Abstract
In wild-type sequences of three paramyxoviruses (measles virus, mumps virus, and Newcastle disease virus), nucleotide diversity at both non-coding sites and at nonsynonymous sites in coding regions was significantly reduced in comparison to that at synonymous sites. Likewise, both the mean and variance of gene diversity at nonsynonymous polymorphic sites were reduced in comparison to non-coding and synonymous sites. Neither of these patterns, which reflect the action of purifying selection against deleterious mutations at nonsynonymous and non-coding sites, was seen in the case of live attenuated vaccine strains, implying that purifying selection has been substantially relaxed on the latter, potentially affecting their biological properties, including antigenicity and vaccine effectiveness. Since the accumulation of mutations increases as s a function of the number of generations of replication, these findings highlight the utility of minimizing the number of generations between the original vaccine master seed and the strains used in vaccination, along with periodic monitoring of the extent of sequence evolution.
Keywords: live attenuated vaccine, measles virus, mumps virus, Newcastle disease virus, paramyxovirus, purifying selection
1. Introduction
Beginning with Jenner’s use of cowpox virus as a vaccination for smallpox over 200 years ago and Pasteur’s discovery of attenuation a century later, the use of live attenuated vaccines has become an important weapon is the fight against a number of viral diseases [1]. Live attenuated vaccines have the advantage over killed-virus vaccines that the former resemble a natural infection and thus interact with all aspects of the host’s immune system; but there are challenges associated with the use of live vaccines [2]. One set of challenges arises from the danger that a live attenuated virus can cause disease in some recipients. For example, in a minority of cases, vaccination with live attenuated virus has caused aseptic meningitis [3]. Likewise, in the case of poliovirus, there is evidence that the live attenuated vaccine virus has mutated in such a way as to regain virulence [4]. Another set of challenges relates to transmission of the live vaccine from a healthy person to one who is immunocompromised [5]. Moreover, studies of live attenuated vaccines often reveal incomplete vaccine effectiveness [6-9].
Live attenuated viruses continue to mutate and thus to evolve. Particularly in the case of RNA viruses, with their inherently high mutation rate, this evolution can be rapid. Evolution of the live vaccine strains can lead to the reacquisition of a virulent phenotype, and evolutionary changes may potentially play a role in the loss of vaccine effectiveness. Because of the importance of these phenomena in the management of vaccination programs, it is important to understand the population processes underlying the evolutionary changes in live attenuated vaccine strains of viruses.
Sequences differences between vaccine strains and their wild-type relatives include those responsible for the attenuated phenotype, which may have been discovered serendipitously [10] or may have arisen after a deliberate series of passages designed to result in attenuation [11]. In addition, there may be certain sequences changes in live attenuated viruses that have been selected because they better adapt the virus to growth under culture conditions [12]. However, all genomes are exposed to deleterious mutations, which natural selection (conservative or “purifying” selection) constantly acts to eliminate [13]. Under the conditions in which live attenuated viruses are cultured, it might be expected that purifying selection will be substantially relaxed, because these viruses are not exposed to many of the selection pressures acting on a free-living virus; for example, the selection pressures arising from natural virus transmission from host to host. In addition, because viruses in culture are expected to have much lower effective population sizes than free-living viruses, it can be expected that selection will be much less effective in removing slightly deleterious mutations in the former than in the latter [14].
Evidence regarding the action of natural selection on protein-coding regions can be obtained by estimating the number of synonymous substitutions per synonymous site (dS) and the number of nonsynonymous (amino acid-altering) substitutions per nonsynonymous site (dN). In most protein-coding regions, dS substantially exceeds dN [15]. This pattern occurs because most nonsynonymous mutations are deleterious to protein function and thus are eliminated by purifying selection [15]. Furthermore, analysis of coding sequences reveals two distinct aspects of purifying selection: (1) past selection against strongly deleterious mutations, which are eliminated quickly; and (2) ongoing selection against slightly deleterious mutations, which may be relatively inefficient if the effective population size is small or recombination is limited [14, 16]. The presence of slightly deleterious nonsynonymous variants, subject to ongoing purifying selection, can be detected by comparing the population frequency patterns of variants at synonymous and nonsynonymous sites [17-20].
The present study uses statistical analysis of published sequences to test the hypothesis that purifying selection has been relaxed in live attenuated vaccine strains. These analyses were applied to three single-stranded RNA negative sense viruses belonging to the viral family Paramyxoviridae: (1) measles virus (MeV; genus Morbillavirus), a pathogen of humans; (2) mumps virus (MuV; genus Rubulavirus), a pathogen of humans; and (3) Newcastle disease virus (NDV; genus Avulavirus), a pathogen of domestic poultry. Applying phylogenetic methods, sequence changes ancestral to clades of vaccine sequences were reconstructed; and patterns of nucleotide sequence polymorphism were compared between samples of wild-type sequences (i.e., those collected from natural infections) and live vaccine sequences.
