative dose of 600 μg. Increasing the dose of salbutamol caused a progressive and significant increase in Vmax30, V part30, FEV1, and FVC, and a decrease in FRC and RV, consistent with effective bronchodilatation and decrease in lung hyperinflation (Table 2). Average DLCO progressively increased with bronchodilatation, but this became significant only with the highest dose of salbutamol (p < 0.05) (Table 2). In contrast, no significant changes occurred in VA and DLCO/VA (Table 2).
Table 2.
Main respiratory functional parameters at baseline and after additional doses of salbutamol
| STEP 1 Baseline | STEP 2 200 μg | STEP 3 600 μg | STEP 4 1000 μg | |
|---|---|---|---|---|
| Subjects, no. | 21 | 21 | 21 | 17 |
| FEV1, L | 1.14 ± 0.40 | 1.26 ± 0.43c | 1.32 ± 0.45c | 1.42 ± 0.50c |
| FVC, L | 2.55 ± 0.88 | 2.83 ± 0.93c | 2.97 ± 0.97c | 3.02 ± 1.03c |
| V˙max30, L/s | 0.24 ± 0.15 | 0.29 ± 0.21 | 0.35 ± 0.27c | 0.36 ± 0.24c |
| V˙part30, L/s | 0.42 ± 0.21 | 0.51 ± 0.24 | 0.54 ± 0.29c | 0.57 ± 0.28c |
| VC, L | 2.65 ± 0.85 | 2.98 ± 0.89c | 3.14 ± 0.97c | 3.36 ± 1.08c |
| FRC, L | 5.28 ± 1.42 | 5.02 ± 1.40c | 4.85 ± 1.34c | 4.72±1.30c |
| RV, L | 4.22 ± 1.35 | 3.96 ± 1.31c | 3.82 ± 1.32c | 3.59 ± 1.35c |
| TLC, L | 6.92 ± 1.54 | 6.94 ± 1.57 | 6.95 ± 1.57 | 6.97 ± 1.50 |
| HbCOa, % | 2.3 ± 0.6 | 3.3 ± 0.6c | 4.3 ± 0.6c | 5.2 ± 0.7c |
| VA, L | 4.93 ± 1.00 | 5.17 ± 1.06 | 5.05 ± 1.03 | 5.15 ± 1.07 |
| DLCO, ml/mmHg/min/L | 14.35 ± 5.07 | 15.12 ± 4.81 | 15.72 ± 4.95 | 16.41 ± 5.02b |
| DLCO/V A, ml/mmHg/min/L | 3.03 ± 1.20 | 3.07 ± 1.17 | 3.21 ± 1.08 | 3.23 ± 1.06 |
Note: HbCO, carboxyhemoglobin measured in 10 patients;
p < 0.05;
p < 0.01 or less versus baseline; Data are means ± SD.
Abbreviations: DLCO, carbon monoxide diffusing capacity; FEV1, forced expiratory volume in one second; FRC, functional residual capacity; FVC, forced vital capacity; RV, residual volume; SD, standard deviation; TLC, total lung capacity; V˙max30 and V˙part30, maximal and partial forced expiratory flows at 30% control FVC; VA, alveolar volume; VC, vital capacity.
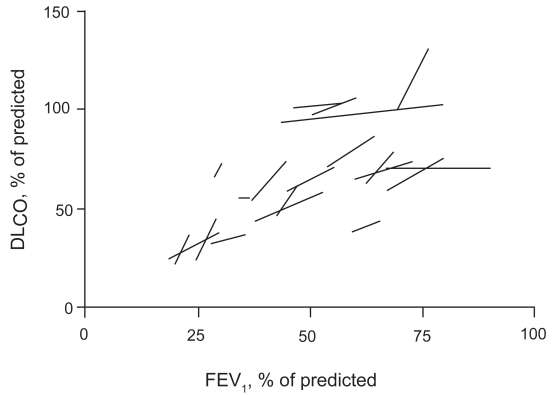
The slopes of linear regression analysis of DLCO plotted against FEV1, VC, and FRC at the four steps in individual patients were significantly different from 0 (Table 3 and Figure 1), thus suggesting that DLCO tended to increase with bronchodilatation and volume recruitment. In contrast, only the slope of the linear regression of DLCO/VA versus FEV1attained statistical significance (Table 3).
