Abstract
Background and Aims
Plants are likely to invest in multiple defences, given the variety of sources of biotic and abiotic damage to which they are exposed. However, little is known about syndromes of defence across plant species and how these differ in contrasting environments. Here an investigation is made into the association between carbon-based chemical and mechanical defences, predicting that species that invest heavily in mechanical defence of leaves will invest less in chemical defence.
Methods
A combination of published and unpublished data is used to test whether species with tougher leaves have lower concentrations of phenolics, using 125 species from four regions of Australia and the Pacific island of New Caledonia, in evergreen vegetation ranging from temperate shrubland and woodland to tropical shrubland and rainforest. Foliar toughness was measured as work-to-shear and specific work-to-shear (work-to-shear per unit leaf thickness). Phenolics were measured as ‘total phenolics’ and by protein precipitation (an estimate of tannin activity) per leaf dry mass.
Key Results
Contrary to prediction, phenolic concentrations were not negatively correlated with either measure of leaf toughness when examined across all species, within regions or within any plant community. Instead, measures of toughness (particularly work-to-shear) and phenolics were often positively correlated in shrubland and rainforest (but not dry forest) in New Caledonia, with a similar trend suggested for shrubland in south-western Australia. The common feature of these sites was low concentrations of soil nutrients, with evidence of P limitation.
Conclusions
Positive correlations between toughness and phenolics in vegetation on infertile soils suggest that additive investment in carbon-based mechanical and chemical defences is advantageous and cost-effective in these nutrient-deficient environments where carbohydrate may be in surplus.
Key words: Antiherbivore defence, leaf toughness, mechanical defence, chemical defence, phenolics, trade-offs
INTRODUCTION
Plant defences come in many forms, including chemical, physical and biotic traits that act directly or indirectly to deter herbivores. Predictive models of optimal defence investment most often focus on total defence or on specific chemical defences (Coley et al., 1985; Gulmon and Mooney, 1986; Herms and Mattson, 1992; van Dam et al., 1996; Iwasa, 2000; Riipi et al., 2002). However, plants are likely to invest in multiple types of defence due to the wide array of sources of biotic and abiotic damage (McKey, 1979; Koricheva et al., 2004; Agrawal and Fishbein, 2006) and to potential benefits of interacting defences (Berenbaum, 1985). Since defences have direct and indirect costs that may constrain investment relative to that in growth and reproduction (McKey, 1979; Rhoades, 1979; Herms and Mattson, 1992), selection for multiple defences may involve trade-offs among forms or syndromes of defence (Kursar and Coley, 2003; Agrawal and Fishbein, 2006).
Here the focus is on associations between two contrasting but widespread (even ubiquitous) forms of carbon-based defence, leaf toughness (mechanical) and phenolics (chemical). It has been shown that investment in chemical defence declines in many species as a leaf develops and toughens (McKey, 1979; Langenheim et al., 1986; Gleadow and Woodrow, 2000; Brunt et al., 2006; Hanley et al., 2007). The question is asked here whether the same trend occurs across species, i.e. whether species that toughen their leaves invest less in chemical defence. All plants have mechanical defences to some degree, in that cell walls provide a significant barrier to herbivores (Abe and Higashi, 1991; Sanson, 2006), and there is considerable evidence that leaf mechanical traits provide a deterrent to a range of herbivores (reviewed by Lucas et al., 2000; Read and Stokes, 2006; Sanson, 2006; Clissold, 2007; Hanley et al., 2007). Cell wall material may also have non-mechanical effects, such as nutrient dilution (Lee et al., 2004) and digestibility reduction (Hagerman and Butler, 1991).
Leaf mechanical traits potentially have both direct and indirect costs for a plant, depending on how the leaf is built. For example, a leaf toughened by sub-epidermal sclerenchyma bears a direct cost of the tissue plus an indirect cost of light attenuation. However, toughening a leaf is not necessarily expensive. A leaf can be strengthened (strength relating to the force to fracture the leaf) and toughened (toughness relating to the energy and propagation of fracture; Sanson et al., 2001) simply by increasing the thickness of the photosynthetic mesophyll. In this case, the costs of thickening a leaf may include negative effects on carbon gain due to internal self-shading and increased resistance to CO2 diffusion, but in a sunlit environment these costs may be small relative to efficiency gains (Roderick et al., 1999; Terashima et al., 2001, 2006). Secondly, in sunny open conditions, relative branching costs might be reduced by concentrating leaf mass into a smaller number of thicker leaves (Givnish, 1979; Read and Stokes, 2006). Hence, even if leaves with higher mass per unit area have lower rates of photosynthesis (Reich et al., 1991) and higher construction costs (Villar and Merino, 2001) per mass, this leaf form may be efficient in poor growing conditions via deterrence of herbivores combined with plant-level efficiency of resource allocation.
Therefore, species adapted to open sunny environments, where costs of thickening leaves may be small, should on average have leaves with higher ‘structural toughness’ (the combined effect of leaf ‘material toughness’ and thickness). It is predicted that these tough-leaved species may rely less on chemical defence. However, carbon-based chemical defences such as phenolics may also be more cost-effective in sunny environments (McKey, 1979; Coley et al., 1985; Herms and Mattson, 1992). Like mechanical defences, phenolics are known to deter a wide range of vertebrate and invertebrate herbivores, with effects including toxicity and reduction in digestibility (Harborne, 1991; Ayres et al., 1997; Hanley and Lamont, 2001; Rafferty et al., 2005). Tannins may be particularly advantageous since they can also have antimicrobial effects (Scalbert, 1991), reduce penetration of UV radiation (Day, 1993; Mazza et al., 2000) and act as antioxidants (Hagerman et al., 1998; Close and McArthur, 2002). Protection against UV radiation may be important in sunny habitats, predicting high efficiency of tannins with multiple protective roles. Hence both these classes of carbon-based defences are potentially broad-spectrum defences (although some herbivores may have overcome these defences) and both potentially act as antiherbivore defences through a variety of similar effects, e.g. digestibility reduction via tannins binding to proteins vs. dilution by cell wall components and associated decreased efficiency of nutrient assimilation (Yang and Joern, 1994, Lee et al., 2004); or toxicity of phenolics preventing or reducing feeding vs. tissue properties that prevent or increase the energy cost of fracture. Both of these are predicted to be efficient in similar (sunny but stressful) environments.
There has been little study of associations between mechanical and chemical defence across species. Here it is asked whether there is evidence of a trade-off between the two ubiquitous defences of leaf toughness and phenolics; specifically, do tough-leaved species invest less in phenolics? Tests are conducted to determine whether there is a negative relationship between foliar phenolic concentration and toughness among evergreen woody species, using data from four regions—temperate south-western and south-eastern Australia, and tropical Queensland (northeast Australia) and New Caledonia (south-west Pacific). These data sets include a range of vegetation types, from Mediterranean-climate shrublands and temperate forest to tropical shrublands, dry forest and rainforest.
MATERIALS AND METHODS
This study uses data from published work, together with new data that broaden the scope of comparisons. Previously reported foliar mechanical properties are included as follows: shrubs and trees in shrubland (kwongan) and eucalypt woodland in a Mediterranean-type climate at Tutanning, south-western Australia (Read et al., 2005); understorey shrubs and small trees in a temperate eucalypt forest at Bunyip State Park, south-eastern Australia (E. Caldwell, J. Read and G. D. Sanson, unpubl. data); and from tropical New Caledonia, shrubs and small trees in shrubland on ultramafic soil (locally known as ‘maquis’), small trees, shrubs and lianes in dry forest (also termed sclerophyll forest: Jaffré, 1993) on non-ultramafic soils (Read et al., 2006a), and canopy trees in rainforest on ultramafic soils (Chatain et al., 2009) (see Appendix for details). Data are also included from tropical rainforest canopy trees growing on basalt soils in northern Queensland (Appendix). For simplicity, to distinguish the shrubland in south-western Australia from that in New Caledonia, they are referred to by their local names (kwongan and maquis). At each study area, mature leaves (approx. 6–12 months old) of 18–44 species were collected from 2–5 replicate plants. The 125 species used in this study (Appendix) include a range of leaf types from soft to very tough.
