Abstract
Study objectives
Patients with chronic obstructive pulmonary disease (COPD) have low exercise capacity and low content of high energetic phosphates in their skeletal muscles. The aim of the present study was to investigate whether creatine supplementation together with exercise training may increase physical performance compared with exercise training in patients with COPD.
Design
In a randomized, double-blind, placebo-controlled study, 23 patients with COPD (forced expiratory volume in one second [FEV1] < 70% of predicted) were randomized to oral creatine (n = 13) or placebo (n = 10) supplementation during an 8-week rehabilitation programme including exercise training. Physical performance was assessed by Endurance Shuttle Walking Test (ESWT), dyspnea and leg fatigue with Borg CR-10, quality of life with St George’s Respiratory Questionnaire (SGRQ). In addition, lung function test, artery blood gases, grip strength test, muscle strength and fatigue in knee extensors were measured.
Results
COPD patients receiving creatine supplementation increased their average walking time by 61% (ESWT) (p < 0.05) after the training period compared with 48% (p = 0.07) in the placebo group. Rated dyspnea directly after the ESWT decreased significantly from 7 to 5 (p < 0.05) in the creatine group. However, the difference between the groups was not statistically significant neither in walking time nor in rated dyspnea. Creatine supplementation did not increase the health related quality of life, lung function, artery blood gases, grip strength and knee extensor strength/fatigue.
Conclusions
Oral creatine supplementation in combination with exercise training showed no significant improvement in physical performance, measured as ESWT, in patients with COPD compared with exercise training alone.
Keywords: COPD, oral creatine supplementation, physical training
Introduction
Patients with chronic obstructive pulmonary disease (COPD) are limited by dyspnea, especially during physical activities (Marcel 1989). As a consequence the patients avoid activities resulting in further impairment of the patients’ physical condition (Olopade et al 1992). Progression of the disease is associated with increased dyspnea, decreased physical performance, skeletal muscular weakness, and high hospitalization rates. All have a negative impact on quality of life (McSweeny et al 1982).
COPD is a disease with effects both in the lungs and organs outside the lungs. For instance skeletal muscle dysfunction, weight loss, cardiovascular and nervous system abnormalities, and osteoporosis are common systemic manifestations of COPD. It is therefore of great importance that therapy for COPD should treat the multicomponent nature of the disease (Agusti 2005). Pulmonary rehabilitation programmes for COPD patients improve the physical performance and the quality of life and are nowadays a cornerstone in the treatment of patients with COPD (Wijkstra et al 1994; Donner and Muir 1997).
The immediate energy source for a muscle contraction is adenosine triphosphate (ATP) which is resynthesized from creatine phosphate (Soderlund and Hultman 1986). Creatine is a naturally occurring compound in the body and is also found in meat and some fishes such as herring (Grzyb and Skorkowski 2005).
Patients with COPD have a lower content of high energetic phosphates in their skeletal and respiratory muscles compared with healthy individuals (Gertz et al 1977; Hughes et al 1983; Jakobsson et al 1990). Among athletics, oral supplementation with creatine has been used in physical training in short-lasting muscle work and is associated with an increase in exercise capacity. Besides this effect, supplementation with creatine has been showed to increased muscular strength and volume (Harris et al 1993).
In patients with chronic heart failure, creatine supplementation has shown significantly increased skeletal muscular performance compared with a placebo group (Gordon et al 1995). Patients with chronic heart failure have similar metabolic changes in their skeletal muscles to patients with COPD (Opasich et al 1996). In the present study the aim was to assess whether creatine supplementation and exercise training could increase physical performance compared with exercise training in patients with COPD.
Materials and methods
Patients
Between January 2001 and March 2003, 23 patients with COPD were recruited to the present study. All patients participated in the pulmonary rehabilitation program including exercise training at the Karolinska University Hospital in Solna or at the University Hospital in Linköping. All patients had COPD according to the British Thoratic Society guidelines (forced expiratory volume in one second [FEV1] < 80% of predicted, FEV1/vital capacity [VC] < 70%) (BTS 1997), and were all in a clinically stable phase. Patients with symptomatic cardiac disease, neurological or orthopedic disability with mobility impairments, and unwillingness to participate in the study were not included. Of the 23 participants, there were 13 women and 10 men, with a mean age of 66 ± 6 (mean % of predicted was ± SD) years. FEV1 was 1.2 ± 0.6, FEV1 43 ± 17 and arterial oxygen partial pressure [PaO2] was 9.6 ± 1.5 kPa.