2. Methods
2.1. Sequences Analyzed
Complete genome sequences of measles virus (MeV), mumps virus (MuV), and Newcastle disease virus (NDV) were aligned using the CLUSTAL X program [21]. Phylogenetic trees were constructed separately for the complete genomes of each of the three viruses by the neighbor-joining (NJ) method [22] on the basis of the maximum composite likelihood (MCL) distance [23]. The reliability of clustering patterns in phylogenetic trees was tested by bootstrapping [24]; 1000 bootstrap pseudo-samples were used. Accession numbers of sequences used in phylogenetic analyses are shown in Figures 1-3. The NDV sequence AY562985, which was shown to be a naturally occurring recombinant by previous phylogenetic analyses [25], was not included in the phylogenetic analysis; however, AY562985 was included in population genetic analyses, which unlike phylogenetic analyses did not depend on the assumption of an absence of recombination between sequences.
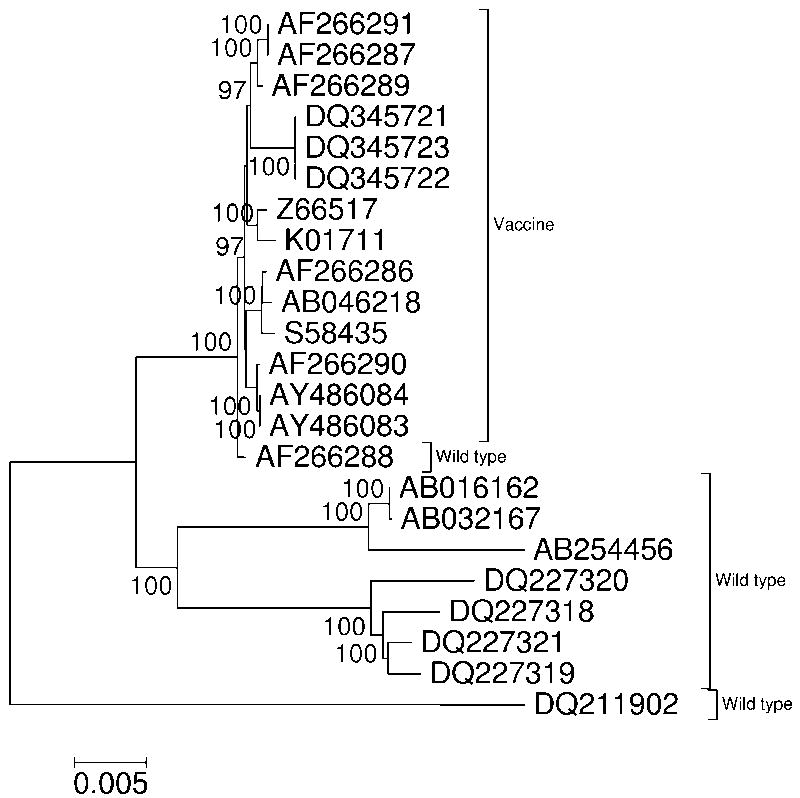
Figure 1.
NJ tree based on MCL distance at 15887 aligned nucleotide sites in selected wild-type and vaccine measles virus (MeV) complete genomes. Numbers on the branches represent the percentage of 1000 bootstrap samples supporting the branch; only values ≥ 95% are shown.
Figure 3.
NJ tree based on MCL distance at 15182 aligned nucleotide sites in selected wild-type and vaccine Newcastle disease virus (NDV) complete genomes. Numbers on the branches represent the percentage of 1000 bootstrap samples supporting the branch; only values ≥ 95% are shown.
For each of the three viruses, genomic sequences used in analyses were chosen to represent both wild-type isolates – i.e., those collected from natural infections – and vaccine strains. The latter were chosen so that they represented a single clade in each of the viral phylogenies, and that clade received 100% bootstrap support in each case (Figures 1-3). In the case of MeV (Figure 1), vaccine sequences used in analyses all belonged to the Edmondston lineage [26]. For MuV (Figure 2), only vaccine sequences belonging to the Urabe lineage [27] were used; sequences from the Jeryl Lynn vaccine strains were not used because fewer complete genomes were available for the latter. In the case of NDV, naturally isolated strains are characterized as velogenic (highly pathogenic), mesogenic (intermediate), or lentogenic (apathogenic; ref. 28). In analyses of NDV (Figure 3), the vaccine sequences used belonged to a clade including both the B1 and LaSota strains. These two vaccine strains were isolated independently from naturally occurring lentogenic strains [10, 29]. Lentogenic strains from outside this clade that have been used for vaccination (e.g., Ulster and V4: ref. 30) were not included in analyses because few genomic sequences were available.
