Abstract
Background
The conventional protein kinase C (PKC) isoform α functions as a proximal regulator of Ca2+ handling in cardiac myocytes (Braz et al., 2004, Nat. Med. 10:248). Deletion of PKCα in the mouse resulted in augmented sarcoplasmic reticulum Ca2+ loading, enhanced Ca2+ transients, and augmented contractility, while overexpression of PKCα in the heart blunted contractility. Mechanistically, PKCα directly regulates Ca2+ handling by altering the phosphorylation status of inhibitor-1, which in turn suppresses protein phosphatase-1 activity, thus modulating phospholamban activity and secondarily, the sarcoplasmic reticulum Ca2+ ATPase (SERCA).
Methods and Results
Here we show that acute inhibition of the conventional PKC isoforms with Ro-32-0432 or Ro-31-8220 significantly augmented cardiac contractility in vivo or in an isolated work performing heart preparation in wildtype mice, but not in PKCα deficient mice. Ro-32-0432 also acutely increased cardiac contractility in two different models of heart failure in vivo. Acute or chronic treatment with Ro-31-8220 in a mouse model of heart failure due to deletion of the muscle lim protein (MLP) gene significantly augmented cardiac contractility and restored pump function. Moreover, adenoviral-mediated gene therapy with a dominant negative PKCα cDNA rescued heart failure in a chronic rat model of post-infarction cardiomyopathy. PKCα was also determined to be the dominant conventional PKC isoform expressed in the adult human heart, providing potential relevance of these findings to human pathophysiology.
Conclusions
Pharmacological inhibition of PKCα, or the conventional isoforms in general, may serve as a novel therapeutic strategy for acutely enhancing cardiac contractility in certain stages of heart failure.
Keywords: Heart failure, contractility, PKC, signaling, cardiomyopathy
Introduction
The protein kinase C (PKC) family of Ca2+ and/or lipid-activated serine-threonine kinases function downstream of many membrane-associated signal transduction pathways1. Approximately 10 different isozymes comprise the PKC family, which are broadly classified by their activation characteristics. The conventional PKC isozymes (α, βI, βII, and γ) are Ca2+- and lipid-activated, while the novel isozymes (ε, θ, η, and δ) and atypical isozymes (ζ, and λ) are Ca2+-independent but activated by distinct lipids2. PKCα is the predominant Ca2+-dependent PKC isoform expressed in the mouse and rabbit heart, while PKCβ and PKCγ are detectable and may have partially overlapping functions3,4.
With respect to the heart, a number of reports have associated PKC activation with hypertrophy, dilated cardiomyopathy, ischemic injury, or mitogen stimulation1. Some evidence also exists implicating PKC isozymes as potential regulators of Ca2+ handling and cardiomyocyte contractility. For example, transient stimulation of PKC activity with phorbol 12-myristate 13-acetate (PMA) in high Ca2+ buffer caused a decrease in cardiac myocyte contraction and a decrease in the magnitude of the Ca2+ transient, which was reversed with a PKC inhibitory agent5,6. PMA stimulation also depressed cardiac contractility in isolated rat hearts and/or isolated cultured-cells, an effect that was also abrogated with PKC inhibitors7–10.
Myocyte contraction and relaxation are directly regulated by intracellular Ca2+ cycling. Ca2+ enters from the voltage-dependent L-type Ca2+ channel within the sarcolemma (plasma membrane), which induces a large release of Ca2+ from the sarcoplasmic reticulum (SR) storage compartment through the ryanodine receptor11. Myocyte relaxation is initiated by sequestration of Ca2+ within the SR through the activity of the sarcoplasmic reticulum Ca2+ ATPase (SERCA2) pump and by Na+/ Ca2+ exchanger activity at the sarcolemma. The magnitude and timing of Ca2+ release, hence the strength of contraction, is dynamically regulated by β-adrenergic receptor signaling to adenylyl cyclase, which produces cAMP resulting in protein kinase A (PKA) activation11. Once activated, PKA directly phosphorylates nodal Ca2+ regulatory proteins such as the L-type Ca2+ channel, the ryanodine receptor, phospholamban (PLN), and inhibitor-1 as a means of regulating protein phosphatase 1 (PP1)11. The failing heart is associated with a dysregulation in Ca2+ handling through many of these proteins and a desensitization in β-adrenergic receptor signaling.
The loss of contractility that accompanies heart failure is also associated with a general increase in PKCα protein content and activity12–19. We have previously shown that PKCα functions as a fundamental regulator of cardiac contractility and Ca2+ handling in myocytes18,20. For example, PKCα gene-deleted mice were shown to be hypercontractile, while transgenic mice overexpressing PKCα were hypocontractile. Enhancement in cardiac contractility associated with PKCα gene deletion protected against pressure overload-induced heart failure and dilated cardiomyopathy associated with deletion of the muscle lim protein (MLP) gene in the mouse18. Here we performed a pre-clinical analysis to determine the efficacy of PKCα inhibition as a means of treating heart failure in adult mice and rats.
Methods
Animal Models
The PKCα−/− and MLP−/− mice were described previously18,21. Equal ratios of males and females were used in all studies for consistency. Animal experiments were approved by the Institutional Animal Care and Use Committee.
Echocardiography and Physiological Preparations
Mice were anesthetized with isoflurane, and echocardiography was performed using a Hewlett Packard 5500 instrument with a 15-MHz compact linear array probe. Echocardiographic measurements were taken on M-mode in triplicate for each mouse. The isolated work-performing heart preparation in the mouse has been described in detail previously22. Acute infusion of Ro-32-0432 in the isolated working heart preparation was performed at a final concentration of 8 ×10−8 µg/ml for 5 minutes with a stock solution made up in DMSO, which was infused with the Krebs solution resulting in a working content of DMSO below 0.05% (infused at 0.2–0.4 ml/min). For invasive hemodynamics in the closed-chest mouse, a 1.4 F Millar catheter was placed into the left ventricle through the right carotid artery to monitor real time heart rate, arterial and left ventricular pressures, and +dP/dt (dP/dtmax) and −dP/dt (dP/dtmin), using MacLab software and interface (Mountain View, CA), as described previously23. In this preparation, dobutamine was given at 32 µg/kg/min, while Ro-32-0432 gave a maximal response at 22.5 µg/kg/min.
Cryoinfarction Model of Heart Failure in the Rat
The rat cryoinfarct model of heart failure was described in detail previously24. Briefly, adult male Sprague-Dawley rats (250–300 g) were anesthetized, mechanically ventilated, and the heart was exposed by a median sternotomy. Twelve of these mice were subjected to cryoinfarction with a liquid nitrogen-cooled probe (8 mm diameter) for 3 freeze-thaw cycles on the left ventricular anterior free wall. Eight other animals underwent a sham procedure.
Rat Catheterization, Invasive Hemodynamics, and Intracoronary Adenoviral Delivery
In vivo cardiac adenoviral gene therapy was performed through an intracoronary route of delivery in the rat as described previously24,25. Adenovirus was given at 4×1010 plaque forming units for Adβgal (N=12, cryoinfarct group) and AdPKCα-dn (N=7, cryoinfarct group) in 1.6 mls of saline injected rapidly while the aorta was cross-clamped. There was also a virus-free sham control group (N=8). One-week afterwards global function was measured in a closed-chest preparation by cardiac catheterization with a 2 F pressure-transducer (Millar Instruments, Houston TX) as described previously25.