Table 3.
Average linear regression slopes of DLCO, and DLCO/VA versus key variables with inhalation of increasing doses of salbutamol.
| Variables | DLCO | DLCO/VA |
|---|---|---|
| FEV1 | 11.8 ± 14.0b | 3.6 ± 5.8a |
| FVC | 1.1 ± 11.6 | 0.01 ± 4.0 |
| V˙max30 | 4.3 ± 25.2 | 1.9 ± 6.6 |
| V˙part30 | 12.3 ± 35.3 | 1.8 ± 8.1 |
| VC | 3.9 ± 6.0b | 1.0 ± 2.6 |
| FRC | −3.9 ± 8.1a | −0.6 ± 2.4 |
Notes: Data are means ± SD; Significant differences from baseline conditions are denoted with symbols:
p < 0.05;
p < 0.01 or less.
Abbreviations: DLCO, carbon monoxide diffusing capacity; FEV1, forced expiratory volume in one second; FRC, functional residual capacity; FVC, forced vital capacity; SD, standard deviation; V˙max30 and V˙part30, maximal and forced expiratory flows at 30% control FVC; VA, alveolar volume; VC, vital capacity.
Figure 1.
Linear regression lines of DLCO versus FEV1 with incremental doses of salbutamol in individual patients. Values are expressed as % of predicted.
Abbreviations: DLCO, carbon monoxide diffusing capacity; FEV1, forced expiratory volume in one second.
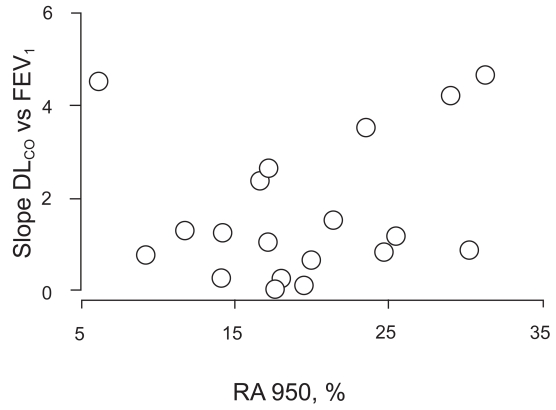
Neither of these slopes correlated with the extent of emphysema as assessed by HRCT scan, thus suggesting that DLCO increased with bronchodilatation irrespective of the extent of emphysema, as assessed with imaging techniques (Figure 2).
Figure 2.
Relationships between linear regression slopes of DLCO versus FEV1 with increasing doses of salbutamol and extent of emphysema, as assessed by HRCT scan quantitative analysis (RA950).
Abbreviations: DLCO, carbon monoxide diffusing capacity; FEV1, forced expiratory volume in one second; HU, Hounsfield units; RA950, lung area with attenuation values lower than −950 HU.
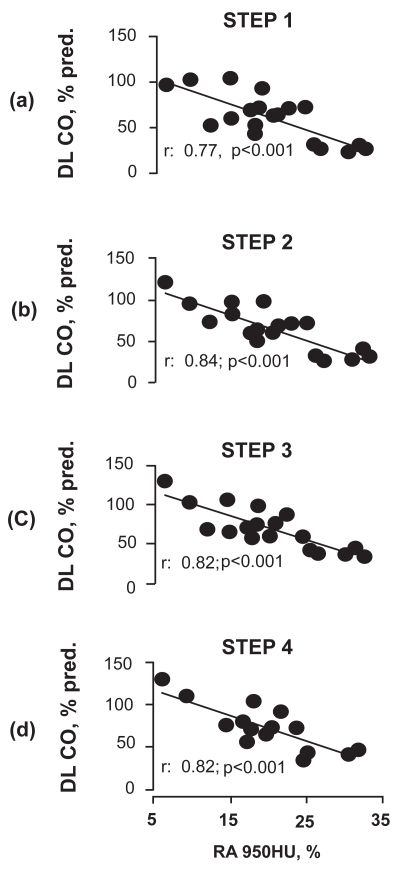
DLCO % pred correlated with the extent of emphysema at all 4 steps of the study (Figure 3).
Figure 3.
