Abstract
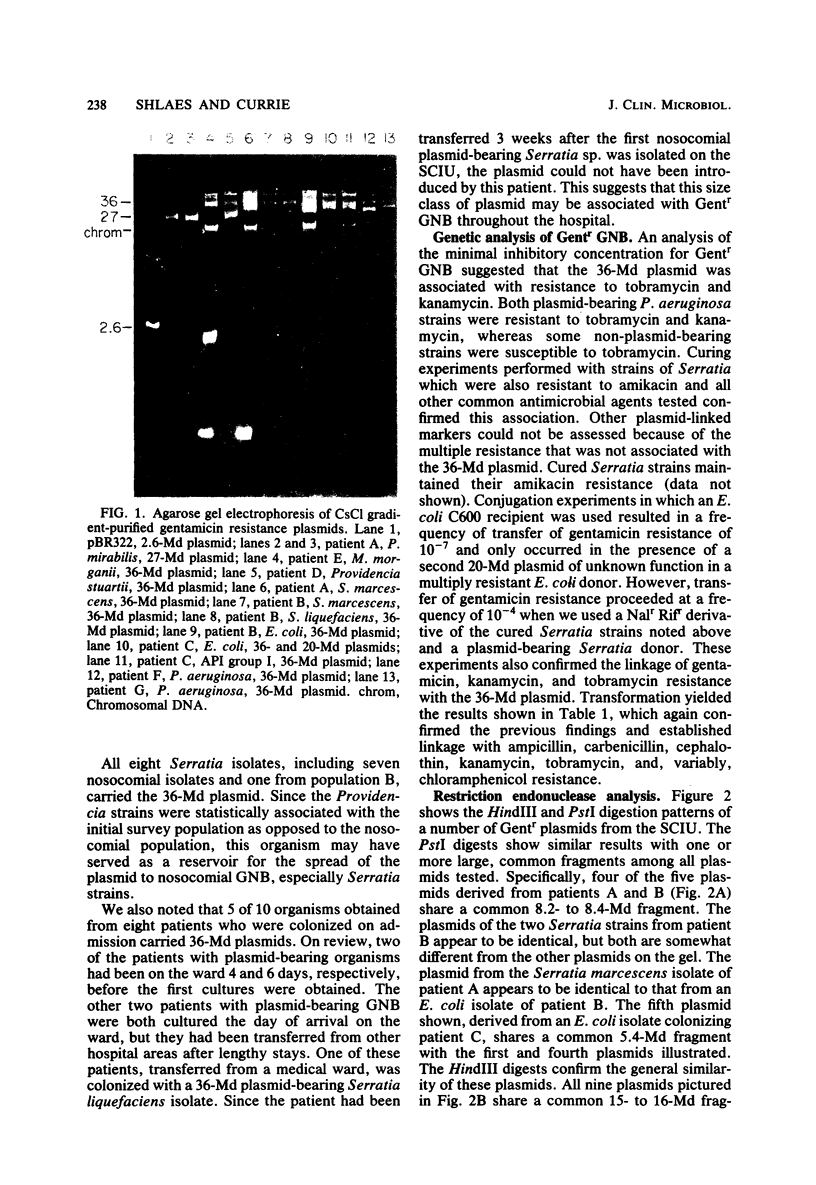
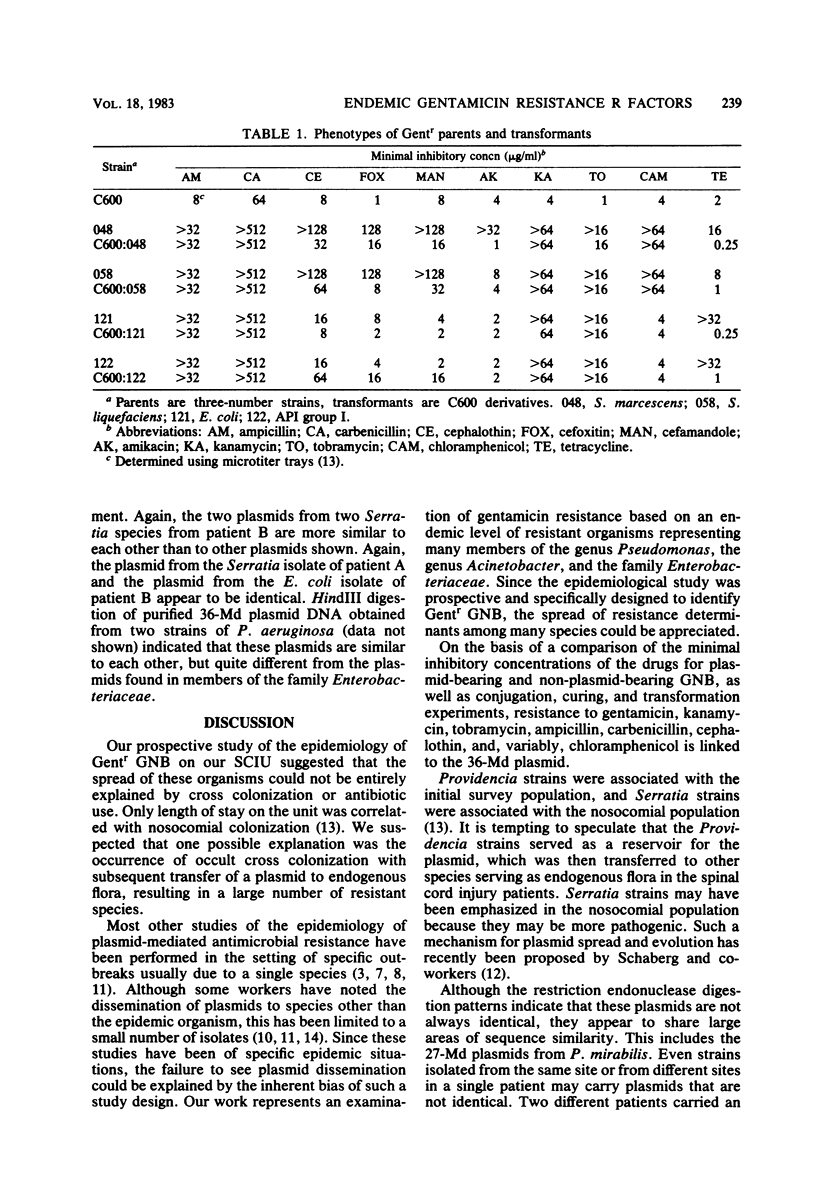
Cleared lysates of gentamicin-resistant, gram-negative bacilli obtained during a prevalence survey and a subsequent prospective study on a spinal cord injury unit were analyzed. Of 105 strains obtained during the epidemiological study, 62 were analyzed for plasmid content. None of the 14 Acinetobacter strains carried plasmids. Of 20 strains from the initial prevalence survey, 9 carried a 36- or (in two cases) a 27-megadalton plasmid. Eight of the nine were Providencia strains; none were Pseudomonas strains. Of 28 nosocomial isolates obtained during the prospective survey, 22 carried plasmids of similar molecular weight (P less than 0.025, compared with the prevalence survey), including 20 of 22 isolates of members of the family Enterobacteriaceae and 2 of 6 Pseudomonas aeruginosa isolates. Conjugation, curing, and transformation indicate that these plasmids carry gentamicin, tobramycin, kanamycin, ampicillin, carbenicillin, cephalothin, and, variably, chloramphenicol resistance. Restriction endonuclease digestion of purified plasmid DNA suggests that the plasmids from multiple species of the family Enterobacteriaceae contain common sequences, whereas those from Pseudomonas spp. do not. This study suggests that an endemic conjugal 36-megadalton gentamicin resistance R factor exists in many nosocomial species of the family Enterobacteriaceae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. D., Gillespie W. A., Richmond M. H. Chemotherapy and antibiotic-resistance transfer between Enterobacteria in the human gastro-intestinal tract. J Med Microbiol. 1973 Nov;6(4):461–473. doi: 10.1099/00222615-6-4-461. [DOI] [PubMed] [Google Scholar]
