Abstract
Background and Aims
Theory for optimal allocation of foliar nitrogen (ONA) predicts that both nitrogen concentration and photosynthetic capacity will scale linearly with gradients of insolation within plant canopies. ONA is expected to allow plants to efficiently use both light and nitrogen. However, empirical data generally do not exhibit perfect ONA, and light-use optimization per se is little explored. The aim was to examine to what degree partitioning of nitrogen or light is optimized in the crowns of three tropical canopy tree species.
Methods
Instantaneous photosynthetic photon flux density (PPFD) incident on the adaxial surface of individual leaves was measured along vertical PPFD gradients in tree canopies at a frequency of 0·5 Hz over 9–17 d, and summed to obtain the average daily integral of PPFD for each leaf to characterize its insolation regime. Also measured were leaf N per area (Narea), leaf mass per area (LMA), the cosine of leaf inclination and the parameters of the photosynthetic light response curve [photosynthetic capacity (Amax), dark respiration (Rd), apparent quantum yield (ϕ) and curvature (θ)]. The instantaneous PPFD measurements and light response curves were used to estimate leaf daily photosynthesis (Adaily) for each leaf.
Key Results
Leaf Narea and Amax changed as a hyperbolic asymptotic function of the PPFD regime, not the linear relationship predicted by ONA. Despite this suboptimal nitrogen partitioning among leaves, Adaily did increase linearly with PPFD regime through co-ordinated adjustments in both leaf angle and physiology along canopy gradients in insolation, exhibiting a strong convergence among the three species.
Conclusions
The results suggest that canopy tree leaves in this tropical forest optimize photosynthetic use of PPFD rather than N per se. Tropical tree canopies then can be considered simple ‘big-leaves’ in which all constituent ‘small leaves’ use PPFD with the same photosynthetic efficiency.
Key words: Optimal resource allocation, nitrogen, photosynthetic capacity, leaf mass per area, tropical trees, radiation use efficiency, scaling, leaf angle, canopy architecture, big leaf model
INTRODUCTION
Within an individual plant, the availability of light (photosynthetic photon flux density, PPFD) declines exponentially with cumulative leaf area index, resulting in strong gradients of PPFD within the canopy (Monsi and Saeki, 1953). Due to their sessile nature, most plants have a high capacity for developmental plasticity and acclimation that allows them to modify the form and function of their leaves to accommodate PPFD gradients within their crown. Adjustments in the physiology of leaves to the PPFD regime (where ‘regime’ is a measure of cumulative PPFD availability over many days or weeks) occurs through changes in leaf mass per area (LMA), leaf anatomy and the differential allocation of nitrogen (N) to carboxylation, electron transfer and light-harvesting processes (Björkman, 1981; Evans, 1989; Hikosaka and Terashima, 1995; Niinemets and Tenhunen, 1997; Oguchi et al., 2003). The principles that organize these adjustments to light availability have been well studied because they are central to our ability to scale photosynthesis from leaf to ecosystems. Over the last 30 years, a theory of optimal nitrogen allocation (ONA; Mooney and Gulmon, 1979; Field, 1983; Farquhar, 1989) based on economic principles has provided valuable explanations of why and how leaf properties should change along light gradients within plant canopies.
The ONA theory is based on the idea that leaf N is a measure of investment in photosynthetic machinery (Field, 1983) because photosynthetic proteins are the dominant fraction of total leaf protein (Evans, 1989). The theory assumes that there are costs and benefits that result from investing a certain amount of N in a leaf and the optimal N invested should depend on the opportunity for carbon gain in the particular micro-environment where a leaf is found (Mooney and Gulmon, 1979; Field, 1983). For instance, ONA predicts that leaves in the sun should have higher N content than leaves in the shade because the opportunity for carbon gain increases with insolation. Several empirical studies have demonstrated such variation of foliar N along PPFD gradients yet not in a perfect match with the theoretical predictions (Field, 1983; Hirose and Werger, 1987a; Evans, 1993; Hollinger, 1996; Dang et al., 1997; Friend, 2001; Kull, 2002; Meir et al., 2002; Wright et al., 2006). In particular, ONA predicts that N allocation is optimal when leaf N changes as a monotonic first degree function of PPFD (Farquhar, 1989); since leaf photosynthetic capacity (Amax) is generally linearly related to leaf N (Field and Mooney, 1986), the same prediction can be applied to changes in leaf Amax along PPFD gradients (e.g. Sands, 1995). Yet, the gradient of N (or Amax) along PPFD gradients in a canopy is often less steep than expected. In general, leaves exposed to a high PPFD regime typically have lower N content than the hypothetical optimum, while leaves in the shade have a higher N content than optimum (Evans, 1993; Hollinger, 1996; Dang et al., 1997; Friend, 2001; Kull, 2002; Meir et al., 2002; Wright et al., 2006). This discrepancy has prompted various explanations consistent with the ONA perspective.
It has been shown that an important assumption of ONA is that both the direct and indirect costs per unit N (i.e. marginal N costs) are the same independent of leaf position in the canopy (Mooney and Gulmon, 1979; Field, 1983; Hirose, 2005). Direct costs such as leaf mass per area (LMA, a measure of construction costs per area) or leaf dark respiration (Rd, a measure of maintenance costs per area) tend to change linearly with both leaf N per area and Amax (e.g. Hirose and Werger, 1987b; Ellsworth and Reich, 1993; Raulier et al., 1999), which supports this ONA assumption. However, there are reasons to question the assumption that indirect marginal costs of N are constant. For instance, when leaf N content increases, the risk of herbivory may increase, which means that indirect costs (i.e. loss of photosynthetic yield because of herbivore damage) may be higher at high rather than low N content (Mooney and Gulmon, 1979; Stockhoff, 1994). Variable marginal costs may also be generated by gradients of abiotic stress within plant canopies (e.g. evaporative demand, temperature and wind) that will not necessarily exactly parallel gradients of light (Niinemets and Valladares, 2004). Incorporating these variable marginal costs in the calculation of net photosynthesis is conceivable in principle (Mooney and Gulmon, 1979; Hollinger, 1996), but difficult in practice.
A second, perhaps more critical, consideration is that optimization of resources other than N may be of greater importance. If N is abundant, then the costs of acquiring it from the environment will be low and plants may ‘prioritize’ either by using other limiting resources optimally or co-optimizing different resources (e.g. Wright et al., 2006). Although N availability is generally expected to be low in temperate and boreal ecosystems, N-availability in the lowland tropics is usually above plant demand (Martinelli et al., 1999; Amundson et al., 2003; Ometto et al., 2006). Several studies have shown that productivity of many tropical forests may be limited by availability of light during the rainy season (Wright et al., 1999; Graham et al., 2003; Nemani et al., 2003; Myneni et al., 2007). Ideally, plants should optimize both N and PPFD use (Haxeltine and Prentice, 1996; Terashima et al., 2005), but there is evidence of possible trade-offs between PPFD and N use at the plant (Hirose and Bazzaz, 1998) and at the leaf scale (Hikosaka et al., 1999). Thus, it is conceivable that tropical canopy trees evolved to optimize the use of light rather than to optimize the use of N.
