Abstract
Background and Aims
Floral scent may play a key role as a selective attractant in plants with specialized pollination systems, particularly in cases where floral morphology does not function as a filter of flower visitors. The pollination systems of two African Eucomis species (E. autumnalis and E. comosa) were investigated and a test was made of the importance of scent and visual cues as floral attractants.
Methods and Key Results
Visitor observations showed that E. autumnalis and E. comosa are visited primarily by pompilid wasps belonging to the genus Hemipepsis. These wasps carry considerably more Eucomis pollen and are more active on flowers than other visiting insects. Furthermore, experiments involving virgin flowers showed that these insects are capable of depositing pollen on the stigmas of E. autumnalis, and, in the case of E. comosa, pollen deposited during a single visit is sufficient to result in seed set. Experimental hand-pollinations showed that both species are genetically self-incompatible and thus reliant on pollinators for seed set. Choice experiments conducted in the field and laboratory with E. autumnalis demonstrated that pompilid wasps are attracted to flowers primarily by scent and not visual cues. Measurement of spectral reflectance by flower petals showed that flowers are cryptically coloured and are similar to the background vegetation. Analysis of headspace scent samples using coupled gas chromatography–mass spectrometry revealed that E. autumnalis and E. comosa scents are dominated by aromatic and monoterpene compounds. One hundred and four volatile compounds were identified in the floral scent of E. autumnalis and 83 in the floral scent of E. comosa, of which 57 were common to the scents of both species.
Conclusions
This study showed that E. autumnalis and E. comosa are specialized for pollination by pompilid wasps in the genus Hemipepsis and achieve specialization through cryptic colouring and the use of scent as a selective floral attractant.
Key words: Eucomis, Pompilidae, wasp pollination, breeding system, pollination syndrome, pollinator shift, floral volatile, floral filter
INTRODUCTION
Plants with specialized pollination systems typically have complex morphology, such as floral spurs, that limits access by certain animals to rewards (Johnson and Steiner, 2000). Specialized pollination systems in plants with open and morphologically unspecialized flowers are more difficult to explain as they usually have rewards which are readily accessible to a range of different potential visitors. Some of these plants have toxic nectar which filters out certain flower visitors (Stephenson, 1981, 1982; Adler 2000; Johnson et al., 2006; Shuttleworth and Johnson, 2006). Another possibility, still poorly documented, is that a combination of cryptic flower colour and a particular scent blend may allow morphologically unspecialized flowers to selectively attract specific pollinators (cf. Johnson, 2005; Johnson et al., 2007; Brodmann et al., 2008).
The genus Eucomis (Hyacinthaceae), commonly known in Africa as ‘pineapple flowers’, contains 11 species that occur in forest, grassland and wetland areas of southern Africa (Williams, 2000). Eucomis flowers are structurally unspecialized (see Fig. 1) and typically produce large amounts of exposed nectar. While these traits would normally be associated with generalist pollination systems, preliminary observations suggest that pollination systems in the genus are usually specialized and remarkably variable among species, ranging from rodent pollination in E. regia (S. D. Johnson, unpubl. res.) to pollination by spider-hunting wasps (Hymenoptera: Pompilidae) in E. autumnalis and E. comosa (this study). We hypothesized that Eucomis autumnalis and E. comosa have specialized pollination systems and achieve specialization through the use of cryptic colouring and floral scent as an attractant.
Fig. 1.
Eucomis species and their visitors: (A) Eucomis autumnalis plant, Vernon Crooks Nature Reserve; (B) Eucomis comosa plants, Gilboa Estate (picture: Jana Jersakova); (C) male Hemipepsis hilaris lapping nectar from E. autumnalis flower, Vernon Crooks Nature Reserve; (D) female H. capensis lapping nectar from Eucomis comosa flower, Gilboa Estate. Note the pollen-covered thorax in contact with the stigma in (C) and (D). Abbreviations: o, ovary; p, petal; s, stigma. Scale bars: (A) = 60 mm; (B) = 200 mm; (C, D) = 10 mm.
The specific aims of this study were: (a) to identify the most effective pollinators of E. autumnalis and E. comosa; (b) to determine the nectar properties of these plants' flowers; (c) to determine whether these plant species have breeding systems that make them reliant on pollinators for reproduction; (d) to determine if pollinators are attracted by floral scent or visual cues; (e) to determine the chemical composition of the floral fragrance; and (f) to determine if the spectral reflectance of the flower petals is similar to that of the background vegetation.
MATERIALS AND METHODS
Study species and field sites
Eucomis autumnalis (Mill.) Chittenden [subsp. clavata (Bak.) Reyneke used in this study] and E. comosa Houtt. ex. Wehrh. (Hyacinthaceae) occur throughout the eastern half of South Africa. Both species are found in damp grasslands or wetlands with dense vegetation (Pooley, 1998). The inflorescences of both species are similar and consist of pale yellow-green flowers arranged around a thick central axis and terminating in leafy bracts (Fig. 1). Eucomis comosa flowers occasionally have purple markings on the ovary and along the edges of the petals. Eucomis autumnalis plants at Vernon Crooks Nature Reserve had 72 ± 8·4 (mean ± s.d., n = 6) flowers per plant and E. comosa plants at Gilboa Estate had 88 ± 12·5 (mean ± s.d., n = 19) flowers per plant. Eucomis autumnalis flowers earlier (October to November) than E. comosa (December to March) (Pooley, 1998). This study was conducted during the flowering seasons between 1999 and 2008 at seven field sites in South Africa (see Table 1).
Table 1.
Details of the seven field sites at which Eucomis autumnalis and E. comosa were studied
| Site name | Co-ordinates and altitude | Habitat | Eucomis species | Approx. no. of plants |
|---|---|---|---|---|
| Gilboa Estate | 29°15′01·6′′S, 30°15′21·6′′E; 1532 m | Wetland surrounded by pine plantation | E. comosa | 100 |
| Highflats | 30°16′10·3′′S, 30°12′09·3′′E; 976 m | Cultivated grassland | E. autumnalis | 100 |
| Howick | 29°27′34·8′′S, 30°14′47·7′′E; 1051 m | Roadside marsh | E. comosa | 30 |
| Midmar Nature Reserve | 29°32′15·8′′S, 30°10′13·1′′E; 1088 m | Moist montane grassland | E. autumnalis | 200 |
| Vernon Crooks Nature Reserve | 30°16′06·5′′S, 30°37′14·5′′E; 447 m | Coastal grassland | E. autumnalis | 50 |
| Wahroonga Farm | 29°36′35·9′′S, 30°07′59·4′′E; 1350 m | Moist montane grassland | E. autumnalis | 10 |
| Wodwo Farm | 29°24′08·1′′S, 29°55′53·2′′E; 1595 m | Montane grassland | E. autumnalis and E. comosa | 10 of each |
Floral visitors and nectar rewards
Floral visitors were recorded at all study sites and representative individuals were collected for later identification and quantification of pollen loads. In some instances, visitors were noted but not collected. Pompilid wasps were identified to species level using keys given in Arnold (1932), Day (1979) and Goulet and Huber (1993). Floral visitors to E. comosa recorded as part of a separate study by Field (2002) are included and presented here. [Note that Field (2002) misidentified E. comosa and referred to it as E. autumnalis in her study.]
