Abstract
Targeting type 4 phosphodiesterase (PDE4) for treatment of COPD has multilevel benefits to patients by reducing inflammation, relieving bronchoconstriction, and improving pulmonary circulation. The isoenzyme-specific narrow spectrum PDE4 inhibitors such as cilomilast and roflumilast may have limited clinical efficacy in managing severe and very severe COPD. Development of dual therapy by combining PDE4 inhibition with Ca2+ channel antagonism may introduce an effective novel armory for physicians to manage patients with severe COPD.
Keywords: COPD, type 4 phosphodiesterase, cilomilast, roflumilast
Introduction
Targeting type 4 phosphodiesterase (PDE4) has been recognized as a promising approach to managing COPD by relieving the symptoms, slowing the progress of the disease, increasing exercise tolerance, reducing exacerbation rate, and improving quality of life (Giembycz 2001, 2005; Mehats et al 2003; Spina 2003, 2004; Lagente et al 2005; Lipworth 2005; Soto and Hanania 2005) The pressing need to develop drugs that control symptoms and reduce mortality (Pauwels et al 2001; GOLD 2005) and the billion-dollar marketing potential for management of COPD have pushed the R&D of PDE4 inhibitors into the product development pipelines of major pharmaceutical companies in the recent years. The early clinical trial data for the second-generation PDE4 inhibitors cilomilast (Ariflo®, GlaxoSmithKline, USA) and roflumilast (Daxas®, Altana, Germany) all pointed to a successful introduction of a novel non-steroid anti-inflammatory therapy to clinicians in combating severe COPD (Gamble et al 2003; Rabe et al 2005) Nevertheless, while the progression of developing cilomilast has idled at the approvable stage for more than two years, the announcement of the termination of the agreement to develop roflumilast between Altana and Pfizer has raised concerns about the therapeutic efficacy of selectively inhibiting one or two isoenzymes in the PDE4 family for COPD management (Pharmiweb 2005).
In the early six-month “RECORD” Phase III trial, roflumilast (500 mg daily) clearly improved lung function (ie, increased FEV1 by +97 mL) and significantly reduced exacerbations (acute worsening of symptoms) compared with placebo (Rabe et al 2005). However, in the follow-up one-year Phase III trials using exacerbations as one of the key endpoints, the results from the European COPD RATIO study that included 1513 patients with severe and very severe COPD have failed to repeat the previously claimed efficacy. In addition, the new trial data confirmed that the PDE4 inhibitor roflumilast’s efficacy was considerably lower than the approved therapies such as fluticasone/salmeterol (a combination therapy of glucocorticosteroid and long-acting β2-agonist) and tiotropium bromide (long-acting anticholinergic). The unexpectedly low long-term efficacy on exacerbation rate from roflumilast therapy made the R&D community re-examine the role of targeting PDE4 in COPD because one of the highest unmet needs in treating the disease is to reduce or eliminate exacerbations (Pharmiweb 2005). In November of 2005, Altana announced the withdrawal of the European Marketing Authorization Application (MAA) for roflumilast and decided to wait for more clinical trial data for submission of a future MAA (Altana 2005a). This holdup no doubt sets back the R&D of the most promising PDE4 inhibitor in development for COPD.
PDE4 inhibition and COPD
COPD is a complex disease with pathophysiological features including inflammation (neutrophils, macrophages, CD8+ lymphocytes infiltration, and inflammatory mediator TNF-α and IL-8 release), airway obstruction (smooth muscle contraction, elevated cholinergic tone), respiratory bronchiolar–alveolar–vasculature remodeling (loss of elastic recoil, alveolar destruction, and fibrosis), pulmonary hyperinflation, gas-exchange abnormalities, and pulmonary hypertension. The progressive loss of lung function leads to reductions in patients’ quality of life and results in exacerbations, cor pulmonale, and death. It is believed that the chronic non-infectious inflammation underlies the pathogenesis and the steady progression of the disease (Pauwels 2001; GOLD 2005). The pathological changes in the patients with COPD are not fully reversible and it often takes many years for a patient at risk (cough, sputum production) to progress into suffering from mild airflow limitation, to moderate, severe, and very severe COPD (with chronic respiratory failure). In the absence of a magical therapy that can stop the disease progression and reverse the abnormalities of pulmonary function, the management, including drug therapy, for COPD is long-term care.
