Abstract
Inducers of fetal hemoglobin (HbF) have shown considerable promise in the treatment of sickle cell disease (SCD). However, the same agents have shown less clinical activity in β-thalassemia (β-Thal). To understand the basis of these differences in clinical effectiveness, we compared the effects of butyrate and hemin on the expression of the different globin genes in progenitors-derived erythroid cells from patients with β-Thal intermedia and SCD. Exposure to butyrate resulted in an augmentation of γ-globin mRNA levels in both SCD and β-Thal. Interestingly, butyrate exposure increased α-globin expression in β-Thal, while α-globin mRNA levels decreased in SCD in response to butyrate. As a result, the favorable effects of the butyrate-induced increase in γ-globin expression on α:β-like globin mRNA imbalance in β-Thal were reduced as a result of the associated increase in α-globin expression. Hemin had similar but less profound effects on all three globin genes in both categories of patients. Although the majority of patients with β-Thal did not correct their globin imbalance in response to butyrate or hemin induction of HbF in a minority of patients resulted in marked reduction in globin imbalance. Thus, we believe that the poor clinical response in a majority of patients with β-Thal to inducers of γ-globin expression may be a reflection of unfavorable effects of these agents on the other globin genes.
Keywords: Thalassemia, Sickle Cells, Hemoglobin Expression, Butyrate, Hemin
INTRODUCTION
β-thalassemia (β-Thal) and sickle cell disease (SCD) are common genetic disorders that cause considerable morbidity and mortality throughout the world. Whereas β-Thal results from quantitative abnormalities of the expression of the adult β-globin genes, SCD results from qualitative abnormalities in the structure of the β-globin chains. These diseases become clinically apparent upon completion of fetal to adult hemoglobin switching after birth. In patients with SCD, substitution of valine for glutamic acid at position 6 of the β-globin chain results in a tendency of sickle hemoglobin (HbS) to polymerize upon deoxygenation, leading to deformed dense red blood cells. Thus, the predominant pathophysiological feature of SCD is vaso-occlusion, which leads to painful crises, acute chest syndrome, strokes and other acute and chronic complications [1]. In patients with β-Thal, a large number of mutations of the β-globin gene result in either partial reduction or complete loss of synthesis of adult β-globin chains, leading to accumulation of aggregates of unpaired, insoluble α-globin chains. The imbalance in the production of α:non-α globin chains is the major factor in the pathophysiology of β-Thal This results in premature destruction of red blood cells, leading to ineffective erythropoiesis and severe anemia [2].
The level of fetal hemoglobin (HbF) in erythrocytes accounts for a large part of the clinical heterogeneity in SCD [3, 4] and β-Thal [5, 6]. Continued HbF expression at high levels in adult life was shown to correlate with milder clinical phenotype in patients with β-globin disorders. In SCD, a high level of HbF interferes with the polymerization of HbS and prevents sickling of red blood cells, while a high level of γ-globin chain synthesis in β-Thal decreases α:non-α chain imbalance and ameliorates the anemia. This provided the rational for the effects to develop of pharmacological inducer of HbF to reactivate γ-globin expression in adult life of patients with SCD and β-Thal.
Several pharmacological agents including hydroxyurea, 5-azacytidine and butyrate that were shown to be effective in clinical trials in SCD were also tested in patients with β-Thal [7, 8]. Although all these agents have shown HbF-inducing activity in β-Thal, clinical response rates have been disappointingly low [9–13]. In other word, in many patients who increased their HbF levels in response to these agents, there was little or no correction of the anemia, which is the end-point of treatment in β-Thal [10, 13, 14]. In addition to increasing γ-globin expression, 5-azacytidine, decitabine & hydroxyurea can suppress hematopoiesis, including erythropoiesis, in patients with hemoglobin disorders [15–17]. This side-effect of HbF induction therapy could counteract the beneficial effects of the increased production of γ-globin chains and prevent the correction of the anemia in patients with β-thalassemia. For this reason, in the studies described below, we investigated the effects of butyrate and hemin in patients with SCD and β-thalassemia at concentrations that do not inhibit erythropoiesis.