Nectar volume and concentration (% sucrose equivalents by weight) were measured using 5-μL capillary tubes and a Bellingham and Stanley 0–50 % or 45–80 % sugar concentration by weight, hand-held refractometer. The 24-h nectar production was measured for E. autumnalis and E. comosa at Vernon Crooks Nature Reserve in October 2006 and at Gilboa Estate in December 2006, respectively. Nectar present at the beginning of the 24-h period was removed and then plants were bagged for 24 h (for E. autumnalis) or cut and kept in water in the laboratory for 24 h (for E. comosa). The standing crop of nectar was measured for E. autumnalis only, at Midmar Nature Reserve at 0730 h in November 2006. Means were calculated per plant and these values used to calculate the population means (presented as the mean per flower per plant). Nectar measurements were taken from relatively few individuals in order to conserve plants for other experiments at the field sites.
Reproductive biology and reliance on pollinators
The breeding systems of E. comosa and E. autumnalis were determined in the 2005–2006 flowering season. For E. autumnalis, three plants were used at Vernon Crooks Nature Reserve and nine at Midmar Nature Reserve and, for E. comosa, ten plants were used at the Howick site. Flowers which had been bagged at the bud stage were randomly assigned to one of three hand-pollination treatments: (1) cross-pollinated, (2) self-pollinated, or (3) unmanipulated control. Pollen used for cross-pollinations was obtained from plants at least 5 m away to minimize bi-parental inbreeding effects. After pollination, flowers were rebagged and left to develop seeds. Once seeds had developed, plants were harvested and the number of seeds per flower in each treatment was counted. Twelve flowers (four per treatment) were used per plant, although some flowers were subsequently destroyed by caterpillars. Differences between treatments in the number of seeds produced per flower were analysed using a one-way ANOVA in conjunction with a Tukey test.
Seed set in naturally pollinated plants was measured as the number of seeds per flower per plant for both E. autumnalis and E. comosa (fruit set was not used as a measure of reproductive success as fruit-like swelling occurs in both unfertilized and fertilized ovaries). For E. autumnalis, natural seed set was measured at Highflats, Vernon Crooks Nature Reserve and Midmar Nature Reserve in the 2005–2006 flowering season. A second measurement was taken at Midmar Nature Reserve as a result of a large number of seed-containing fruits having been eaten by caterpillars. In the second sample, only undamaged fruits were selected. For E. comosa, natural seed set was measured at Gilboa Estate in the 2004–2005 flowering season and Howick in the 2005–2006 flowering season. Seed set was measured by dissecting individual fruits and counting the number of seeds present. Differences in seed set were compared using t-tests assuming unequal variance.
The number of ovules per flower was counted using a dissecting microscope (seeds present were counted as one ovule). This was measured on five E. comosa plants and six E. autumnalis plants.
Pollinator effectiveness
Pollen loads and placement were determined for all insect visitors (except where no individuals were collected) to E. autumnalis and E. comosa. Pollen was removed from each insect's body with fuchsin gel (Beattie, 1971) and a light microscope used to estimate the total Eucomis pollen loads per individual. In instances where individuals were carrying low amounts of pollen, the number of pollen grains was estimated directly on the insect using a dissecting microscope. Pollen loads for E. comosa visitors measured by Field (2002) are included here.
As Hemipepsis wasps appeared to be the most important pollinators of both Eucomis species (they were the most abundant visitors, carried the highest pollen loads and were the only insects that moved frequently between inflorescences; Tables 2 and 6), further experiments were conducted to establish their effectiveness in transferring pollen to stigmas. For E. comosa, this was done by conducting an experiment to determine the effectiveness of single visits by Hemipepsis wasps for seed set in the 2003–2004 flowering season. Flowers on 14 inflorescences which had been bagged at the bud stage at Gilboa Estate were exposed in the field to visits by Hemipepsis wasps. After being visited by at least one wasp, the flowers were rebagged. A further 13 inflorescences were bagged at the bud stage but were not exposed, and served as controls. After 15 d, all inflorescences were removed to the laboratory and the number of seeds per exposed flower was counted. Seed set in exposed and control flowers was compared using a Mann–Whitney U-test as data were not normally distributed.
Table 2.
Insects observed visiting flowers of Eucomis autumnalis and E. comosa at the study sites
| Number observed |
|||||
|---|---|---|---|---|---|
| Visitors | Total | Captured | Functional group | Field site* | Reference |
| Eucomis autumnalis | |||||
| Hymenoptera | |||||
| Hemipepsis capensis (Pompilidae) | 11 | 6 | Pompilid | VC, W | This study |
| H. errabunda | 1 | 1 | Pompilid | M | This study |
