Abstract
How endosomes contribute to the maintenance of vesicular structures at presynaptic terminals remains controversial and poorly understood. Here, we have investigated synaptic endosomal compartments in the presynaptic terminals of C. elegans GABAergic motor neurons. Using RAB reporters, we find that several subsynaptic compartments reside in, or near, presynaptic regions. Loss of function in the C. elegans JIP3 protein, UNC-16, causes a RAB-5-containing compartment to accumulate abnormally at presynaptic terminals. Ultrastructural analysis shows that synapses in unc-16 mutants contain reduced number of synaptic vesicles, accompanied by an increase in the size and number of cisternae. FRAP analysis revealed a slow recovery of RAB-5 in unc-16 mutants, suggestive of an impairment of RAB-5 activity state and local vesicular trafficking. Overexpression of RAB-5:GDP partially suppresses, whereas overexpression of RAB-5:GTP enhances, the synaptic defects of unc-16 mutants. Our data demonstrate a novel function of UNC-16 in the regulation of synaptic membrane trafficking and suggest that the synaptic RAB-5 compartment contributes to synaptic vesicle biogenesis or maintenance.
Keywords: synaptic vesicle, unc-16, JIP3, kinesin, Rab5, endosome
INTRODUCTION
In non-neuronal cells, the sorting and maturation of various cargos generally occur through the endosomal network. Each of the endosomal compartments differs in composition and function, and can be defined by the presence of small Rab GTPases (Segev, 2001; Zerial and McBride, 2001). For example, Rab5 is commonly found at the early endosome (Chavrier et al., 1990; Mu et al., 1995), while RAB7 and Rab11 are predominantly on the late and recycling endosomes, respectively (Ullrich et al., 1996). GTP bound Rabs recruit effector proteins to contribute to the organization of endosomal compartments (Zerial and McBride, 2001; Jahn, 2004).
Neuronal synapses are vesicular-rich subcellular structures. Synaptic vesicles (SV) are unique organelles defined by size, morphology and the competence of fusion and release (Gray, 1959; Sudhof, 2004). Extensive biochemical, morphological and physiological studies suggest that SV precursors must undergo a transformation or maturation to become functional mature SVs at the presynaptic terminal (Bonanomi et al., 2006). At least two pathways are implicated in SV maturation: SV precursors may mature following an initial fusion with the plasma membrane, or through an intermediate endosomal compartment. The second pathway is supported by a variety of observations. First, at nerve terminals, Rab5a is present on endosomal compartments, and an endosome-like compartment expands after strong stimulation (Heuser and Reese, 1973; de Hoop et al., 1994; Fischer von Mollard et al., 1994). In addition, purified synaptosomal endocytic vesicles can also fuse with early endosomal acceptor compartments (Rizzoli et al., 2006). Lastly, in Drosophila neuromuscular junctions (NMJs), Rab5 is involved in the maintenance of synaptic endosomal integrity (Wucherpfennig et al., 2003), and may also regulate SV size uniformity (Shimizu et al., 2003). However, the roles of endosomal intermediates in SV maturation and the regulation of endosomes at synapses are not well understood.
In this study, we have examined candidate endosomal compartments in C. elegans motor neurons. We show that the presynaptic terminals of these neurons likely contain multiple endosomal-like compartments and that the C. elegans JIP3 (JNK interacting protein 3) protein, UNC-16, specifically regulates a RAB-5-containing compartment. UNC-16, Drosophila Sunday Driver (SYD) and mammalian JIP3 are previously known to act as scaffolding proteins for the JNK kinases, bind the light chain of kinesin-1 (KLC), and regulate the transport of SV precursors in mature neurons (Bowman et al., 2000; Byrd et al., 2001; Verhey et al., 2001; Sakamoto et al., 2005). Mouse JIP3/SYD also interacts with the dynactin complex, and upon sciatic nerve injury, appears to mediate retrograde transport of the damage signal (Cavalli et al., 2005). In C. elegans, the kinesin heavy chain, UNC-116 and the light chain, KLC-2 constitute a conventional kinesin-1 complex. Loss of function in unc-116/KHC, klc-2 and unc-16 each results in mislocalization of SV markers (Sakamoto et al., 2005). Here, we find that loss of function in unc-16 also causes enlargement of a RAB-5-containing compartment at synapses, accompanied by a reduction of morphologically defined SVs. This effect of UNC-16 on RAB-5 containing compartments is distinct from that of the kinesin motors and the SV endocytosis machinery. Overexpression of constitutively active RAB-5 enhances, while overexpression of inactive RAB-5 partially suppresses, the synaptic defects in unc-16 mutants. We propose that UNC-16 contributes to SV maturation and maintenance in part via regulation of the synaptic endosomes involving RAB-5.
METHODS
Strains and Genetics
All C. elegans strains were generated from N2 (Bristol) and maintained at 20-22.5°C as described (Brenner, 1974). The following mutations were used in this study: unc-16(ju146), unc-16(e109), unc-116(e2281), unc-104(e1265), unc-11(e47), unc-26(e205), unc-26(e1196), unc-57(e406), and dhc-1(or195ts). The following markers were used: Punc-25 SNB-1∷GFP(juls1), Punc-25 YFP∷RAB-5(juls198), Punc-25 CFP∷RAB-7(juEx989), Punc-25 CFP∷RAB-10(juEx1144), Punc-25 CFP∷RAB-11(juEx1145), Punc-25 YFP∷RAB-5; Punc-25SNB-1∷CFP (juEx993), Punc-25mcherry∷RAB-3 (juEx1368), and Punc-25YFP∷RAB-5(Q78L) (juEx1447). Homozygous mutants were recognized based on behavior or were confirmed by allele-specific PCR and sequencing. Strains containing dhc-1(or195ts) were maintained at 15°C. For analysis, adults were lysed on plates at 15°C, plates were then transferred to 25°C as L1 stage larvae. Validation of the temperature shift was confirmed by monitoring brood size and sterility of dhc-1(or195) animals at 25°C.
Plasmids and Transgenic Strains
Punc-25YFP∷rab-5 plasmid (pCZ677) contained 1.3 kb rab-5 genomic DNA including the ATG through the Stop codon with YFP (from A. Fire’s vector pPD136.64) in frame at the 5’ end of rab-5, driven by the unc-25 promoter. Punc-25CFP∷rab-7(pCZGY168), Punc-25CFP∷rab-10(pCZGY170), Punc-25CFP∷rab-11(pCZGY171), Punc-25rab-5(Q78L)(pCZGY172), Punc-25rab5(S33N) (pCZGY173) and Punc-25YFP∷rab-5(Q78L) (pCZGY452) and Punc-25mCherry∷RAB-3 (pCZGY405) were generated using cDNAs for each rab gene and the Gateway cloning technology (Invitrogen, Carlsbad, CA).