Previous studies of the activities of several HbF inducing agents in progenitor-derived erythroid cells from normal volunteers showed different effects on the expression of the different globin genes. These studies showed that although 5-azacytidine, hydroxyurea and butyrate increased γ-globin expression, these agents had different effects on β-globin gene expression. Whereas hydroxyurea decreased β-globin expression, butyrate increased its expression and 5-azacytidine had no effect on its expression [23]. The molecular bases for these different effects are entirely unclear. It is also not clear whether the effects of HbF inducing agents on the expression of the α- and β-like globin genes will be different in patients with SCD and β-thalassemia. We believe that comparing the activities of different HbF inducing agents side-by-side in erythroid cells from patients with SCD and β-thalassemia might shed some light on the differences in the clinical responses of patients with these globin disorders. Thus, in the studies described in this report, we compared the effects of two HbF inducers; butyrate, a histone deacetylase inhibitor, and hemin, an erythropoiesis inducer, on expression of all major globin genes (i.e. γ-, β- and α-globin genes) in progenitor-derived erythroid cells from different patients with SCD and β-thalassemia.
DESIGN AND METHODS
Cell Cultures
Peripheral blood samples were obtained from five patients with SCD who signed an informed consent forms approved by the IRB of Mount Sinai School of Medicine, NY, USA and from nine patients with β-Thal intermedia (Table 1) from the Chronic Care Center, Hazmieh, Lebanon, following the guidelines of the local IRB. None of the patients enrolled in this study were on chronic transfusion regimens at the time they were enrolled in the study. Furthermore, any patient who received a blood transfusion during the 3 months prior to enrollment was excluded from participation in the study to avoid the confounding effects of transfusions on the response to HbF induction [7]. Peripheral blood mononuclear cells were isolated on a ficoll-hypaque gradient and cultured into burst-forming unit-erythroid (BFU-E) colonies as previously described [18], either in the absence (control) or presence of butyrate (50μM or 150μM, Sigma, Saint Louis, MO, USA) or hemin (100μM, Sigma). These drug concentrations appear to be non-toxic since they did not inhibit colony formation or decrease the number of cells per colony [18].
Table 1.
Characteristics of patients with β-Thal intermedia
| Patient# |
Age*/Gender |
α-globin genotype |
β-globin genotype |
Type of mutation |
% Hb F |
Symbols& |
|---|---|---|---|---|---|---|
| 1 | 21/M | αα/αα | IVSI-6/IVSI-6 | Splicing | 8.7 | ▵ |
| 2 | 52/M | αα/αα | IVSI-6/IVSI-6 | 10.5 | • | |
| 3 | 27/F | αα/αα | IVSI-6/IVSI-6 | 16.6 | ⋄ | |
| 4 | 24/F | αα/αα | IVSI-6/IVSI-6 | 17.3 | □ | |
| 5 | 42/F | αα/αα | IVSI-6/IVSI-6 | 60.0 | × | |
| 6 | 43/F | αα/αα | IVSI-1/IVSI-6 | RNA processing/splicing | nd | + |
| 7 | 24/F | αα/αα | IVSI-5/IVSI-5 | RNA processing | 80.1 | * |
| 8 | 62/M | αα/αα | −88/−88 | Transcription | 28.2 | ▴ |
| 9 | 50/F | αα/αα | −88/−88 | 55.5 | ○ |
Quantitative Real-Time Reverse-Transcription-PCR Analysis
Total RNA was extracted from cells using TRIzol kit (Invitrogen, Carlsbad, CA, USA) and treated with RNase-free DNase I (Ambion, Austin, TX, USA) for 30 min at 37°C and 5 min at 75°C. cDNA was synthesized using oligo (dT) primers from the Omniscript RT Kit (Qiagen, Valencia, CA) in 25 μl reaction volumes. Quantitative real-time reverse transcription-PCR assays were performed using specific primers and TaqMan probes for different globin genes as previously described [18, 19]. The expression of the different globin genes was normalized to the level of expression of 18S rRNA (Applied Biosystems, Foster City, CA) and the data expressed either as globin mRNA/18S rRNA, fold change relative to the control or % γ/(β+γ), % β/(β+γ) and α/(β+γ). The γ-, β- and α-globin primers and probes were previously described [18, 19].
Statistical analysis
Student’s t-test was used to determine statistical significance. A P≤0.05 was considered statistically significant.
