Abstract
Epidermal fatty acid binding protein, E-FABP, a lipid chaperone, has been shown to regulate the inflammatory function of macrophages and dendritic cells. Herein, we demonstrate that T cell expression of E-FABP promotes Th17 differentiation, while counter-regulating development of FoxP3+ regulatory T cells (Treg). In response to immunization with myelin oligodendrocyte glycoprotein peptide (MOG35–55), E-FABP-deficient mice generated reduced levels of Th17 cells and elevated levels of Tregs, as compared to wild-type mice. Likewise, naïve CD4+ T cells isolated from E-FABP-deficient mice showed reduced expression of IL-17 and enhanced expression of FoxP3, in vitro, when subjected to Th17 or Treg polarizing conditions, respectively. It has been demonstrated previously that IL-21, induced by IL-6, stimulates the expression of the nuclear receptors RORγt and RORα, which in turn induce expression of IL-17. We found that the impaired Th17 differentiation by E-FABP-deficient CD4+ T cells was associated with lower levels of IL-21 expression in response to IL-6, and reduced expression of RORγt and RORα. However, E-FABP-deficient CD4+ T cells expressed significantly higher levels of the nuclear receptor PPARγ than wild-type CD4+ T cells and treatment with the PPARγ antagonist GW9662 restored expression of IL-21, RORγt, RORα and IL-17 by E-FABP-deficient T cells to wild-type levels. The negative influence of E-FABP-deficiency on IL-17 expression was attributed to PPARγ-mediated suppression of IL-6-induced STAT3 activity. Thus, taken together, our data indicate that expression of E-FABP by CD4+ T cells contributes to the control of IL-6 stimulation of the IL-21/ROR/IL-17 pathway and to the Th17/Treg counter-balance.
Keywords: T cells, EAE/MS, Cytokines, Transcription Factors
Introduction
Fatty acid-binding proteins, FABPs3, consist of a family of small, highly homologous cytosolic proteins that display distinct patterns of tissue distribution (1). FABPs are capable of binding a variety of hydrophobic compounds, with the specificity and affinity of ligand binding varying amongst the various family members. Evidence suggests that a general function of FABPs is to shuttle lipid ligands to intracellular compartments or enzyme systems within the cell (1,2). Studies of FABP deficient mice have revealed a role of epithelial FABP (E-FABP) and adipose FABP (A-FABP) in the development of obesity, insulin resistance and atherosclerosis (3). Recent work has revealed a role of these 2 FABPs in the regulation of macrophage and dendritic cell (DC) inflammatory function. We have shown that A-FABP-deficiency in macrophages results in elevated activity of the lipid-liganded nuclear receptor, PPARγ. Elevated PPARγ activity, in turn, upregulates expression of genes involved in cholesterol trafficking, while downregulating expression of genes encoding proinflammatory proteins (4). DC deficient for A-FABP and/or E-FABP do not effectively promote Th1 differentiation in vitro (5,6), and FABP-deficient mice immunized with MOG35–55, show protection from development of experimental autoimmune encephalomyelitis (EAE), with reduced levels of IFNγ, and IL-17 in CNS tissue as compared to wild-type mice.
Development of EAE has been shown to involve both Th1 and Th17 T helper cell subsets, each of which play distinct roles in disease pathogenesis (7–9). Development and progression of EAE is attenuated by FoxP3+ regulatory T cells (Treg) (10) and recent studies have shown that Th17 and Treg differentiation are reciprocally regulated (11,12). Th17 differentiation can be initiated by a combination of TGFβ and IL-6 in mouse (13,14), and is maintained by IL-23 (15). IL-21, induced by IL-6, acts in an autocrine fashion to promote Th17-cell differentiation (16). IL-21 induces expression of the of the nuclear receptor superfamily members, RORγt and RORα (15,17). Both RORγt and RORα contribute to the induction of T cell expression of IL-17 (17,18) and double deficiency of these two transcription factors impairs Th17 development and protects mice against development of EAE (17). Interestingly, IL-6, which promotes Th17 differentiation when in combination with TGFβ, inhibits the generation of FoxP3+ Tregs (11), whereas TGFβ-induced FoxP3 has been shown to antagonize RORγt and RORα function (12,19). Thus, the Th17 and Treg differentiation programs are antagonistic and the polarization towards one or the other functional phenotype can be modulated by the cytokine milieu (12).
In addition to the role of RORγt and RORα in Th17 differentiation, there is evidence that other members of the nuclear receptor superfamily influence the Th17/Treg balance. For example, the retinoic acids (RAs), all-trans-retinoic acid (ATRA), a ligand for RAR nuclear receptor family members, and 9-cis-retinoic acid (9-cis-RA), a ligand for both retinoid-X-receptors (RXRs) and RARs, have been shown to both induce Treg differentiation and inhibit Th17 differentiation (20). In addition, there is evidence that PPARγ expression by Tregs is essential for their regulatory function (21). Interestingly, E-FABP is capable of binding RAs and there is overlap in ligand binding specificities between E-FABP and PPAR family members (22–24). These observations led to a number of studies that have linked FABP expression with RA transport, and with PPAR activity, in a variety of cell culture systems. For example, there is evidence for E-FABP transport of RA to the nucleus (24), and there are studies suggesting that FABPs may act to sequester PPAR ligands in the cytoplasm, hence inhibiting nuclear entry and PPAR activity (4,25,26).
In our previous study of the impact of deficiency of A-FABP and E-FABP on the development of EAE we focused on the role of macrophages and DC (6). Although A-FABP expression is primarily restricted to macrophages, DC, and adipocytes, E-FABP is the most widely expressed of the various FABP family members and is expressed in most leukocyte populations, including CD4+ T cells (5). Given the dramatic differences in IL-17 expression between FABP-deficient mice and wild-type mice at the sites of inflammation in the EAE model (6), we explored the possibility that the presence of E-FABP in T cells may influence IL-17 expression. We provide evidence that E-FABP contributes to the regulation of Th17 differentiation in vitro and during the development of EAE in vivo.
Materials and Methods
Mice
Mice deficient for E-FABP were generated as previously described (27) and backcrossed >10 generations onto a C57BL/6 background. The E-FABP-deficient mice were re-derived, bred, and maintained in the University of Louisville Research Resources Facility. All animal care and experimental procedures used in this study were approved by the University of Louisville Institutional Animal Care and Use Committee.
Induction of EAE
MOG35–55 peptide corresponding to the sequence MEVGWYRSPFSRVVHLYRNGK was purchased from Bio-Synthesis. Mice were injected in the flank with a 100µl emulsion containing 150µg of MOG35–55 in CFA (Sigma-Aldrich) supplemented with 500ng of Mycobacterium tuberculosis H37Ra (Difco Laboratories). Mice were injected i.p. with 500 ng of pertussis toxin (List Biological Laboratories) immediately following MOG35–55 injection and again 2 days post-immunization. For adoptive transfer of EAE, C57BL/6 female mice were immunized as described above. Draining lymph nodes were harvested 11 d post-immunization and cultured for 5 days in RPMI 1640 supplemented with 10% FCS, penicillin/streptomycin, L-glutamine, HEPES, sodium pyruvate, and 2-ME, in the presence of MOG35–55 (25 µg/ml) and recombinant murine IL-23 (10 ng/ml; R&D Systems) for maintenance of the Th17 phenotype, as previously described (28,29). Following in vitro culture, the cells were analyzed by flow cytometry, which confirmed the presence of Th17 (CD4+, IL-17+), but not Th1 (CD4+, IFNγ+) cells, as has been reported previously (28). Cells were harvested, washed, and 1.5 × 107 cells were transferred to wild type and E-FABP-deficient female mice via the tail vein. Mice were given 250 ng of pertussis toxin i.p. on days 0 and 2 post transfer. The animals were scored daily for clinical symptoms of EAE. Clinical scores were designated numerically according to the following: 0, no detectable EAE symptoms; 1, tail paralysis/loss of tonicity; 2, abnormal gait; 3, hind limb paralysis; 4, hind and forelimb paralysis; 5, moribund or dead; 0.5 gradations were assigned for intermediate scores.