Cardiac Histological Analysis
Hearts were collected at the indicated times, fixed in 10% formalin containing PBS, and embedded in paraffin. Serial 9-µm heart sections from each group were analyzed. Samples were stained with H&E or Masson’s trichrome.
Primary Cardiomyocyte Culture
Primary cultures of cardiomyocytes were obtained by enzymatic dissociation of 1–2 day-old Sprague-Dawley rat neonates as described previously, as well as adenoviral infection conditions26. Cardiomyocytes were cultured under serum-free conditions in M199 media supplemented with penicillin/streptomycin (100 U/ml) and L-glutamine (2 mmol/L). Cells were subsequently treated with Ro-32-0432 or Ro-31-8220 at a concentration of 50 nM for 1.5 hrs. PMA (200 nM) was also given 1 hr before harvest.
Replication-Deficient Adenoviruses
Dominant negative PKCα was described previously as a L368R mutation18,27. AdPKCα-dn or an adenovirus encoding β-galactosidase (Adβgal) were plaque purified, expanded, titered in HEK293 cells, and banded in CsCl for gene therapy in the rat cryoinfarct model described above.
Western Blot Analysis
Western blotting was performed as described previously with primary antibodies against Phospho-MARCKS, PKCα, βI, βII, γ, and ε (Santa Cruz)26,27. Recombinant PKC isozyme standards were loaded between 0.1 ng – 15 ng (Upstate Biotechnologies). Chemifluorescent detection was performed with the Vistra ECF reagent (RPN 5785; Amersham Pharmacia Biotech) and scanned with a PhosphorImager.
Statistical Analysis
Two-sample Student’s t tests were used to compare means between two independent groups/samples, and analysis of variance (ANOVA) was used to compare means among three or more independent groups. Paired t tests were used to compare values within mice before and after treatment. Repeated measures ANOVA was used for analysis of dose-response data. A Newman-Keuls post test was applied whenever multiple comparisons were conducted. Note that caution is warranted when using parametric tests such as t tests or ANOVA since those are not robust in small samples as shown in figures 1C and 1D.
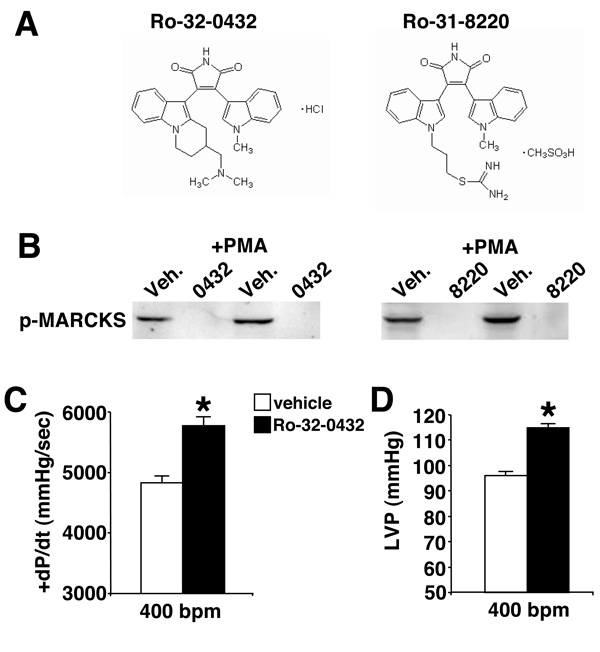
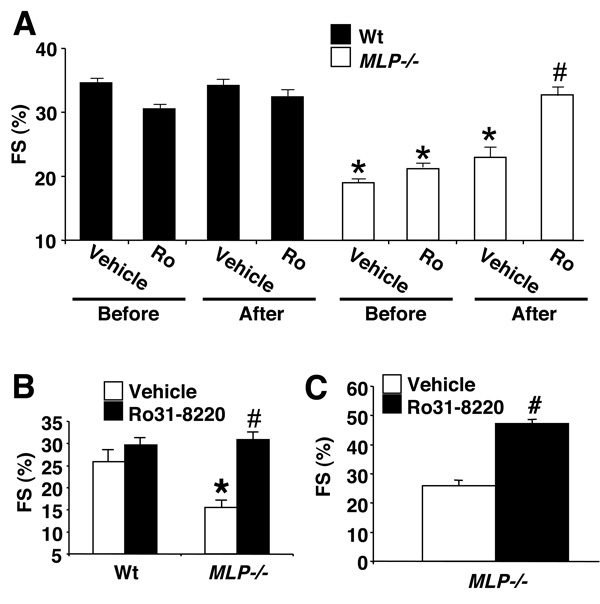
Figure 1. Functional assessment of Ro-32-0432 and Ro-31-8220 in the heart.
(A) Chemical structures of Ro-32-0432 and Ro-31-8220 used in this study. (B) Western blot analysis for phosphoMARCKS protein to analyze the inhibitory ability of Ro-32-0432 (50 nM) and Ro-31-8220 (50 nM) compared with vehicle (Veh) in cultured neonatal rat cardiomyocytes infected with AdPKCα and treated with PMA. (C) Isolated working heart preparation of cardiac contractility (+dP/dt) *P=0.0052 or (D) left ventricular pressure developed following vehicle infusion (N=3) or Ro-32-0432 (N=4) at 8 × 10−8 µg/ml *P=0.0012. Statistical significance was assessed by two-sample t tests.
Statement of Responsibility
All authors had full access to the data and take full responsibility for its integrity.
Results
Acute Infusion of Ro-32-0432 Increases Cardiac Contractility in Mice
Loss or inhibition of PKCα augments cardiac contractile function at baseline and in models of heart failure18,20. These results suggested that a non-toxic and tissue-available pharmacologic inhibitor with selectivity towards PKCα, or the classical isoforms of PKC (α, β and γ) might be of significant therapeutic value. Here we investigated two different bisindolylmaleimide compounds, Ro-32-0432 and Ro-31-8220, previously shown to have selectivity and efficacy for classical PKC isoforms, especially PKCα28–30 (Fig 1A). To address the ability of these compounds to inhibit PKCα in cardiomyocytes, phosphorylation of the MARCKS protein was investigated given its description as a PKC target and its ability to specifically bind the PKCα isozyme31,32. Both compounds specifically inhibited the levels of phosphorylated MARCKS protein at serine 152/156 in cardiomyocytes compared with vehicle after PMA stimulation (Fig 1B). We were unable to identify any phosphorylation target that was absolutely specific to PKCα, nor was intracellular translocation of PKCα altered by the Ro compounds (data not shown). While other endogenous PKC isoforms could also phosphorylate MARCKS, this assay was made relatively specific by overexpression of a given PKC isozyme (Supplemental Figure). Using this assay, Ro-31-8220 and Ro-32-0432 were determined to be mostly specific for PKCα and β, but not ε and δ (Supplemental Figure).