All patients inhaled short- and long-acting brochodilators, 13 patients inhaled corticosteroid, two patients had both oral and inhaled steroids, and one patient only had oral steroids. Four patients were treated with diuretics, nine with oral N-acetylcysteine and five with oral theophylline. The patients’ medical treatment was unchanged during the study period. One patient had never smoked (COPD caused by alpha-1-antitrypsin deficiency), four were current smokers, and 19 patients were ex-smokers.
The study was approved by the Local Ethics Committee at the Karolinska University Hospital (Dnr: 00-404).
Spirometry and arterial blood samples
Spirometry was performed by a Vitalograph Compact C (Förbandsmaterial AB, Gothenburg, Sweden). Normal values were calculated according to European Coal and Steel Union guidelines (ECSU 1983). An arterial blood sample was taken with the patient seated and spontaneously breathing air. The samples were analyzed within 15 minutes. Both spirometry and arterial blood samples were assessed before and after the rehabilitation programme.
Pulmonary rehabilitation programme and health-related quality of life
The eight-week rehabilitation programme consisted of exercise training and education, with two training sessions a week supervised by of a specially-trained physiotherapist. Each training sessions consisted of eight different exercises: ergometer cycling, arm muscle training with dumbbells, rising from a stool, theraband exercises for the shoulder girdle, thigh muscle training with weight cuffs, getting up onto a low stool, abdominal muscle training, and flexibility exercises for the thorax and adjacent joints.
The aim of the ergometer cycling was to be able to exercise without interruption for 30 minutes. The load was chosen by the physiotherapist based on the degree of dyspnea and leg fatigue rated on a Borg CR-10 scale and/or whether the patient was forced to stop pedaling at very short intervals (Borg 1982). The maximum tolerated degree of dyspnea was 7 out of 10. The load during cycling was increased when the patient could exercise without interruption for 30 minutes. Oxygen saturation and heart rate were recorded via pulse oximetry before, during, and at the end of every ergometer cycling session.
In the other exercises, the patients performed 15 repetitions for each exercise repeated three times. In the exercises which required different loads such as weight cuffs, the physiotherapist guided the patients to identify the weight that after 15 repetitions was so heavy that it could not be lifted once more. During the training session, patients were given instructions in pursed lips breathing. The length of the training sessions varied between 60–75 minutes depending on need of recovery between the different exercises. During the 8 week programme the patients also took part in an educational programme including lung physiology, pulmonary disease, breathing techniques, secretion clearance techniques, medications, and nutrition. all patients received a home training programme after the training period and were encouraged to be physically active in daily life. Health-related quality of life was assessed with St George’s Respiratory Questionnaire (SGRQ) (Jones et al 1992) at baseline and after the training programme. A decrease in score indicates a better health-related quality of life and a change of 4 units is of clinical relevance according to Jones (2002).
Creatine supplementation
Thirteen patients were randomized to the creatine group and ten to the placebo group. There were no differences between the groups regarding clinical background data (Table 1). All patients received oral and written instructions on how to ingest the compound. All involved investigators were blinded to patients receiving creatine supplementation. The powder (glucose or creatine) was delivered in small test tubes and was taken orally during 8 weeks. The dosage of creatine was 0.3 g/kg body weight/day during seven days and then 0.07 g/kg body weight/day during the remaining 7 weeks. The patients dissolved the powder in hot liquid. The intake was divided in four doses per day during the first week and one dose per day during the remaining weeks.
Table 1.
Patient characteristics at baseline
| Placebo group n = 10 | Creatine group n = 13 | |
|---|---|---|
| Men/women | 4/6 | 6/7 |
| Age, years | 64 ± 6 | 67 ± 6 |
| FEV1 % pred | 42 ± 12 | 44 ± 21 |
| PaO2, kPa | 9.7 ± 1.5 | 9.5 ± 1.5 |
| PCO2, kPa | 5.0 ± 0.7 | 4.9 ± 0.3 |
| Weight, kg | 63 ± 14 | 72 ± 16 |
| BMI, kg/m2 | 22 ± 3 | 25 ± 4 |
| ISWT, m | 290 ± 67 | 305 ± 107 |
Note: Values are presented as mean ± SD.