RESULTS
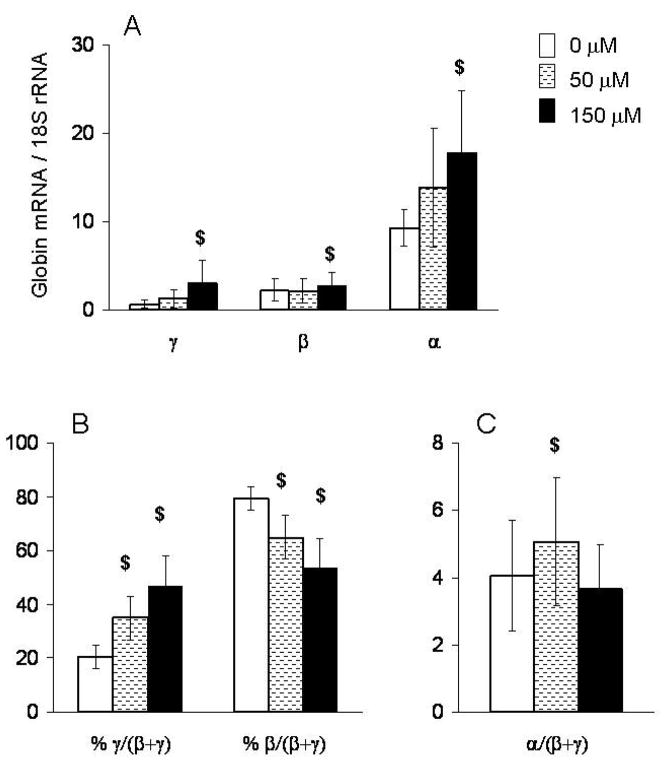
We compared the effects of 50μM or 150μM of butyrate in primary erythroid cells of patients with β-Thal intermedia and SCD. We used quantitative real-time PCR to measure the effects of increasing concentrations of butyrate on γ-, β- and α-globin mRNA in these cells. The detailed data presented in figure 1 and table 2 are from a representative patient homozygous for a splicing mutation of IVSI-6 that allows a significant level of normal globin mRNA splicing resulting in a β-Thal intermedia phenotype. In the absence of butyrate, the levels of γ- and β-globin mRNA were 15.5 fold and 4.2 fold less than α-globin mRNA, respectively (Fig 1A & table 2). Exposure to 50μM butyrate had no effect on β-globin mRNA levels, but exposure to 150μM butyrate resulted in a slight increase in β-globin mRNA. Furthermore, butyrate increased γ-globin mRNA levels by 2–4 fold in a dose-dependent manner. Thus, the γ/(β+γ) ratio increased in a dose-dependent manner by 15% and 27% in response to butyrate at 50μM and 150 μM, respectively (Fig 1B). Interestingly, the levels of α-globin mRNA also increased in a dose-dependent manner. Thus, the effects of butyrate on α/(β+γ) imbalance were very small at the lower concentration, and were completely lost at the higher concentration (Fig 1C).
Figure 1. Effects of different concentrations of butyrate on the expression of γ-, β- and α-globin genes.
Mononuclear cells from patient #4 with β-Thal intermedia were cultured at 50 μM and 150μM concentrations of butyrate. mRNA levels in BFU-E derived colonies were measured by quantitative real-time RT-PCR and expressed as (A) absolute levels, (B) % γ/(β+ γ), % β/(β+ γ) and (C) ratio α/(β+γ). In each experiment, each sample had triplicates $: p≤0.05.
Table 2.
Values of mRNA globin expression in progenitor-derived cells from patient #4
| Control |
Butyrate 150μM |
Increments |
|
|---|---|---|---|
| γ-globin* | 0.6 | 3.1 | 2.5 |
| β-globin* | 2.2 | 2.8 | 0.6 |
| α-globin* | 9.3 | 17.9 | 8.6 |
| α/γ ratio | 15.5 | 5.8 | |
| α/β ratio | 4.2 | 6.4 | 2.2 |
| α/(β+γ) ratio | 3.3 | 3.0 |
Globin mRNA/18S rRNA
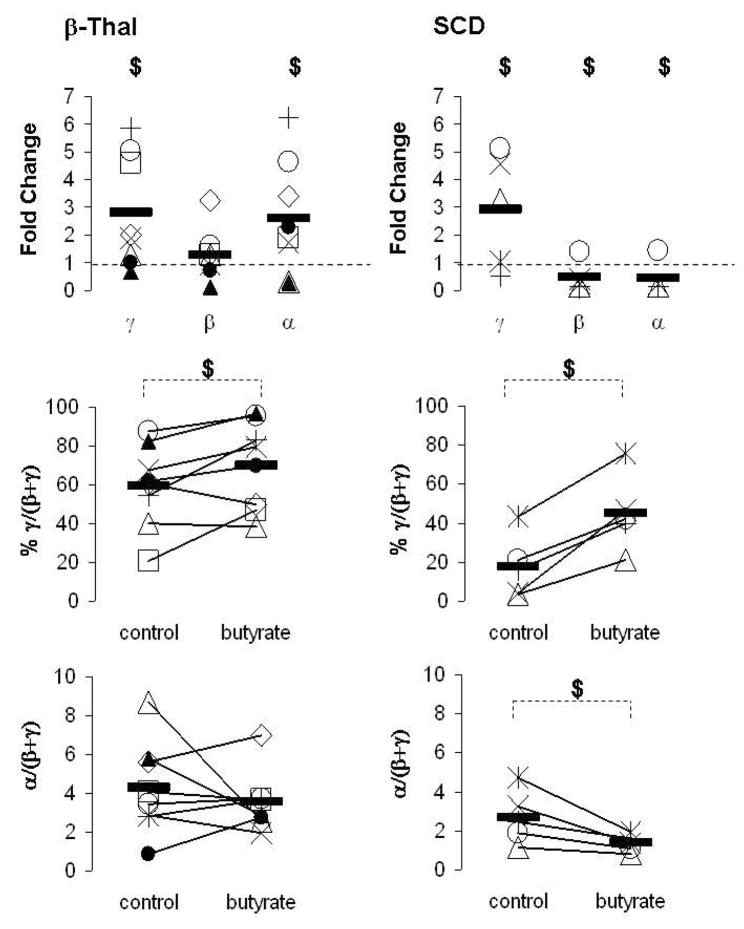