Relationships between DLCO as % of predicted and extent of emphysema, as assessed by HRCT scan quantitative analysis (RA950), at the four steps of the study.
Abbreviations: DLCO, carbon monoxide diffusing capacity; HRCT, high resolution computed tomography; HU, Hounsfield units; RA950, lung area with attenuation values lower than −950 HU.
Discussion
The major findings of the present study on COPD patients are that DLCO progressively increased with cumulative doses of inhaled salbutamol to become significant at doses higher than those commonly used for clinical treatments. In addition, this occurred independently of the presence and extent of emphysema, as assessed by radiological parameters. VA tended to increase, so that DLCO/VA remained unchanged.
Theory predicts that VA, permeability characteristics of the alveolar-to-capillary barrier, and ability of hemoglobin to combine with CO are the main determinants of DLCO (Krogh 1915; Cadigan et al 1961; Chinn et al 1988). Therefore, any intervention resulting in recruitment of lung volume would be expected to increase DLCO. Unexpectedly however, the substantial increase in slow VC observed with salbutamol in our COPD patients was not associated with a significant increase in DLCO. The increment in VC was due to a decrease in RV and not to an increase in TLC, which suggests opening of airways previously closed. Had the lack of increase in DLCO mostly manifested in patients with low lung elastic recoil as in emphysema despite the decrease in RV, this would have been presumably the result of opening of airways subtending enlarged emphysematous units with disrupted vascular component unable to subtract CO from the alveoli. The absence of any relationship between changes in DLCO and DLCO/VA after salbutamol and HRCT data suggests, therefore, additional and/or alternative mechanisms. Among them is the possibility that VA was already fully recruited at maximal inflation even before inhaling the bronchodilator. Under these conditions, the effects of the medication on bronchial tone were negligible to recruit new VA useful for gas exchange. Alternatively, alveolar recruitment with salbutamol in COPD occurred in the airways subtending alveolar units with hypoxic vasoconstriction. As documented in many animal species including humans, a decrease in O2 tension in the alveolar compartment would cause a local increase in pulmonary vascular resistance, as a result of narrowing of pre-capillary arteries by mechanisms involving K+ and Ca++ channels (Post et al 1992) and enhanced by local acidosis (Enson et al 1964). With bronchodilatation and ensuing alveolar recruitment, hypoxic vasoconstriction might have disappeared and DLCO increased, unless vascular reopening lagged behind alveolar reopening.
An additional cause for an increased DLCO with high doses of salbutamol might have been the increase in cardiac output and the resulting distension of the pulmonary bed. Though we do not bring direct evidence for this, the increase in heart rate reported in healthy volunteers after doses of salbutamol similar to ours (Bremner et al 1993) would suggest that the cardiovascular side-effects of high doses of β2 agonists might be indeed capable of increasing cardiac output, thus possibly contributing to increase DLCO. Yet, it remains to demonstrate that these doses of salbutamol can really do that.
Previous studies have documented that imposing an inspiratory resistance in healthy subjects is associated with an increase in DLCO, as a result of an increase in pleural pressure and ensuing amount of blood shifted from the abdomen to the chest wall and lungs (Cotes et al 1960; Smith et al 1969). By converse, a decreased inspiratory resistance would be expected to lower DLCO. Whether the bronchodilatation observed in the present study might have affected DLCO is a matter of speculation, though it could have also contributed to blunt the expected increase in DLCO.
The fact that DLCO increased with progressive reduction in bronchial tone may have some clinical relevance. For instance, in clinical trials using this parameter as a functional outcome for follow-up or evaluation of the effects of new, nonbronchodilator treatments in emphysema, any significant change of DLCO should be taken with cautiousness before concluding that this reflects a clear disease modification. In contrast, the maintenance of the correlation between DLCO as % pred and extent of emphysema (Figure 3) at all 4 steps would apparently suggest that the effect of bronchial tone on DLCO is insignificant with respect to the diagnosis of the disease.
In conclusion, the present study documents that improved lung function induced with cumulative doses of inhaled salbutamol is associated with a slight but significant increase in DLCO irrespective of the presence and extent of emphysema. These findings implicate that changes in DLCO over time, as observed in clinical or therapeutic trials, may be at least in part affected by changes in airway caliber.