To address the ability of this class of compound to augment intrinsic cardiac contractility in the absence of neurohumoral influences, an isolated working heart preparation was performed. After equilibration, acute infusion of Ro-32-0432 significantly enhanced contractility and left ventricular developed pressure in hearts from mice in the FVB genetic background (Fig 1C, D).
PKCα null mice demonstrate specificity of the inhibitory effect on contractility
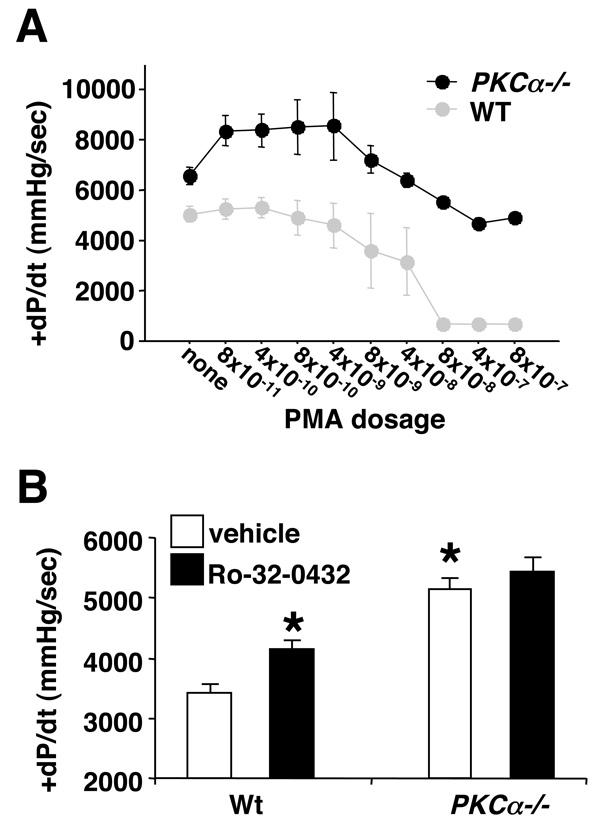
The data presented above suggested that Ro-32-0432 and Ro-31-8220 could enhance cardiac contractile function, although the relative specificity of this effect as being mediated through conventional PKC isozyme inhibition was uncertain. To more directly ascertain specificity, acute infusion of PMA or Ro-32-0432 was performed in wildtype or PKCα null hearts in an isolated work performing heart preparation. PMA functions as a potent activator of PKC isozymes in the heart, largely producing a negative effect on contractility5–10. In theory, if PKCα is the primary regulator of the contractility data presented above, mice deficient in PKCα should not respond to Ro-32-0432, or possibly PMA. PMA infusion had no effect on contractility in isolated wildtype hearts from a concentration of 8×10−11 µg/ml through 4×10−9 µg/ml (Fig 2A). Increasing PMA concentration above 8×10−9 severely reduced or completely arrested cardiac contractility (Fig 2A). However, PMA infusion in isolated hearts from PKCα null mice had a noticeably different effect. PMA concentrations of 8×10−11 through 4×10−9 produced a mild, but significant positive inotropic effect, and even at the highest concentration of PMA did not reduce contractility in PKCα null hearts below untreated wildtype hearts (Fig 2A).
Figure 2. PKCα is the primary contractility regulating PKC isozyme in the heart.
(A) Isolated working heart preparation from wildtype (N=4) and PKCα−/− (N=4) mice. Contractility (+dP/dt) was continuously measured as increasing concentration of PMA was infused at the indicated dosages (µg/ml) for 5 minutes each. These data were evaluated for significance using a repeated-measure analysis of variance. All measurements are +/−SEM. (B) Isolated working heart preparation in wildtype (N=6) and PKCα−/− (N=6) mice. Hearts were infused with vehicle (N=6) or Ro-32-0432 (N=6, 8 × 10−8 µg/ml). Significance was assessed by ANOVA (P=0.0002) followed by a Newman-Keuls post test. *P<0.05 versus Wt vehicle.
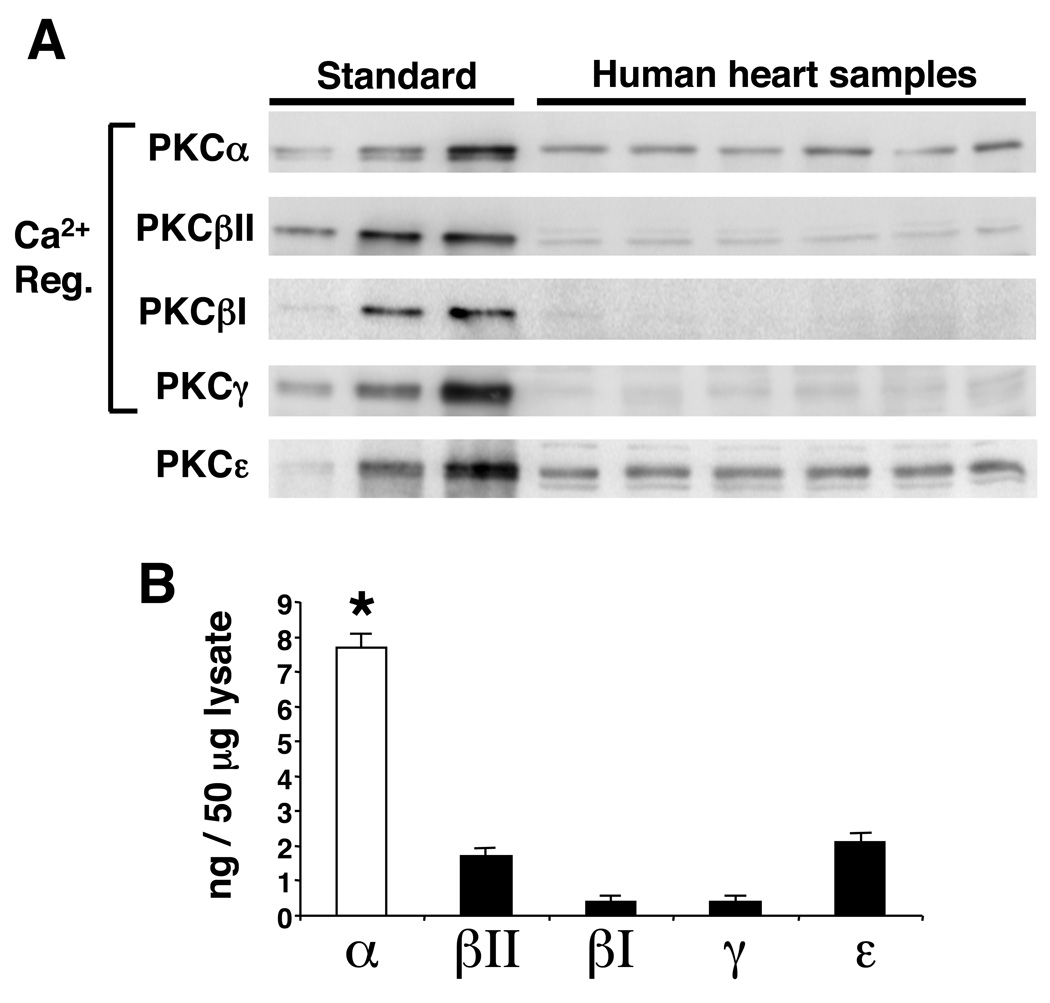
Consistent with the PMA infusion data discussed above, infusion of Ro-32-0432 (8 × 10−8 µg/ml) did not significantly enhance cardiac contractility in work performing isolated hearts from PKC α−/− mice, while hearts from wildtype mice (C57BL/6 background) showed a significant increase upon infusion (P<0.05) (Fig 2B). As expected, hearts from PKC α−/− mice continued to show enhanced cardiac contractility at baseline or with vehicle infusion (Fig 2A, B). Taken together, these data suggest that the overall contractility effects observed for the bisindolylmaleimide compounds are largely mediated through PKCα. While these compounds can also inhibit PKCβ and PKCγ, these isoforms are significantly lower in total content in the heart compared with PKCα, further suggesting PKCα as a biologically important target3,4. PKCα levels also dominate in the human heart compared with PKCβ, PKCγ, and PKCε when normalized to total protein content with recombinant standards (Fig 3A, B). This result suggests that pharmacologic inhibition of PKCα with compounds of the bisindolylmaleimide class might also be efficacious as a selective inotrope in heart failure patients.