Figure 2.
NJ tree based on MCL distance at 15376 aligned nucleotide sites in selected wild-type and vaccine mumps virus (MuV) complete genomes. Numbers on the branches represent the percentage of 1000 bootstrap samples supporting the branch; only values ≥ 95% are shown.
In each of the three viruses, the genome encodes seven major proteins (listed in 5’ to 3’ order): NP; V/P; M; F, HN; and L. MuV also encodes SH between F and HN. In MeV and MuV, the V/P gene expresses alternative products with the help of RNA editing. For the purposes of analyzing the pattern of nucleotide diversity in coding sequences as described below, the coding sequence of the longest non-edited open reading frame was analyzed.
2.2. Statistical Analyses
The number of synonymous substitutions per synonymous site (dS) and the number of nonsynonymous (amino acid-altering) substitutions per nonsynonymous site (dN) were estimated by Nei and Gojobori’s [31] method. Estimation of these quantities by more complex methods [32, 33] yielded essentially identical results, as is expected because the number of substitutions per site was generally low [34]; therefore, we used Nei and Gojobori’s method because it has a lower variance [35]. The synonymous nucleotide diversity (symbolized πS) is defined as the mean of dS for all pairwise comparisons among a set of sequences, while the nonsynonymous nucleotide diversity (symbolized πN) is the mean of dN for all pairwise comparisons among a set of sequences. The Jukes-Cantor method [35] was used to estimate the number of nucleotide substitutions per site (d) in non-coding regions; the mean of all pairwise comparisons of d is the nucleotide diversity (π). Ancestral nucleotide sequences were reconstructed by the maximum parsimony method [36] on the basis of the NJ trees. In each case, the tree was highly consistent, with consistency indices of 0.925 for MeV, 0.905 for MuV, and 0.612 for NDV. By comparison of reconstructed ancestral sequences, dS, dN, and d were estimated along branches within the tree. In each phylogeny, there was a wild-type sequence that formed a sister group to the vaccine clade: AF266288 in MeV (Figure 1); AY309060 in MuV (Figure 2); and DQ060053 in NDV (Figure 3). Mean dS, dN, and d were estimated between the members of each vaccine clade and the wild-type sister. The standard errors of mean numbers of nucleotide substitutions, including both nucleotide diversity within sets of sequences and comparisons of between sets of sequences, were estimated by the bootstrap method implemented in the MEGA 4.0 program [23].
Gene diversity (“heterozygosity”) at individual polymorphic nucleotide sites (SNP sites) was estimated by the formula:
where n is the number of alleles and xi is the population frequency of the ith allele (ref. 37, p. 177). In coding regions, single nucleotide polymorphisms were classified either as synonymous or nonsynonymous depending on their effect of the encoded nucleotide sequence. Ambiguous sites were excluded from these analyses; i.e., sites at which both synonymous and nonsynonymous variants occurred or at which the polymorphism could be considered synonymous or nonsynoymous depending on the pathway taken by evolution. Gene diversity was not normally distributed. Therefore, in testing for differences in the mean and variance of gene diversity among categories (synonymous, nonsynonymous, and non-coding) of SNP sites, randomization tests were used. In each test, 1000 pseudo-data sets were created by sampling (with replacement) from the data; a difference between two categories was considered significant at the α level if it was greater than the absolute value of 100(1-α) % of the differences observed between the same categories in the pseudo-data sets.
3. Results
3.1. Nucleotide Diversity
In the wild-type isolates of MeV, MuV, and NDV, both nonsynonymous nucleotide diversity (πN) and nucleotide diversity in non-coding regions (π) were significantly lower than synonymous nucleotide diversity (πS ; Table 1). In wild-type sequences of all three viruses, the ratio πN : πS was less than 1:10 (Table 1). This pattern is evidence of past purifying selection both on the amino acid sequence of encoded proteins and on non-coding regions. By contrast, in the vaccine sequences from MeV and MuV, neither πN nor π in non-coding regions significantly lower than synonymous nucleotide diversity (πS ; Table 1). The ratio πN : πS was over seven times as high in the vaccine sequences of MeV as in wild-type MeV sequences (Table 1). Similarly, the ratio πN : πS was over 10 times as high in the vaccine sequences of MuV as in wild-type MuV sequences (Table 1).
Table 1.