Leaf toughness was measured as work-to-fracture using shearing tests (Read and Sanson, 2003; Read et al., 2005). Species were included for which a leaf strip was cut or analysed from one side of a leaf (to avoid influence of leaf margin or midrib) and sheared by a guillotine blade (15–66 species per region), using a custom-built portable force-tester or a modified Chatillon UTSE Universal Force Tester with identical shearing blade characteristics. The area under the generated force–displacement curve was used as the measure of work-to-shear (‘structural toughness’), standardized to strip width (the width of tissue cut), and also as specific work-to-shear (work-to-shear per unit leaf thickness), a measure of ‘material toughness’ (Read and Sanson, 2003). Specific work-to-shear (sometimes referred to as ‘toughness’ or ‘tissue toughness’) has been suggested as a key mechanical trait in herbivore deterrence and other aspects of plant performance (Choong et al., 1992; Choong, 1996; Lucas et al., 2000; Wright and Westoby, 2002). Both mechanical properties are likely to affect herbivores, but the effects of each may differ among guilds of herbivores (where guild differences include body size and mode of feeding) (Choong et al., 1992, Choong, 1996; Lucas et al., 2000; Peeters et al., 2001; Sanson et al., 2001; E. Caldwell, J. Read and G. D. Sanson, unpubl. data).
Leaves were freeze-dried and ground to a powder, and ‘total phenolics’ were assayed by the Prussian-blue method (Price and Butler, 1977) as modified by Graham (1992), following extraction in 50% acetone (Cork and Krockenberger, 1991). Concentration was expressed as gallic acid equivalents (GAE) per leaf dry mass. Tannin activity was estimated from precipitation of protein using the blue BSA (bovine serum albumin) method (Asquith and Butler, 1985) with bovine γ-globulin as the standard. As tannins vary in their capacity to bind proteins, the results are reported as the amount of protein bound per unit leaf dry mass, rather than concentration of tannins. For both measures of phenolics, it is acknowledged that since different compounds can give different colour yields per mass (Mueller-Harvey, 2001), the estimates are only semi-quantitative. Mean values for maquis and dry forest in New Caledonia have been reported previously (Read et al., 2006a).
Comparison of chemistry with mechanics in a way that is relevant to a herbivore and to the evolved plant defence ‘strategy’ is not straightforward. Herbivores cut leaves in such a way that the toughness (both work-to-shear and specific work-to-shear) of the cut region may not have a necessary or predictable relationship with the volume or mass of tissue acquired. What is most relevant to a herbivore is likely to include the amount of phenolics consumed, or amount consumed per unit mass ingested and per mass of nutrient ingested, per effort of fracturing the leaf. Here phenolics are compared on a mass basis (percentage leaf dry mass), with toughness expressed per width of the cut (measured as work-to-shear) and per unit cross-sectional area of the cut (specific work-to-shear). Pearson's correlation was used to test the degree of association between leaf toughness and phenolics across all species and separately for each vegetation type. Analysis of variance (ANOVA), followed by Tukey's post hoc comparisons, was used to compare levels of phenolics and toughness among vegetation types. Data were transformed when necessary and SYSTAT v. 11 was used for data analysis. Phylocom 4·0© (Webb et al., 2008) was used to compute phylogenetic independent contrasts (PICs) from a phylogeny developed by Phylomatic based on the angiosperm consensus tree from Davies et al. (2004) (with subfamilial resolution where necessary and available), allowing correlation of traits independently of relatedness among species. A critical level of α = 0·05 was used for all hypothesis tests.
RESULTS
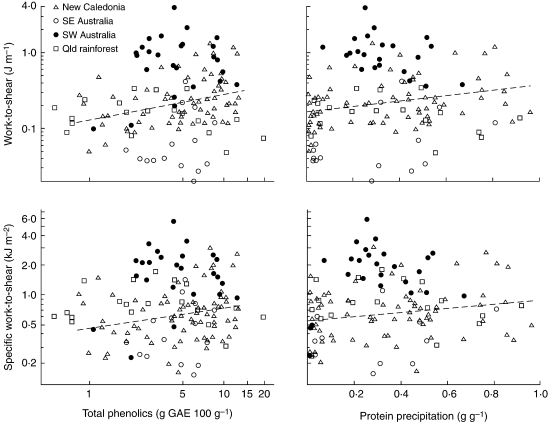
There was a high degree of variation among species in all four traits (35- to 191-fold), including within vegetation types (Fig. 1). No significant correlation was recorded between phenolics (both total phenolics and tannin activity) and leaf toughness (work-to-shear or specific work-to-shear) across the full set of species (Fig. 1, Table 1). Similarly, when associations between phenolic concentration and leaf toughness were investigated within regions, no correlations were recorded for woodland and kwongan species in south-western Australia, for forest understorey species in south-eastern Australia, or for tropical or for tropical rainforest trees in Queensland. However, for New Caledonian species, a positive correlation was recorded between total phenolics and both measures of toughness, and a positive correlation and near-significant correlation was recorded between tannin activity and work-to-shear and specific work-to-shear, respectively, although these correlations did not remain significant when the data were re-analysed using PICs (Table 1).
Fig. 1.
The relationship between leaf toughness (work-to-shear and specific work-to-shear, both plotted on a log scale) and phenolic concentration (total phenolics and tannin activity, the latter measured as protein precipitation) across all species. Species from New Caledonia (maquis, dry forest and rainforest), south-eastern Australia (eucalypt forest understorey species), south-western Australia (kwongan and eucalypt woodland) and tropical rainforest in north Queensland. Each data point is the mean of 2–5 replicate plants. The line of best fit is shown (derived by Model 1 regression) for New Caledonian species (Table 1).
Table 1.
Correlations (r) between phenolic concentration (total phenolics and tannin activity, the latter measured as protein precipitation) and leaf toughness (measured as work-to-shear of a leaf strip and as specific work-to-shear)
| Total phenolics |
Tannin activity (protein precipitation) |
|||
|---|---|---|---|---|
| Work-to-shear | Specific work-to-shear | Work-to-shear | Specific work-to-shear | |
| All species (n = 125, 96) | 0·166 (0·060) | 0·105 (0·236) | 0·163 (0·065) | 0·135 (0·126) |
| Australia | ||||
| Kwongan and woodland (n = 25, 19) | 0·225 (0·280) | 0·172 (0·410) | 0·257 (0·215) | 0·273 (0·187) |
| Eucalypt forest understorey (n = 15, 12) | 0·223 (0·424) | 0·205 (0·464) | 0·395 (0·145) | 0·313 (0·256) |
| Tropical rainforest (n = 19, 18) | −0·280 (0·245) | −0·117 (0·634) | −0·229 (0·346) | −0·150 (0·539) |
| New Caledonia (all species) (n = 66, 58) | 0·343 (0·003) | 0·245 (0·039) | 0·321 (0·006) | 0·230 (0·053) |
| Dry forest (n = 22, 21) | −0·022 (0·923) | −0·128 (0·572) | 0·098 (0·664) | −0·037 (0·871) |
| Rainforest (n = 27, 24) | 0·497 (0·007)* | 0·443 (0·018) | 0·471 (0·012)* | 0·387 (0·042) |
| Maquis (n = 21, 20) | 0·251 (0·272) | 0·430 (0·052) | 0·490 (0·024)* | 0·538 (0·012)* |
All data were log-transformed for analysis, except tannin activity. The associated P-values are given in parentheses, with significant values (P < 0·05) indicated in bold. An asterisk indicates significant correlations using phylogenetic independent contrasts. For n, the first value is the number of species, followed by the number of phylogenetic contrasts tested.