Figure 3. Western blot analysis of PKC isozyme content in the adult human heart.
(A) Western blot panels and (B) quantitation of the indicated PKC isozymes in the human heart normalized against recombinant protein standards for each. Significance was assessed by ANOVA (P<0.0001) followed by Dunnett’s post test. *P<0.01 α versus βII, βI, γ, and ε.
Ro-32-0432 increases cardiac contractility in heart failure models
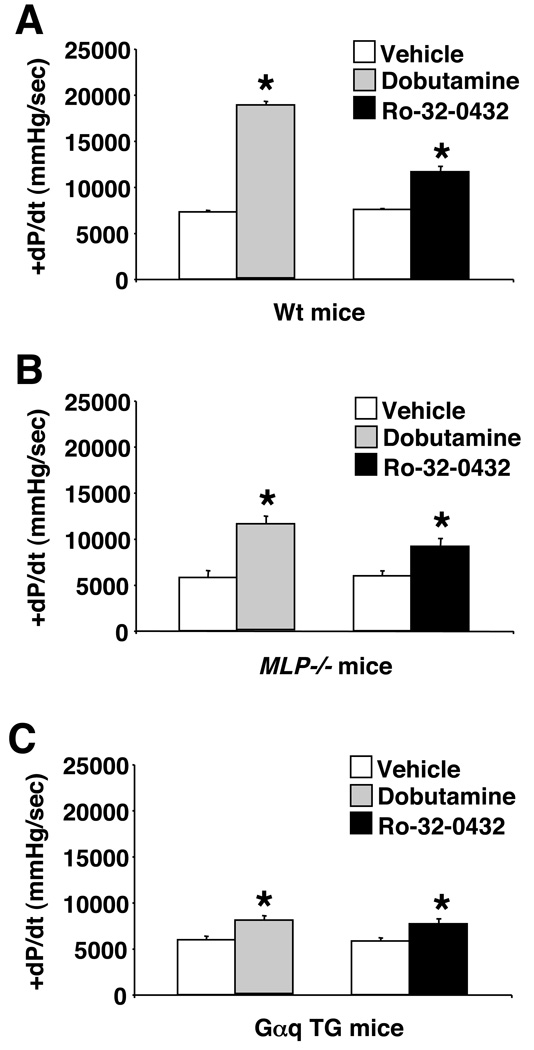
In addition to increasing the cardiac contractile response in an isolated working heart preparation, catheter-based infusion of Ro-32-0432 in an invasive preparation in anesthetized wildtype mice also increased cardiac contractility (Fig 4A). In this preparation, mice were first challenged with a β-adrenergic agonist, dobutamine, to assess the upper maximal limit of inotropic effect, equilibrated, then challenged with Ro-32-0432. While the contractile effect associated with Ro-32-0432 was less robust than dobutamine, it was nonetheless significant (P<0.001) (see discussion). The same infusion protocol was also employed in two models of heart failure due to deletion of the MLP gene and overexpression of Gαq in the heart (Fig 4B, C). Importantly, while the response to dobutamine was severely blunted in the two heart failure models, consistent with previously reported desensitization of the β-adrenergic receptors, the inotropic response to Ro-32-0432 was more maintained when compared with wildtype mice (P<0.05). For example, dobutamine challenge in Gαq mice was only 16% that of wildtype mice, while the remaining inotropic effect was 57% with Ro-32-0432 infusion (Fig 4C).
Figure 4. Ro-32-0432 increases cardiac contractility in Wt and failing mice.
(A) Assessment of contractility by invasive hemodynamics in a closed-chest preparation in wildtype mice following vehicle (N=5), dobutamine (N=5, 32 µg/kg/min), or Ro-32-0432 infusion (N=5, 22.5 µg/kg/min). *P<0.001 versus vehicle. (B) Invasive hemodynamic assessment in MLP−/− mice following vehicle (N=3), dobutamine (N=3, 32 µg/kg/min), or Ro-32-0432 infusion (N=3, 22.5 µg/kg/min). *P<0.001 versus vehicle. (C) Invasive hemodynamic assessment in Gαq transgenic mice following vehicle (N=4), dobutamine (N=4, 32 µg/kg/min), or Ro-32-0432 infusion (N=4, 22.5 µg/kg/min). *P<0.001 versus vehicle infusion. Statistical significance was assessed by ANOVA (P<0.0001 for A, B, and C) followed by Newman-Keuls post test.
Pharmacologic inhibition of PKCα restores cardiac function in MLP−/− mice
Since chronic treatment with traditional inotropes is associated with adverse outcomes in heart failure patients, here we investigated the affects of chronic Ro-31-8220 administration over 4–6 weeks in MLP−/− heart failure mice. All mice were assessed for ventricular performance by echocardiography at the beginning of the study and 6 weeks later. Ro-31-8220 (or vehicle) was injected once a day at a dosage of 6 mg/kg/day, s.q. The Ro-31-8220 compound was employed for all in vivo studies over the Ro-32-0432 compound only because of issues related to expense surrounding large-scale synthesis and the quantity that was needed for long-term use in mice. Pharmacokinetic analysis of Ro-31-8220 in the rat showed a half-life of 5.7 hours and plasma concentrations of drug were 100-fold greater than the IC50 for PKCα at 6 hrs (see discussion). This dosage of Ro-31-8220 was well tolerated in the mouse and had no observable detrimental effects over 6 weeks, nor was body-weight affected. Wildtype mice injected with vehicle or Ro-31-8220 showed no change in fractional shortening or any other measures of ventricular dimensions over the 6-week period (Fig 5A, and Table 1). In contrast, MLP−/− mice showed a dramatic rescue in fractional shortening after 6 weeks of Ro-31-8220 compared with vehicle treatment or baseline values before treatment (Fig 5A, Table 1). The study shown in Figure 5A was performed in 6-month old wildtype and MLP−/− mice, although a similar improvement in fractional shortening was observed in aged MLP−/− mice (14 months) compared with vehicle-treated mice over four-weeks of treatment (Fig 5B, Table 1). Wildtype and MLP−/− mice were also subjected to isolated, working heart preparation, demonstrating a significant increase in cardiac contractility in MLP−/− mice chronically treated with Ro-31-8220, comparable to wildtype mice (Table 2). A similar rescue in diastolic function (−dP/dt) and left ventricular pressure developed was also observed (Table 2).