Synonymous (πS) and nonsynonymous (πN) nucleotide diversity in coding regions and nucleotide diversity (π) in non-coding regions in vaccine and wild-type (wt) genomic sequences of paramyxoviruses.
| Virus | Genome set (no.) | Coding | Non-coding | ||
|---|---|---|---|---|---|
| πS ± S.E. | πN ± S.E. | πN : πS | π ± S.E. | ||
| MeV | wt (9) | 0.0984 ± 0.0038 | 0.0089 ± 0.0006*** | 0.09 | 0.0708 ± 0. 0039*** |
| vaccine (14) | 0.0042 ± 0.0005 | 0.0027 ± 0.0003 | 0.64 | 0.0050 ± 0.0010 | |
| MuV | wt (6) | 0.1616 ± 0.0048 | 0.0078 ± 0.0006*** | 0.05 | 0.0914 ± 0. 0057*** |
| vaccine (8) | 0.0007 ± 0.0003 | 0.0004 ± 0.0001 | 0.57 | 0.0018 ± 0.0006 | |
| NDV | wt (27) | 0.4533 ± 0.0103 | 0.0308 ± 0.0010*** | 0.07 | 0.0914 ± 0. 0057*** |
| vaccine (5) | 0.0200 ± 0.0019 | 0.0033 ± 0.0004*** | 0.17 | 0.0153 ± 0.0023 | |
| B1 (2) | 0.0070 ± 0.0015 | 0.0029 ± 0.0005 | 0.41 | 0.0098 ± 0.0026 | |
| Lasota (3) | 0.0043 ± 0.0010 | 0.0026 ± 0.0004 | 0.61 | 0.0065 ± 0.0015 | |
Tests of the hypothesis that πN or π equals the corresponding value of πS:
P < 0.001 (Z-test).
In the case of NDV, the vaccine sequences showed a distinctive pattern (Table 1). In the coding regions, πN was significantly lower than πS, although the ratio πN : πS was greater (0.17) than that seen in wild-type NDV (0.07; Table 1). On the other hand, π in the non-coding regions did not differ significantly from πS in the case of NDV vaccine strains (Table 1). However, when nucleotide diversities were computed separately for the B1 and LaSota strains, although the numbers of sequences were small, the pattern was similar to that seen in vaccine strains of MeV and MuV (Table 1). For comparisons within B1 and LaSota strains, neither πN nor π in non-coding regions significantly lower than synonymous nucleotide diversity (πS ; Table 1). Likewise, the ratios πN : πS computed within B1 and LaSota, were much greater than those for wild-type NDV (Table 1).
3.2. Ancestral Sequence Comparisons
The pattern of nucleotide substitution along the branch ancestral to the clade of vaccine sequences was examined within each of the three viruses. In MeV, dN was actually greater than dS on the ancestral branch, although the difference was not significant due to the small number of substitutions (Table 2). In MuV, dS was slightly greater than dN on the ancestral branch, but the difference was not significant (Table 2). In both MeV and MuV, d in non-coding regions on the ancestral branch was slightly but not significantly greater than dS (Table 2). NDV showed a striking difference from the other two viruses, with both dN in coding regions and d in non-coding regions significantly lower than dS and on the ancestral branch (Table 2).
Table 2.
Estimated number of synonymous (dS) and nonsynonymous (dN) nucleotide substitutions per site in coding regions and nucleotide substitutions per site (d) in non-coding regions on the branch leading to the ancestor of paramyxovirus vaccine clades; and mean dS, dN, and d in comparisons between vaccine strains and the nearest wild-type sister sequence.
| Virus | Genome set (no.) | Coding | Non-coding | ||
|---|---|---|---|---|---|
| dS ± S.E. | dN ± S.E. | dN : dS | d ± S.E. | ||
| MeV | branch to ancestor | 0.0003 ± 0.0003 | 0.0005 ± 0.0002 | 1.67 | 0.0012 ± 0. 0008 |
| vs. sister group | 0.0036 ± 0.0007 | 0.0024 ± 0.0003 | 0.67 | 0.0054 ± 0.0014 | |
| MuV | branch to ancestor | 0.0114 ± 0.0019 | 0.0103 ± 0.0011 | 0.90 | 0.0189 ± 0. 0041 |
| vs. sister group | 0.0241 ± 0.0029 | 0.0260 ± 0.0016 | 1.08 | 0.0523 ± 0.0069*** | |
| NDV | branch to ancestor | 0.0363 ± 0.0034 | 0.0034 ± 0.0006*** | 0.09 | 0.0196 ± 0. 0037*** |
| vs. sister group | 0.0735 ± 0.0043 | 0.0072 ± 0.0007*** | 0.10 | 0.0430 ± 0.0047*** | |
Tests of the hypothesis that dN or d equals the corresponding value of dS:
P < 0.001 (Z-test).
In the case of MeV, mean dN did not differ significantly from mean dS in comparisons between the members of the vaccine clade and a wild-type sister sequence (Table 2). Likewise, mean d in non-coding regions of MeV did not differ significantly from mean dS in coding regions in comparisons between the vaccine clade and wild-type sister (Table 2). Likewise in MuV, mean dN was slightly but not significantly greater than mean dS in comparisons between the members of the vaccine clade and the wild-type sister sequence (Table 2). On the other hand, mean d in non-coding regions of MuV was actually significantly greater than mean dS in coding regions in comparisons between the vaccine clade and wild-type sister (Table 2). As with the pattern of reconstructed sequence evolution on the ancestral branch, NDV differed from the other two viruses in the comparison with the nearest wild-type sister sequence, with both dN in coding regions and d in non-coding regions significantly lower than dS (Table 2).