| H. hilaris | 35 | 11 | Pompilid | Hi, M, VC | This study |
| H. capensis/H. errabunda/H. hilaris† | 29 | 0 | Pompilid | VC | This study |
| Hemipepsis sp. 1 | 1 | 1 | Pompilid | VC | This study |
| Cryptochilus sp.1 (Pompilidae) | 7 | 7 | Pompilid | VC | This study |
| Priocnemis sp. 1 (Pompilidae) | 1 | 1 | Pompilid | M | This study |
| Pompilidae sp. 1 | 3 | 0 | Pompilid | Hi | This study |
| Tiphia sp. 1 (Tiphiidae) | 7 | 2 | Tiphiid | Hi, VC | This study |
| Apis mellifera (Apidae) | 1 | 0 | Bee | Hi | This study |
| Halictidae sp. 1 | 1 | 0 | Bee | VC | This study |
| Tenthredinidae sp. 1 | 2 | 0 | Tenthredinid | VC | This study |
| Coleoptera | |||||
| Atrichelaphinis tigrina (Scarabaeidae: Cetoniinae) | 31 | 15 | Cetoniin | M, W, VC | This study |
| Cyrtothyrea marginalis (Scarabaeidae: Cetoniinae) | 10 | 8 | Cetoniin | M, VC | This study |
| Leucocelis haemorrhoidalis (Scarabaeidae: Cetoniinae) | 1 | 1 | Cetoniin | M | This study |
| Elateridae sp. 1 | 175 | 8 | Elaterid | VC | This study |
| Chrysomelidae sp. 2 | 1 | 1 | Chrysomelid | M | This study |
| Diptera | |||||
| Sarcophagidae sp. 2 | 4 | 0 | Short-tongued fly | Hi | This study |
| Sarcophagidae sp. 3 | 1 | 0 | Short-tongued fly | M | This study |
| Calliphoridae sp. 1 | 2 | 0 | Short-tongued fly | Hi | This study |
| Calliphoridae sp. 2 | 3 | 0 | Short-tongued fly | M | This study |
| Calliphoridae sp. 3 | 3 | 3 | Short-tongued fly | M | This study |
| Muscidae sp. 1 | 1 | 0 | Short-tongued fly | Hi | This study |
| Muscidae sp. 2 | 1 | 0 | Short-tongued fly | M | This study |
| Hemiptera | |||||
| Reduviidae sp. 1 | 1 | 0 | Hemiptera | Hi | This study |
| Lygaeidae sp. 1 | 1 | 1 | Hemiptera | M | This study |
| Eucomis comosa | |||||
| Hymenoptera | |||||
| Hemipepsis capensis (Pompilidae) | 34 | 34 | Pompilid | G, Ho, Wo | Field (2002), This study |
| H. dedjas | 3 | 1 | Pompilid | Ho | This study |
| H. errabunda | 7 | 7 | Pompilid | G, Ho | Field (2002), This study |
| H. hilaris | 27 | 27 | Pompilid | G, Ho, Wo | Field (2002), This study |
| H. capensis/H. errabunda/H. hilaris† | 86 | 0 | Pompilid | G, Ho, Wo | Field (2002), This study |
| Cyphononyx sp. 1 (Pompilidae) | 3 | 1 | Pompilid | Ho | This study |
| Pepsinae sp.1 (Pompilidae) | 8 | 5 | Pompilid | G | This study |
| Pompilinae sp. 1 (Pompilidae) | 1 | 1 | Pompilid | G | This study |
| Pompilidae sp. 2 | 1 | 1 | Pompilid | Ho | This study |
| Polistes sp. 1 (Vespidae) | 1 | 1 | Vespid | G | This study |
| Polistes sp. 2 (Vespidae) | 1 | 1 | Vespid | G | This study |
| Eumenidae sp. 1 | 1 | 0 | Eumenid | Ho | This study |
| Halictus sp. 1 (Halictidae) | 1 | 1 | Bee | G | Field (2002) |
| Apidae sp. 1 | 1 | 1 | Bee | G | This study |
| Apis mellifera (Apidae) | 49 | 7 | Bee | Ho | This study |
| Tiphia sp. 1 (Tiphiidae) | 8 | 0 | Tiphiid | G | This study |
| Tiphiidae sp. 1 | 1 | 1 | Tiphiid | Ho | This study |
| Tenthredinidae sp. 1 | 1 | 1 | Tenthredinid | G | This study |
| Formicidae sp. 1 | 2 | 0 | Ant | G | This study |
| Coleoptera | |||||
| Lycidae sp. 1 | 2 | 2 | Lycid | G | Field (2002); this study |
| Lycidae sp. 2 | 7 | 7 | Lycid | Ho | This study |
| Lycidae sp. 3 | 3 | 3 | Lycid | G, Ho | This study |
| Lycidae sp. 4 | 5 | 5 | Lycid | G | Field (2002); this study |
| Lycidae sp. 5 | 7 | 7 | Lycid | G, Ho | Field (2002); this study |
| Unidentified Lycidae | 17 | 0 | Lycid | G | This study |
| Atrichelaphinis tigrina (Scarabaeidae: Cetoniinae) | 15 | 9 | Cetoniin | G, Ho | Field (2002); this study |
| Cyrtothyrea marginalis (Scarabaeidae: Cetoniinae) | 2 | 2 | Cetoniin | G | This study |
| Cetoniinae sp. 1 (Scarabaeidae) | 1 | 1 | Cetoniin | G | This study |
| Cetoniinae sp. 2 (Scarabaeidae) | 1 | 1 | Cetoniin | G | Field (2002) |
| Chrysomelidae sp. 1 | 2 | 2 | Chrysomelid | Ho | This study |
| Cerambycidae sp. 1 | 1 | 1 | Cerambycid | G | This study |
| Cantharidae sp. 1 | 1 | 1 | Cantharid | Ho | This study |
| Elateridae sp. 1 | 1 | 1 | Elaterid | G | This study |
| Coccinelidae sp. 1 | 1 | 1 | Coccinelid | Ho | This study |
| Diptera | |||||
| Calliphoridae sp. 4 | 1 | 1 | Short-tongued fly | Ho | This study |
| Tabanocella denticornis (Tabanidae) | 8 | 8 | Short-tongued fly | G | Field (2002); this study |
| Tabanus taeniatus (Tabanidae) | 2 | 2 | Short-tongued fly | G | Field (2002) |
| Sarcophagidae sp. 1 | 1 | 1 | Short-tongued fly | G | Field (2002) |
| Hemiptera | |||||
| Lygaeidae sp. 2 | 1 | 1 | Hemiptera | Ho | This study |
| Lepidoptera | |||||
| Catacroptera cloanthe (Nymphalidae) | 1 | 0 | Butterfly | Ho | This study |
| Danaus chrysippus (Danaidae) | 1 | 0 | Butterfly | Ho | This study |
| Stygionympha vigilans (Nymphalidae) | 1 | 1 | Butterfly | G | Field (2002) |
| Vanessa cardui (Nymphalidae) | 1 | 0 | Butterfly | Ho | This study |
| Nymphalidae sp. 1 | 1 | 1 | Butterfly | G | Field (2002) |
| Nymphalidae sp. 2 | 1 | 1 | Butterfly | Ho | This study |
| Nymphalidae sp. 3 | 1 | 0 | Butterfly | Ho | This study |
*G, Gilboa Estate; Hi, Highflats; Ho, Howick; M, Midmar Nature Reserve; VC, Vernon Crooks Nature Reserve; W, Wahroonga Farm; Wo, Wodwo Farm.
† These were individuals of these three Hemipepsis species but could not be identified to species as they were observed but not captured.
Table 6.