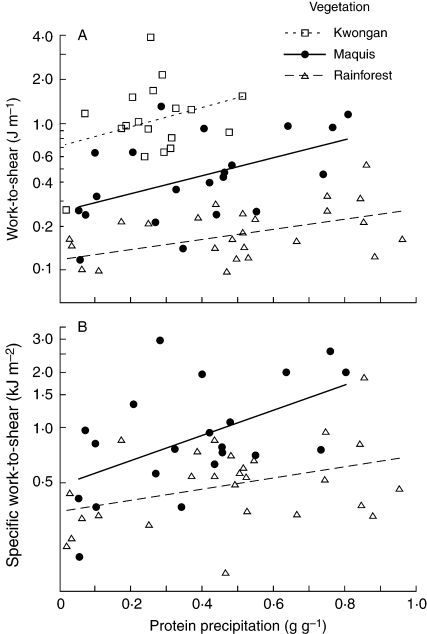
When New Caledonian species were analysed separately by vegetation type, no correlation was recorded between phenolics and leaf toughness for dry forest species (Table 1). For maquis and rainforest species (both on low-nutrient ultramafic soils), however, there was a positive correlation of both work-to-shear and specific work-to-shear with tannin activity, and for rainforest species there was a similar relationship with total phenolics (Fig. 2, Table 1). The positive correlations between work-to-shear and both measures of phenolics, and of specific work-to-shear with tannin activity in maquis, remained using PICs (Table 1).
Fig. 2.
The relationship between leaf toughness (work-to-shear and specific work-to-shear, both plotted on a log scale) and tannin activity (measured as protein precipitation) for rainforest and maquis species in New Caledonia. Kwongan species in south-western Australia are included in (A). Each data point is the mean of 3–5 replicate plants. The line of best fit is shown for each vegetation type (derived by Model 1 regression).
Leaves of maquis species were tougher on average, in terms of both work-to-shear and specific work-to-shear, than New Caledonian rainforest trees, including when analysed by PICs (Table 2, Fig. 2). Levels of total phenolics and tannin activity were higher in rainforest and maquis species on ultramafic soils than in dry forest species on sedimentary soils, including when analysed by PICs (Table 2). Leaves of New Caledonian rainforest trees had higher levels of total phenolics than Queensland rainforest trees, but did not differ in work-to-shear and specific work-to-shear, despite lower soil P concentration in the New Caledonian rainforest (Appendix). Levels of total phenolics and tannin activity were higher in New Caledonian rainforest, but specific work-to-shear was lower than in Queensland rainforest when analysed by PICs (Table 2).
Table 2.
Phenolic concentration (total phenolics and tannin activity, the latter measured as protein precipitation) and leaf toughness (work-to-shear and specific work-to-shear) for each vegetation type
| Total phenolics (g GAE 100 g−1) | Protein precipitation (g g−1) | Work-to-shear (J m−1) | Specific work-to-shear (kJ m−2) | |
|---|---|---|---|---|
| Australia | ||||
| Kwongan | 4·5 ± 0·5apq | 0·26 ± 0·03apq | 1·27 ± 0·20p | 2·31 ± 0·30pq |
| Eucalypt forest understorey | 4·9 ± 0·5apq | 0·29 ± 0·06abpq | 0·09 ± 0·03 | 0·51 ± 0·09apq |
| Tropical rainforest | 4·7 ± 1·1aq | 0·33 ± 0·07abq | 0·17 ± 0·02aq | 0·86 ± 0·09bcp |
| New Caledonia | ||||
| Dry forest | 4·1 ± 0·1aq | 0·16 ± 0·04aq | 0·19 ± 0·03aq | 0·67 ± 0·08abp |
| Rainforest | 6·2 ± 0·5bp | 0·48 ± 0·05bp | 0·19 ± 0·02aq | 0·56 ± 0·06acq |
| Maquis | 7·6 ± 0·7bp | 0·38 ± 0·05abp | 0·53 ± 0·08p | 1·09 ± 0·17bp |
| F, P | 5·0, <0·001 | 5·2, <0·001 | 45·6, <0·001 | 18·5, <0·001 |
The data are means ± s.e. For the south-western Australian vegetation, only kwongan species are included (n = 18). The results of ANOVA are given, with shared superscript letters indicating no significant difference among vegetation types, and shared subscripts indicating the results of PICs analysis. All data were log-transformed for analysis, except tannin activity (protein precipitation). Sources of data are given in the text.
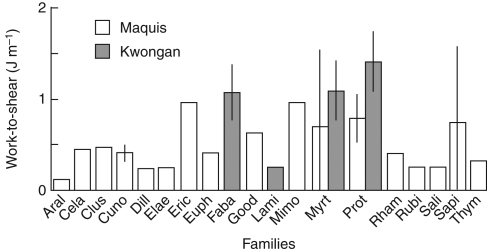
In addition to having tougher leaves, the maquis species had higher work-to-shear and specific work-to-shear relative to tannin activity than New Caledonian rainforest species (Fig. 2). No difference in foliar N was recorded between maquis and rainforest (both 1·0 ± 0·1 %: Read et al., 2006a; Chatain et al., 2009), i.e. there was no difference in concentration of this major nutritional reward for herbivores, although the tougher leaves of maquis species may make N less accessible. A similar trend of high toughness in open sunny communities is apparent among the other communities studied. Leaves of all the forest species invested similarly in work-to-shear relative to investment in phenolics; only the eucalypt forest understorey trees and shrubs showed a lower investment (Table 2). In contrast, leaves of kwongan species were not only tougher (higher work-to-shear and specific work-to-shear) compared with other communities studied (Table 2), but tougher relative to tannin activity (Fig. 2A). Furthermore, when kwongan species were investigated for correlations between work-to-shear and tannin activity, a similar trend (but not significant: r = 0·343, P = 0·178) to maquis species was recorded (Fig. 2A). Some of these trends were lost using PICs analysis (Table 2). Species of Fabaceae, Myrtaceae and Proteaceae were particularly common in the kwongan, but the maquis contained a higher diversity of common families. Comparison of broad-leaved Myrtaceae and Proteaceae in kwongan and maquis shows the higher work-to-shear on average in kwongan species (Fig. 3).
Fig. 3.
Comparison of work-to-shear by family in shrub-dominated vegetation in New Caledonia (maquis) and southwestern Australia (kwongan). Family names are abbreviated to the first four letters.
DISCUSSION
Correlations between foliar toughness and phenolics
No evidence was obtained that tough-leaved species generally invested less in chemical defence. Instead, positive correlations were recorded between measures of leaf toughness and phenolics in maquis and rainforest in New Caledonia, with a similar trend suggested in kwongan of south-western Australia. From the data it is concluded that within any community the levels of defences can be highly variable, as has been found previously (Coley, 1983; Kursar and Coley, 2003; Mali and Borges, 2003). More significantly, it was found that among co-occurring species in some environments, as foliar toughness increases, so do concentrations of phenolics, suggesting that additive investment in these contrasting forms of defence is cost-effective. Some of these relationships hold true using phylogenetically independent contrasts.
Other forms of chemical defence were not measured, so more general conclusions about relationships between mechanical and chemical defence among species cannot be drawn. Furthermore, individual phenolics may have varying roles (Harborne, 1991; Kraus et al., 2003), as may the leaf traits that contribute to mechanical properties. However, the positive correlations between toughness and phenolics on some sites warrant exploration. Significant positive correlations between leaf toughness and phenolics were confined to vegetation on ultramafic soils. These soils have low levels of macronutrients, particularly P, K and Ca, as well as high levels of heavy metals such as Ni (Read et al., 2006a, b). Foliar N : P ratios are 29 ± 1 (mean ± s.e.) in the maquis (Read et al., 2006a) and 31 ± 1 in the rainforest trees (Chatain et al., 2009), suggesting P limitation (more than approx. 20, following Güsewell, 2004), compared with 19 ± 1 in the dry forest on sedimentary soils (Read et al., 2006a) and approx. 20 in the Queensland rainforest trees (Gleason et al., 2009). Kwongan species have N : P ratios of 34 ± 3 (Read et al., 2005), and also show a positive trend between leaf toughness and phenolics, similar to that in vegetation on ultramafic soils. The question was asked as to whether the marked positive trends between leaf toughness and phenolics on these low-nutrient soils relate to these potentially P-limiting conditions. It has been hypothesized that when environmental conditions limit rates of growth relatively more than photosynthesis, assimilates may accumulate in a plant and may be used for defence potentially at less cost to fitness [i.e. the ecological side of the expanded growth–differentiation balance (GDB) hypothesis: Herms and Mattson, 1992; Stamp, 2003]. However, this hypothesis relates to intraspecific variation along a resource gradient, rather than to trends in defence allocation among species.
