Abstract
To define the effects of pregnancy on mechanical properties and reactivity, mesenteric veins from late pregnant (LP) and virgin control (NP) rats were pressurized to determine gestational changes in size and distensibility. Reactivity studies used an adrenergic constrictor (norepinephrine, NE) and an endothelium-mediated vasodilator (acetylcholine, ACh). The contribution of nitric oxide (NO) to endothelial function was evaluated with pharmacologic inhibition of NO synthase. Roles of NO and cGMP in smooth muscle vasodilation were determined by using an NO donor with and without cGMP inhibition using ODQ, a selective inhibitor of guanylyl cyclase. In pregnancy, endothelium-dependent vasodilation markedly increased (largely due to endogenous NO), smooth muscle response to NO decreased (primarily related to cGMP production), and NE sensitivity decreased considerably, with no changes in vessel size or distensibility. Our results identify a pro-vasodilatory state in the systemic venous system which would serve to facilitate the accommodation to plasma volume expansion requisite for normal pregnancy.
Keywords: mesenteric vein, endothelium, nitric oxide, rat, cGMP
During pregnancy, the maternal cardiovascular system undergoes profound changes to allow for reproductive success1, 2. At the level of the maternal vasculature, these adaptations require changes in both structure and behavior (reactivity) 3,4,5,6 which assist in tolerance of a significant increase in intravascular volume. Likewise, failure to establish complete cardiovascular adaptation to plasma volume expansion can have profound implications for developing pregnancy, as seen in pathologic states such as pre-eclampsia and intrauterine growth restriction7, 8, and 9.
The mesenteric circulation, which includes the blood supply to the large and small intestines as well as to the liver and spleen, has been found to hold up to one fourth of the total blood volume10,11.12,13. Studies focused on the arterial side of the mesenteric circulation have documented significant changes during pregnancy, including decreased adrenergic (sympathetic) reactivity3, 14, and 15 and enhanced endothelium-dependent vasodilation16.
Much less is known about venous adaptations, although changes in the compliance and reactivity of this circuit could cause marked changes in cardiac output and capacitance. The limited data which are available show increased adrenergic sensitivity17, 18, and there have been no published studies which address changes in endothelial or smooth muscle function.
This study was therefore designed to investigate the effects of pregnancy on mechanical properties and endothelial and smooth muscle responsiveness of systemic veins. The use of isolated, pressurized venous segments allowed us to evaluate changes in size and distensibility, and to probe endothelial vs. smooth muscle mechanisms involved in the regulation of venous tone. In view of previously reported changes in mesenteric arterial reactivity and biomechanics, as well as documented tolerance of large plasma volume expansion in normal pregnancy19, we hypothesized that pregnancy would increase venous compliance (and therefore size), and augment vasodilatory reserve.
MATERIALS AND METHODS
Animals
Nonpregnant age-matched cycling virgin (NP, n = 20) and timed-pregnant (late pregnant; LP, n = 21) female Sprague-Dawley rats, (Charles River, Canada), were housed at the University of Vermont College of Medicine Animal Facility and were studied at 15.2 ± 0.8 weeks of age. Rats were maintained on a 12 hour light and dark photoperiod, and were provided with food and water ad libitum. Estrous cycle stage was determined in a subset of the nonpregnant rats (15 animals) by examining the cell composition of vaginal smears on the day of vessel harvest. The study protocol was approved by the University of Vermont Animal Care and Use Committee. Pregnant animals were studied on day 20 of a 22 day gestation.
Animals were killed by decapitation after a surgical plane of anesthesia was induced using an intraperitoneal injection of pentobarbital sodium (Nembutal 50mg/mL, Ovation Pharmaceuticals, Deerfield, IL). The abdominal cavity was then opened, and a section of gut 5–10 cm distal to the pylorus, and its entire mesenteric circulation was excised and pinned in a Petri dish containing cold (4°C) physiologic HEPES-buffered saline solution (pH = 7.4).
Tissue preparation and vasograph system
Second-order mesenteric vein branches from an area 5–10 cm from the pylorus were dissected free of surrounding fat and connective tissue. Vein segments 2–3 mm long were mounted on two glass microcannulas within the chamber of a 5-mL vasograph filled with HEPES buffer. The vein was secured with one strand of nylon suture (diameter 10 µm) to the proximal microcannula which was connected to an in-line pressure transducer and servo system (Living Systems Instrumentation, Burlington, VT). The vein was initially perfused with physiologic HEPES saline solution (37 °C) with a proximal pressure of 2 mm Hg to flush any residual luminal contents. The distal end of the vein was then cannulated and secured in the same manner. A stopcock that was distal to the cannula remained closed throughout the experiment to prevent flow. Venous wall integrity was assessed by ensuring that the system would maintain a transmural pressure of 6 mm Hg for 2 minutes under no flow conditions with the servo null pressure feedback system turned off. If pressure decreased during that time, the vein segment was removed and another segment from the same region of the mesentery was dissected and cannulated.
Vessel diameter was defined as the distance between the internal edges of each vessel wall, and measured perpendicular to the long axis of the wall. A complete description of the lumen-diameter analyzing system has been previously published20, 21.
Several specimens were also submitted to the University of Vermont Imaging Center for evaluation of venous microstructure via transmission electron microscopy. The tissue preparation protocol has been discussed in detail in a previous publication from our laboratory5.
Experimental Protocol
Vessels were equilibrated for 45 minutes in a physiologic HEPES saline solution with propranolol (1 µM) added to block β-receptors and to ensure the response seen with norepinephrine was due to α-stimulation only. The concentration of propranolol used has been established in previous mesenteric venous studies to effectively block venous β-adrenergic receptors17. During this time, the solution was warmed and maintained at 37 °C. All experiments were performed at 6 mm Hg intraluminal pressure and without intraluminal flow.
After vessel equilibration, norepinephrine (NE) was added to the circulating solution in increasing concentrations between 1 nM and 10 µM. After 5–15 minutes at each concentration, the stable vessel diameter was recorded on the video imaging system. Pharmacologic efficacy (NP, n = 7; LP, n = 8) was evaluated by comparing maximal constriction to NE to that obtained in a depolarizing high potassium (80 mM) solution at the end of each experiment, using the formula: (øi−øNE)/ (øi−øK+) × 100, where øi is the initial diameter, øNE is the diameter at each NE concentration, and øK+ is the diameter in high potassium solution. Pharmacologic sensitivity was also determined by calculating the concentration of NE required to produce half of the response seen at the maximal concentration of NE (EC50).
In additional experiments (NP, n = 6; LP, n = 6), vessels were preconstricted to 50–70% of the initial diameter with NE, and a concentration-response curve to ACh was obtained (1 nM to 10 µM). As with NE, efficacy was determined by relating the diameter at each ACh concentration to the maximal diameter possible, measured in a vessel relaxing solution (diltiazem 10 µM and papaverine 100 µM) at the end of the experiment, using the formula: (øACh−øi)/ (ødp−øi) × 100, where øACh is the diameter at each ACh concentration, øi is the initial constricted diameter, and ødp is the fully relaxed diameter. Sensitivity was then calculated as already described and expressed by determining the EC50.