Pollen loads for insects visiting Eucomis autumnalis and E. comosa, measured per functional group
| Functional group | No. examined for pollen | Pollen load: mean (range) | Pollen placement on body |
|---|---|---|---|
| E. autumnalis | |||
| Hymenoptera | |||
| Pompilid | 13 | 964 (0–5000) | Head, thorax |
| Tiphiid | 2 | 55 (10–100) | Head, thorax |
| Coleoptera | |||
| Cetoniin | 13 | 208 (0–800) | Head, thorax, legs |
| Elaterid | 8 | 0 (0) | |
| Chrysomelid | 1 | 0 (0) | |
| Diptera | |||
| Short-tongued fly | 3 | 4 (0–12) | Thorax |
| Hemiptera | |||
| Lygaeid bug | 1 | 0 (0) | |
| E. comosa | |||
| Hymenoptera | |||
| Pompilid | 13 | 1362 (100–5000) | Head, thorax |
| Vespid | 2 | 20 (0–40) | Thorax |
| Bee | 9 | 154 (0–700) | Head, thorax, abdomen, legs, wings |
| Tiphiid | 1 | 0 (0) | |
| Tenthredinid | 1 | 80 (80) | Head, thorax |
| Coleoptera | |||
| Lycid | 18 | 367 (10–1500) | Head, thorax, legs |
| Cetoniin | 13 | 43 (0–200) | Thorax, abdomen |
| Chrysomelid | 2 | 0 (0) | |
| Cerambycid | 1 | 50 (50) | Head, thorax, elytra |
| Cantharid | 1 | 0 (0) | |
| Elaterid | 1 | 0 (0) | |
| Diptera | |||
| Short-tongued fly | 7 | 68 (0–150) | Thorax |
| Lepidoptera | |||
| Butterfly | 3 | 7 (0–10) | Thorax |
Logistical constraints prevented the use of a similar field-based single visit experiment with E. autumnalis. However, an experiment was conducted to determine the effectiveness of Hemipepsis wasps in depositing pollen on stigmas of E. autumnalis in the 2003–2004 flowering season. Flowers on seven inflorescences which had been bagged at the bud stage in Vernon Crooks Nature Reserve were placed in an 80-cm3 wood and mesh cage in the laboratory. Stigmas of all flowers were examined with a dissecting microscope to ensure that they contained no pollen and flowers in which the stigmas contained pollen grains were removed. Six pompilids (five H. capensis and one H. hilaris), collected on Pachycarpus asperifolius (Apocynaceae) at Vernon Crooks Nature Reserve, were then placed in the cage with the virgin inflorescences. After 24 h, all flowers were removed and their stigmas inspected for pollen deposition using a dissecting microscope. The number of pollen grains on each flower was estimated to one of four categories: 0; 1–10; 10–100 and >100 pollen grains.
Responses of wasps to scent and visual cues
The flowers of E. autumnalis and E. comosa are morphologically similar (Figs 1 and 3) and the inflorescences of these two species are functionally identical. Experiments investigating the functional importance of scent and visual cues as attractants were thus conducted only with E. autumnalis. To do this, two field-based choice experiments (in October 2004) and a laboratory-based Y-maze choice experiment (in October 2007) were conducted. In all these experiments, Hemipepsis wasps and plants from Vernon Crooks Nature Reserve were used.
Fig. 3.
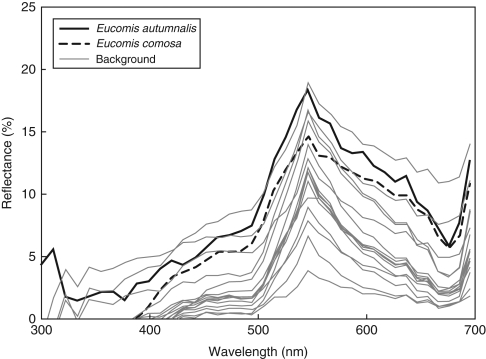
Reflectance spectra for Eucomis autumnalis and E. comosa floral petals and background vegetation. Each curve represents the mean spectrum calculated from individual replicates.
In the first field-based choice experiment, two plants were uprooted and placed in vases 2 m apart at right angles to the oncoming breeze. Flowers were removed from one inflorescence until both inflorescences had equal numbers of flowers. One of the inflorescences was then covered with the plant's own leaves as well as with leaves of a common sympatric plant species, Gerbera ambigua (Asteraceae), so that the inflorescence was completely concealed from view. The second inflorescence was left unmanipulated. Both the upper- and under-side of E. autumnalis leaves exhibit similar spectral reflectance properties to the flower petals, and also appear dull green in colour (see Fig. S1 in Supplementary Data, available online). Gerbera ambigua leaves are dull green on the upper side, with a similar spectral reflectance curve to the E. autumnalis flower petals and leaves (see Fig. S1). The underside of the G. ambigua leaves appears as a dull white colour with a maximum reflectance of approx. 50 % (see Fig. S1). The number of inspections (approached to within at least 15 cm but did not alight) or visits (actually landed) by Hemipepsis wasps to covered and unmanipulated inflorescences was recorded over a period of approx. 3 h. In addition, the direction of approach with respect to the prevailing breeze was recorded for 45 of the wasps observed in these experiments.
In the second field-based choice experiment, one of the inflorescences in a pair was enclosed in a transparent plastic ziplock bag to prevent it from emitting scent. Silica gel was added to the ziplock bag to prevent condensation as the plant transpired. A ziplock bag was placed in front of the second inflorescence such that it still emitted scent but approaching wasps would see both inflorescences through plastic. Covering flower petals with plastic reduced overall reflectance, but did not alter the hue of the petals (see Fig. S1 in Supplementary Data, available online).
Each of the above experiments was replicated and differences between the number of visits to each inflorescence in the two experiments were tested using a goodness-of-fit test. To quantify visual cues of flowers and leaves used in these experiments, the spectral reflectance of leaves of G. ambigua, and leaves, flowers and plastic-enclosed flowers of E. autumnalis were measured across the 300–700 nm range using the methods described below (see Fig. S1).
In the laboratory-based choice experiment, a 20-mm-diameter glass Y-maze placed on a light table was used. Each arm of the Y-maze was 90 mm long and the main arm was 170 mm long. The main arm of the Y-maze was connected to a suction pump such that air was drawn along each arm of the Y. One arm of the Y was then attached to a polyacetate bag containing an E. autumnalis inflorescence and the other arm attached to an empty polyacetate bag. A small hole was made in each bag to allow airflow through the bag. Wasps were inserted at the entrance to the Y-maze and allowed to walk down the Y-maze and select one of the arms. In total, 20 runs were made with four Hemipepsis wasps (five runs per wasp). The side containing the inflorescence was selected randomly for each run. As responses were identical for all wasps, individual choices were pooled and the number of choices made in favour of the arm with flowers was compared with the number of choices in favour of the arm without flowers using a binomial test to establish if wasps showed overall preference.
Spectral reflectance analysis of flowers and background vegetation
Spectral reflectance across the 300–700 nm range was determined using an Ocean Optics S2000 spectrometer (Ocean Optics Inc., Dunedin, FL, USA) and fibre optic reflection probe (QR-400-7-UV-VIS; 400 µm) held at 45° to the petal surface. The light source used was an Ocean Optics DT-mini deuterium tungsten halogen light source with an approx. 200- to 1100-nm spectral range. An Ocean Optics WS-1 diffuse reflectance standard was used to calibrate the spectrometer (Johnson and Andersson, 2002). Spectral reflectance was measured for petals of E. autumnalis from Vernon Crooks Nature Reserve in October 2004, and E. comosa from Gilboa Estate in November 2006. Spectral reflectance of background vegetation was measured from the upper surface of green leaves of 16 different plant species (various grasses, forbs and herbs) from Vernon Crooks Nature Reserve in November 2006. Four replicates were taken for each of the Eucomis species and three replicates for each of the background species. A mean spectrum was calculated for each plant species.
