Abstract
Background and Aims
The clone EMB-2 of the interspecific hybrid Helianthus annuus × H. tuberosus provides an interesting system to study molecular and physiological aspects of somatic embryogenesis. Namely, in addition to non-epiphyllous (NEP) leaves that expand normally, EMB-2 produces epiphyllous (EP) leaves bearing embryos on the adaxial surface. This clone was used to investigate if the ectopic expression of H. annuus LEAFY COTYLEDON1-LIKE (Ha-L1L) gene and auxin activity are correlated with the establishment of embryogenic competence.
Methods
Ha-L1L expression was evaluated by semi-quantitative RT-PCR and in situ hybridization. The endogenous level and spatial distribution of free indole-3-acetic acid (IAA) were estimated by a capillary gas chromatography–mass spectrometry–selected ion monitoring method and an immuno-cytochemical approach.
Key Results
Ectopic expression of Ha-L1L was detected in specific cell domains of the adaxial epidermis of EP leaves prior to the development of ectopic embryos. Ha-L1L was expressed rapidly when NEP leaves were induced to regenerate somatic embryos by in vitro culture. Differences in auxin distribution pattern rather than in absolute level were observed between EP and A-2 leaves. More precisely, a strong IAA immuno-signal was detected in single cells or in small groups of cells along the epidermis of EP leaves and accompanied the early stages of embryo development. Changes in auxin level and distribution were observed in NEP leaves induced to regenerate by in vitro culture. Exogenous auxin treatments lightly influenced Ha-L1L transcript levels in spite of an enhancement of the regeneration frequency.
Conclusions
In EP leaves, Ha-L1L activity marks the putative founder cells of ectopic embryos. Although the ectopic expression of Ha-L1L seems to be not directly mediated by auxin levels per se, it was demonstrated that localized Ha-L1L expression and IAA accumulation in leaf epidermis domains represent early events of somatic embryogenesis displayed by the epiphyllous EMB-2 clone.
Key words: Helianthus annuus × H. tuberosus, auxin, epiphylly, LEAFY COTYLEDON1-LIKE gene, somatic embryogenesis
INTRODUCTION
In higher plants, embryogenesis has been largely characterized at the morphological and physiological levels (West and Harada, 1993; Jürgens, 2001; Laux et al., 2004). Also the genetic network that controls embryogenic processes is becoming better understood because of the identification of several genes that play regulatory roles either in specific embryogenesis phases (reviewed in Jenik et al., 2007) or during the whole process (reviewed in Park and Harada, 2008). For example, the class of LEAFY COTYLEDON (LEC) genes, such as LEC1, LEC1-LIKE (L1L), LEC2 and FUSCA3 (FUS3) all encoding regulatory proteins involved in embryogenesis (Laurssen et al., 1998; Lotan et al., 1998; Stone et al., 2001; Kwong et al., 2003).
Mutational analyses showed that the LEC genes function early in embryogenesis to maintain suspensor cell fate and specify cotyledon identity (Meinke, 1992; Meinke et al., 1994; West et al., 1994). Late in embryogenesis, the LEC genes are required for initiating and/or maintaining maturation phase and repressing precocious germination (Parcy et al., 1997; Lotan et al., 1998; Stone et al., 2001). There is also evidence of a mutual interaction among different LEC genes, during embryo development (Parcy et al., 1997; Kagaya et al., 2005; Santos Mendoza et al., 2005; To et al., 2006). An upstream gene, PICKLE (PKL), represses LEC gene expression outside of the embryo (Ogas et al., 1999; Dean Rider et al., 2003). In addition, members of the VIVIPAROUS1 (VP1)/ABSCISIC ACID INSENSITIVE (ABI3)-LIKE (VAL) family of B3 domain transcription factors function as global repressors of the LEC1/B3 transcription factors network in germinating seedlings (Suzuki et al., 2007).
Plant embryogenesis is not always strictly dependent on fertilization because a variety of dicot and monocot species reproduce asexually by apomixis (Koltunow and Grossniklaus, 2003). Moreover, gametophytic and somatic cells can be induced in vitro to undergo embryogenic development. Embryos can also develop ectopically from single cells of stem or leaf epidermis (Konar et al., 1972; Dickinson, 1978), a process that can occur naturally in epiphyllous species (Dickinson, 1978), as well as in somaclonal variants (Konar et al., 1972; Fambrini et al., 2000).
Although sexual, apomictic and in vitro embryos often arise from different starting tissues and are activated by different signals, it is likely that at a very early stage all three depend on the same signalling pathway (Mordhorst et al., 1997; Lotan et al., 1998; Koltunow and Grossniklaus, 2003; Tucker et al., 2003; Xu et al., 2006). This is consistent with the initiation of somatic embryos from vegetative cells mis-expressing embryo-specific genes LEC1 (Lotan et al., 1998), LEC2 (Stone et al., 2001), BABY BOOM (Boutilier et al., 2002), Ha-L1L (Fambrini et al., 2006b) and PGA37/MYB118 (Wang et al., 2008). Further support is provided by the involvement of SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE (SERK) genes in both zygotic and somatic embryo initiation (Hecht et al., 2001) as well as in the development of embryo sacs from nucellar tissue in apomictic species (Albertini et al., 2005). The conclusion from these studies is that zygotic, apomictic and in vitro embryogenesis are morphogenetic end points of closely related pathways. However, relatively little is known about the early inductive events underlying the transition of a somatic cell into an embryogenic mode of development.
It is generally believed that in vitro embryogenesis is mediated by a signalling cascade triggered by auxin and/or stressful conditions (Dodeman et al., 1997; Mordhorst et al., 1997; Ikeda et al., 2006). In particular, in Helianthus annuus the transient but non-uniform accumulation of endogenous auxin is among the first signals leading to the induction of somatic embryogenesis from immature zygotic embryos (Thomas et al., 2002). On the other hand, LEC genes directly interact with hormone-response genes (Brocard-Gifford et al., 2003; Gazzarrini et al., 2004; Kagaya et al., 2005; Braybrook et al., 2006; Stone et al., 2008). For example, the penetrance of the turnip (tnp) mutant phenotype of Arabidopsis, a gain-of-function mutant of LEC1, is strongly enhanced or antagonized in the presence of exogenous auxin and cytokinins, respectively (Casson and Lindsey, 2006).