Cell isolation and cell culture conditions
Single cells from CNS were isolated using Neural Tissue Dissociation Kits (Miltenyi Biotec) per manufacturer’s instructions. For analysis of Th17/Treg differentiation, naïve CD4+ T cells were purified from spleen of C57BL/6 and E-FABP-deficient female mice by using CD4+ CD62L+ T cell isolation kits (Miltenyi Biotec). Purified T cells were plated at a ratio of 1:5 with irradiated (3000 rads) splenic feeder cells in RPMI medium supplemented with 5% FBS, 2mM L-glutamine, 10mM Hepes, 2.5 µM 2-ME, 5 µg/ml insulin-transferring-selenium, 10µg/ml gentamycin, 100 µg/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich). Cells were stimulated with 2.5 µg/ml anti-CD3 (clone 145-11). For Th17 promoting conditions, cultures were supplemented with 5 ng/ml rhTGFβ1 (R&D Systems), 20 ng/ml rmIL-6 (R&D Systems). Treg differentiation was promoted by supplementation of cell cultures with 100nM 9-cis-RA (Biomol). Three days after initiation of the cultures, T cells were collected for the flow cytometric analysis. Supernatants were harvested and assayed by ELISA for IL-17 (R&D systems) and IFNγ (BD Biosciences). The FABP inhibitor, designated UMN2421 was identified using high throughput screening procedures designed to identify small molecules that displace endogenous FABP ligands. Using the 1,8-ANS displacement assay, UMN2421 binds to EFABP in a 1:1 stoichiometry with an apparent Kd of ~ 3.4 ± 0.6 µM. The details of the screening procedures and the crystal structure of the UMN2421-AFABP complex will be published elsewhere (manuscript in preparation). The PPARγ antagonist, GW9662, was purchased from Cayman Chemical. UMN2421, 9-cis-RA, and GW9662 were dissolved in DMSO and stored at −80°C in light-proof containers.
Flow cytometric analysis
For analysis of cytokine synthesis via flow cytometry, cells were re-stimulated for 5–6 h with 50 ng/ml PMA (Sigma) and 750 ng/ml ionomycin (Calbiochem). After 1 h, GolgiStop (BD Biosciences) was added to block cytokine secretion. Cells were surface stained for 15–30 min at 4°C with APC-conjugated anti-CD4 mAb (clone RM45, BD Biosciences) in PBS supplemented with 1% BSA and 0.2% sodium azide. T cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences) and stained intracellularly with PE-conjugated anti-IL-17 (anti-IL-17A clone TC11-18H10, BD Biosciences), FITC-labeled anti-IFNγ (clone XMG1.2, BD Biosciences), or PE-Foxp3 (clone FJK-16s, eBioscience). Samples were acquired on a FACSCalibur flow cytometer and data analysis was conducted using FlowJo software (Tree Star Inc.).
Quantitative Real-time RT PCR analysis
mRNA was isolated from cultured cells and converted to cDNA using MACS One-step cDNA columns (Miltenyi Biotec). Real-time RT PCR was performed using a DNA-Opticon 3 monitor (MJ Research, currently Bio-Rad) using SYBR Green (Qiagen). RORγt, RORα, IL-17, IL-21, RARα, RXRα, PPARα, PPARδ/β, PPARγ, and β-actin expression was analyzed by Quantitect Primer Assays (Qiagen). Results were normalized to β-actin. Relative expression of RNA transcripts was quantified using the relative expression software tool, REST (30).
Western blot analysis
Naïve or activated CD4+ T cells were lysed in buffer containing 25 mM Tris-HCl, 1% deoxycholate, 0.35 M NaCl, phosphatase inhibitor solution (Cayman Chemical) and 1% Triton X-100 (Fischer Scientific). Protein quantity was assayed by bicinchoninic acid (Pierce) and 20µg of protein was loaded per well on a 15% Tris-HCl gel (Bio-Rad). The contents of the gel were transferred in a Trans-Blot SemiDry Transfer Cell (Bio-Rad) onto nitrocellulose membranes (Amersham Biosciences). The membranes were incubated with different antibody. Ab-bound proteins were detected using an ECL Western blotting analysis system (Amersham Biosciences), and the membranes were exposed to Kodak Biomax XL X-ray film (Eastman Kodak).
PPARγ activity and STAT3 DNA Binding assays
Nuclear proteins isolated from 5 × 106 naïve CD4+ T cells or differentiated Th17 cells from wild-type (WT) and E-FABP-deficient mice were extracted using nuclear extraction kits purchased from Cayman Chemical. PPARγ activity was measured using Cayman’s transcription factor assay kits. Briefly, 10µg of the extracted nuclear proteins was added to wells containing immobilized specific double stranded DNA sequences corresponding to the peroxisome proliferator response element (PPRE). Bound PPARγ was detected by addition of specific antibody to PPARγ. Relative PPARγ activity was measured by comparison with an unstimulated wild-type control. Activation of STAT3 in nuclear lysates was measured using 10 µg of nuclear extract protein per sample and TransAM STAT3 ELISA (Active Motif). Activated STAT3 in nuclear extracts bound to immobilized STAT3 consensus oligonucleotide was detected using an antibody directed against STAT3 followed by a HRP-conjugated secondary antibody and colorimetric analysis at 450nm.
Analysis of E-FABP, PPARγ and 13-HODE by confocal microscopy
Th17 cells were transferred to poly-D-lysine-coated microslides (Santa Cruz Biotechnology) and 13-HODE-biotin (Cayman Chemical) was added at 5µM for 3 h. Cells were washed with PBS then fixed for 30 min at ambient temperature with 4% paraformaldehyde. The cells were permeabilized for 2 min with 0.1% Triton X-100 (Fisher) in PBS and blocked for 30 min with BSA (Fisher). The cells were labeled with streptavidin Alexa Fluor® 568 for detection of biotinlylated 13-HODE, and FITC-conjugated anti-PPARγ antibody (Santa Cruz Biotechnology). For E-FABP staining, the cells were treated with a primary rabbit anti-E-FABP (31) in PBS, followed by Alexa Fluor® 488 conjugated chicken anti-rabbit IgG (Invitrogen). The cells were incubated with 300 nM 4,6-diamidine-2-phenylindol, dihydrochloride (DAPI, Invitrogen) for 5 min. Coverslips were mounted onto glass microscopy slides (Fisher) with Dako fluorescent mounting medium (Dako North America). Confocal microscopy was performed using an Olympus 3 Laser Scanning Microscope (Olympus America Inc.) at 120 x magnification.
Statistical analysis
The Student t test was performed for comparisons of disease clinical score, levels of cytokine production, and ratios of IL-17+ or Foxp3+ cells. A p value of <0.05 was considered significant.
Results
E-FABP-deficient mice subjected to EAE have reduced T cell expression of IL-17 concomitant with enhanced expression of FoxP3
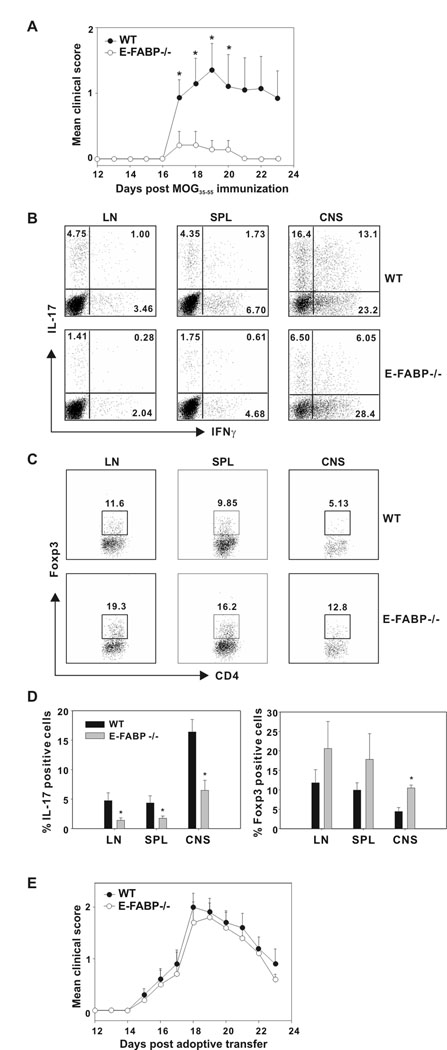
In previous work, we demonstrated that E-FABP-deficient macrophages and DC are defective in expression of inflammatory cytokines and are inefficient in the promotion of proinflammatory T cells responses during antigen presentation (6). In mice deficient for both A-FABP and E-FABP, we saw greatly reduced IL-17 expression in CNS tissue, as compared to WT mice following induction of EAE (6). Since E-FABP is expressed in T cells (5), we tested the possibility that E-FABP deficiency may affect T cell IL-17 expression in a manner that contributes to the disease-protective effect of FABP-deficiency. To address this question, we examined T cells isolated from MOG35–55 - immunized E-FABP-deficient and WT mice. As found previously (6), E-FABP-deficient mice showed reduced clinical symptoms of EAE as compared to WT mice (Fig. 1A). Cells isolated from lymph node, spleen and CNS were analyzed by flow cytometry for expression of IL-17 and IFNγ. As shown in Fig. 1B, E-FABP-deficient mice produced greatly reduced numbers of IL-17+ CD4+ T cells, as well as cells expressing both IL-17 and IFNγ, which have been identified previously in the EAE model (32) and in human memory T cell populations (33). In each of these tissues, the reduction in IL-17+ T cells associated with E-FABP deficiency ranged from approximately 60–70%. The percentages of IFNγ+ CD4+ T cells were not significantly different, and overall percentages of CD4+ T cells did not differ, as previously reported (6).