Germline transformation was performed following standard procedures (Mello and Fire, 1991). All XFP-RAB markers were generated by injecting the plasmid DNA of interest at 1-5 ng/μl into N2 animals using either Pttx-3RFP or Pttx-3GFP at 50 ng/μl as a coinjection marker. Multiple lines were obtained and screened for any abnormalities in axon and synapse morphology. Only lines that showed no discernable abnormalities were used. juls198 [Punc-25YFP∷RAB-5] was generated by UV/trimethylpsoralen mutagenesis, and backcrossed against N2 at least four times. pCZGY172 (RAB-5(Q78L)) and pCZGY173 (RAB-5(S33N)) were injected at 50-100 ng/μl into CZ333 juls1 [Punc-25SNB-1∷GFP] animals using Pttx-3RFP as coinjection marker at 50 ng/μl. Two to three transgenic lines were analyzed for each construct.
XFP Analysis
In general, the XFP markers were observed using a 63X Plan-apochromat objective and appropriate filter set (Chroma, Battleboro, VT) on a Zeiss Axioplan 2 microscope. At least two independent lines were analyzed. Quantitative analysis was obtained from one representative line, with the exception of the mutant RAB-5 transgenes, where two individual lines were used for each analysis and the data pooled. The effect of unc-16 was observed with two additional transgenes of YFP∷RAB-5 (juEx857 and juEx897), and quantitative analysis done on the integrated line, juls198. We also analyzed trans-heterozygous unc-16(e109)/unc-16(ju146) and observed indistinguishable effects from those of homozygous mutants for each allele, both by juls198 expression pattern and locomotion. To score the effect of RAB-5(Q78L) or RAB-5(S33N) on the pattern of Punc-25 SNB-1∷GFP (juls1), the anterior one third of the dorsal nerve cord (representing axons of DD1-2) was visually scored for phenotypes, genotype blind. For all image analysis, animals were anesthetized using 1% phenoxy propanol. Statistical analysis was conducted in MedCalc using a Fisher’s exact test.
Confocal Image analysis
Images of XFP markers were collected from the dorsal nerve cords using a 63X objective on a Zeiss LSM5 PASCAL laser scanning confocal microscope. Confocal stacks of 5-8 0.4 μm sections were merged into a single plane and exported as a tiff file with a scale bar. The Metamorph image analysis software (Molecular Devices, Sunnyvale, Ca) was used for quantification of puncta size, intensity and number. The images were thresholded against background to select for total puncta fluorescence, a region of interest (ROI) was drawn and data collected for total object number, pixel area, perimeter and total length measured. Intensity measurements were collected using Metamorph linescans through the nerve cord and background corrected. For image collection and analysis of YFP∷RAB-5, Argon laser output was set to 40% current, and transmission at 514 nm was set to 20% for WT, or 5% for unc-16 and all double mutants containing unc-16 to capture the full dynamic range of the fluorescence. unc-16 data was then normalized to WT levels. This normalization was done based on the following analysis: we collected and analyzed WT YFP∷RAB-5 data sets at both 20% and 5% laser transmission at 514nm, and found that the relative intensity (RIU) of YFP∷RAB-5 puncta at 20% laser transmission was 4.5 fold of those at 5% laser transmission. To correct for the difference in the RIU, all YFP∷RAB-5 unc-16 data sets collected at 5% laser transmission at 514nm were multiplied by 4.5. For Fig. 2 E, a linescan was drawn through the mid region of the dorsal nerve cord (30-120 μm per animal) and data collected for puncta number. The resulting measurements were analyzed by a two-tailed Student’s t test. Colocalization images were collected as single plane scans, or z-axis projections, of the central region of the nerve cords, and areas analyzed ranged from 35-120μm. Laser and image capture settings were optimized using animals carrying only a single transgene (either CFP or YFP or mCherry fusions) to avoid bleed through and areas of large aggregation were avoided.
Figure 2. Endosomal compartments are differentially regulated by kinesin-1 and UNC-104 motor proteins.

Images are confocal z-stack projections of WT, unc-104 and unc-116 mutant animals showing expression of (A) YFP∷RAB-5, (B) CFP∷RAB-7, (C) CFP∷RAB-10 and (D) CFP∷RAB-11. In unc-116 mutants, RAB-5 and RAB-7 puncta intensity and size decrease. In unc-104 mutants, RAB-5 becomes entirely diffuse, puncta are rarely detectable; RAB-7 shows similar, but weaker, defects. Quantitation of XFP∷RAB puncta per 100 μm is shown in (E) as mean ± SEM(*P< 0.05, **P< 0.01 compared to WT, n = 8-10 animals for each genotype, n/s = not significant). Scale bars are 10 μm.
FRAP analysis was performed on a Zeiss LSM510 confocal microscope following the general procedures described in (Reits et al., 2000; Jordens et al., 2001). Image series were collected using a 63X oil immersion Planapochromat objective and a zoom of 3. Argon laser output was set to 40% current and transmission at 514 nm was set to 10% for WT and 5% for unc-16 to yield a similar dynamic range per puncta. For the bleach, an ROI was drawn around the puncta of interest and laser transmission increased to 100%. 100 scanning iterations resulted in roughly 90% loss of fluorescence in the bleached area. Time series were collected at 10s intervals. To calculate percent of recovery after photobleaching, intensity profiles for the bleach spot, background region and unbleached puncta within the same time-lapse series were collected using Zeiss LSM510 image analysis software. Background was subtracted from all data and unbleached puncta intensities were used to correct for fluorescence loss during image acquisition. To avoid movement artifacts during FRAP, images were collected from animals that were fully immobilized. Under these conditions, soluble free GFP recovered immediately (data not shown).
Electron Microscopy
Young adult hermaphrodites of genotype CZ333 juls1 ([Punc-25SNB-1∷GFP), CZ2018 unc-16(ju146); juls1, CZ1125 unc-16(e109); juls1 were fixed in parallel using glutaraldehyde and osmium fixative. 300-500 serial 45 nm sections were collected from two WT (juls1), one unc-16(ju146), and one unc-16(e109) mutant worms. Nerve cords were photographed with a Gatan digital camera on a JEOL 1200 electron microscope. NMJs were identified as synapses with muscles as postsynaptic partners (White et al., 1986). GABAergic NMJs have muscles as sole postsynaptic partners, whereas cholinergic NMJs usually have two postsynaptic partners, a muscle and a neuronal process. Small, round, clear vesicles of 30-35nm diameter were counted as SVs. Presynaptic densities were calculated as the number of sections that contained recognizable electron-density, multiplied by 45nm. Membrane structures of irregular shape and of diameter larger than 60nm were counted as cisternae. Statistical analysis was conducted using student t-test.