In order to add further insights into molecular and physiological aspects of somatic embryogenesis, in the present study an epiphyllous clone (EMB-2) derived by in vitro tissue culture of the interspecific hybrid Helianthus annuus × H. tuberosus was used. This clone produces epiphyllous (EP) leaves, both in vitro and in vivo, that exhibit prominent ectopic embryo- and shoot-like structures (Fambrini et al., 2000; Chiappetta et al., 2006). EP leaves are produced alongside normal non-epiphyllous (NEP) leaves. In previous work, we demonstrated that Ha-L1L, a L1L gene of H. annuus, was highly expressed in epiphyllous embryos of the EMB-2 clone. In the present study, the extent to which auxin and Ha-L1L expression were integrated in the initial stages of EMB-2 epiphyllous embryo development was investigated. To this end, both endogenous amount and spatial distribution of indole-3-acetic acid (IAA) were determined in EP and NEP leaves of EMB-2, as well in control leaves of a non-epiphyllous interspecific hybrid (A-2). Spatial expression of Ha-L1L was also monitored during the early stages of ectopic embryo development. Finally, the relationship between Ha-L1L and endogenous IAA together with spatial distribution of each were monitored in NEP leaves of EMB-2 cultured in vitro on different media.
MATERIALS AND METHODS
Plant material and growth conditions
The somaclone EMB-2 was previously induced in vitro from leaf explants of the tetraploid (2n = 4x = 68) interspecific hybrid Helianthus annuus × H. tuberosus (A-2 clone; Fambrini et al., 2000, 2006b). To obtain clones under sterile conditions, both A-2 and EMB-2 plants were multiplied by culturing single-nodes on solidified (8 g L−1 Bactoagar; Oxoid, Basingstoke, UK) MS basal medium (Murashige and Skoog, 1962) without growth regulators in 150-mL Erlenmeyer flasks (Fambrini et al., 2000). The cultures were incubated in a growth chamber at 25 ± 1 °C in a 16-h daily photoperiod. Irradiance intensity during the light period was 30 µmol m−2 s−1, by means of cool-light fluorescent lamps (Philips TLD 36W/33; Philips, Eindhoven, The Netherlands). EMB-2 plants were also grown in a growth chamber in pots containing a mixture of vermiculite, peat and soil at 23 ± 1 °C under a 16-h daily photoperiod (Fambrini et al., 2000). Irradiation was 200 µmol m−2 s−1 provided by mercury vapour lamps (Osram HQI-TS 250 W/NDN, Wembley, UK).
Histological analysis
Leaves from in vitro and in vivo grown EMB-2 plants were collected and fixed for 24 h in FAA (formalin/glacial acetic acid/ethanol/distilled water, 10 : 5 : 50 : 35, v/v) at room temperature before being transferred into 70 % ethanol (Fambrini et al., 2006a). Water was removed by a graded ethanol series while the dehydrated material was cleared in xylene according to Ruzin (1999). Paraffin-embedded tissues were sectioned (10 µm thick) using a rotary microtome (Reichert, Vienna, Austria). The serial sections obtained were then stained in Delafield's haematoxylin (BDH Chemicals Ltd, Poole, UK).
IAA analysis
Leaves of control (A-2) and epiphyllous clone (EMB-2) were collected from different in vitro grown plantlets (n = 3). For EMB-2 plants, EP leaves were separated from NEP ones. Freeze-dried samples (300 mg f. wt) were extracted in 65 % isopropanol (v/v) with 0·02 m imidazole buffer at pH 7 to which [3H]IAA as radiotracer and [13C6]IAA (kindly provided by J. D. Cohen, Department of Horticultural Science, Saint Paul, MN, USA), as an internal standard for quantitative mass-spectral analysis, were added. After overnight isotope equilibration, free IAA was measured according to Baraldi et al. (1995). Quantitative IAA analysis was done by a capillary gas chromatography–mass spectrometry–selected ion monitoring using a Hewlett Packard 5890–5970 System equipped with a 30-m HP 1 capillary column (i.d. 0·25 mm; film thickness 0·25 µm), carrier gas He (1 mL min−1), injector at 280 °C, oven temperature increased from 50 °C to 110 °C at 30 °C min−1, then 6 °C min−1 to 280 °C, source temperature 270 °C, ionizing voltage 70 eV. Ions monitored were m/z 130 and 136 for the base peak (quinolinium ion) and 189 and 195 for the molecular ion of the methyl-IAA and methyl-[13C6]IAA, respectively (Rapparini et al., 2002). The ratios of 130 : 136 and 189 : 195 were used to calculate endogenous levels of IAA. The data are presented as means (± s.d.) of three independent experiments with three replicates.
IAA immuno-cytolabelling
Control (A-2) and epiphyllous clone (EMB-2) leaves were collected from in vitro grown plantlets (n = 3). For EMB-2 plants, EP leaves were separated from NEP ones. Excised tissue samples were immediately fixed in a 0·5 % (v/v) glutaraldehyde and 3 % (w/v) paraformaldehyde mixture in PBS (135 mm NaCl, 2·7 mm KCl, 1·5 mm KH2PO4, pH 7·2) for 3 h at 4 °C. EDAC pre-treatment was substituted with a pre-embedding procedure (Chiappetta et al., 2006) and fixed samples were cut using the vibratome (Leica VT1000E, Bensheim, Germany). The floating sections (30–35 µm) were processed for the immuno-localization as described in Moctezuma (1999) and Thomas et al. (2002), and incubated overnight at 4 °C with anti-IAA monoclonal primary antibody (Sigma-Aldrich, Milan, Italy) diluted 1 : 200 in PBS/BSA solution (10 mm phosphate solution, 0·8 % BSA). Subsequently, sections were incubated with the secondary antibody (anti-mouse IgG alkaline phosphatase conjugate (Promega Italia, Milan, Italy) diluted 1 : 100 in PBS/BSA solution for 4 h. After washing, the sections were developed with NBT (nitro blue tetrazolium) and BCIP (5-bromo-4-chloro-3-indolylphosphate) mixture for 5 min, then rinsed with stop buffer (100 mm Tris–HCl, pH 8·0; 1 mm EDTA), mounted on slides and immediately observed and photographed. To verify the effectiveness of the immuno-localization technique, sections were processed with the omission of the primary IAA antibody and with a competition assay control as described by Kerk and Feldman (1995).