If the positive correlations between phenolics and toughness among species on nutrient-poor sites are a consequence of carbohydrate surplus, there should be variation among species in the extent to which nutrients constrain growth relative to photosynthesis (e.g. due to variation in physiology and resource allocation traits), and growth constraint should correlate positively with levels of both toughness and phenolics. This requires further investigation. The suggested role of soil nutrients cannot be simply tested by examining the relationship between defence and foliar nutrients across species within a community. For example, species with high levels of foliar nutrients may have expended more resources to acquire them, potentially leading to a positive correlation of foliar nutrients with defence investment among species within a site. Indeed, there is no evidence of consistent simple and direct relationships between defence investments and nutrient limitations within these sites: rainforest trees in New Caledonia show negative correlations of work-to-shear (not using PICs), total phenolics and tannin activity with foliar N; maquis species in New Caledonia show a weak positive correlation of tannin activity (and total phenolics using PICs) with foliar N : P; and the kwongan species show a positive correlation of phenolics and tannin activity with foliar P (and negative with N : P using PICs; Table 3).
Table 3.
Correlations (r) between putative defences and foliar nutrient concentrations across species in kwongan in southwestern Australia and vegetation on ultramafic soils in New Caledonia
| P | N | N:P | |
|---|---|---|---|
| Kwongan, Western Australia (n = 18, 14) | |||
| Total phenolics | 0·587 (0·010)* | 0·145 (0·566) | −0·216 (0·389)* |
| Tannin activity | 0·641 (0·004)* | 0·102 (0·688) | −0·272 (0·275)* |
| Work-to-shear | 0·014 (0·956) | −0·259 (0·299) | −0·230 (0·359) |
| Specific work-to-shear | 0·127 (0·615) | −0·161 (0·523) | −0·208 (0·407) |
| Maquis, New Caledonia (n = 21, 20) | |||
| Total phenolics | −0·205 (0·374) | −0·030 (0·897) | 0·296 (0·192)* |
| Tannin activity | −0·253 (0·264) | −0·003 (0·990) | 0·434 (0·049)* |
| Work-to-shear | −0·150 (0·517) | 0·002 (0·993) | 0·233 (0·309) |
| Specific work-to-shear | 0·031 (0·895) | 0·153 (0·508) | 0·170 (0·462) |
| Rainforest, New Caledonia (n = 27, 24) | |||
| Total phenolics | −0·364 (0·057) | −0·393 (0·039)* | 0·082 (0·677) |
| Tannin activity | −0·353 (0·065) | −0·402 (0·034)* | 0·046 (0·817) |
| Work-to-shear | −0·100 (0·614) | −0·415 (0·028) | 0·155 (0·431) |
| Specific work-to-shear | −0·292 (0·132) | −0·137 (0·488) | 0·269 (0·166) |
All data were log-transformed, except tannin activity (protein precipitation). The associated P-values are given in parentheses, with significant values indicated in bold. An asterisk indicates significant correlations using PICs. Nutrient concentrations are taken from Read et al. (2005, 2006a) and Chatain et al. (2009). For n, the first value is the number of species, followed by the number of contrasts using PICs.
The trends may also reflect interactive effects of nutrient availability with other environmental variables on patterns of defence investment among species (and high soil concentrations of heavy metals may play a role in species growing on ultramafic soils). Foliar nutrient concentration may be a proxy for growth rate, such that species with high N : P values also have low maximal growth rates (Güsewell, 2004) and by the growth rate hypothesis (Coley et al., 1985; Stamp, 2003) and the evolutionary side of the GDB hypothesis (Herms and Mattson, 1992; Stamp, 2003) are more heavily defended, with leaves of longer lifespan in which immobile quantitative defences such as toughness and polyphenols are particularly efficient (Coley et al., 1985; Witkowski et al., 1992). The positive trend between foliar P and phenolics in kwongan species appears to contradict this hypothesis, but there are no data for growth rates.
Correlations of leaf mechanical traits were recorded with both tannin activity and total phenolics. Tannins have a clearer role in antiherbivore defence than other phenolics, and, given their multiple roles, including protection from photodamage (Day, 1993; Close and McArthur, 2002), may occur at higher concentrations when there are efficiency gains from multiple benefits. Types of tannins have not been analysed separately. Condensed tannins have negative effects on a range of herbivores, including reduced protein digestion (Hagerman and Robbins, 1993; Ossipov et al., 2001) and toxicity (Berenbaum, 1985). The role of hydrolysable tannins and other phenolics is less clear—the former have a high capacity to precipitate proteins (Ossipov et al., 2001; Ossipova et al., 2001), but effects on herbivores are inconsistent, including among insects (Karowe, 1985; Behmer et al., 2002). If hydrolysable tannins are metabolically cheaper than condensed tannins, for example (Riipi et al., 2002), correlations with toughness may vary among phenolic compounds. It is also noted that work-to-shear (the combined effect of specific work-to-shear and leaf thickness) generally correlated more strongly with measures of phenolics than specific work-to-shear. How these and other leaf mechanical traits influence guilds of herbivores (e.g. Peeters et al., 2007) warrants further investigation in this regard.
No measures of spinescence, a common attribute of kwongan species (Hanley et al. 2007), have been included in this study. It is also noted that a few species excluded from the data sets of the kwongan and the eucalypt understorey in south-eastern Australia (because their leaf morphology did not allow the measurement protocol) were spinescent. A negative association has been recorded between spinescence and foliar fluoroacetate concentrations among Gastrolobium (Fabaceae) species (Twigg and Socha 1996), and with phenolic concentrations in seedlings of Hakea (Proteaceae) species (Hanley and Lamont 2002) in south-western Australia. Within some taxa, therefore, there appear to be trends that are not manifest or even occur in an opposite direction from those recorded across sets of diverse species. There were insufficient data to test for linear correlations effectively in spinescent vs. non-spinescent kwongan shrubs, but no significant difference in levels of total phenolics, tannin activity or either measure of toughness were found between these groups (P >0·05, all data log-transformed).
Comparisons of defence among vegetation types
Differences in defence investment patterns were recorded among those plant communities where phenolics and toughness were correlated. In the sunny conditions experienced by canopy species of shrublands and forests, where there may be little cost in having a thicker (or possibly denser) leaf, both tannins and toughness may be efficient means of providing protection from a range of forms of damage, including herbivores, and so may form part of a ‘defence syndrome’ (Kursar and Coley, 2003; Agrawal and Fishbein, 2006). However, leaves of New Caledonian rainforest canopy trees invested similarly to maquis plants in total phenolics and tannin activity, but with, on average, half the toughness (both work-to-shear and specific work-to-shear). That is, maquis species invest ‘preferentially’ in toughness compared with rainforest. This is not simply due to thickening of leaves since a similar trend is shown in both work-to-shear and specific work-to-shear, i.e. the leaves are built differently in terms of cell wall chemistry and/or internal architecture. Whether this apparent difference in physical:chemical defence investment between rainforest and maquis indeed reflects different adaptations (both levels and ratios) to reduce herbivore damage is unclear. Even though rainforest and maquis are often contiguous in the wetter regions of New Caledonia, often on the same soils (Read et al., 2006b), the maquis canopy is commonly more open and exposed to stronger evaporative loads. In addition, the extent of maquis in wet regions is related to the wildfire regime, maquis being a primary formation only on drier sites (Jaffré, 1980, 1993; Morat et al., 1986; McCoy et al., 1999), and so some maquis species may have evolved under drier conditions. Hence the higher degree of leaf toughening relative to phenolics in maquis species may reflect a higher investment in anatomical and morphological mechanisms of drought resistance that affect leaf mechanics (e.g. Heide-Jørgensen, 1990; Oertli et al., 1990), either coincidentally or because of the efficiency conferred by multiple benefits. Even if drought resistance is the primary selecting force for these traits, the toughening should provide some defence against herbivory [‘neutral resistance’, as defined by Edwards (1988)], and so potentially reduce the need for investment in additional chemical defence.