Figure 1. Protection from EAE is associated with reduced levels of Th17 cells and enhanced levels of Foxp3 cells in E-FABP deficient mice.
A, WT and E-FABP-deficient mice (n=7/group) were MOG35–55-immunized and scored daily for 23 days post-immunization (* p < 0.05). B,C, Lymph node (LN), spleen (SPL) and CNS tissue was harvested from MOG35–55-immunized WT and E-FABP-deficient mice on day 20 post-immunization. Isolated cells were re-stimulated with PMA plus ionomycin and analyzed for IL-17, IFNγ and Foxp3 expression by intracellular labeling and flow cytometric analysis. Dot plots were gated on CD4+ T cells and the percentiles of cells staining positively are shown. D, The mean values and standard deviation of IL-17+ (left panel) or Foxp3+ CD4+ T cells (right panel) shown are based on three independent experiments (* p < 0.05). E, WT MOG35–55-specific Th17 cells were transferred in to naïve WT or E-FABP-deficient recipients (n=5/group) and scored daily for 23 d post-transfer.
Recent work has shown that IL-17 and FoxP3 are counter-regulated via transcription factor antagonism (12). Therefore, we considered it likely that the decreased IL-17 expression by T cells isolated from E-FABP-deficient mice may be accompanied by enhanced expression of FoxP3. Expression of FoxP3 in T cells isolated from lymph node, spleen and CNS of MOG35–55 immunized mice (as above) was evaluated via flow cytometry. Indeed, FoxP3 expression was found to be higher in E-FABP-deficient mice than their WT counterparts (Fig. 1C.). The results of 3 separate experiments in which expression of IL-17 and FoxP3 was evaluated are shown as histograms in Fig. 1D. The reciprocal expression of FoxP3 versus IL-17 was clearly apparent, particularly in the CNS compartment. These results suggest that E-FABP deficiency favors FoxP3 expression/Treg differentiation over IL-17 expression, thus resulting in a T cell functional profile that is protective from development of disease in the EAE model. Therefore, we speculated that the protection from disease displayed by E-FABP-deficient mice may be overridden by transfer of WT Th17 T cells. To test this possibility, WT MOG35–55-specific Th17 cells, expanded in vitro via antigen stimulation in the presence of IL-23 (28,29) were transferred into naïve WT and E-FABP-deficient recipients. As shown in Figure 1E, naïve E-FABP-deficient mice developed disease with identical kinetics and achieving similar clinical scores, as did naïve WT mice upon transfer of WT MOG35–55-specific Th17 cells.
Cytokine driven Th17 differentiation in vitro is impaired by E-FABP deficiency
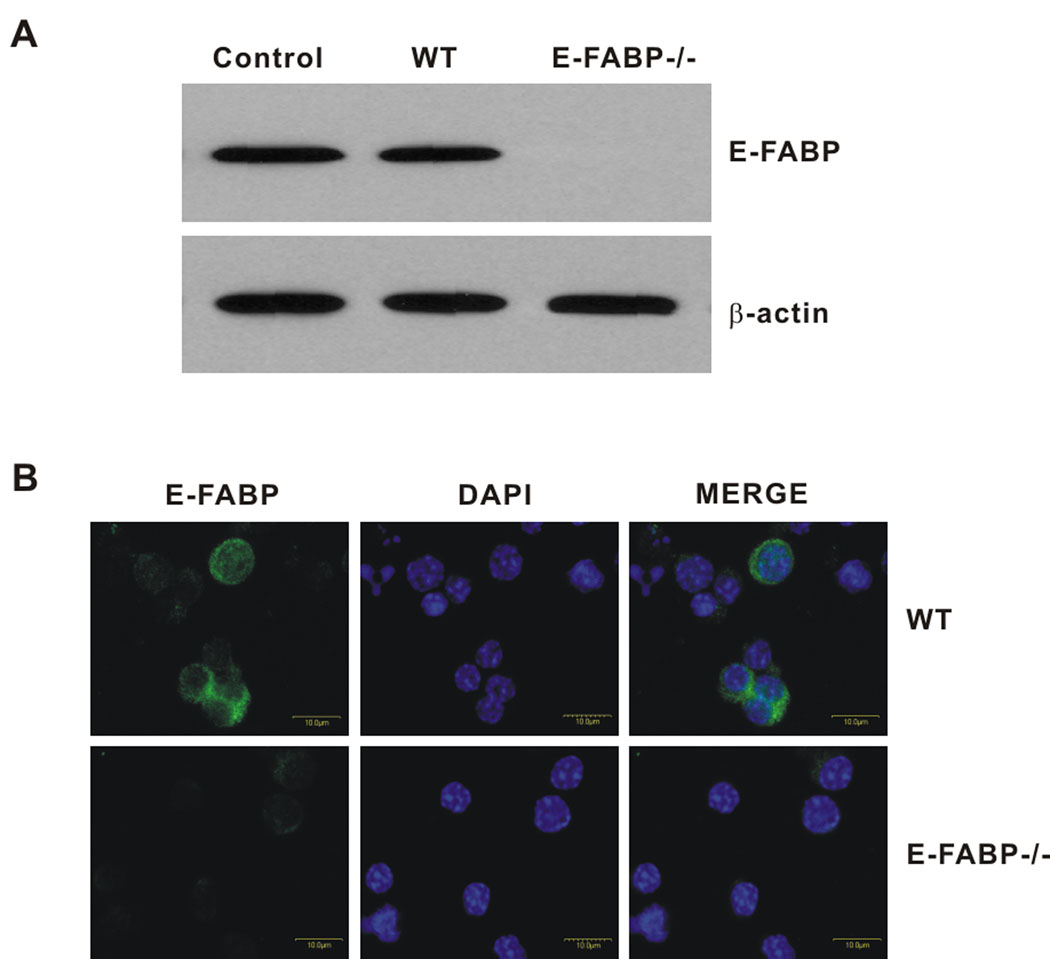
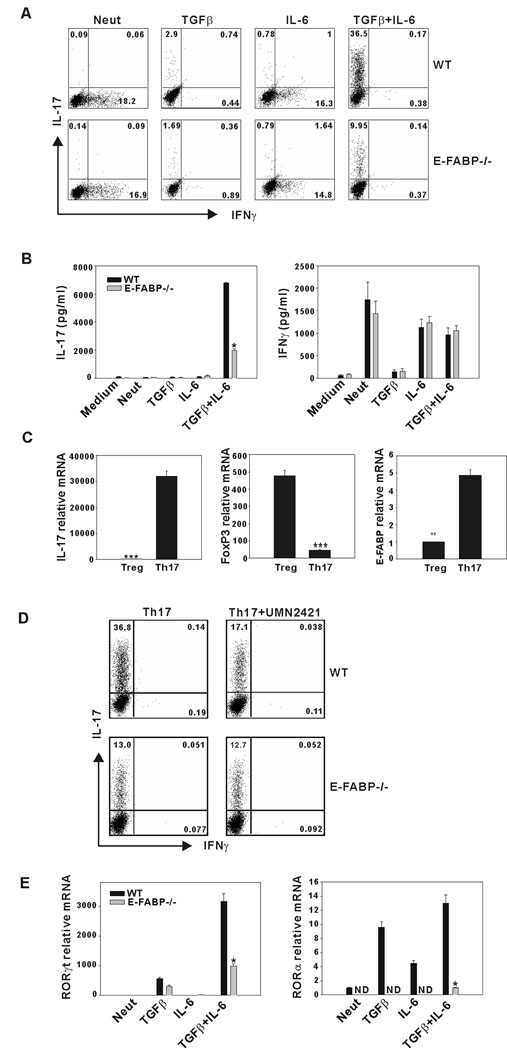
In the experiments shown in Fig. 1, a defect in Th17 differentiation during development of disease in vivo was revealed. To further address the mechanisms underlying this defect, we evaluated the influence of E-FABP deficiency on Th17 differentiation in vitro. CD4+ T cells were purified from spleens of WT and E-FABP-deficient mice and expression of E-FABP in WT CD4+ T cells was confirmed by Western blot analysis and confocal microscopy (Figs. 2A and B). Confocal microscopy indicated a cytoplasmic localization of E-FABP, as we have observed previously in macrophages and DC (unpublished data). Purified CD4+,CD62L+ naïve T cells isolated from spleens of WT and E-FABP-deficient mice were cultured under conditions shown to promote Th17 differentiation, i.e., with antigen receptor ligation in the presence of TGFβ and IL-6 (14). Following a 3 d culture period, IL-17 and IFNγ-expressing cells were identified by intracellular staining and flow cytometric analysis. Activation of T cells with anti-CD3 alone, or with IL-6 resulted in induction of modest populations of IFNγ producing cells by both WT and E-FABP-deficient T cells. As anticipated, addition of both IL-6 and TGFβ induced a significant population of Th17 cells (~ 37%) in the cultures of WT T cells. However, cultures of E-FABP-deficient T cells under these same conditions resulted in ~ 10% Th17 cells (Fig. 3A). Consistent with this observation, there was also a greatly reduced level of IL-17 in the culture supernatants of E-FABP-deficient CD4+ T cells subjected to Th17 polarizing conditions as compared to WT T cells, whereas there was no significant difference in IFNγ production (Fig. 3B).