3.3. Analysis of SNPs
Gene diversity was computed at individual polymorphic sites in order to test for ongoing purifying selection. In the wild-type populations of MeV, MuV, and NDV, both mean and variance of gene diversity were significantly lower at nonsynonymous SNP sites than at synonymous SNP sites or at non-coding SNP sites (Table 3). In the case of the vaccine strains of MeV and MuV, there was not a significance difference in mean or variance of gene diversity between nonsynonymous SNPs and synonymous or non-coding SNPs (Table 3). In the case of NDV, by contrast, the vaccine strains, like wild-type strains, showed significantly lower mean and variance of gene diversity at nonsynonymous SNPs than at either synonymous or non-coding SNPs (Table 3).
Table 3.
Means and standard deviations (S.D.) of gene diversity at non-coding, synonymous, and nonsynonymous sites in wild-type and vaccine genomic sequences of paramyxoviruses.
| Wild-type |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Virus | Non-coding | Synonymous | Nonsynonymous | ||||||
| N | Mean | S.D. | N | Mean | S.D. | N | Mean | S.D. | |
| MeV | 357 | 0.294a | 0.130e | 899 | 0.295b | 0.128e | 310 | 0.264 | 0.122 |
| MuV | 239 | 0.338b | 0.094e | 1209 | 0.326a | 0.086c | 211 | 0.309 | 0.072 |
| NDV | 965 | 0.306b | 0.175e | 3298 | 0.282b | 0.172d | 1274 | 0.209 | 0.165 |
| Vaccine |
|||||||||
| Non-coding | Synonymous | Nonsynonymous | |||||||
| N | Mean | S.D. | N | Mean | S.D. | N | Mean | S.D. | |
| MeV | 27 | 0.294 | 0.116 | 53 | 0.248 | 0.094 | 97 | 0.279 | 0.116 |
| MuV | 9 | 0.219 | 0.000 | 9 | 0.236 | 0.052 | 15 | 0.256 | 0.080 |
| NDV | 44 | 0.396a | 0.088e | 120 | 0.431b | 0.077e | 79 | 0.347 | 0.063 |
Randomization tests of the hypothesis that the mean of gene diversity at non-coding or synonymous polymorphic sites equals that at nonsynonymous sites:
P < 0.01;
P < 0.001.
Randomization tests of the hypothesis that the variance of gene diversity at non-coding or synonymous polymorphic sites equals that at nonsynonymous sites:
P < 0.05;
P < 0.01;
P < 0.001.
In coding regions of MeV, of 1182 sites that were polymorphic in wild-type sequences but not in vaccine sequences, 296 (25.0 %) were nonsynonymous. By contrast, of 123 sites that were polymorphic in vaccine sequences but not in wild-type sequences, 83 (67.5%) were nonsynonymous. The difference in proportions was highly significant (χ2 = 97.36; 1 d.f.; P < 0.001). There was a similar pattern in the case of MuV, although the number of polymorphic sites in the vaccine sequences was small. Of 1413 coding sites polymorphic in the wild-type but not the vaccine MuV sequences, 210 (14.9%) were nonsynonymous. But 13 of 17 (76.5%) coding sites polymorphic in vaccine but not in wild-type sequences were nonsynonymous.
In MeV, 25 coding sites that were polymorphic in both wild-type and vaccine sequences, 13 (52.0%) were nonsynonymous. The latter proportion differed significantly from that found among coding sites polymorphic only in wild-type sequences (χ2 = 9.34; 1 d.f.; P < 0.002). In the case of MuV, there were only 6 coding sites (only one of which was nonsynonymous) that were polymorphic in both wild-type and vaccine sequences; so comparison with sites polymorphic in wild-type sequences only was not meaningful.
Of coding sites polymorphic in wild-type but not vaccine sequences of NDV, 1255 of 4455 (28.2 %) were nonsynonymous. By contrast, 54 of 82 (65.9 %) coding sites polymorphic in vaccine but not wild-type NDV sequences were nonsynymous. The difference in proportions was highly significant (χ2 = 55.70; 1 d.f.; P < 0.001). In this respect, NDV resembled MeV and MuV. On the other hand, of 111 coding sites polymorphic in both wild-type and vaccine sequences of NDV, only 19 (17.1%) were nonsynonymous. The latter proportion was actually lower than that in coding sites polymorphic in wild-type but not vaccine sequences of NDV (Figure 7A); and the difference was statistically significant (χ2 = 6.58; 1 d.f.; P = 0.01).