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in one second; ISWT, Incremental Shuttle Walking Test; m, metres; PaO2, arterial oxygen partial pressure; PCO2, arterial carbon dioxide partial pressure; SD, standard deviation.
On three occasions, at one week from start and at eight weeks and two months after the end of the creatine intake, the patients collected the urine during 24 hours to get a rough indication that the patients had ingested creatine. These data were not presented.
Walking tests
The patients performed an Incremental shuttle walking test (ISWT) (Singh et al 1992). Before the test and directly after, oxygen saturation, heart and breathing rates were measured as well as dyspnea and leg fatigue. Oxygen saturation and heart rate were measured using pulse oximeters (Model 8500, Nonin Medical Inc, MN, USA/Model 512, Novametrix Medical inc. Wallingford, CT, USA) (Wahr et al 1995). The patients rated their dyspnea and leg fatigue according to a Borg CR-10 scale (Borg 1982).
Depending on the walking distance during the ISWT, walk speed was correlated for the Endurance Shuttle Walking Test (ESWT) (Revill et al 1999). The patients performed both an ISWT and an ESWT at baseline, but only ESWT at the 8 weeks follow up after the training programme.
Measurements of muscle strength and fatigue
The grip strength was measured with a Jamar Dynamometer/Grippit (Jamardynamometer standard, BB44JR, WS Routband Comp LTD, Albionmille, Helmshore, Rossendale, UK/Grippit type G100, serial number 197100, AB detector, Gothenburg, Sweden) at baseline and after 8 weeks. The patients were told to squeeze maximally during five seconds. After resting for one minute the patients repeated the test with the same instructions for the second and third trial. The mean value of the three trials was recorded (Mathiowetz et al 2000).
In 12 patients (7 women, mean age 63 ± 8 years and 5 men 70 ± 2 years), maximal voluntary strength and fatigue in right knee extensors muscles were assessed; seven from the creatine group and five from the placebo group. Maximal voluntary strength and fatigue in right knee extensors muscles were measured with an isokinetic dynamic dynamometer (Kin-Com 500H, Chattecx Corp., Chattanooga TN, USA) during standardized perpetual verbal encouragement (Stahle and Tollback 2001). Maximal voluntary concentric strength was measured at 30°/s angular velocity in a movement range from 90° to 30° knee flexion. Mean peak torque of the three curves with good reproducibility was calculated. After a five minutes rest, muscle fatigue was evaluated by 3 bouts of 30 maximal consecutive concentric repetitions at 180°/s angular velocity in a movement range from 90° to 30° knee flexion (Colliander et al 1988). Fatigue was evaluated by calculating the decline in strength within and between bouts. Thus, the declines of mean peak torque (PT) for repetitions 1–10, 11–20, and 21–30 were calculated. Further, the decline of the summed PT per bout was calculated and compared between bouts. One learning-session was performed at least two days or within one week before the actual baseline testing.
Statistics
The primary outcome was physical performance measured with ESWT. The sample size was powered as other studies showing effects of pulmonary rehabilitation programmes (Lacasse et al 1996).
The group results are presented as arithmetical means (m) and confidence intervals or median and range unless otherwise stated. To test differences between groups unpaired Student’s t-test and Wilcoxon Rank sum were used, and within group analysis by paired wise Student’s t-test.
Results
Effect of creatine supplementation on lung function, arterial blood gases, and body weight
No significant changes in FEV1 and arterial blood gases neither within or between the creatine and the placebo group before or after the training programme were found. Body weight was unchanged after creatine supplementation in both groups.
Effect of creatine supplementation on Endurance Shuttle Walking Test and Health-Related Quality of Life
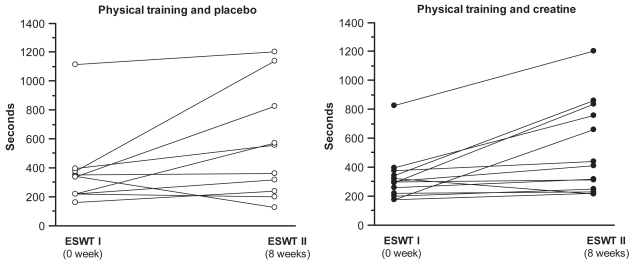
Patients with creatine supplementation increased their average walking time by 61% from 320 (95% confidence interval [CI] 218.9 to 421.4) to 515 (95% CI 324 to 705.9) seconds in ESWT (p < 0.01) after the training programme, while the group with placebo treatment increased theirs by 48% from 372 (95% CI 177.2 to 567.4) to 552 seconds (95% CI 276.6 to 828.2) (p = 0.07). The difference in walking time (ESWT) between the two groups from baseline and after 8 weeks training programme was not statistically significant (p = 0.8) (Figure 1).