Figure 5. Ro-31-8220 reverses loss of ventricular performance in MLP−/− mice.
(A) Assessment of fractional shortening (FS) in Wt and MLP−/− mice by echocardiography before vehicle (N=8 MLP−/− , N=8 Wt) or Ro-31-8220 (N=10 MLP−/−, N=9 Wt) treatment or 6 weeks after daily s.q injections of vehicle or Ro-31-8220 at 6 mg/kg/day. *P<0.05 MLP−/− versus Wt mice before treatment of after vehicle treatment. #P=0.008 MLP−/− after Ro-31-8220 versus MLP−/− before Ro-31-8220 treatment, #P=0.0036 versus MLP−/− before vehicle treatment, or #P=0.009 versus MLP−/− after vehicle treatment. Statistical significance was assessed by paired t tests for comparisons within the same groups and by two-sample t tests between Ro versus vehicle. ANOVA was used for any comparisons among groups (P<0.0001) followed by a Newman-Keuls post test. (B) Fractional shortening in a separate 4-week study in old Wt (N=8) and MLP−/− mice (14 months, N=8) after vehicle (N=8) or Ro-31-8220 treatment (N=8). Statistical significance was assessed by ANOVA (P=0.04) followed by Newman-Keuls post test. *P<0.05 MLP−/− versus Wt mice. #P<0.05 MLP−/− after Ro-31-8220 versus MLP−/− after vehicle treatment. (C) Fractional shortening in a separate 3-day study in MLP−/− mice treated with vehicle (N=6) or Ro-31-8220 (N=6). #P=0.01 MLP−/− after Ro-31-8220 versus MLP−/− after vehicle treatment. Statistical significance was assessed by a two-sample t test.
Table 1.
Echocardiographic assessment of cardiac structure-function in Wt and MLP−/− mice treated with vehicle or Ro-31-8220.
| Septum (d) | LVED | LV wall (d) | Septum (s) | LVES | LV wall (s) | FS (%) | ||
|---|---|---|---|---|---|---|---|---|
| 6 mo. | Wt pre−VehN=8 | 0.76+/−0.109 | 3.82+/−0.244 | 0.69+/−0.104 | 1.16+/−0.135 | 2.59+/−0.197 | 1.00+/−0.125 | 35.05+/−0.74 |
| Wt pre−RoN=9 | 0.71+/−0.094 | 4.08+/−0.224 | 0.69+/−0.093 | 1.09+/−0.116 | 2.84+/−0.187 | 1.00+/−0.044 | 30.43+/−0.613 | |
| Wt VehN=8 | 0.67+/−0.031 | 3.95+/−0.051 | 0.70+/−0.031 | 1.01+/−0.032 | 2.54+/−0.051 | 0.94+/−0.031 | 35.78+/−0.192 | |
| Wt RoN=9 | 0.65+/−0.03 | 3.94+/−0.06 | 0.70+/−0.03 | 0.96+/−0.03 | 2.66+/−0.07 | 0.95+/−0.03 | 32.85+/−0.24 | |
| MLP−/−pre−VehN=8 | 0.63+/−0.099 | 4.30+/−0.259 | 0.61+/−0.097* | 0.86+/−0.116* | 3.52+/−0.234* | 0.76+/−0.109* | 17.92+/−0.529* | |
| MLP−/−pre−RoN=10 | 0.65+/−0.008 | 3.98+/−0.199 | 0.63+/−0.079* | 0.89+/−0.094* | 3.17+/−0.178* | 0.83+/−0.091* | 20.57+/−0.452* | |
| MLP−/− Veh N=8 | 0.62+/−0.03 | 4.37+/−0.07* | 0.57+/−0.03* | 0.77+/−0.03* | 3.35+/−0.07* | 0.80+/−0.04* | 23.31+/−0.23* | |
| MLP−/− Ro N=10 | 0.63+/−.023 | 4.20+/−0.046 | 0.64+/−0.028 | 0.92+/−0.032 | 2.92+/−0.052# | 0.90+/−0.031 | 30.34+/−0.232# | |
| >1 yr. | Wt Veh | 0.81+/−0.05 | 4.55+/−0.17 | 0.84+/−0.07 | 1.23+/−0.09 | 3.39+/−0.36 | 1.39+/−0.06 | 25.87+/−5.10 |
| Wt Ro | 0.75+/−0.01 | 4.31+/−0.35 | 0.83+/−0.09 | 1.18+/−0.02 | 3.05+/−0.33 | 1.13+/−0.12 | 29.65+/−2.60 | |
| MLP−/− Veh | 0.64+/−0.03 | 5.01+/−0.15 | 0.71+/−0.08 | 0.97+/−0.10 | 4.24+/−0.26 | 0.98+/−0.02 | 15.48+/−3.17* | |
| MLP−/− Ro | 0.80+/−0.03# | 4.64+/−0.41 | 0.69+/−0.05 | 1.23+/−0.07 | 3.24+/−0.42 | 1.13+/−0.08 | 30.83+/−2.93# | |
Ro-31-8220 reverses loss of ventricular performance in MLP−/− mice. (Top) Assessment of cardiac function in 6 mo. old mice by echocardiography before vehicle or Ro-31-8220 treatment or 6 weeks after daily s.q injections of vehicle or Ro-31-8220 at 6 mg/kg/day. Statistical significance was assessed by paired t tests for comparisons within the same groups. ANOVA was used for comparisons among the four treated groups (P<0.0001) followed by a Newman-Keuls post test.
P<0.05 MLP−/− versus Wt mice before treatment of after vehicle treatment.
P<0.05 MLP−/− after Ro-31-8220 versus MLP−/− before treatment of after vehicle treatment. (Bottom) Echocardiography in a separate 4-week study in old Wt (N=8) and MLP−/− mice (14 months, N=8) and after vehicle (N=8) or Ro-31-8220 treatment (N=8). Statistical significance was assessed by ANOVA among the groups (P=0.0212) followed by a Newman-Keuls post test. Note that due to design, with factors WT/MLP and vehicle/Ro, a two-factor ANOVA with interaction term is equivalent to ANOVA on four independent groups.
P<0.05 MLP−/− versus Wt mice.
P<0.05 MLP−/− after Ro−31−8220 versus MLP−/− after vehicle treatment. All measurements are means ± SEM. Septal and left ventricular (LV) wall thicknesses were assessed in diastole (d) and systole (s) in millimeters (mm). Additional abbreviations used: LVED, left ventricular end-diastolic dimension; LVES, left ventricular end-systolic dimension; FS, fractional shortening; Ro, Ro-31-8220; Veh, vehicle; Wt, wildtype.