Inhibition of PDE4 has been established as an effective and reliable approach to increasing intracellular cAMP (Conti et al 2003) that underlines the signaling mechanisms for the treatment of COPD. In recent years, numerous in vitro, in vivo, and clinical trial studies demonstrated that PDE4 inhibitors (eg, rolipram, cilomilast, and roflumilast) relax airway smooth muscles to increase air flow (Holbrook et al 1996; Bundschuh et al 2001) and improve pulmonary circulation (Schermuly 2000; de Witt 2000), inhibit bronchiolar–alveolar–vasculature remodeling, and fibrosis (Kumar et al 2003), reduce neutrophils–macrophages/CD8+ T cells infiltration and pro-inflammatory mediator release (Kumar et al 2003; Profita et al 2003; Wollin et al 2005), improve patients’ exercise capacity and quality of life, and prevent the progressive loss of pulmonary function (Rabe et al 2005; Gamble et al 2005). With all these preferred outcomes, it seems that the PDE4 inhibitors in development (cilomilast and roflumilast) would be an ideal armory for the healthcare community to combat COPD. Why, then, has the long-term trial with roflumilast failed to produce the expected results? It could be due to a dose regimen (500 mg daily) that was effective for patients with moderate to severe COPD (Rabe et al 2005) but not adequate for those patients suffering from severe and very severe COPD (Altana 2005b) or the intrinsic low efficacy of the narrow-spectrum PDE4 inhibitors.
Subtype specific PDE4 inhibition and COPD
Developing PDE4 inhibitor as a therapy for COPD is based on the fact that theophylline dilates airway smooth muscles and improves pulmonary function by inhibition of PDE activity (Barnes 2003; Spina 2004) The dose-limiting adverse reactions (nausea, emesis, cardiac arrhythmias) with the non-selective PDE inhibitor theophylline and the first-generation PDE4 inhibitor rolipram (Huang et al 2001; Lagente et al 2005) directed the R&D of PDE inhibitors to discover the second-generation of PDE4 inhibitors cilomilast and roflumilast that have been successfully brought to the final stage for administration approval (Spina 2003, 2004; Lipworth 2005)
Based on the fact that the emetogenic reaction to PDE4 inhibition is due to reticence of the PDE4D isoenzyme (Lamontagne et al 2001; Robichaud et al 2002), several researchers in the field proposed to develop isoform-specific PDE4 inhibitors that reduce or completely avoid disturbing PDE4D activity and therefore do not trigger the emetic responses in the nervous system (Giembycz 2002; Robichaud et al 2002; Card et al 2004).
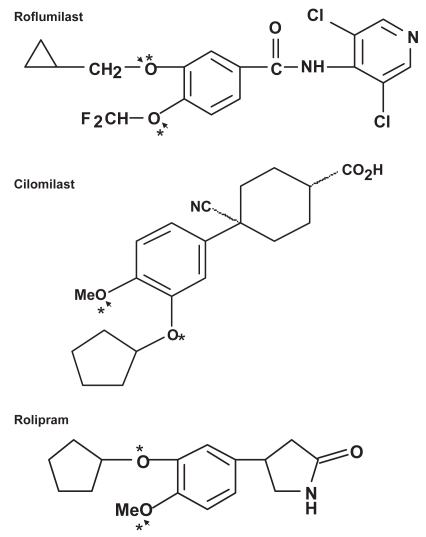
Structural studies have provided evidence that the folding of catalytic domains of PDE4 has a conformation involved in binding of selective inhibitors with a common scheme: (i) a hydrophobic sub-pocket sandwiching an inhibitor in the active site; (ii) hydrogen bond(s) to an invariant glutamine controlling the orientation inhibitor binding (therefore the affinity or potency) (Lee et al 2002; Huai et al 2003; Card et al 2004) (Figure 1). The scaffold of individual PDE4 isoenzyme and the structure of a given selective inhibitor govern isoenzyme-selective inhibition, depict the binding affinity, and determine the therapeutic window and rank order of potency in clinical use for the treatment of COPD. Enhancement of isoenzyme selectivity is critical for reducing side-effects of the PDE4 inhibitors. The strength of the interaction (hydrogen bond) between the oxygen group(s) of an inhibitor and the amide nitrogen group of glutamine (Gln) 369 for PDE4D and Gln 443 for PDE4B plays a pivotal role in determination of the potency and isoenzyme selectivity of an inhibitor (Lee et al 2002; Huai et al 2003; Card et al 2004). In addition, selective PDE4 inhibitors such as cilomilast and roflumilast have additional functional groups that can utilize the remaining empty space of the pocket to yield extra binding energy (increasing potency) and result in greater isoenzyme selectivity (Lee et al 2002; Huai et al 2003; Card et al 2004)
Figure 1.
