Abstract
Background
Increased intra-subject response time standard deviations (RT-SD) discriminate children with Attention-Deficit/Hyperactivity Disorder (ADHD) from healthy controls. RT-SD is averaged over time, thus it does not provide information about the temporal structure of response time variability. We previously hypothesized that such increased variability may be related to slow spontaneous fluctuations in brain activity occurring with periods between 15s and 40s. Here, we investigated whether these slow response time fluctuations add unique differentiating information beyond the global increase in RT-SD.
Methods
We recorded RT at 3s intervals for 15 minutes during an Eriksen flanker task for 29 children with ADHD and 26 age-matched typically developing controls (TDC) (mean ages 12.5 ± 2.4 and 11.6 ± 2.5; 26 and 12 boys, respectively). The primary outcome was the magnitude of the spectral component in the frequency range between 0.027 and 0.073 Hz measured with continuous Morlet wavelet transform.
Results
The magnitude of the low frequency fluctuation was greater for children with ADHD compared to TDC (p=0.02, d= 0.69). After modeling ADHD diagnosis as a function of RT-SD, adding this specific frequency range significantly improved the model fit (p=0.03; odds ratio= 2.58).
Conclusions
Fluctuations in low frequency response time variability predict the diagnosis of ADHD beyond the effect associated with global differences in variability. Future studies will examine whether such spectrally specific fluctuations in behavioral responses are linked to intrinsic regional cerebral hemodynamic oscillations which occur at similar frequencies.
Keywords: ADHD, inattention, response time standard deviation, variability, multisecond oscillations, children
Introduction
The diagnosis of Attention-Deficit/Hyperactivity Disorder (ADHD) is based primarily on historical reports of symptoms, usually from parents and educators, which although reliable, are necessarily subjective (1). Neuropsychological tests designed to assess executive functions have been studied extensively in attempts to discover objective markers to substantiate the diagnosis and constrain models of pathophysiology. Metaanalyses confirm that most such measures differentiate ADHD from healthy comparison groups, but with only moderate effect sizes (2, 3).
The observation that individuals with ADHD are consistently inconsistent, reflected experimentally as increased intra-subject variability in response time (RT-ISV), led to the suggestion that RT-ISV should be considered an objective index of ADHD (4, 5). Klein et al. (6) examined four commonly used tasks and found that response time standard deviation (RT-SD) best discriminated children with ADHD from healthy controls, with substantially larger effect sizes than mean response time, directional errors, omission, or inhibitory control measures.
Increased RT-ISV has been repeatedly documented in ADHD (4, 6), but trial-to-trial variations in performance, which are also found in aging, dementia, and traumatic brain injury (7), are generally assumed to reflect primarily stochastic factors. An open question is whether additional systematic processes underlie elevated RT-ISV. Our interest in the temporal characteristics of RT-ISV stemmed from two converging lines of evidence. First, basal ganglia neurons recorded in the awake locally anesthetized rat exhibit intrinsic rhythmic activity in the range of 0.028-0.05 Hz (8). These fluctuations are selectively modulated by dopaminergic medications which are the first drugs of choice in the treatment of ADHD (8). Second, intrinsic brain hemodynamic oscillations of the putative default-mode network (9, 10, 11, 12), which has been posited to underlie attentional lapses characteristic of ADHD (13, 14, 15), occur at similarly low frequencies. These observations led us to hypothesize that fluctuations in RT should exhibit an oscillatory pattern in the low frequency range (i.e., one cycle occurring ∼ every 15-40s) reflecting “a failure to fully and effectively transition from a baseline default-mode to an active processing mode during performance of cognitive tasks” (p.2; 14). We reasoned that these fluctuations would be significantly more prominent in individuals with ADHD than in healthy comparison subjects (4). To investigate such a hypothesis, frequency analyses which break up a function into the frequencies that compose it are useful methods to examine fluctuations in RT-ISV. Applying such approaches in a secondary analysis of previously collected Eriksen-flanker task data (16, 17, 18), we found that the power of the frequency band (i.e., a range of frequencies with an upper and lower limit) centered at 0.05 Hz was significantly higher (p=0.01) in 24 boys with ADHD compared to 18 matched controls (4). However, those data had been collected discontinuously in six 180 s blocks, limiting the observable lower range of frequencies. In the present study, we prospectively administered the same task continuously for 900 s to quantify RT-ISV in a broader range of lower frequencies.
Examining the frequency characteristics of RT-ISV could provide a means for linking this cognitive measure to underlying neurophysiological processes (4, 19). In order to select externally validated frequency bands, we made use of the observation that the frequency ranges of neuronal oscillations (i.e., frequency bands) and their center frequencies “form a linear progression on the natural logarithmic scale.” (p. 1926; 19) Signals at frequency bands so defined have been hypothesized to be generated by distinct, independent mechanisms (20). The frequency band targeted here (0.027–0.073 Hz), corresponds to the slowest of the 10 putative oscillation bands defined by Penttonen and Buzsaki (20). We selected it based on our prior data (4) and the modal frequency of brain resting state networks (21, 22, 23). This study was designed to determine whether such multisecond oscillations in RT-ISV differentiate groups of children with ADHD from typically developing control children, beyond the spectrally non-specific effects of RT-ISV which are easily quantified by RT-SD (6). To address the spectral specificity of our findings, we also examined the neighboring slow frequency bands (20).