RESULTS
Endosomal components localize to the presynaptic region of D motor neurons
It remains uncertain what kind of endosomes are present in presynaptic terminals and what roles they have in presynaptic development and function (Murthy and De Camilli, 2003). To explore the nature of endosomal compartments in C. elegans synapses, we began by expressing fluorescently tagged RAB-5, RAB-7, RAB-10 and RAB-11 in the D-type GABAergic motor neurons (see Materials and Methods). We also expressed EEA-1, the C. elegans ortholog of a known Rab5 effector (Simonsen et al., 1998). The D neurons form synapses en passant along the axons, and the presynaptic sites are visualized by a fluorescently-tagged synaptobrevin (SNB-1) marker (Fig. 1 A and B) (Hallam and Jin, 1998). In wild type (WT) young adult animals each of the endosomal markers form a punctate pattern along the nerve processes (Fig. 1 BE). To examine the spatial relationship of the RAB markers with respect to synapses, we performed co-localization studies with SV markers. A collective analysis of confocal images revealed extensive colocalization of YFP∷RAB-5 and SNB-1∷CFP puncta (Fig. 1 B), as well as CFP∷RAB-10 or CFP∷RAB-11 with mCherry∷RAB-3 (Fig. 1 C and D). Importantly, the synaptic morphology seen with SNB-1∷CFP or mCherry∷RAB-3 was normal in animals co-expressing each RAB marker (Fig. 1 B-D, data not shown), indicating that the expression of these transgenic RAB markers does not interfere with synapse development. Further co-localization studies of CFP∷RAB-7 and YFP∷RAB-5 revealed extensive overlaps of these two RAB compartments in nerve processes (Fig. 1 E). In contrast, YFP∷EEA-1 puncta showed irregular size and distribution, only partially overlapping with CFP∷RAB-7 puncta (Fig. S 1A). This partial co-localization of EEA-1 with RAB-7 is consistent with the reported study that in cultured hippocampal neurons EEA-1 is not present at presynaptic sites (Wilson et al., 2000). Because the resolution of the confocal microscope does not allow us to discern the subsynaptic localization of the RABs, we examined the expression of the RAB markers in the cell bodies of the D neurons. Each RAB marker showed a discrete localization pattern within the cell body and displayed a variable degree of overlap with each other and with mCherry∷RAB-3 (Fig. S 2), suggesting that the RAB markers likely label distinct compartments and/or domains. From the analysis of the RAB reporters, we infer that the presynaptic terminals of the D neurons likely contain an endosomal network.
Figure 1. Endosomal markers localize to synapses.
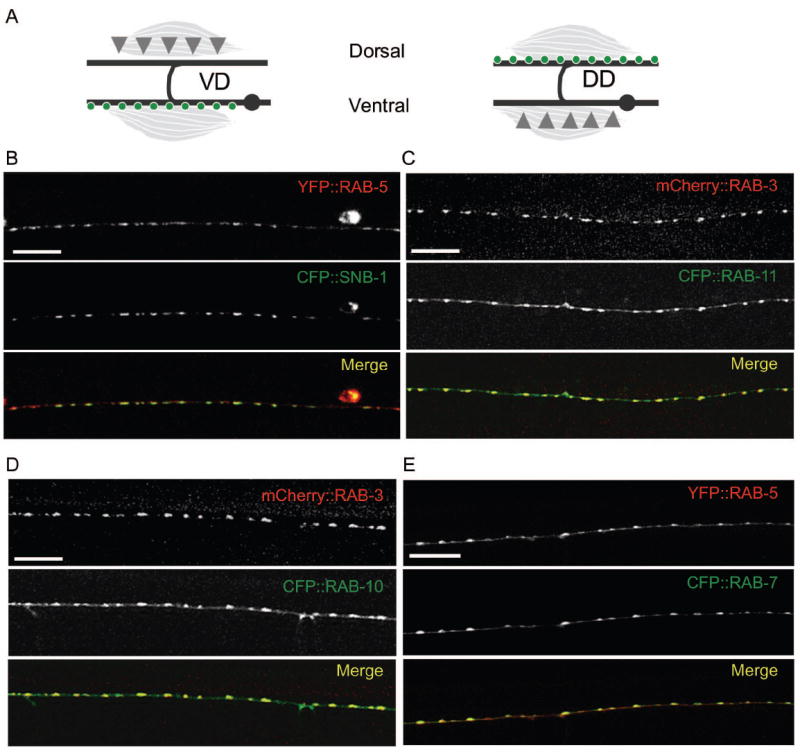
(A) GABAergic DD and VD neurons are pseudo-unipolar such that their cell bodies (gray dots) are on the ventral side of the animal and synapses (green circles) form en passant on the dorsal process of the DD neurons and the ventral process of the VD neurons (gray lines). Synaptic inputs (gray triangles) are made onto the ventral processes of the DD or the dorsal processes of the VD neurons
(B-E) Confocal images of animals co-expressing XFP∷RAB, or SNB-1∷CFP transgenes. (B) Punc-25-YFP∷RAB-5 (red) and Punc-25-SNB-1∷CFP (green) expression in the ventral process of VD neurons. (C) Punc-25-mCherry∷RAB-3 (red) and Punc-25-CFP∷RAB-11 (green) expression in the dorsal process of the DD neurons. (D) Punc-25-mCherry∷RAB-3 (red) and Punc-25-CFP∷RAB-10 (green) expression in the dorsal process of the DD neurons. (E) Punc-25-YFP∷RAB-5 (red) and Punc-25-CFP∷RAB-7 (green) expression in the dorsal process of the DD neurons. Scale bars are 10μm.
RAB compartments are differentially regulated by kinesin-1 and UNC-104 motors
We next asked how the synaptic endosomal compartments might be established by examining the pattern of the RAB markers in unc-116/KHC and unc-104/KIF1A kinesin mutants. unc-116(e2281) and unc-104(e1265) are partial loss of function mutations and reduce anterograde transport (Hall and Hedgecock, 1991; Byrd et al., 2001). In both kinesin mutants, YFP∷RAB-5 and CFP∷RAB-7 fluorescence was very faint and diffuse, forming few puncta (Fig. 2 A-B). For example, the puncta density of YFP∷RAB-5 was decreased from 26.0 ± 1.4 puncta/100 μm in WT animals to 0.1 ± 0.1 in unc-104 mutants and 20.1 ± 1.2 in unc-116 mutants (Fig. 2 E). Additionally, unc-116 mutants frequently showed a discontinuous distribution of YFP∷RAB-5 puncta such that axonal regions of 10 to 50 μm frequently lacked any fluorescence (data not shown). These observations suggest that both UNC-116/KHC and UNC-104/KIF1A promote establishment of the RAB-5 and RAB-7 compartment at synapses. In contrast, the overall puncta density of CFP∷RAB-10 and CFP∷RAB-11 was not significantly altered in unc-104 mutants, although the puncta distribution and size was more irregular than in WT animals (Fig. 2 C-E). In unc-116 mutants the puncta density of CFP∷RAB-10 and CFP∷RAB-11 was slightly, but significantly, reduced, and the puncta also displayed irregular distribution and size (Fig. 2 C-E). This analysis suggests that each RAB marker likely represents a distinct subcompartment in the D neurons and that their establishment and maintenance are differentially regulated by anterograde transport via the kinesin-1 and UNC-104 motor proteins.