We performed similar studies in 8 patients with β-Thal intermedia and 5 patients with SCD. Butyrate increased γ-globin expression in 75% of patients with β-Thal and 60% of patients with SCD (Fig 2). However, butyrate exposure had markedly different effects on the expression of the β- and α-globin genes in the two categories of patients. While, butyrate had minor effects on β-globin expression in 63% of patients with β-Thal, β-globin mRNA levels decreased in 80% of patients with SCD (Fig 2). This butyrate-mediated decrease in β-globin expression resulted in a marked increase in the mean γ/(β+γ) mRNA ratios in patients with SCD while the mean increase in γ/(β+γ) ratios in patients with β-Thal was more modest (Fig 2). More importantly, the expression of the α-globin genes increased following butyrate exposure in 63% of patients with β-Thal, while α-globin mRNA levels decreased in 80% of patients with SCD (Fig 2). Thus, in the 8 patients with β-Thal that we studied, butyrate improved α/(β+γ) mRNA imbalance in 2 patients (i.e. patients # 1&9), worsened the imbalance in 2 patients (i.e. patients # 3&6) and did not affect the imbalance in 4 patients (Fig 2).
Figure 2. Effects of butyrate on the expression of the γ-, β- and α-globin genes.
Mononuclear cells from patients with β-Thal (×,+, ◇, □, ▵, ○, ●, ▲) and SCD (*, ×, +, ▵, ○) were cultured into BFU-E derived colonies in absence (control) or presence of 150μM butyrate. mRNA levels were measured by quantitative real-time RT-PCR and expressed as fold change relative to the control, % γ/(β+ γ) and ratio α/(β+ γ). Solid horizontal lines represent the mean value for every condition. In each experiment, each sample had triplicates $: p≤0.05
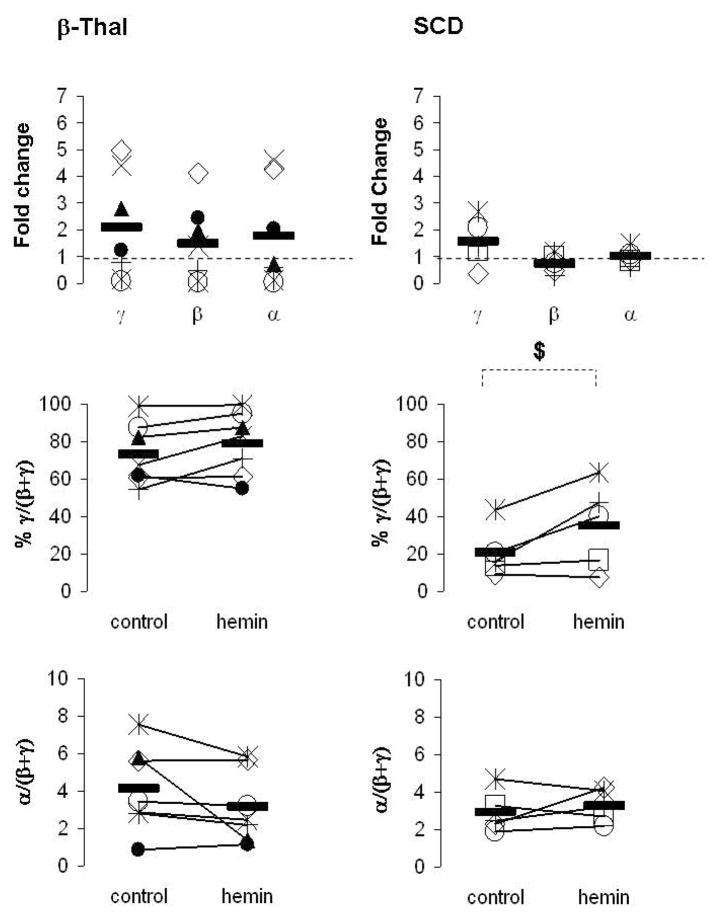
We also investigated the effects of hemin in primary erythroid cells from patients with β-Thal intermedia and SCD. We performed these studies in 7 patients with β-Thal intermedia and 5 patients with SCD. Hemin increased γ-globin expression in 57% and 40% of patients with β-Thal and SCD, respectively (Fig 3). Interestingly, hemin exposure had minor effects on β-globin expression in a majority of patients