References
- [ATS] American Thoracic Society. Single breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique. Statement of the American Thoracic Society. Am Rev Respir Dis. 1987;136:1299–307. doi: 10.1164/ajrccm/136.5.1299. [DOI] [PubMed] [Google Scholar]
- Åkesson U, Dahlström J-A, Wollmer P. Changes in transfer factor of the lung in response to bronchodilatation. Clin Physiol. 2000;20:14–18. doi: 10.1046/j.1365-2281.2000.00213.x. [DOI] [PubMed] [Google Scholar]
- Anthonisen NR, Wright EC. Bronchodilator response in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986;133:814–19. [PubMed] [Google Scholar]
- Bremner P, Woodman K, Burgess C, et al. A comparison of the cardiovascular effects of formoterol, salbutamol and fenoterol. Eur Respir J. 1993;6:204–10. [PubMed] [Google Scholar]
- Cadigan JB, Marks A, Ellicott MF, et al. An analysis of factor affecting the measurement of pulmonary diffusing capacity by the single breath method. J Clin Invest. 1961;40:1495–514. doi: 10.1172/JCI104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley PMA, Burge PS, Spencer S, et al. the Isolde Study Investigators. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58:659–64. doi: 10.1136/thorax.58.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveri I, Pellegrino R, Dore R, et al. Mechanisms for isolated volume response to a broncho-dilator in patients with COPD. J Appl Physiol. 2000;88:1989–95. doi: 10.1152/jappl.2000.88.6.1989. [DOI] [PubMed] [Google Scholar]
- Chinn DJ, Askew J, Rowley L, et al. Measurement technique influences the response of transfer factor (TLCO) to salbutamol in patients with airflow limitation. Eur Respir J. 1988;1:15–21. [PubMed] [Google Scholar]
- Cotes JE, Snidal DP, Shepard RH. Effect of negative intra-alveolar pressure on pulmonary diffusing capacity. J Appl Physiol. 1960;15:372–6. doi: 10.1152/jappl.1960.15.3.372. [DOI] [PubMed] [Google Scholar]
- Cotes JE. Lung function: assessment and application in medicine. 4th ed. Oxford: Blackwell Scientific Publication; 1979. [Google Scholar]
- Crapo RO, Hankinson JL, Irvin C, et al. Single-breath carbon monoxide diffusing capacity (Transfer factor). Recommendations for a standard technique - 1995 Update. Am J Respir Crit Care Med. 1995;152:2185–98. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]
- Enson Y, Giuntini C, Lewis ML, et al. The influence of hydrogen ion concentration and hypoxemia on the pulmonary circulation. J Clin Invest. 1964;43:1146–62. doi: 10.1172/JCI104999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevenois PA, De Maertelaer V, DeVuyst P, et al. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–7. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- Gevenois PA, DeVuyst P, De Maertelaer V, et al. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–92. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- Jones RS, Meade F. A theoretical and experimental analysis of anomalies in the estimation of pulmonary diffusing capacity by single breath method. Q J Exp Physiol. 1961;46:131–43. doi: 10.1113/expphysiol.1961.sp001525. [DOI] [PubMed] [Google Scholar]
- Krogh M. The diffusion of gases through the lungs of man. J Physiol London. 1915;49:271–300. doi: 10.1113/jphysiol.1915.sp001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels RA, Buist S, Calverley PMA, et al. Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global initiative for chronic obstructive lung disease (GOLD) Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- Post JM, Hume JR, Archer SL, et al. Direct role for the potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992;262:C882–90. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- Quanjer PhH, Tammeling GJ, Cotes JE, et al. 1993Standardized lung function testing Eur Respir J 65–40.8381090 [Google Scholar]
- Rose GL, Cassidy SS, Johnson RL., Jr Diffusing capacity at different lung volumes during breath holding and rebreathing. J Appl Physiol. 1979;47:32–7. doi: 10.1152/jappl.1979.47.1.32. [DOI] [PubMed] [Google Scholar]
- Smith TC, Rankin J. Pulmonary diffusing capacity and the capillary bed during Valsalva and Müller maneuvers. J Appl Physiol. 1969;27:826–33. doi: 10.1152/jappl.1969.27.6.826. [DOI] [PubMed] [Google Scholar]