A third consideration is the role of adjustments in branch and leaf angles along PPFD gradients, which are often treated as fixed constants and not directly considered in ONA theory. A leaf can potentially alter its cost–benefit relationships by changing its spatial configuration without any change in its N content. For instance, a leaf blade can be oriented to intercept more PPFD (Ehleringer and Forseth, 1980; Forseth and Ehleringer, 1982; Galvez and Pearcy, 2003) or, conversely, to intercept less PPFD and hence reduce photoxidative, temperature or water stresses associated with high irradiance (Forseth and Ehleringer, 1982; Kao and Forseth, 1992; Feng et al., 2002; Galvez and Pearcy, 2003; Jiang et al., 2006; Kadioglu and Terzi, 2007). In addition, leaf orientation can homogenize the gradient of PPFD in the crown through an influence on self-shading (Terashima and Hikosaka, 1995). Thus, leaf orientation may help explain deviations between observed and expected Narea and Amax distributions.
Considering the points raised in the preceding paragraphs as well as many published empirical data (Evans, 1993; Hollinger, 1996; Dang et al., 1997; Bond et al., 1999; for a review see Kull, 2002), it was expected that in the present study system of tropical trees, Narea and Amax vs. PPFD might not strictly follow the 1 : 1 relationship predicted by ONA theory. Given the overall importance of light limitation during the rainy season on tropical tree canopies, we hypothesized that light-use efficiency may be of greater priority than N-use efficiency. To avoid potential bias from estimating PPFD due to variations in leaf angle, the ONA predictions were tested using incident PPFD in situ on leaves in their natural orientation. Optimal light use was then evaluated by looking at the relationship between average leaf daily photosynthesis (Adaily) and the average daily PPFD incident on individual leaves over the day (i.e. PPFD regime). The Adaily is a measure of time-integrated photosynthesis that incorporates the effects of both leaf angle and foliar physiology. Thus, a 1 : 1 relationship between Adaily and incident PPFD regime would suggest that leaf angle and physiology are co-ordinated to optimize light use. If the optimization of light use and N are in fact simultaneous, then a linear relationship between leaf Amax and Narea with the PPFD regime would be expected. Under the assumption of constant direct marginal costs in ONA theory, it would also be expected that leaf Rd and LMA would scale linearly with Narea. To evaluate all these possibilities, we studied how Narea, LMA, Amax, Rd, apparent quantum yield (ϕ), curvature (θ) and the cosine of leaf angle changed along vertical PPFD regimes within the canopy of individual tropical canopy trees; the PPFD regime was estimated as average daily PPFD incident on the adaxial surface of leaves over periods between 9 and 17 d. The overarching goal of the study was to assess possible explanations for the difference between observed and expected N and Amax distributions along PPFD gradients in the crown of tropical canopy trees.
MATERIALS AND METHODS
Study site
The study was conducted in Parque Natural Metropolitano, a reserve located on the Pacific coast of the Republic of Panama, near Panama City (9°04′N, 79°23′W, 100 m a.s.l.). This 265-ha reserve protects a 75–150-year-old tropical dry forest. The dry season occurs between January and April and the wet season between June and November; May and December are transition months. The forest canopy was reached via a 42-m free-standing construction crane with a 52-m-long jib (Parker et al., 1992). A tubular one-man gondola (diameter approx. 0·7 m) was used to access the inside of tree crowns. The small size of the gondola allowed repeated and non-destructive access to leaves in a broad range of microenvironments within tree crowns.
Species
Two adult canopy trees of Ficus insipida Willd (Moraceae), two of Castilla elastica Sessé (Moraceae), and one of Luehea seemannii Planch. & Triana (Tiliaceae), were sampled. The tops of all trees were fully exposed to sunlight and were situated at or near the upper edge of the forest canopy. Ficus insipida is a fast-growing, early-successional species (Croat, 1978) that has the highest reported leaf Amax of any tree (Zotz et al., 1995). Ficus insipida has an open crown with a leaf area index (LAI) between 2 and 4. Castilla elastica is also an early-successional species, but has a denser crown than F. insipida, with an LAI ≈ 5·0. Luehea seemannii is a secondary- to late-successional tree, with an LAI ≈ 4·0, and has leaves with pronounced heliotropic movements (E. A. Graham, University of California, Los Angeles, CA, unpubl. res.). Castilla elastica drops its leaves in the dry season, while both F. insipida and L. seemannii are evergreen.
Procedure
All measurements were made during the wet season of 2000, between early June and mid-November. Within each tree, 15 leaves were randomly selected from a broad range of PPFD regimes. Approximately one-third of the leaves were fully exposed to direct sunlight, one-third to intermediate light conditions, and one-third to shaded micro-environments. Each leaf was marked at emergence and measurements were made on young, fully developed leaves. During the observations, all measured leaves remained in a micro-environment similar to that during their development.
PPFD on the adaxial leaf surface at natural leaf orientation was measured with small G1118 GaAsP (gallium arsenide phosphide) photodiodes (Hamamatsu Corp., Bridgewater, NJ, USA), which operate in a broad range of temperatures with minimum drift (Fielder and Comeau, 2000). All sensors were calibrated every 1–2 months under a large range of natural daylight conditions against LI-190SA quantum sensors (Li-Cor, Lincoln, NE, USA). To determine the effect of changes in spectral quality, calibration constants for 18 sensors in the open (R : FR = 1·13) and under a leaf canopy (R : FR = 0·58) were compared. No significant effect of changes in the spectra on the calibration constants was found (t = 0·362, P = 0·722), although a small 3·2 % drift in the response has been reported for measurements made in the forest understorey (Pearcy, 1989; Fielder and Comeau, 2000). Although cosine errors can occur when direct beam PPFD is incident near the ‘horizons’ of the plane of the GaAsP sensors, this type of error is small for solar angles greater than 15° (Pearcy et al., 1990). Additionally, this study was carried out during the wet season when above-canopy, direct-beam PPFD only occurred 16·2 % of the time, while fully diffuse PPFD regimes (including overcast conditions plus early mornings and late afternoons) were the most common, occurring 46·9 % of the time. The remaining 36·8 % corresponded to intermediate conditions, when the sky was partially overcast and some direct beam PPFD was still present. The sensor, which weighs only 0·13 g, and its connector caused no visible effect on leaf orientation. The sensors were connected to a data logger (23X; Campbell Scientific Instruments, Logan, UT, USA), with cables 10–20 m long. Instantaneous PPFD was measured simultaneously for 15 leaves within the same tree and data were recorded every 2 s for 9–17 d. A few leaves were lost to herbivory or disease, or were torn during the photosynthesis measurements but the final sample size was between 10 and 14 leaves per tree. Daylength was calculated assuming a sun trajectory of 182°, which included PPFD when the sun was 1° below the horizon at sunrise and sunset.