Gas chromatography–mass spectrometry (GC-MS) analysis of floral scent
Floral scent was collected using dynamic headspace extraction methods and analysed by coupled GC-MS. In total, eight samples for E. autumnalis and six samples for E. comosa were obtained. Two of the E. autumnalis samples and one of the E. comosa samples were analysed by Dr Roman Kaiser (Givaudan, Switzerland) as per the methods described in Kaiser and Tollsten (1995). For these three samples, inflorescences were cut and removed to the laboratory where they were enclosed in a glass vessel (excluding damaged plant tissue) and the air from the vessel pumped through a filter containing 3 mg Porapak™ Q for 6–7 h at a realized flow rate of 50 mL min−1. One of the two E. autumnalis samples was taken from a single inflorescence from Vernon Crooks Nature Reserve in the 2004–2005 season (sample S1 in Table S1 in Supplementary Data available at AoB online), and the second E. autumnalis sample was taken from three inflorescences from Midmar Nature Reserve in the 2005–2006 season (sample S2 in Table S1). The E. comosa sample was taken from three inflorescences from Gilboa Estate in the 2005–2006 season. These samples were then eluted with approx. 30 µL of 9:1 hexane:acetone solvent and analysed by GC-MS using a DB-WAX column (J & W Scientific) and the instrumentation and temperature programmes as described in Kaiser and Tollsten (1995).
The remaining six E. autumnalis and five E. comosa samples were taken by enclosing inflorescences in polyacetate bags. Air from these bags was then pumped through small cartridges filled with 1 mg of Tenax® and 1 mg of Carbotrap™ activated charcoal at a realized flow rate of 50 mL min−1. Controls were taken from an empty polyacetate bag sampled for the same duration. The E. autumnalis samples were taken in the field at Vernon Crooks Nature Reserve on 24 October 2007. Each sample was taken from a single inflorescence for a duration of 20 min. The E. comosa samples were taken from cut inflorescences collected at Gilboa Estate on 10 January 2008. Both these Eucomis species have thick fleshy stems and cut inflorescences survive for several weeks in vases without wilting. Each sample was taken from four inflorescences for a duration of 2 h. To minimize contamination by green leaf volatiles (as a result of using cut inflorescences), care was taken to bag only undamaged plant tissue. As previous attempts to sample this species had produced weak samples, the scent was accumulated in the bags for 3 h before the sample was taken. This served to minimize contamination from continuous pumping of background air. GC-MS analysis of these samples was carried out using a Varian CP-3800 GC (Varian, Palo Alto, CA, USA) with a 30 m × 0·25 mm internal diameter (film thickness 0·25 µm) Alltech EC-WAX column coupled to a Varian 1200 quadrupole mass spectrometer in electron-impact ionization mode. Cartridges were placed in a Varian 1079 injector equipped with a ‘Chromatoprobe’ thermal desorbtion device. The flow of helium carrier gas was 1 ml min−1. The injector was held at 40 °C for 2 min with a 20:1 split and then increased to 200 °C at 200 °C min−1 in splitless mode for thermal desorbtion. After a 3 min hold at 40 °C, the temperature of the GC oven was ramped up to 240 °C at 10 °C min−1 and held there for 12 min. Compounds were identified using the Varian Workstation software with the NIST05 mass spectral library and verified, where possible, using retention times of authentic standards and published Kovats indices. Compounds present at similar abundance in the controls were considered to be contaminants and excluded from analyses. For quantification, 68 different standards (comprising representatives from all compound classes) were injected into cartridges (200 ng of each) and thermally desorbed under identical conditions to the samples. Eucomis inflorescences are racemose with flowers maturing acropetally from the base. Individual inflorescences consequently contain a range of different aged flowers. We considered individual inflorescences to be complete functional units. The age and number of flowers per inflorescence in each sample were thus not recorded and emission rates were calculated per inflorescence.
RESULTS
Floral visitors and nectar rewards
Pompilid wasps (of both sexes) were the most abundant visitors to flowers of both Eucomis autumnalis and E. comosa (Table 2 and Fig. 1C, D). The most abundant pompilid species were identified as Hemipepsis capensis, H. errabunda and H. hilaris. Eucomis autumnalis was also visited by relatively large numbers of two cetoniin beetle species (Atrichelaphinis tigrina and Cyrtothyrea marginalis) and a single unidentified elaterid beetle species. Eucomis comosa was also visited by relatively large numbers of honeybees (Apis mellifera), lycid beetles (five species) and a single cetoniin beetle species (A. tigrina; Table 2). All other visitors to both plant species were observed infrequently and in relatively low numbers (Table 2). Of the visitors to E. autumnalis, Hemipepsis wasps were the only insects that were observed to move frequently between separate plants. This was in contrast to the cetoniin beetle species and the elaterid beetle species which spent long periods of time on each inflorescence and were seldom observed moving between plants. Likewise, Hemipepsis wasps were the only insects that moved frequently between E. comosa plants, although honeybees were also occasionally observed to move between plants. Floral visitors were active throughout the day on inflorescences of both plant species.
Eucomis autumnalis flowers produced a greater volume of more dilute nectar over a 24-h period, when compared with E. comosa flowers (Table 3). The standing crop of nectar on E. autumnalis flowers was considerably more concentrated than nectar that accumulated over 24 h (Table 3).
Table 3.
Nectar properties for Eucomis autumnalis and E. comosa
| Species | Sampling method | n* | Volume (μL): mean ± s.d. | n* | Concentration (%): mean ± s.d. |
|---|---|---|---|---|---|
| E. autumnalis | 24 h production | 16 (3) | 15·8 ± 5·44 | 15 (3) | 19 ± 4·8 |
| Standing crop | 20 (4) | 2·0 ± 1·04 | 20 (4) | 71 ± 4·9 | |
| E. comosa | 24 h production | 25 (5) | 2·8 ± 0·98 | 25 (5) | 62 ± 3·8 |
*The numbers in parentheses refer to the number of plants.
Reproductive biology and reliance on pollinators
Seed set was significantly higher in outcrossed flowers than in selfed or unmanipulated controls for both E. autumnalis and E. comosa (Table 4). Natural seed set was higher for E. comosa than for E. autumnalis (Table 5). For E. autumnalis, seed set was not significantly different between sites (F2,13 = 2·72, P = 0·10; for the Midmar Nature Reserve site, this analysis only included the seed set obtained by measuring all flowers on a plant). The two sampling methods used for measuring natural seed set in E. autumnalis at Midmar Nature Reserve yielded results which were not significantly different (t = 0·75, P = 0·48). For E. comosa there was no significant difference between seed set at Gilboa Estate in 2004–2005 and Howick in 2005–2006 (t = 0·55, P = 0·59).
Table 4.