The high level of toughness relative to phenolics in broad-leaved kwongan species (Fig. 2) is consistent with the trends in New Caledonian vegetation. The particularly high values of toughness in kwongan species may reflect the more severe dry season, the lower level of soil nutrients such as P (Appendix), and also the presence of efficient native vertebrate browsers [native vertebrate browsers are currently absent in New Caledonia, but were possibly present (but unlikely) in the past, cf. Balouet (1991)]. Some mechanisms that increase mechanical defence may also reduce UV-B penetration, e.g. leaf thickness, particularly epidermal thickening, and possibly lignification (Day, 1993), and protect against excess light of other wavelengths (Jordan et al., 2005), and against freezing (Larcher, 2005), in addition to protection from herbivores and damage by storms, etc. Hence mechanical defence, or the underlying leaf traits conferring mechanical properties, may provide a highly efficient means of protection (Grubb, 1986; Read and Stokes, 2006) against multiple stresses in this severe environment.
Tropical rainforest trees may experience many of the stresses experienced by tropical maquis, but invested less in toughness relative to phenolics. It is also noted that New Caledonian rainforest trees on ultramafic soils (low total P concentrations) have a similar foliar work-to-shear to trees of Queensland rainforest, but have higher levels of phenolics (Table 2). Elevation of defences above a certain level in a rainforest environment may be more efficiently based on chemistry because of the likely inefficiencies associated with increasing levels of mechanical defence (cumulative direct and indirect costs) in microhabitats that are more prone to shading. In particular, the juveniles of rainforest canopy species may have to establish in more shaded environments than juveniles of shrubland species, particularly as the shrubland species commonly establish following wildfire. Thus, adaptive leaf traits of rainforest juveniles may impose constraints on leaf traits of the adult (and vice versa). However, developmental plasticity allows adjustment to local environments (Kalisz and Kramer, 2008). For rainforest canopy species, it is predicted that leaf toughening occurs most often by anatomical means that are relatively plastic (e.g. by thickening the mesophyll, rather than by a subepidermal layer of sclerenchyma), or by greater reliance on phenotypically plastic chemical defences, to optimize performance across contrasting microhabitats (within and between individuals of a species). High plasticity has been recorded in the diversity and levels of foliar secondary compounds (Mole et al., 1988; Downum et al., 2001). Dominy et al. (2003) showed on average 1·7 times higher specific work-to-shear (termed ‘leaf fracture toughness’) in canopy leaves of tropical rainforest canopy trees than understorey plants of the same species, but 2·4 times higher phenolics and 4·3 times higher tannins. Boege (2005) recorded no difference in leaf toughness between saplings and reproductive trees of the tropical tree Casearia nitida, but higher concentrations of phenolics in foliage of reproductive trees. Hence, it is predicted that the advantages of plasticity may contribute significantly to the profile of defences used by species in vegetation such as rainforest where markedly different environments can be experienced among individuals, and among ontogenetic stages and foliage location within individuals.
ACKNOWLEDGEMENTS
We thank Sean Gleason for use of leaf samples from his study sites (Queensland EPA permit no. WITK03219805), Peter Grubb and an anonymous referee for helpful comments on the manuscript, and Frank Zich (Australian Tropical Herbarium, CSIRO) and Peter Lowry (Missouri Botanical Garden) for advising on nomenclature of some Queensland and New Caledonian species, respectively. This research was supported in part by Monash Small Grants (to J.R. and G.D.S.) and the Programme de Conservation des Forêts Sèches (to M.G.).
APPENDIX
The species used in this study, their site and vegetation of origin
Species are given by alphabetical listing of family within each region and vegetation type. For species at Tutanning, Western Australia, D indicates species growing in woodland on dolerite, S indicates those in kwongan on grey sands, and L indicates plants growing in kwongan on laterite. The references indicate where study site and vegetation descriptions can be found. Species nomenclature generally follows Jaffré et al. (2004) for New Caledonia, Western Australian Herbarium (1998–) for Western Australia, and the Australian Plant Census (2008) for Victoria and Queensland.
Kwongan and woodland at Tutanning, Western Australia, Australia (32°S, 117°E; 300 m asl): mean annual rainfall of 448 mm, total soil N of 0·01, 0·14 and 0·22 %, and total soil P of 54, 124 and 330 mg kg−1 for grey sands, laterite and dolerite, respectively (details given in Read et al., 2005)
| Daviesia rhombifolia (L) | Fabaceae |
| Gastrolobium parviflorum (D,L) | Fabaceae |
| Gastrolobium spinosum (L,S) | Fabaceae |
| Gastrolobium trilobum (D) | Fabaceae |
| Jacksonia floribunda (L) | Fabaceae |
| Microcorys capitata (L) | Lamiaceae |
| Acacia acuminata (D) | Mimosaceae |
| Acacia meisneri (D) | Mimosaceae |
| Eucalyptus accedens (L) | Myrtaceae |
| Eucalyptus astringens subsp. astringens (D) | Myrtaceae |
| Eucalyptus drummondii (L,S) | Myrtaceae |
| Eucalyptus pachyloma (L) | Myrtaceae |
| Eucalyptus wandoo subsp. wandoo (D) | Myrtaceae |
| Banksia armata var. ignicida (L) | Proteaceae |
| Banksia attenuata (S) | Proteaceae |
| Banksia nobilis subsp. nobilis (L) | Proteaceae |
| Banksia proteoides (L) | Proteaceae |
| Banksia rufa subsp. tutanningensis (L) | Proteaceae |
| Banksia sessilis var. sessilis (S) | Proteaceae |
| Hakea ferruginea (L) | Proteaceae |
| Hakea ruscifolia (S) | Proteaceae |
| Persoonia quinquenervis (S) | Proteaceae |
| Stirlingia latifolia (S) | Proteaceae |
| Dodonaea bursariifolia (D) | Sapindaceae |
| Solanum oldfieldii (D) | Solanaceae |
Rainforest at Wooroonooran National Park, Atherton Tablelands, Queensland, Australia (17°S, 146°E, 700–800 m asl): mean annual rainfall of ∼3500 mm, total soil N of 0·32 %, total soil P of 1515 mg kg−1 on basalt soils (Gleason et al., 2009)
| Schefflera actinophylla | Araliaceae |
| Homalanthus novoguineensis | Euphorbiaceae |
| Elaeocarpus angustifolius | Elaeocarpaceae |
| Apodytes brachystylis | Icacinaceae |
| Cryptocarya mackinnoniana | Lauraceae |
| Litsea leefeana | Lauraceae |
| Neolitsea dealbata | Lauraceae |
| Argyrodendron peralatum | Malvaceae |
| Argyrodendron trifoliolatum | Malvaceae |
| Franciscodendron laurifolium | Malvaceae |
| Aglaia tomentosa | Meliaceae |
| Myristica insipida | Myristicaceae |
| Pilidiostigma tropicum | Myrtaceae |
| Rhodomyrtus pervagata | Myrtaceae |
| Cardwellia sublimis | Proteaceae |
| Alphitonia sp. | Rhamnaceae |
| Acronychia acidula | Rutaceae |
| Melicope xanthoxyloides | Rutaceae |
| Castanospora alphandii | Sapindaceae |
Understorey shrubs and small trees of eucalypt forest at Bunyip State Park, Victoria, Australia (37°S, 145°E; approx. 180 m asl): mean annual rainfall of 937 mm at Labertouche (approx.12 km south at 77 m asl) (Bureau of Meteorology, Australia; no soil data available)
| Olearia lirata | Asteraceae |
| Goodenia ovata | Goodeniaceae |
| Prostanthera lasianthos | Lamiaceae |
| Acacia myrtifolia | Mimosaceae |