In vitro hormonal treatments of NEP explants of EMB-2 plants
Explants from NEP leaves of EMB-2 were used as starting material for in vitro experiments. A-2 leaves were used as controls. EMB-2 leaves were selected using a reflecting stereoscope, before the in vitro culture, to exclude the presence of epiphyllous structures (NEP leaves). Explants were placed in Petri dishes (100 mm × 15 mm) on basal medium without growth regulators (MS) and on MS medium supplemented with different growth regulators: trans-zeatin riboside, gibberellic acid, IAA, 2,4-dichlorophenoxyacetic acid (2,4-D), abscisic acid and an inhibitor of auxin transport, 2,3,5-triiodobenzoic acid (TIBA). In a first experiment, all substances were added at 10 µm. In a second experiment different concentrations of 2,4-D were used. The media, supplemented with 30 g L−1 sucrose, were solidified with 8 g L−1 Bactoagar (Oxoid). Cultures were incubated in a growth chamber under the same conditions as described above. The regeneration frequencies (number of explants developing embryogenic structures on the total of cultured explants) were estimated from 3-week-old cultures of three independent experiments each with five to six replications (Petri dishes). Each Petri dish contained 15–18 explants. NEP leaves, at 1 or 3 d from the start of the in vitro culture on MS medium, were collected for both quantitative (n = 6) and immuno-cytolabelling (n = 5) IAA analysis as described above.
RNA isolation from in vitro-cultured explants and semi-quantitative RT-PCR analyses of Ha-L1L expression
Total RNA extractions were carried out from A-2 and NEP explants collected after 0, 1 or 3 d of culture on media described above. Total RNA was extracted from samples with the TriPure Isolation Reagent, according to the manufacturer's instructions (Roche Diagnostics GmbH, Mannheim, Germany). The total RNA (4 µg) from each sample was used, with the Superscript pre-amplification kit (Invitrogen S.R.L., Life Technologies, Milan, Italy), to produce the first-strand cDNA in conditions recommended by the manufacturer. PCRs were performed using gene-specific primers from cDNA of Ha-L1L (CHI-TCDNA, 5′-CTAGAGAGAGACAATTCC-3′ and CHI-REL, 5′-GAAGACAATGAGTGCATTG-3′) and β-actin (ACT5, 5′-GATTCCGTTGCCCA/TGAGGTC/T-3′ and ACT3, 5′-TC/TTCTGGA/TGGA/TGCAACCACC-3′). Primers were designed to yield 175-bp and 243-bp fragments for Ha-L1L and β-actin, respectively. PCRs were carried out for 30 cycles where the cDNAs were exponentially amplified. PCRs were performed in 20 µL of 10 × buffer (Euroclone Spa – Life Division Sciences, Pero, Italy) containing 0·1 mm dNTPs, 0·75 or 1·50 µm of each primer for Ha-L1L, 0·15 µM of each primer for β-actin, 1 mm MgCl2, 1·25 U EuroTaq DNA polymerase (Euroclone) and 1 µL of single-stranded cDNA. Amplifications were carried out according to the following temperature profile: 94 °C for 5 min for denaturation, then 94 °C for 30 s, 53 °C for 25 s, 72 °C for 25 s and a final extension of 7 min at 72 °C. The amplified fragments were quantified using Quantity One (BioRad, Hercules, CA, USA). Each Ha-L1L signal was normalized to the β-actin signal for calculation of relative amounts. Each value was the mean of four independent experiments.
Construction of DIG-RNA probe
A clone HaK-4, containing partly the 3′-non-coding region (157 bp), spanning across the Ha-L1L-specific primers, CHI-SF: 5′-TATGCTCAGTGTAAAGAC-3′ (located 18 nucleotides upstream the translational stop codon), and CHI-SR: 5′-GTAGATGGAGAGTGTCAG-3′, was linearized with appropriate restriction enzymes and used as template to synthesize digoxigenin-labelled RNA sense and antisense probes, according to the DIG-RNA labelling kit protocol (Roche Diagnostics GmbH, Mannheim, Germany).
In situ hybridization
Excised tissues of EMB-2 and A-2 leaves were fixed, dehydrated, embedded in paraffin, cut into 8-μm sections and hybridized (55 °C) to a digoxigenin-labelled antisense HaK-4 RNA probe according to Cañas et al. (1994). For immunological detection, the slides were incubated in buffer 1 [1 % (w/v) blocking solution, 100 mm Tris, pH 7·5, and 150 mm NaCl] for 1 h and then equilibrated with buffer 2 [100 mm Tris, pH 7·5, and 150 mm NaCl, 0·5 % (w/v) BSA, and 0·3 % (w/v) Triton X-100]. Tissue sections were then incubated with anti-digoxigenin-alkaline-phosphatase conjugate diluted 1 : 5000 in buffer 2 in a humidified box for 2 h and then washed three times for 20 min in buffer 100 mm Tris, pH 7·5, and 150 mm NaCl. The tissue sections were equilibrated in buffer 3 (100 mm Tris, pH 9·5, and 100 mm NaCl and 50 mm MgCl2) for 10 min and then incubated for 72 h in 3·2 µg mL−1 5-bromo-4-chloro-3-indolyl-phosphate : 6·6 µg mL−1 nitroblue tetrazolium salt in buffer 3 in a humidified box. Accumulation of Ha-L1L transcripts is visualized as a violet-brownish staining.
Statistical analysis
Data were treated using analysis of variance procedures, and means were compared by Tukey's test (P = 0·05). Homogeneity of variances was evaluated using Bartlett's test (P = 0·05). Statistical analyses on percentage data were performed after arc sine transformation.