Loss of function in unc-16 causes increased accumulation of synaptic YFP∷RAB-5
The JIP3 family of proteins has been characterized for their role in regulating vesicular transport via kinesin-1 (Bowman et al., 2000; Byrd et al., 2001; Verhey et al., 2001; Sakamoto et al., 2005). We therefore tested how the JIP3 protein UNC-16 might regulate the presynaptic endosomal compartments. In D neurons, functional UNC-16∷CFP showed a punctate pattern, largely colocalizing with YFP∷RAB-5 (Fig. S3). In unc-16(e109) and unc-16(ju146) mutant animals, both of which are strong loss of function and likely null mutations (Byrd et al., 2001; see Materials and Methods), we observed a striking increase in the area and peak intensity of YFP∷RAB-5 puncta (Fig. 3 A and B, and data not shown for unc-16(e109)). The puncta size increased from 1.25 ± .04 μm2 in WT to 1.56 ± .07 μm2 in unc-16(ju146). The relative intensity of YFP∷RAB-5 puncta was dramatically increased, from 167.4 ± 6.5 relative intensity units (RIU) in WT to 950.0 ± 27.2 RIU in unc-16(ju146) mutants (Fig. 3 B, see Materials and Methods). In contrast, none of the other three RAB markers or EEA-1, were altered in unc-16 mutants (Fig. 3 C, D, S1B) nor were other synaptic and subcellular markers tested (SupplementaryTable S1 and data not shown).
Figure 3. Loss of function in unc-16 specifically alters synaptic endosomal YFP∷RAB-5.
(A) Images are confocal z-stack projections of YFP∷RAB-5 in the dorsal cords of WT and mutants as labeled. In order to show the full dynamic range of the YFP∷RAB-5 puncta fluorescence all images for unc-16 containing animals were taken with reduced laser power (see Methods). In WT, YFP∷RAB-5 is localized along the nerve processes in a punctate distribution. In unc-16 mutants puncta size and intensity are increased, and the punctate pattern along the nerve process becomes irregular in size and distribution. In dhc-1 mutant animals, YFP∷RAB-5 fluorescence becomes more dispersed with decreased puncta size and intensity. unc-16 unc-116, unc-16; unc-104, and unc-16;dhc-1 double mutants show an additive phenotype, with YFP∷RAB-5 puncta seen as increased intensity but variable size.
(B) Quantitation of YFP∷RAB-5 puncta area and intensity. For puncta area (μm2), data is depicted as average puncta area (bars) and individual puncta area (black circles). For puncta intensity, RIU refers to Relative Intensity Units of peak puncta intensities. All data are shown as mean ± SEM, statistical comparison is made pairwise with WT (red lines) or pairwise with each other (black lines) (n=10 animals for each genotype, *P< 0.05 and **P<0.01).
(C-D) CFP∷RAB-7 is largely unaltered in unc-16 mutants. (C). Images are confocal z-stack projections of CFP∷RAB-7 in the dorsal cords of WT and unc-16(ju146). (D) Quantitation of CFP∷RAB-7 puncta size and intensity are shown as mean ± SEM (*P< 0.05 compared to WT, n=10 animals for each genotype, n/s = not significant). Scale bars are 10μm.
We sought to address if the effect of unc-16 on the RAB-5 compartment involved KHC and KIF1A. In both unc-16 unc-116 and unc-16; unc-104 double mutants, the spatial distribution of YFP∷RAB-5 puncta was irregular and the puncta size was smaller than those in either single mutant alone. However, the YFP∷RAB-5 intensity resembled that of unc-16 single mutants (Fig. 3 A and B). We infer that loss of unc-16 function causes abnormal accumulation of RAB-5-labeled compartments even when anterograde transport is compromised. Additionally, we examined whether the effect of unc-16 on the RAB-5 compartment might be due to reduced retrograde transport via dynein, using a temperature sensitive mutation in the dynein heavy chain dhc-1(or195) (Hamill et al., 2002). YFP∷RAB-5 puncta were decreased in size and intensity in dhc-1 single mutants relative to WT animals (Fig. 3A and B). unc-16; dhc-1 double mutants showed an additive effect such that YFP∷RAB-5 puncta size and intensity displayed an intermediate level compared with either single mutant alone, implying that the effect on YFP∷RAB-5 by loss of unc-16 function is unlikely to be mediated via dynein. Together, these observations suggest that UNC-16 regulates RAB-5 mediated membrane trafficking events at synapses, likely independent of these three motors.
UNC16 affects the RAB-5 compartment independent of, or in parallel to, the synaptic endocytic pathway
The endosomal system receives materials via both anterograde transport from the trans-Golgi network biosynthetic pathway and retrogradely from endocytic vesicles derived from the plasma membrane. To determine whether the role of UNC-16 on the synaptic RAB-5 compartment requires SV endocytosis, we examined the genetic interactions between unc-16 and the SV endocytic machinery. In C. elegans, the SV endocytic machinery includes UNC-11 AP180 clathrin adaptor protein, UNC-57 endophilin and UNC-26 synaptojanin (Nonet et al., 1999; Harris et al., 2000; Schuske et al., 2003). In mutant animals for each of the three genes, synaptic endocytosis is severely compromised, and coated vesicles and invaginated pits accumulate at the synaptic terminals. We found that YFP∷RAB-5 puncta size and intensity were reduced to different degrees in each mutant (Fig. 4 A and B), indicating that SV endocytosis is required for maintaining the integrity of the RAB-5 compartment in these presynaptic terminals. The double mutants of unc-16 with unc-11, unc-57 or unc-26 showed an overall additive phenotype (Fig. 4 A and B). The size and intensity of YFP∷RAB-5 puncta in the double mutants were different from those in unc-11, unc-26 or unc-57 alone, but were also significantly different from that in unc-16 mutants (Fig. 4 B). This analysis suggests that UNC-16 and the SV endocytic machinery have independent roles at the RAB-5 compartment.
Figure 4. The effect of unc-16 on the RAB-5 compartment is distinct from those in mutants defective in synaptic vesicle endocytosis.

(A) Confocal z-stack projections of YFP∷RAB-5 in the dorsal cords of AP180/unc-11(e47), endophilin/unc-57(e406), synaptojanin/unc-26(e205), unc-16; unc-11, unc-16; unc-57, and unc-16; unc-26. In unc-11 mutants YFP∷RAB-5 puncta size and intensity are decreased, while in unc-57 and unc-26 mutants YFP∷RAB-5 puncta are dispersed and diffuse. The double mutants with unc-16 show an additive phenotype with increased intensity. In order to show the full dynamic range of the YFP∷RAB-5 puncta fluorescence all images for unc-16 containing animals were taken with reduced laser power (see Methods)
(B) Quantitation of puncta area and intensity. Data presentation is the same as in Fig. 2 B; red lines, pairwise comparison with WT, black lines, pairwise comparison with each other (n=10 animals for each genotype, *P< 0.05 and **P<0.01).
(C) Confocal z-stack projections of SNB-1∷GFP in the dorsal cords of WT, unc-16, unc-57 and unc-16; unc-57 mutants. In unc-57 mutants SNB-1∷GFP puncta become dispersed with few distinct puncta remaining. unc-16; unc-57 double mutants show an intermediate phenotype with puncta number more closely resembling that of unc-16 single mutants, however, puncta area and distribution are irregular compared with either single mutant alone. (D) Quantitation of SNB-1∷GFP puncta number per 100 μm in unc-16, unc-57 and the double mutant (mean ± SEM, *P< 0.05, n=7-10 animals for each genotype); red lines, pairwise comparison with WT; black lines, pairwise comparison with each other. Scale bars are 10μm.