Figure 2. Expression of E-FABP in CD4+ T cells.
A, E-FABP expression in naïve CD4+ T cells was evaluated by Western blot. Adipocyte lysate was used as a positive control for E-FABP expression. B, Confocal analysis of E-FABP expression in T cells. E-FABP is labeled in green (AlexaFluor 488) nuclei are DAPI stained (blue).
Figure 3. Development of Th17 cells in vitro is impaired in E-FABP-deficient mice.
A, Naive CD4+ T cells were stimulated with soluble anti-CD3 plus irradiated antigen-presenting cells. The percentages of IL-17+ and IFNγ+ cells in T cells from WT or E-FABP-deficient mice were determined after 3 d of culture via intracellular labeling flow cytometric analysis. B, CD4+ T cells were stimulated with plate-bound anti-CD3 plus soluble anti-CD28 and the indicated recombinant cytokines. Production of IL-17 (left panel) and IFNγ (right panel) were measured by ELISA after activation for 3 d (* p < 0.01). C, Naive CD4+ T cells purified from WT mice were cultured under Th17 or Treg conditions for 3 d. Relative expression of IL-17, Foxp3 and E-FABP was analyzed by real-time RT PCR. Expression was normalized to beta-actin and presented as the levels of mRNA expression relative to a non-stimulated T cell control (**p < 0.01, *** p < 0.001). D, Purified naïve CD4+ T cells were stimulated as in A, under Th17-favoring conditions with or without UMN2421 (10µM). IL-17 and IFNγ expression was assessed by intracellular labeling and flow cytometric analysis. E, CD4+ T cells were cultured as in B and levels of RORγt, (left panel) and RORα (right panel) mRNA expression were determined by quantitative real-time RT PCR. The results shown are representative of three independent experiments (* p < 0.01).
These data suggested that the levels of E-FABP expression in CD4+ T cells may reflect their polarized state, i.e., that polarization towards a Treg phenotype may be associated with a downregulation of E-FABP expression. To test this possibility, naïve WT CD4+ T cells were cultured under either Th17 or Treg promoting conditions for 3 d and analyzed for expression of IL-17, FoxP3 and E-FABP by real-time RT PCR (Fig. 3C). Expression of E-FABP was significantly reduced in T cells cultured under Treg-promoting conditions, a finding that supports a functional relationship between E-FABP expression and Th17 differentiation. For further confirmation of the role of E-FABP, we cultured naïve CD4+ T cell under Th17 polarizing conditions in the presence or absence of the FABP inhibitor UMN2421. UMN2421 was titrated at a range of 1–30 µM (not shown), with a concentration of 10 µM found to be optimal and subsequently used in the experiments shown. Addition of UMN2421 reduced IL-17 expression by WT CD4+ T cells to very near the levels of E-FABP-deficient T cells (a 54% reduction) whereas the inhibitor had no impact the percentage of IL-17+ cells in the E-FABP-deficient cultures (Fig. 3D) DMSO alone was used as a carrier control and had no effect on IL-17 expression by either group (not shown).
The reduced IL-17 production by E-FABP-deficient T cells in response to TGFβ + IL-6, and the ability of an E-FABP inhibitor to reduce IL-17 expression, indicated that E-FABP promotes the outcome(s) of TGFβ + IL-6 signaling that lead to IL-17 expression. These outcomes include induction of the retinoic acid receptor-related orphan receptors, RORγt and RORα, which have been shown to drive IL-17 expression (17). We cultured naïve CD4+ T cells isolated from E-FABP-deficient and WT as in the experiments presented in Fig. 3A–C, and evaluated the expression of RORγt and RORα. Expression of RORγt was very effectively induced by the combination of TGFβ and IL-6 in WT T cells, but only very modestly in T cells from E-FABP-deficient cells (Fig. 3E left panel). RORα expression was induced in WT cells via TGFβ and IL-6 alone, and more so by the combination of cytokines. However, overall, the expression of RORα was much less than RORγt. Expression of RORα by E-FABP-deficient T cells was only detectable when the cells were cultured with both TGFβ and IL-6 (Fig. 3E right panel).
