Abstract
Background and Aims
Changes in rainfall and temperature brought about through climate change may affect plant species distribution and community composition of grasslands. The primary objective of this study was to test how manipulation of water and temperature would influence the plasticity of stomatal density and leaf area of bluebunch wheatgrass, Pseudoroegneria spicata. It was hypothesized that: (1) an increased water supply will increase biomass and leaf area and decrease stomatal density, while a reduced water supply will cause the opposite effect; (2) an increase in temperature will reduce biomass and leaf area and increase stomatal density; and (3) the combinations of water and temperature treatments can be aligned along a stress gradient and that stomatal density will be highest at high stress.
Methods
The three water supply treatments were (1) ambient, (2) increased approx. 30 % more than ambient through weekly watering and (3) decreased approx. 30 % less than ambient by rain shades. The two temperature treatments were (1) ambient and (2) increased approx. 1–3 °C by using open-top chambers. At the end of the second experimental growing season, above-ground biomass was harvested, oven-dried and weighed, tillers from bluebunch wheatgrass plants sampled, and the abaxial stomatal density and leaf area of tillers were measured.
Key Results
The first hypothesis was partially supported – reducing water supply increased stomatal density, but increasing water supply reduced leaf area. The second hypothesis was rejected. Finally, the third hypothesis could not be fully supported – rather than a linear response there appears to be a parabolic stomatal density response to stress.
Conclusions
Overall, the abaxial stomatal density and leaf area of bluebunch wheatgrass were plastic in their response to water and temperature manipulations. Although bluebunch wheatgrass has the potential to adapt to changing climate, the grass is limited in its ability to respond to a combination of reduced water and increased temperature.
Key words: Bluebunch wheatgrass, Pseudoroegneria spicata, biomass, climate change, grassland, open top chamber, rain shade, stomata
INTRODUCTION
Recent predictions of global climate change suggest that there will be an increased frequency of heat waves, increased areas of drought, increased frequency of heavy precipitation events, and increased winter temperatures (Intergovernmental Panel on Climate Change, 2007). The ability of species to respond and adapt to changing environments will therefore be critical. Some terrestrial plant populations have been extending their ranges toward the poles or to higher elevations since the retreat of the last ice age, but within the past several decades the rate of range extension has increased due to changing climate (Parmesan and Yohe, 2003; Tape et al., 2006). In addition, certain plant traits, such as phenology and leaf morphology, appear to have changed in some cases, with leaf expansion and flowering occurring earlier in the spring (Parmesan and Yohe, 2003; Root et al., 2003), and evidence that increases in atmospheric CO2 concentration causes a reduction in stomatal density (Woodward, 1987; Woodward and Kelly, 1995).
For many of the world's grasslands, climate change models predict hotter drier summers along with warmer winters and increased rainfall (Environment Canada, 2003; Intergovernmental Panel on Climate Change, 2007). The amount and timing of precipitation is likely to be important especially for those species near their limits of distribution. The interior grasslands of British Columbia (BC), Canada represent the northern limit of many grassland species (Tisdale, 1947) and individuals at the limits of a species range are important indicators of genetic variation and potential for adaptation (Lesica and Allendorf, 1995; Tape et al., 2006). In these grasslands, bluebunch wheatgrass (Pseudoroegneria spicata) is one of the dominant native species (van Ryswyk et al., 1966) and is considered one of the most important forage grass species on western rangelands for livestock and wildlife (Parish et al., 1996; Bawtree et al., 1998). The range of bluebunch wheatgrass extends into Alaska, but only along the coast. In the interior of the continent, the northern limits of its range do not extend beyond BC.
To assess bluebunch wheatgrass' response to the predicted changes in climate, three parameters – stomatal density, leaf area and plant above-ground biomass – were measured under manipulated temperature and water conditions at three experimental sites. Stomata have been described as ‘the necessary evil’ (Sutcliffe, 1974); they are essential for carbon dioxide acquisition but at the cost of water loss (Beerling et al., 1993). The development of stomata is considered a critical stage in the evolution of advanced land plants (Hetherington and Woodward, 2003). Stomatal diffusion resistance, and hence conductance, is directly related to the size and spacing of stomata on the leaf surface, i.e. a trade-off between size and number of stomata (Jones, 1992; Beerling et al., 1993; Wang et al., 2007). A leaf with many, small stomata can reduce potential conductance and increase water-use efficiency (Poulos et al., 2007). Compared with stomatal length (e.g. guard cell length or stomatal aperture length), stomatal density is relatively plastic (Richardson et al., 2001) and potentially adaptive to environmental change (Carpenter and Smith, 1975; Woodward, 1987; Poulos et al., 2007; Lake and Woodward, 2008; Sekiya and Yano, 2008). Studies have shown that stomatal density is affected by soil water (Bañon et al., 2004; Sekiya and Yano, 2008; Xu and Zhou, 2008), light (Schoch et al., 1980; Thomas et al., 2003), CO2 (Woodward, 1987; Woodward and Kelly, 1995; Woodward et al., 2002), O2 (Ramonell et al., 2001), soil phosphorus (Sekiya and Yano, 2008) and UV-B (Gitz et al., 2004). Due to the relationship between stomata and the amount of water lost, the density of stomata is an important ecophysiological trait, especially in water-limited environments (Peat and Fitter, 1994; Croxdale, 2000; Sack et al., 2003; Poulos et al., 2007; Xu and Zhou, 2008).