Methods
Thirty-three children with ADHD (27 boys) and 26 typically developing comparison children (TDC; 12 boys) between the ages of 7.5 and 16.4 years participated in this study. Children with ADHD were recruited through referrals from the NYU Child Study Center Child & Family Associates, parent support groups, newsletters, flyers, and web/newspaper ads. We recruited TDC from the local community through flyers/ads, and word of mouth. Families received $60 for participating in the study. Written informed consent was obtained from parents and assent from children, as approved by the institutional review board. Estimated full-scale IQ ≥ 80 and absence of known neurological or chronic medical diseases were required of all subjects. DSM-IV diagnosis of ADHD (1) was based upon parent interviews using the Schedule of Affective Disorders and Schizophrenia for Children — Present and Lifetime Version (K-SADS-PL) (24). Diagnosis of psychotic disorders, major depressive disorder, conduct disorder, tic disorders, and pervasive developmental disorders were exclusionary. Children were excluded if they were being treated with psychoactive medications except for psychostimulants which were withheld for at least 24 hours prior to testing. Inclusion as a TDC required T-scores below 60 on all four Conners’ Parent Rating Scale-Revised: Long Version ADHD-summary scales (25, 26).
Symptom severities were obtained from the Conners’ Parent Rating Scale-Revised: Long Version, the Child Behavior Checklist (27), and the Conners’ Teacher Rating Scale-Revised: Long Version (25, 26). Parents provided demographic information and socio-economic status was estimated using the Hollingshead Index of Social Position (28). The Wechsler Abbreviated Scale of Intelligence (29) provided estimates of IQ. The Wechsler Individual Achievement Test Second Edition (30) provided standardized measures of Word Reading, Numerical Operations, and Spelling.
Experimental Procedure
Participants completed the same arrow version of the Eriksen Flanker task used in Scheres et al. (16) except that stimuli were presented continuously for 930 s. Task stimuli consisted of a horizontal array composed of a target central arrow with four flanking arrows (two per side) pointing either to the same direction (congruent trials) or the opposite direction (incongruent trials) as the center arrow (e.g., >>>>> and <<><<, respectively) or, on neutral trials, four rectangles (e.g., □□>□□). Within each trial, a warning fixation cross (500ms) was followed by a stimulus (1000ms), then a blank screen (1500ms). Inter-trial intervals were constant at 3000ms. Trial types occurred with equal frequency, as did the direction of the target arrow (right or left) in a block-randomized order within each of five 60-trial blocks. To minimize transition effects, the 15-minute task was preceded by 10 trials (30s) that were discarded from analysis. All participants received the same pseudo-randomized sequence. Children were instructed to press the right or left button corresponding to the direction of the target arrow as quickly and accurately as possible. Children completed at least one 90s-practice session which could be repeated until criterion of 80% correct responses was attained (six children with ADHD and one TDC required a second practice session).
Data Analyses
Time Domain
Responses below the minimal physiological response time of 100ms (31) were discarded. For each subject, we then computed the mean RT and the RT-SD over all remaining responses. Number of omissions and directional errors (no button pressed and incorrect button pressed, respectively) were also computed. Subjects with omissions exceeding 15% of trials (45/300) were excluded from further analyses, as they were considered insufficiently engaged in the task. Four children with ADHD (ages 7.6 to 9.5 years, three girls) were excluded for omission rates of 21% to 41%. A 2-tailed Mann-Whitney test indicated that these children were significantly younger (8.2 ± 0.8 vs. 12.4 ± 2.5 years; p=.008) and more hyperactive (e.g., ADHD Index T-score 77 ± 5 vs. 69 ± 6; p=.03) than the remaining children with ADHD.
Data Preparation for Frequency Domain Analyses
First, a displaced logarithmic transformation, i.e., f(x)=ln(x-a) with displacement parameter a=100, was applied to each subject’s observed RTs using the S-PLUS/R function (32) “logtrans,” to obtain approximate normality of the distribution. Second, since trial type, a factor with six levels (three stimulus types and two directions), impacts mean RTs, we regressed it out and used the timeseries of residuals in our analyses. Third, to maintain the temporal structure of the timeseries, missing and impossible responses ≤100 ms were interpolated by averaging the two immediate neighboring trial responses.
Frequency Domain
We examined a frequency interval ranging from 0.0052 Hz to 0.17 Hz as appropriate with our 900 s task duration and 3000 ms inter-trial interval (see Supplementary Text). Within this frequency range we identified four bands based on the approach of Penttonen and Buzsaki (20). Following their formula, we extended the oscillation classes down to Slow-6. Accordingly, we selected Slow-6 (0.0052 Hz – 0.010 Hz, centered at 0.006 Hz [period 101-192s]), Slow-5 (0.010 – 0.027 Hz, centered at 0.016 Hz [37-101s]), Slow-4 (0.027 – 0.073 Hz, centered at 0.044 Hz [14-37s]), and Slow-3 (0.073 – 0.17 Hz, centered at 0.12 Hz [6-14s]); (see Supplementary Table 1).
Spectral Measures
We were interested in the contribution of each frequency component as a proportion of total variance. As such, for each frequency band we computed the normalized spectral density (see Supplementary Text). Normalized spectral density is usually estimated with the periodogram method via fast Fourier transform (FFT) (33). An alternative method for decomposing variability into oscillatory components, which avoids the stationarity assumption, is the time-frequency representation of timeseries using the continuous wavelet transform (CWT) (33, 34, 35) (see Supplementary Text). We applied the CWT (35) using Morlet wavelets (half-length 25), implemented in the Matlab (The MathWorks, Natick, MA) Time-Frequency Toolbox (http://tftb.nongnu.org), to each subject’s normalized timeseries (RT timeseries divided by RT-SD). Following other authors (e.g., Humeau et al.(36)), we averaged the scalogram at a given frequency band over time to represent the average relative energy of the timeseries within this frequency band over the whole task interval. To examine the effect of time on task, we computed the average relative energy separately for the five 180s-blocks, each of which included equal proportions of all trial types. We also estimated the spectral density function with FFT using a 15-point Hamming window on the residual data after resampling to create timeseries of 1024 evenly spaced data points. The area under the curve of the normalized spectral density within each target frequency was the resulting outcome variable. In this paper, the term relative spectrum refers to both the relative energy measured by CWT and the normalized spectral density measured by FFT.