Structures of representative PDE4 inhibitors. Asterisks indicate the oxygens that form hydrogen-bonds with Gln 369 of PDE4D and arrows indicate the hydrogen-bond(s) with Gln 443 of PDE4B.
For example, while cilomilast’s additional functional groups interact with 10 almost identical residues that form the hydrophobic sub-pocket in PDE4D and PDE4B, the oxygen atoms of the cyclopentyloxy and methoxy groups of cilomilast form two hydrogen bonds with the Gln369 of PDE4D, whereas there is only one hydrogen bond being formed between the methoxy group of cilomilast and the Gln 443 of PDE4B (Card et al 2004; comparing http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1xom&template=ligands.html&l=1.1 with http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1xlx&template=ligands.html&l=1.1). This difference may partially explain the fact that cilomilast is approximately 10-fold more selective for PDE4D than for PDE4B, despite over 90% identity between the PDE4B and PDE4D catalytic domains. (Giembycz 2001; Odingo 2005)
Roflumilast shows a better fitting to the hydrophobic sub-pocket in the PDE4D catalytic site than cilomilast (Card et al 2004; comparing http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1xom&template=ligands.html&l=1.1 with http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1xoq&template=ligands.html&l=2.1), which depicts the experimental finding that roflumilast inhibits PDE4D 338-fold more potently than cilomilast (Table 1). As for PDE4B inhibition, roflumilast’s cyclopropylmethoxy and difluoromethoxy oxygen groups form two hydrogen bonds with Gln 443 of PDE4B (Card et al 2004; comparing http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1xmu&template=ligands.html&l=1.1 with http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1xlx&template=ligands.html&l=1.1), which may partially explain its 585-fold greater inhibition potency than cilomilast toward PDE4b (Table 1). The dichloropyridyl substitution of roflumilast also increases its potency compared with cilomilast in terms of PDE4B inhibition (Card et al 2004).
Table 1.
value comparison between roflumilast and IC50 cilomilast for phosphodiesterase (PDE) 4B and PDE4D inhibition
| PDE4B2 inhibition (IC50, nM) | PDE4D5 inhibition (IC50, nM) | |
|---|---|---|
| Roflumilast | 0.41 | 0.81 |
| Cilomilast | 240 | 61 |
Data from Plexxikon, Inc. (2005).
The rank order of potency for inhibition of PDE4 activity and lipopolysaccharide-stimulated TNFa release, for relaxing bronchoconstriction in guinea pigs, and the daily doses for treatment of COPD by roflumilast, cilomilast, rolipram, and theophylline are summarized in Table 2. By improving the inhibition ratio on PDE4B/PDE4D, roflumilast indeed lowers the emetic profile without compromising its therapeutic efficacy in comparison to cilomilast (Huang et al 2001; Spina 2003)
Table 2.
Potency comparison between phosphodiesterase inhibitors
| PDE4 inhibition (IC50, nM)a | TNF-α release (IC50, nM)a | Bronchorelaxation (EC50, μmol/kg)b | Dosage for COPD (mg, daily)c | |
|---|---|---|---|---|
| Roflumilast | 0.8 | 20 | 1.5 | 0.5 |
| Cilomilast | 120 | 1300 | 52.2 | 15 twice |
| Rolipram | 1100 | 30 | 32.5 | - |
| Theophylline | >10 000 | >10 000 | >300 | 100–600 |
LeadDiscovery; Yoshimura et al (1995); Tenor et al (1996).
The PDE4B-specific single-molecule targeting can undoubtedly reduce or eliminate an inhibitor’s unwanted effects. However, this approach may compromise the efficacy of a PDE4 inhibitor because airway and vascular smooth muscles express multiple PDE4D isoforms (eg, D1, D2, and D5) and PDE4D plays a crucial role in bronchoconstriction and vascular smooth muscle contraction (Liu et al 2000; Baillie et al 2001; Mehats et al 2003). An agent without 4D inhibition may end up lacking sufficient therapeutic efficacy for controlling COPD (Spina 2004).