Figure 1.
Individual walking time during Endurance Shuttle Walking Test (ESWT) for the creatine (n = 13) and the placebo groups (n = 10) before and after creatine/placebo supplementation and the training programme. Within the creatine group difference: p < 0.01; within the placebo group difference: p = 0.0741. Creatine group versus placebo group (p = 0.8).
The patients’ median rated dyspnea directly after the ESWT, before and after the 8 week programme, decreased significantly from 7 to 5 (p < 0.05) in the creatine group. In the placebo group the dyspnea index decreased from 5 to 4 (p = 0.28). The median rated leg fatigue tended to decrease from 3 to 2 (p = 0.06) in the creatine group (n = 10) and from 2 to 0.25 in the placebo group (n = 6) (p = 0.36). In comparison between the two groups there was no significant difference in median rated dyspnea or leg fatigue after the 8 weeks programme.
Oxygen saturation, heart rate and breathing after the 8 weeks programme, assessed directly after the ESWT, showed no significant difference within or between the groups.
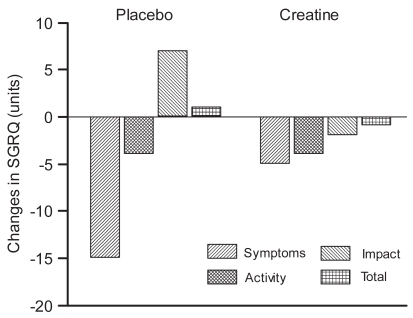
Regarding health-related quality of life no significant differences in the various dimensions (symptoms, activity, and impacts) or in total score after the training programme were found. The dimension of symptom decreased with a significant difference (p < 0.05) within the placebo group since two patients had 40 units in difference from baseline compared with a mean of 7 units change for the rest of the group. The creatine group also decreased in this dimension with a mean value of 5 units, but did not reach significant difference. The change in units, at baseline, and after the training programme regarding the dimensions and total score are shown in Figure 2.
Figure 2.
Change in St George’s Respiratory Questionnaire (SGRQ) in the Creatine group (n = 9) and Placebo group (n = 8) after oral supplementation of creatine/placebo and the training programme. Mean values are presented.
Note:*p < 0.05 within the group.
Effect of creatine supplementation on muscle strength and fatigue
There were no significant differences in grip strength between the creatine group (n = 12) and the placebo group (n = 10) before and after the training programme.
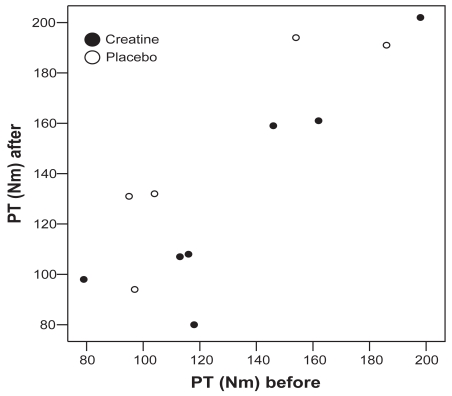
No differences in maximal knee extensor strength within or between the creatine (n = 7, p = 0.74) and placebo (n = 5, p = 0.07) groups before or after the training programme (p = 0.08) (Figure 3). Maximal knee extensor strength for the total group was 131 Nm (95% CI 106 to 155) and 138 Nm (95% CI 111 to 165), before and after the training programme (p = 0.26).
Figure 3.
Knee extensor peak torque (PT) (Nm) at 30°/s, for the creatine (n = 7) and the placebo group (n = 5) before and after an 8 weeks training period.