Statistical Analyses
Group Characteristics
We compared the two groups on demographic and clinical characteristics using chi-square tests for sex, ethnic group, and socioeconomic class and analysis of covariance (ANCOVA) adjusting for sex for continuous clinical measures.
Task Group Comparisons
The comparison between ADHD and TDC groups with respect to the time and frequency domain measures averaged over the 900s task period was based on ANCOVA. Effect of time on task was examined based on time and frequency domain measures computed over the five 180 s blocks using repeated measures ANCOVA. Age was included as a covariate because it showed a significantly linear negative relation with RT-SD within our sample age-range as expected (37, 38). Sex was included because of our group differences in sex distribution and a prior report of sex differences in RT-SD (39). We report F-tests, p-values and Cohen’s d effect size.
Predicting ADHD Diagnosis
To test whether the relative spectrum of Slow-4 oscillations contributes to the diagnostic classification of ADHD, independent of and in addition to RT-SD, we carried out a series of logistic regressions. First, we modeled ADHD diagnosis (yes/no) as a function of RT-SD; we then added Slow-4 relative spectrum as a predictor and assessed its contribution to the deviance from the model using a likelihood ratio test. Second, to confirm that the findings are specific to Slow-4 variations, we repeated the same analysis separately with Slow-3, Slow-5 and Slow-6. All logistic regression models controlled for age and sex. The estimated effects are expressed as odds ratios, i.e., the proportional change in odds of ADHD associated with one-standard-deviation change in the measure of relative spectrum. All tests were performed at the 0.05 level of significance, two-sided.
Correlations
To explore the relation between Slow-4 measures and indices of task performance, Spearman correlation coefficients were computed between unadjusted Slow-4 spectrum and the number of omission and directional errors within each group. Additionally, we computed correlations between unadjusted Slow-4 spectrum and symptom severity ratings within each group.
Results
Group Characteristics
Twelve out of 29 children with ADHD included in the analysis met current criteria for Combined type (ADHD-C), 16 for Predominantly Inattentive type (ADHD-I), and one for Predominantly Hyperactive/Impulsive type (ADHD-H/I). Eleven children with ADHD (six ADHD-C, six ADHD-I, and one ADHD-H/I) presented with comorbid disorders: four with Oppositional Defiant Disorder, four with anxiety disorders (one with Generalized Anxiety Disorder and Social Phobia, one with Social Phobia, and two with Anxiety Disorder Not Otherwise Specified, one of whom also had dysthymia), two with nocturnal enuresis, and one with an Adjustment Disorder with Depressive Symptoms. Sixteen were drug naïve at the time of testing (nine ADHD-I, six ADHD-C, and one ADHD-H/I) and two (ADHD-I) had discontinued stimulant treatment within three months prior to this study. The eleven children undergoing stimulant treatment (five ADHD-I and six with ADHD-C) reported discontinuing medication 24 hours prior to testing.
As shown in Table 1, the two groups of children differed in sex distribution. They did not differ significantly on estimated IQ, academic achievement, age, socioeconomic status (85% of TDC and 87% of ADHD were from the two highest SES classes) or parent-identified ethnicity (Caucasian 54%, and 48%, African-American 8% and 17%, Hispanic/Latino 11% and 28%, “others,” including Asian, Native American and mixed ethnic group, 27% and 7%, in TDC and ADHD, respectively; χ2(3)=6.19; p=.10). Symptom ratings differed significantly, as expected.
Table 1.
Clinical Characteristics
| TDC | ADHD | Group Comparisons | |||||
|---|---|---|---|---|---|---|---|
| (n=26) | (n=29) | ||||||
| Males, Number (%) | 12 (46) | 26 (90) | χ12 =12.15 p<.001 | ||||
| ANCOVAsex | |||||||
| Mean | SD | Mean | SD | F | p | df | |
| Age (years) | 11.6 | 2.5 | 12.5 | 2.4 | 0.68 | 0.36 | 1,52 |
| FIQ | 114 | 14 | 106 | 13 | 3.75 | 0.06 | 1,52 |
| VIQ | 119 | 18 | 109 | 16 | 2.89 | 0.09 | 1,50 |
| PIQ | 107 | 11 | 102 | 12 | 3.24 | 0.08 | 1,50 |
| WIAT composite | 113 | 16 | 103 | 17 | 2.99 | 0.19 | 1,52 |
| CPRS-R:L | |||||||
| ADHD Index | 45.0 | 4.1 | 69.1 | 6.2 | 228.94 | <.001 | 1,52 |
| DSM-IV Inattention | 45.2 | 4.3 | 68.5 | 5.7 | 228.70 | <.001 | 1,52 |
| DSM-IV Hyper./Impuls. | 45.4 | 4.1 | 65.7 | 13.3 | 40.34 | <.001 | 1,52 |
| DSM-IV Total | 44.8 | 4.4 | 69.9 | 6.7 | 185.22 | <.001 | 1,52 |
| CTRS-R:L* | |||||||
| ADHD Index | 47.3 | 7.3 | 66.5 | 15.2 | 19.86 | <.001 | 1,39 |
| DSM-IV Inattention | 46.1 | 5.8 | 64.1 | 11.8 | 30.66 | <.001 | 1,39 |
| DSM-IV Hyper./Impuls. | 48.4 | 8.1 | 60.6 | 16.1 | 5.55 | .008 | 1,39 |
| DSM-IV Total | 47.1 | 7.4 | 65.6 | 14.2 | 20.05 | <.001 | 1,39 |
| CBCL parent | |||||||
| Total Problems | 39.8 | 8.3 | 61.2 | 7.1 | 72.95 | <.001 | 1,52 |
| Externalizing | |||||||
| Problems | 42.6 | 8.6 | 56.8 | 10.0 | 18.65 | <.001 | 1,52 |
| Internalizing Problems | 43.5 | 7.4 | 56.7 | 11.4 | 14.27 | <.001 | 1,52 |
Number and percentage of males in each group and Mean and Standard Deviation (SD) in typically developing children (TDC), and children with Attention Deficit Hyperactivity Disorder (ADHD) included in the task analyses (i.e., omissions < 15%).