PDE4 inhibition and pulmonary circulation
The beneficial effects on COPD via relaxing airway smooth muscles and anti-inflammation mediated by PDE4 inhibition with cilomilast and roflumilast have been emphasized and reviewed extensively (Spina 2004; Lagente et al 2005; Lipworth 2005; Soto 2005). Improving pulmonary circulation has not been considered as an important therapeutic approach for managing patients with COPD. However, the facts are 1) during COPD exacerbations, pulmonary hypertension (PH) is increased, 2) the presence of PH is recognized as the single strongest indicator of prognosis in COPD patients among the numerous clinically used lung function parameters (Doi et al 2003; Alp et al 2006), and 3) clinically, the higher the pulmonary arterial pressure, the shorter the life expectancy of COPD patients (Barbera et al 2003). Although inhaling nitric oxide vasodilator can worsen gas exchange because of altered hypoxic regulation of ventilation-balance (Barbera 1996) in patients with stable COPD, and vasodilators are considered as contraindications for COPD patients, in their preliminary clinical trial, Alp et al (2006) demonstrated that reduction of pulmonary vasculature resistance using the PDE5 inhibitor sildenafil was able to significantly improve the exercise capacity (6-minute walk test) of patients suffering from severe COPD. A double-blinded, placebo-controlled, crossover clinical trial of sildenafil in patients (n=10) with COPD is going on to evaluate the effect of PDE5 inhibition on patients’ exercise function, pulmonary function, and quality of life (National Institutes of Health).
Although there is a lack of reports about cilomilast- or roflumilast-caused PDE4 inhibition on the improvement of pulmonary circulation, in isolated perfused lung preparations, intravascular or transbronchial administration of subthreshold doses of the wide-spectrum PDE4 inhibitor rolipram synergistically amplified the pulmonary vasodilatory response to inhaled PGI2 and concomitantly improved ventilation–perfusion matching and relieved pulmonary hypertension (Schermuly et al 2000). More interestingly, in anesthetized cats, De Witt et al (2000) found that rolipram was more potent than either siguazodan (PDE3 inhibitor) or zaprinast (PDE5 inhibitor) in reducing pulmonary lobar arterial pressure. When the tone in the pulmonary vascular bed was raised to a high steady level with a constant infusion of the thromboxane mimic U46619 (9,11-dideoxy-11, alpha9alpha-epoxymethano prostaglandin F2α), intralobar injections of rolipram caused dose-related decreases in systemic arterial pressure and pulmonary arterial pressure. The preference reduction in pulmonary arterial pressure by inhibition of PDE4 suggests that administration of PDE4 inhibitors may benefit COPD patients by increasing ventilation (airway relaxation) and improving alveolar perfusion (vasodilation), therefore improving blood–gas exchange capacity and patients’ pulmonary function.
Thus, it is highly likely that the maximal therapeutic efficacy of targeting PDE4 in the treatment of severe COPD depends upon three effectors of downstream intracellular cAMP elevation: 1) anti-inflammation (Lipworth 2005), 2) airway relaxation (Spina 2004), and 3) vasodilation. Sacrificing any one of these effectors with an isoenzyme-specific narrow-spectrum PDE4 inhibitor will compromise the effectiveness of the therapy. How, then, is the dose-limiting dilemma (nausea, vomiting, diarrhea, and arteritis) (Giembycz 2005; Spina 2004; Lipworth 2005) associated with the wide-spectrum PDE4 inhibition overcome?
Approaches for improving the therapeutic efficacy of PDE4 inhibitors
The fact that there are over 60 PDE isoenzymes encoded by 21 human PDE genes and at least 16 PDE4 isoenzymes from 4 PDE4 genes (Soderling and Beavo 2000; Conti et al 2003; Houslay and Adams 2003; Huai et al 2004) may render it highly possible that searching for isoenzyme-specific PDE4 inhibitors will yield low-efficacy agents. In addition, mechanisms for upregulation of PDE4 activity by cAMP-induced PDE4 gene expression and PKA-catalyzed phosphorylation–activation of PDE4 isoforms (Conti et al 2003; Wallace et al 2005) may very likely reverse an isoenzyme-specific, PDE4 inhibitor-produced elevation of intracellular cAMP level, and therefore the associated biological beneficial effects.