RESULTS
Development of ectopic embryos in EMB-2 leaves
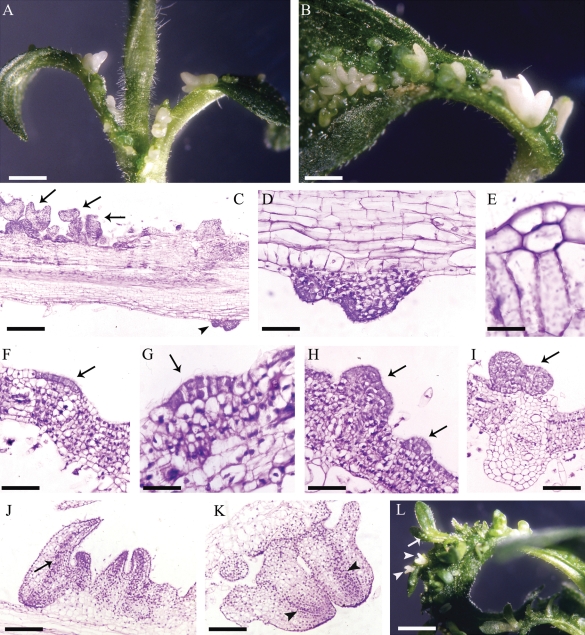
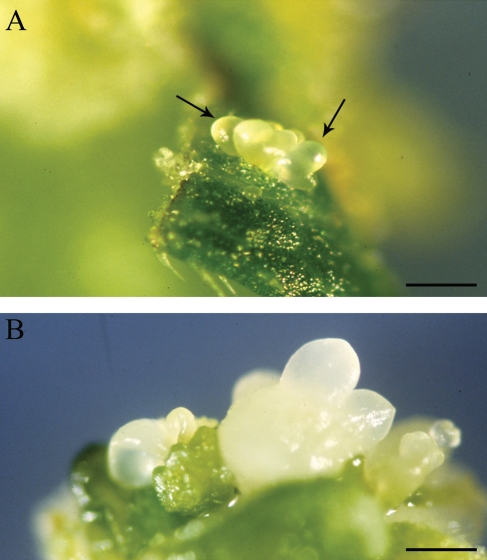
In EMB-2 plants, ectopic embryos develop profusely from the adaxial surface of EP leaves (Fambrini et al., 2000; Chiappetta et al., 2006; Fig. 1A and B). Only on the petiole, can ectopic structures occasionally originate from the abaxial leaf surface (Fig. 1C and D). The first sign of somatic embryo induction is a shift from anticlinal to periclinal divisions in epidermal cell clusters (Fig. 1E). Multicellular budding and single-cell initiation of ectopic structures apparently both occur, with multicellular budding being the more frequent pattern in the present study. This multicellular origin was resolved in some leaf cross-sections, where linear clusters of epidermal cells, with densely stained cytoplasm (Fig. 1F), initiated cell division to form small protuberances (Fig. 1G, H). These structures rapidly developed into clumps of epiphyllous embryos (Fig. 1I–L). It is hypothesized that these adaxial epidermal cells are putative founders of somatic embryos. Epiphyllous embryos formed directly opposite to the xylem pole of the veins (Fig. 1I). Phenomena of recurrent embryogenesis from primary ectopic embryos were also observed (Fig. 1L).
Fig. 1.
Development of epiphyllous structures in EMB-2 plants of the interspecific hybrid Helianthus annuus × H. tuberosus. (A) An epiphyllous EMB-2 plant grown in vitro. (B) Ectopic embryos can be seen on both adaxial surface and petiole of an epiphyllous (EP) leaf. (C) Longitudinal section of an EP leaf, proximal to the petiole, along the main vein; arrows indicate ectopic structures on the adaxial surface while the arrowhead indicates an ectopic structure on the abaxial surface. (D) Magnification of (C) showing the ectopic structure originated on the abaxial surface. (E) A periclinal division in the adaxial epidermis. (F) A linear cluster of epidermal cells with densely stained cytoplasm (arrow). (G, H) Initial stages of ectopic structures (arrows). (I) An ectopic embryo formed directly opposite a xylem pole of a vein (arrow). (J, K) Epiphyllous cotyledonary embryos showing a procambial strand (arrow) and embryo axes (arrowheads). (L) Globular (arrow) and cotyledonary embryos (arrowheads) developed from primary ectopic embryos. Scale bars: (A) = 3·5 mm; (B) = 2·5 mm; (C) = 370 µm; (D) = 90 µm; (E) = 20 ρm; (F) = 100 µm; (G) = 50 µm; (H) = 85 µm; (I) = 120 µm; (J) = 210 µm; (K) = 190 µm; (L) = 4·2 mm.
In EMB-2 plants endogenous levels of free IAA are different in epiphyllous versus non-epiphyllous leaves
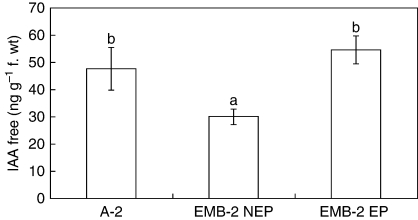
Given the clear link between auxin and plant embryogenesis (Zimmerman, 1993; Thomas et al., 2002; Friml et al., 2003; Jenik and Barton, 2005; Ikeda et al., 2006), the endogenous level of free IAA was estimated in EP and NEP leaves of EMB-2 plants compared with that of a non-epiphyllous genotype (A-2), used as control. The amount of endogenous IAA in EP leaves was 1·8-fold higher than in NEP ones (Fig. 2), in spite of a quite comparable IAA level in EP and A-2 leaves that do not show this epiphyllous condition. Hence, total amount of IAA per se is not necessarily a sensitive marker of the epiphyllous morphogenetic condition.
Fig. 2.
Level of endogenous free IAA in the leaf from in vitro-grown plants of the interspecific hybrid Helianthus annuus × H. tuberosus. Free mean (± s.d.) IAA concentrations in three independent experiments each with three replicates. Columns marked with the same letter are not significantly different at the P = 0·05 level according to Tukey's test. Abbreviations: A-2, leaves of the non-epiphyllous clone; EMB-2 EP, epiphyllous leaves of the EMB-2 clone; EMB-2 NEP, non-epiphyllous leaves of the EMB-2 clone.
A discontinuous IAA distribution marks epidermis of EP leaves
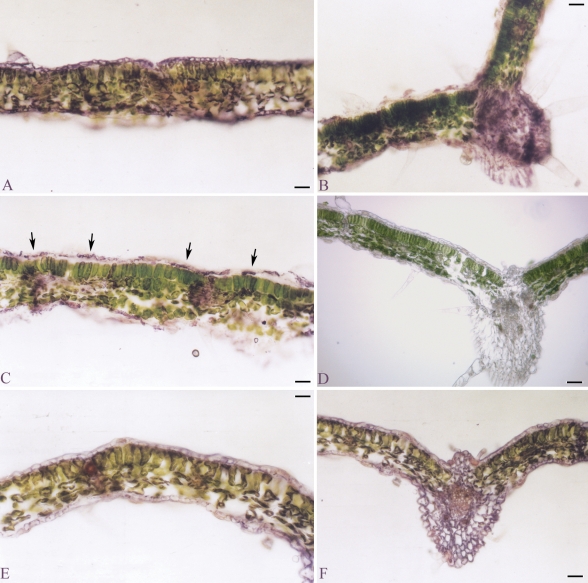
Several observations support the hypothesis that embryogenic cells originate by change of cell polarity and subsequent asymmetric division (Zimmerman, 1993; Dodeman et al., 1997). The induction and the co-ordination of cell polarity have classically been attributed to auxin and its flow (reviewed in Dhonukshe et al., 2005a; Jenik and Barton, 2005; Boutté et al., 2007). In order to verify whether in EMB-2 the development of somatic embryos could be related to a localized accumulation of this hormone, IAA spatial distribution was analysed through immuno-cytolocalization.