Leaf area was also measured because there is evidence of a phenotypic response in relation to photosynthetic potential and growth – the larger the leaf the greater the growth rate (Grime, 1979; Raven et al., 1999; Gianoli and Gonzalez-Teuber, 2005; Maseda and Fernández, 2006; Xu and Zhou, 2008). Because grasses die back to their base each year, leaf area is a comparative measure of the current year's growth (Gurevitch et al., 2002).
Above-ground biomass was measured as an assessment of overall plant growth and performance, and to determine if the two plant leaf traits (stomatal density and leaf area) correlate with growth. These three parameters – stomatal density, leaf area, and biomass – were used to provide a quantitative assessment of the plants' response to drought and increased temperature.
The objective of this study was to test adaptive phenotypic plasticity of bluebunch wheatgrass to temperature and water manipulations in the field. It was hypothesized that: (a) an increased water supply will increase biomass and leaf area and decrease stomatal density in bluebunch wheatgrass; while a reduction in water supply will have the opposite effect; (b) an increase in temperature will reduce biomass and leaf area and increase stomatal density; and (c) the combinations of water and temperature treatments can be aligned along a stress gradient and that stomatal density will be highest at high stress and lowest at low stress.
MATERIALS AND METHODS
Site description
The field site was in the Pseudoroegneria spicata (bluebunch wheatgrass) and Artemisia tridentata (big sagebrush) grasslands of the Lac Du Bois Grassland Provincial Park north of Kamloops, British Columbia, Canada described by van Ryswyk et al. (1966). Soil organic carbon is approx. 1·75 % and there is an approximate annual precipitation of 270 mm (van Ryswyk et al., 1966). Pseudoroegneria is the dominant species with approx. 50 % cover (C. Carlyle, unpubl. res.). Three study sites were sampled: Site One (56·22751°N, 68·0766°E) at an elevation of 559 m a.s.l.; Site Two (56·25974°N, 68·0692°E) at an elevation of 723 m a.s.l.; and Site Three (56·25976°N, 68·0480°E) at an elevation of 736 m a.s.l. Plant community composition analysis showed no significant differences between the sites (C. Carlyle, unpubl. res.), suggesting that sites could be used as replicates. Other than bluebunch wheatgrass and big sagebrush, common plants included Poa sandbergii, P. pratensis, Koeleria macrantha, Bromus tectorum and Hesperostipa comata.
Experimental design
Above-ground biomass and leaf samples were collected between 26 July and 1 August 2006 from a pre-existing, continuing climatic manipulation experiment that began in April 2005. The 1-m2 experimental plots were part of a fully factorial design with three water and two temperature manipulations at three sites. The three water treatments were (1) water added, (2) water reduced and (3) an unmanipulated control. The additional precipitation was 30 % of the monthly average administered weekly; this amount varied by month but ranged from 1·1 to 2·4 L plot−1 week−1. Precipitation was reduced using rain shades (Kochy and Wilson, 2004) placed over the plots in the direction of the prevailing wind. Temperature was either ambient, or increased by using open top chambers (Zavaleta et al., 2003; Klein et al., 2004). Each of the water × temperature treatment combinations was replicated three times at each of the three sites for a total of 54 experimental plots. The water × temperature treatment combinations represented a stress gradient, from low stress (increased water and control temperature) to high stress (decreased water and increased temperature).
Six bluebunch wheatgrass tillers were harvested from the fully developed (i.e. ligule present) leaves of two plants within all plots for a total of 324 tillers. The air-dried samples were stored at room temperature in paper bags until processing.