E-FABP deficiency promotes RA-induced Foxp3 expression
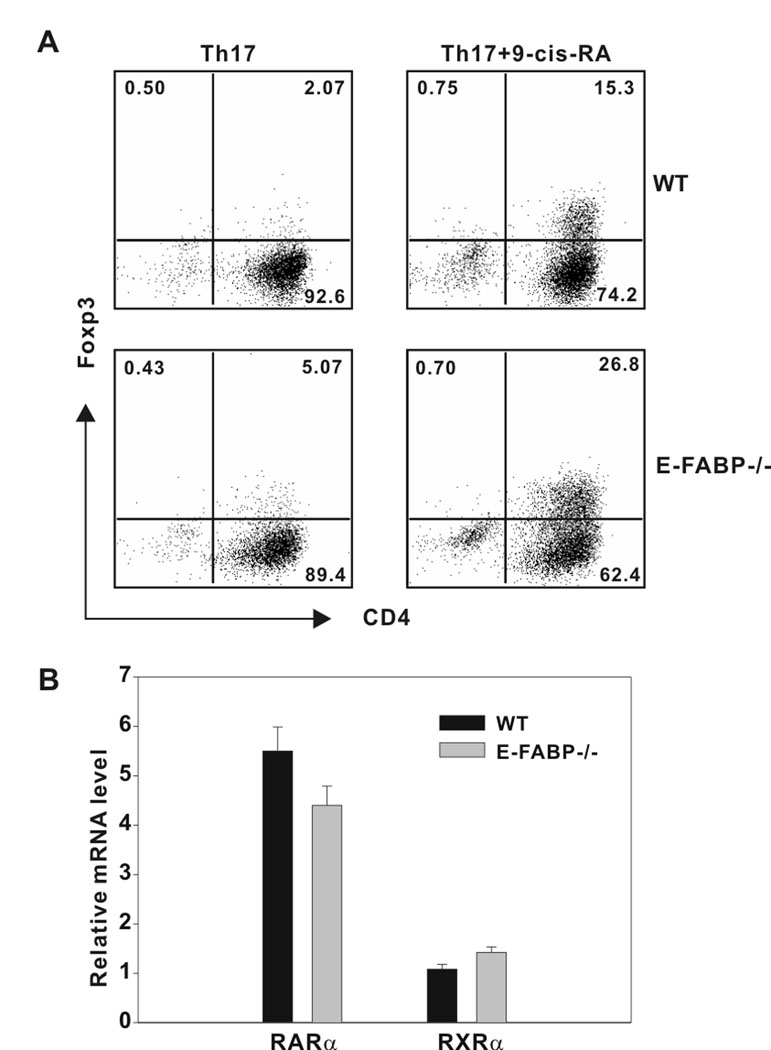
Since we had observed an enhancement of Tregs in vivo in E-FABP-deficient mice undergoing EAE, we performed in vitro experiments that represented the counterpoint to those presented in Fig. 3, in that naïve CD4+ T cells were driven in vitro towards a Treg phenotype. RAs have been shown to drive FoxP3 expression in response to TGFβ, and to counter Th17 culture conditions to favor FoxP3 expression (20). Although ATRA, through RARα, has been shown to influence Treg differentiation indirectly via its influence on an accompanying T cell population (34), we have found that 9-cis-RA acts directly on cultures of purified naïve CD4+ T cells. Naïve CD4+ cells isolated from WT or E-FABP-deficient mice were cultured under Th17 conditions in the presence or absence of 9-cis-RA. Following 3 d in culture FoxP3 expression was evaluated via intracellular labeling and flow cytometric analysis. As shown in Fig. 4A, treatment with 9-cis-RA resulted in the induction of ~ 15% FoxP3+ T cells in the WT cultures, whereas the same treatment resulted in ~ 27% FoxP3+ T cells in the E-FABP-deficient cultures. These data show a similar trend as was seen in our in vivo experiments (Fig.1C) and indicate that absence of E-FABP promotes Treg differentiation. Given the dramatic differences in the expression of the nuclear receptors RORγt and RORα in E-FABP-deficient versus wild-type cells, we considered the possibility that differences may also exist between the two genotypes in expression of RAR and RXRα, both of which can bind 9-cis-RA. As shown in Fig. 4B, no differences in expression of these nuclear receptors between WT and E-FABP-deficient T cells were observed.
Figure 4. E-FABP deficiency enhances FoxP3 expression in response to RA stimulation.
A, Naïve CD4+ T cells were cultured for 3 days under Th17 conditions in the presence or absence of 100 nm 9-cis-RA. T cells were recovered and re-stimulated with PMA plus ionomycin and analyzed for Foxp3 expression by intracellular labeling and flow cytometric analysis. B, Naïve CD4+ T cells isolated from WT and E-FABP-deficient mice were cultured with anti-CD3 plus anti-CD28 for 3 d. Relative expression of RARα and RXRα was analyzed by real-time PCR. Expression was normalized to β-actin and presented as the level of expression relative to a non-stimulated WT naïve T cell control. The data shown are representative of three independent experiments.
E-FABP-deficient CD4+ T cells display enhanced PPARγ expression and activity
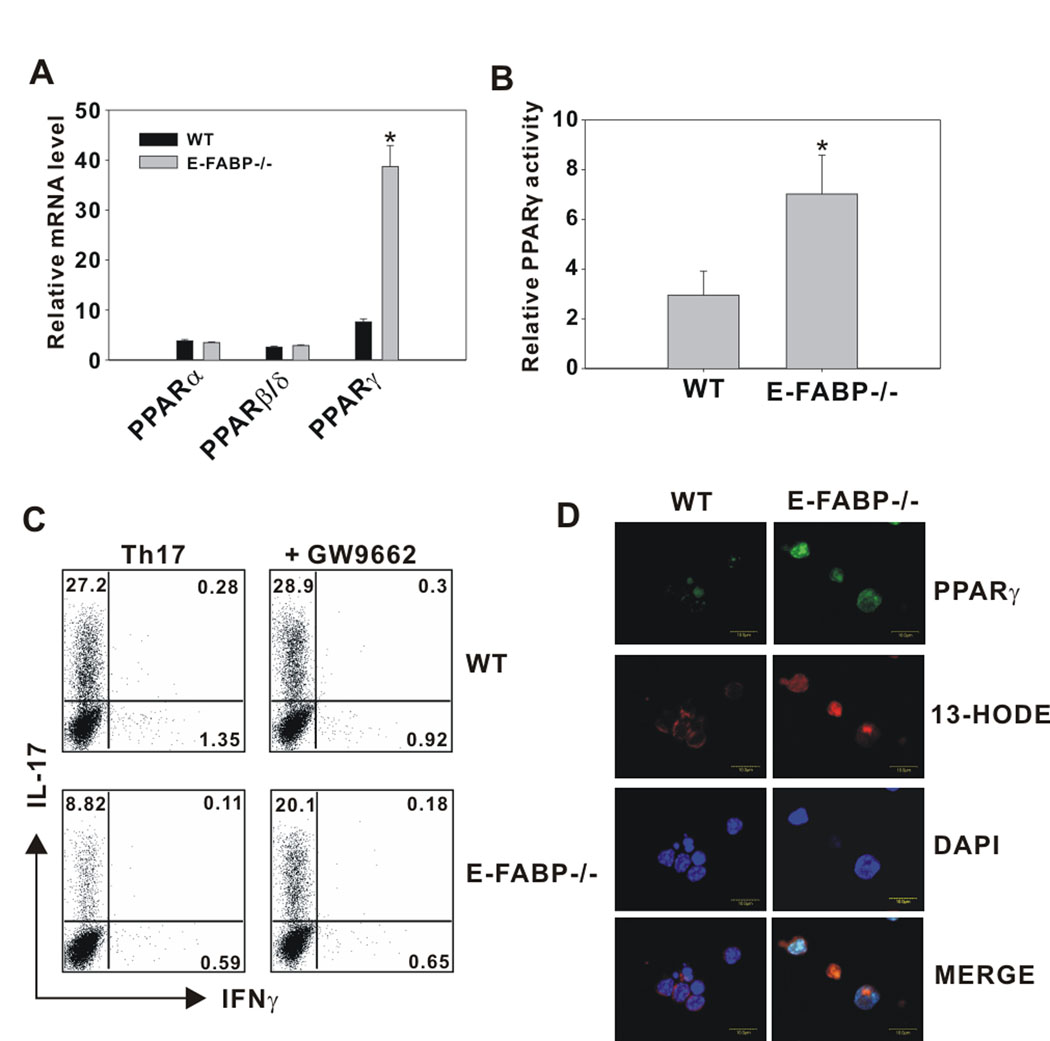
The data shown in Figure 4 suggest that E-FABP-deficiency enhances FoxP3 expression independent of RAR or RXR expression, and therefore this effect likely occurs via an enhancement of RA availability (i.e., trafficking to the nucleus) or function. For example, 9-cis-RA binding to RXR could provide support for the activity of other nuclear receptors, such as PPARs, through the formation of PPAR/RXR heterodimers. In our previous studies of FABP function in macrophages, we noted an increase in PPARγ activity in FABP-deficient macrophages (4). Thus, we considered PPARs as a candidates for the altered regulation of Th17/FoxP3 expression in E-FABP-deficient T cells. To address this possibility, we first analyzed the expression of PPARα, PPARβ/δ and PPARγ in WT and E-FABP-deficient CD4+ T cells and found that although equivalent levels of PPARα and PPARβ/δ were expressed by T cells of both genotypes, PPARγ expression was approximately 10-fold higher in E-FABP-deficient CD4+ T cells (Fig. 5A). As expected, the higher level of PPARγ in E-FABP-deficient T cells was accompanied by a significantly higher level of PPARγ activity (Fig. 5B). These results implicated PPARγ as a potential mediator of the functional phenotype associated with FABP-deficiency in CD4+ T cells. To address this directly, WT and E-FABP-deficient naïve CD4+ T cells were cultured in Th17-driving conditions in the presence or absence of the PPARγ antagonist GW9662. The defective Th17 production in E-FABP-deficient T cells was successfully restored to near WT levels by inhibition of PPARγ activity with GW9662 treatment, whereas WT T cells were unaffected (Fig. 5C). The role of PPARγ was also assessed by confocal microscopy in which the localization of a PPARγ/E-FABP ligand, 13-HODE, and its interaction with nuclear PPARγ was examined. The enhanced expression of PPARγ (labeled in green) in E-FABP-deficient CD4+ T cells as compared to WT CD4+ T cells was apparent (Fig. 5D). Localization of 13-HODE (labeled in red) was largely cytoplasmic in the WT cells, where it is capable of binding to cytoplasmic E-FABP. However, in the absence of E-FABP, 13-HODE freely enters the nucleus. Merging these two labels (Fig, 5D, bottom panel) reveals an enhanced interaction of 13-HODE with nuclear PPARγ in the E-FABP-deficient CD4+ T cells as compared with WT CD4+ T cells, in which 13-HODE remains cytoplasmic. Thus, thus combination of E-FABP deficiency and elevated PPARγ expression promote PPARγ ligand nuclear entry and receptor binding.