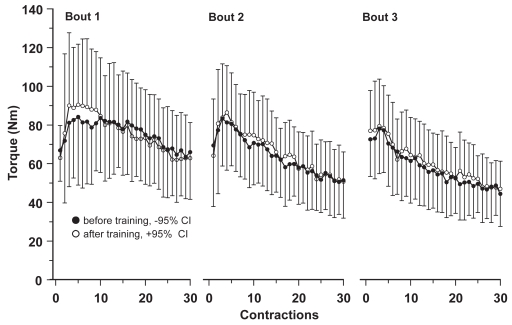
The decline in mean and PT during the fatigue tests did not differ within or between the groups before or after the training programme. The results from the leg muscle fatigue test in the creatine group (n = 7) are shown in Figure 4.
Figure 4.
Mean peak torque (Nm) for 3 bouts of 30 consecutive maximal concentric knee extensor contractions for the creatine group before (n = 7) (solid dots) and after (n = 6) (open dots) an 8 week training period. Each data point represents group mean values (with 95% confidence intervals) per contraction.
All results are summarized in Table 2.
Table 2.
Effects of creatine supplementation during 8 weeks rehabilitation compared with placebo and rehabilitation in patients with COPD. Data are presented as group mean values (with 95% confidence intervals) from baseline and after 8 weeks rehabilitation. Data for estimating dyspnoea and leg fatigue are presented as median and range. Statistically significant differences, $:p < 0.05; $$: p < 0.01(within group difference from baseline)
| Placebo group n = 10
|
Creatine group n = 13
|
P-value
|
|||
|---|---|---|---|---|---|
| Baseline | 8 weeks rehab | Baseline | 8 weeks rehab | Between-groups differences | |
| Pulmonary function | |||||
| FEV1, l | 1.17(0.89 to 1.44) | 1.22(0.91 to 1.54) | 1.27(0.82 to 1.73) | 1.33(0.86 to 1.80) | 0.9664 |
| FEV1% pred | 41.9(33.08 to 50.72) | 43.9(33.74 to 54.06) | 44(31.51 to 57.11) | 46.2(33.48 to 58.82) | 0.8600 |
| Arterial blood gases | |||||
| PaO2, kPa | 9.69(8.65 to 10.74) | 9.73(8.27 to 11.19) | 9.51(8.59 to 10.12) | 9.32(8.61 to 10.03) | 0.9947 |
| PaCO2, kPa | 5.04(4.54 to 5.54) | 5.28(4.68 to 5.88)$ | 4.93(4.77 to 5.08) | 4.98(4.77 to 5.19) | 0.1191 |
| Body weight | |||||
| Kg | 63.32(53.57 to 73.07) | 64.36(54.52 to 74.20)$ | 72.11(62.58 to 81.64) | 73.35(63.24 to 83.47)$ | 0.645 |
| BMI | 22.3(20 to 24.5) | 22.6(20.5 to 24.7) | 24.8(22.2 to 27.3) | 25.2(22.4 to 28.1)$ | 0.766 |
| Walking test | |||||
| ESWT, s | 372.3(177.2 to 567.4) | 552.4(276.6 to 828.2) | 320.2(219 to 421.4) | 514.9(324 to 705.9)$$ | 0.8913 |
| Directly after ESWT | 106.2(88.8 to 123.6) | 110.5(93.2 to 127.8)$$ | 108(97.8 to 118.2) | 113.4(107.2 to 119.6)$$ | 0.3911 |
| Heart rate/min | 24.6(22 to 27.2) | 23.6(21.8 to 25.4)$$ | 26.7(22 to 31) | 25.9(22.2 to 29.5)$$ | 0.7773 |
| Breathing rate/min | 90.6(86.9 to 94.2) | 88.6(82.6 to 94.6)$ | 88.2(84.7 to 91.7) | 88.9(85.3 to 92.4)$$ | 0.6725 |
| SaO2, % dyspnea, Borg 0–10 | 5(1–9) | 4(0.5–8) | 7(0–10) | 5(4–7)$ | 0.2166 |
| Leg fatigue, Borg 0–10 | 2(0–5)(n = 12) | 0.25(0–5)(n = 6) | 3(0–10)(n = 10) | 2(0–9)(n = 6) | 0.9131 |
| Muscle strength | |||||
| Grip strength, Nm n = 12/10 | 270.5(201.7 to 339.3) | 287.1(202.8–371.4)$ | 304(257.3 to 350.8) | 310.8(268.1 to 353.4)$ | 0.9151 |
| Leg muscle strength, Nm n = 7/5 | 127(77 to 178) | 148(95 to 202) | 133(97 to 169) | 131(90 to 171) | 0.0820 |
| SGRQ (n = 9/8) | |||||
| Total score | 24(30.1 to 54.7) | 43.1(27.7 to 58.5)$ | 45.2(30.9 to 59.5) | 43.8(29.6 to 57.9) | 0.7726 |
| Symptoms | 44.6(21.8 to 67.4) | 27.6(10 to 45.3) | 48.1(29.1 to 67.1) | 43(26 to 60) | 0.1779 |
| Activity | 59(48.4 to 69.6) | 56.8(45.1 to 68.4) | 62.1(47.7 to 76.5) | 57.7(40.7 to 74.6) | 0.6987 |
| Impact | 31.8(17 to 46.5) | 38.5(20.6 to 56.5) | 34.8(19.9 to 49.7) | 32.6(17.7 to 47.4) | 0.3595 |
Abbreviations: BMI, body mass index; ESWT, Endurance Shuttle Walking Test; FEV1, forced expiratory volume in one second; PaO2, arterial oxygen partial pressure; PCO2, arterial carbon dioxide partial pressure; SaO2, arterial oxygen saturation; SGRQ, St George’s Respiratory Questionnaire.