Sample processing
Soil moisture and temperature were measured in 18 plots representing three replicates of the six treatment combinations at a single site (Site Three). The cost of the sensors prevented us from sampling more than one site, and Site Three was randomly selected to test the effect of the climate treatments. Soil moisture [% volumetric water content (m3 water m−3 soil)] was measured in the top 10 cm of the soil layer (Onset Soil Moisture Smart sensors S-SMx-M005; Decagon Devices, Inc., Pullman, WA, USA). Soil temperature was measured at 5 cm below the soil surface (Onset Soil Temperature sensor TMC50 – HD) (Decagon Devices, Inc.). Sensors were located in the centre of the experimental plots. Readings were taken continuously every half hour from May to September 2006.
In August 2006 the above-ground biomass above 5 cm was harvested from 0·5 × 0·5 m plots, sorted to species, oven-dried at 80 °C for at least 48 h, and weighed. However, before clipping the entire plot, the lowest, and therefore oldest, leaf of the six bluebunch wheatgrass tillers were removed for the stomata counts and leaf area measurements. The middle portion of each of these leaves was removed and soaked in a 0·025 m phosphate buffer for a minimum of 48 h. The phosphate buffer rehydrated the cells, making the stomata easier to locate and count. There was a slight variation in the width of the leaves, but because the stomata occurred in two rows, one on each side of the mid-rib, the counting protocol was standardized to a 2·2-cm linear transect along the length of the leaf, counting all stomata on both sides of the mid-rib, rather than trying to identify a per unit area of leaf. Redmann (1985) found a ratio of stomata at 65 : 35 on the adaxial and abaxial leaf surface. The stomata were counted on the abaxial surface (using a Fisher Science Micromaster compound microscope at ×100 magnification; Fisher Scientific Co., Pittsburgh, PA, USA), because there is a greater potential for changes in stomatal number on this side (Redmann, 1985; Gurevitch et al., 2002). The adaxial surface is conducive to retaining a high humidity level due to the morphology of the leaf and the tendency of the leaf to roll inwards, shielding the adaxial surface from wind and sunlight. Whereas the climate boundary layer of the abaxial surface is smaller causing lower humidity levels and a potentially higher rate of evapotranspiration. Therefore, stomatal density on the abaxial surface is more likely to be variable and affected by atmospheric temperature and humidity and water use efficiency. After stomatal counts, the leaf area was measured for each tiller using a LI-COR model LI-3000A portable area meter (LI-COR Inc., Lincoln, NE, USA).
Statistical analysis
Statistical analyses were done using SYSTAT 8·0 for Windows. A regression was done comparing stomatal density to leaf area. Two General Linear Models (GLM) were performed on stomatal density and leaf area with site as the blocking variable and water and temperature as the treatment factors. To meet the assumptions of a normal distribution, stomatal density was transformed to the natural logarithm of the value + 1. Tukey's post-hoc analysis was used to determine significant differences between means. Three GLMs were done on bluebunch wheatgrass biomass, total biomass and the proportion of the total biomass that was bluebunch wheatgrass with site as the blocking variable, and water and temperature as the treatment factors. Bluebunch wheatgrass and total biomass variables were transformed using the natural logarithm. Proportion data were transformed using the square root arcsine function. Tukey's post-hoc analysis was used to determine significant differences between means.
RESULTS
The soil temperature of control plots ranged from 4·1 °C to 42 °C and the open top chambers increased mean soil temperatures 0·5–1·2 °C above control temperatures. The mean daily maximum temperature increase due to the open top chambers ranged from 2·3 to 3·6 °C with an absolute maximum increase of 9·1° C. The soil moisture of control plots ranged from 0 to 17 % and water treatments decreased, and increased, the soil moisture by as much as 2·1 % and 0·3 %, respectively, from control conditions (Table 1). The mean daily maximum increase of soil moisture due to watering was about 0·6 % with an absolute maximum increase of 6·9%. The mean daily maximum decrease of soil moisture due to the rain shades was about 1·6 % with an absolute maximum decrease of 8·1 % below the control plot (Table 1).
Table 1.