In those experiments where NO inhibition was undertaken (NP, n = 4; LP, n = 6), vessels were initially treated (after NE preconstriction) with a test dose of 1µM ACh to confirm that a functional vascular endothelium was present. Following washout and re-equilibration, vessels were then incubated with a combination of N-Nitro-L-arginine (L-NNA, 200 µM) and Nω-nitro-L-arginine methyl ester (L-NAME, 200 µM) to provide inhibition of NO production22 for 20 minutes, re-constricted with NE and tested with ACh as detailed above.
The effects of pregnancy on venous smooth muscle sensitivity to nitric oxide was directly assessed by obtaining dilatory responses to an NO donor 2,2’-(Hydroxynitrosohydrazono)-bis-ethanimine (DETA/NO)23,24. As detailed above, vessels were equilibrated (NP, n = 6; LP, n = 6), preconstricted with NE to 50–70% of the initial diameter, subjected to eNOS inhibition (L-NNA) for 20 minutes prior to the administration of DETA/NO; pharmacologic sensitivity to DETA/NO was calculated by comparing vessel diameters at each concentration of DETA/NO to the maximal response seen with DETA/NO, using the formula (øD−øi)/(øDmax−øi)×100, where øD is the diameter at each DETA/NO concentration, øi is the initial constricted diameter, and øDmax is the maximum diameter response seen to DETA/NO. Efficacy was compared by determining maximal relaxation in DETA/NO to the fully relaxed diameter in a relaxing solution of papaverine + diltiazem.
Several recent reports have documented NO effects being mediated independently of the cGMP-PKG signaling pathway (e.g. p38-MAPK activation25 and K channels26) in other cell types27, 28. Hence, we used a selective inhibitor of soluble guanylyl cyclase, ODQ (1H-[1,2,4]oxadiazolo[4,3-α-]quinoxalin-1-one, 10µM)29,30,31,32 which was added to the superfusate after maximal dilation with DETA/NO was observed (NP, n = 6; LP, n = 6), to determine proportional inhibition of DETA/NO dilation.
Following determinations of reactivity, distensibility (NP, n = 7; LP, n = 9) was evaluated in the relaxing solution by lowering transmural pressure until the vessel began to collapse, usually at 1 mm Hg, and then increasing pressure just enough to determine the diameter of the vessel under inflated but unstressed conditions at 2 mm Hg. Transmural pressure was then increased in a stepwise fashion over a range of 2–10 mm Hg. Lumen diameter was allowed to stabilize for five minutes after each increase in pressure. Distensibility (%) was calculated by using the following equation: øTMP/øinitial, where øTMP is the diameter at that particular transmural pressure and øinitial at the lowest pressure at which inflation was observed.
Drugs and solutions
HEPES physiologic salt solution contained the following (in mM): sodium chloride 141.8, potassium chloride 4.7, magnesium sulfate 1.7, calcium chloride 2.8, potassium phosphate 1.2, HEPES 10.0, EDTA 0.5, and dextrose 5.0). High potassium (80 mM) HEPES solution contained the following (in mM): sodium chloride 63, potassium chloride 78.8, magnesium sulfate 1.7, calcium chloride 2.8, potassium phosphate 1.2, HEPES 10.0 L, EDTA 0.5, and dextrose 5.0. Both solutions were prepared in deionized water and titrated with sodium hydroxide to a physiologic pH of 7.4. Norepinephrine hydrochloride, and acetylcholine hydrochloride (Sigma, St. Louis, Mo., USA) were administered from stock solutions prepared daily in deionized water. All chemicals were purchased from Fisher Scientific (Fair Lawn, NJ) and Sigma unless otherwise specified.
Statistical analysis
All data are expressed as means ± SE. Norepinephrine efficacy is presented relative to maximal vessel constriction to a depolarizing high potassium (80 mM) HEPES solution, and sensitivity is presented relative to maximal vessel constriction to NE, as already described. ACh and DETA/NO efficacies are presented relative to maximal vessel dilation with relaxing solution, and sensitivities are presented relative to maximal dilation to each agent. Sensitivity was determined by constructing a concentration-response curve for each vessel, and then extrapolating to the concentration that produced 50% of the maximum effect (EC50). Data are shown as the average of the individual EC50 values ± SE. The results were analyzed with Student t-test or Mann-Whitney rank sum for paired observations, and a one-way ANOVA for multiple comparisons. A p-value of < 0.05 was considered statistically significant.
RESULTS
Vessel parameters
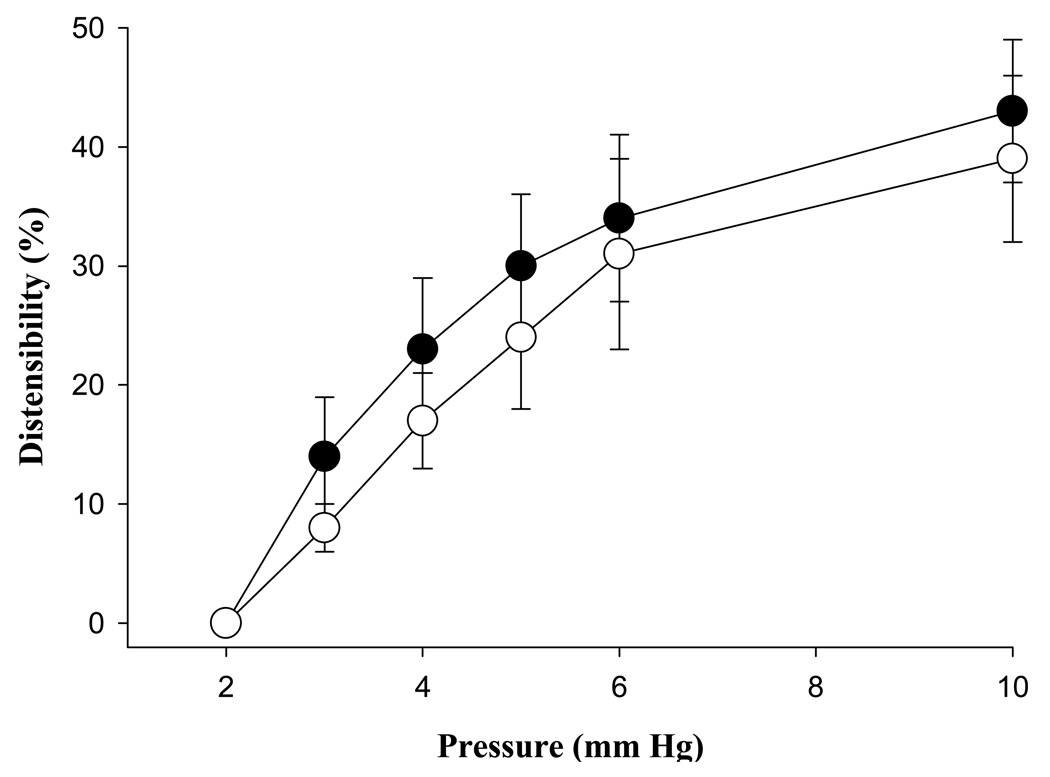
Venous diameter at a physiologic pressure (6 mm Hg) was unchanged in pregnancy (NP = 426 ± 30, LP = 403 ± 25 µm, p = 0.58). Likewise, venous distensibility over a physiologic range of pressure was similar in veins from both pregnant and nonpregnant animals (p > 0.05, Figure 1).