To further evaluate how the changes on YFP∷RAB-5 in double mutants between unc-16 and the endocytic machinery are related to the SV cycle, we examined the expression of SNB-1∷GFP. In unc-57 single mutants, SNB-1∷GFP showed strong but diffuse fluorescence, and few normal puncta remained (Fig. 4 C and D), reflecting the endocytosis defect in which most SNB-1∷GFP is retained on the plasma membrane in the endocytosis defective mutants (Dittman and Kaplan, 2006). In unc-16 mutants SNB-1∷GFP puncta size was increased, while puncta number was decreased (Fig. 5 A, 5 D, see below). unc-16; unc-57 double mutants showed an intermediate phenotype with SNB-1∷GFP puncta interspersed at irregular intervals along the axons (Fig. 4 C). Within the stretches of puncta, the average SNB-1∷GFP puncta number was comparable to that of unc-16 single mutants (Fig. 4 D). We interpret that the SNB-1∷GFP defect in unc-57 mutants may be partially suppressed in the double mutants. Because double mutants between unc-16 and the endocytosis mutants show YFP∷RAB-5 and SNB-1∷GFP phenotypes that more closely resemble those in unc-16, we speculate that UNC-16 likely influences the synaptic RAB-5 compartment independent of SV retrieval from plasma membrane.
Figure 5. Vesicular structures at the synapses of unc-16 mutant animals are altered.

(A-D) SNB-1∷GFP, not mCherry∷RAB-3, puncta are altered in unc-16 mutants. (A) Images are confocal z-stack projections of SNB-1∷GFP in the dorsal cords of WT, unc-16(ju146) and unc-16(e109) mutant animals. (B) Images are single-plane confocal scan of YFP∷RAB-5 and SNB-1∷CFP co-expression in unc-16 mutants, showing that these markers are affected similarly. (C) mCherry∷RAB-3 puncta are unaltered in unc-16(ju146) animals. Images are confocal z-stack projections of the dorsal cord in young adults. (D) Quantitation of SNB-1∷GFP and mCherry∷RAB-3 in WT and unc-16 mutant animals (puncta area μm2, mean ± SEM, *P< 0.05 compared to WT). Scale bars are 10μm.
(E) Shown are TEM images of ventral cord GABAergic synapses of WT and two examples of unc-16. Overall synaptic morphology is normal but vesicular structures are altered in unc-16(ju146) mutant animals. Dense core vesicles (dcv), synaptic vesicles (sv), the active zone (AZ) and cisternae (c) are labeled with black arrows. Abnormal cisternal structures are labeled with a red arrow. Scale bar is 0.5 μm.
(F) Quantitation of the number of SV per synapse and of total cisternae per 45 nm section in WT, unc-16(ju146) and unc-16(e109) (mean ± SEM, GABAergic: WT n= 21, unc-16(ju146) n= 6 and unc-16(e109) n= 10; Cholinergic: WT n= 46, unc-16(ju146) n= 14 and unc-16(e109) n= 14)
The SV number is reduced in unc-16 mutants
Several studies have implicated Rab5 and its associated endosomal compartment in SV recycling following exocytosis (Fischer von Mollard et al., 1994; Rizzoli et al., 2006). Drosophila Rab5 has been shown to regulate the size of SVs (Shimizu et al., 2003). We asked how the effects of UNC-16 on the RAB-5 compartment might affect SVs. We first analyzed two transgenic SV markers, SNB-1∷GFP and mCherry∷RAB-3. Synaptobrevin (SNB-1) is an SV membrane anchored SNARE, and is also known to be a component of SV precursors derived from the early stages of the secretory pathway and is present in a population of transport packets (Mundigl et al., 1993). Rab3A (RAB-3) is involved in SV exocytosis, is transiently associated with SVs at the late stages of the secretory pathway, and therefore has been used to identify the ‘mature’ SVs that undergo rapid recycling (Matteoli et al., 1991). In WT animals, both markers form uniform-shaped fluorescent puncta of similar size (Fig. 5 A and C). SNB-1∷GFP puncta were larger in unc-16 mutants than in WT animals (Fig. 5 A and D),1.06 ± 0.74 μm2 and 1.08 ± 0.51 μm2 in unc-16(ju146) and unc-16(e109) respectively, versus 0.86 ± 0.43 μm2 in WT (P < 0.05). This enlarged pattern of SNB-1∷GFP was essentially parallel to that of YFP∷RAB-5, as shown by simultaneous detection of YFP∷RAB-5 and SNB-1∷CFP coexpressed in unc-16 mutants (Fig. 5 B). In contrast, mCherry∷RAB-3 puncta in unc-16 mutant animals were not significantly different from WT (Fig. 5 C and D, P=0.12). This analysis suggests that lack of UNC-16 may alter a subpopulation of the SVs, particularly at early stages of the secretory pathway.
Since UNC-16 also functions in kinesin-1 mediated transport (Byrd et al., 2001), we addressed whether the SV defect could be due to a defect in transport by performing fluorescence recovery after photobleaching (FRAP) analysis of SNB-1∷GFP. We reasoned that the recovery rate of SNB-1∷GFP would be due to several factors, including transport of new SV components and diffusion from neighboring synapses into the bleached region. We found that in WT animals, SNB-1∷GFP recovered to reach 50% initial intensity by 150s post bleach (Fig. 6 A). In unc-16 mutant animals, the rate of recovery was unaltered (Fig. 6 A, p>0.3 at all time points, n=5). These data suggest that a defect in transport is unlikely to account for the enlarged SNB-1∷GFP puncta in unc-16 mutant animals.
Figure 6. Loss of unc-16 function impairs RAB-5 recovery rates after photobleaching.
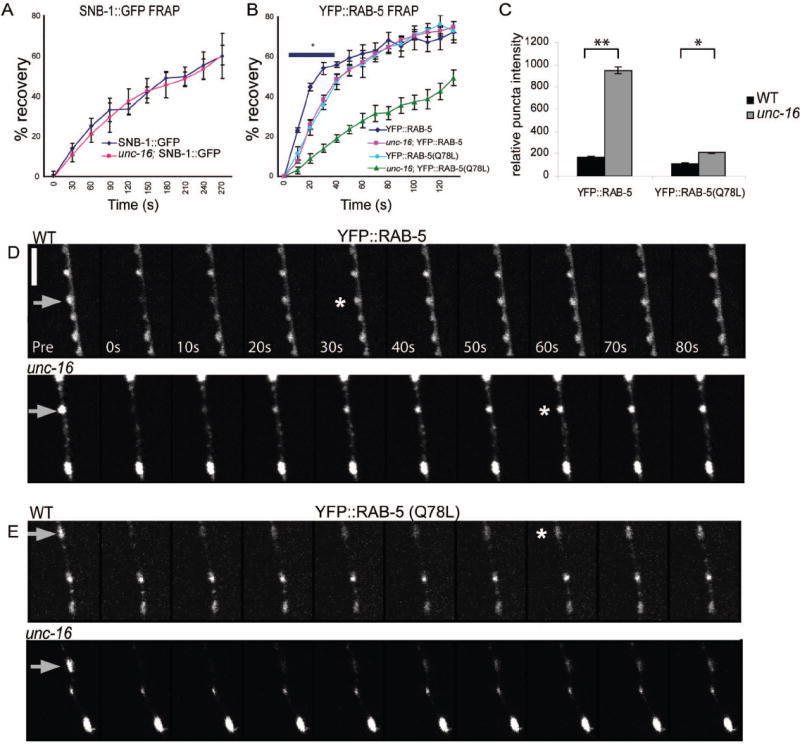
(A) Averaged FRAP recovery profiles of SNB-1∷GFP (juls1) in WT (diamonds) and unc-16 (squares) (mean ± SEM, n=5). Recovery rates of SNB-1∷GFP are unaltered in unc-16 mutants.