Mean (±s.e.) soil moisture and soil temperature measurements in water and temperature treatment plots
| Treatments |
Soil moisture (% volumetric water content; m3 water m−3) | ||
|---|---|---|---|
| Water | Temperature | Soil temperature (°C) | |
| Decrease | Increase | 0·03 (0·00002) | 20·0 (0·05) |
| Control | 0·7 (0·0001) | 20·1 (0·05) | |
| Control | Increase | 1·1 (0·0002) | 19·4 (0·05) |
| Control | 2·4 (0·0002) | 18·9 (0·05) | |
| Increase | Increase | 2·7 (0·0003) | 19·3 (0·05) |
| Control | 2·7 (0·0003) | 18·8 (0·05) | |
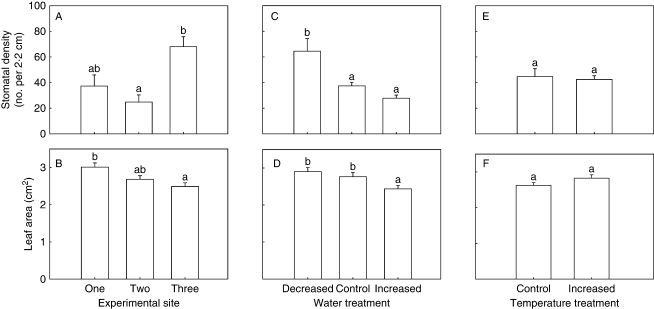
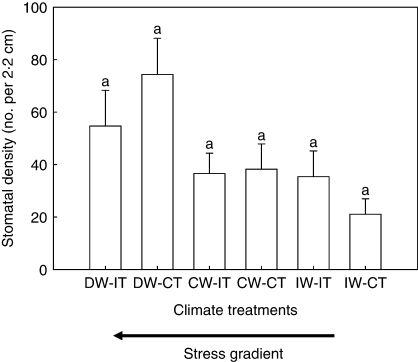
There was no significant relationship between stomatal density and leaf area (R2 = 0·006; P = 0·252). The block effect (site) was significant for stomatal density and leaf area (Table 2). Stomatal density in Site Three was higher than Site Two (Fig. 1A). Plants at Site Three had the lowest leaf areas, while Site One had the highest (Fig. 1B). Stomatal density was affected by water (Table 2 and Fig. 1C), but not temperature (Table 2 and Fig. 1E). An increase in water supply resulted in a reduction in stomatal density (Fig. 1C). Leaf area was affected by water treatment (Table 2 and Fig. 1D), but not temperature (Table 2 and Fig. 1E). An increase in water supply caused a reduction in leaf area (Fig. 1D). The two-way interaction effect between water and temperature was not significant for stomatal density or leaf area (Table 2), but aligning the climate treatments along a stress gradient shows a trend of increasing stomatal density with increasing stress (Fig. 2).
Table 2.
Summary of General Linear Models for bluebunch wheatgrass stomatal density (n = 314) and leaf area (n = 304) with site as the blocking variable and water and temperature as the treatment factors
| Stomatal density |
Leaf area |
||||
|---|---|---|---|---|---|
| Source | d.f. | F-ratio | P | F-ratio | P |
| Block (site) | 2 | 22·173 | <0·001 | 5·859 | 0·003 |
| Water | 2 | 6·048 | 0·003 | 4·039 | 0·019 |
| Temperature | 1 | 0·469 | 0·494 | 2·279 | 0·132 |
| Water × temperature | 2 | 1·096 | 0·336 | 0·097 | 0·907 |
| Error for stomatal density | 306 | ||||
| Error for leaf area | 296 | ||||
Values in bold are significant at P < 0·10.
Fig. 1.
Effects of main treatments (+s.e.) where (A), (C) and (E) are the effects of site, water and temperature, respectively on stomatal density (number of stomata per 2·2-cm length of leaf; n = 314), and (B), (D) and (F) are the effects of site, water and temperature, respectively on leaf area (n = 304). Bars sharing the same letters are not significantly different (P > 0·10) using Tukey's test.
Fig. 2.
Effects of water and temperature treatments (+s.e.) on stomatal density (number of stomata per 2·2-cm length of leaf). D, Decreased; C, control; I, increased; W, water; T, temperature. n = 314. Bars sharing the same letters are not significantly different (P > 0·10) using Tukey's test. The arrow below the x-axis shows the directional increase in climate stress.
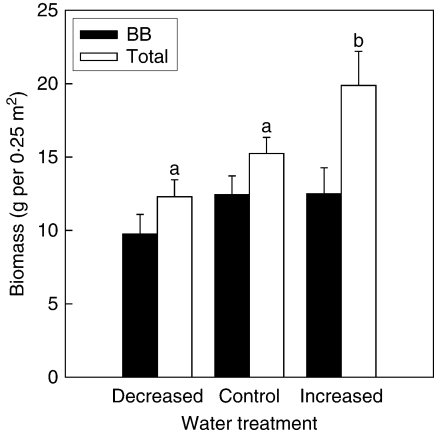
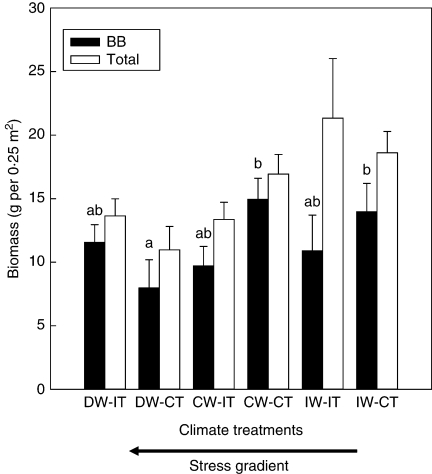
Total biomass of the treatment plots, which included all the plant species, had no blocking effect and no temperature effect, but was affected by the water treatment (Table 3), with increased water supply increasing total biomass (Fig. 3). No interaction effect was detected for total biomass. The biomass of bluebunch wheatgrass within the treatment plots was affected by the interaction between water and temperature treatments, with no blocking effects or main effects (Table 3). One treatment combination, decreased water supply and ambient temperature, had lower amounts of bluebunch wheatgrass than the ambient water and ambient temperature, and the increased water and ambient temperature combination (Fig. 4).