Teacher questionnaires not available for 5 TDC and 9 children with ADHD. CBCL: Child Beha vior Checklist; CPRS-R: L Conners’ Parent Rating Scale-Revised-Long version; CTRS-R:L: Conners’ Teachers Rating Scale-Revised Long version; df: degree of freedom; DSM-IV Hyper./Impuls.: DSM-IV Hyperactive/Impulsive scale; FIQ: estimated full-scale IQ; PIQ: estimated Performance IQ; VIQ: estimated verbal IQ; WIAT composite: Wechsler Individual Achievement Test-II Composite Standard Score.
Task Results
Group Comparisons
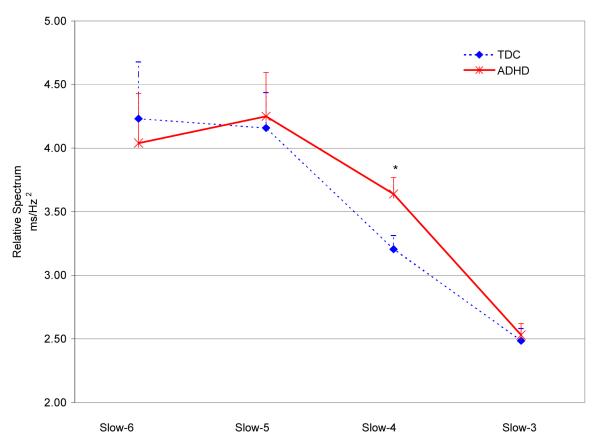
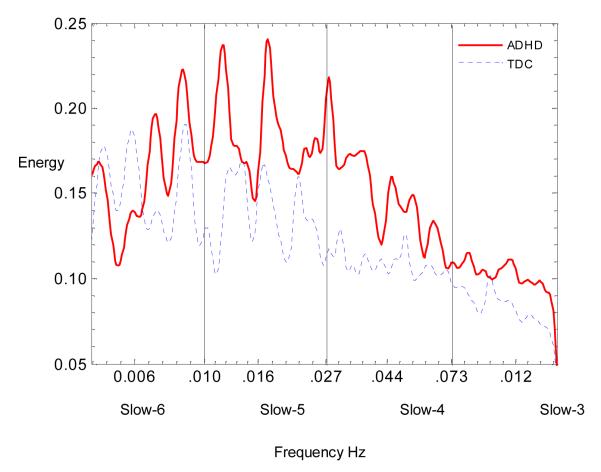
The groups did not differ in mean-RT. Children with ADHD had significantly more errors and greater RT-SD than controls, although both groups were accurate (96% overall for TDC, 95% for ADHD; see Table 2). As Figure 1 shows, children with ADHD had greater relative spectrum in the Slow-3, Slow-4, and Slow-5 frequency bands, but the difference reached statistical significance only in the Slow-4 band. Figure 2 illustrates the between-group differences in spectrum across the sampled frequency bands. Similar results were obtained for the Slow-4 relative spectrum measured with FFT (F(1,51)=4.03, p=0.05; see Supplementary Table 2).
Table 2.
Group Comparisons on Time and Frequency Domain Task Variables
| TDC | ADHD | Group Comparisons |
|||||
|---|---|---|---|---|---|---|---|
| (n=26) | (n=29) | ANCOVAsex/age | |||||
| Mea n |
SD | Mea n |
SD | F(1,51) | P | d | |
| Time Domain | |||||||
| Mean-RT (ms) | 863.1 | 146.8 | 845.4 | 111.2 | 2.17 | 0.47 | 0.41 |
| RT-SD | 155.5 | 34.8 | 170.0 | 32.3 | 4.74 | 0.03 | 0.60 |
| # Omission Errors | 8.5 | 9.9 | 9.5 | 11.3 | 3.86 | 0.05 | 0.54 |
| # Directional Errors | 2.8 | 3.1 | 6.2 | 9.3 | 5.62 | 0.02 | 0.66 |
| Frequency Domain (MWT) ms/Hz 2 | |||||||
| Slow-3 (0.12 Hz) | 2.49 | 0.50 | 2.53 | 0.50 | <1 | 0.70 | 0.11 |
| Slow-4 (0.044 Hz) | 3.21 | 0.55 | 3.64 | 0.70 | 6.27 | 0.02 | 0.69 |
| Slow-5 (0.016 Hz) | 4.16 | 1.43 | 4.25 | 1.90 | 3.12 | 0.58 | 0.15 |
| Slow-6 ( 0.006 Hz) | 4.23 | 2.28 | 4.04 | 2.14 | <1 | 0.97 | 0.01 |
Group Mean and Standard Deviation (SD) of time and frequency domain task variables averaged over the 15 min task, and results of the analysis of covariance adjusting for age and sex (ANCOVA sex/age). Time Domain: Mean RT: mean response time. Frequency Domain: for each frequency band, the group average am plitude resulting from the Morlet w avelet transform (MWT) of the norm alized residuals of ln(RT) is reported. Frequencies in parentheses are the central frequencies for thefour bands. d: Cohen’s d.