Table 2.
Ex vivo working heart analysis form wildtype and MLP−/− mice injected vehicle or Ro-31-8220 for 4-weeks.
| Parameters | Wt |
MLP−/− Vehicle |
% change Wt vs MLP |
MLP−/− Ro-31-8220 |
% change MLP-v vs MLP-Ro |
|---|---|---|---|---|---|
| N=7 | N=7 | N=8 | |||
| Heart rate (bpm) | 408 ± 3 | 404 ± 1 | n.s | 404 ± 1 | n.s |
| +dP/dt (mmHg/sec) | 6702 ± 202 | 6133 ± 128 | 9* | 7099 ± 157 | 16† |
| −dP/dt (mmHg/sec) | 4814 ± 107 | 3189 ± 103 | 34* | 3962 ± 85 | 24† |
| LVP (mmHg) | 126 ± 2 | 111 ± 3 | 12* | 132 ± 3 | 19† |
All results are shown as standard error of the mean. +/−dP/dt represents a measure of contractility based on the derivative of pressure change (mmHg) versus time (sec). LVP represents left ventricular pressure developed. All hearts were paced, (bpm, beats per minute). Statistical significance was assessed by ANOVA (P=0.002) followed by a Newman-Keuls post test.
P≤0.05 Wildtype versus MLP−/− vehicle;
P≤0.01 MLP−/− vehicle versus MLP−/− Ro-31-8220.
While Ro-31-8220 rescued cardiac contractile performance in MLP−/− mice over 4 or 6 weeks of chronic administration, it did not alter heart weight, or reverse cardiac chamber dilation as assessed by echocardiography, or improve histo-pathology (data not shown). These observations suggest that part of the increase in cardiac performance associated with chronic Ro-31-8220 treatment might involve an acute influence on contractility itself. Indeed, injection of Ro-31-8220 in MLP−/− mice for only 3 days improved fractional shortening compared with vehicle treatment (Fig 5C).
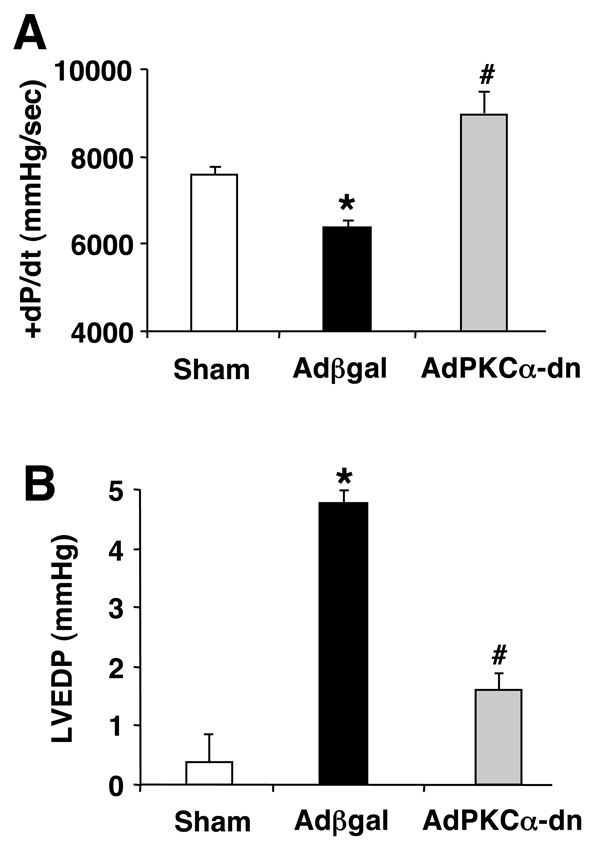
Cardiac adenoviral-mediated gene delivery of dominant negative PKCα attenuates heart failure in a chronic rat cryo-infarction injury model
While relatively selective for the conventional PKC isoforms, the bisindolylmaleimide compounds employed herein have the potential to inhibit other kinases. To further investigate PKCα as a primary determinant of the affects described above, we employed a dominant negative PKCα adenovirus for gene therapy in rats 12 weeks after cryo-mediated infarction injury. Rats were assessed for ventricular performance by invasive hemodynamics 1 week after adenoviral-mediated gene transfer of a control virus (Adβgal) or AdPKCα-dn. Cryo-infarcted rats treated with Adβgal showed a marked depression in cardiac contractility (P<0.05), consistent with our previous observations24, while AdPKCα-dn treated rats showed an augmentation in ventricular performance (P<0.05)(Fig 6A). Moreover, elevated left ventricular diastolic pressure that typifies heart failure was largely reversed with AdPKCα-dn treatment, without an affect on heart rate (Fig 6B, and data not shown). These data support the conclusion that inhibition of PKCα in the adult heart augments cardiac contractility, partially relieving heart failure.
Figure 6. Dominant negative PKCα gene transfer reverses heart failure in a rat cryoinfarct model.
(A) Invasive hemodynamic assessment of cardiac contractility (+dP/dt) and (B) left ventricular end diastolic pressure using a closed-chest preparation in sham (N=8), Adβgal treated (N=12), or AdPKCα-dn treated rats (N=7). Statistical significance was assessed by ANOVA (P=0.0014 in A, P<0.0001 in B) followed by a Newman-Keuls post test. *P<0.05 Adβgal versus sham. #P<0.05 AdPKCα-dn versus Adβgal.
Discussion
One potential issue associated with the data presented here is the specificity of the bisindolylmaleimide compounds, Ro-32-0432 and Ro-31-8220. While both compounds have excellent potency for PKCα, each can also inhibit other kinases28–30, especially the two other classic PKC isozymes, PKCβ and PKCγ. However, given the high degree of sequence conservation amongst the three classic PKC genes, PKCβ and PKCγ may function analogous to PKCα in regulating cardiac contractility, so partial or complete inhibition of these isozymes may also be desirable. Indeed, we also observed that LY333531, a PKCα/β selective inhibitory compound of the dimethylamine class, lead to a 28% increase in +dP/dt in wildtype rats (data not shown). Even though this compound was claimed to be PKCβ-specific13, we see relatively equal inhibitory activity towards PKCα (data not shown). While all three conventional isozymes may regulate cardiac contractility, other lines of evidence suggest that PKCα is potentially the most critical. For example, PKCα is the dominant conventional PKC isozyme expressed in the mouse, rabbit and human heart, although this observation does not take into account potential differences in activity (Ref 3, 4 and Fig 3). Ro-31-0432 did not significantly augment cardiac contractility in mice lacking PKCα, further supporting the conclusion that the biologic effect of Ro-31-0432 on contractility is largely due to PKCα. Moreover, general activation of both classic and novel PKC isozymes in the heart by acute infusion of PMA produced a dramatic decrease in contractility in wildtype mice, but not PKCα−/− mice. This result suggests that PKCα is a primary negative regulator of cardiac contractility following a global activation of PKC isozymes in the heart. The dominance of PKCα in regulating cardiac contractility is also supported by adenoviral-mediated gene therapy with dominant negative PKCα in the rat heart. These results are also consistent with our previous observations whereby AdPKCα (wildtype) reduced contractility in isolated adult myocytes, while AdPKCα-dn enhanced it18. Finally, PKC α−/− mice continued to show increased cardiac contractility at baseline, as previously described18.