Table 3.
Summary of General Linear Models for bluebunch wheatgrass biomass total biomass, and the proportion of bluebunch wheatgrass to total biomass (n = 50) with site as the blocking variable and water and temperature as the treatment factors
| Biomass |
Total biomass |
Proportion of biomass |
|||||
|---|---|---|---|---|---|---|---|
| Source | d.f. | F-ratio | P | F-ratio | P | F-ratio | P |
| Block (site) | 2 | 0·119 | 0·888 | 0·761 | 0·473 | 1·112 | 0·338 |
| Water | 2 | 0·720 | 0·493 | 4·892 | 0·012 | 0·895 | 0·416 |
| Temperature | 1 | 0·337 | 0·565 | 0·000 | 0·997 | 0·152 | 0·699 |
| Water × temperature | 2 | 2·672 | 0·081 | 1·473 | 0·241 | 1·862 | 0·168 |
| Error | 42 | ||||||
Values in bold are significant at P < 0·10.
Fig. 3.
Effects of water treatment (+s.e.) on bluebunch wheatgrass (BB) and total biomass (n = 50). Total biomass bars sharing the same letters are not significantly different (P > 0·10) using Tukey's test. There was no significant water treatment effect on bluebunch wheatgrass; therefore no letters are displayed.
Fig. 4.
Effects of the water and temperature treatment combinations (+s.e.) on bluebunch wheatgrass (BB) and total biomass (n = 50). D, Decreased; C, control; I, increased; W, water; T, temperature. Bars sharing the same letters are not significantly different (P > 0·10) using Tukey's test. The arrow below the x-axis shows the directional increase in climate stress. There was no significant interaction treatment effect on total biomass; therefore no letters are displayed.
DISCUSSION
The present study has confirmed that stomatal density in bluebunch wheatgrass varies according to water manipulations, but not to temperature. Studies have shown that stomatal density is affected by abiotic parameters (Woodward, 1987; Ramonell et al., 2001; Thomas et al., 2003; Gitz et al., 2004; Sekiya and Yano, 2008), but with the exception of the CO2 study in which field-collected herbarium samples were measured (Woodward, 1987), all of these studies were conducted on a single species grown in greenhouses. Here, it was found that experimentally reducing water supply in the field caused an increase in stomatal density of bluebunch wheatgrass, despite other uncontrolled biotic and abiotic factors in the community. Increasing water supply, however, did not reduce stomatal density. In contrast, a study of 32 xerophytic tree species in Israel showed that irrigation significantly reduced stomatal density, but the amount of water added was much greater than the current study with bluebunch wheatgrass (Gindel, 1969).
Stomatal density varied by site, with mean stomatal numbers of Site Three plants approximately three times that of Site Two plants (Fig. 1A). There is nothing suggestive of differences in site characteristics (e.g. elevation or plant community composition) to explain these differences. It may be that stomatal density is not only a phenotypically plastic plant trait but also genotypically differentiated. Further genetic work is needed to understand this. However, despite site differences, and blocking for site in our general linear models, we still found significant water treatment effects on stomatal density, but not temperature treatment effects.
A study of the growth and development of Quercus robur leaves under experimentally increased temperatures showed a reduction in stomatal density (Beerling and Chaloner, 1993). It is suspected that Beerling and Chaloner (1993) detected a significant temperature effect because the range in mean temperature for their study was approx. 8 °C, whereas the range in temperature in the present study was only approx. 2 °C, which represents a more realistic climate change scenario over the next few decades (Intergovernmental Panel on Climate Change, 2007). It is possible that if temperature was elevated by approx. 4 °C plants might respond with an increase in stomatal density; i.e. there may be a threshold along an environmental gradient where stomatal density will cease to increase and then decrease (see Xu and Zhou, 2008).