Figure 1. Group Differences in Slow-6, 5, 4, 3 Relative Spectra.
Relative spectra in the Slow-6, 5, 4, and 3 averaged over time resulting from continuous Morlet wavelet transform of normalized residuals of response time (RT) timeseries. ADHD = Attention-Deficit/Hyperactivity Disorder; TDC = typically developing children (dotted-line). Although the ADHD group showed increased relative spectrum in Slow-5, Slow-4, and Slow-3, group differences were statistically significant only for Slow-4 (*: p=0.02).
Figure 2. Group Differences in Frequency Spectrum.
ADHD = Attention-Deficit/Hyperactivity Disorder (continuous line); TDC = typically developing children (dotted-line). The X-axis represents the frequencies on the natural logarithmic scale included between 0.0052 and 0.17 Hz grouped in four ranges: Slow-6 centered at 0.006 Hz, Slow-5 at 0.016 Hz, and Slow-4 at 0.044 Hz and Slow-3 at 0.12 Hz. The Y-axis represents the magnitude of the frequency spectra measured with continuous Morlet wavelet transform.
The only difference between groups with respect to time-on-task effects was observed on number of directional errors (F(2.3, 117.3)=2.94, Greenhouse-Geisser corrected, p=0.049). The TDC children had the fewest directional errors in the first three-minute interval (0.38 ± 0.70), and a slightly higher but stable number of such errors in blocks 2-5 (0.69 ± 1.09; 0.54 ± 0.90; 0.58 ± 0.64; 0.61 ± 0.80, respectively), while the children with ADHD showed a more robust increase over time (0.48 ± 1.06; 1.17 ± 1.92; 1.80 ± 3.19; 1.14 ± 1.96; 1.65 ± 2.74 for blocks 1-5, respectively). There were no significant differences in the three-way interaction between group by frequency band by time-on-task (F(12, 1007)=0.82, p=0.63) nor in the two-way interaction between group and time-on-task (F(4,1019)=0.66, P=0.62). The two-way interaction between frequency band and time-on-task (F(12, 468)=4.64, p<0.001) and the main effect of time-on-task (F(4,216)=17.76, p<0.001) were significant, indicating non-stationarity of the RT timeseries and differences between frequency bands with respect to deviations from stationarity. Specifically, Slow-4, Slow-5 and Slow-6 showed quadratic time effects (e.g., Slow-4 relative spectrum: 3.41 ± 0.95; 4.04 ± 1.16; 3.76 ± 1.15; 3.90 ± 1.18; 3.30 ± .89 for blocks 1-5, respectively; post-hoc pairwise comparisons between blocks 1 and 2 and blocks 4 and 5 differed significantly; p<0.001 for both). By contrast, Slow-3 relative spectrum increased only slightly from block 1 to 4 with a slight decrease at block 5 (Slow-3: 2.53 ± .62, 2.66 ± .60, 2.77 ± .66, 2.85 ± .65, 2.57 ± .66 for blocks 1-5, respectively).
Prediction of ADHD Diagnosis
After adjusting for age and sex, RT-SD significantly predicted diagnosis (p=0.04; see Table 3). The deviance significantly decreased after adding Slow-4 relative spectrum to the model RT-SD + age + sex (p=0.03). Separately adding the relative spectrum measure of any of the other frequency bands to the initial model did not significantly improve the prediction of diagnosis. The same analyses repeated with relative spectrum of each low frequency band computed with FFT yielded similar but non-significant results (Slow-4 odds ratio =1.89, see Supplementary Table 3).
Table 3.
Contributions of Slow-3, -4, -5, -6 to ADHD-related Intra-Subject Variability
| Model | Added variable |
Deviance | LR | df | p | OR** (95%CI) |
|---|---|---|---|---|---|---|
| (age + sex)* + | RT-SD | 58.64 | 4.39 | 1 | 0.04 | 1.97 (1.04, 4.05) |
| age + sex + RT-SD + | Slow-3 | 58.56 | 0.12 | 1 | 0.78 | 1.11 (0.57, 2.49) |
| age + sex + RT-SD + | Slow-4 | 54.10 | 4.65 | 1 | 0.03 | 2.58 (1.08, 7.50) |
| age + sex + RT-SD + | Slow-5 | 58.49 | 0.13 | 1 | 0.72 | 1.14 (0.58, 2.39) |
| age + sex + RT-SD + | Slow-6 | 58.43 | 0.21 | 1 | 0.65 | 0.86 (0.44, 1.65) |
Logistic regressions modeling ADHD diagnosis as a function of RT-SD adjusting for age and sex. Each Slow- frequency band relative spectrum is added separately. Only Slow-4 significantly adds to the prediction of ADHD in the presence of RT-SD.
Deviance with model limited to (age + sex) = 63.106; df =53.
Odds ratio (OR) of ADHD diagnosis for increase in the added predictor by 1 SD unit and 95% CI based on profile likelihood.