Fig. 1.
Distensibility of mesenteric veins from nonpregnant (●, n = 7) and late pregnant (○, n = 9) animals. Distensibility was similar in vessels from both groups, all p-values > 0.05. Vertical lines delineate standard error.
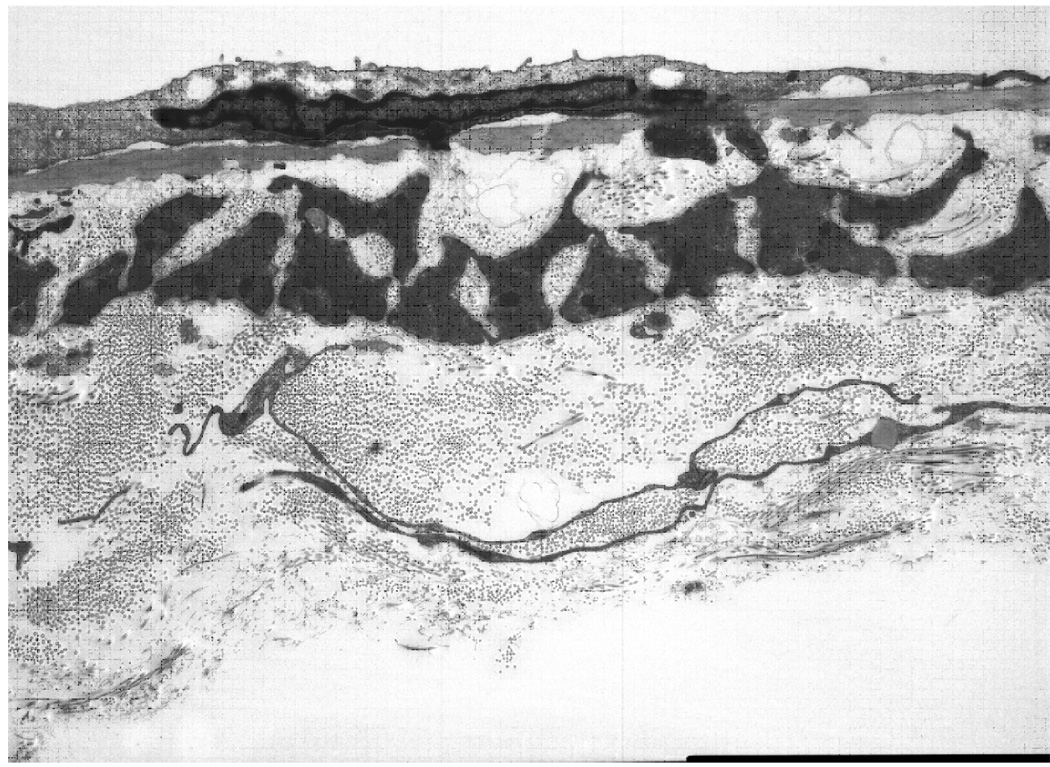
Transmission electron microscopy (TEM) was performed on vein segments from both pregnant and nonpregnant animals to evaluate wall ultrastructure. Appearance was similar in both, and a photomicrograph of a longitudinal section through the wall of a vein from a pregnant animal is shown in Figure 2. Examination revealed an endothelial layer separated from a single layer of vascular smooth muscle by a well-defined elastic lamina, and a thick adventitial layer composed of loosely-arranged bundles of collagen fibers.
Fig. 2.
Transmission electron micrograph of pressurized (6 mm Hg) NP mesenteric vein showing the endothelium (top) separated from a single layer of vascular smooth muscle by the internal elastic lamina. Note endothelial foot process (center) penetrating the lamina. The scale bar at the lower right corner of the image equals 5 µm.
Alpha-adrenergic reactivity
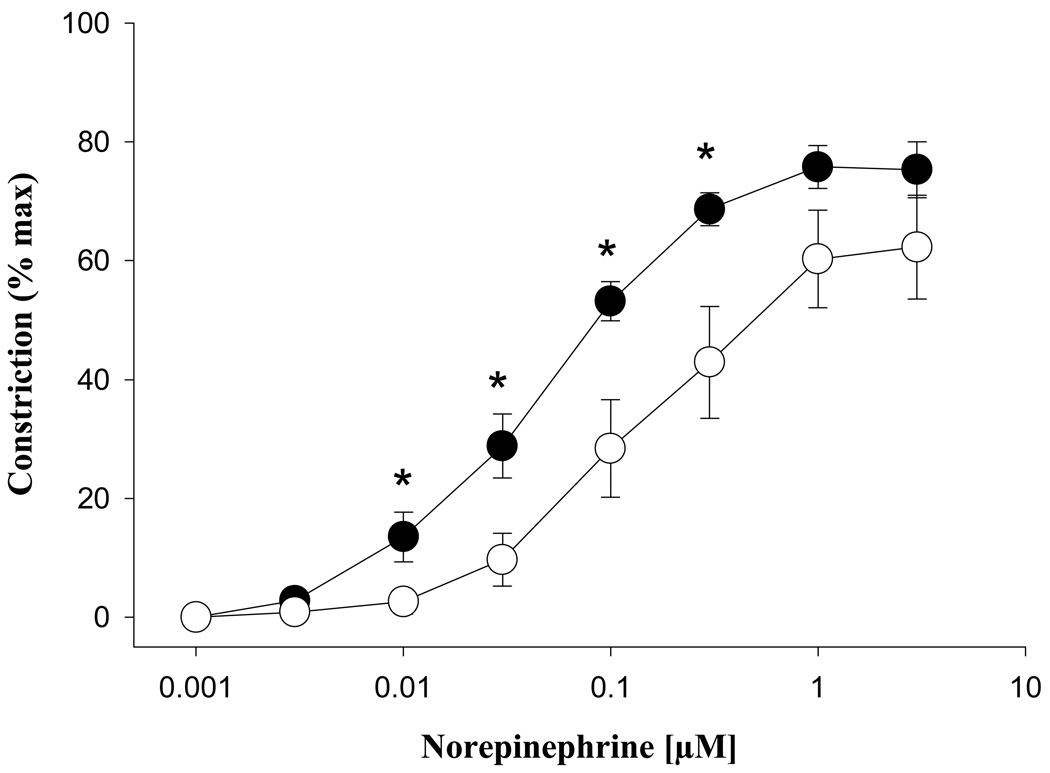
Norepinephrine (NE) produced marked reductions in the lumen diameters of veins from both groups, with similar efficacies (NP = 75.8 ± 3.6, LP = 62.3 ± 8.8%, p > 0.05; Figure 3). Sensitivity to NE was significantly decreased in pregnancy (EC50: NP = 56 ± 12, LP = 209 ± 57 nM, p = 0.04). When the NP group was separated by estrous cycle stage (proestrus = 4, estrus = 7), the differences in reactivity remained significant. The maximal constriction response to a depolarizing high potassium (80 mM) solution remained unchanged in pregnancy, (maximum constriction NP = 76 ± 6%, LP = 75 ± 7%, p= 0.60).