Although no other studies that have investigated stomatal density following controlled water supply manipulations in the field are known, leaf adaptations can be compared across elevation gradients, with the assumption that elevation is negatively correlated with water availability. The results of such studies are varied but Körner et al. (1989) and Körner (1999) concluded that there is a general, but inconsistent, trend towards increased stomatal density with increasing elevation – at high elevations there is relatively lower water availability and plants have higher stomatal density. However, the problem with elevational analyses is that many environmental factors that have the potential to influence stomatal density might also be changing along the gradient, with drought and temperature being two of the more important factors.
The x-axis of Fig. 2 was arranged along a gradient of climate stress, with the left-hand side representing the greatest stress (decreased water and increased temperature) and the right-hand side the least stress (increased water and control temperature). The trend shows increased stomatal density from the right to the left, peaking with decreased water and control temperature. Why wasn't the highest stomatal density found in the extreme climate treatment condition? A recent study by Xu and Zhou (2008) also showed a parabolic response of stomatal number – intermediate levels of water deficit increased stomatal numbers but higher levels of water deficit caused a reduction in stomatal number. It was suggested, therefore, that plants may have reached a physiological threshold, where increased climatic stress prevented the plants from responding by increasing stomatal density, or there may have been biotic interactions affecting phenotypic response.
It was decided to measure stomatal density on the abaxial surface of the leaf. Redmann (1985) reported that the ratio of the percentage adaxial stomata to percentage abaxial stomata for grasses ranges from 100 : 0 to 50 : 50 in boreal forests, from 100 : 0 to 60 : 40 in mesic grasslands, and from 85 : 15 to 65 : 35 in xeric grasslands. He also reported that bluebunch wheatgrass had a 65 : 35 stomatal distribution. Redmann (1985) reported that as temperature increased, the range in stomatal distribution decreased and stomata were more consistently found on the abaxial surface. Perhaps in the present study if stomatal density had been measured on the adaxial surface an effect of temperature may have been detected.
A recent study showed that stomatal density of cowpea (Vigna sinensis) was affected by soil phosphorus (P), but that there was a complex interaction between soil water and soil P (Sekiya and Yano, 2008). There was an increase in stomatal density under low soil water at low and medium soil P (50 and 150 mg superphosphate per 5 cm diameter and 15 cm high pot), but this effect was reversed under high soil P (450 mg per pot). Such high soil P is not found in the present study site, where the average soil P is approx. 10 mg L−1 (C. Carlyle, unpubl. res.), otherwise very different results for stomatal density might have been expected.
The hypothesis regarding leaf area was rejected: an increased water supply actually decreased leaf area in bluebunch wheatgrass (Fig. 1D). However, a reduction in water supply had no effect on leaf area, nor did an increase in temperature. Change in leaf area in an Israeli study was also not consistent – irrigation caused an increase in leaf area in five tree species but a decrease in eight tree species (Gindel, 1969). Bluebunch wheatgrass is adapted to the temperate, semi-arid steppe and open woodland regions of western North America. As a xerophyte, it is adapted to drought conditions and is less sensitive to reductions in water supply (Kobl and Robberecht, 1996), i.e. when experiencing drought imposed by the rain shades, the plants may not need to alter their gross leaf morphology to survive. However, with an increase in water supply the response of bluebunch wheatgrass to the resource opportunity does not seem to reflect a potential for increased growth rate. Indeed, there was no significant increase in bluebunch wheatgrass biomass with increased water supply (Fig. 3).
Bluebunch wheatgrass biomass was affected by the interaction between water and temperature (Fig. 4). The fact that biomass is reduced in decreased water supply at ambient temperature compared with control water with ambient temperature and increased water with ambient temperature indicates that decreasing water will reduce the relative abundance of the grass within the community. Therefore, the adaptive phenotypic plasticity of leaf traits, such as stomatal density and leaf area, is critical for the plant's survival.
Why wasn't the least amount of bluebunch wheatgrass biomass found in the low water-increased temperature treatment (Fig. 4); i.e. the treatment with the greatest climatic stress? It is possible that bluebunch wheatgrass may be able to tolerate the extreme climate conditions better than its neighbours. If so, bluebunch wheatgrass could be released from interspecific competition at extreme drought.
CONCLUSIONS
Stomatal density and leaf area are plastic in their response to climate manipulations but the response is limited to specific treatments. The present results regarding stomatal density suggest that bluebunch wheatgrass may be capable of adapting to a changing climate through changes in the number of stomata. These results are all the more significant because they document stomatal density response of plants growing in the field to manipulated climate treatments, rather than a correlation analysis along a pre-existing environmental gradient. The changes in stomatal density are also related to changes in bluebunch wheatgrass biomass; with greater biomass there was lower stomatal density. It might also be useful to think of increasing stomatal density as an indicator of climate change on plant function but, to detect such an effect, a comprehensive stomatal density database for many species across a wide geographic range would be needed. This study provides support for controlled climate experiments, rather than only relying on elevational gradients to interpret ecophysiological adaptation.