| Hedycarya angustifolia | Monimiaceae |
| Banksia marginata | Proteaceae |
| Banksia spinulosa | Proteaceae |
| Grevillea barklyana | Proteaceae |
| Lomatia fraseri | Proteaceae |
| Pomaderris aspera | Rhamnaceae |
| Spyridium parvifolium | Rhamnaceae |
| Boronia muelleri | Rutaceae |
| Correa reflexa var. reflexa | Rutaceae |
| Leionema bilobum | Rutaceae |
| Zieria arborescens subsp. arborescens | Rutaceae |
New Caledonia
| (A) Rainforest on ultramafic soils (22°S, 167° E; 250–940 m asl): mean annual rainfall of approx. 2425 mm, total soil N of 0·24 %, total soil P of 93 mg kg−1 (averaged across sites) on ultramafic soils formed over peridotite and gabbro (details given in Read et al., 2000, 2006b; Chatain et al., 2009) | |
| Semecarpus neocaledonica | Anacardiaceae |
| Cerberiopsis candelabra var. candelabra | Apocynaceae |
| Myodocarpus fraxinifolius | Araliaceae |
| Deplanchea speciosa | Bignoniaceae |
| Calophyllum caledonicum | Clusiaceae |
| Codia discolor | Cunoniaceae |
| Hibbertia lucens | Dilleniaceae |
| Diospyros parviflora | Ebenaceae |
| Elaeocarpus yateensis | Elaeocarpaceae |
| Styphelia pancheri | Ericaceae |
| Neoguillauminia cleopatra | Euphorbiaceae |
| Flindersia fournieri | Flindersiaceae |
| Cryptocarya guillauminii | Lauraceae |
| Acropogon dzumacensis | Malvaceae |
| Ficus austrocaledonica | Moraceae |
| Arillastrum gummiferum | Myrtaceae |
| Nothofagus aequilateralis | Nothofagaceae |
| Nothofagus balansae | Nothofagaceae |
| Nothofagus codonandra | Nothofagaceae |
| Nothofagus discoidea | Nothofagaceae |
| Alphitonia neocaledonica | Rhamnaceae |
| Crossostylis grandiflora | Rhizophoraceae |
| Guettarda eximia | Rubiaceae |
| Storthocalyx chryseus | Sapindaceae |
| Planchonella kuebiniensis | Sapotaceae |
| Gastrolepis austrocaledonica | Stemonuraceae |
| Strasburgeria robusta | Strasburgeriaceae |
| (B) Maquis on ultramafic soils (22°S, 166°E; 30–100 m asl): mean annual rainfall of approx. 1820 mm, total soil N of 0·08 %, total soil P of 157 mg kg−1 (averaged across sites; Read et al., 2006a). The vegetation ranged from shrubby maquis (‘le maquis arbustif’) on brown eutrophic hypermagnesian soils formed over serpentinite to ligno-herbaceous maquis on ferrallitic soils formed over peridotite (Jaffré, 1980; Read et al., 2006a). | |
| Polyscias pancheri | Araliaceae |
| Peripterygia marginata | Celastraceae |
| Montrouziera sphaeroidea | Clusiaceae |
| Codia spathulata | Cunoniaceae |
| Pancheria alaternoides | Cunoniaceae |
| Hibbertia lucens | Dilleniaceae |
| Dubouzetia campanulata | Elaeocarpaceae |
| Styphelia cymbulae | Ericaceae |
| Longetia buxoides | Euphorbiaceae |
| Scaevola beckii | Goodeniaceae |
| Acacia spirorbis | Mimosaceae |
| Cloezia artensis var. artensis | Myrtaceae |
| Xanthostemon pubescens | Myrtaceae |
| Grevillea gillivrayi var. gillivrayi | Proteaceae |
| Stenocarpus umbelliferus var. billardieri | Proteaceae |
| Alphitonia neocaledonica | Rhamnaceae |
| Normandia neocaledonica | Rubiaceae |
| Homalium betulifolium | Salicaceae |
| Dodonaea viscosa | Sapindaceae |
| Cupaniopsis tontoutensis | Sapindaceae |
| Solmsia calophylla | Thymelaeaceae |
| (C) Dry forest (22°S, 166°E; 5–60 m asl): mean annual rainfall of approx. 945 mm, total soil N of 0·49 %, total soil P of 534 mg kg−1 (averaged across sites; Read et al., 2006a). The forest occurred on brown eutrophic soils (eutric cambisols) derived largely from basic sedimentary rock (Read et al., 2006a). Recent work suggests that the forest type includes dry and mesic sub-types (Jaffré et al., 2008) | |
| Alyxia tisserantii | Apocynaceae |
| Carissa ovata | Apocynaceae |
| Melodinus celastroides | Apocynaceae |
| Capparis artensis | Capparaceae |
| Diospyros fasciculosa | Ebenaceae |
| Cleistanthus stipitatus | Euphorbiaceae |
| Codiaeum peltatum | Euphorbiaceae |
| Croton insularis | Euphorbiaceae |
| Fontainea pancheri | Euphorbiaceae |
| Premna serratifolia | Lamiaceae |
| Dysoxylum bijugum | Meliaceae |
| Acacia spirorbis | Mimosaceae |
| Malaisia scandens | Moraceae |
| Cloezia artensis var. artensis | Myrtaceae |
| Eugenia sp. | Myrtaceae |
| Jasminum didymum | Oleaceae |
| Gardenia urvillei | Rubiaceae |
| Psydrax odorata | Rubiaceae |
| Homalium deplanchei | Salicaceae |
| Arytera arcuata | Sapindaceae |
| Solanum pancheri | Solanaceae |
| Wikstroemia indica | Thymelaeaceae |
LITERATURE CITED
- Abe T, Higashi M. Cellulose centered perspective on terrestrial community structure. Oikos. 1991;60:127–133. [Google Scholar]
- Agrawal AA, Fishbein M. Plant defense syndromes. Ecology. 2006;87:S132–S149. doi: 10.1890/0012-9658(2006)87[132:pds]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Asquith TN, Butler LG. Use of dye-labeled protein as spectrophotometric assay for protein precipitants such as tannin. Journal of Chemical Ecology. 1985;11:1535–1544. doi: 10.1007/BF01012199. [DOI] [PubMed] [Google Scholar]
- Australian Plant Census. IBIS database. Centre for Plant Biodiversity Research, Council of Heads of Australian Herbaria; 2008. http://www.chah.gov.au/apc/index.html. accessed 28 August 2008. [Google Scholar]
- Ayres MP, Clausen TP, MacLean SF, Redman AM, Reichardt PB. Diversity of structure and antiherbivore activity in condensed tannins. Ecology. 1997;78:1696–1712. [Google Scholar]
- Balouet JC. The fossil vertebrate record of New Caledonia. In: Vickers-Rich P, Monaghan JM, Baird RF, Rich TH, editors. Vertebrate palaeontology of Australasia. Melbourne: Pioneer Design Studio in cooperation with the Monash University Publications Committee; 1991. pp. 1383–1409. [Google Scholar]
- Behmer ST, Simpson SJ, Raubenheimer D. Herbivore foraging in chemically heterogeneous environments: nutrients and secondary metabolites. Ecology. 2002;83:2489–2501. [Google Scholar]
- Berenbaum M. Brementown revisited: interactions among allelochemicals in plants. Recent Advances in Phytochemistry. 1985;19:139–169. [Google Scholar]
- Boege K. Herbivore attack in Casearia nitida influenced by plant ontogenetic variation in foliage quality and plant architecture. Oecologia. 2005;143:117–125. doi: 10.1007/s00442-004-1779-9. [DOI] [PubMed] [Google Scholar]
- Brunt C, Read J, Sanson GD. Changes in resource concentration and defence during leaf development in a tough-leaved (Nothofagus moorei) and soft-leaved (Toona ciliata) species. Oecologia. 2006;148:583–592. doi: 10.1007/s00442-006-0369-4. [DOI] [PubMed] [Google Scholar]
- Chatain A, Read J, Jaffré T. Does leaf-level nutrient-use-efficiency explain Nothofagus-dominance of some tropical rain forests in New Caledonia? Plant Ecology. 2009 doi: 10.1007/s11258-008-9477-z. [Google Scholar]
- Choong MF. What makes a leaf tough and how this affects the pattern of Castanopsis fissa leaf consumption by caterpillars. Functional Ecology. 1996;10:668–674. [Google Scholar]
- Choong MF, Lucas PW, Ong JSY, Pereira B, Tan HTW, Turner IM. Leaf fracture toughness and sclerophylly: their correlations and ecological implications. New Phytologist. 1992;121:597–610. [Google Scholar]
- Clissold FJ. The biomechanics of chewing and plant fracture: mechanisms and implications. Advances in Insect Physiology. 2007;34:317–372. [Google Scholar]
- Close DC, McArthur C. Rethinking the role of many plant phenolics – protection from photodamage not herbivores? Oikos. 2002;99:166–172. [Google Scholar]
- Coley PD. Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecological Monographs. 1983;53:209–233. [Google Scholar]