Noticing the low therapeutic ratio and insufficient clinical efficacy of the current generation of PDE4 inhibitors (cilomilast and roflumilast), Giembycz (2005) assumed that one potential means of improving the therapeutic ratio and safety of PDE4 inhibitors may lie in the development of compounds that have broader phosphodiesterase specificity and suggested dually targeting PDE4 and PDE1, PDE3, or PDE7 to enhance clinical efficacy. This approach seems to revisit the previously well-described PDE inhibitors such as theophylline or zardvarine (Schmidt et al 2000; Barnes 2003). It may lead to the dose-limiting drawback cycle again, because it is known that targeting cAMP-specific PDE3 is associated with an increase in morbidity and mortality in heart failure patients (Packer et al 1991).
In a news feature on Pharmiweb.com, there is a remark about Pfizer’s development of an inhaled dual-action PDE4/Spiriva® (long-lasting muscarinic antagonist tiotropium) combination product for COPD (Pharmiweb 2005). This combined dual action modality is a favorable approach for managing patients with severe COPD considering the presence of bronchoconstricting and inflammatory pathologies in the disease. In general, moderately targeting two mechanisms to reach the therapeutic goal should be more effective and safer than exploiting a single mechanism to its extended degree.
We have proposed to co-administer Ca2+ channel antagonist (CCA) to overcome PDE4 inhibitor-caused adverse effects, especially emetic responses (Wang and Wang 2005) because 1) stimuli that upregulate the cAMP pathway can increase the excitability of the neurons in the locus coeruleus (LC) that plays an important role in mediating neuronal emesis (Nestler et al 1999; Takeda et al 2001); 2) the PDE4D isoform is localized to neurons in the structures of the medulla including LC (Lamontagne et al 2001), which are consistent with a role for PDE4D in the emetic response; and 3) the LC neurons fire action potentials spontaneously, resulting from endogenous properties of the membrane conductance to a persistent inward Ca2+ current, which can be blocked by diltiazem (Williams et al 1984; Tokuyama and Ho 1996; Filosa and Putnam 2003). Thus, in the presence of CCA, even when a complete inhibition of PDE4D leads to an elevation of cAMP in the LC neurons, the LC cells will not be able to fire action potentials due to a blockade of the depolarizing L-type Ca2+ currents, therefore eliminating the intrinsic dose-limiting emetic pharmacological profile of the wide-spectrum PDE4 inhibition. In addition, CCAs also relax airway smooth muscles and exhibit anti-inflammatory effects, which may synergistically augment a PDE4 inhibitor’s therapeutic effects on COPD (Worley and Kotlikoff 1990; Szabo et al 1997; Brown et al 2004). The clinical use of CCAs in treatment of pulmonary hypertension in patients with COPD further support the combination therapy of a PDE4 inhibitor and a CCA (Sajkov et al 1997). One concern regarding the combined therapy is the possible difference between the pharmacokinetics of the two drugs that may compromise the expected outcomes. This pitfall can be eliminated by developing an agent with two pharmacophores in one chemical structure, therefore being able to simultaneously target two therapeutic mechanisms such as L-type Ca2+ channels and PDE4s (Wang and Wang 2005). This design should greatly improve the tolerability of PDE4 inhibition in COPD patients. We believe that it is worthwhile to carry out a randomized clinical trial to evaluate the safety and efficacy of dually targeting PDE4 and Ca2+ channels in managing the patients with severe COPD.
Conclusion
The unsatisfied efficacy of using PDE4 inhibitor roflumilast in treatment of severe and very severe COPD has raised concerns in the R&D community about the administrative approvability for the highly expected novel therapeutic modality in combating COPD. The extensive in vitro, in vivo, and clinical trial study data and the established clinical beneficial effects (anti-inflammation, bronchorelaxation, pulmonary vasodilation) associated with PDE4 inhibition strongly validate targeting PDE4 for controlling COPD. Development of an inhaled dual-action therapy such as PDE4 inhibitor and muscarinic antagonist may be a correct approach to bringing a PDE4 inhibitor to the demanding market. The other approach is using CCA to overcome PDE4 inhibitor-caused adverse effects, especially emetic responses and simultaneously enhance PDE4 inhibitor’s anti-inflammatory and bronchorelaxation, pulmonary vasodilation effects. Of course, development of a dual agent that has with two pharmacophores in one chemical structure, therefore being able to target PDE4 and L-type Ca2+ channels should also be able to improve the therapeutic window of PDE4 inhibition and may make available a new therapeutic approach to managing COPD.