ACKNOWLEDGEMENTS
We thank Montana Burgess for her help in the field and the laboratory. Don Thompson from Agriculture and Agri-Food Canada allowed access to field research sites. Bruno Delesalle from British Columbia Grasslands Conservation Council provided use of facilities. This work was funded by a National Sciences and Engineering Research Council (NSERC) Undergraduate Research Award to A. Greenall, a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant, a Canadian Foundation for Innovation grant, and a British Columbia Forest Science Program grant to L. Fraser, and an NSERC Discovery Grant to C. Ross Friedman.
LITERATURE CITED
- Bañon S, Fernandez JA, Franco JA, Torrecillas A, Alarcón JJ, Sánchez-Blanco MJ. Effects of water stress and night temperature preconditioning on water relations and morphological and anatomical changes of Lotus creticus plants. Scientia Horticulturae. 2004;101:333–342. [Google Scholar]
- Bawtree AH, Campbell CW, Hennan EG, Spalding DJ, Withler IL. History of range use and related activities. In: Campbell CW, Bawtree AH, editors. Rangeland Handbook for BC. Kamloops, BC: British Columbia Cattlemen's Association; 1998. pp. 1–21. [Google Scholar]
- Beerling DJ, Chaloner WG. The impact of atmospheric CO2 and temperature changes on stomatal density: observation from Quercus robur Lammas leaves Annals of Botany. 1993;71:231–235. [Google Scholar]
- Beerling DJ, Chaloner WG, Huntley B, Pearson JA, Tooley MJ. Stomatal density responds to the glacial cycle of environmental change. Proceedings of the Royal Society: Biological Sciences. 1993;251:133–138. [Google Scholar]
- Carpenter SB, Smith ND. Stomatal distribution and size in southern Appalachian hardwoods. Canadian Journal of Botany. 1975;53:1153–1156. [Google Scholar]
- Croxdale JL. Stomatal patterning in angiosperms. American Journal of Botany. 2000;87:1069–1080. [PubMed] [Google Scholar]
- Environment Canada. 20th Century Trends in the Earth's Climate and Bio-physical System. 2003. Government of Canada. Available at: http://www.msc-smc.ec.gc.ca/education/scienceofclimatechange/understanding/trends/index_e.html .
- Gianoli E, González-Teuber M. Environmental heterogeneity and population differentiation in plasticity to drought in Convolvulus chilensis (Convolvulaceae) Evolutionary Ecology. 2005;19:603–613. [Google Scholar]
- Gindel I. Stomatal number and size as related to soil moisture in tree xerophytes in Israel. Ecology. 1969;50:263–267. [Google Scholar]
- Gitz DC, III, Liu-Gitz L, Britz SJ, Sullivian JH. Ultraviolet-B effects on stomatal density, water-use efficiency, and stable carbon isotope discrimination in four glasshouse-grown soybean (Glycine max) cultivars. Environmental and Experimental Botany. 2004;53:343–355. [Google Scholar]
- Grime JP. Plant strategies and vegetation processes. Chichester: John Wiley and Sons; 1979. [Google Scholar]
- Gurevitch J, Scheiner SM, Fox GA. The ecology of plants. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change. Climate change 2007: the physical science basis. Geneva, Switzerland: Intergovernmental Panel on Climate Change; 2007. Available at: http://ipcc-wg1.ucar.edu/index.html . [Google Scholar]
- Jones HG. Plants and microclimate. 2nd edn. New York, NY: Cambridge University Press; 1992. [Google Scholar]
- Klein JA, Harte J, Zhao XQ. Experimental warming causes large and rapid species loss, dampened by simulated grazing, on the Tibetan Plateau. Ecology Letters. 2004;7:1170–1179. [Google Scholar]
- Kobl PF, Robberecht R. Pinus ponderosa seedlings establishment and the influence of competition with the bunchgrass Agropyron spicatum. International Journal of Plant Science. 1996;157:509–515. [Google Scholar]
- Kochy M, Wilson SD. Semiarid grassland response to short-term variation in water availability. Plant Ecology. 2004;174:197–203. [Google Scholar]
- Körner C. Alpine plant life. Berlin: Springer-Verlag; 1999. [Google Scholar]
- Körner C, Neumayer M, Menendez-Riedl SP, Smeets-Scheel A. Functional morphology of mountain plants. Flora. 1989;182:353–383. [Google Scholar]
- Lake JA, Woodward FI. Response of stomatal numbers to CO2 and humidity: control by transpiration rate and abscisic acid. New Phytologist. 2008;179:397–404. doi: 10.1111/j.1469-8137.2008.02485.x. [DOI] [PubMed] [Google Scholar]