- Coley PD, Bryant JP, Chapin FS. Resource availability and plant antiherbivore defense. Science. 1985;230:895–899. doi: 10.1126/science.230.4728.895. [DOI] [PubMed] [Google Scholar]
- Cork SJ, Krockenberger AK. Methods and pitfalls of extracting condensed tannins and other phenolics from plants: insights from investigations on Eucalyptus leaves. Journal of Chemical Ecology. 1991;17:123–134. doi: 10.1007/BF00994426. [DOI] [PubMed] [Google Scholar]
- van Dam NM, de Jong TJ, Iwasa Y, Kubo T. Optimal distribution of defences: are plants smart investors? Functional Ecology. 1996;10:128–136. [Google Scholar]
- Davies TJ, Barraclough TG, Chase MW, Soltis PS, Soltis DE, Savolainen V. Darwin's abominable mystery: insights from a supertree of the angiosperms. Proceedings of the National Academy of Sciences, USA. 2004;101:1904–1909. doi: 10.1073/pnas.0308127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TA. Relating UV-B radiation screening effectiveness of foliage to absorbing-compound concentration and anatomical characteristics in a diverse group of plants. Oecologia. 1993;95:542–550. doi: 10.1007/BF00317439. [DOI] [PubMed] [Google Scholar]
- Dominy NJ, Lucas PW, Wright SJ. Mechanics and chemistry of rain forest leaves: canopy and understorey compared. Journal of Experimental Botany. 2003;54:2007–2014. doi: 10.1093/jxb/erg224. [DOI] [PubMed] [Google Scholar]
- Downum K, Lee D, Hallé F, Quirke M, Towers N. Plant secondary compounds in the canopy and understorey of a tropical rain forest in Gabon. Journal of Tropical Ecology. 2001;17:477–481. [Google Scholar]
- Edwards PJ. Insect herbivory and plant defence theory. In: Grubb PJ, Whittaker JB, editors. Toward a more exact ecology. Oxford: Blackwell Scientific Publications; 1988. pp. 275–297. [Google Scholar]
- Givnish T. On the adaptive significance of leaf form. In: Solbrig OT, Raven PH, Jain S, Johnson GB, editors. Topics in plant population biology. New York: Columbia University Press; 1979. pp. 375–407. [Google Scholar]
- Gleadow RM, Woodrow IE. Temporal and spatial variation in cyanogenic glycosides in Eucalyptus cladocalyx. Tree Physiology. 2000;20:591–598. doi: 10.1093/treephys/20.9.591. [DOI] [PubMed] [Google Scholar]
- Gleason SM, Williams LJ, Read J, Metcalfe DJ, Baker PJ. Cyclone effects on the structure and production of a tropical upland rainforest: implications for life-history tradeoffs. Ecosystems. 2008;11:1277–1290. [Google Scholar]
- Graham HD. Stabilization of the Prussian blue color in the determination of polyphenols. Journal of Agricultural and Food Chemistry. 1992;40:801–805. [Google Scholar]
- Grubb PJ. Sclerophylls, pachyphylls and pycnophylls: the nature and significance of hard leaf surfaces. In: Juniper B, Southwood R, editors. Insects and the plant surface. London: Edward Arnold; 1986. pp. 137–150. [Google Scholar]
- Gulmon SL, Mooney HA. Costs of defense and their effects on plant productivity. In: Givnish TJ, editor. On the economy of plant form and function. Cambridge: Cambridge University Press; 1986. pp. 681–698. [Google Scholar]
- Güsewell S. N:P ratios in terrestrial plants: variation and functional significance. New Phytologist. 2004;164:243–266. doi: 10.1111/j.1469-8137.2004.01192.x. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Butler LG. Tannins and lignins. In: Rosenthal GA, Berenbaum MR, editors. Herbivores: their interactions with secondary plant metabolites. 2nd edn. Vol. 1. New York: Academic Press; 1991. pp. 355–388. [Google Scholar]
- Hagerman AE, Robbins CT. Specificity of tannin-binding salivary proteins relative to diet selection by mammals. Canadian Journal of Zoology. 1993;71:628–633. [Google Scholar]
- Hagerman AE, Riedl KM, Jones GA, et al. High molecular weight plant polyphenolics (tannins) as biological antioxidants. Journal of Agricultural and Food Chemistry. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB. Herbivory, serotiny and seedling defence in Western Australian Proteaceae. Oecologia. 2001;126:409–417. doi: 10.1007/s004420000538. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB. Relationships between physical and chemical attributes of congeneric seedlings: how important is seedling defence? Functional Ecology. 2002;16:216–222. [Google Scholar]
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematics. 2007;8:157–178. [Google Scholar]
- Harborne JB. The chemical basis of plant defense. In: Palo RT, Robbins CT, editors. Plant defenses against mammalian herbivory. Boca Raton, FL: CRC Press; 1991. pp. 45–59. [Google Scholar]
- Heide-Jørgensen HS. Xeromorphic leaves of Hakea suaveolens R. Br. IV. Ontogeny, structure and function of the sclereids. Australian Journal of Botany. 1990;38:25–43. [Google Scholar]
- Herms DA, Mattson WJ. The dilemma of plants: to grow or defend. Quarterly Review of Biology. 1992;6:283–335. [Google Scholar]
- Iwasa Y. Dynamic optimization of plant growth. Evolutionary Ecology Research. 2000;2:437–455. [Google Scholar]
- Jaffré T. Étude écologique du peuplement végétal des sols dérivés de roches ultrabasiques en Nouvelle Calédonie. 1980 Travaux et Documents de l'Orstom no. 124. [Google Scholar]
- Jaffré T. The relationship between ecological diversity and floristic diversity in New Caledonia. Biodiversity Letters. 1993;1:82–87. [Google Scholar]
- Jaffré T, Morat P, Veillon J.-M, Rigault F, Dagostini G. Composition et caractérisation de la flore indigène de Nouvelle-Calédonie. 2004 IRD Documents Scientifiques et Techniques II 4, Noumea. [Google Scholar]
- Jaffré T, Rigault F, Munzinger J. Identification and characterization of floristic groups in dry forests relicts of a West Coast region of New Caledonia. Pacific Conservation Biology. 2008;14:128–145. [Google Scholar]
- Jordan GJ, Dillon RA, Weston PH. Solar radiation as a factor in the evolution of scleromorphic leaf anatomy in Proteaceae. American Journal of Botany. 2005;92:789–796. doi: 10.3732/ajb.92.5.789. [DOI] [PubMed] [Google Scholar]
- Kalisz S, Kramer EM. Variation and constraint in plant evolution and development. Heredity. 2008;100:171–177. doi: 10.1038/sj.hdy.6800939. [DOI] [PubMed] [Google Scholar]
- Karowe DN. Differential effect of tannic acid on two tree-feeding Lepidoptera: implications for theories of plant anti-herbivore chemistry. Oecologia. 1985;80:507–512. doi: 10.1007/BF00380074. [DOI] [PubMed] [Google Scholar]
- Koricheva J, Nykänen H, Gianoli E. Meta-analysis of trade-offs among plant antiherbivore defenses: are plants jacks-of-all-trades, masters of all? American Naturalist. 2004;163:E64–E75. doi: 10.1086/382601. [DOI] [PubMed] [Google Scholar]
- Kraus TEC, Dahlgren RA, Zasoski RJ. Tannins in nutrient dynamics of forest ecosystems – a review. Plant and Soil. 2003;256:41–66. [Google Scholar]
- Kursar TA, Coley PD. Convergence in defense syndromes of young leaves in tropical rainforests. Biochemical Systematics and Ecology. 2003;31:929–949. [Google Scholar]
- Langenheim JH, Macedo CA, Ross MK, Stubblebine WH. Leaf development in the tropical leguminous tree Copaifera in relation to microlepidopteran herbivory. Biochemical Systematics and Ecology. 1986;14:51–59. [Google Scholar]
- Larcher W. Climatic constraints drive the evolution of low temperature resistance in woody plants. Journal of Agricultural Meteorology. 2005;61:189–202. [Google Scholar]
- Lee KP, Raubenheimer D, Simpson SJ. The effects of nutritional imbalance on compensatory feeding for cellulose-mediated dietary dilution in a generalist caterpillar. Physiological Entomology. 2004;29:108–117. [Google Scholar]