Results of Eucomis autumnalis and E. comosa breeding system experiments
| Seed set (mean ± s.d.) |
|||
|---|---|---|---|
| Species | Cross | Self | Control |
| E. autumnalis | 5·8 ± 4·89 (n = 46)a | 0·1 ± 0·25 (n = 45)b | 0·02 ± 0·07 (n = 48)b |
| E. comosa | 5·6 ± 4·23 (n = 38)a | 0·1 ± 0·12 (n = 40)b | 0·4 ± 0·39 (n = 40)b |
Sample sizes refer to the number of flowers in each treatment.
Treatments with different letters are significantly different at the 5 % level.
Table 5.
Natural seed set for Eucomis autumnalis and E. comosa measured at different sites
| Field site | n* | Percentage of flowers that set seed | No. of ovules: mean ± s.d. per flower (n) | Seed set: mean ± s.d. per flower per plant |
|---|---|---|---|---|
| Eucomis autumnalis | ||||
| Highflats | 439 (6)† | 62 | 29·0 ± 1·62 (20) | 1·6 ± 0·73 |
| Midmar | 239 (4)† | 54 | Not measured | 3·7 ± 2·83 |
| Midmar‡ | 90 (9) | 77 | Not measured | 5·0 ± 3·02 |
| Vernon Crooks | 429 (6)† | 48 | 31·1 ± 4·46 (40) | 1·4 ± 1·15 |
| Eucomis comosa | ||||
| Gilboa | 47 (5) | 98 | 27·2 ± 2·91 (28) | 7·4 ± 2·13 |
| Howick | 72 (9) | 96 | Not measured | 8·3 ± 4·28 |
* The numbers in parentheses refer to the number of plants.
† Sampled all flowers on each plant.
‡ This sample excluded fruits damaged by caterpillars (see Materials and Methods).
Pollinator effectiveness
Pompilid wasps as a functional group carried considerably higher loads of E. autumnalis (mean = 964 grains per wasp) and E. comosa (mean = 1362 grains per wasp) pollen than all other visitors (Table 6). Pollen was located on the head and thorax of pompilid wasps (see Fig. 1C, D). The only other insects which carried appreciable amounts of pollen were cetoniin beetles (mean = 208 grains per beetle) in the case of E. autumnalis and lycid beetles (mean = 367 grains per beetle) in the case of E. comosa (Table 6).
Eucomis comosa flowers which were exposed to visits by pompilid wasps (n = 332 flowers on 14 inflorescences) set significantly more seeds (mean ± s.d. = 0·14 ± 0·21 seeds per flower per plant, range = 0–5) than flowers that were not exposed (n = 130 flowers on 13 inflorescences; no seeds were produced; Mann–Whitney U, Z = 3·38, P = 0·003).
Pollen grains were deposited on 42 % of virgin E. autumnalis stigmas exposed to visits by Hemipepsis wasps in a cage (Table 7). Of the flowers which received pollen, most received between one and ten grains (Table 7).
Table 7.
Pollen deposition on the stigmas of virgin Eucomis autumnalis flowers placed in a cage with Hemipepsis wasps
| Number of flowers in each pollen load category |
||||||
|---|---|---|---|---|---|---|
| Flowers exposed | Stigmas with pollen deposited | 0 pollen grains | 1–10 pollen grains* | 10–100 pollen grains | >100 pollen grains | |
| Number | 155 | 65 | 90 | 48 | 14 | 3 |
| Percentage | 100 | 42 | 58 | 30 | 9 | 2 |
*Category containing the median value.
Responses of wasps to scent and visual cues
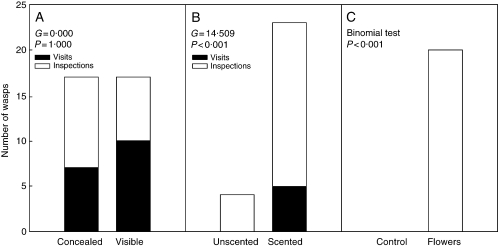
There was no difference between the number of visits by pompilid wasps to E. autumnalis inflorescences concealed from view using leaves and to exposed inflorescences (Fig. 2). Of the 45 wasps that approached plants, 41 (91 %) approached from downwind, four (9 %) approached from 90° to the wind, and no wasps approached plants from upwind. Pompilid wasps visited the E. autumnalis inflorescence situated behind a plastic bag significantly more than they visited the inflorescence contained within a plastic bag to prevent emission of scent (Fig. 2). No other insects (aside from the pompilid wasps) were attracted to E. autumnalis flowers during these experiments.
Fig. 2.
Field-based and laboratory (Y-maze) choice experiments with E. autumnalis and Hemipepsis wasps: (A) number of visits and inspections by Hemipepsis wasps to inflorescences concealed from view (covered with leaves) compared with visible inflorescences; (B) number of visits and inspections by Hemipepsis wasps to unscented inflorescences (covered with a plastic ziplock bag) compared with scented inflorescences (placed behind a plastic ziplock bag, but not covered); (C) number of choices by wasps in favour of the arm containing flowers compared with the control (empty) arm in a Y-maze (n = 20 runs with four wasps).
In the Y-maze experiment, the Hemipepsis wasps selected the arm with the flowers in all 20 runs (Fig. 2).
Spectral reflectance analysis of flowers and background vegetation
Eucomis autumnalis petals are typically dull green in colour with maximum reflectance of approx. 20 % at 550 nm and low overall reflectance (Fig. 3). Eucomis comosa petals have a similar colouring to E. autumnalis, but had slightly lower overall reflectance (Fig. 3). The spectral reflectance of both Eucomis species was very similar to that of the background vegetation, and, although slightly brighter than the average background, fell within the range of green background reflectance (Fig. 3).
GC-MS analysis of floral scent
To the human nose the scents of both E. autumnalis and E. comosa are similar and have a sweet-spicy fragrance. The floral scent of E. autumnalis was dominated by monoterpenes and, to a lesser extent, benzenoids (see Table S1 in Supplementary Data, available online). The scent of E. comosa was dominated by monoterpenes and benzenoid compounds, with this species producing a comparatively larger proportion of benzenoids than E. autumnalis (see Table S1). Linalool and 3,5-dimethoxy toluene were the predominant monoterpene and benzenoid compounds in E. comosa and E. autumnalis, respectively, with 3,5-dimethoxy toluene forming a relatively higher proportion of the scent of E. comosa. Both species produced a large number of compounds (ranging from 41 to 61 among individual samples; see Table S1). Overall, a total of 104 compounds were identified in E. autumnalis samples and 83 compounds in E. comosa samples with 57 compounds occurring in samples from both species (see Table S1). Eucomis autumnalis samples contained 47 unique compounds (not found in any E. comosa samples) while E. comosa samples contained 26 unique compounds (see Table S1).