References
- Alp S, Skrygan M, Schmidt WE, et al. Sildenafil improves hemodynamic parameters in COPD-an investigation of six patients. Pulm Pharmacol Ther. 2006;19:386–90. doi: 10.1016/j.pupt.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Altana. [Accessed 16 June 2006];ALTANA has withdrawn the EU marketing authorisation application for Daxas. [online] 2005a URL: http://www.altana.com/files/pressemeldungen/altana_pm_2005-11-15_b2771aa087.pdf.
- Altana. [Accessed 16 June 2006];Top line results of first one-year COPD study with Daxas [online] 2005b URL: http://www.altana.com/files/pressemeldungen/altana_pm_2005-07-01_d26089802f.pdf.
- Baillie G, MacKenzie SJ, Houslay MD. Phorbol 12-myristate 13-acetate triggers the protein kinase A-mediated phosphorylation and activation of the PDE4D5 cAMP phosphodiesterase in human aortic smooth muscle cells through a route involving extracellular signal regulated kinase (ERK) Mol Pharmacol. 2001;60:1100–11. doi: 10.1124/mol.60.5.1100. [DOI] [PubMed] [Google Scholar]
- Barbera JA, Roger N, Roca J, et al. Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet. 1996;347:436–40. doi: 10.1016/s0140-6736(96)90011-2. [DOI] [PubMed] [Google Scholar]
- Barbera JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21:892–905. doi: 10.1183/09031936.03.00115402. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Theophylline: new perspectives for an old drug. Am J Respir Crit Care Med. 2003;167:813–18. doi: 10.1164/rccm.200210-1142PP. [DOI] [PubMed] [Google Scholar]
- Brown DM, Donaldson K, Borm PJ, et al. Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiology Lung Cell Mol Physiol. 2004;286:L344–53. doi: 10.1152/ajplung.00139.2003. [DOI] [PubMed] [Google Scholar]
- Bundschuh DS, Eltze M, Barsig J, et al. In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther. 2001;297:280–90. [PubMed] [Google Scholar]
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233–47. doi: 10.1016/j.str.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Conti M, Richter W, Mehats C, et al. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–6. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- de Witt BJ, Marrone JR, Kadowitz PJ. Comparison of responses to siguazodan, rolipram, and zaprinast in the feline pulmonary vascular bed. Eur J Pharmacol. 2000;406:233–8. doi: 10.1016/s0014-2999(00)00501-x. [DOI] [PubMed] [Google Scholar]
- Doi M, Nakano K, Hiramoto T, et al. Significance of pulmonary artery pressure in emphysema patients with mild-to-moderate hypoxemia. Respir Med. 2003;97:915–20. doi: 10.1016/s0954-6111(03)00115-x. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol Cell Physiol. 2003;284:C145–55. doi: 10.1152/ajpcell.00346.2002. [DOI] [PubMed] [Google Scholar]
- Gamble E, Grootendorst DC, Brightling CE, et al. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:976–82. doi: 10.1164/rccm.200212-1490OC. [DOI] [PubMed] [Google Scholar]
- Giembycz MA. Cilomilast: a second generation phosphodiesterase 4 inhibitor for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2001;10:1361–79. doi: 10.1517/13543784.10.7.1361. [DOI] [PubMed] [Google Scholar]
- Giembycz MA. 4D or not 4D - the emetogenic basis of PDE4 inhibitors uncovered? Trends Pharmacol Sci. 2002;23:548. doi: 10.1016/s0165-6147(02)02089-8. [DOI] [PubMed] [Google Scholar]
- Giembycz MA. Phosphodiesterase-4: selective and dual-specificity inhibitors for the therapy of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:326–33. doi: 10.1513/pats.200504-041SR. discussion 340–1. [DOI] [PubMed] [Google Scholar]
- GOLD. [Accessed 16 June 2006];Executive summary: Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease [online] 2005 URL: http://www.goldcopd.org/Guidelineitem.asp?l1=2&l2=1&intId=996.