Figure 5. E-FABP deficiency is associated with elevated PPARγ expression and activity.
A, Naïve CD4+ T cells purified from WT and E-FABP-deficient mice were cultured with anti-CD3 plus anti-CD28 for 3 d. Relative expression of PPARα, PPARδ/β and PPARγ was analyzed by quantitative real-time PCR. Expression was normalized to β-actin and presented as the level of mRNA expression relative to a non-stimulated WT naïve T cell control. Data shown are representative of three similar experiments (* p< 0.05). B, In vitro cultured T cells from WT and E-FABP-deficient mice were stimulated for 3 d. PPARγ activity in nuclear lysates was measured by relative comparison with an unstimulated WT control (* p < 0.05). C, Naïve CD4+ T cells were cultured for 3 d under Th17 conditions in the presence or absence of the PPARγ antagonist GW9662 (5µM). T cells were recovered and re-stimulated with PMA plus ionomycin for 5 h and analyzed for IL-17 and IFNγ expression by intracellular labeling and flow cytometric analysis. D, In vitro cultured T cells from WT and E-FABP-deficient mice were incubated with 13-HODE-biotin and labeled with streptavidin (red, Alexa Fluor 5680) and FITC conjugated anti- PPARγ (green). Nuclei were stained with DAPI (blue).
PPARγ inhibits the IL-21/ROR/IL-17 axis via inhibition of IL-6-mediated STAT3 activity in E-FABP-deficient CD4+ T cells
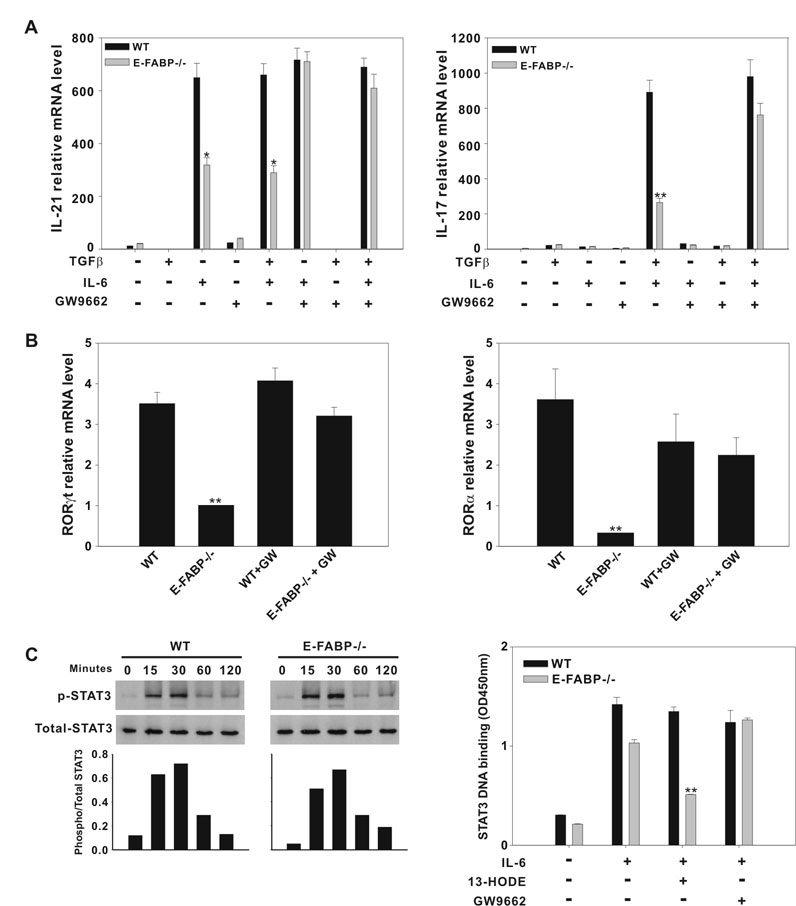
Expression of IL-17 by T cells subjected to Th17 polarizing conditions entails IL-6 induction of IL-21, which, in turn, induces expression of RORγt and RORα (15) (17). The activities of both IL-6 and IL-21 are STAT3-dependent (15). PPARγ has been shown to down-regulate the expression of proinflammatory cytokines by antagonizing the function of transcription factors such as NF-κB, AP-1, and STATs (35). PPARγ interference with STAT3 activity has been described in a variety of systems and has been identified as a mechanism of PPARγ inhibition of IL-6-mediated cell signaling (36). Thus, we speculated that the reduced RORγt, RORα and IL-17 expression displayed by E-FABP-deficient CD4+ T cells is mediated PPARγ, possibly via inhibition of STAT3 activity. To test this hypothesis, naïve CD4+ T cells isolated from WT and E-FABP-deficient mice were stimulated with anti-CD3 and anti-CD28 in the presence of Th17-driving cytokines with or without the addition of the PPARγ antagonist GW9662. We found that production of IL-21 by E-FABP-deficient CD4+ T cells, induced by IL-6, was significantly reduced as compared to WT CD4+ T cells, and that this deficiency was restored completely by addition of GW9662 (Fig. 6A, left panel). Likewise, GW9662 restored the IL-17 production by E-FABP-deficient CD4+ T cells to WT levels (Fig. 6A, right panel). We also evaluated the effect of PPARγ antagonism on RORγt and RORα expression induced under Th17 polarizing conditions, with similar results. Both in the case of RORγt (Fig. 6B, left panel) and RORα (Fig. 6B, right panel), addition of GW9662 brought the level of expression of these nuclear receptors by E-FABP-deficient CD4+ T cells to near the levels expressed by WT CD4+ T cells. Interestingly, GW9662 reduced expression of RORα by WT CD4+ T cells, although this effect was not statistically significant.
Figure 6. The reduced Th17 differentiation exhibited by E-FABP-deficient T cells is mediated by PPARγ.
A, WT and E-FABP-deficient naïve T cells were stimulated with anti-CD3 and anti-CD28 in the presence of combinations of cytokines +/− GW9662 (GW), as indicated. Data shown are representative of three similar experiments (* p < 0.05, ** p < 0.01). B, Expression of RORγt (left panel) and RORα (right panel) mRNA by naïve T cells, cultured as under Th17 conditions +/− GW9662 , was analyzed by real-time RT PCR (** p < 0.01). C, Left panel, naïve CD4+ T cells from WT and E-FABP were purified and stimulated with IL-6 at the indicated time points on plate-bound anti-CD3 and soluble anti-CD28. Cell lysates were analyzed by Western blot using Abs specific for the phosphorylated form of STAT3, or total STAT3, as indicated. The histogram represents ratio of phosphorylated STAT3 to total STAT3 band density. Right panel, CD4+ T cells from WT and E-FABP-deficient mice were treated without or with 13-HODE +/− GW9662 for 3h followed by a 20 min stimulation with IL-6. STAT3 DNA binding was assayed as described in Material and Methods. The data are presented as mean ± standard deviation of triplicate samples (** p < 0.01).