DISCUSSION
The results of this study indicate that both Eucomis autumnalis and E. comosa are pollinated primarily by Hemipepsis pompilid wasps and that scent is the key floral attractant. Field observations indicate that these wasps are the most abundant visitors to both plant species (Table 2) and, as a functional group, carry considerably higher pollen loads than any other visitors (Table 6). Exposure of virgin flowers of both species to visits from only Hemipepsis wasps showed that these wasps are capable of depositing pollen on the stigmas of E. autumnalis (Table 7), and, in the case of E. comosa, pollen deposited during a single visit is sufficient to result in seed set (although the low deposition rates and seed set compared with pollen loads suggest that these wasps are not very efficient in this respect). Experimental hand-pollinations showed that both E. autumnalis and E. comosa are genetically self-incompatible and thus reliant on pollinators for seed set. GC-MS analysis showed that both plant species produce a large number of floral volatiles and behavioural experiments showed that the Hemipepsis wasps are attracted by scent rather than visual cues. Measurement of spectral reflectance showed that both E. autumnalis and E. comosa flowers have similar colouring to background vegetation (Fig. 3).
Aside from pompilid wasps, both Eucomis species were visited by several insects which may also contribute to pollination. In the case of E. autumnalis, the only other moderately abundant visitors were cetoniin beetles and a single species of elaterid beetle (Table 2). The cetoniin beetles, however, tend to spend long periods of time on a single plant and were seldom observed moving between plants [pers. obs.; see fig. 2 in Shuttleworth and Johnson (2008) for a comparison between floral visiting times of Hemipepsis wasps and Atrichelaphinis tigrina, one of the beetles observed here, on milkweed flowers]. In addition, the cetoniin beetles carried considerably less pollen than the pompilid wasps (Table 6). Individuals of the elaterid species, although abundant, were small enough to access nectar without contacting anthers or stigmas and thus did not carry pollen. In the case of E. comosa, flowers were also visited by relatively large numbers of honeybees, lycid beetles and cetoniin beetles (Table 2). Although these visitors did carry some pollen (Table 6), their presence on flowers was inconsistent between flowering seasons and field sites (pers. obs.; Table 2). The cetoniin beetles were also seldom observed moving between plants. These insects are thus unlikely to make significant contributions to outcross pollination in either E. autumnalis or E. comosa, suggesting that both Eucomis species are specialized for pollination by pompilid wasps.
Natural seed set in both E. autumnalis and E. comosa was notably low (Table 5). This may, to some extent, reflect pollen limitation. The single visit and pollen deposition experiments suggest that Hemipepsis wasps, although capable of depositing pollen on stigmas, are not very efficient in this respect (Table 7). The low seed set recorded could thus reflect the inefficiency of these wasps in terms of transferring pollen to stigmas. However, seed set of outcrossed flowers in the hand-pollination experiments (Table 4) was similar to natural seed set (Table 5), suggesting that natural seed set might be limited by physiological resources or genetic factors.
It appears that E. autumnalis and E. comosa achieve some degree of specialization through the combination of cryptic colouring and scent as a selective floral attractant. Choice experiments clearly demonstrated that wasps can find inflorescences by fragrance alone and do not require visual cues (Fig. 2). Indeed, flowers of the two study species are cryptic because of their dull yellow-green colour which reflects <20 % of visible light and is similar to the background vegetation (Fig. 3). Furthermore, the scents of E. autumnalis and E. comosa are different to the scents of two morphologically similar congeners (E. bicolor and E. humilis) which are also cryptically coloured but pollinated by carrion flies and have very different scent composition (A. Shuttleworth and S. D. Johnson, unpubl. res.). This suggests some degree of adaptation to different pollinators in terms of the volatile compounds produced.
The absence of direct floral filters in either of these Eucomis species is intriguing. The flowers of both species produce relatively large amounts of concentrated nectar (Table 3). Without morphological filters this nectar is freely available to a wide variety of flower visitors, including nectar robbers. In some plants with exposed nectar, specialization can be achieved by distasteful compounds in nectar which renders it unpalatable to non-pollinating insects. This has been demonstrated in a milkweed pollinated by the same Hemipepsis wasps (Shuttleworth and Johnson, 2006) and in a bird-pollinated Aloe (Johnson et al., 2006), both of which have nectar which is distasteful to bees. Although nectar palatability was not investigated in this study, we believe the production of toxic nectar is unlikely in these Eucomis species since the flowers were also visited by generalist nectar-feeding insects. Indeed, the relatively high number of non-pollinating visitors to the E. autumnalis and E. comosa flowers (Table 2) compared with other pompilid-pollinated flowers (see Ollerton et al., 2003; Johnson, 2005; Shuttleworth and Johnson, 2006, 2008, 2009; Johnson et al., 2007) suggests that the nectar of Eucomis flowers is at least partially palatable to other insects. The high concentration of nectar in E. autumnalis and E. comosa flowers is consistent with the nectars of other pompilid-pollinated flowers (Ollerton et al., 2003; Shuttleworth and Johnson, 2006, 2008, 2009; Johnson et al., 2007; but see Johnson, 2005), suggesting that Hemipepsis wasps have driven the evolution of at least some of the nectar characteristics in these species.
The high number of monoterpene and aromatic volatile compounds common to the scents of both species (see Table S1 in Supplementary Data available at AoB online) may be a result of common descent (the phylogenetic relatedness of the two species is unknown) or it could indicate that a blend of these compounds plays a key function for the attraction of pompilid wasps. Two orchid species, Disa sankeyi and Satyrium microrrhyncum, specialized for pollination by these same pompilid wasps also emit a large number of different floral volatile compounds (Johnson, 2005; Johnson et al., 2007). The scent of Disa sankeyi is dominated by monoterpene and aromatic compounds, while that of S. microrrhynchum is composed almost entirely of monoterpenes, sesquiterpenes and a few aromatics (Johnson, 2005; Johnson et al., 2007). Overall, only five compounds (elemicin, α-pinene, β-pinene, limonene and α-terpineol) were common to the scents of these two orchids and both Eucomis species. However, most of these are common floral volatiles and are unlikely to be a specific signal for wasps (see Knudsen et al., 2006). It is possible that broad suites of compounds within particular classes will be found to characterize pompilid-pollinated plants, as in the example of scents of moth-pollinated flowers which often contain high proportions of terpenoid and aromatic alcohols with small amounts of nitrogen-containing compounds (Knudsen and Tollsten, 1993).