- Anderson J. D., Ingram L. C., Richmond M. H., Wiedemann B. Studies on the nature of plasmids arising from conjugation in the human gastro-intestinal tract. J Med Microbiol. 1973 Nov;6(4):475–486. doi: 10.1099/00222615-6-4-475. [DOI] [PubMed] [Google Scholar]
- Courtney M. A., Miller J. R., Summersgill J., Melo J., Raff M. J., Streips U. N. R-factor responsible for an outbreak of multiply antibiotic-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 1980 Dec;18(6):926–929. doi: 10.1128/aac.18.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Iflah Y., Raibaud P., Tancrede C., Rousseau M. R-plasmic transfer from Serratia liquefaciens to Escherichia coli in vitro and in vivo in the digestive tract of gnotobiotic mice associated with human fecal flora. Infect Immun. 1980 Jun;28(3):981–990. doi: 10.1128/iai.28.3.981-990.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. E., Jr, Eidson M., Guerry P., Falkow S., Drusin L. M., Roberts R. B. Interbacterial transfer of R factor in the human intestine: in-vivo acquisition of R-factor-mediated kanamycin resistance by a multiresistant strain of Shigella sonnei. J Infect Dis. 1972 Jul;126(1):27–33. doi: 10.1093/infdis/126.1.27. [DOI] [PubMed] [Google Scholar]
- Ingram L. C., Richmond M. H., Sykes R. B. Molecular characterization of the R factors implicated in the carbenicillin resistance of a sequence of Pseudomonas aeruginosa strains isolated from burns. Antimicrob Agents Chemother. 1973 Feb;3(2):279–288. doi: 10.1128/aac.3.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J. F., Jr, McNeill W. F. Characteristics of Serratia marcescens containing a plasmid coding for gentamicin resistance in nosocomial infections. J Infect Dis. 1981 Jun;143(6):810–817. doi: 10.1093/infdis/143.6.810. [DOI] [PubMed] [Google Scholar]