These data indicated that PPARγ was responsible for the reduced IL-21, RORγt, RORα and IL-17 expression exhibited by E-FABP-deficient CD4+ T cells. Given the known influence of PPARγ on STAT3 signaling, we evaluated STAT3 activation following IL-6 stimulation in E-FABP-deficient and WT CD4+ T cells. We found by Western blot analysis that IL-6-induced STAT3 phosphorylation in E-FABP-deficient and WT CD4+ T cells was indistinguishable (Fig. 6C, left panel), suggesting that any impact of PPARγ on STAT3 signaling occurred downstream of STAT3 phosphorylation. In other systems, PPARγ has been shown to disrupt STAT3 DNA binding (36). Therefore, we evaluated IL-6-induced STAT3 DNA binding activity in E-FABP-deficient and WT CD4+ T cells in the presence and absence of GW9662. As shown in Figure 6C, right panel, E-FABP-deficient T cells displayed reduced STAT3 DNA binding in response IL-6 stimulation. Although this difference did not quite achieve statistical significance (p = 0.058 in the experiment shown) this result was consistent. Importantly, addition of the PPARγ agonist 13-HODE further reduced STAT3 DNA binding in E-FABP-deficient CD4+ T cells to levels to less than 50% that of WT, whereas addition of 13-HODE had no impact on STAT3 DNA binding in WT CD4+ T cells. Addition of GW9662 to IL-6 stimulated CD4+ T cells (in the absence of 13-HODE) restored the levels of STAT3 DNA binding in E-FABP-deficient CD4+ T cell to the levels displayed by WT CD4+ T cells. These data demonstrate that, although STAT3 phosphorylation and nuclear translocation occur in response to IL-6 in E-FABP-deficient T cells, STAT3 DNA binding is impaired and PPARγ is indicated as a mediator of this impairment.
Discussion
Recent work has revealed a role of the FABPs, A-FABP and E-FABP in potentiating inflammatory responses (4–6). We demonstrated previously that mice deficient for A-FABP, E-FABP, or both (A,E-FABP-deficient) have reduced macrophage and DC inflammatory function which results in impaired Th1 generation and protection from development of EAE (6). In this prior study, we found that purified T cells derived from A,E-FABP-deficient mice had equivalent proliferative responses to anti-CD3 stimulation in vitro, as well as equivalent IFNγ production. This indicated that there were no intrinsic defects in the T cells, themselves, in terms of Th1 generation, and that the poor Th1 generation in vivo and during antigen recall responses in vitro, was attributed to the faulty APC activity associated with FABP-deficiency. However, we also observed greatly reduced levels of IL-17 present in the CNS of A,E-FABP-deficient mice as compared to WT mice during the course of disease. In the current study, we focused exclusively on E-FABP-deficient mice to investigate the possibility that E-FABP, expressed in T cells, may influence Th17 differentiation and in this process we have made the novel discovery that, indeed, E-FABP contributes to the Th17/Treg differentiation program.
In the examination of CD4+ T cells in tissues of mice undergoing EAE, we found reduced levels of CD4+ T cells expressing IL-17 in the E-FABP-deficient mice, which included reduced levels of the CD4+ T cell population that expressed both IL-17 and IFNγ (Fig.1B), which has been characterized recently as a significant population arising during active EAE (32). Given the reports of reciprocal regulation of IL-17 and FoxP3 expression (12), we also examined expression of FoxP3 by CD4+ T cells from tissues of mice undergoing EAE, and confirmed that the reduced IL-17 production by T cells from E-FABP-deficient mice is accompanied by enhanced FoxP3 expression (Fig. 1C). These results indicate that absence of E-FABP in CD4+ T cells shifts the program of regulation of IL-17 and FoxP3 gene expression, favoring the Treg phenotype, a phenomenon that was corroborated by experiments in which Th17 and Treg phenotypes were driven in vitro (Fig. 3 and Fig. 4). The ability of an FABP inhibitor to mimic the E-FABP-deficient state (Fig. 3D) promotes the concept that functional E-FABP, i.e., E-FABP capable of lipid ligand binding, influences this gene regulation program. Also of importance is the finding that levels of E-FABP expression in WT CD4+ T cells diminish with differentiation to the Treg phenotype (Fig.3C). If the presence of E-FABP promotes IL-17 expression, as is indicated by the data, then one would expect that signals that promote Treg differentiation would reduce E-FABP expression. Currently, studies of the regulation of E-FABP expression are very limited. The data presented herein suggest that mediators that promote anti-inflammatory responses and Treg differentiation are likely to suppress E-FABP expression.
Interestingly, accompanying the reduced propensity for Th17 differentiation by E-FABP-deficient CD4+ T cells was the enhanced propensity for Treg differentiation induced by addition of 9-cis-RA (Fig. 4A). This difference was not associated with differences in expression of the nuclear receptors capable of binding 9-cis-RA, i.e., RAR and RXR family members (Fig. 4B), suggesting that E-FABP affects the interaction of 9-cis-RA with its cognate receptors, or the ability of ligand-bound receptors to interact with their heterodimeric partners or gene targets. This phenomenon was reminiscent of our studies of the relationship between FABP expression and PPARγ function in macrophages (4). Our data suggested a scenario by which FABPs restrict PPARγ ligand accessibility to PPARγ via binding and maintaining them in the cytoplasmic compartment. Given that E-FABP has the capacity to bind both retinoids as well as PPAR ligands, we considered the possibility that a similar scenario may exist in CD4+ T cells. Both 9-cis-RA and PPARγ ligands have been shown to be protective against EAE, and the combination of both provides additive protection from disease (37,38). The amelioration of EAE by PPARγ agonists and by 9-cis-RA has been largely attributed to their ability to inhibit production of Th1 promoting cytokines by antigen presenting cells, and to reduce inflammatory function of glial cells (34,37,39). However, recent studies have also provided evidence for direct effects of retinoids, including 9-cis-RA, on T cell cytokine production (40,41) and a direct influence of PPARγ expressed by Treg on their functional capacity (21). Interestingly, in our assessment of a potential influence of PPARs in E-FABP-deficient CD4+ T cells, we saw enhanced expression of PPARγ, accompanied by enhanced activity (Fig. 5A), whereas the enhanced effect of 9-cis-RA was independent of RAR or RXR receptor expression (Fig. 4B).
The enhanced PPARγ expression/activity was a likely candidate as a mediator of the reduced IL-17 expression by E-FABP-deficient CD4+T cells based on the previous publications demonstrating an inhibitory effect of PPARγ on STAT3 activity (36,42). Indeed, we found that antagonism of PPARγ activity effectively reversed the reduced IL-21, RORγt, RORα and IL-17 expression exhibited by E-FABP-deficient T cells (Fig. 6A and B), which confirmed the role of PPARγ in suppression of Th17 differentiation. We also present evidence that this effect of PPARγ was due to inhibition of STAT3 DNA binding, a phenomenon that has been previously reported (36). Given that RAR and RXR expression are equivalent between E-FABP-deficient and WT CD4+ T cells, we believe that the enhanced effect of 9-cis-RA on FoxP3 expression is likely due to the provision of RXR heterodimeric partnering to PPARγ, which is enhanced in E-FABP-deficient cells. This concept is supported by preliminary data demonstrating that GW9662 reduces FoxP3 expression in E-FABP-deficient CD4+ T cells (not shown).