The dosages of Ro-32-0432 and Ro-31-8220 that were selected for use in vivo were in excess of the calculated in vitro IC50 values. However, calculation of the IC50 value for a given compound does not account for ATP concentration in a cell (which competes with the bisindolylmaleimide compounds), the organ availability of the compound, membrane permeability characteristics, its metabolism, and its selectivity/binding for other kinases, all of which can influence its efficacious dosage in vivo. Hence, an empirical examination of biologic effectiveness is probably more important than a rigid adherence to an arbitrary IC50 value.
Ro-32-0432 and Ro-31-8220 each increased cardiac contractility in wildtype and MLP−/− mice without toxic effect or lethality. In fact, none of the 27 wildtype and MLP−/− mice given Ro-31-8220 died during 4 or 6 weeks of chronic treatment at 6 mg/kg/day, nor was any lethargy or overt symptoms of drug intolerance observed. Also of interest, chronic and acute administration of Ro-31-8220 in MLP−/− mice each had an identical effect on contractility, suggesting that PKC inhibition is not subject to significant desensitization as characteristic of β-agonists. Indeed, augmented contractility associated with dobutamine administration in the MLP−/− and Gαq heart failure models was dramatically blunted compared with wildtype mice, yet the same general contractility enhancement was largely preserved between wildtype mice and the two heart failure models when the PKCα inhibitory compound was used. Thus, selectively targeting PKCα may provide a unique therapeutic approach in patients that are desensitized to β-agonists, or for more chronic indications where desensitization would normally occur.
One unique aspect associated with PKCα inhibition, whether due to gene ablation or the bisindolylmaleimide compounds, is that contractility is only moderately increased. By comparison, β-agonist or phosphodiesterase inhibitors tend to have a more pronounced effect on cardiac contractility, effects that have been associated with increased incidences of sudden death and more rapid decompensation in heart failure patients33. While such data warrant caution when inotropic support is prescribed, there may be important differences in the means of providing such support. The significant, yet less robust augmentation in cardiac contractility associated with PKCα inhibition may have a safer profile. PKCα also regulates one particular subset of effects that is initiated by β-adrenergic receptors and cAMP. Specifically, PKCα functions through direct phosphorylation of I-1, resulting in altered PP1 activity, which in turn regulates PLN phosphorylation. Alterations in PLN phosphorylation regulate SERCA2 function in the heart, which controls Ca2+ loading and the magnitude of the Ca2+ transient11. Larger Ca2+ transients directly enhance myocardial contractility by augmenting the number of cross-bridges generated between the myosin heads and actin-containing thin filaments, generating the power stroke. Thus, pharmacologic inhibition of PKCα activity would function at the level of SR Ca2+ handling, possibly providing a safer inotropic profile. Indeed, deletion of PLN in the mouse augments cardiac contractility only through increased loading of the SR without reducing viability or increasing the propensity towards arrhythmia34. These observations suggest that inotropic support through PKCα inhibition could be safer than agents that directly activate the β-adrenergic receptor or increase cAMP. Finally, inhibition of PKCα may also benefit a failing myocardium independent of contractility, since PKCα is involved in reactive signaling within the heart that participates in hypertrophy, negative remodeling, and decompensation. In conclusion, PKCα represents a novel target for treating human heart failure by modulating contractility within a more restrained physiologic window, by only mediating inotropic effects specifically through SR Ca2+ loading, and by possibly reducing reactive signaling through neuro-endocrine pathways.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (SCCOR grant P01HL077101) and an Established Investigator Grant from the American Heart Association (J.D.M.). M.H was supported by a National Institutes of Health training grant #T32 HL007752. W.J.K. was supported by R01 HL56205 and S.T.P was supported by a fellowship form the Pennsylvania Delaware Affiliate of the American Heart Association. We thank Dr. Jane F. Djung for pharmacologic kinetic analyses.
Footnotes
Conflict of Interest Statement:
A.N.C. is an employee of Procter and Gamble Pharmaceuticals. No other significant financial relationships exist with the remaining authors.
References
- 1.Molkentin JD, Dorn GWII. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 2.Dempsey EC, Newton AC, Mochley-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am. J. Physiol. Lung Mol. Physiol. 2000;279:L429–L438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 3.Pass JM, Gao J, Jones WK, Wead WB, Wu X, Zhang J, Baines CP, Bolli R, Zheng YT, Joshua IG, Ping P. Enhanced PKC beta II translocation and PKC beta II-RACK1 interactions in PKC epsilon-induced heart failure: a role for RACK1. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H2500–H2510. doi: 10.1152/ajpheart.2001.281.6.H2500. [DOI] [PubMed] [Google Scholar]
- 4.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ. Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 5.Capogrossi MC, Kaku T, Filburn CR, Pelto DJ, Hansford RG, Spurgeon HA, Lakatta EG. Phorbol ester and dioctanoylglycerol stimulate membrane association of protein kinase C and have a negative inotropic effect mediated by changes in cytosolic Ca2+ in adult rat cardiac myocytes. Circ. Res. 1990;66:1143–1155. doi: 10.1161/01.res.66.4.1143. [DOI] [PubMed] [Google Scholar]
- 6.Capogrossi MC, Kachadorian WA, Gambassi G, Spurgeon HA, Lakatta EG. Ca2+ dependence of alpha-adrenergic effects on the contractile properties and Ca2+ homeostasis of cardiac myocytes. Circ. Res. 1991;69:540–550. doi: 10.1161/01.res.69.2.540. [DOI] [PubMed] [Google Scholar]
- 7.Ward CA, Moffat MP. Positive and negative inotropic effects of phorbol 12-myristate 13-acetate: relationship to PKC-dependence and changes in [Ca2+]i. J. Mol. Cell. Cardiol. 1992;24:937–948. doi: 10.1016/0022-2828(92)91861-x. [DOI] [PubMed] [Google Scholar]
- 8.Watson JE, Karmazyn M. Concentration-dependent effects of protein kinase C-activating and - nonactivating phorbol esters on myocardial contractility, coronary resistance, energy metabolism, prostacyclin synthesis, and ultrastructure in isolated rat hearts. Effects of amiloride. Circ. Res. 1991;69:1114–1131. doi: 10.1161/01.res.69.4.1114. [DOI] [PubMed] [Google Scholar]