Leaf orientation was measured just before the beginning of the PPFD measurements. Two angle measurements were made to estimate the inclination of the leaf lamina. The first was the angle between the leaf mid-vein and the horizontal plane (i.e. a completely horizontal mid-vein had an angle of 0°). The second was the rotational angle of the leaf lamina around the mid-vein, measured such that a leaf lamina that was parallel to the direction of gravity had an angle of 90°. After the PPFD measurements were completed, in situ net CO2 fixation rates were measured as a function of instantaneous PPFD (A-PPFD curves) for each leaf with a portable infrared gas analyser (LI-6400, Li-Cor Instruments) with red-blue light-emitting diodes (6400-02B, Li-Cor). Leaf temperature, CO2 concentration, and flow rate were maintained constant at 29 °C, 370 µmol mol−1, and 500 ml min−1, respectively. To obtain A-PPFD curves, leaves were first induced at 500–750 µmol m−2 s−1 for 5–10 min. Secondly, net photosynthesis was briefly measured at 2000 µmol m−2 s−1 and then PPFD was progressively decreased to 1500, 1000, 750, 500, 250, 100, 50 and 0 µmol m−2 s−1. Net photosynthesis was allowed to stabilize at each value of PPFD and a complete photosynthetic light curve (including induction time) was obtained in 25–45 min. Relative humidity in the leaf chamber was controlled manually to ≈70–80 % during measurements. Most leaves were measured in the morning to prevent photoprotection or midday stomatal closure, although a few shade leaves were measured around noon on very cloudy/rainy days.
After the A-PPFD curves were completed, leaves were collected and brought back to the laboratory to measure their different properties. The leaf section used to measure photosynthesis (approx. 8–12 cm2) was cut and its exact area was measured with a leaf area meter. Next, these leaf sections were oven dried at 60 °C until they reached constant mass. Nitrogen content (gN g−1 leaf dry mass) was measured with an elemental analyser (ECS 4010, Costech Technologies, Inc., Valencia, CA, USA).
Data analysis
Data processing was done with the package MathCad Plus 6·0 (Mathsoft, Cambridge, MA, USA, 1996) and R (R Development Core Team, 2006). Photosynthetic light response curves were fitted to a non-rectangular hyperbola (Thornley, 1976). Parameters of the photosynthetic light response curve were estimated with Photosyn Assistant (Dundee Scientific, Dundee, Scotland, UK). The non-rectangular hyperbola function is given by
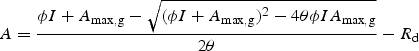 |
where A is net CO2 assimilation, I is instantaneous PPFD incident on the leaf, ϕ is the apparent quantum yield, Amax,g is the light saturated rate of gross assimilation, θ is the curvature, and Rd is dark respiration. In this study, Amax values correspond to the maximum rate of net assimilation, which was calculated as Amax,g – Rd. To estimate Adaily, the PPFD measurements and the photosynthetic light response curves of leaves were used and it was assumed that leaf assimilation rate was a function of instantaneous PPFD throughout the day. Since these curves were measured under optimal conditions and no stress factors were included in the calculations, Adaily was a measure of potential carbon fixation. The assumption of no-stress was reasonable as previous measurements done on the same F. insipida trees reported here showed that there was little mid-day stomatal closure during the wet season (Zotz et al., 1995). An important consideration when calculating Adaily was whether the PPFD regime above the canopy was different among trees, since each individual tree was measured at a different time during the rainy season. No differences were found in average above-canopy daily PPFD among periods of measurements (n = 5 periods, F = 0·952, P = 0·440).
Leaf mass per area was calculated as the ratio between the dry mass (g) of the leaf section used to measure photosynthesis and its area (m2). Leaf nitrogen per unit area (gN m−2) was calculated as the product between leaf nitrogen content (gN g−1 leaf dry mass) and LMA (g leaf dry mass m−2). The cosine of leaf inclination was calculated by taking the product of the cosines of the two leaf angle measurements.
The data were fitted to linear and exponential models and the parameters estimated analytically using minimum least squares. An analysis of covariance was used to test for significant differences between slopes and intercepts of the relationship between Adaily and PPFD regime among trees (Zar, 1996). A hyperbolic model with two parameters and zero intercept was used to describe changes in several leaf functional traits as a function of the PPFD regime
where y is a leaf trait, x is the PPFD regime and a and b are parameters. The parameters of the hyperbolas were estimated with an iterative Gauss–Newton method with step halving implemented in JMP (JMP 5·0; SAS Institute, Inc., NC, USA, 2002). To determine the significance of the parameters, likelihood profiles were used to estimate 95 % confidence limits for each individual parameter. The models (linear, exponential, hyperbolic) were selected based on their applicability to all individual trees, the distributions of their residuals and their r2 value.
RESULTS
Leaf Amax and leaf Narea were positively correlated in all trees. The correlations were highly significant (highest P < 0·004) and the coefficient of correlation (r) varied between 0·85 and 0·96. The same was observed for the correlation between leaf Amax and LMA (highest P < 0·03; r between 0·69 and 0·97) and between Narea and LMA (highest P < 0·0004; r between 0·91 and 0·99). The relationship of leaf Rd with the other traits was generally positive but more variability was observed in the data set. Leaf Rd was positively correlated with Amax in both C. elastica (minimum P < 0·002, r between 0·76 and 0·95) and L. seemannii (P < 0·001, r = 0·84) but was not significantly correlated to Amax in either F. insipda tree (P = 0·088 for F. insipida-1 and P = 0·363 for F. insipida-2). Leaf Rd was positively correlated to leaf Narea in all trees (highest P < 0·003; r between 0·84 and 0·86) except F. insipida 2 (P = 0·121, r = 0·55). Similarly, Rd was positively correlated to LMA in all trees (highest P < 0·001; r between 0·81 and 0·94) but F. insipida-2 (P = 0·198, r = 0·45). Overall, these different correlations suggest that Amax, Narea, LMA and Rd covaried linearly, so that an increment in any one of these traits was related to a proportional increment in the other traits.