Still under investigation is the mechanism by which PPARγ expression is elevated in E-FABP-deficient T cells. PPARγ expression in other cell types (e.g., adipocytes) has shown to be influenced by cholesterol levels and dietary fat intake (43). Thus, it is likely that dysregulated lipid trafficking in the absence of E-FABP may impact lipid availability in a manner that acts to boost PPARγ expression. In addition, the studies performed herein used exclusively female mice, due to their enhanced susceptibility to development of disease in the EAE model. Preliminary data suggest that the reduced IL-17 associated with E-FABP-deficiency in less pronounced in male mice, indicative of a gender influence of E-FABP. Previous studies have found gender differences in PPAR family member expression (44) which we will explore as a possible contributor to this observed phenomenon. In summary, we have made the novel observation that expression of the lipid chaperone E-FABP in CD4+ T cells promotes IL-17 expression and that absent or reduced E-FABP expression is associated with enhanced expression of FoxP3. E-FABP expression in CD4+ T cells is negatively correlated with expression of PPARγ, which is capable of suppressing IL-6 activation of the STAT3/IL-21/ROR/IL-17 pathway. The finding that the E-FABP expression modulates the Th17/Treg balance suggests that mediators and mechanisms that regulate E-FABP expression or function may serve as targets for manipulation of this balance, thereby regulating outcomes of autoimmune and inflammatory disease.
Acknowledgements
The authors wish to acknowledge the excellent technical assistance of Lihua Zhang and Meena Vanchinathan.
Footnotes
This work was supported by National Institutes of Health grants AI048850 (to J.S.), and DK053189 (to D.A.B.), National Multiple Sclerosis Society Grant RG 3374 (to J.S.), by an American Heart Association Predoctoral Fellowship (to J.M.R.), and in part, by the Commonwealth of Kentucky Research Challenge Trust Fund (to J.S. and R.D.S.).
Abbreviations used in this paper: FABP, fatty acid-binding protein; PPAR, peroxisome proliferator-activating receptor; Treg, regulatory T; ATRA, all-trans-retinoic acid; 9-cis-RA, 9- cis-retinoic acid; ROR, retinoic acid-related orphan receptor; RAR, retinoic acid receptor; RXR, retinoid X receptor; EAE, experimental autoimmune encephalomyelitis; MOG, myelin oligodendrocyte glycoprotein.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Hertzel AV, Bernlohr DA. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol. Metab. 2000;11:175–180. doi: 10.1016/s1043-2760(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 2.Smith AJ, Thompson BR, Sanders MA, Bernlohr DA. Interaction of the adipocyte fatty acid-binding protein with the hormone-sensitive lipase: regulation by fatty acids and phosphorylation. J. Biol. Chem. 2007;282:32424–32432. doi: 10.1074/jbc.M703730200. [DOI] [PubMed] [Google Scholar]
- 3.Makowski L, Hotamisligil GS. The role of fatty acid binding proteins in metabolic syndrome and atherosclerosis. Curr. Opin. Lipidol. 2005;16:543–548. doi: 10.1097/01.mol.0000180166.08196.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J. Biol. Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolph MS, Young TR, Shum BO, Gorgun CZ, Schmitz-Peiffer C, Ramshaw IA, Hotamisligil GS, Mackay CR. Regulation of dendritic cell function and T cell priming by the fatty acid-binding protein AP2. J. Immunol. 2006;177:7794–7801. doi: 10.4049/jimmunol.177.11.7794. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds JM, Liu Q, Brittingham KC, Liu Y, Gruenthal M, Gorgun CZ, Hotamisligil GS, Stout RD, Suttles J. Deficiency of fatty acid-binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:313–321. doi: 10.4049/jimmunol.179.1.313. [DOI] [PubMed] [Google Scholar]
- 7.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12-and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat. Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox CA, Shi G, Yin H, Vistica BP, Wawrousek EF, Chan CC, Gery I. Both Th1 and Th17 are immunopathogenic but differ in other key biological activities. J. Immunol. 2008;180:7414–7422. doi: 10.4049/jimmunol.180.11.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor RA, Anderton SM. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J. Neuroimmunol. 2008;193:1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 12.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangan PR, Harrington LE, O′Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 14.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 16.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 21.Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptor gamma is required for regulatory CD4+ T cell-mediated protection against colitis. J. Immunol. 2007;178:2940–2949. doi: 10.4049/jimmunol.178.5.2940. [DOI] [PubMed] [Google Scholar]
- 22.Kane CD, Coe NR, Vanlandingham B, Krieg P, Bernlohr DA. Expression, purification, and ligand-binding analysis of recombinant keratinocyte lipid-binding protein (MAL-1), an intracellular lipid-binding found overexpressed in neoplastic skin cells. Biochemistry. 1996;35:2894–2900. doi: 10.1021/bi952476e. [DOI] [PubMed] [Google Scholar]
- 23.Simpson MA, LiCata VJ, Ribarik CN, Bernlohr DA. Biochemical and biophysical analysis of the intracellular lipid binding proteins of adipocytes. Mol. Cell Biochem. 1999;192:33–40. [PubMed] [Google Scholar]
- 24.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helledie T, Antonius M, Sorensen RV, Hertzel AV, Bernlohr DA, Kolvraa S, Kristiansen K, Mandrup S. Lipid-binding proteins modulate ligand-dependent trans-activation by peroxisome proliferator-activated receptors and localize to the nucleus as well as the cytoplasm. J. Lipid Res. 2000;41:1740–1751. [PubMed] [Google Scholar]
- 26.Helledie T, Jorgensen C, Antonius M, Krogsdam AM, Kratchmarova I, Kristiansen K, Mandrup S. Role of adipocyte lipid-binding protein (ALBP) and acyl-coA binding protein (ACBP) in PPAR-mediated transactivation. Mol. Cell Biochem. 2002;239:157–164. [PubMed] [Google Scholar]
- 27.Maeda K, Uysal KT, Makowski L, Gorgun CZ, Atsumi G, Parker RA, Bruning J, Hertzel AV, Bernlohr DA, Hotamisligil GS. Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes. 2003;52:300–307. doi: 10.2337/diabetes.52.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das SJ, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 30.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hertzel AV, naars-Eiden A, Bernlohr DA. Increased lipolysis in transgenic animals overexpressing the epithelial fatty acid binding protein in adipose cells. J. Lipid Res. 2002;43:2105–2111. doi: 10.1194/jlr.m200227-jlr200. [DOI] [PubMed] [Google Scholar]
- 32.Suryani S, Sutton I. An interferon-gamma-producing Th1 subset is the major source of IL-17 in experimental autoimmune encephalitis. J. Neuroimmunol. 2007;183:96–103. doi: 10.1016/j.jneuroim.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 33.costa-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Drew PD. 9-Cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes. J. Neuroimmunol. 2006;171:135–144. doi: 10.1016/j.jneuroim.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 36.Wang LH, Yang XY, Zhang X, Huang J, Hou J, Li J, Xiong H, Mihalic K, Zhu H, Xiao W, Farrar WL. Transcriptional inactivation of STAT3 by PPARgamma suppresses IL-6-responsive multiple myeloma cells. Immunity. 2004;20:205–218. doi: 10.1016/s1074-7613(04)00030-5. [DOI] [PubMed] [Google Scholar]
- 37.Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- 38.Diab A, Hussain RZ, Lovett-Racke AE, Chavis JA, Drew PD, Racke MK. Ligands for the peroxisome proliferator-activated receptor-gamma and the retinoid X receptor exert additive anti-inflammatory effects on experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2004;148:116–126. doi: 10.1016/j.jneuroim.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Natarajan C, Bright JJ. Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1 differentiation. Genes Immun. 2002;3:59–70. doi: 10.1038/sj.gene.6363832. [DOI] [PubMed] [Google Scholar]
- 40.Dawson HD, Collins G, Pyle R, Key M, Taub DD. The Retinoic Acid Receptor-alpha mediates human T-cell activation and Th2 cytokine and chemokine production. BMC. Immunol. 2008;9:16. doi: 10.1186/1471-2172-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dawson HD, Collins G, Pyle R, Key M, Weeraratna A, ep-Dixit V, Nadal CN, Taub DD. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC. Immunol. 2006;7:27. doi: 10.1186/1471-2172-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang LH, Yang XY, Zhang X, Farrar WL. Nuclear receptors as negative modulators of STAT3 in multiple myeloma. Cell Cycle. 2005;4:242–245. [PubMed] [Google Scholar]
- 43.Vidal-Puig A, Jimenez-Linan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J. Clin. Invest. 1996;97:2553–2561. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, Crowell A, Loh J, Oksenberg J, Steinman L. Peroxisome proliferator-activated receptor (PPAR) alpha expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J. Exp. Med. 2007;204:321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]