(B) Averaged FRAP recovery profiles of YFP∷RAB-5 (juls198) in WT (diamonds) and unc-16 (squares) and of YFP∷RAB-5(Q78L) in WT (circles) and unc-16 (triangles) ((mean ± SEM, * p< 0.01, n=5). Recovery rates of YFP∷RAB-5 after photobleaching are significantly slower in unc-16 mutants than in WT animals. Recovery rates of YFP∷RAB-5(Q78L) in WT are similar to those of YFP∷RAB-5 in unc-16 mutants. YFP∷RAB-5(Q78L) is slowed further in unc-16 mutants.
(C) Quantitation of YFP∷RAB-5 and YFP∷RAB-5(Q78L) puncta intensity in WT and unc-16 mutants are shown as mean ± SEM.) (n=10 animals for each genotype, *P< 0.05).
(D-E) Representative time course is shown for YFP∷RAB-5 recovery in WT and unc-16 mutant, and YFP∷RAB-5(Q78L) in WT and unc-16 mutant. Gray arrows indicate bleached puncta, asterix shows time point where recovery has reached 55%. Scale bar is 5μm.
To further evaluate the alterations in SV morphology in unc-16 mutants, we performed a serial ultrastructural analysis of NMJs on two unc-16 mutant animals. These synapses are identified as a single cluster of SVs surrounding one prominent electron-dense presynaptic specialization. The overall synaptic organization was normal in unc-16 mutant animals (Fig. 5 E). However, significantly fewer 30-40 nm diameter SVs were present in unc-16 than in WT (Fig. 5 F). For example, the mean SV number in GABAergic synapses in both unc-16(ju146) and unc-16(e109) animals was 352.5 ± 89.4 and 253.7 ± 31.25, respectively, compared to 513.31 ± 49.63 in WT (P<0.05). In addition, we noticed an increase in irregularly shaped vesicle profiles that had a diameter larger than 60 nm and spanned more than one section (Fig. 5 E and F). We refer to these structures as endosomal cisternae. In unc-16 mutants there was a significant increase in the number of cisternae per section both within a SV cluster and immediately adjacent to the synaptic sections (Fig. 5 F). The average diameter of these cisternae was also significantly larger in unc-16 than in WT animals (89 ± 1.4 nm in WT and 103 ± 2.2 nm in unc-16 animals, p<0.01, n=310 for each genotype). Thus, loss of unc-16 function causes an increase in the size and number of cisternae. For technical reasons, we are currently unable to determine the molecular identity of these cisternae. However, it is conceivable that the cisternae may correspond to the RAB-5 compartments accumulated in unc-16 mutants. Taken together, the reduction in SV number but parallel increases of both SNB-1∷GFP and YFP∷RAB-5 puncta size in unc-16 mutants suggest that alterations in the RAB-5 compartment lead to an impairment in SV formation.
The RAB-5-containing compartment is less dynamic in unc-16 mutants
Rab5 cycles between an active membrane associated GTP-bound form and a cytosolic GDP-bound form (Zerial and McBride, 2001). Therefore, we hypothesized that the increased size and intensity of YFP∷RAB-5 in unc-16 mutants may reflect a defect in RAB-5 cycling, resulting in a prolonged association of YFP∷RAB-5 with endosomal compartments. We performed FRAP analysis to evaluate the dynamics of RAB-5 at synapses. In WT animals, YFP∷RAB-5 recovered to approximately 55% of its initial intensity within 30s and reached a maximal recovery of 70% by 120s (Fig. 6 B and C). This recovery suggests that there are pools of RAB-5 rapidly cycling within and between nearby synapses. In unc-16 mutants, the recovery rates of YFP∷RAB-5 levels were significantly lower, reaching 7-19% in the initial 40s of recovery (p<0.01) (Fig. 6 B and D). By 50s post-bleach, however, YFP∷RAB-5 intensity levels in unc-16 mutants were comparable to those in WT animals. The slower recovery of YFP∷RAB-5 in unc-16 could be due to an impairment in RAB-5 cycling state, hence the membrane-associated state, or slower diffusion and transport of RAB-5-containing vesicles. To distinguish between these possibilities, we performed FRAP analysis on a YFP∷RAB-5(Q78L) reporter, which locks the YFP∷RAB-5 in the GTP-bound, membrane-associated state (Lichtenstein et al., 1998). We reasoned that the recovery of YFP∷RAB-5(Q78L) would represent diffusion and transport of membrane-bound RAB-5 from nearby synapses. In WT animals, the recovery rates of YFP∷RAB-5(Q78L) were slower than those of YFP∷RAB-5, and were similar to that of YFP∷RAB-5 in unc-16 mutants (Fig. 6 B and E, P> 0.05 at all time points). Moreover, the overall puncta intensity of this activated YFP∷RAB-5(Q78L) reporter was only slightly increased in unc-16 mutants compared to that in WT animals, which is in contrast to the dramatic effects of loss of unc-16 function on YFP∷RAB-5 (Fig. 6 C). These observations support an interpretation that in unc-16 mutants the RAB-5 population is likely shifted to the GTP-bound state, leading to preferential membrane association of RAB-5. We also observed that the recovery rate of YFP∷RAB-5(Q78L) in unc-16 mutants was slower than that in WT (Fig. 6 B, P< 0.05). Together, this analysis reveals that the dynamic state of RAB-5 is impaired in unc-16 mutants, and that UNC-16 is also required for transport and local diffusion of RAB-5 membrane vesicles.
Overexpression of RAB-5:GDP suppresses, whereas overexpression of RAB-5:GTP enhances, the synaptic defects in unc-16 mutants
To functionally test whether alteration in RAB-5 activity might in part underlie the mutant phenotypes of unc-16, we expressed either GTP bound RAB-5, RAB-5(Q78L), or GDP bound RAB-5, RAB-5(S33N) (Lichtenstein et al., 1998), and evaluated their effects on the pattern of SNB-1∷GFP in WT and unc-16 animals. Transgenic overexpression of either RAB-5 variant did not cause any discernable abnormalities in WT (Fig. 6 G). However, overexpression of RAB-5(S33N) partially rescued the enlarged SNB-1∷GFP puncta defect of unc-16 animals. 42% of the unc-16; Ex[RAB-5(S33N)] animals showed an overall pattern of SNB-1∷GFP similar to that of WT, while none of the unc-16 animals showed WT puncta distribution (Fig. 7A and B, p<0.01). In contrast, overexpression of RAB-5(Q78L) significantly enhanced the SNB-1∷GFP phenotypes in unc-16 as the puncta showed an aggregated appearance and the distribution was more disorganized in 69% of unc-16; Ex[RAB-5(Q78L)] animals, compared to 9% in unc-16(ju146) animals alone (Fig. 7A and B, p<0.01). These observations strengthen our conclusion that UNC-16 functions in part to promote the dynamics of the synaptic RAB-5 compartment.