- Holbrook M, Gozzard N, James T, et al. Inhibition of bronchospasm and ozone-induced airway hyperresponsiveness in the guinea-pig by CDP840, a novel phosphodiesterase type 4 inhibitor. Br J Pharmacol. 1996;118:1192–200. doi: 10.1111/j.1476-5381.1996.tb15523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai Q, Wang H, Sun Y, et al. Three-dimensional structures of PDE4D in complex with roliprams and implication on inhibitor selectivity. Structure. 2003;11:865–73. doi: 10.1016/s0969-2126(03)00123-0. [DOI] [PubMed] [Google Scholar]
- Huai Q, Liu Y, Francis SH, et al. Crystal structures of phosphodiesterases 4 and 5 in complex with inhibitor 3-isobutyl-1-methylxanthine suggest a conformation determinant of inhibitor selectivity. J Biol Chem. 2004;279:13095–101. doi: 10.1074/jbc.M311556200. [DOI] [PubMed] [Google Scholar]
- Huang Z, Ducharme Y, Macdonald D, et al. The next generation of PDE4 inhibitors. Curr Opin Chem Biol. 2001;5:432–8. doi: 10.1016/s1367-5931(00)00224-6. [DOI] [PubMed] [Google Scholar]
- Kumar RK, Herbert C, Thomas PS, et al. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J Pharmacol Exp Ther. 2003;307:349–55. doi: 10.1124/jpet.103.053819. [DOI] [PubMed] [Google Scholar]
- Lagente V, Martin-Chouly C, Boichot E, et al. Selective PDE4 inhibitors as potent anti-inflammatory drugs for the treatment of airway diseases. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):131–6. doi: 10.1590/s0074-02762005000900023. [DOI] [PubMed] [Google Scholar]
- Lamontagne S, Meadows E, Luk P, et al. Localization of phosphodiesterase-4 isoforms in the medulla and nodose ganglion of the squirrel monkey. Brain Res. 2001;920:84–96. doi: 10.1016/s0006-8993(01)03023-2. [DOI] [PubMed] [Google Scholar]
- LeadDiscovery. [Accessed 16 June 2006];Phosphodiesterase 4 (PDE4) & phosphodiesterase 5 (PDE5) inhibitors [online] 2006 URL: http://www.leaddiscovery.co.uk/reports/Phosphodiesterase-library.html.
- Lee ME, Markowitz J, Lee JO, et al. Crystal structure of phosphodi-esterase 4D and inhibitor complex(1) FEBS Lett. 2002;530:53–8. doi: 10.1016/s0014-5793(02)03396-3. [DOI] [PubMed] [Google Scholar]
- Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365(9454):167–75. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- Liu H, Palmer D, Jimmo SL, et al. Expression of phosphodiesterase 4D (PDE4D) is regulated by both the cyclic AMP-dependent protein kinase and mitogen-activated protein kinase signaling pathways. A potential mechanism allowing for the coordinated regulation of PDE4D activity and expression in cells. J Biol Chem. 2000;275:26615–24. doi: 10.1074/jbc.M001634200. [DOI] [PubMed] [Google Scholar]
- Mehats C, Jin SL, Wahlstrom J, et al. PDE4D plays a critical role in the control of airway smooth muscle contraction. FASEB J. 2003;17:1831–41. doi: 10.1096/fj.03-0274com. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. [Accessed 16 June 2006];Sildenafil for Chronic Obstructive Pulmonary Disease [online] 2006 URL: http://www.clinicaltrials.gov/ct/gui/show/NCT00104637.
- Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biol Psychiatry. 1999;46:1131–9. doi: 10.1016/s0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- Odingo JO. Inhibitors of PDE4: a review of recent patent literature. Expert Opinion on Therapeutic Patents. 2005;15:773–87. [Google Scholar]
- Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure: the PROMISE Study Research Group. N Engl J Med. 1991;325:1468–75. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Buist AS, Calverley PM, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- Pharmiweb. [Accessed 16 June 2006];Daxas deal leaves Altana short of breath [online] 2005 URL: http://www.pharmiweb.com/features/feature.asp?ROW_ID=624.
- Plexxikon, Inc. [Accessed 16 June 2006];PDE4 inhibitor factor sheet [online] 2005 URL: http://www.plexxikon.com/plx-pde4.pdf.