LR: Likelihood ratio; RT-SD: response time standard deviation; Likelihood ratio; RT-SD: response time standard deviation; Slow-3:0.073 - 0.170 Hz, center frequency 0.120 Hz; Slow-4: 0.027-0.073 Hz, center frequency 0.044; Slow-5: 0.010-0.027 Hz, center frequency 0.016; Slow-6: 0.0052-0.010, center frequency 0.006 Hz.
Correlations
As shown in Figure 3, unadjusted Slow-4 spectrum was significantly correlated with omission errors within the ADHD and TDC groups (r=0.81, and r=0.62, respectively; p<0.001), and with directional errors in ADHD (r=0.60, p<0.001) but not in TDC (r=-0.17, NS). None of the symptom severity ratings correlated significantly with unadjusted Slow-4 spectrum within groups. However, across the entire sample, unadjusted Slow-4 spectrum was significantly correlated with Child Behavior Checklist attention problems parent ratings (r=0.44, n=55, p=0.001).
Figure 3. Correlations Between Errors and Unadjusted Slow-4 Spectrum.
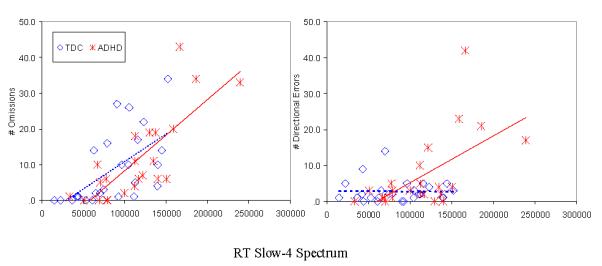
Errors of omission (left) and directional errors (right). The X-axis represents the magnitude of the frequency spectra measured with continuous Morlet wavelet transform. The significant correlation between Slow-4 spectrum and number of omission errors is nearly identical in the two groups (r=.81 and r=.62 for ADHD and TDC, respectively).
Discussion
The purpose of this study was to determine whether measuring response time variability in children with ADHD within the Slow-4 frequency band (i.e., 0.027-0.073 Hz) would provide greater diagnostic information than RT-SD alone. Having confirmed prior reports (6, 40, 41, 42, 43, 44, 45) that children with ADHD exhibit greater RT-SD than age-matched TDC, we also found that they showed significantly greater variability specifically in the Slow-4 frequency range. Furthermore, when variability in the Slow-4 frequency band was isolated by normalizing the spectral density functions, it was the only low frequency band tested which significantly contributed to RT-SD in differentiating the ADHD from the TDC group.
Our results are in broad agreement with recent findings by Johnson et al. (46, 47). Using fixed and random order versions of the Sustained Attention Response Task, with instructions to respond to all digits from 1 to 9 except to the digit 3, presented every 1.4 s, they found that children with ADHD show greater spectrum in frequencies from 0.004 to 0.35 Hz. Their task allowed them to differentiate a “fast” variability component (0.0772-0.35 Hz), which they suggested reflects sustained attention mechanisms, and a slower variability component (below 0.0772 Hz), ascribed to arousal processes. While the increased magnitude in the lower frequency band is broadly in agreement with our findings, differences in task designs do not allow us to determine the extent of convergence or disagreement. For instance, we did not find group differences in frequencies higher than Slow-4. However, our fixed 3 s inter-trial interval limited the extent to which we could examine the entire range of Slow-3 and the even faster frequencies analyzed by Johnson et al. (46, 47). On the other hand, because our task duration was nearly three times longer (15 min), we could explore the slower frequency bands, Slow-5 and Slow-6. The groups did not differ significantly in these two frequency bands.
The present work confirms our previous findings (4) despite several methodological refinements. First, we performed frequency analysis in residualized RT timeseries demonstrating that group differences in Slow-4 are not due to RT fluctuations driven by the trial types. Second, by normalizing the spectrum by total variance, we measured the proportional contribution of each frequency band separately from global spectrally non-specific variability. Thus, our findings highlight a more delimited low frequency band, Slow-4. Third, we decomposed variability into both time and frequency domains using the Morlet CWT, thus avoiding violations of the assumption of stationarity of the RT timeseries required by FFT. We found equivalent nonstationarity in both diagnostic groups; thus, analyzing relative spectrum over time did not lead to a loss of information comparing groups. By contrast, computing spectral density via FFT under the assumption that the RT timeseries are stationary, is strictly speaking, not appropriate in this case. Still, FFT measures provided for comparability with the literature showed rough agreement with our CWT results.
In contrast with the frequency domain and other time domain measures, children with ADHD worsened over time in number of directional errors, suggesting a possible additional impairment in sustained attention. RT Slow-4 was positively correlated with the number of omission and directional errors, except in TDC where directional errors were minimal. Better characterization of the relation between errors and RT spectral measures, as well as of the frequency pattern with which errors occur, would require designs that can elicit more frequent errors.
Our results should be interpreted in light of study limitations. The two groups, although matched for age, socio-economic status, ethnicity and IQ, were not matched for sex distribution. Accordingly, all our analyses were adjusted for sex. However, comparisons limited to the 26 boys with ADHD and 12 TDC boys showed identically significant results for Slow-4 (F(36)=5.98, p=0.02, Cohen’s d = 0.80). We excluded children who omitted over 15% of responses. The four excluded children with ADHD were among the most severely affected, and conservatively removing them reduced our statistical power. Further our sample size was not adequate to test the possible effects of specific clinical characteristics such as ADHD group subtype, comorbidity, history of medication treatment, current medication status, and whether effects may have been exacerbated by pharmacological rebound.