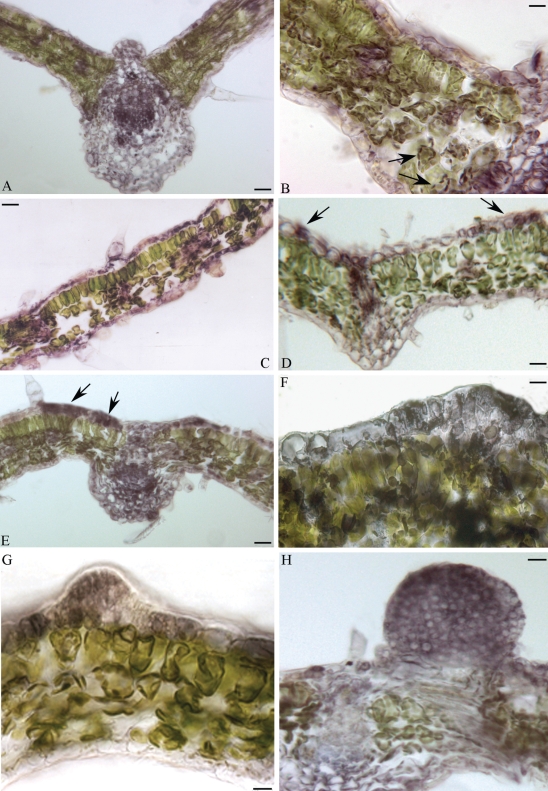
In both A-2 and NEP leaves immuno-reaction was observed in the epidermis, strongly in vascular bundles, and scattered in mesophyll tissue (Fig. 3A, C). Moreover, in mesophyll cells the immuno-reaction was mainly localized in chloroplasts (Fig. 3B), as observed in peach (Ohmiya et al., 1990) and sunflower (Thomas et al., 2002). In EP leaves strongly immuno-reactive cell clusters were frequently identified along the adaxial epidermis (Fig. 3D, E). More precisely, immuno-staining was detected in single cells or in a small group of cells (linear cluster) from which ectopic embryo formation would be predicted (compare Fig. 3D, E with Fig. 1F, G). These results suggest strongly an auxin pulse in the putative founder cells of ectopic embryos.
Fig. 3.
Immuno-localization of free IAA in leaf cross-sections of A-2 and EMB-2 plants of the interspecific hybrid Helianthus annuus × H. tuberosus. Immuno-reaction evidenced as purple-violet staining. (A) Leaf of the non-epiphyllous A-2 clone. (B) Higher magnification of (A); arrows indicate labelled chloroplasts. (C) A non-epiphyllous (NEP) leaf of the EMB-2 clone. (D–H) Epiphyllous leaves (EP) of the EMB-2 clone. Arrows in (D) and (E) indicate strongly labelled cells. (G, H) Immuno-stained embryogenic structures from the first cell divisions. Scale bars: (A) = 35 µm; (B) = 15 µm; (C) = 40 µm; (D) = 20 µm; (E) = 30 µm; (F) = 8 µm; (G) = 12 µm; (H) = 15 µm.
An intense immuno-staining marked the development of whole embryogenic structure from the initial cell divisions (Fig. 3F, G) through to advanced globular embryo-like structures (Fig. 3H). No immuno-reaction was observed in EP leaves processed without primary antibody (data not shown).
Ha-L1L is highly expressed in early developmental stages of somatic embryo-like structures
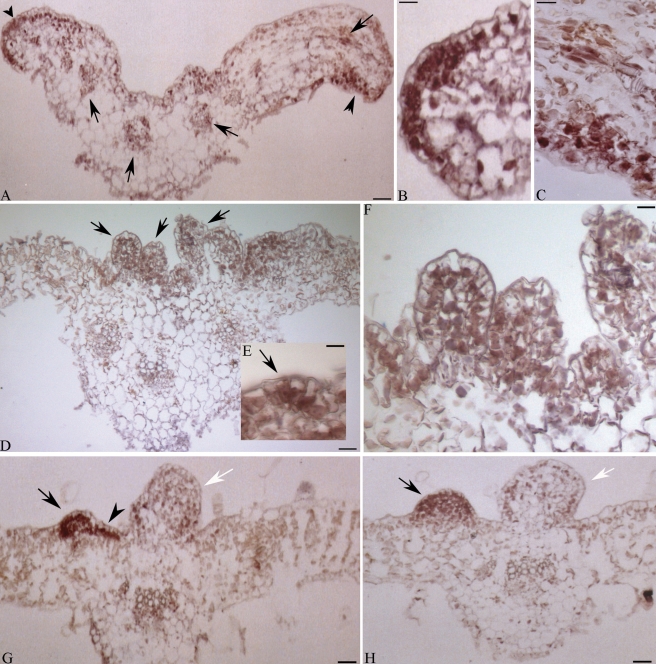
To dissect in more detail the relationship between Ha-L1L and the embryogenic competence usually exhibited by EP leaves, the spatial localization of transcripts at the very early stages of ectopic embryo development was investigated. In the part of the EP leaves proximal to the petiole junction (Fig. 4A), transcripts were detected in both the adaxial (Fig. 4B) and abaxial (Fig. 4C) epidermis as well in parenchyma cells of vascular bundles (Fig. 4A). Notably, labelled cells were grouped at the leaf margin (Fig. 4B and C) and were discontinuously distributed along the blade (Fig. 4A). In more distal sections, exhibiting ectopic structures at different developmental stages (Fig. 4D, G, H), a strong accumulation of Ha-L1L mRNA occurred in linear cluster of cells (Fig. 4G), incipient somatic embryos (Fig. 4E), as well as developing globular embryo-like structures (Fig. 4F, G and H). Notably, in structures clearly arrested in development and showing enlarged vacuolated cells, there was a clear decline of the hybridization signal (Fig. 4G, H). Signal was not detected in leaves processed with the sense probe (data not shown). Moreover, in both A-2 and NEP leaves, in situ hybridization did not reveal any Ha-L1L transcript accumulation (Fig. S1 in Supplementary Data, available online).
Fig. 4.