4. Discussion
Analysis of the patterns of nucleotide sequence polymorphism in wild-type isolates of three species of Paramyxoviridae showed reduced nucleotide diversity at nonsynonymous sites in comparison to synonymous sites in coding regions, as is seen in most genes [15]. This pattern is evidence of the action of purifying selection against a substantial fraction of nonsynonymous mutations. In addition, nucleotide diversity in non-coding regions was reduced in comparison to synonymous sites in coding regions, implicating purifying selection on the former. In vaccine strains of measles virus (MeV) and mumps virus (MuV), there was no evidence of reduced nucleotide diversity at either nonsynonyous sites in coding regions or at noncoding sites. Thus, there was evidence that a striking characteristic of the vaccine strains of these viruses is relaxation of purifying selection.
Examination of gene diversity at polymorphic nucleotide sites showed that, in the case of wild-type MeV and MuV, both the mean and variance of gene diversity at nonsynonymous SNP sites was reduced in comparison to that at either synonymous SNP sites or those in non-coding regions (Table 3). This pattern is indicative of ongoing purifying selection on nonsynonymous sites because it shows that there are few nonsynonymous polymorphisms occurring at high frequency [8]. Again, no such pattern was seen in the case of the vaccine strains of MeV and Muv (Table 3). Since the polymorphic variants currently found in a vaccine strain must have arisen after the most recent common ancestor of that strain, it is significant that there is evidence of a relaxation of purifying selection against such newly arisen variants. Thus, the relaxation of purifying selection did not simply occur during the attenuation process that gave rise to the vaccine strains of MeV and MuV, but rather it is ongoing.
The results obtained for Newcastle disease virus (NDV) differed from those for MeV and MuV; but these differences are easily explainable by the fact that the NDV vaccine sequences analyzed were derived from two related but independent field isolates, B1 and LaSota [10, 29]. When analyses included both B1 and LaSota strains, they did not reveal evidence of a relaxation of selection (Table 1 and 3). This result can be explained by the fact that the original B1 and LaSota, lineages evolved independently in a natural environment since their most recent common ancestor, prior to the separate isolation and maintenance of each lineage in culture as a vaccine. Thus, sequence comparisons between B1 and LaSota should be expected to show the effects of purifying selection that occurred in the natural state, whereas sequence comparisons within each strain should reveal the relaxation of purifying selection after each lineage was cultured as a vaccine. Consistent with this interpretation, when NDV vaccine sequences were analyzed within strains, they showed a pattern of nucleotide diversity indicating a relaxation of purifying selection similar to that seen in vaccine strains of MeV and MuV (Table 1). Given the independent isolation of the NDV vaccine strains, the observed differences between NDV and the other two viruses strongly support the hypothesis that the relaxation of purifying selection occurs as a consequence of culture of a live vaccine and not for some other reason.
Vaccine strains of MeV, MuV, and NDV all showed an excess of polymorphic nonsynonymous sites in comparison to wild-type sequences, and the bulk of these nonsynonymous polymorphisms were unique to the vaccine strains. This pattern provides further evidence in support of the hypothesis that purifying selection is relaxed in the vaccine strains. Moreover, because the polymorphisms involved introduced numerous amino acid substitutions unique to the vaccine strains, they suggest that the latter are capable of following evolutionary trajectories substantially different from those of the ancestral viruses.
In the case of MeV, a wild-type sequence close to the Edmonston strain ancestor (AF266288; ref. 38) enabled reconstruction of the small number of changes ancestral to the vaccine clade. A total of six changes were reconstructed, five nonsynonymous and one synonymous; all of these changes corresponded to differences between the Edmondson strain and its wild-type sister sequence that were previously described [38]. Of the five amino acid substitutions, four were fixed in all available sequences in the vaccine clade and in none of the wild-type sequences. These four changes involve the substitutions E→K at position 89 of the M protein; S→G at position 211 and N→Y at position 481 of the H protein; and D→A at position 1717 of the L protein. Because the latter four amino acid changes were fixed in the vaccine clade, they appear likely to contribute to the phenotypic properties selected for during the attenuation process. There were just two reconstructed changes in the non-coding region on the branch ancestral to the MeV vaccine clade, both 5’ to the start site of the NP gene: A →T at site 23 and C→A at site 43. Since these changes also were fixed in all available members of the vaccine clade, they also are candidates for contributing to the attenuated phenotype.
However, the sequence changes apparently fixed during the attenuation process of MeV vaccine were far fewer in number than the polymorphic sites unique to the vaccine sequences. This implies that most of the evolutionary change observed in the vaccine sequences is due to mutations that occurred after the vaccine strain was established, including many mutations that would be deleterious in a free-living virus and are found in the vaccine strain only because of the relaxation of purifying selection. Thus, the relaxation of purifying selection is likely to be the primary factor giving rise to sequence diversity in the vaccine strain of MeV.