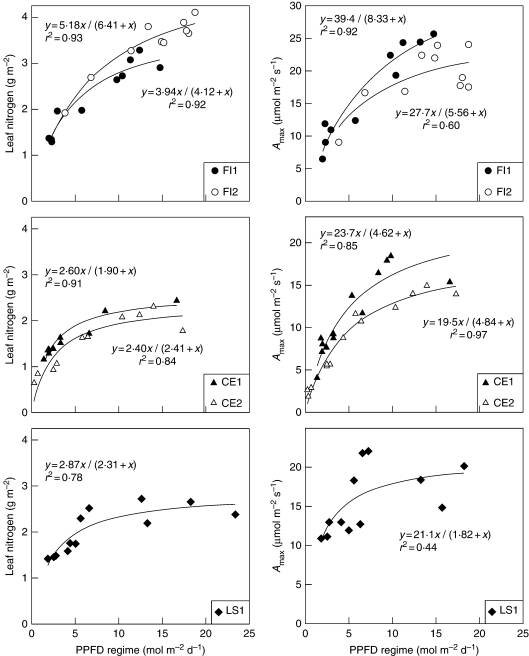
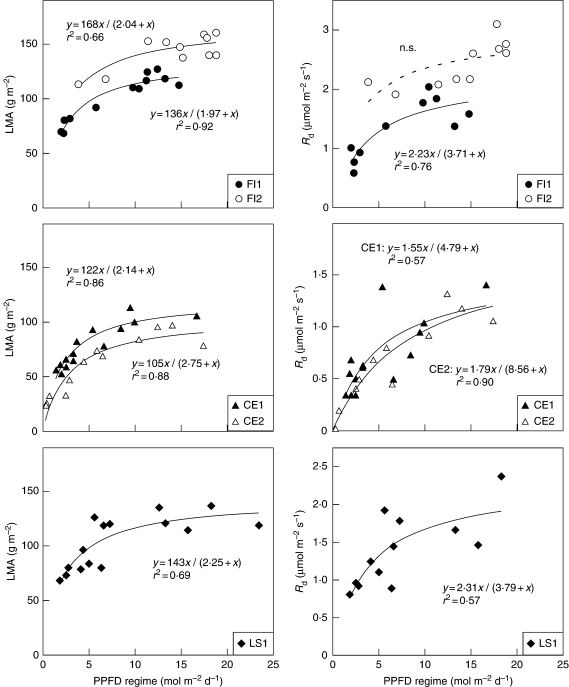
In contrast, leaf Narea and leaf Amax changed as a hyperbolic function of the PPFD regime (Fig. 1). All hyperbola parameters were positive and their 95 % confidence intervals did not include zero. The curvature of the hyperbola was similar between trees, species and traits, and showed that the rate of change in these traits decreased with increasing PPFD regime. Above a PPFD of approx. 10 mol m−2 s−1, the change in the traits was relatively small. In contrast, the most important changes in trait values occurred in leaves exposed to a PPFD regime below approx. 5–7 mol m−2 d−1. The hyperbolic relationships explained a very large proportion of the variability in Narea along the PPFD regime gradients in F. insipida (92–93 %), C. elastica (84–91 %) and L. seemannii (78 %). Similarly, most of the variation in Amax was described by a hyperbola in F. insipida (60–92 %) and in C. elastica (85–97 %), while in L. seemannii leaf Amax was more variable and PPFD explained less variation (44 %). The traits related to the construction and maintenance costs, LMA and Rd, respectively, also changed as a hyperbolic function of the PPFD regime and the shape of the curvatures were comparable to that of Narea and Amax (Fig. 2). A large proportion of the variation in LMA along the PPFD gradient was also explained by the hyperbolic model in F. insipida (66–92 %), C. elastica (86–88 %) and L. seemannii (69 %). The relationship between leaf Rd and PPFD was not significant for F. insipida-2 because the leaves had similar Rd and no leaves were sampled in the most shaded portions of the crown. Yet, the hyperbola explained 76 % of the variation in Rd in F. insipida-1, between 57 and 90 % of the variation in C. elastica and 57 % of the variation in L. seemannii. Simple linear regressions were also fitted to these four traits but the linear models were less appropriate than the hyperbolas due to curvature in the residuals. Also, the hyperbolic models explained considerably more variability in Narea (14·4 % more on average), Amax (10·3 %), LMA (13·9 %) and Rd (15·2 %) relationships than the linear models. The relationship between the apparent quantum yield (ϕ) and the PPFD regime was not significant in any tree (data not shown). The relationship between the curvature (θ) parameter and the PPFD regime was not significant for all trees but C. elastica-2 for which θ decreased linearly with PPFD (P = 0·008, r2 = 0·52) (not shown). Overall, the results for Narea, Amax, LMA and Rd indicate that there was great consistency in how these traits change along PPFD regime gradients. These traits did not change much in the upper parts of the crown where PPFD availability was higher while leaf phenotypic change was more pronounced in the lowest and shadier parts of the crown.
Fig. 1.
Relationships between leaf nitrogen content (g m−2) of individual leaves vs. PPFD regime (mol m−2 d−1; left-hand panels) and leaf photosynthetic capacity (Amax, μmol m−2 s−1) of individual leaves vs. PPFD regime (right-hand panels) in five tropical canopy trees. The PPFD regime was calculated as average daily PPFD incident on the adaxial surface of individual leaves at their natural orientation. The continuous lines correspond to two-parameter hyperbolas with both parameters significantly different from zero. ‘FI’ refers to Ficus insipida (individuals 1 and 2), ‘CE’ to Castilla elastica (individuals 1 and 2) and ‘LS’ to Luehea seemannii.
Fig. 2.
Relationships between leaf mass per area (LMA, g m−2) of individual leaves vs. PPFD regime (mol m−2 d−1; left-hand panels) and leaf dark respiration (Rd, μmol m−2 s−1) vs. PPFD regime (right-hand panels) in five tropical canopy trees. The continuous lines show the best fit to a two-parameter hyperbola with both parameters significantly different from zero, while the dashed line is for relationships with at least one parameter not different from zero. See Fig. 1 for species’ names.
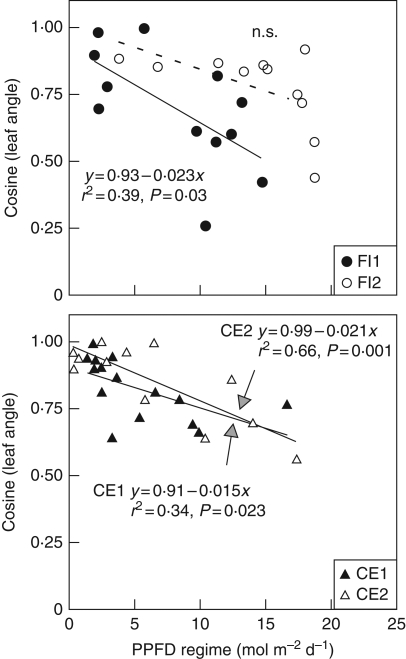
In contrast to the above traits, the cosine of leaf angle decreased proportionally to the PPFD regime in F. insipida-1 and both C. elastica (Fig. 3). The cosine of leaf angle corresponded to the proportion of the leaf surface (one-sided) that was projected on a horizontal plane, i.e. equivalent to the shade cast by a leaf on a horizontal plane if the leaf illumination comes from a source placed vertically above it. Under low PPFD regime the values were near 1, which indicated that leaves were oriented horizontally. The monotonic linear decrease in the cosine of leaf angle shows that leaves were gradually becoming more inclined as PPFD incident on their adaxial surface increased. The amount of variation explained by the PPFD regime varied between 34 % and 66 % and for F. insipida-2 the relationship was not significant. Results were not reported for L. seemannii as this species presents heliotropic leaf movements with leaves changing their angle within a time scale of minutes (E. A. Graham, unpubl. res.).
Fig. 3.
Relationships between cosine of leaf angle and PPFD regime (mol m−2 d−1) of individual leaves vs. PPFD regime in five tropical canopy trees. Cosine of leaf angle was calculated by taking the product of the cosine of the angle of rotation and the cosine of the angle of inclination of the leaf blade (see Materials and Methods). Leaves with a cosine of 1·0 were horizontal while leaves with a cosine of 0·0 were fully vertical. Continuous lines correspond to statistically significant least square linear regressions. The dashed line is for a non-significant relationship. See Fig. 1 for species’ names.
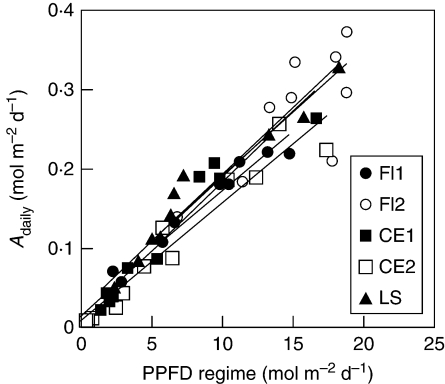
It was found that leaf Adaily was proportional to the PPFD regime on leaves in all trees (Fig. 4). This result is in contrast with the hyperbolic change that was observed between Amax and the PPFD regime (Fig. 1). Most of the variation in Adaily was explained by the linear relationship with the PPFD regime in F. insipida (75–94 %), C. elastica (94 % for both trees) and L. seemannii (94 %). Moreover, an analysis of covariance could not detect a difference between slopes (F = 0·51, P = 0·72) or elevations (i.e. intercepts; F = 2·00, P = 0·11) when comparing the different individual trees; the common slope was 0·016 and was highly significant (t = 21·89, P < 0·001). The common intercept was 0·017 mol m−2 d−1 and was significant (t = 2·45, P = 0·018), although its absolute value was close to zero. The common linear relationship indicated that there was a strong convergence in photosynthetic leaf light use efficiency (calculated as the ratio between leaf Adaily and PPFD regime) for all sampled leaves in this forest.