Discussion
The aim of the present study was to investigate whether creatine supplementation may have an additive effect on physical performance in COPD patients participating in a pulmonary rehabilitation programme including exercise training. We failed to prove any differences in walking time (ESWT) after the training period between the groups receiving creatine supplementation or placebo. Our data did not show that exercise training and creatine supplementation improved walking time in ESWT in relative to exercise training and placebo.
All patients included in the study were referred from the Primary Health Care in Stockholm and Linköping County and seemed to be representative for a group of patients with moderate to severe COPD. Today the effect of exercise training in patients with COPD is well established and there are several studies including patients with moderate to severe stage of COPD showing benefits of pulmonary rehabilitation (Wijkstra et al 1994; Donner and Muir 1997; Tiep 1997). When we formed our programme we followed the evidence-based guidelines as outlined in “Pulmonary rehabilitation” (ACCP/AACVPR 1997). There were no difficulties for the patients to accomplish the exercise programme and reach the desired levels of dyspnea. The ESWT test is a submaximal test of physical performance and should therefore reflect a person’s daily physical performance better than a maximal test. The test has also showed sensitivity to changes after exercise training in COPD patients (Revill et al 1999).
In the present study, ESWT improved in both groups. However, the improvement was significant only in the creatine group. There was no significant difference when comparing the results of the ESWT between the groups. Hence, we failed to show any benefit of creatine supplementation in combination with exercise training.
In healthy subjects an increase of 25%–30% of total creatine concentration in the muscles was found after oral creatine supplementation, as well as a significant increase in maximal short-lasting muscle work after oral creatine supplementation. The size of the increase in strength power was approximately 4%–6% (Balsom et al 1993).
Gordon and colleagues (1995) found significantly increased skeletal muscular performance in patients with chronic heart failure compared with a placebo group after oral supplementation of creatine during ten days without any exercise training. They concluded that both patients with heart failure and healthy individuals with low levels of creatine in the muscles show better muscle function after oral supplementation.
Studies of skeletal muscle metabolite concentrations in leg muscle of patients with COPD have shown deranged muscle metabolism with decreased concentrations of ATP and creatine phosphate (Jakobsson and Jorfeldt 1995). A positive effect in physical performance was therefore expected when creatine was supplemented to training.
A recently reported study, also investigating the effect of creatine supplementation on physical performance in patients with COPD, Fuld and colleagues (2005), reports an increase in fat-free-mass, upper and lower limb muscle strength and endurance, but no improvement in whole body exercise capacity. In this study the patients had a loading phase of supplements for 14 days, then a maintenance phase during a 10 week training programme.
In contrary to Fuld and colleagues (2005) our patients started supplementation with creatine/placebo and exercise training at the same time with one week higher intake of creatine and then a maintaining phase during the rest of the training period, ie, seven weeks. The quantity, duration, and intensity of the training sessions seem to be equal to Fuld’s study except for the fact that we did not provide a home training program to the participants during the exercise programme.