- Lucas PW, Turner IM, Dominy NJ, Yamashita N. Mechanical defences to herbivory. Annals of Botany. 2000;86:913–920. [Google Scholar]
- Mali S, Borges RM. Phenolics, fibre, alkaloids, saponins, and cyanogenic glycosides in a seasonal cloud forest in India. Biochemical Systematics and Ecology. 2003;31:1221–1246. [Google Scholar]
- Mazza CA, Boccalandro HE, Giordano CV, Battista D, Scopel AL, Ballaré CL. Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiology. 2000;122:117–125. doi: 10.1104/pp.122.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy S, Jaffré T, Rigault F, Ash JE. Fire and succession in the ultramafic maquis of New Caledonia. Journal of Biogeography. 1999;26:579–594. [Google Scholar]
- McKey DD. The distribution of secondary compounds within plants. In: Rosenthal GA, Janzen DH, editors. Herbivores: their interaction with secondary plant metabolites. New York: Academic Press; 1979. pp. 55–133. [Google Scholar]
- Mole S, Ross JAM, Waterman PG. Light-induced variation in phenolic levels in foliage of rain-forest plants. I. Chemical changes. Journal of Chemical Ecology. 1988;14:1–21. doi: 10.1007/BF01022527. [DOI] [PubMed] [Google Scholar]
- Morat Ph, Jaffré T, Veillon J-M, MacKee HS. Affinités floristiques et considérations sur l'origine des maquis miniers de la Nouvelle-Calédonie. Bulletin du Museum National de Histoire Naturelle, Paris 4e sér. 8 section B, Adansonia. 1986;2:133–182. [Google Scholar]
- Mueller-Harvey I. Analysis of hydrolysable tannins. Animal Feed Science and Technology. 2001;91:3–20. [Google Scholar]
- Oertli JJ, Lips SH, Agami M. The strength of sclerophyllous cells to resist collapse due to negative turgor pressure. Acta Œcologica. 1990;11:281–289. [Google Scholar]
- Ossipov V, Haukioja E, Ossipova S, Hanhimäki S, Pihlaja K. Phenolic and phenolic-related factors as determinants of suitability of mountain birch leaves to an herbivorous insect. Biochemical Systematics and Ecology. 2001;29:223–240. doi: 10.1016/s0305-1978(00)00069-7. [DOI] [PubMed] [Google Scholar]
- Ossipova S, Ossipov V, Haukioja E, Loponen J, Pihlaja K. Proanthocyanidins of Mountain Birch leaves: quantification and properties. Phytochemical Analysis. 2001;12:128–133. doi: 10.1002/pca.568. [DOI] [PubMed] [Google Scholar]
- Peeters PJ, Read J, Sanson GD. Variation in the guild composition of herbivorous insect assemblages among co-occurring plant species. Austral Ecology. 2001;26:385–399. [Google Scholar]
- Peeters PJ, Sanson G, Read J. Leaf biomechanical properties and the densities of herbivorous insect guilds. Functional Ecology. 2007;21:246–255. [Google Scholar]
- Price ML, Butler LG. Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. Journal of Agricultural and Food Chemistry. 1977;25:1268–73. [Google Scholar]
- Rafferty C, Lamont BB, Hanley ME. Selective feeding by kangaroos (Macropus fuliginosus) on seedlings of Hakea species: effects of chemical and physical defences. Plant Ecology. 2005;177:201–208. [Google Scholar]
- Read J, Sanson GD. Characterising sclerophylly: the mechanical properties of a diverse range of leaf types. New Phytologist. 2003;160:81–99. doi: 10.1046/j.1469-8137.2003.00855.x. [DOI] [PubMed] [Google Scholar]
- Read J, Stokes A. Plant biomechanics in an ecological context. American Journal of Botany. 2006;93:1546–1565. doi: 10.3732/ajb.93.10.1546. [DOI] [PubMed] [Google Scholar]
- Read J, Jaffré T, Godrie E, Hope GS, Veillon J-M. Structural and floristic characteristics of some monodominant and adjacent mixed rainforests in New Caledonia. Journal of Biogeography. 2000;27:233–250. [Google Scholar]
- Read J, Sanson GD, Lamont BB. Leaf mechanical properties in sclerophyll woodland and shrubland on contrasting soils. Plant and Soil. 2005;276:95–113. [Google Scholar]
- Read J, Sanson GD, de Garine-Wichatitsky M, Jaffré T. Sclerophylly in two contrasting tropical environments: low nutrients vs. low rainfall. American Journal of Botany. 2006;a 93:1601–1614. doi: 10.3732/ajb.93.11.1601. [DOI] [PubMed] [Google Scholar]
- Read J, Jaffré T, Ferris JM, McCoy S, Hope GS. Does soil determine the boundaries of monodominant rain forest with adjacent mixed rain forest and maquis on ultramafic soils in New Caledonia? Journal of Biogeography. 2006;b 33:1055–106. [Google Scholar]
- Reich PB, Uhl C, Walters MB, Ellsworth DS. Leaf lifespan as a determinant of leaf structure and function among 23 amazonian tree species. Oecologia. 1991;86:16–24. doi: 10.1007/BF00317383. [DOI] [PubMed] [Google Scholar]
- Rhoades DF. Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Janzen DH, editors. Herbivores: their interaction with secondary plant metabolites. New York: Academic Press; 1979. pp. 3–54. [Google Scholar]
- Riipi M, Ossipov V, Lempa K, et al. Seasonal changes in birch leaf chemistry: are there trade-offs between leaf growth and accumulation of phenolics? Oecologia. 2002;130:380–390. doi: 10.1007/s00442-001-0826-z. [DOI] [PubMed] [Google Scholar]
- Roderick ML, Berry SL, Noble IR, Farquhar GD. A theoretical approach to linking the composition and morphology with the function of leaves. Functional Ecology. 1999;13:683–695. [Google Scholar]
- Sanson G. The biomechanics of browsing and grazing. American Journal of Botany. 2006;93:1531–1545. doi: 10.3732/ajb.93.10.1531. [DOI] [PubMed] [Google Scholar]
- Sanson G, Read J, Aranwela N, Clissold F, Peeters P. Measurement of leaf biomechanical properties in studies of herbivory: opportunities, problems and procedures. Austral Ecology. 2001;26:535–546. [Google Scholar]
- Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–3883. [Google Scholar]
- Stamp N. Out of the quagmire of plant defense hypotheses. Quarterly Review of Biology. 2003;78:23–55. doi: 10.1086/367580. [DOI] [PubMed] [Google Scholar]
- Terashima I, Miyazawa S-I, Hanba YT. Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. Journal of Plant Research. 2001;114:93–105. [Google Scholar]
- Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S. Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. Journal of Experimental Botany. 2006;57:343–354. doi: 10.1093/jxb/erj014. [DOI] [PubMed] [Google Scholar]
- Twigg LE, Socha LV. Physical versus chemical defence mechanisms in toxic Gastrolobium. Oecologia. 1996;108:21–28. doi: 10.1007/BF00333210. [DOI] [PubMed] [Google Scholar]
- Villar R, Merino J. Comparisons of leaf construction costs in woody species with differing leaf life-spans in contrasting ecosystems. New Phytologist. 2001;151:213–226. doi: 10.1046/j.1469-8137.2001.00147.x. [DOI] [PubMed] [Google Scholar]
- Webb CO, Ackerly DD, Kembel SW. Phylocom: software for the analysis of phylogenetic community structure and character evolution. 2008. Version 4.0 URL: http://phylodiversity.net/phylocom/ [DOI] [PubMed]
- Western Australian Herbarium. FloraBase – the Western Australian Flora. Department of Environment and Conservation; 1998. http://florabase.calm.wa.gov.au/ accessed 18 August 2008. [Google Scholar]
- Witkowski ETF, Lamont BB, Walton CS, Radford S. Leaf demography, sclerophylly and ecophysiology of two Banksia species with contrasting leaf life spans. Australian Journal of Botany. 1992;40:849–862. [Google Scholar]
- Wright IJ, Westoby M. Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. New Phytologist. 2002;155:403–416. doi: 10.1046/j.1469-8137.2002.00479.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Joern A. Influence of diet quality, developmental stage, and temperature on food residence time in the grasshopper Melanoplus differentialis. Physiological Zoology. 1994;67:598–616. [Google Scholar]