Fig. 4.
Linear regressions between the PPFD regime (mol m−2 d−1) of individual leaves and average daily net leaf photosynthesis (Adaily, mol m−2 d−1) for five tropical canopy trees. Regression slopes and intercepts did not differ among the five trees (see Results). Each point corresponds to an individual leaf for which average daily net photosynthesis was calculated for the period of PPFD measurements (between 9 and 17 d). See Fig. 1 for species’ names.
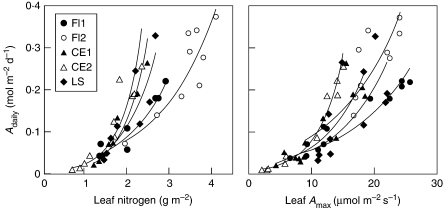
When comparing leaf Narea with leaf Adaily, the relationship was best described with an increasing exponential function (Fig. 5). These exponential relationships were highly significant (P < 0·001) and they explained between 81·4 % and 92·2 % of the variation. These plots demonstrate that the ratio Adaily:N was lower for shaded leaves at the lower positions in the crown than for sunnier leaves. The relationship between leaf Amax and leaf Adaily followed a similar type of increase. All exponential relationships were significant (highest P < 0·010) and explained between 51·2 % and 97·5 % of the variation. It was also found that the relationships between Adaily vs. Narea and Amax could be fitted to linear relationships. However, the average fit was better with the exponential model and it explained more variation (3·13 % better for the Adaily–Narea relationship and 5·33 % better for the Adaily–Amax relationship).
Fig. 5.
Relationship between leaf nitrogen content (g m−2) and leaf daytime carbon gain (Adaily, mol m−2 d−1; left panel) and between leaf photosynthetic capacity (Amax, μmol m−2 s−1) and Adaily (right panel). The continuous lines correspond to statistically significant least square exponential regressions. See Fig. 1 for species’ names.
DISCUSSION
The prediction from ONA theory that there should be a monotonic first-order increase in Amax or Narea with PPFD regime is not generally supported by empirical data (e.g. Evans, 1993; Hollinger, 1996; Dang et al., 1997; Bond et al., 1999; for a review see Kull, 2002). In earlier studies and here as well, shaded leaves lower in the canopy exhibit higher Narea or Amax than expected while sun leaves in the upper canopy exhibit lower trait values than expected. In several studies, linear models have been fitted to the relationships between Narea, Amax or carboxylation capacity (Vcmax) vs. PPFD regime, and these regressions had elevations higher than zero in the majority of cases (Kull, 2002). Although linear models were also fitted to the present data and significant positive intercepts were found in all trees (data not shown), pattern in the residuals indicated a simple linear model was not statistically valid for the trees studied. Because of the broad range of PPFD regimes assessed (16-fold on average and up to 39-fold), it was observed that changes in Narea and Amax were ascending at low PPFD regime and tended towards an asymptote at intermediate and high PPFD regimes.
Several assumptions of ONA theory hold in the present data, and canopy leaves do partition N in the direction predicted by ONA, albeit imperfectly. There is a positive correlation between Narea and Amax, which supports the assumption that leaf Narea is a valid measure of investment in the photosynthetic enzymes. This agrees with earlier reports for many species of plants (e.g. Field and Mooney, 1986; Hirose and Werger, 1987a; Field, 1991; Ellsworth and Reich, 1993; Anten et al., 1995). Also there are linear correlations between Narea and LMA and between Narea and Rd, which show that the present measures of construction (LMA) and maintenance costs (Rd) changed in direct proportion with N, in accord with the assumption of constant marginal costs (i.e. constant cost per unit N). Positive linear Narea–LMA and Narea–Rd relationships also have been commonly reported (e.g. Hirose and Werger, 1987b; Ellsworth and Reich, 1993; Raulier et al., 1999). There are, however also some assumptions that are not satisfied in the present data and that may account for the imperfect fit to ONA predictions.
There is reason to question the assumption that indirect marginal costs are the same for all leaves irrespective of their N content or position in the canopy. Indirect marginal costs may well be higher for leaves with high N content because these leaves are usually found in exposed parts of the canopy where environmental stresses are higher (e.g. Niinemets and Valladares, 2004). Similarly, indirect marginal costs may be higher if herbivores show preferences for leaves with a higher N content (e.g. Mooney and Gulmon, 1979; Stockhoff, 1994). The present study was not designed to measure how indirect costs changed as a function of leaf Narea or with the physical location of a leaf in the crown. However, part of the key cost–benefit relationship in the ONA theory was directly evaluated (Hirose, 2005) – how gains (i.e. Adaily) changed with allocation to Narea. It was found that Adaily scaled as an exponential function of Narea or Amax indicating that marginal gains were higher in the upper part of the tree crowns, where the highest leaf Amax and Narea were also found; in other words, photosynthetic N-use efficiency (PNUE, photosynthetic gains per unit N) was higher in the sunnier than in the shadier parts of the trees. Since this disproportionate increase in marginal benefits could compensate, or even exceed, any increase in indirect marginal costs, variable indirect marginal costs may not necessarily explain the hyperbolic change in Narea and Amax with PPFD regime.
It should therefore be considered that deviations from ONA predictions may arise simply because the most fundamental assumption in the theory is violated. Perhaps N is not the focus of optimization, particularly in many tropical forests. In lowland tropical forests isotopic fractionation of N indicates that N availability is generally above plant demand (Martinelli et al., 1999; Amundson et al., 2003; Ometto et al., 2006) and that some of these forests may be in a non-Liebig condition where multiple nutrients co-limit productivity (Kaspari et al., 2008). Under these conditions, natural selection may not be able to favour the evolution of mechanisms that allow tropical plants to maximize the efficient use of one mineral resource over another. On the other hand, there is evidence that the productivity of lowland tropical forests is limited by PPFD availability during the cloudy, rainy seasons (Wright et al., 1999; Graham et al., 2003; Nemani et al., 2003; Myneni et al., 2007). The strong convergence of variation in leaf function within the canopy among three species varying in canopy architecture and leaf display patterns suggests that the tropical tree species studied evolved to use PPFD efficiently, not N. The monotonic first-order function between leaf Adaily and PPFD regime that were observed shows that all leaves in the crown used PPFD with the same photosynthetic efficiency (εleaf), which was calculated as the ratio between leaf Adaily and leaf PPFD regime (Rosati and DeJong, 2003). This proportionality was maintained because most photosynthetic activity was concentrated on the ascending portion of the photosynthetic light response curves of leaves (Monteith, 1994; Rosati and DeJong, 2003) and, in particular, in the section of the curves where photosynthetic PPFD use efficiency was maximal (J. M. Posada, unpubl. res.), i.e. constant εleaf was also related to maximum PPFD-use efficiency. A 1 : 1 relationship between leaf Adaily and the PPFD regime has also been observed in cultivated plants in temperate latitudes (Rosati and DeJong, 2003; Rosati et al., 2004), suggesting that optimization of εleaf may be a more widespread phenomenon than previously acknowledged.