In MuV, nucleotide substitutions in non-coding regions were significantly elevated in comparison to those at synonymous sites in the comparison between vaccine sequences and the nearest wild-type sister sequence (Table 2). This suggests that certain changes in non-coding regions may have been selectively favored during the attenuation process. In contrast to MeV, the nearest available wild-type sister sequence to the MuV vaccine sequence clade was not very closely related; and all available sequences from the MuV Urabe vaccine lineage were very closely related to one another (Tables 1 and 2). Nonetheless, in the vaccine strains of MuV, there were several nonsynonymous polymorphisms unique to the vaccine strains, implying that the relaxation of purifying selection has played a role the diversification of MuV vaccine strain sequences.
In conclusion, the present analyses demonstrate that the extent of continuing molecular evolution in live attenuated vaccine strains of MeV, MuV, and NDV has been substantial. Obviously, empirical studies will be required to test for phenotypic effects of any individual mutation occurring in vaccine lineages. However, the present analyses demonstrate that, as a consequence of the relaxation of purifying selection, vaccine strains can accumulate numerous amino acid sequence differences from free-living strains, thereby potentially affecting their biological properties, including antigenicity and vaccine effectiveness. The accumulation of selectively neutral or nearly neutral mutations increases with the number of generations of replication that the virus undergoes [13]. Therefore, minimizing the number of generations between the original vaccine master seed and the strains used in vaccination would seem a practical step to minimize the accumulation of mutations in vaccine strains, along with monitoring of evolutionary change in live attenuated vaccines by periodic sequence analysis [39].
Acknowledgments
This research was supported by grant GM43940 from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCullers JA. Evolution, benefits, and shortcomings of vaccine management. J Managed Care Pharm. 2007;13(7 Suppl B):S2–S6. doi: 10.18553/jmcp.2007.13.s7-b.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melnick JL. Conventional viral vaccines and their influence on the epidemiology of disease. In: Dimmock NJ, Griffiths PD, Madeley CR, editors. Control of Virus Diseases. Cambridge: Cambridge University Press; 1999. pp. 3–19. [Google Scholar]
- 3.Bonnet MC, Dutta A, Weinberger C, Plotkin SA. Mumps vaccine strains and aseptic meningitis. Vaccine. 2006;24(4950):7037–7045. doi: 10.1016/j.vaccine.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet MC, Dutta A. World wide experience with inactivated poliovirus vaccine. Vaccine. 2008;26(39):4978–4983. doi: 10.1016/j.vaccine.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control and Hospital Immunol. 2007;28(6):702–707. doi: 10.1086/517952. [DOI] [PubMed] [Google Scholar]
- 6.Cohen C, White JM, Savage EJ, Glynn JR, Choi Y, Andrews N, et al. Vaccine effectiveness estimates, 2004-2005 mumps outbreak, England. Emerging Infect Dis. 2007;13(1):12–17. doi: 10.3201/eid1301.060649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marin M, Nguyen HQ, Langidrik JR, Edwards R, Briand K, Papiana MJ, et al. Measles transmission and vaccine effectiveness during a large outbreak on a densely populated island: implications for vaccination policy. Clin Infect Dis. 2006;42(3):315–319. doi: 10.1086/498902. [DOI] [PubMed] [Google Scholar]
- 8.Marin M, Quinlisk P, Shimabukuru T, Sahney C, Brown C, LeBaron CW. Mumps vaccination coverage and vaccine effectiveness in a large outbreak among college students – Iowa, 2006. Vaccine. 2008;26(2930):3601–3607. doi: 10.1016/j.vaccine.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 9.Velicko I, Müller LL, Pebody R, Gergonne B, Aidyralieva C, Kostiuchenko N, et al. Nationwide measles epidemic in Ukraine: the effect of low vaccine effectiveness. Vaccine. 2008 doi: 10.1016/j.vaccine.2008.09.012. in press. [DOI] [PubMed] [Google Scholar]
- 10.Hitchner SB. Serendipity in science: discovery of the B-1 strain of Newcastle disease virus. Avian Dis. 1975;19(2):215–223. [PubMed] [Google Scholar]
- 11.Enders JF, Katz SL, Milovanovic ML, Holloway A. Studies of an attenuated measles virus vaccine. N Engl J Med. 1960;263:153–159. doi: 10.1056/NEJM196007282630401. [DOI] [PubMed] [Google Scholar]
- 12.Boroskin YS, Yamade A, Kaptsova TI, Skvortsova OI, Sinitsyna OA, Takeuchi K, et al. Genetic evidence for variant selection in the course of dilute passaging of mumps vaccine virus. Res Virol. 1992;143(4):279–283. doi: 10.1016/s0923-2516(06)80116-0. [DOI] [PubMed] [Google Scholar]