with β-Thal and SCD (Fig 3). Overall, hemin-exposure resulted in an increase in γ/(β+γ) mRNA ratios in only 29% and 40% of patients with β-Thal and SCD, respectively (Fig 3). The hemin-mediated increase in γ-globin expression was associated with an increase in the expression of the α-globin genes in patients with β-Thal, while the levels of α-globin mRNA were relatively unchanged in patients with SCD (Fig 3). Thus, hemin did not have significant favorable effects on the α/(β+γ) mRNA imbalance in the 7 patients with β-Thal that we studied (Fig 3).
Figure 3. Effects of hemin on the expression of the γ-, β- and α-globin genes.
Mononuclear cells from patients with β-Thal (*, ×, +, ◇, ○, ●, ▲) and SCD (*, +, □, ◇, ○) were cultured into BFU-E derived colonies in absence (control) or presence of 100μM hemin. mRNA levels were measured by quantitative real-time RT-PCR and expressed as fold change relative to the control, % γ/(β+ γ) and ratio α/(β+ γ). Solid horizontal lines represent the mean value for every condition. In each experiment, each sample had triplicates $: p≤0.05
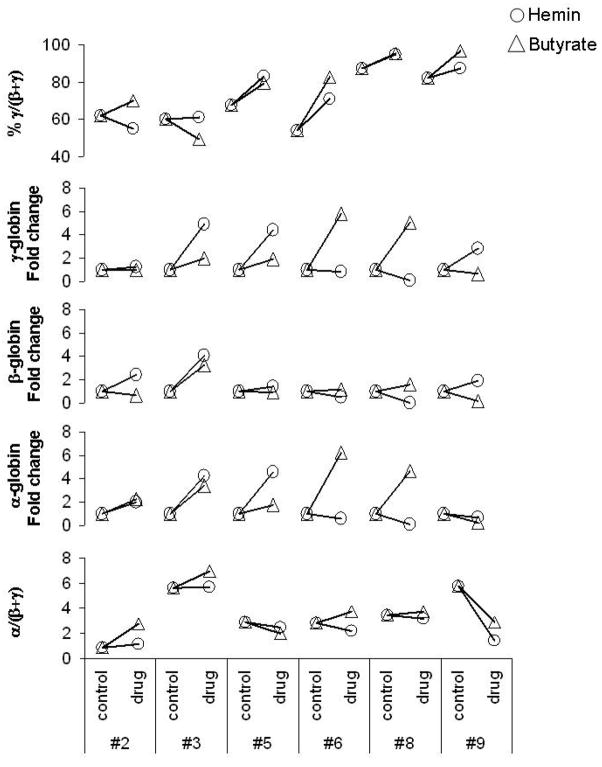
Our in vitro assay makes it possible to compare the effects of different HbF inducers in BFU-E from the same patient. Thus, we compared the effects of butyrate and hemin on the expression of different globin genes in six patients with β-thal intermedia (Fig 4). In some patients, butyrate resulted in more effective induction of γ-globin genes (i.e. patients #6&8) while in others, hemin was more effective (i.e. patients # 3, 5&9) (Fig 4). Similarly, butyrate resulted in more induction of α- and β-globin expression in some patients while in others, hemin resulted in more induction than butyrate. More importantly, in one patient, hemin resulted in an improvement in α/(β+γ) chain imbalance while butyrate worsened the chain imbalance (i.e. patient #6). In another patient, both agents resulted in a marked improvement in the α/(β+ γ) chain imbalance (i.e. patient #9)
Figure 4. Comparison of the effects of butyrate and hemin on the expression of the γ-, β- and α-globin genes in the progenitor cells of the same patients with β-thal.