Localization of Ha-L1L transcripts in cross-sections of epiphyllous leaves (EP) of the interspecific hybrid Helianthus annuus × H. tuberosus. The presence of Ha-L1L mRNAs is evidenced as a violet-brownish staining. (A) Leaf blade proximal to the petiole junction; arrowheads indicate labelled cells grouped along the leaf margin and discontinuously distributed along the blade; arrows indicate labelled vascular bundles. (B, C) Higher magnification of (A). (D) Distal leaf; arrows indicate ectopic embryos. (E, F) Higher magnification of (D); arrow in (E) indicates incipient ectopic structure. (G, H) Distal leaf blades; arrowhead indicates a linear cluster of labeled cells; arrows indicate advanced globular embryos; white arrows indicate ectopic structures arrested in development. Scale bars: (A) = 35 µm; (B, C) = 11 µm; (D) = 45 µm; (E) = 10 µm; (F) = 16 µm; (G, H) = 35 µm.
Ha-L1L activation and changes in IAA level and distribution occurred in NEP leaves cultured in vitro
A clear regeneration competence characterized all EMB-2 leaves since explants of NEP leaves cultured on media without growth regulators were able to differentiate somatic embryos and shoot-like structures (Chiappetta et al., 2006). This prompted us to verify whether embryogenic competence in explanted NEP leaves could be related to either an activation of Ha-L1L transcription or changes in IAA level or distribution. To this end, both NEP and A-2 leaves were cultured on MS basal medium without growth regulators. After 21 d, a high percentage of NEP explants developed embryo-like structures on the adaxial leaf surface (Table 1 and Fig. 5A, B), whereas A-2 explants did not.
Table 1.
Regeneration from non-epiphyllous (NEP) leaves of the EMB-2 clone of Helianthus annuus × H. tuberosus cultured for 3 weeks on different media
| Treatment | Regeneration (%) |
|---|---|
| MS | 54·05 ± 3·98a |
| MS + IAA | 67·96 ± 3·10b |
| MS + TIBA | 70·94 ± 1·23b |
The growth regulators were added at 10 µm.
The data are means (± s.e.) from three independent experiments each with five or six replications (Petri dishes).
Values followed by a common superscript letter are not different at the 0·05 level of significance, according to Tukey's test.
Fig. 5.
Regeneration of somatic embryos from in vitro-cultured NEP leaves of the EMB-2 clone of the interspecific hybrid Helianthus annuus × H. tuberosus. Somatic embryos on the adaxial surface of NEP explants cultured for 9 d (A) and/or 14 d (B) on MS (Murashige and Skoog, 1962) basal medium. Arrows in (A) indicates globular somatic embryos. Scale bars: (A) = 1·7 mm; (B) = 1·2 mm.
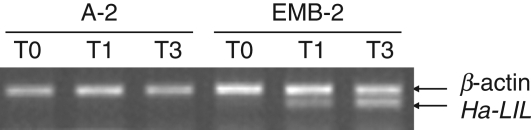
After 1 d of in vitro culture (T1), Ha-L1L transcripts accumulated in NEP leaves (Fig. 6), suggesting that the up-regulation of the gene precedes somatic embryo initiation. By contrast, in cultured A-2 leaves Ha-L1L mRNA was not detected.
Fig. 6.
RT-PCR analysis of Ha-L1L mRNA accumulation. The amplification of Ha-L1L and β-actin transcripts was performed from total RNA isolated from leaves of A-2 and non-epiphyllous (NEP) leaves of the EMB-2 clone cultured in vitro for 1 or 3 d on MS basal medium without growth regulators. The RT-PCR products were resolved on TAE 2·0 % agarose gel. Abbreviations: T0, untreated leaves; T1, leaves cultured for 1 d on MS medium; T3, leaves cultured for 3 d on MS medium.
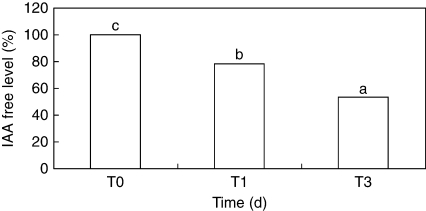
A significant decline of free IAA content also occurred in NEP leaves during the first 3 d of culture (Fig. 7), which resulted in a progressive reduction of IAA immuno-reaction in vascular bundles, mesophyll tissue and epidermis (Fig. 8A–F). A more discontinuous pattern of cell labelling was frequently detected along the adaxial epidermis in leaves cultured for 24 h (T1) compared with the uniform distribution observed in leaves detached at time 0 (Fig. 8A, C). A very faint and homogenous IAA signal was also detected in the adaxial epidermis of leaves cultured for 72 h (Fig. 8E). An immuno-reaction was not observed in EP leaves processed without primary antibody (Fig. 8D).
Fig. 7.
Level of endogenous IAA free in non-epiphyllous (NEP) leaves of the interspecific hybrid Helianthus annuus × H. tuberosus cultured in vitro for 1 or 3 d on MS basal medium without growth regulators. Data were registered as percentage of IAA free with respect to control (T0, untreated leaves). Leaves cultured for 1 d (T1) or 3 d (T3) on MS medium. Data are means of six replicates. Columns marked with different letters are significantly different (P = 0·05).
Fig. 8.
Immuno-localization of free IAA in leaf cross-sections of non-epiphyllous (NEP) leaves of the interspecific hybrid Helianthus annuus × H. tuberosus cultured in vitro for 1 or 3 d on MS basal medium without growth regulators. Immuno-reaction is evidenced as purple-violet staining. (A) Untreated leaf (T0). (B) Untreated leaf (T0) showing the central vascular bundle. (C) Leaf cultured for 1 day on MS basal medium (T1). (D) Leaf processed without primary antibody (control). (E) Leaf cultured for 3 d on MS basal medium (T3). (F) Leaf cultured for 3 d on MS basal medium (T3) showing the central vascular bundle. Arrows in (C) indicate strongly labelled cells. Scale bars: (A, C, E) = 45 µm; (B, D, F) = 70 µm.
Somatic embryogenesis and Ha-L1L transcription are slightly influenced by auxin treatments
The transcription of the Ha-L1L gene was monitored in explants of NEP leaves cultured in the presence of IAA and TIBA. After 3 weeks culture, a slight increase in the frequency of somatic embryo regeneration was detected on both IAA- and TIBA-supplemented media compared with MS basal medium (Table 1).