The lacking effect in our study may be due to the fact that we used the submaximal performance test and that the sample size in measuring lower limb muscle strength was low. Also, our programme was not formed to train specified muscle groups. Furthermore, we have no data of skeletal muscle metabolic concentration to show that oral creatine supplementation was accompanied by increased concentration of high-energy phosphates in the leg muscles.
The questionnaire for measuring health-related quality of life (SGRQ) was found suitable for the group of COPD-patients included in the present study. That the degree of physical ability affects the quality of life, and that rehabilitation programmes for COPD patients have a positive effect on physical performance and quality of life has been shown by Jones and colleagues (1992). In the creatine group, all three dimensions (symptoms, activity and impact) and the total score in SGRQ changed in a positive direction, but did not reach clinical significance (Jones 2002). In the placebo group there was a surprisingly large improvement in the dimension of symptoms (15 units) and when we analyzed the data we found that two patients had 40 units in difference from baseline compared with a mean of 7 units change for the rest of the group (n = 6). We speculate that these two patients have had an exacerbation close to baseline and recovered during the training programme.
There is to our knowledge only one study published investigating the effects of creatine supplementation in patients with COPD (Fuld et al 2005). The results in the present study failed to show an effect of oral creatine supplementation in contrast to Fuld and colleagues (2005). Further research in this field to find individual modalities of treatment for patients with COPD is needed (Griffiths and Proud 2005).
Our conclusion is that because COPD patients are a very heterogeneous group, they could probably respond very differently to oral creatine supplementation and we need larger studies to find whether oral creatine supplementation is of any benefit for patients with COPD or not.
References
- [ACCP/AACVPR] American College of Chest Physicians; American Association of Cardiovascular and Pulmonary Rehabilitation. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based guidelines. ACCP/AACVPR Pulmonary Rehabilitation Guidelines Panel. American College of Chest Physicians. American Association of Cardiovascular and Pulmonary Rehabilitation. Chest. 1997;112:1363–96. [PubMed] [Google Scholar]
- Agusti AG. COPD, a multicomponent disease: implications for management. Respir Med. 2005;99:670–82. doi: 10.1016/j.rmed.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Balsom PD, Ekblom B, Soderlund K, et al. Creatine supplementation and dynamic high-intensity intermittent exercise. Scand J Med Sci Sports. 1993;3:143–9. [Google Scholar]
- Borg G. A category scale with ratio properties for intermodel and interindividual comparisons. In: Geissler HG, Petzolds P, editors. Psychophysical judgement and the process of perception. Amsterdam: North-Holland Publ Co; 1982. pp. 25–34. [Google Scholar]
- [BTS] British Thoracic Society. BTS guidelines for the management of chronic obstructive pulmonary disease. The COPD Guidelines Group of the Standards of Care Committee of the BTS. Thorax. 1997;52:S1–28. [PMC free article] [PubMed] [Google Scholar]
- Colliander EB, Dudley GA, Tesch PA. Skeletal muscle fiber type composition and performance during repeated bouts of maximal, concentric contractions. Eur J Appl Physiol Occup Physiol. 1988;58:81–6. doi: 10.1007/BF00636607. [DOI] [PubMed] [Google Scholar]
- Donner CF, Muir JF. Selection criteria and programmes for pulmonary rehabilitation in COPD patients. Rehabilitation and Chronic Care Scientific Group of the European Respiratory Society. Eur Respir J. 1997;10:744–57. [PubMed] [Google Scholar]
- [ECSU]European Coal and Steel Union. Standardized lung function testing. Report working party. Bull Eur Physiopathol Respir. 1983;S19(5):1–95. [PubMed] [Google Scholar]
- Fuld JP, Kilduff LP, Neder JA, et al. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax. 2005;60:531–7. doi: 10.1136/thx.2004.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz I, Hedenstierna G, Hellers G, et al. Muscle metabolism in patients with chronic obstructive lung disease and acute respiratory failure. Clin Sci Mol Med. 1977;52:396–403. [PubMed] [Google Scholar]