- Lesica P, Allendorf FW. When are peripheral populations valuable for conservation? Conservation Biology. 1995;9:753–760. [Google Scholar]
- Maseda PH, Fernández RJ. Stay wet or else: three ways in which plants can adjust hydraulically to their environment. Journal of Experimental Botany. 2006;57:3963–3977. doi: 10.1093/jxb/erl127. [DOI] [PubMed] [Google Scholar]
- Parish R, Coupe R, Lloyd D. Plants of southern interior British Columbia and the inland Northwest. Vancouver, BC: Lone Pine Publishing; 1996. [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Peat HJ, Fitter AH. A comparative study of the distribution and density of stomata in the British flora. Biological Journal of the Linnean Society. 1994;52:377–393. doi: 10.1111/j.1095-8312.1994.tb00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos HM, Goodale UM, Berlyn GP. Drought response of two Mexican oak species, Quercus laceyi and Q. sideroxyla (Fagaceae), in relation to elevational position. American Journal of Botany. 2007;94:809–818. doi: 10.3732/ajb.94.5.809. [DOI] [PubMed] [Google Scholar]
- Ramonell KM, Kuang A, Porterfield DM, et al. Influence of atmospheric oxygen on leaf structure and starch deposition in Arabidopsis thaliana. Plant, Cell & Environment. 2001;24:419–428. doi: 10.1046/j.1365-3040.2001.00691.x. [DOI] [PubMed] [Google Scholar]
- Raven PH, Evert RF, Eichhorn SE. Biology of plants. 6th edn. New York, NY: W.H. Freeman and Company Worth Publishers; 1999. [Google Scholar]
- Redmann RE. Adaptation of grasses to water stress-leaf rolling and stomate distribution. Annals of the Missouri Botanical Garden. 1985;72:833–842. [Google Scholar]
- Richardson AD, Ashton PMS, Berlyn GP, McGroddy ME, Cameron IR. Within-crown foliar plasticity of western hemlock, Tsuga heterophylla, in relation to stand age. Annals of Botany. 2001;88:1007–1015. [Google Scholar]
- Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- van Ryswyk AL, McLean A, Marchand LS. The climate, native vegetation, and soils of some grasslands at different elevations in British Columbia. Canadian Journal of Plant Science. 1966;46:35–50. [Google Scholar]
- Sack L, Grubb PJ, Marañón T. The functional morphology of juvenile plants tolerant of strong summer drought in shaded forest understories in southern Spain. Plant Ecology. 2003;168:139–163. [Google Scholar]
- Schoch P-G, Zinsou C, Sibi M. Dependence of the stomatal index on environmental factors during stomatal differentiation in leaves of Vigna sinensis L. 1. Effect of light intensity. Journal of Experimental Botany. 1980;31:1211–1216. [Google Scholar]
- Sekiya N, Yano K. Stomatal density of cowpea correlates with carbon isotope discrimination in different phosphorus, water and CO2 environments. New Phytologist. 2008;179:799–807. doi: 10.1111/j.1469-8137.2008.02518.x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. Plants and water. Studies in biology. London: Arnold; 1974. Vol. 14. [Google Scholar]
- Tape K, Sturm M, Racine C. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Global Change Biology. 2006;12:686–702. [Google Scholar]
- Thomas PW, Woodward FI, Quick WP. Systemic irradiance signalling in tobacco. New Phytologist. 2003;161:193–198. [Google Scholar]
- Tisdale EW. The grasslands of the southern interior of British Columbia. Ecology. 1947;28:346–382. [Google Scholar]
- Wang Y, Chen X, Xiang C. Stomatal density and bio-water saving. Journal of Integrative Plant Biology. 2007;49:1435–1444. [Google Scholar]
- Woodward FI. Stomatal numbers are sensitive to increase in CO2 from pre-industrial levels. Nature. 1987;18:617–618. [Google Scholar]
- Woodward FI, Kelly CK. The influence of CO2 concentration on stomatal density. New Phytologist. 1995;131:311–327. [Google Scholar]
- Woodward FI, Lake JA, Quick WP. Stomatal development and CO2: ecological consequences. New Phytologist. 2002;153:477–484. doi: 10.1046/j.0028-646X.2001.00338.x. [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhou G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany. 2008;59:3317–3325. doi: 10.1093/jxb/ern185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaleta ES, Shaw MR, Chiariello NR, et al. Grassland responses to three years of elevated temperature, CO2, precipitation, and N deposition. Ecological Monographs. 2003;73:585–604. [Google Scholar]