- Markowitz S. M., Veazey J. M., Jr, Macrina F. L., Mayhall C. G., Lamb V. A. Sequential outbreaks of infection due to Klebsiella pneumoniae in a neonatal intensive care unit: implication of a conjugative R plasmid. J Infect Dis. 1980 Jul;142(1):106–112. doi: 10.1093/infdis/142.1.106. [DOI] [PubMed] [Google Scholar]
- Roe E., Jones R. J., Lowbury E. J. Transfer of anibioic resistanceetween Pseudomonas aeruginosa, Escherichia coli, and other gram-negative bacilli in rns. Lancet. 1971 Jan 23;1(7691):149–152. doi: 10.1016/s0140-6736(71)91930-1. [DOI] [PubMed] [Google Scholar]
- Rubens C. E., Farrar W. E., Jr, McGee Z. A., Schaffner W. Evolution of a plasmid mediating resistance to multiple antimicrobial agents during a prolonged epidemic of nosocomial infections. J Infect Dis. 1981 Feb;143(2):170–181. doi: 10.1093/infdis/143.2.170. [DOI] [PubMed] [Google Scholar]
- Sadowski P. L., Peterson B. C., Gerding D. N., Cleary P. P. Physical characterization of ten R plasmids obtained from an outbreak of nosocomial Klebsiella pneumoniae infections. Antimicrob Agents Chemother. 1979 Apr;15(4):616–624. doi: 10.1128/aac.15.4.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Rubens C. E., Alford R. H., Farrar W. E., Schaffner W., McGee Z. A. Evolution of antimicrobial resistance and nosocomial infection. Lessons from the Vanderbilt experience. Am J Med. 1981 Feb;70(2):445–448. doi: 10.1016/0002-9343(81)90786-5. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Currie C. A., Rotter G., Eanes M., Floyd R. Epidemiology of gentamicin-resistant, gram-negative bacillary colonization in a spinal cord injury unit. J Clin Microbiol. 1983 Aug;18(2):227–235. doi: 10.1128/jcm.18.2.227-235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins L. S., Plorde J. J., Falkow S. Molecular analysis of R-factors from multiresistant nosocomial isolates. J Infect Dis. 1980 May;141(5):625–636. doi: 10.1093/infdis/141.5.625. [DOI] [PubMed] [Google Scholar]