Mononuclear cells from patients with β-Thal (#2, 3, 5, 6, 8&9) were cultured into BFU-E derived colonies in absence (control) or presence (drug) of 150 μM butyrate or 100μM hemin. mRNA levels were measured by quantitative real-time RT-PCR and expressed as % γ/(β+ γ), fold change relative to the control and ratio α/(β+γ). In each experiment, each sample had triplicates
DISCUSSION
All the pharmacological agents that have been tested in clinical trials to induce HbF production in patients with SCD were also tested in β-Thal and Hb E/β-Thal [20]. The first study of hydroxyurea in thalassemia major showed that administration of the drug to six transfusion-dependent patients did not lead to a reduction in the frequency of transfusion [13]. Other studies that followed in different patient populations with β-Thal major showed either no effect or a modest effect of hydroxyurea on transfusion requirements in the studied patients [9, 21]. Moreover, a study of hydroxyurea in β-Thal intermedia demonstrated a significant increase in HbF levels without a corresponding amelioration of the anemia [14]. As a result of these studies that showed modest effects of hydroxyurea in a small minority (10–20%) of the treated patients, this drug was not approved by the FDA for the treatment of β-Thal. Butyrate was also tested in patients with β-Thal. Although administration of arginine butyrate was shown to result in a significant improvement in the anemia in a few patients with β-Thal, the majority of the studied patients continued to manifest profound anemia [10]. The activity of decitabine is currently under investigation in patients with β-Thal. Although all these agents have shown some HbF inducing activity in patients with β-Thal, meaningful hematological and/or clinical responses have been much more difficult to demonstrate. In contrast all these agents have shown marked hematological and/or clinical effects in patients with SCD [20].
As a first step towards understanding why the current drugs and therapeutic regimens are not as effective in β-Thal as in SCD, we performed a comprehensive analysis of the effects of butyrate and hemin on the expression of the α-, β- and γ-globin genes. In contrast, the vast majority of previous studies evaluated the effect of the different agents on the relative levels of the HbF in patients with β-Thal. Our data in progenitor cells from patients with β-Thal intermedia showed that although butyrate and hemin were effective inducers of the expression of the γ-globin genes, they also induced the expression of the α-globin genes. These observations are in agreement with our previous report that showed an increase in α-globin mRNA in human erythroleukemic K562 cells following butyrate and hemin exposure [19]. Thus, our data show that an increase in γ-globin expression does not necessarily result in improvement in globin imbalance in β-Thal. The beneficial effects of γ-globin induction on the chain imbalance (i.e. α/(β+ γ)) were neutralized, at least in part, by the corresponding increase inα-globin expression in a large fraction of patients with β-Thal. Interestingly, the small fraction of patients with β-Thal that corrected their chain imbalance in vitro are very similar to the low response rates observed in vivo following treatment of patients with β-Thal with HbF inducers. Interestingly, these drugs had a different effect on α-globin expression in patient with SCD. Butyrate exposure resulted in a significant decrease in α-globin mRNA levels, while hemin had no effect on α-globin expression in patients with SCD. A decrease in α-globin mRNA levels has favorable effect in patients with SCD since it will mimic the favorable effect of α-thalassemia in these patients.
Moreover, our studies showed that when these agents induced a real reversal of the fetal to adult globin switch, the effects of this switch on the disease pathophysiology are different in SCD and β-Thal. The observed decrease in the expression of the β-globin gene in SCD would lead to a decrease in the effective concentration of HbS and a further increase in the relative HbF levels, resulting in more potent anti-sickling effects. In contrast, a similar decrease in the expression of β-globin gene in β-Thal intermedia in which the β-globin gene is partially active will have a negative effect on chain imbalance (i.e. α/(β+ γ)) and reduce the benefit from the increase in γ-globin gene expression.