Although one might expect plants to use both N and light efficiently (Haxeltine and Prentice, 1996; Terashima et al., 2005), it may be impossible to simultaneously achieve perfect optimization of both (Hirose and Bazzaz, 1998; Hikosaka et al., 1999; Terashima et al., 2005). Here, it was not directly evaluated whether the observed leaf Narea deviated from the N content that would maximize PNUE (e.g. Hikosaka et al., 1999). However, the lack of support for the 1:1 Narea vs. PPFD relationship as well as the exponential Adaily vs. Narea relationship strongly suggested that maximum PNUE was not attained by the entire crown in these trees. It was also emphasized that leaf Amax was not in simple proportionate relationship to the time-integral of leaf Adaily. Leaf Amax is a measure of the maximum instantaneous C-gain, while Adaily incorporates both the physiology and the modulating effect of leaf angle on the PPFD environment. The present data therefore suggest that the spatial arrangement of leaves played a central role in the optimization of PPFD-use and should be explicitly considered when establishing a mechanistic link between the allocation to Narea or Amax and the time-integral of leaf carbon gain.
It was found that the cosine of leaf angle declined monotonically along the entire PPFD gradient for the three of our four study trees that had no heliotropic leaf movements. Above the mid-range of PPFD regime, where Narea and Amax changed little, leaf angle appeared to play a central role in maintaining Adaily proportional to the PPFD regime, while at low PPFD regime both leaf angle and leaf physiology changed. This suggests that leaves optimized their photosynthetic use of PPFD thanks to a leaf-level mechanism that co-ordinated their spatial orientation and their physiology. Previous studies also have long recognized the contribution of leaf angle to an efficient use of PPFD as tilted leaves allow greater sharing of light within the plant canopy (Boysen-Jensen, 1932; Monsi and Saeki, 1953; Monteith, 1994; Terashima and Hikosaka, 1995; Terashima et al., 2005). Also recognized in large canopy tree crowns is the importance of not only leaf-angle variations, but also shoot and branch architecture on light gradients generated by self-shading (Kitajima et al., 2005). Several studies have included, with different levels of complexity, the effect that canopy architecture has on within-canopy PPFD gradients and optimal resource allocation (e.g. Hirose and Werger, 1987b; Gutschick and Wiegel, 1988; Hikosaka et al., 1999; Schieving and Poorter, 1999). It is possible that a relatively small gain in whole plant carbon gain through perfect ONA, or perfect carbon allocation that is reflected in LMA (Gutschick and Wiegel, 1988), are less important than increased leaf area index in maintaining competitiveness with neighbouring plants (Gutschick and Wiegel, 1988; Schieving and Poorter, 1999). This has been considered as an explanation for retaining lower shade leaves in trees despite their low N-use efficiency and small contribution to whole-canopy photosynthesis (Monsi and Saeki, 1953; Kitajima et al., 2005). In herbaceous canopies, models that consider the dynamics of leaf production and death generate more realistic leaf trait distributions in the canopy (Franklin and Ågren, 2002; Hikosaka, 2003, 2005). However, in crowns of many canopy trees, lower shade leaves develop simultaneously with higher sun leaves, unlike sequential development of leaves typical in herbaceous canopies.
Finally, the observed convergence in optimal PPFD use has important implications for the scaling of photosynthesis from leaves to canopy (Dewar, 1996; Haxeltine and Prentice, 1996). Estimating overall εleaf as the linear slope between Adaily and the PPFD regime, no significant differences were found in slopes among the study trees. There was a strong convergence in daily photosynthetic light-use efficiency despite substantial species differences in canopy architecture, life history and successional status. These results were particularly surprising for L. seemannii, which has heliotropic leaves that can change their angle in a matter of minutes (E. A. Graham, unpubl. res.). The common slope value, 0·016 mol CO2 · mol photons−1, was well within the range estimated in cultivated plants, although higher values were reported during cloudy days (Rosati and DeJong, 2003; Rosati et al., 2004). The maintenance of this constant εleaf supports the use of ‘big-leaf’ models to simulate whole plant or canopy photosynthesis. Big-leaf models consider the canopy as a single leaf and, due to their simplicity, they have been used extensively (e.g. Amthor, 1994; Dang et al., 1997; Friend, 2001). These models generally assume that leaf Amax changes in a 1 : 1 ratio with the PPFD gradients, which, as shown in this and other studies, is usually not the case (e.g. Friend, 2001; Kull, 2002; Wright et al., 2006). The present results suggest that it is possible to use an even simpler big-leaf approach where the Amax–PPFD gradients can be ignored. The constant εleaf means that all leaves, regardless of their position along the PPFD gradient or taxonomic identity, used light with the same efficiency. This constancy also indicates that εleaf is equal to the light-use efficiency of the whole forest canopy (εcanopy). Although the common elevation was statistically significant, its absolute value was close to zero suggesting that this approximation is reasonable. Hence, it should be possible to estimate net photosynthesis of a forest canopy (Acanopy) by combining εcanopy with measures of PPFD absorbed (PPFDA) by leaves using the simple equation:
| 1 |
This equation is analogous to the well-know relationship between plant growth and absorbed photosynthetic radiation (APAR) where growth is proportional to εg · APAR (Monteith, 1977; Monteith, 1994); in this equation εg is a constant defining the light-use efficiency of biomass production. The two equations cannot be compared directly because growth (i.e. biomass production) is the result of plant gross photosynthesis minus plant respiration. The results of the present study, however, show how leaf-level optimization of light use can help explain the proportional relationship between biomass production and APAR. This conclusion agrees with previous predictions (Monteith, 1994; Dewar, 1996; Haxeltine and Prentice, 1996) and empirical data for cultivated plants (Rosati and DeJong, 2003; Rosati et al., 2004). An advantage of eqn (1) is that it can be used to estimate tree and forest net photosynthesis as well as gross primary production by taking a few gas exchange measures at leaf level.
In summary, it was found that individual leaves optimized the photosynthetic use of light along PPFD regime gradients in the crown of tropical trees. We suggest that this optimization was possible through unidentified mechanisms that co-ordinate the angle and the physiology of individual leaves so that most instantaneous PPFD incident on a leaf is concentrated on the ascending part of the A–PPFD curve (Rosati and DeJong, 2003; J. M. Posada, unpubl. res.). Although the leaves exhibit variation in the direction of ONA, they did not exhibit a perfect match to the prediction of a 1:1 relationship between Narea or Amax with the PPFD regime. In contrast, daily photosynthetic light-use efficiency exhibited convergent constancy among leaves in a manner to achieve optimization of light use for the whole crown. In other words, N allocation was a subordinate response driven by leaves within the plant canopy using PPFD efficiently. The present study provides evidence of a strong convergence towards the optimization of εleaf, which potentially can simplify the scaling of photosynthesis from leaves to plants and ecosystems. The generality of the results should be tested, especially in systems where insolation may not be the primary factor limiting productivity but where plants still present non-optimal leaf N distributions along PPFD gradients.