Figure 7. RAB-5:GDP suppresses and RAB-5:GTP enhances the SNB-1∷GFP phenotype in unc-16.

(A). Shown are dorsal cord epifluorescence images of SNB-1∷GFP in WT and unc-16 mutant animals. unc-16 animals expressing RAB-5(S33N) show an improved SNB-1∷GFP pattern; and unc-16 animals expressing RAB-5(Q78L) show disorganization and aggregation of the SNB-1∷GFP pattern. Scale bar is 10μm.
(B) Quantitation of SNB-1∷GFP phenotypes The morphology of SNB-1∷GFP puncta was categorized as normal (~0.86 μm2), enlarged (~1.1 μm2) or aggregate (observed as a stretch of irregularly distributed puncta with varying size up to 3.5 μm2) * p< 0.01, n= 34-49 animals per genotype.
DISCUSSION
Most of what we know about the endosomal network in presynaptic terminals has come from studies in neuroendocrine cells, such as PC12 cells, which produce synaptic like microvesicles (SLMVs) but do not form axons and have few synaptic specializations. SLMVs form from the plasma membrane and through the early endosome (Schmidt et al., 1997; Lichtenstein et al., 1998; Shi et al., 1998; de Wit et al., 1999). In PC12 cells early endosomes are described as clathrin coated vacuolar and tubular structures that have diameters of 100-500 nm, but can also reach a length of up to 3 μm (Stoorvogel et al., 1996). Cisternal, endosome-like, structures that contain Rab5 are also observed in Drosophila NMJs and in cultured hippocampal neuron synapses (de Hoop et al., 1994; Wucherpfennig et al., 2003). However, it remains uncertain what kind of endosomes may exist in other types of synapses and what roles they may have in synapse development and function (Murthy and De Camilli, 2003). Recently, a systematic proteomic analysis revealed an abundant representation of Rab proteins in rat brain SV membrane preparations (Takamori et al., 2006). Rab GTPases have emerged as distinct “addresses” for intracellular organelles (Zerial and McBride, 2001), raising the possibility that synapses contain diverse membrane compartments in addition to well-characterized SVs. Understanding the nature of such compartments and their relationship to SVs should add further insight into the regulation of synapses.
In this study, we have examined the distribution of synaptic endosomal components in the C. elegans D-type motor neurons. Our ultrastructural analysis revealed cisternal structures that are approximately 90nm in diameter both within the SV clusters and in regions immediately surrounding the synapse. We find that the transgenically expressed RAB-5, RAB-7, RAB-10 and RAB-11 markers label compartments that are present at the vicinity of the presynaptic terminals. We do not yet know the direct relationship of these RAB-labeled compartments to those anatomically observed cisternal structures. Nonetheless, the observation that the RAB markers are differentially altered in kinesin and dynein mutants suggests that each RAB compartment is likely uniquely established and maintained. Our data further reveal a specific regulation of the RAB-5 positive compartment by the JIP3 protein UNC-16.
Rab5 has long been known to be present in presynaptic terminals, and early biochemical studies suggest that synaptic Rab5 is predominantly in the GDP-bound state (de Hoop et al., 1994; Fischer von Mollard et al., 1994a; Fischer von Mollard et al., 1994b; Stahl et al., 1994). Perturbing Rab5 activity by overexpression of WT or dominant negative Rab5 affects SV release in cultured hippocampal neurons and Drosophila NMJs (Star et al., 2005; Wucherpfennig et al., 2003). Yet, how Rab5 activity at synapses is regulated and how such regulation influences SV trafficking remains poorly understood.
We find that loss of unc-16 function causes a specific accumulation of a RAB-5 compartment without affecting other RAB labeled compartments at motor neuron synapses. Ultrastructurally, we observed an increase in the size and number of cisternal structures at synapses in unc-16 mutants. Persistent action of GTP bound Rab5 is known to cause enlarged endosomes (Stenmark et al., 1994). We assessed the dynamics of the RAB-5 compartment using FRAP analysis and found that the recovery rates of YFP∷RAB-5 in unc-16 mutants are slower than those in WT, and are similar to that of the GTP bound YFP∷RAB-5(Q78L). The transgenic overexpression of a GDP-locked RAB-5(S33N) can partially rescue the synaptic SNB-1∷GFP defects of unc-16 mutants, which is possibly due to excess RAB-5:GDP acting as a sink to the RAB GEFs, thus impairing the production of RAB-5:GTP. In contrast, overexpression of RAB-5(Q78L) enhances the SNB-1∷GFP defects in unc-16 mutants. From these observations we infer that in unc-16 mutants RAB-5 appears to be abnormally maintained in the GTP bound state, which results in a prolonged association of RAB-5 with endosomal membranes. Our expression analysis of UNC-16 and RAB-5 showed a high degree of co-localization along the nerve processes. However, UNC-16 is unlikely to act as a direct or sole regulator of RAB-5 cycling. UNC-16 does not have known domains implicated in GTPase binding. We have tested multiple candidate GEFs and GAPs by mutations or by RNAi, but observed no obvious effects on YFP∷RAB-5 (Table S2, Fig. S4). Additionally, mutant animals for the JNK kinase pathway, such as jnk-1, jkk-1 or sek-1, also do not accumulate RAB-5 at synapses (Table S2 and Fig. S4), making the JNK signaling cascade unlikely to account for the effect of UNC-16 on RAB-5. We thus speculate that UNC-16 could act through several downstream effectors, each of which may act redundantly to regulate RAB-5. The observation that the recovery rate of YFP∷RAB-5(Q78L) in unc-16 mutants is slower than that in WT implies an additional defect in local diffusion or transport of RAB-5 containing vesicles. Taken together, we propose that UNC-16 may influence the dynamics of the RAB-5 compartment via recruitment of RAB-5 regulators to the synapse and by stimulating local membrane trafficking.