We would like to point out that for logistical and technical reasons, the studies described in this report were limited to the measurements of globin mRNA levels and did not include measurements of globin chain synthesis in progenitor derived cells from patients with β-thal intermedia. We had previously shown that butyrate increases γ-globin chain synthesis in patients with SCD in part by increasing the efficiency of translation of γ-globin mRNA [22]. Thus, we are fully aware that the agents that we are using to induce the transcription of the γ-globin genes may also have different effects on the efficiency of translation of globin mRNAs. Nonetheless, the studies we described above make it possible to conclude that these agents do have different effects on the levels of the different globin mRNA levels. It is reasonable to assume that these changes in globin mRNA levels will be associated with corresponding changes in the levels of globin chain synthesis. As a matter of fact, in experiments we did not present in this report, we found that in erythroid cells from patients with SCD, the levels of mRNA (% γ/(γ+β)) measured by real time PCR corresponded well with the levels of globin chains (%γ/(γ+β)) measured by polyacrylamide gel electrophoresis and fluorography in presence or absence of butyrate or hemin (data not shown). Thus, we believe it is very likely that the differences we observed in globin mRNA levels in response to butyrate and hemin will be associated with similar but not necessarily identical changes in globin chain synthesis.
The studies described in this report emphasize the importance of quantifying the effects of inducers of HbF on all the relevant globin genes, not only on the targeted γ-globin genes. These effects might explain, at least in part, the more favorable effects of the inducers of HbF in SCD than in β-Thal. It is clear from our data that an increase in γ-globin expression does not always result in an improvement in the chain imbalance in β-Thal. As a result, it should not be surprising that a significant fraction of patients with β-Thal who increase their HbF levels in response to pharmacological agents do not respond by correcting their anemia [20].
Although we have only tested two pharmacological agents in a relatively small number of patients, our data shows that the different agents can affect the expression of globin genes differently in the same patient and in different patients. These observations support and extend previous observations by Smith et al in which 5-azacytidine, butyrate and hydroxyurea had different effects on mRNA levels of the different β-like globin genes in erythroid cells from normal donors [23]. Our studies showed that even when two agents induce γ-globin expression in the same patient, one agent may correct the chain imbalance and another may either not correct it or make it worse. Thus, although the fraction of patients who respond to a particular agent by correcting their chain imbalance may be small, the fraction of patients with β-Thal who might correct their chain imbalance in response to a battery of different agents could be much larger. Thus, if it were possible to predict the in vivo effects on chain imbalance by testing these agents in vitro, it might be possible to “personalize” HbF induction therapy by selecting the best agent for a particular patient based on in vitro activity. In vitro testing might also predict responsiveness to a combination of pharmacological agents that could result in maximal correction of the chain imbalance in vivo. This hypothesis will need to be tested in clinical trials in a larger number of patients with β-Thal. Furthermore, it is entirely unclear why different patients may respond to inducers of HbF differently. This will undoubtedly require further laboratory investigation to determine whether the epigenetic configuration of the globin gene clusters will determine responsiveness to the different drugs. It is becoming clear that the idea of finding a single agent that will correct the pathophysiology of the disease in all patients with SCD and β-Thal may be too optimistic. The approval of the use of hydroxyurea for the treatment of SCD should be considered the beginning of the era of pharmacological induction of fetal hemoglobin rather than its end. Research in the laboratory for the clinic drug the next two decades will likely illustrate the full potential of this therapeutic approach to hemoglobin disorders.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (HL-073438) to GFA and another grant from Ovation Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 2.Weatherall DJ. Pathophysiology of thalassaemia. Baillieres Clin Haematol. 1998;11:127–146. doi: 10.1016/s0950-3536(98)80072-3. [DOI] [PubMed] [Google Scholar]