ACKNOWLEDGEMENTS
We thank Stephen Mulkey, Joe Wright, Thomas Sinclair, Jack Putz, Tim Martin, Edward Schuur, Fernando Valladares and Grace Crummer for providing ideas and suggestions during the different stages of this research. We are grateful to Ben Bolker and Toshi Okuyama for their help with statistical theory and R programming. Eric Graham, Deborah Buehler and Anouk St-Arnaud provided valuable help in the field and during data processing. This work was supported by a Colciencias Ph.D. Fellowship; a Russell Dissertation Fellowship (University of Florida); a fellowship from the World Ecology Center (University of Missouri St-Louis); a Sigma Xi Grant-in-Aid-of-Research and a short-term postdoctoral fellowship from the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT) to J.M.P. The Smithsonian Tropical Research Institute supported fieldwork in their canopy crane. A Natural Sciences and Engineering Research Council of Canada (NSERC) grant to M.J.L. supported manuscript preparation.
LITERATURE CITED
- Amthor JS. Scaling CO2 photosynthesis relationships from the leaf to the canopy. Photosynthesis Research. 1994;39:321–350. doi: 10.1007/BF00014590. [DOI] [PubMed] [Google Scholar]
- Amundson R, Austin AT, Schuur EAG, et al. Global patterns of the isotopic composition of soil and plant nitrogen. Global Biogeochemical Cycles. 2003;17:1030. [Google Scholar]
- Anten NPR, Schieving F, Werger MJA. Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C3 and C4 mono- and dicotyledonous species. Oecologia. 1995;101:504–513. doi: 10.1007/BF00329431. [DOI] [PubMed] [Google Scholar]
- Björkman O. Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Zeigler H, editors. Encyclopedia of plant physiology. Berlin: Springer-Verlag; 1981. New Series. [Google Scholar]
- Bond BJ, Farnsworth BT, Coulombe RA, Winner WE. Foliage physiology and biochemistry in response to light gradients in conifers with varying shade tolerance. Oecologia. 1999;120:183–192. doi: 10.1007/s004420050847. [DOI] [PubMed] [Google Scholar]
- Boysen-Jensen P. Die stoffproduktion der pflanzen. Jena: Verlag von Gustav Fischer; 1932. [Google Scholar]
- Croat TB. The flora of Barro Colorado Island. Palo Alto, CA: Stanford University Press; 1978. [Google Scholar]
- Dang QL, Margolis HA, Sy M, Coyea MR, Collatz GJ, Walthall CL. Profiles of photosynthetically active radiation, nitrogen and photosynthetic capacity in the boreal forest: implications for scaling from leaf to canopy. Journal of Geophysical Research–Atmospheres. 1997;102:28845–28859. [Google Scholar]
- Dewar RC. The correlation between plant growth and intercepted radiation: an interpretation in terms of optimal nitrogen content. Annals of Botany. 1996;78:125–136. [Google Scholar]
- Ehleringer JR, Forseth IN. Solar tracking by plants. Science. 1980;210:1094–1098. doi: 10.1126/science.210.4474.1094. [DOI] [PubMed] [Google Scholar]
- Ellsworth DS, Reich PB. Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia. 1993;96:169–178. doi: 10.1007/BF00317729. [DOI] [PubMed] [Google Scholar]
- Evans JR. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia. 1989;78:9–19. doi: 10.1007/BF00377192. [DOI] [PubMed] [Google Scholar]
- Evans JR. Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. II. Stability through time and comparison with a theoretical optimum. Australian Journal of Plant Physiology. 1993;20:69–82. [Google Scholar]
- Farquhar GD. Models of integrated photosynthesis of cells and leaves. Philosophical Transactions of the Royal Society of London, Series B. 1989;323:357–367. [Google Scholar]
- Feng YL, Cao KF, Feng ZL. Thermal dissipation, leaf rolling and inactivation of PSII reaction centres in Amomum villosum. Journal of Tropical Ecology. 2002;18:865–876. [Google Scholar]
- Field C. Allocating leaf nitrogen for the maximization of carbon gain: leaf age as a control on the allocation program. Oecologia. 1983;56:341–347. doi: 10.1007/BF00379710. [DOI] [PubMed] [Google Scholar]
- Field CB. Ecological scaling of carbon gain to stress and resource availability. In: Mooney HA, Winner WE, Pell EJ, editors. Integrated responses of plants to stress. London: Academic Press; 1991. [Google Scholar]
- Field C, Mooney HA. The photosynthesis–nitrogen relationship in wild plants. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- Fielder P, Comeau P. Construction and testing of an inexpensive PAR sensor. Victoria, BC: Research Branch, Ministry of Forests; 2000. [Google Scholar]
- Forseth IN, Ehleringer JR. Ecophysiology of two solar tracking desert winter annuals. II. Leaf movements, water relations and microclimate. Oecologia. 1982;54:41–49. doi: 10.1007/BF00541105. [DOI] [PubMed] [Google Scholar]
- Franklin O, Ågren GI. Leaf senescence and resorption as mechanisms of maximizing photosynthetic production during canopy development at N limitation. Functional Ecology. 2002;16:727–733. [Google Scholar]
- Friend AD. Modelling canopy CO2 fluxes: are ‘big-leaf’ simplifications justified? Global Ecology and Biogeography. 2001;10:603–619. [Google Scholar]
- Galvez D, Pearcy RW. Petiole twisting in the crowns of Psychotria limonensis: implications for light interception and daily carbon gain. Oecologia. 2003;135:22–29. doi: 10.1007/s00442-002-1158-3. [DOI] [PubMed] [Google Scholar]
- Graham EA, Mulkey SS, Kitajima K, Phillips NG, Wright SJ. Cloud cover limits net CO2 uptake and growth of a rainforest tree during tropical rainy seasons. Proceedings of the National Academy of Sciences of the USA. 2003;100:572–576. doi: 10.1073/pnas.0133045100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschick VP, Wiegel FW. Optimizing the canopy photosynthetic rate by patterns of investment in specific leaf mass. The American Naturalist. 1988;132:67–86. [Google Scholar]
- Haxeltine A, Prentice IC. A general model for the light-use efficiency of primary production. Functional Ecology. 1996;10:551–561. [Google Scholar]
- Hikosaka K. A model of dynamics of leaves and nitrogen in a plant canopy: an integration of canopy photosynthesis, leaf life span, and nitrogen use efficiency. The American Naturalist. 2003;162:149–164. doi: 10.1086/376576. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. Leaf canopy as a dynamic system: ecophysiology and optimality in leaf turnover. Annals of Botany. 2005;95:521–533. doi: 10.1093/aob/mci050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K, Terashima I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant, Cell & Environment. 1995;18:605–618. [Google Scholar]
- Hikosaka K, Sudoh S, Hirose T. Light acquisition and use by individuals competing in a dense stand of an annual herb. Xanthium canadense. Oecologia. 1999;118:388–396. doi: 10.1007/s004420050740. [DOI] [PubMed] [Google Scholar]
- Hirose T. Development of the Monsi–Saeki theory on canopy structure and function. Annals of Botany. 2005;95:483–494. doi: 10.1093/aob/mci047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Bazzaz FA. Trade-off between light- and nitrogen-use efficiency in canopy photosynthesis. Annals of Botany. 1998;82:195–202. [Google Scholar]