Fig. 3.
Constriction of mesenteric veins from nonpregnant (●, n = 7) and late pregnant (○, n = 7) animals in response to norepinephrine. Efficacy (shown relative to maximal constriction with a high potassium depolarizing solution) was unchanged in pregnancy, p > 0.05. Asterisks denote statistically significant comparisons (p < 0.05).
Endothelium-dependent vasodilation
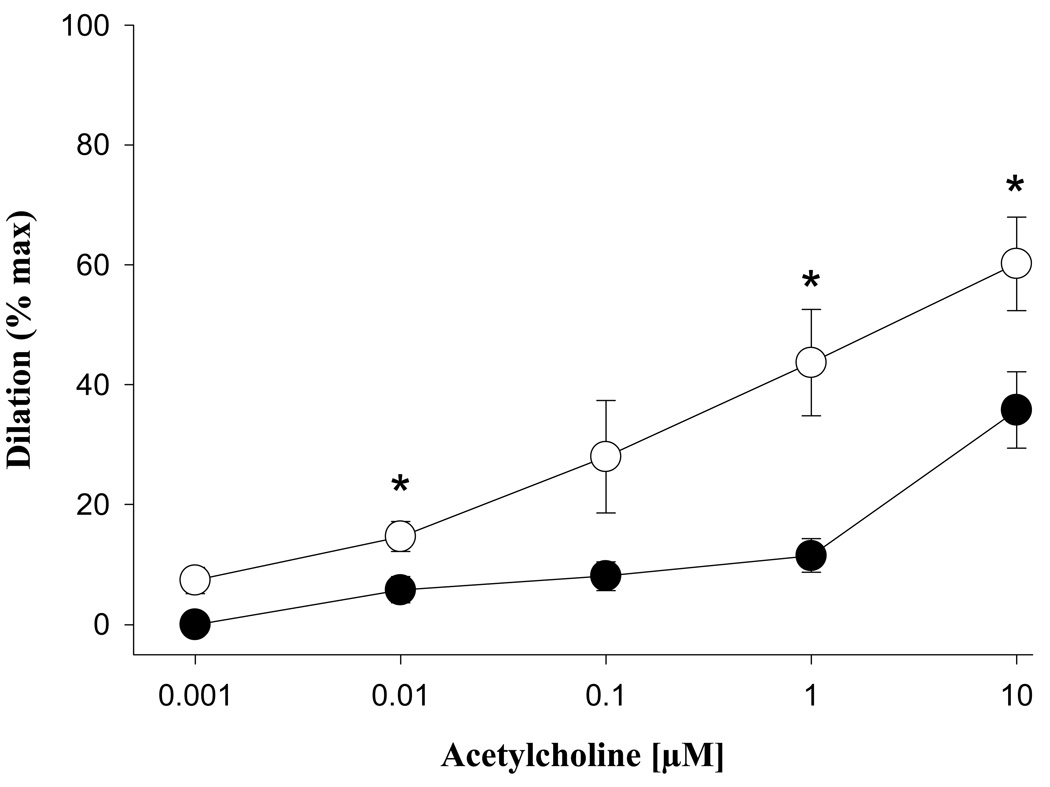
The maximal dilation seen in response to acetylcholine (ACh) increased significantly during pregnancy (NP = 35.8 ± 6.4, LP = 60.2 ± 7.8 % p = 0.04, Figure 4) as did sensitivity (EC50: NP = 935 ± 422, LP = 194 ± 60, nM, p = 0.03). In response to ACh, neither group reached the maximum dilation response as seen with diltiazem and papaverine.
Fig. 4.
Dilation of mesenteric veins from nonpregnant (●, n = 6) and pregnant (○, n = 6) animals in response to acetylcholine. Efficacy (shown relative to maximal dilation measured in relaxing solution) significantly increased in pregnancy. Asterisks denote statistically significant comparisons (p < 0.05).
Contribution of nitric oxide to ACh-induced reactivity
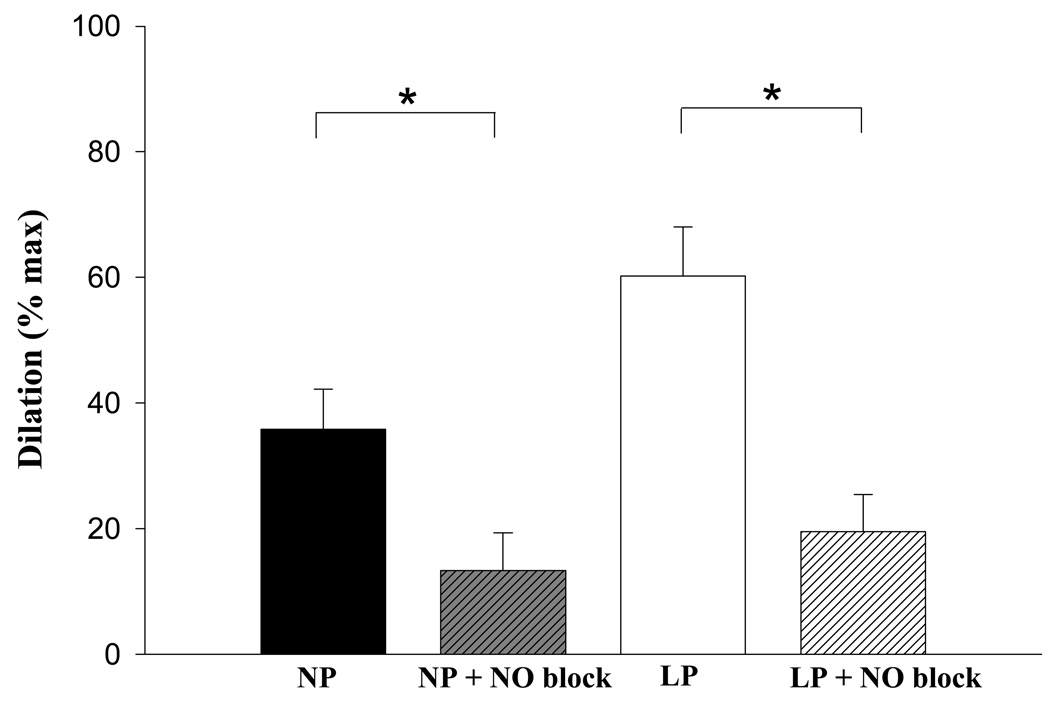
After preincubation with nitric oxide synthase inhibitors L-NNA and L-NAME, the maximal dilatory response to ACh was significantly attenuated in both study groups (NP = 63 + 6.0%, LP = 67.7 + 6.0% reduction in response to ACh, P < .05, Figure 5), although there was no statistically significant difference in the residual dilation after NO blockade between groups (P > .05).