- 3.Bailey K, Morris JS, Thomas P, Serjeant GR. Fetal haemoglobin and early manifestations of homozygous sickle cell disease. Arch Dis Child. 1992;67:517–520. doi: 10.1136/adc.67.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens MC, Hayes RJ, Vaidya S, Serjeant GR. Fetal hemoglobin and clinical severity of homozygous sickle cell disease in early childhood. J Pediatr. 1981;98:37–41. doi: 10.1016/s0022-3476(81)80529-x. [DOI] [PubMed] [Google Scholar]
- 5.Bollekens JA, Forget BG. Delta beta thalassemia and hereditary persistence of fetal hemoglobin. Hematol Oncol Clin North Am. 1991;5:399–422. [PubMed] [Google Scholar]
- 6.Serjeant GR. Natural history and determinants of clinical severity of sickle cell disease. Curr Opin Hematol. 1995;2:103–108. doi: 10.1097/00062752-199502020-00001. [DOI] [PubMed] [Google Scholar]
- 7.Fathallah H, Sutton M, Atweh GF. Pharmacological Induction of Fetal Hemoglobin: Why Haven’t We Been More Successful in Thalassemia? Ann N Y Acad Sci. 2005;1054:228–237. doi: 10.1196/annals.1345.029. [DOI] [PubMed] [Google Scholar]
- 8.Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. J Clin Invest. 2007;117:850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajjar FM, Pearson HA. Pharmacologic treatment of thalassemia intermedia with hydroxyurea. J Pediatr. 1994;125:490–492. doi: 10.1016/s0022-3476(05)83304-9. [DOI] [PubMed] [Google Scholar]
- 10.Ikuta T, Atweh G, Boosalis V, White GL, Da Fonseca S, Boosalis M, Faller DV, Perrine SP. Cellular and molecular effects of a pulse butyrate regimen and new inducers of globin gene expression and hematopoiesis. Ann N Y Acad Sci. 1998;850:87–99. doi: 10.1111/j.1749-6632.1998.tb10466.x. [DOI] [PubMed] [Google Scholar]
- 11.Ley TJ, DeSimone J, Anagnou NP, Keller GH, Humphries RK, Turner PH, Young NS, Keller P, Nienhuis AW. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982;307:1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- 12.Ley TJ, DeSimone J, Noguchi CT, Turner PH, Schechter AN, Heller P, Nienhuis AW. 5-Azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patients with sickle cell anemia. Blood. 1983;62:370–380. [PubMed] [Google Scholar]
- 13.Nienhuis AW, Ley TJ, Humphries RK, Young NS, Dover G. Pharmacological manipulation of fetal hemoglobin synthesis in patients with severe beta-thalassemia. Ann N Y Acad Sci. 1985;445:198–211. doi: 10.1111/j.1749-6632.1985.tb17189.x. [DOI] [PubMed] [Google Scholar]
- 14.Loukopoulos D, Voskaridou E, Stamoulakatou A, Papassotiriou Y, Kalotychou V, Loutradi A, Cozma G, Tsiarta H, Pavlides N. Hydroxyurea therapy in thalassemia. Ann N Y Acad Sci. 1998;850:120–128. doi: 10.1111/j.1749-6632.1998.tb10469.x. [DOI] [PubMed] [Google Scholar]
- 15.Charache S, Dover GJ, Moyer MA, Moore JW. Hydroxyurea-induced augmentation of fetal hemoglobin production in patients with sickle cell anemia. Blood. 1987;69:109–116. [PubMed] [Google Scholar]
- 16.Fibach E, Prasanna P, Rodgers GP, Samid D. Enhanced fetal hemoglobin production by phenylacetate and 4-phenylbutyrate in erythroid precursors derived from normal donors and patients with sickle cell anemia and beta-thalassemia. Blood. 1993;82:2203–2209. [PubMed] [Google Scholar]
- 17.Humphries RK, Dover G, Young NS, Moore JG, Charache S, Ley T, Nienhuis AW. 5-Azacytidine acts directly on both erythroid precursors and progenitors to increase production of fetal hemoglobin. J Clin Invest. 1985;75:547–557. doi: 10.1172/JCI111731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fathallah H, Weinberg RS, Galperin Y, Sutton M, Atweh GF. Role of epigenetic modifications in normal globin gene regulation and butyrate-mediated induction of fetal hemoglobin. Blood. 2007;110:3391–3397. doi: 10.1182/blood-2007-02-076091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fathallah H, Portnoy G, Atweh GF. Epigenetic analysis of the human alpha- and beta-globin gene clusters. Blood Cells Mol Dis. 2008;40:166–173. doi: 10.1016/j.bcmd.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atweh GF, Loukopoulos D. Pharmacological induction of fetal hemoglobin in sickle cell disease and beta-thalassemia. Semin Hematol. 2001;38:367–373. doi: 10.1016/s0037-1963(01)90031-9. [DOI] [PubMed] [Google Scholar]
- 21.Zeng YT, Huang SZ, Ren ZR, Lu ZH, Zeng FY, Schechter AN, Rodgers GP. Hydroxyurea therapy in beta-thalassaemia intermedia: improvement in haematological parameters due to enhanced beta-globin synthesis. Br J Haematol. 1995;90:557–563. doi: 10.1111/j.1365-2141.1995.tb05584.x. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg RS, Ji X, Sutton M, Perrine S, Galperin Y, Li Q, Liebhaber SA, Stamatoyannopoulos G, Atweh GF. Butyrate increases the efficiency of translation of gamma-globin mRNA. Blood. 2005;105:1807–1809. doi: 10.1182/blood-2004-02-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith RD, Li J, Noguchi CT, Schechter AN. Quantitative PCR analysis of HbF inducers in primary human adult erythroid cells. Blood. 2000;95:863–869. [PubMed] [Google Scholar]