- 13.Kimura M. The Neutral Theory of Molecular Evolution. Cambridge: Cambridge University Press; 1983. [Google Scholar]
- 14.Ohta T. Slightly deleterious mutant substitutions in evolution. Nature. 1973;246(5428):96–98. doi: 10.1038/246096a0. [DOI] [PubMed] [Google Scholar]
- 15.Hughes AL. Adaptive Evolution of Genes and Genomes. New York: Oxford University Press; 1999. [Google Scholar]
- 16.Hughes AL. Near neutrality: leading edge of the neutral theory of molecular evolution. Ann NY Acad Sci. 2008;1133:162–179. doi: 10.1196/annals.1438.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes AL, Packer B, Welsch R, Bergen AW, Chanock SJ, Yeager M. Widespread purifying selection at polymorphic sites in human protein-coding loci. Proc Natl Acad Sci USA. 2003;100(26):15754–15757. doi: 10.1073/pnas.2536718100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes AL. Evidence for abundant slightly deleterious polymorphisms in bacterial populations. Genetics. 2005;169(2):553–558. doi: 10.1534/genetics.104.036939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes AL, Hughes MK. More effective purifying selection on RNA viruses than in DNA viruses. Gene. 2007;404(12):117–125. doi: 10.1016/j.gene.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes AL, Piontkivska H. Nucleotide sequence polymorphism in circoviruses. Infect Genet Evol. 2008;8(2):130–138. doi: 10.1016/j.meegid.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Diggins DG. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 23.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 24.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):83–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 25.Han G-Z, He C-Q, Ding N-Z, Ma LY. Identification of a natural multi-recombinant of Newcastle disease virus. Virology. 2008;371(1):54–60. doi: 10.1016/j.virol.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 26.Parks CL, Lerch RA, Walpita PA, Wang H-P, Sidhu MS, Udem SA. Analysis of the noncoding regions of measles virus strains in the Edmonston lineage. J Virol. 2001;75(2):921–933. doi: 10.1128/JVI.75.2.921-933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amexis G, Fineschi N, Chumakov K. Correlation of genetic variability with safety of mumps vaccine Urabe AM9 strain. Virology. 2001;287(1):234–241. doi: 10.1006/viro.2001.1009. [DOI] [PubMed] [Google Scholar]
- 28.Hanson RP. Heterogeneity within strains of Newcastle disease virus: key to survival. In: Alexander DJ, editor. Newcastle Disease. Boston: Kluwer Academic Publishers; 1988. pp. 112–120. [Google Scholar]
- 29.Goldhaft TM. Historical note on the origin of the LaSota strain of Newcastle disease virus. Avian Dis. 1980;24(2):297–301. [PubMed] [Google Scholar]
- 30.Miller PJ, King DJ, Afonso CL, Suarez DL. Antigenic differences among Newcastle disease virus strains of different genotypes used in vaccine formulation affect viral shedding after a virulent challenge. Vaccine. 2007;25(41):7238–7246. doi: 10.1016/j.vaccine.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3(5):418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 32.Li W-H. Unbiased estimates of the rates of synonymous and nonsynonymous substitution. J Mol Evol. 1993;36(1):96–99. doi: 10.1007/BF02407308. [DOI] [PubMed] [Google Scholar]
- 33.Yang Z, Nielsen R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol Biol Evol. 2000;17(1):32–43. doi: 10.1093/oxfordjournals.molbev.a026236. [DOI] [PubMed] [Google Scholar]
- 34.Hughes AL, French JO. Homologous recombination and the pattern of nucleotide substitution in Ehrlichia ruminantium. Gene. 2007;387(12):31–37. doi: 10.1016/j.gene.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Nei M, Kumar S. Molecular Evolution and Phylogenetics. New York: Oxford University Press; 2000. [Google Scholar]
- 36.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) Sunderland, Mass.: Sinauer; 2000. [Google Scholar]
- 37.Nei M. Molecular Evolutionary Genetics. New York: Columbia University Press; 1987. [Google Scholar]
- 38.Baričević M, Forčević D, Gulija TK, Jug R, Mažuran R. Determination of the coding and non-coding nucleotide sequences of genuine Edmonston-Zagreb master seed and current working seed lot. Vaccine. 2005;23(8):1072–1078. doi: 10.1016/j.vaccine.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Amexis G, Rubin S, Chizhikov V, Pelloquin F, Carbone K, Chumakov K. Sequence diversity of Jeryl Lynn strain of mumps virus: quantitative mutant analysis for vaccine quality control. Virology. 2002;300:171–179. doi: 10.1006/viro.2002.1499. [DOI] [PubMed] [Google Scholar]