- Profita M, Chiappara G, Mirabella F, et al. Effect of cilomilast (Ariflo) on TNF-alpha, IL-8, and GM-CSF release by airway cells of patients with COPD. Thorax. 2003;58:573–9. doi: 10.1136/thorax.58.7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe KF, Bateman ED, O’Donnell D, et al. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366(9485):563–71. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- Robichaud A, Stamatiou PB, Jin SL, et al. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110:1045–52. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajkov D, Wang T, Frith PA, et al. A comparison of two long-acting vasoselective calcium antagonists in pulmonary hypertension secondary to COPD. Chest. 1997;111:1622–30. doi: 10.1378/chest.111.6.1622. [DOI] [PubMed] [Google Scholar]
- Schmidt DT, Watson N, Dent G, et al. The effect of selective and non-selective phosphodiesterase inhibitors on allergen- and leukotriene C(4)-induced contractions in passively sensitized human airways. Br J Pharmacol. 2000;131:1607–18. doi: 10.1038/sj.bjp.0703725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly RT, Roehl A, Weissmann N, et al. Subthreshold doses of specific phosphodiesterase type 3 and 4 inhibitors enhance the pulmonary vasodilatory response to nebulized prostacyclin with improvement in gas exchange. J Pharmacol Exp Ther. 2000;292:512–20. [PubMed] [Google Scholar]
- Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000;12:174–9. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Soto FJ, Hanania NA. Selective phosphodiesterase-4 inhibitors in chronic obstructive lung disease. Curr Opin Pulm Med. 2005;11:129–34. doi: 10.1097/01.mcp.0000151715.58124.9e. [DOI] [PubMed] [Google Scholar]
- Spina D. Phosphodiesterase-4 inhibitors in the treatment of inflammatory lung disease. Drugs. 2003;63:2575–94. doi: 10.2165/00003495-200363230-00002. [DOI] [PubMed] [Google Scholar]
- Spina D. The potential of PDE4 inhibitors in respiratory disease. Curr Drug Targets Inflamm Allergy. 2004;3:231–6. doi: 10.2174/1568010043343822. [DOI] [PubMed] [Google Scholar]
- Szabo C, Hasko G, Nemeth ZH, et al. Calcium entry blockers increase interleukin-10 production in endotoxemia. Shock. 1997;7:304–7. doi: 10.1097/00024382-199704000-00011. [DOI] [PubMed] [Google Scholar]
- Takeda N, Morita M, Horii A, et al. Neural mechanisms of motion sickness. J Med Invest. 2001;48:44–59. [PubMed] [Google Scholar]
- Tenor H, Hatzelmann A, Church MK, et al. Effects of theophylline and rolipram on leukotriene C4 (LTC4) synthesis and chemotaxis of human eosinophils from normal and atopic subjects. Br J Pharmacol. 1996;118:1727–35. doi: 10.1111/j.1476-5381.1996.tb15598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama S, Ho IK. Inhibitory effects of diltiazem, an L-type Ca2+ channel blocker, on naloxone-increased glutamate levels in the locus coeruleus of opioid-dependent rats. Brain Res. 1996;722:212–6. doi: 10.1016/0006-8993(96)00187-4. [DOI] [PubMed] [Google Scholar]
- Wallace DA, Johnston LA, Huston E, et al. Identification and characterization of PDE4A11, a novel, widely expressed long isoform encoded by the human PDE4A cAMP phosphodiesterase gene. Mol Pharmacol. 2005;67:1920–34. doi: 10.1124/mol.104.009423. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang T. Novel approaches to using PDE4 inhibitors for antihypertensive therapy. Curr Opin Investig Drugs. 2005;6:283–8. [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner SA, et al. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13:137–56. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- Worley JF, 3rd, Kotlikoff MI. Dihydropyridine-sensitive single calcium channels in airway smooth muscle cells. Am J Physiol. 1990;259:L468–80. doi: 10.1152/ajplung.1990.259.6.L468. [DOI] [PubMed] [Google Scholar]
- Wollin L, Bundschuh DS, Wohlsen A, et al. Inhibition of airway hyperresponsiveness and pulmonary inflammation by roflumilast and other PDE4 inhibitors. Pulm Pharmacol Ther. 2005 Oct 27; doi: 10.1016/j.pupt.2005.09.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]