- 9.McKenna TM, Li S, Tao S. PKC mediates LPS- and phorbol-induced cardiac cell nitric oxide synthase activity and hypocontractility. Am. J. Physiol. 1995;269:H1891–H1898. doi: 10.1152/ajpheart.1995.269.6.H1891. [DOI] [PubMed] [Google Scholar]
- 10.Wang YG, Benedict WJ, Huser J, Samarel AM, Blatter LA, Lipsius SL. Brief rapid pacing depresses contractile function via Ca(2+)/PKC-dependent signaling in cat ventricular myocytes. Am. J. Physiol. Heart. Circ. Physiol. 2001;280:H90–H98. doi: 10.1152/ajpheart.2001.280.1.H90. [DOI] [PubMed] [Google Scholar]
- 11.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat. Rev. Mol. Cell. Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 12.Neumann J. Altered phosphatase activity in heart failure, influence on Ca2+ movement. Basic Res. Cardiol. 2002;97:I91–I95. doi: 10.1007/s003950200036. [DOI] [PubMed] [Google Scholar]
- 13.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of calcium-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 14.Bayer AL, Heidkamp MC, Patel N, Porter M, Engman S, Samarel AM. Alterations in protein kinase C isoenzyme expression and autophosphorylation during progression of pressure overload-induced left ventricular hypertrophy. Mol. Cell. Biochem. 2003;242:145–152. [PubMed] [Google Scholar]
- 15.Wang J, Liu X, Sentex E, Takeda N, Dhalla NS. Increased expression of protein kinase C isoforms in heart failure due to myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H2277–H2287. doi: 10.1152/ajpheart.00142.2002. [DOI] [PubMed] [Google Scholar]
- 16.Braun M, Simonis G, Birkner K, Pauke B, Strasser RH. Regulation of protein kinase C isozyme and calcineurin expression in isoproterenol induced cardiac hypertrophy. J. Cardiovasc. Pharmacol. 2003;41:946–954. doi: 10.1097/00005344-200306000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Koide Y, Tamura K, Suzuki A, Kitamura K, Yokoyama K, Hashimoto T, Hirawa N, Kihara M, Ohno S, Umemura S. Differential induction of protein kinase C isoforms at the cardiac hypertrophy stage and congestive heart failure stage in Dahl salt-sensitive rats. Hypertens. Res. 2003;26:421–426. doi: 10.1291/hypres.26.421. [DOI] [PubMed] [Google Scholar]
- 18.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Iodi B, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKCα regulates cardiac contractility and propensity towards heart failure. Nat. Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 19.Johnsen DD, Kacimi R, Anderson BE, Thomas TA, Said S, Gerdes AM. Protein kinase C isozymes in hypertension and hypertrophy: insight from SHHF rat hearts. Mol. Cell. Biochem. 2005;270:63–69. doi: 10.1007/s11010-005-3781-x. [DOI] [PubMed] [Google Scholar]
- 20.Hahn HS, Marreez Y, Odley A, Sterbling A, Yussman MG, Hilty KC, Bodi I, Liggett SB, Schwartz A, Dorn GW., 2nd Protein kinase Calpha negatively regulates systolic and diastolic function in pathological hypertrophy. Circ. Res. 2003;93:1111–1119. doi: 10.1161/01.RES.0000105087.79373.17. [DOI] [PubMed] [Google Scholar]
- 21.Arber S, Hunter JJ, Ross J, Jr, Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. doi: 10.1016/s0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- 22.Gulick J, Hewett TE, Klevitsky R, Buck SH, Moss RL, Robbins J. Transgenic remodeling of the regulatory myosin light chains in the mammalian heart. Circ. Res. 1997;80:655–664. doi: 10.1161/01.res.80.5.655. [DOI] [PubMed] [Google Scholar]
- 23.D'Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW. Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc. Natl. Acad. Sci. U S A. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Most P, Pleger ST, Volkers M, Heidt B, Boerries M, Weichenhan D, Loffler E, Janssen PM, Eckhart AD, Martini J, Williams ML, Katus HA, Remppis A, Koch WJ. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J. Clin. Invest. 2004;114:1550–1563. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pleger ST, Remppis A, Heidt B, Völkers M, Chuprun JK, Kuhn MC, Zhou RH, Gao E, Szabo G, Weichenhan D, Müller OJ, Eckhart AD, Katus HA, Koch WJ, Most P. S100A1 Gene Therapy Preserves in vivo Cardiac Function after Myocardial Infarction. Mol.Ther. 2005 doi: 10.1016/j.ymthe.2005.08.002. in press. [DOI] [PubMed] [Google Scholar]
- 26.De Windt LJ, Lim HW, Haq S, Force T, Molkentin JD. Calcineurin promotes protein kinase C and c-Jun NH2-terminal kinase activation in the heart. Cross-talk between cardiac hypertrophic signaling pathways. J. Biol. Chem. 275:13571–13579. doi: 10.1074/jbc.275.18.13571. 200. [DOI] [PubMed] [Google Scholar]
- 27.Braz JC, Bueno OF, De Windt LJ, Molkentin JD. PKC alpha regulates the hypertrophic growth of cardiomyocytes through extracellular signal-regulated kinase1/2 (ERK1/2) J. Cell. Biol. 2002;156:905–919. doi: 10.1083/jcb.200108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem. J. 1993;294:335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bit RA, Davis PD, Elliott LH, Harris W, Hill CH, Keech E, Kumar H, Lawton G, Maw A, Nixon JS. Inhibitors of protein kinase C. 3. Potent and highly selective bisindolylmaleimides by conformational restriction. J. Med. Chem. 1993;36:21–29. doi: 10.1021/jm00053a003. [DOI] [PubMed] [Google Scholar]
- 30.Davis PD, Elliott LH, Harris W, Hill CH, Hurst SA, Keech E, Kumar MK, Lawton G, Nixon JS, Wilkinson SE. Inhibitors of protein kinase C. 2. Substituted bisindolylmaleimides with improved potency and selectivity. J. Med. Chem. 1992;35:994–1001. doi: 10.1021/jm00084a004. [DOI] [PubMed] [Google Scholar]
- 31.Poussard S, Dulong S, Aragon B, Jacques Brustis J, Veschambre P, Ducastaing A, Cottin P. Evidence for a MARCKS-PKCalpha complex in skeletal muscle. Int. J. Biochem. Cell. Biol. 2001;33:711–721. doi: 10.1016/s1357-2725(01)00045-0. [DOI] [PubMed] [Google Scholar]
- 32.Heemskerk FM, Chen HC, Huang FL. Protein kinase C phosphorylates Ser152, Ser156 and Ser163 but not Ser160 of MARCKS in rat brain. Biochem. Biophys. Res. Commun. 1993;190:236–241. doi: 10.1006/bbrc.1993.1036. [DOI] [PubMed] [Google Scholar]
- 33.Warner Stevenson L. Clinical Use of Inotropic Therapy for Heart Failure: Looking Backward or Forward?: Part II: Chronic Inotropic Therapy. Circulation. 2003;108:492–497. doi: 10.1161/01.CIR.0000078349.43742.8A. [DOI] [PubMed] [Google Scholar]
- 34.Slack JP, Grupp IL, Dash R, Holder D, Schmidt A, Gerst MJ, Tamura T, Tilgmann C, James PF, Johnson R, Gerdes AM, Kranias EG. The enhanced contractility of the phospholamban-deficient mouse heart persists with aging. J. Mol. Cell. Cardiol. 2001;33:1031–1040. doi: 10.1006/jmcc.2001.1370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.