Fig. 5.
Nitric oxide (NO) inhibition (N-nitro-L-arginine [L-NNA] + Nω-nitro-L-arginine methyl ester [L-NAME]) significantly (P < .05) reduces dilation in vessels from both nonpregnant (NP, n = 6; NP + NO blockade, n = 4) and late pregnant (LP, n = 6; LP + NO blockade, n = 6) animals. Asterisks denote statistically significant comparisons (P < 0.05). There was no significant difference in residual dilation between groups.
Smooth muscle response to nitric oxide and inhibition of cGMP
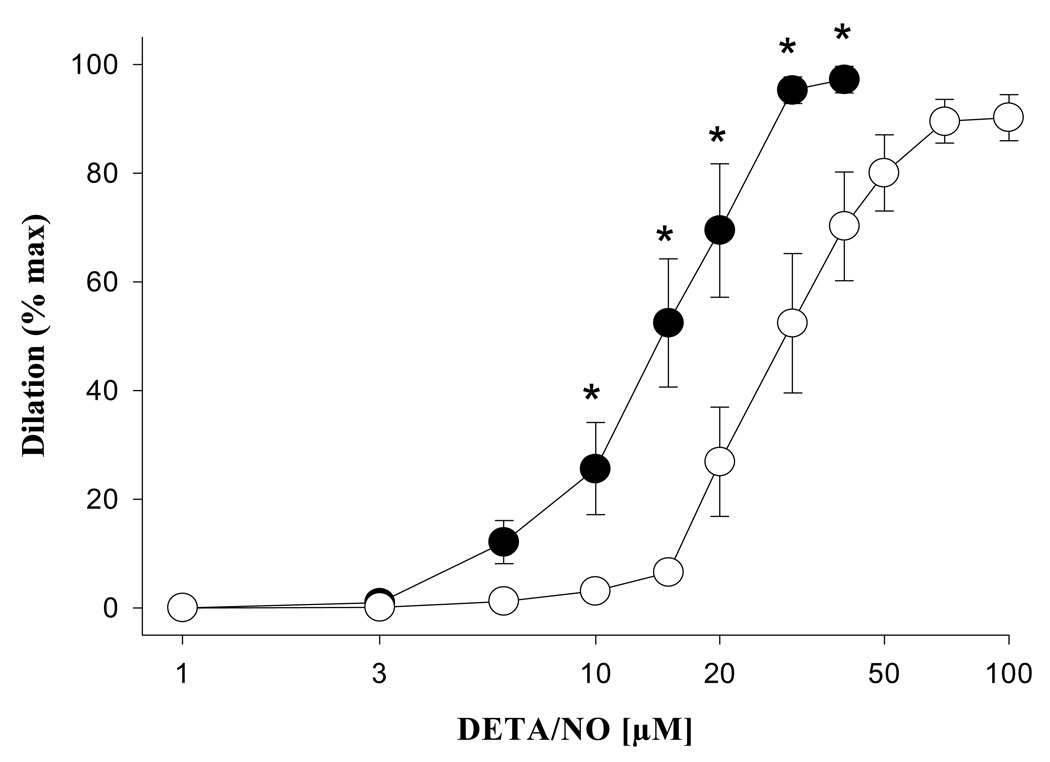
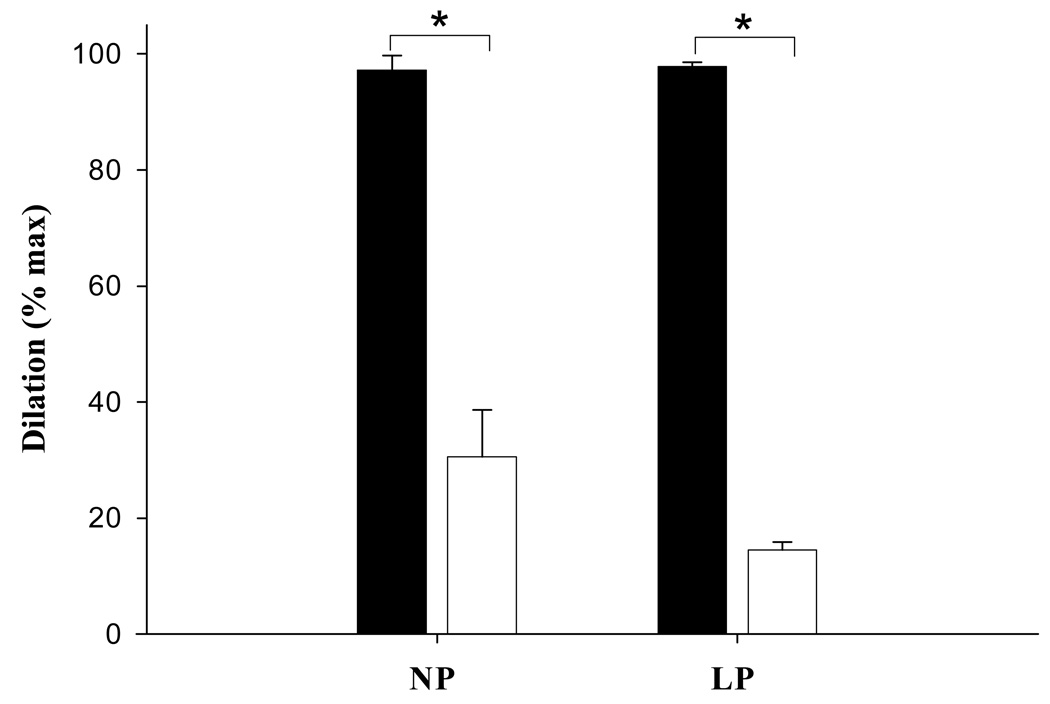
A significant decrease in vascular smooth muscle sensitivity to DETA/NO was observed during pregnancy with a significant increase in the amount of DETA/NO required to produce 50% of the maximal dilatory response (EC50: NP = 16 ± 0.9, LP = 27 ± 0.9 µM, p < 0.005, Figure 6). The effects of DETA/NO administration were largely reversed by ODQ in both the pregnant (p < 0.05) and nonpregnant groups (p < 0.001), although some residual dilation was present, and did not differ between groups (p > 0.05, Figure 7).