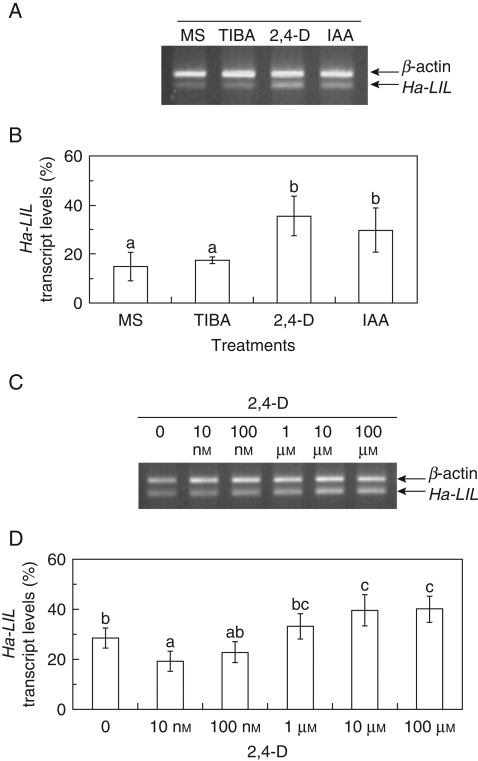
Semi-quantitative RT-PCR analysis of Ha-L1L transcription was carried out in NEP leaves cultured in vitro for 3 d on the different media (Fig. 9). The transcript levels did not change in response to TIBA supply, whereas a slight increase of Ha-L1L mRNAs was detected in auxin-treated NEP leaves (IAA and 2,4-D; Fig. 9A, B). In addition, a low different dose exposure to 2,4-D revealed that Ha-L1L transcript levels decreased or remained unchanged in comparison to those of leaves grown on basal medium (Fig. 9C, D). Only a slight increase of Ha-L1L mRNA steady state levels was detected at high auxin concentrations (Fig. 9C, D). Other hormones, i.e. t-zeatin, abscisic acid and gibberellic acid, did not affect the Ha-L1L mRNA steady-state levels (Fig. S2 in Supplementary Data, available online).
Fig. 9.
RT-PCR analysis of Ha-L1L mRNA accumulation. (A, C) The amplification of Ha-L1L and β-actin transcripts was performed from total RNA isolated from non-epiphyllous (NEP) leaves of the EMB-2 clone cultured in vitro for 3 d on different media. The RT-PCR products were resolved on a TAE 2·0 % agarose gel. MS, Murashige and Skoog (1962) basal medium; TIBA, 2,3,5-triiodobenzoic acid; IAA, indol-3-acetic acid; 2,4-D, 2,4-dichlorophenoxyacetic acid. (B, D) The bar graphs show the relative levels of Ha-L1L transcripts, normalized with respect to the β-actin product, estimated by quantitative densitometry of the PCR products resolved by gel electrophoresis in (A) and (C), respectively. The columns in (B) and (D) marked by the same letter are not significantly different at the P < 0·05 level according to Tukey's test. Values are shown ± s.d. (n = 4).
DISCUSSION
In the present work it is shown that a localized Ha-L1L mis-expression occurred in epidermal cell clusters before ectopic embryos became evident in EP leaves of EMB-2 plants. These ectopic structures initiate through periclinal divisions of adaxial epidermis cells. Moreover, after only 1 d of in vitro culture Ha-L1L transcripts accumulated in excised NEP leaves which later (i.e. after 14 d culture) will develop ectopic embryos, thus confirming that the up-regulation of the gene precedes somatic embryo initiation. Note that in EP leaves of the EMB-2 clone, Ha-L1L transcripts highly accumulated in ectopic embryos at heart and early cotyledon stages (Fambrini et al., 2006b). Analogously, in maize and carrot, LEC1 mRNA was detected in embryogenic cell clusters as well as in the first stage of somatic embryo development (Zhang et al., 2002; Yazawa et al., 2004). Direct somatic embryogenesis was strongly impaired in Arabidopsis lec mutants (Gaj et al., 2005). At-LIL expression was detected in Arabidopsis zygote (Kwong et al., 2003) and Ha-L1L was strongly expressed in early stages of Helianthus zygotic embryogenesis (Fambrini et al., 2006b). More recently, L1L expression has been detected in both zygotic and somatic embryogenesis of Theobroma cacao (Alemanno et al., 2008) and in somatic embryogenesis of Vitis vinifera (Schellenbaum et al., 2008). In particular, a strong expression of VvL1L occurred during the early induction phase of V. vinifera somatic embryos while in developing embryos the accumulation of VvL1L transcripts decreased markedly (Schellenbaum et al., 2008). Therefore, it is hypothesized that the LEC1-like homologue in Helianthus, Ha-L1L, might be a component of the molecular pathway that marks putative founder cells for ectopic embryogenesis.
Among the promoting factors of somatic embryogenesis, auxin has been long regarded as a key factor (Mordhorst et al., 1997; Ikeda et al., 2006). Here it is shown that ectopic embryogenesis of EMB-2 clone was related to auxin distribution pattern rather than the hormone's absolute level. Note that, auxin level was quite comparable in A-2 and EP leaves, in spite of non-epiphyllous behaviour of the A-2 clone. By contrast, a strong IAA accumulation in linear cell clusters occurred along the adaxial epidermis of EP leaves as compared with the homogenous reaction in the epidermis of both A-2 and NEP leaves. A strong and heterogeneous accumulation of auxin was also detected in the protodermis of explants from immature zygotic embryos of Arabidopsis thaliana transgenic lines undergoing direct somatic embryogenesis (Kurcyńska et al., 2007). Interestingly, the pattern of hormone distribution correlated with the histological observations on an embryogenic-like pattern of cell divisions (Kurcyńska et al., 2007). Hence, it seems that in the EMB-2 clone, the strongly IAA-reactive cells observed along adaxial epidermis of EP and NEP leaves cultured in vitro, also correspond to the putative founder cells of ectopic embryos.
Asymmetric distribution of auxin across plant tissues, as well as the auxin accumulation in small cell clusters, are features that accompany and mediate developmental events (reviewed in Woodward and Bartel, 2005; Tanaka et al., 2006). Periclinal division of primordia founder cells is strongly indicative of the initiation of primordia (Benková et al., 2003) and polar auxin transport is strongly linked to changes in division plane (Dhonukshe et al., 2005a, b). In Helianthus, a transient localized auxin pulse is among the first signals leading to cell divisions that initiate somatic embryogenesis (Thomas et al., 2002). Recently, live imaging of in vitro regeneration systems with the fusion construct pPIN1::PIN1-GFP have further supported the key role of asymmetric distribution of auxin in de novo morphogenesis of both adventitious meristems (Gordon et al., 2007) and somatic embryos (Zhao et al., 2008). However, IAA accumulation in EP leaves was not restricted to founder cells of ectopic embryos (i.e. adaxial epidermal cells). The palisade and lacunose tissues also showed an IAA immuno-reaction, although embryo-like structures did not occur from these domains (Fambrini et al., 2000). Analogously, in direct somatic embryogenesis from immature zygotic embryo of Arabidopsis thaliana, not all cells showing auxin signal were embryogenic (Kurcyńska et al., 2007). Therefore, perhaps an auxin transient pulse is conditional for events leading to the development of somatic embryos but other unknown signals are required to trigger this event in some but not all cells.