Increased ISV in ADHD has been a recent focus of active study (45, 4, 6). Leth-Steensen et al. suggested that exponentially prolonged RTs contributed to increased RT-ISV and are uniquely responsible for the group differences observed in such tasks (48). The contribution of such prolonged RTs can be measured by analyzing ex-Gaussian distributions (49), which decompose the RT distribution into a Gaussian normal component (indexed by mu and sigma, representing the mean and SD of the normal distribution) and an exponential component (indexed by tau representing both mean and SD of the exponential distribution). Hervey et al. (41) confirmed that children with ADHD differ markedly in having prolonged RTs, indexed by larger tau (48, 41). Increased variability can also include a higher proportion of extremely rapid RTs (45, 38).
From a theoretical perspective, increased RT-ISV in ADHD has been attributed to a deficient allocation of effort in accordance with the cognitive energetic state regulation deficit model (50, 51). Independent confirmation that Slow-4 fluctuations in RT contribute independently to differentiating individuals with ADHD would support focusing on this easily collected measure as an objective index that could be linkable to underlying neurophysiological processes (19, 4). Independent neuronal oscillation bands have been defined from ultra fast (1-4 ms/cycle) to very slow (15-40 s/cycle) frequencies (19). Although their nature, relation, and specific physiological functions have yet to be fully clarified, in general high frequency oscillations are hypothesized to provide high spatial resolution, whereas slow oscillations involve larger neuronal areas and are better suited for regulating dynamic relationships between and within brain networks (19, 20).
Episodic prolongations of RT were predicted by periodically decreased BOLD fMRI signal in any of three loci, including right dorsal anterior cingulate (52). In our present data, the association between the magnitude of Slow-4 fluctuations and errors supports the interpretation that these fluctuations in RT reflect episodic lapses of attention which may result from the interplay of intrinsic brain rhythms fluctuating at low frequencies and spanning large expanses of brain (9, 23, 53, 15, 13, 12). In a recent resting state fMRI study, we found that the temporal coherence between the right dorsal anterior cingulate cortex (52) and precuneus/posterior cingulate cortex was significantly decreased in adults with ADHD (13). A planned study will test whether increased Slow-4 fluctuations in RT are linked to decreased functional connectivity of this circuit in ADHD. Additional study designs, electrophysiological approaches, and pharmacological probes are needed to examine whether increased Slow-4 variability may have a greater effect on incongruent trials requiring inhibitory control, or on presumably more boring neutral or congruent trials (14).
In summary, our findings indicate that fluctuations in Slow-4 RT variability predict the diagnosis of ADHD beyond the effects associated with differences in global variability. Future studies will examine whether such spectrally specific fluctuations in behavioral responses are linked to intrinsic regional cerebral hemodynamic oscillations (12) occurring in similar frequency bands.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Dr. Judith Walters for helpful considerations on frequency analyses, Drs. Edmund Sonuga-Barke and Anouk Scheres for helpful discussions of earlier versions of this work; Drs. Glenn Hirsh, Steven Kurtz and Amy Krain for assistance in subject recruitment; Daniel Margulies and Weijin Gan for assistance in data analyses and Snigdha Rathor for technical assistance in the initial design of the database. Most of all, sincere gratitude goes to the children and parents who dedicated their time for this study.
Support: This work was supported by the Stavros S. Niarchos Foundation and the National Alliance for Research on Schizophrenia and Depression.
Footnotes
Financial disclosure: A. Di Martino, M. Ghaffari, J. Curchack, P. Reiss, M. Vannucci, E. Petkova, D. F. Klein, and F.X. Castellanos have no conflicts of interest to declare. Christopher Hyde is employed by BioAssessments, L.L.C., which conducted fast Fourier transform analyses under contract.
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Association; Washington, D.C.: 2000. Text Revision. [Google Scholar]
- 2.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- 4.Castellanos FX, Sonuga-Barke EJS, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellanos FX, Tannock R. Neuroscience of attention-deficit hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 6.Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald SW, Nyberg L, Backman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Ruskin DN, Bergstrom DA, Shenker A, Freeman LE, Baek D, Walters JR. Drugs used in the treatment of attention-deficit/hyperactivity disorder affect postsynaptic firing rate and oscillation without preferential dopamine autoreceptor action. Biol Psychiatry. 2001;49:340–350. doi: 10.1016/s0006-3223(00)00987-2. [DOI] [PubMed] [Google Scholar]
- 9.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raichle ME, Gusnard DA. Intrinsic brain activity sets the stage for expression of motivated behavior. J Comp Neurol. 2005;493:167–176. doi: 10.1002/cne.20752. [DOI] [PubMed] [Google Scholar]
- 12.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 13.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007 doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Scheres A, Oosterlaan J, Sergeant JA. Response execution and inhibition in children with AD/HD and other disruptive disorders: the role of behavioural activation. J Child Psychol Psychiatry. 2001;42:347–357. [PubMed] [Google Scholar]
- 17.Scheres A, Oosterlaan J, Swanson J, Morein-Zamir S, Meiran N, Schut H, et al. The effect of methylphenidate on three forms of response inhibition in boys with AD/HD. J Abnorm Child Psychol. 2003;31:105–120. doi: 10.1023/a:1021729501230. [DOI] [PubMed] [Google Scholar]
- 18.Scheres A, Oosterlaan J, Geurts H, Morein-Zamir S, Meiran N, Schut H, et al. Executive functioning in boys with ADHD: primarily an inhibition deficit? Archives of Clinical Neuropsychology. 2004;19:569–594. doi: 10.1016/j.acn.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 20.Penttonen M, Buzsaki G. Natural logarithmic relationship between brain oscillators. Thalamus & Related Systems. 2003;2:145–152. [Google Scholar]
- 21.Horovitz SG, Fukunaga M, de Zwart JA, van GP, Fulton SC, Balkin TJ, et al. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukunaga M, Horovitz SG, van GP, de Zwart JA, Jansma JM, Ikonomidou VN, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Diagnostic interview. 1996. Kiddie-Sads-Present and Lifetime Version (K-SADS-PL) - Screen interview.