Fig. 6.
Dilation of mesenteric veins from nonpregnant (●, n = 6) and late pregnant animals (○, n = 6) in response to DETA/NO, a nitric oxide donor. Note the significant reduction in sensitivity during pregnancy, as evidenced by the right-ward shift in the concentration-response curve. EC50 values (p < 0.05) are presented in text of Results section. Asterisks denote statistically significant comparisons (p < 0.05).
Fig. 7.
Effect of cyclic guanosine monophosphate (cGMP) inhibition via 1H-[1,2,4]oxadiazolo[4,3-α-]quinoxalin-1-one (ODQ) on venous smooth muscle response to DETA/NO in nonpregnant (NP, n = 6) and late pregnant animals (LP, n = 6). Solid bars denote response in the absence of ODQ, and open bars show response after administration of a maximal concentration of ODQ (10 mmol/L). Although there was a significant (P < .05) decrease in dilation in both groups in the presence of ODQ, the extent of residual dilation was similar (P > .05).
DISCUSSION
Maternal plasma volume expands significantly during pregnancy, with average increases of 40–50% for singleton pregnancies33, and much higher values in multiple gestations34. Although changes in the arterial system to allow for tolerance of this volume overload have been well studied, much less is known about the venous side of the circulation, which contains more than two thirds of total blood volume. In addition to their roles in capacitance, venous tone and distensibility are also important determinants of cardiac return and output. And although only a handful of studies have examined changes in venous distensibility or reactivity during gestation, an association between a failure to adapt and preeclampsia has already been noted.
To our knowledge, this is the first study to specifically identify a significant increase in systemic venous endothelium-dependent vasodilation in pregnancy. This was evidenced by increases in both the sensitivity and magnitude of effect of acetylcholine in veins from pregnant animals. These findings are consistent with pregnancy-associated increases in endothelium-dependent vasodilation previously noted in mouse14 and rat15,35 uterine and mesenteric arteries as well as in ovine36 and human37 uterine arteries. Although the underlying mechanism is not known, these adaptive changes may be hormonally regulated, as the vasodilation seen exclusively in pregnancy in the guinea pig uterine artery can be reproduced in the nonpregnant state by chronic administration of estradiol4,38.
Endothelium-induced vasodilation involves multiple pathways which include nitric oxide, cyclooxygenase and endothelium-derived hyperpolarizing factor (EDHF)39,40. Using ACh, a standard probe for endothelial vasodilator function, our data indicate that NO plays a primary role, with a >60% decrease in vasodilatory response observed in the presence of NO inhibition. These findings are consistent with experiments on human uterine arteries which have shown an increase in nitric oxide-dependent dilator responses during pregnancy associated with both increased expression and activity of endothelial NO synthase (eNOS) 41,42. The residual (non-NO) component was similar in vessels from pregnant and nonpregnant animals and further experiments are required to identify the specific contributions of prostanoids vs. EDHF in this effect.
The importance of augmented NO release by the endothelium is underscored by the fact that vascular smooth muscle sensitivity to DETA/NO, an NO donor, was reduced in veins from pregnant animals. This compensatory downregulation of smooth muscle NO sensitivity has been noted previously in the uterine artery in response to sodium nitroprusside, and may represent a form of nitrate tolerance in response to increased endothelial NO signaling in the pregnant state43.
As seen in the TEM image, second-order mesenteric veins contain only a single layer of smooth muscle cells. In spite of this, the contractile ability of isolated veins was impressive, as evidenced by the approximately 75% decreases in lumen diameter (in both groups) in response to an 80 mM potassium depolarizing solution, and ~60% constriction noted in response to the maximal concentrations of norepinephrine. Other studies have documented a rich sympathetic innervation of mesenteric veins, lending physiological credibility to sympathetic regulation of tone44. In addition, one report demonstrated NO-containing nerves as well, suggesting that the level of tone may be determined by a balance of vasodilatory and vasoconstrictor nerves45, in addition to endothelial and humoral influences.
Although vasodilation to NO is thought to be primarily mediated by cGMP signaling, several recent studies have documented activation of non-cGMP pathways in response to NO stimulation of cardiac myocytes26,28 and bovine granulosa cells27. To explore this possibility further, we used ODQ, a well established inhibitor of guanylyl cyclase. Small (<20%) but measurable residual dilation was observed in the presence of supra-maximal concentrations of ODQ, raising the possibility that non-cGMP mechanisms may operate in venous vascular smooth muscle as well, and contribute to venodilation.
Also consistent with a pro-vasodilatory state was our finding of a blunted adrenergic venous constrictor response. Our results do differ from earlier work in our laboratory, which showed an increased constrictor response to NE in mesenteric veins18. This may be largely explained by our inclusion of β-adrenergic blockade in our protocol. We were trying to specifically identify the effect of α-adrenergic stimulation, and β-receptor stimulation of mesenteric vessels has been shown to cause vasodilation46 and may thereby have altered our earlier results. In addition, results similar to our current finding of decreased venous α-adrenergic sensitivity have been seen in in vivo studies of human dorsal hand veins in pregnancy. There was no evidence for an effect of estrus cycle on venous NE sensitivity in this study, although the n values were relatively low, and all the animals were only in estrus or proestrus. Estrogen is known to affect arterial vasodilation to other agonists, for example, vascular endothelial growth factor,47 however, and measurement of serum estradiol would allow for potential correlation between estradiol levels and decreasing a-adrenergic sensitivity.
Increased endothelial vasodilatory influence may be the primary venous adaptation to volume expansion in pregnancy, as there were no parallel changes in venous mechanical or structural characteristics. There was no change in vessel diameter, which contrasts with the changes seen in uterine veins in late pregnancy5 but is consistent with previous data on mesenteric veins18,48. Likewise, there were no significant differences in passive distensibility, although the trend towards less distensibility was similar to that seen previously in mesenteric veins in both pregnancy and the postpartum period.17,48
Despite the limitations of isolated vessel experiments, we are encouraged by the identification of vessel changes in vitro which support the maternal tolerance of large plasma volume increase as identified in normal pregnancy in vivo46,49. It appears that venous adaptations in pregnancy favor a pro-vasodilatory state in which α-adrenergic responsiveness is decreased, coupled with an increase in endothelial-dependent vasodilatory responsiveness which appears to be primarily mediated by an NO-dependent mechanism. In addition, there is downregulation of smooth muscle response to NO which is largely related to cGMP production, and this is likely compensated for by large increases in nitric oxide production and/or availability. The combination of increased vasodilation and decreased α-adrenergic sensitivity would enhance venous capacitance and thereby accommodate the plasma volume increases seen in normal pregnancy.