- Gordon A, Hultman E, Kaijser L, et al. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc Res. 1995;30:413–18. [PubMed] [Google Scholar]
- Griffiths TL, Proud D. Creatine supplementation as an exercise performance enhancer for patients with COPD? An idea to run with. Thorax. 2005;60:525–6. doi: 10.1136/thx.2004.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzyb K, Skorkowski EF. Characterization of creatine kinase isoforms in herring (Clupea harengus) skeletal muscle. Comp Biochem Physiol B Biochem Mol Biol. 2005;140:629–34. doi: 10.1016/j.cbpc.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Harris RC, Viru M, Greenhaff PL, et al. The effect of oral creatine supplementation on running performance during maximal short term exercise in man. J Physiology. 1993;467:74. [Google Scholar]
- Hughes RL, Katz H, Sahgal V, et al. Fiber size and energy metabolites in five separate muscles from patients with chronic obstructive lung diseases. Respiration. 1983;44:321–8. doi: 10.1159/000194564. [DOI] [PubMed] [Google Scholar]
- Jakobsson P, Jorfeldt L. Long-term oxygen therapy may improve skeletal muscle metabolism in advanced chronic obstructive pulmonary disease patients with chronic hypoxaemia. Respir Med. 1995;89:471–6. doi: 10.1016/0954-6111(95)90122-1. [DOI] [PubMed] [Google Scholar]
- Jakobsson P, Jorfeldt L, Brundin A. Skeletal muscle metabolites and fibre types in patients with advanced chronic obstructive pulmonary disease (COPD), with and without chronic respiratory failure. Eur Respir J. 1990;3:192–6. [PubMed] [Google Scholar]
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19:398–404. doi: 10.1183/09031936.02.00063702. [DOI] [PubMed] [Google Scholar]
- Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–7. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- Lacasse Y, Wong E, Guyatt GH, et al. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet. 1996;348:1115–19. doi: 10.1016/S0140-6736(96)04201-8. [DOI] [PubMed] [Google Scholar]
- Hensley JM, Saunders NA, editors. Clinical epidemology of chronic obstructive pulmonary disease. New York: Marcel Dekker; 1989. [Google Scholar]
- Mathiowetz V, Vizenor L, Melander D. Comparison of Baseline instruments to the Jamar dynamometer and the B and L Engineering pinch gauge. Occ Ther J Research. 2000;20:147–62. [Google Scholar]
- McSweeny AJ, Grant I, Heaton RK, et al. Life quality of patients with chronic obstructive pulmonary disease. Arch Intern Med. 1982;142:473–8. [PubMed] [Google Scholar]
- Olopade CO, Beck KC, Viggiano RW, et al. Exercise limitation and pulmonary rehabilitation in chronic obstructive pulmonary disease. Mayo Clin Proc. 1992;67:144–57. doi: 10.1016/s0025-6196(12)61316-0. [DOI] [PubMed] [Google Scholar]
- Opasich C, Aquilani R, Dossena M, et al. Biochemical analysis of muscle biopsy in overnight fasting patients with severe chronic heart failure. Eur Heart J. 1996;17:1686–93. doi: 10.1093/oxfordjournals.eurheartj.a014752. [DOI] [PubMed] [Google Scholar]
- Revill SM, Morgan MD, Singh SJ, et al. The endurance shuttle walk: a new field test for the assessment of endurance capacity in chronic obstructive pulmonary disease. Thorax. 1999;54:213–22. doi: 10.1136/thx.54.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SJ, Morgan MD, Scott S, et al. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax. 1992;47:1019–24. doi: 10.1136/thx.47.12.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund K, Hultman E. Effects of delayed freezing on content of phosphagens in human skeletal muscle biopsy samples. J Appl Physiol. 1986;61:832–5. doi: 10.1152/jappl.1986.61.3.832. [DOI] [PubMed] [Google Scholar]
- Stahle A, Tollback A. Effects of aerobic group training on exercise capacity, muscular endurance and recovery in elderly patients with recent coronary events: a randomized, controlled study. Adv Phys. 2001;3:29–37. [Google Scholar]
- Tiep BL. Disease management of COPD with pulmonary rehabilitation. Chest. 1997;112:1630–56. doi: 10.1378/chest.112.6.1630. [DOI] [PubMed] [Google Scholar]
- Wahr JA, Tremper KK, Diab M. Pulse oximetry. Respir Care Clin N Am. 1995;1:77–105. [PubMed] [Google Scholar]
- Wijkstra PJ, Van Altena R, Kraan J, et al. Quality of life in patients with chronic obstructive pulmonary disease improves after rehabilitation at home. Eur Respir J. 1994;7:269–73. doi: 10.1183/09031936.94.07020269. [DOI] [PubMed] [Google Scholar]