- Hirose T, Werger MJA. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia. 1987;a 72:520–526. doi: 10.1007/BF00378977. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA. Nitrogen use efficiency in instantaneous and daily photosynthesis of leaves in the canopy of a Solidago altissima stand. Physiologia Plantarum. 1987;b 70:215–222. [Google Scholar]
- Hollinger DY. Optimality and nitrogen allocation in a tree canopy. Tree Physiology. 1996;16:627–634. doi: 10.1093/treephys/16.7.627. [DOI] [PubMed] [Google Scholar]
- Jiang CD, Gao HY, Zou Q, Jiang GM, Li LH. Leaf orientation, photorespiration and xanthophyll cycle protect young soybean leaves against high irradiance in field. Environmental and Experimental Botany. 2006;55:87–96. [Google Scholar]
- Kadioglu A, Terzi R. A dehydration avoidance mechanism: leaf rolling. Botanical Review. 2007;73:290–302. [Google Scholar]
- Kao W-Y, Forseth IN. Diurnal leaf movements, chlorophyll fluorescence and carbon assimilation in soybean grown under different nitrogen and water availabilities. Plant, Cell & Environment. 1992;15:703–710. [Google Scholar]
- Kaspari M, Garcia MN, Harms KE, Santana M, Wright SJ, Yavitt JB. Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecology Letters. 2008;11:35–43. doi: 10.1111/j.1461-0248.2007.01124.x. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Mulkey SS, Wright SJ. Variation in crown light utilization characteristics among tropical canopy trees. Annals of Botany. 2005;95:535–547. doi: 10.1093/aob/mci051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull O. Acclimation of photosynthesis in canopies: models and limitations. Oecologia. 2002;133:267–279. doi: 10.1007/s00442-002-1042-1. [DOI] [PubMed] [Google Scholar]
- Martinelli LA, Piccolo MC, Townsend AR, et al. Nitrogen stable isotopic composition of leaves and soil: tropical versus temperate forests. Biogeochemistry. 1999;46:45–65. [Google Scholar]
- Meir P, Kruijt B, Broadmeadow M, et al. Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant, Cell & Environment. 2002;25:343–357. [Google Scholar]
- Monsi M, Saeki T. Uber der Lichtfaktor in den Pflanzengesellschaften und seine Bedeutung fur die Stoffproduktion. Japanese Journal of Botany. 1953;14:22–52. [Google Scholar]
- Monteith JL. Climate and the efficiency of crop production in Britain. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 1977;281:277–294. [Google Scholar]
- Monteith JL. Validity of the correlation between intercepted radiation and biomass. Agricultural and Forest Meteorology. 1994;68:213–220. [Google Scholar]
- Mooney HA, Gulmon SL. Environmental and evolutionary constraints on the photosynthetic characteristics of higher plants. In: Solbrig OT, Jain S, Johnson GB, Raven PH, editors. Topics in plant population biology. New York, NY: Columbia University Press; 1979. [Google Scholar]
- Myneni RB, Yang W, Nemani RR, et al. Large seasonal swings in leaf area of Amazon rainforests. Proceeding of the National Academy of Sciences of the USA. 2007;104:4820–4823. doi: 10.1073/pnas.0611338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani RR, Keeling CD, Hashimoto H, et al. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science. 2003;300:1560–1563. doi: 10.1126/science.1082750. [DOI] [PubMed] [Google Scholar]
- Niinemets U, Tenhunen JD. A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant, Cell & Environment. 1997;20:845–866. [Google Scholar]
- Niinemets Ü, Valladares F. Photosynthetic acclimation to simultaneous and interacting environmental stresses along natural light gradients: optimality and constraints. Plant Biology. 2004;6:254–268. doi: 10.1055/s-2004-817881. [DOI] [PubMed] [Google Scholar]
- Oguchi R, Hikosaka K, Hirose T. Does the photosynthetic light-acclimation need change in leaf anatomy? Plant, Cell & Environment. 2003;26:505–512. [Google Scholar]
- Ometto JPHB, Ehleringer JR, Domingues TF, et al. The stable carbon and nitrogen isotopic composition of vegetation in tropical forests of the Amazon Basin, Brazil. Biogeochemistry. 2006;79:251–274. [Google Scholar]
- Parker GG, Smith AP, Hogan KP. Access to the upper forest canopy with a large tower crane. BioScience. 1992;42:664–670. [Google Scholar]
- Pearcy RW. Radiation and light measurements. In: Pearcy RW, Ehleringer JR, Mooney HA, Rundel PW, editors. Plant physiological ecology: field methods and instrumentation. London: Chapman and Hall; 1989. [Google Scholar]
- Pearcy RW, Roden JS, Gamon JA. Sunfleck dynamics in relation to canopy structure in a soybean (Glycine max (L.) Merr.) canopy. Agricultural and Forest Meteorology. 1990;52:359–372. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- Raulier F, Bernier PY, Ung C-H. Canopy photosynthesis of sugar maple (Acer saccharum): comparing big-leaf and multilayer extrapolations of leaf-level measurements. Tree Physiology. 1999;19:407–420. doi: 10.1093/treephys/19.7.407. [DOI] [PubMed] [Google Scholar]
- Rosati A, DeJong TM. Estimating photosynthetic radiation use efficiency using incident light and photosynthesis of individual leaves. Annals of Botany. 2003;91:869–877. doi: 10.1093/aob/mcg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati A, Metcalf SG, Lampinen BD. A simple method to estimate photosynthetic radiation use efficiency of canopies. Annals of Botany. 2004;93:567–574. doi: 10.1093/aob/mch081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands JP. Modelling canopy production. I. Optimal distribution of photosynthetic resources. Australian Journal of Plant Physiology. 1995;22:593–601. [Google Scholar]
- Schieving F, Poorter H. Carbon gain in a multispecies canopy: the role of specific leaf area and photosynthetic nitrogen-use efficiency in the tragedy of the commons. New Phytologist. 1999;143:201–211. [Google Scholar]
- Stockhoff BA. Maximization of daily canopy photosynthesis: effects of herbivory on optimal nitrogen distribution. Journal of Theoretical Biology. 1994;169:209–220. [Google Scholar]
- Terashima I, Hikosaka K. Comparative ecophysiology of leaf and canopy photosynthesis. Plant, Cell & Environment. 1995;18:1111–1128. [Google Scholar]
- Terashima I, Araya T, Miyazawa S-I, Sone K, Yano S. Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: an eco-developmental treatise. Annals of Botany. 2005;95:507–519. doi: 10.1093/aob/mci049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley JHM. Mathematical models in plant physiology. London: Academic Press; 1976. [Google Scholar]
- Wright IJ, Leishman MR, Read C, Westoby M. Gradients of light availability and leaf traits with leaf age and canopy position in 28 Australian shrubs and trees. Functional Plant Biology. 2006;33:407–419. doi: 10.1071/FP05319. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Carrasco C, Calderon O, Paton S. The El Niño southern oscillation, variable fruit production, and famine in a tropical forest. Ecology. 1999;80:1632–1647. [Google Scholar]
- Zar JH. Biostatistical analysis. NJ: Prentice Hall; 1996. [Google Scholar]
- Zotz G, Harris G, Königer M, Winter K. High rates of photosynthesis in the tropical pioneer tree, Ficus insipida Willd. Flora. 1995;190:265–272. [Google Scholar]