Contributor Information
Maurizio Mandala, Email: Maurizio.Mandala@med.uvm.edu.
Carolyn Barron, Email: Carolyn.Barron@med.uvm.edu.
Ira Bernstein, Email: Ira.Bernstein@vtmednet.org.
George Osol, Email: George.Osol@uvm.edu.
References
- 1.Capeless EL, Clapp JF. Cardiovascular changes in the early phase of pregnancy. Am J Obstet Gynecol. 1989;161:1449–1453. doi: 10.1016/0002-9378(89)90902-2. [DOI] [PubMed] [Google Scholar]
- 2.Duvekot JJ, Peeters LLH. Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstet Gynecol Surv. 1994;49(12 Suppl):S1–S14. doi: 10.1097/00006254-199412011-00001. [DOI] [PubMed] [Google Scholar]
- 3.Ralevic V, Burnstock G. Mesenteric arterial function in the rat in pregnancy: role of sympathetic and sensory-motor perivascular nerves, endothelium, smooth muscle, nitric oxide and prostaglandins. Br J Pharmacol. 1996;117(7):1463–1470. doi: 10.1111/j.1476-5381.1996.tb15307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell C, Brown MJ. Arteriographic evidence for a cholinergic dilator mechanism in uterine hyperaemia of pregnancy in the guinea-pig. J Reprod Fert. 1971;27:59–65. doi: 10.1530/jrf.0.0270059. [DOI] [PubMed] [Google Scholar]
- 5.Page KL, Celia G, Leddy G, Taatjes DJ, Osol G. Structural remodeling of rat uterine veins in pregnancy. Am J Obstet Gynecol. 2002;187:1647–1652. doi: 10.1067/mob.2002.127599. [DOI] [PubMed] [Google Scholar]
- 6.D’Angelo G, Osol G. Regional variation in resistance artery diameter responses to α-adrenergic stimulation during pregnancy. Am J Physio. 1993;264:H78–H85. doi: 10.1152/ajpheart.1993.264.1.H78. (Heart Circ Physiol 33) [DOI] [PubMed] [Google Scholar]
- 7.Alexander BT, Bennett WA, Khalil RA, Granger JP. Preeclampsia: Linking placental ischemia with cardiovascular-renal dysfunction. News Physio Sci. 2001;16:282–286. doi: 10.1152/physiologyonline.2001.16.6.282. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein IM, Meyer MC, Osol G, Ward K. Intolerance to volume expansion: a theorized mechanism for the development of preeclampsia. Obstet Gynecol. 1998;92(2):306–308. doi: 10.1016/s0029-7844(98)00207-5. [DOI] [PubMed] [Google Scholar]
- 9.Goolin RC. Maternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardation. Obstet Gynecol. 1993;82(4 Pt 1):636–637. [PubMed] [Google Scholar]
- 10.Pang CCY. Autonomic control of the venous system in health and disease. Effects of drugs. Pharmacol Ther. 2001;90:179–230. doi: 10.1016/s0163-7258(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 11.Rothe CF. Reflex control of veins and vascular capacitance. Physiol Rev. 1983;63(4):1281–1342. doi: 10.1152/physrev.1983.63.4.1281. [DOI] [PubMed] [Google Scholar]
- 12.Rowell LB. Editorial: Importance of scintigraphic measurement of human splanchnic blood volume. Jl Nucl Med. 1990;31(2):160–162. [PubMed] [Google Scholar]
- 13.Greenway CV. Role of splanchnic venous system in overall cardiovascular homeostasis. Fed Proc. 1983;42(6):1678–1684. [PubMed] [Google Scholar]
- 14.Crandall ME, Keve TM, McLaughlin MK. Characterization of norepinephrine sensitivity in the maternal splanchnic circulation during pregnancy. Am J Obstet Gynecol. 1990;162(5):1296–1301. doi: 10.1016/0002-9378(90)90040-e. [DOI] [PubMed] [Google Scholar]
- 15.Cooke CM, Davidge ST. Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biology of Reproduction. 2003;68:1072–1077. doi: 10.1095/biolreprod.102.009886. [DOI] [PubMed] [Google Scholar]
- 16.Gerber RT, Anwar MA, Poston L. Enhanced acetylcholine induced relaxation in small mesenteric arteries from pregnant rats: an important role for endothelium-derived hyperpolarizing factor (EDHF) Br Jl Pharm. 1998;125:455–460. doi: 10.1038/sj.bjp.0702099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhawan V, Brookes ZLS, Kaufman S. Repeated pregnancies (multiparity) increases venous tone and reduces compliance. Am J Phyiol Regul Integr Comp Physiol. 2005;289:R23–R28. doi: 10.1152/ajpregu.00034.2005. [DOI] [PubMed] [Google Scholar]
- 18.Hohmann M, Keve TM, Osol G, McLaughlin MK. Norepinephrine sensitivity of mesenteric veins in pregnant rats. Am J Physio Regul Integr Comp Physiol. 1990;259:R753–R759. doi: 10.1152/ajpregu.1990.259.4.R753. [DOI] [PubMed] [Google Scholar]
- 19.Monga M. Maternal Cardiovascular and Renal Adaptation to Pregnancy. In: Creasy RK, Resnik R, editors. Maternal-Fetal Medicine: Principles and Practice. 5th ed. Philadelphia, Pa: Saunders; pp. 111–120. [Google Scholar]
- 20.Halpern W, Osol G, Coy GS. Mechanical behavior of pressurized in vitro prearteriolar vessels determined with a video system. Am J Physio. 1985;249:H914–H921. doi: 10.1007/BF02363917. [DOI] [PubMed] [Google Scholar]
- 21.Halpern W, Osol G. Blood vessel diameter measurement. Prog Appl Microcirc. 1985;8:32–39. [Google Scholar]
- 22.Williams JM, Hull AD, Pearce WJ. Maturational modulation of endothelium-dependent vasodilatation in ovine cerebral arteries. Am J Physiol Regul Integr Comp Physiol. 2005;288:R149–R157. doi: 10.1152/ajpregu.00427.2004. [DOI] [PubMed] [Google Scholar]
- 23.Bivalacqua TJ, Champion HC, De Witt BJ, et al. Analysis of vasodilator responses to novel nitric oxide donors in the hindquarters vascular bed of the cat. J Cardiovasc Pharmacol. 2001;38:120–129. doi: 10.1097/00005344-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Cawley SM, Sawyer CL, Brunelle KF, van der Vliet A, Dostmann WR. Nitric oxide-evoked transient kinetics of cyclic GMP in vascular smooth muscle cells. Cell Signal. 2007;19:1023–1033. doi: 10.1016/j.cellsig.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Rabkin SW, Klassen SS, Tsang MY. Sodium nitroprusside activates p38 mitogen activated protein kinase through a cGMP/PKG independent mechanism. Life Sci. 2007;81(8):640–646. doi: 10.1016/j.lfs.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Garreffa AM, Woodman OL, Cao AH, Ritchie RH. Sodium nitroprusside protects adult rat cardiac myocytes from cellular injury induced by simulated ischemia: role for a non-cGMP-dependent mechanism of nitric oxide protection. J Cardiovasc Pharmacol. 2006;47(1):1–8. doi: 10.1097/01.fjc.0000189601.12276.8b. [DOI] [PubMed] [Google Scholar]