- 25.Conners CK. Conners’ Rating Scales-Revised Technical Manual. Multi-Health Systems, Inc.; North Tonawanda, NY: 1997. [Google Scholar]
- 26.Conners CK. Conners’ Rating Scales-Revised User’s Manual. Multi-Health Systems, Inc.; North Tonawanda, NY: 1997. [Google Scholar]
- 27.Achenbach T, Rescorla L. Manual for the ASEBA school-age forms and profile. University of Vermont, rsearch center for Children, Youth and Families; Burlington, VT: 2001. [Google Scholar]
- 28.Hollingshead AB. Four factor index of social status. New Haven: 1975. [Google Scholar]
- 29.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 30.Wechsler D. Wechsler Individual Achievement Test. 2nd ed. The Psychological Corporation; New York: 2001. (WIAT-II) [Google Scholar]
- 31.Luce RD. Response times: their role in inferring elementary mental organization. Oxford Univeristy Press; New York: 1986. [Google Scholar]
- 32.Venables WN, Ripley BD. Modern Applied Statistic with S* 4th ed Springer; New York: 2002. [Google Scholar]
- 33.Rioul O, Flandrin P. Time-Scale Energy-Distributions - A General-Class Extending Wavelet Transforms. Ieee Transactions on Signal Processing. 1992;40:1746–1757. [Google Scholar]
- 34.Percival DB, Walden AT. Wavelet methods for time series analysis. Cambridge University Press; Cambridge: 2000. [Google Scholar]
- 35.Vidakovic B. Statistical Modeling by Wavelets. Wiley; New York: 1999. [Google Scholar]
- 36.Humeau A, Koitka A, Abraham P, Saumet JL, L’Huillier JP. Time-frequency analysis of laser Doppler flowmetry signals recorded in response to a progressive pressure applied locally on anaesthetized healthy rats. Physics in Medicine and Biology. 2004;49:843–857. doi: 10.1088/0031-9155/49/5/014. [DOI] [PubMed] [Google Scholar]
- 37.Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- 38.Williams BR, Strauss EH, Hultsch DF, Hunter MA. Reaction time inconsistency in a spatial stroop task: age-related differences through childhood and adulthood. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:417–439. doi: 10.1080/13825580600584590. [DOI] [PubMed] [Google Scholar]
- 39.Conners CK, Epstein JN, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. J Abnorm Child Psychol. 2003;31:555–562. doi: 10.1023/a:1025457300409. [DOI] [PubMed] [Google Scholar]
- 40.Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Hervey AS, Epstein JN, Curry JF, Tonev S, Eugene AL, Keith CC, et al. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- 42.Mullins C, Bellgrove MA, Gill M, Robertson IH. Variability in time reproduction: difference in ADHD combined and inattentive subtypes. J Am Acad Child Adolesc Psychiatry. 2005;44:169–176. doi: 10.1097/00004583-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Rubia K, Smith A, Taylor E. Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychol. 2007;13:276–304. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- 44.Teicher MH, Ito Y, Glod CA, Barber NI. Objective measurement of hyperactivity and attentional problems in ADHD. J Am Acad Child Adolesc Psychiatry. 1996;35:334–342. doi: 10.1097/00004583-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Williams BR, Strauss EH, Hultsch DF, Hunter MA, Tannock R. Reaction time performance in adolescents with attention deficit/hyperactivity disorder: evidence of inconsistency in the fast and slow portions of the RT distribution. J Clin Exp Neuropsychol. 2007;29:277–289. doi: 10.1080/13803390600678020. [DOI] [PubMed] [Google Scholar]
- 46.Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M, et al. Response variability in attention deficit hyperactivity disorder: evidence for neuropsychological heterogeneity. Neuropsychologia. 2007;45:630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 47.Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Daibhis A, et al. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia. 2007;45:2234–2245. doi: 10.1016/j.neuropsychologia.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst) 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 49.Heathcote A. Fitting wald and ex-Wald distributions to response time data: an example using functions for the S-PLUS package. Behav Res Methods Instrum Comput. 2004;36:678–694. doi: 10.3758/bf03206550. [DOI] [PubMed] [Google Scholar]
- 50.Sergeant J, Oosterlaan J, Van der Meere J. Information processing and energetic factors in attention-deficit/hyperactivity disorder. In: Quay HC, Hogan AE, editors. Handbook of disruptive behavior disorders. Plenum Press; New York: 1999. pp. 75–104. [Google Scholar]
- 51.Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biol Psychiatry. 2005;57:1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 53.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 54.Brockwell PJ, Davis RA. Time series: Theory and methods. 2nd ed Springer; New York: 2006. [Google Scholar]
- 55.Muller T, Reinhard M, Oehm E, Hetzel A, Timmer J. Detection of very low-frequency oscillations of cerebral haemodynamics is influenced by data detrending. Med Biol Eng Comput. 2003;41:69–74. doi: 10.1007/BF02343541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.