SV components are transported as precursor forms, which are morphologically and compositionally distinct from SVs at the synapses (Tsukita and Ishikawa, 1980; Bonanomi et al., 2006). Current models of SV maturation include at least two pathways, directly from the plasma membrane or indirectly through an intermediate endosomal compartment (Cremona and De Camilli, 1997; Hannah et al., 1999). Several studies have implicated Rab5 and its associated endosomal compartment in SV recycling following exocytosis (Fischer von Mollard et al., 1994; Rizzoli et al., 2006). Our observation that YFP∷RAB-5 synaptic puncta are diminished in mutants that are defective in synaptic endocytosis is consistent with these studies, and support a conclusion that the integrity or maintenance of the RAB-5 compartment requires SV recycling. However, we further find that in double mutants of unc-16 with those that are defective in SV endocytosis, the RAB-5 compartment remains abnormally accumulated at synapses. These results suggest that UNC-16 influences trafficking of RAB-5 containing membranous organelles independent of, or in parallel to, SV cycling from the plasma membrane. Our ultrastructural analysis shows that in unc-16 mutants the SV number per synapse is significantly reduced, while cisternal structures are increased both within the SV cluster and the surrounding perisynaptic area. While we cannot yet identify the molecular nature of these cisternae, it is conceivable that they may correspond to some of the RAB-5 compartments accumulated in unc-16 mutants. At the light microscope level, we observed that the puncta size of SNB-1∷GFP, which represents both precursor SVs and mature SVs, is increased, without changing the total amount of GFP fluorescence. In contrast, mCherry∷RAB-3, which is incorporated into SVs at later stages in the secretory pathway and preferentially associates with the active SV cycling pool (Matteoli et al., 1991; Star et al., 2005), is not altered. The latter observation argues that an impairment in synaptic exocytosis is unlikely to be the primary cause of RAB-5 dysfunction in unc-16 mutants. Instead, we interpret these results to mean that in unc-16 mutants, a fraction of SNB-1∷GFP reflects SV precursors that are abnormally accumulated in the RAB-5 positive compartment.
In summary, neuronal function depends on the proper sorting of synaptic components to the distinct compartments. This process requires long-range transport and regulation of local vesicular trafficking. JIP3 proteins have previously been characterized as cargo adaptors to the kinesin-1 complex in the selective transport and localization of SV precursors. In this study, we have uncovered an intriguing role of UNC-16 in regulating the synaptic RAB-5-containing compartment. We suggest that through yet unidentified molecules UNC-16 contributes to SV maturation from synaptic endosomes, by, modulating RAB-5 activity state and vesicular trafficking at synaptic endosomal organelles.
Supplementary Material
(A) Confocal images of CFP∷RAB-7(green) and YFP∷EEA-1 (red) coexpressed in the dorsal cord of the D-neurons. EEA-1 only partially overlaps with RAB-7. (B) Epifluorescence images of YFP∷EEA-1 in WT and unc-16 mutant animals. YFP∷EEA-1 is unaltered by unc-16 mutations. Scale bars are 10μm.
Single plane confocal images of D neuron cell bodies in animals co-expressing SNB-1∷CFP; YFP∷RAB-5, CFP∷RAB-7; YFP∷EEA-1, CFP∷RAB-7; YFP∷RAB-5, CRP∷RAB-10; mCherryRAB-3 and CFP∷RAB-11; mCherryRAB-3. Each pair of markers show only partial colocalization, suggesting that they each label distinct domains or compartments within the cell body. Scale bar is 5 μm.
Images are single plane confocal scans of animals co-expressing YFP∷RAB-5 (red) and UNC-16∷CFP (green). YFP∷RAB-5 and UNC-16∷CFP colocalize along the nerve processes and co-expression of RAB-5 with UNC-16 causes RAB-5 to be recruited to the enlarged UNC-16 puncta (white arrowheads). Scale bar is 10μm.
Phenotypes from Table S 2. Shown are dorsal cord epifluorescence images of YFP∷RAB-5 (juls198) in WT and various mutant animals. Scale bar is 10μm
Acknowledgments
We thank J. Kanbar for technical assistance, K. Shen for the mCherry∷RAB-3, A. D. Chisholm, S. Sann, and members of the Jin lab for comments on the manuscript. This work was supported by grants from the US National Institutes of Health (GM67237 to B.D.G, NS35546 to Y.J.). H.V.E. was supported by a UC President’s Postdoctoral Fellowship. A.G. is an Associate, and Y.J. is an Investigator, of the Howard Hughes Medical Institute.
References
- Bonanomi D, Benfenati F, Valtorta F. Protein sorting in the synaptic vesicle life cycle. Prog Neurobiol. 2006;80:177–217. doi: 10.1016/j.pneurobio.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Cavalli V, Kujala P, Klumperman J, Goldstein LS. Sunday Driver links axonal transport to damage signaling. J Cell Biol. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Kaplan JM. Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc Natl Acad Sci U S A. 2006;103:11399–11404. doi: 10.1073/pnas.0600784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam SJ, Jin Y. lin-14 regulates the timing of synaptic remodelling in Caenorhabditis elegans. Nature. 1998;395:78–82. doi: 10.1038/25757. [DOI] [PubMed] [Google Scholar]
- Hamill DR, Severson AF, Carter JC, Bowerman B. Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev Cell. 2002;3:673–684. doi: 10.1016/s1534-5807(02)00327-1. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Takei K, Cameron R, Hurlbut P, Johnston PA, Sudhof TC, Jahn R, De Camilli P. Association of Rab3A with synaptic vesicles at late stages of the secretory pathway. J Cell Biol. 1991;115:625–633. doi: 10.1083/jcb.115.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Bethani I, Zwilling D, Wenzel D, Siddiqui TJ, Brandhorst D, Jahn R. Evidence for early endosome-like fusion of recently endocytosed synaptic vesicles. Traffic. 2006;7:1163–1176. doi: 10.1111/j.1600-0854.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Kawamura S, Ozaki K. An essential role of Rab5 in uniformity of synaptic vesicle size. J Cell Sci. 2003;116:3583–3590. doi: 10.1242/jcs.00676. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. Embo J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Ishikawa H. The movement of membranous organelles in axons. Electron microscopic identification of anterogradely and retrogradely transported organelles. J Cell Biol. 1980;84:513–530. doi: 10.1083/jcb.84.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, de Hoop M, Zorzi N, Toh BH, Dotti CG, Parton RG. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts. Mol Biol Cell. 2000;11:2657–2671. doi: 10.1091/mbc.11.8.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Confocal images of CFP∷RAB-7(green) and YFP∷EEA-1 (red) coexpressed in the dorsal cord of the D-neurons. EEA-1 only partially overlaps with RAB-7. (B) Epifluorescence images of YFP∷EEA-1 in WT and unc-16 mutant animals. YFP∷EEA-1 is unaltered by unc-16 mutations. Scale bars are 10μm.
Single plane confocal images of D neuron cell bodies in animals co-expressing SNB-1∷CFP; YFP∷RAB-5, CFP∷RAB-7; YFP∷EEA-1, CFP∷RAB-7; YFP∷RAB-5, CRP∷RAB-10; mCherryRAB-3 and CFP∷RAB-11; mCherryRAB-3. Each pair of markers show only partial colocalization, suggesting that they each label distinct domains or compartments within the cell body. Scale bar is 5 μm.
Images are single plane confocal scans of animals co-expressing YFP∷RAB-5 (red) and UNC-16∷CFP (green). YFP∷RAB-5 and UNC-16∷CFP colocalize along the nerve processes and co-expression of RAB-5 with UNC-16 causes RAB-5 to be recruited to the enlarged UNC-16 puncta (white arrowheads). Scale bar is 10μm.
Phenotypes from Table S 2. Shown are dorsal cord epifluorescence images of YFP∷RAB-5 (juls198) in WT and various mutant animals. Scale bar is 10μm