- 27.Basini G, Grasselli F, Ponderato N, Bussolati S, Tamanini C. Lipid hydroperoxide and cGMP are not involved in nitric oxide inhibition of steroidogenesis in bovine granulosa cells. Reprod Fertil Dev. 2000;12(5–6):289–295. doi: 10.1071/rd00089. [DOI] [PubMed] [Google Scholar]
- 28.Paolocci N, Ekelund UE, Isoda T, et al. cGMP-independent inotropic effects of nitric oxide and peroxynitrate donors: potential role for nitrosylation. Am J Physiol Heart Circ Physiol. 2000;279(4):H1982–H1988. doi: 10.1152/ajpheart.2000.279.4.H1982. [DOI] [PubMed] [Google Scholar]
- 29.Young HM, Ciampoli D, Johnson PJ, Stebbing MJ. Inhibitory transmission to the longitudinal muscle of the mouse caecum is mediated largely by nitric oxide acting via soluble guanylyl cyclase. J Auton Nerv Syst. 1996;61:103–108. doi: 10.1016/s0165-1838(96)00064-1. [DOI] [PubMed] [Google Scholar]
- 30.Fedele E, Jin Y, Varnier G, Raiteri M. In vivo microdialysis study of a specific inhibitor of soluble guanylyl cyclase on the glutamate receptor/nitric oxide/cyclic GMP pathway. Br Jl Pharmacol. 1996;119:590–594. doi: 10.1111/j.1476-5381.1996.tb15713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cellek S, Kasakov L, Moncada S. Inhibition of nitrergic relaxations by a selective inhibitor of the soluble guanylyl cyclase. Br Jl Pharmacol. 1996;118:137–140. doi: 10.1111/j.1476-5381.1996.tb15376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1 H-[1,2,4]oxadiazolo[4,3-α]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 33.Pritchard JA. Changes in the blood volume during pregnancy and delivery. Anesthesiology. 1965;26:393. doi: 10.1097/00000542-196507000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Rovinsky JJ, Jaffin H. Cardiovascular hemodynamics in pregnancy. I. Blood and plasma volumes in multiple pregnancy. Am J Obstet Gynecol. 1965;93:1. doi: 10.1016/0002-9378(65)90288-7. [DOI] [PubMed] [Google Scholar]
- 35.Wight E, Kung CF, Moreau P, Takase H, Bersinger NA, Lusher TF. Aging, serum estradiol levels and pregnancy differentially affect vascular reactivity of the rat uterine artery. J Soc Gynecol Investig. 2000;7:106–113. [PubMed] [Google Scholar]
- 36.Janowiak MA, Magness RR, Habermehl DA, Bird IM. Pregnancy increases ovine uterine artery endothelial cyclooxygenase-1 expression. Endocrinology. 1998;139:767–771. doi: 10.1210/endo.139.2.5739. [DOI] [PubMed] [Google Scholar]
- 37.Nelson SH, Steinsland, Suresh MS, Lee NM. Pregnancy augments nitric oxide-dependent dilator response to acetylcholine in the human uterine artery. Hum Reprod. 1998;13(5):1361–1367. doi: 10.1093/humrep/13.5.1361. [DOI] [PubMed] [Google Scholar]
- 38.Ni Y, Meyer M, Osol G. Gestation increases nitric oxide-mediated vasodilation in rat uterine arteries. Am J Obstet Gynecol. 1997;176:856–864. doi: 10.1016/s0002-9378(97)70611-2. [DOI] [PubMed] [Google Scholar]
- 39.Xiao D, Pearce WJ, Zhang L. Pregnancy enhances endothelium-dependent relaxation of ovine uterine artery: role of NO and intracellular Ca2+ doi: 10.1152/ajpheart.2001.281.1.H183. [DOI] [PubMed] [Google Scholar]
- 40.Bell C. Oestrogen-induced sensitization of the uterine artery of the guinea-pig to acetylcholine. Br J Pharmac. 1973;49:595–601. doi: 10.1111/j.1476-5381.1973.tb08535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson SH, Steinsland OS, Suresh MS, Lee NM. Pregnancy augments nitric oxide-dependent dilator response to acetylcholine in the human uterine artery. Human Reproduction. 1998;13(5):1361–1367. doi: 10.1093/humrep/13.5.1361. [DOI] [PubMed] [Google Scholar]
- 42.Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong Y, Sanchez JM. Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circ Res. 2000;87:406–411. doi: 10.1161/01.res.87.5.406. [DOI] [PubMed] [Google Scholar]
- 43.Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci. 1995;16(1):23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- 44.Okamura T, Uchiyama M, An J, Toda N. Nitric-oxide mediated neurogenic relaxation in monkey mesenteric veins. Jpn J Pharmacol. 1995;68(4):405–411. doi: 10.1254/jjp.68.405. [DOI] [PubMed] [Google Scholar]
- 45.Miller VM. Selective production of endothelium-derived nitric oxide in canine femoral veins. Am J Physiol. 1991;261(3 Pt 2):H677–H682. doi: 10.1152/ajpheart.1991.261.3.H677. [DOI] [PubMed] [Google Scholar]
- 46.Landau R, Dishy V, Wood AJJ, Stein CM, Smiley RM. Disproportionate decrease in α- compared with β-adrenergic sensitivity in the dorsal hand vein in pregnancy favors vasodilation. Circulation. 2002;106:1116–1120. doi: 10.1161/01.cir.0000028334.32833.b0. [DOI] [PubMed] [Google Scholar]
- 47.Storment JM, Meyer M, Osol G. Estrogen augments the vasodilatory effects of vascular endothelial growth factor in the uterine circulation of the rat. Am J Obstet Gynecol. 2000;183:449–453. doi: 10.1067/mob.2000.105910. [DOI] [PubMed] [Google Scholar]
- 48.Hohmann M, McLaughlin MK, Kunzel W. Direct assessment of mesenteric vein compliance in the rat during pregnancy. Z Geburtshilfe Perinatol. 1992;196(1):33–40. [PubMed] [Google Scholar]
- 49.Ford GA, Robson SC, Mahdy ZA. Superficial hand vein responses to NG-monomethyl-L-arginine in post-partum and non-pregnant women. Clin Sci. 1996;90(6):493–497. doi: 10.1042/cs0900493. [DOI] [PubMed] [Google Scholar]