Alternatively, pompilid-pollinated plants may rely on specific prey-related compounds to attract spider-hunting wasps. A recent study by Brodmann et al. (2008) found that two European orchids (Epipactis helleborine and E. purpurata) have developed a chemical-mimicry system in which they utilize green-leaf volatiles to attract their vespid pollinators. Green-leaf volatiles are produced by damaged plant tissues and the vespid wasps typically use these volatiles as a cue to find their herbivorous caterpillar prey. The key difference between the Epipactis–vespid system and this system is that pompilid wasps hunt spiders and not caterpillars. However, it is likely that pompilid wasps use chemical cues to locate their spider prey, as has been demonstrated for other prey-hunting wasps (Hendrichs et al., 1994; Brodmann et al., 2008). Pompilid-pollinated plants may thus be mimicking compounds produced by spiders which are attractive to these spider-hunting wasps. Unfortunately, the specific spiders used by these Hemipepsis wasps are not yet established, making it difficult to explore this idea further. Furthermore, pompilid-pollinated plants are visited by both male and female wasps, while only females hunt prey, suggesting that these wasps are not attracted solely by prey-related volatiles. Further research exploring the floral scents of other non-pompilid pollinated Eucomis species in conjunction with gas chromatography–electro-antennogram detection and bioassay experiments are ultimately required to fully understand the scent cues used by E. autumnalis and E. comosa to attract Hemipepsis wasps.
This study shows a clear role for floral scent in the specialized pollination of two Eucomis species. In future, we intend to use GC-EAD and bioassays to determine which of the dozens of floral volatiles produced by these flowers are attractive to pompilid wasps. Further research on other members of the genus may provide interesting insights into the role of floral scent in pollinator shifts by morphologically similar plant species.
SUPPLEMENTARY DATA
Supplementary data is available online at www.aob.oxfordjournals.org and consists of the following. Fig. S1: Reflectance spectra measured in the field-based choice experiments. Table S1: The floral volatiles identified by GC-MS.
ACKNOWLEDGEMENTS
We thank A-L. Wilson for assistance in the field, and C. E. F. Anderson, J. Bailey, A. D. Zinn and K. Todd for conducting the field-based choice experiments. We thank Dr R. Kaiser for analysing solvent scent samples and Dr A. Jürgens for assistance with the remainder of the fragrance analysis. Dr R. Kaiser, Dr A. Jürgens and three anonymous reviewers are thanked for providing valuable comments on an earlier draft of the manuscript. Prof. D. Brothers is thanked for assistance with the identification of the wasps. G. and L. Walker, A. and H. Shuttleworth and R. Kunhardt are thanked for permission to work on their respective properties. This study was supported by the National Research Foundation of South Africa.
LITERATURE CITED
- Adler LS. The ecological significance of toxic nectar. Oikos. 2000;91:409–420. [Google Scholar]
- Arnold G. The Psammocharidae (olim Pompilidae) of the Ethiopian region. Annals of the Transvaal Museum. 1932;14:284–396. [Google Scholar]
- Beattie AJ. A technique for the study of insect-borne pollen. Pan Pacific Entomologist. 1971;47:82. [Google Scholar]
- Brodmann J, Twele R, Francke W, Hölzler G, Zhang Q-H, Ayasse M. Orchids mimic green-leaf volatiles to attract prey-hunting wasps for pollination. Current Biology. 2008;18:740–744. doi: 10.1016/j.cub.2008.04.040. [DOI] [PubMed] [Google Scholar]
- Day MC. The species of Hymenoptera described by Linnaeus in the genera Sphex, Chrysis, Vespa, Apis and Mutilla. Biological Journal of the Linnean Society. 1979;12:45–84. [Google Scholar]
- Field LF. Consequences of habitat fragmentation for the pollination of wildflowers in moist upland grasslands of KwaZulu-Natal. South Africa: University of Natal; 2002. MSc Thesis. [Google Scholar]
- Goulet H, Huber JT, editors. Hymenoptera of the world: an identification guide to families. Ontario: Centre for Land and Biological Resources Research; 1993. [Google Scholar]
- Hendrichs J, Katsoyannos BI, Wornoayporn V, Hendrichs MA. Odour-mediated foraging by yellowjacket wasps (Hymenoptera: Vespidae): predation on leks of pheromone-calling Mediterranean fruit fly males (Diptera: Tephritidae) Oecologia. 1994;99:88–94. doi: 10.1007/BF00317087. [DOI] [PubMed] [Google Scholar]
- Johnson SD. Specialized pollination by spider-hunting wasps in the African orchid Disa sankeyi. Plant Systematics and Evolution. 2005;251:153–160. [Google Scholar]
- Johnson SD, Andersson S. A simple field method for manipulating ultraviolet reflectance of flowers. Canadian Journal of Botany. 2002;80:1325–1328. [Google Scholar]
- Johnson SD, Steiner KE. Generalization versus specialization in plant pollination systems. Trends in Ecology and Evolution. 2000;15:140–143. doi: 10.1016/s0169-5347(99)01811-x. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Hargreaves AL, Brown M. Dark bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology. 2006;87:2709–2716. doi: 10.1890/0012-9658(2006)87[2709:dbnfaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Ellis A, Dotterl S. Specialization for pollination by beetles and wasps: the role of lollipop hairs and fragrance in Satyrium microrrhynchum (Orchidaceae) American Journal of Botany. 2007;94:47–55. doi: 10.3732/ajb.94.1.47. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Tollsten L. An introduction to the scent of cacti. Flavour and Fragrance Journal. 1995;10:153–164. [Google Scholar]
- Knudsen JT, Tollsten L. Trends in floral scent chemistry in pollination syndromes: floral scent composition in moth-pollinated taxa. Botanical Journal of the Linnean Society. 1993;113:263–284. [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Stahl B. Diversity and distribution of floral scent. Botanical Review. 2006;72:1–120. [Google Scholar]
- Ollerton J, Johnson SD, Cranmer L, Kellie S. The pollination ecology of an assemblage of grassland asclepiads in South Africa. Annals of Botany. 2003;92:807–834. doi: 10.1093/aob/mcg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley E. A field guide to wildflowers of KwaZulu-Natal and the Eastern Region. Durban: Natal Flora Publications Trust; 1998. [Google Scholar]
- Shuttleworth A, Johnson SD. Specialized pollination by large spider-hunting wasps and self-incompatibility in the African milkweed Pachycarpus asperifolius. International Journal of Plant Sciences. 2006;167:1177–1186. [Google Scholar]
- Shuttleworth A, Johnson SD. Bimodal pollination by wasps and beetles in the African milkweed Xysmalobium undulatum. Biotropica. 2008;40:568–574. [Google Scholar]
- Shuttleworth A, Johnson SD. Environmental Entomology. 2009. Palp-faction: an African milkweed dismembers its wasp pollinators. (in press) [DOI] [PubMed] [Google Scholar]
- Stephenson AG. Toxic nectar deters nectar thieves of Catalpa speciosa. American Midland Naturalist. 1981;105:381–383. [Google Scholar]
- Stephenson AG. Iridoid glycosides in the nectar of Catalpa speciosa are unpalatable to nectar thieves. Journal of Chemical Ecology. 1982;8:1025–1034. doi: 10.1007/BF00987883. [DOI] [PubMed] [Google Scholar]
- Williams R. Hyacinthaceae. In: Leistner OA, editor. Seed plants of southern Africa: families and genera. Strelitzia. Vol. 10. Pretoria: National Botanical Institute; 2000. pp. 610–621. [Google Scholar]