An open question of behaviour of the EMB-2 clone is whether the localized auxin accumulation in EP leaves is due to local biosynthesis or re-distribution through polar transport. Although the present data are not sufficient to discriminate between these hypotheses, the recent evidence showing that polar auxin transport and YUCCA-mediated auxin biosynthesis have strong genetic interactions can be recalled (Cheng et al., 2007). It is likely that both events (i.e. auxin transport and biosynthesis) could be involved to exceed the hormonal threshold required for organ initiation (Zhao, 2008). In this context, the effect on somatic embryogenesis observed in NEP leaves cultured on medium enriched with TIBA, an auxin-transport inhibitor (Geldner et al., 2001; Dhonukshe et al., 2008), deserves a specific comment. It is known that TIBA treatments promote or negatively affect adventitious regeneration in vitro in relation to the species and/or the concentration used (Laublin et al., 1991; Nissen and Minocha, 1993). Taking into account that NEP leaves showed a significant reduction of IAA content in the course of in vitro culture on MS basal medium, the positive effect of TIBA-enriched medium in promoting somatic embryogenesis could be related to a local auxin accumulation, as demonstrated in tobacco cells after TIBA treatment (Petrášek et al., 2002). It can be predicted that both IAA and TIBA media were more suitable than MS basal medium to influence the auxin gradient in NEP leaves, enhancing the hormone accumulation in discrete regions of leaf explants through a differential uptake or transport inhibition, respectively. Moreover, TIBA could act altering the endogenous content of a different growth regulator with morphogenetic effects, as observed in the leaf of Echinacea purpurea (Jones et al., 2007).
Finally, a basic issue is the nature of the interaction between auxin activity and the genetic control of embryogenic competence. To date, the most evident result is that the auxin biosynthetic gene YUCCA4 is a direct target of LEC2 (Stone et al., 2008). However, although the existing data are not plentiful neither univocal (Zhang et al., 2002; Alemanno et al., 2008; Schellenbaum et al., 2008; Sharma et al., 2008), the hypothesis that in the induction of embryogenesis, LEC1/L1L genes function downstream from auxin remains possible (Gaj et al., 2005). Only a slight increase of Ha-L1L mRNA steady-state levels was detected in NEP leaves exposed to auxin treatments as compared with leaves grown on basal medium. However, post-transcriptional or -translational effects cannot be excluded and alternatively auxin could act by altering the expression of other key embryonic regulators to modify in vitro response as observed in the turnip mutant of Arabidopsis (Casson and Lindsey, 2006).
In summary, it was clearly demonstrated that localized Ha-L1L expression and IAA accumulation in leaf epidermis domain represent early events of the embryogenesis displayed by the epiphyllous EMB-2 clone. Further studies are required to fully understand the mechanisms underlying the relationship between these two key factors as well as the temporal correlation between ectopic Ha-L1L expression and IAA pulse in epidermal cells.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org/ and consist of the following two figures. Fig S1: Ha-L1L in situ hybridization in A-2 and EMB-2 NEP of the interspecific hybrid, and cross-sections of NEP and A-2 leaves incubated with the sense and antisense probes. Fig. S2: RT-PCR analysis of Ha-L1L mRNA accumulation.
ACKNOWLEDGEMENTS
We thank Lucia Giorgetti for assistance with the in situ hybridization and Giuliano Cionini for technical assistance with the histological analyses. This work was supported by a grant from Ministero Italiano dell'Università e Ricerca (PRIN 2006-2008).
LITERATURE CITED
- Albertini E, Marconi G, Reale L, et al. SERK and APOSTART: candidate genes for apomixis in Poa pratensis. Plant Physiology. 2005;138:2185–2199. doi: 10.1104/pp.105.062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemanno L, Devic M, Niemenak N, et al. Characterization of leafy cotyledon1-like during embryogenesis in Theobroma cacao L. Planta. 2008;227:853–866. doi: 10.1007/s00425-007-0662-4. [DOI] [PubMed] [Google Scholar]
- Baraldi R, Bertazza G, Bregoli AM, et al. Auxins and polyamines in relation to differential in vitro root induction on microcuttings of two pear cultivars. Journal of Plant Growth Regulation. 1995;14:49–59. [Google Scholar]
- Benková E, Michniewicz M, Sauer M, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. The Plant Cell. 2002;14:1737–1749. doi: 10.1105/tpc.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutté Y, Ikeda Y, Grebe M. Mechanisms of auxin-dependent cell and tissue polarity. Current Opinion in Plant Biology. 2007;10:616–623. doi: 10.1016/j.pbi.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Braybrook SA, Stone SL, Park S, et al. Genes directly regulated by LEAFY COTYLEDON2 provide insight into the control of embryo maturation and somatic embryogenesis. Proceedings of the National Academy of Sciences of the USA. 2006;103:3468–3473. doi: 10.1073/pnas.0511331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard-Gifford IM, Linch TJ, Finkelstein RR. Regulatory networks in seeds integrating developmental, abscisic acid, sugar and light signaling. Plant Physiology. 2003;131:78–92. doi: 10.1104/pp.011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañas LA, Busscher M, Angenent GC, Beltrán J-P, van Tunen AJ. Nuclear localization of petunia MADS box protein FBP1. The Plant Journal. 1994;6:597–604. [Google Scholar]
- Casson SA, Lindsey K. The turnip mutant of Arabidopsis reveals that LEAFY COTYLEDON1 expression mediates the effect of auxin and sugars to promote embryonic cell identity. Plant Physiology. 2006;142:526–541. doi: 10.1104/pp.106.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappetta A, Michelotti V, Fambrini M, et al. Zeatin accumulation and misexpression of a class I knox gene are intimately linked in the epiphyllous response of the interspecific hybrid EMB-2 (Helianthus annuus × H. tuberosus) Planta. 2006;223:917–931. doi: 10.1007/s00425-005-0150-7. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. The Plant Cell. 2007;19:2430–2439. doi: 10.1105/tpc.107.053009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean Rider S, Jr, Henderson JT, Jerome RE, Edenberg HJ, Romero-Severson J, Ogas J. Coordinate repression of regulators of embryonic identity by PICKLE during germination in Arabidopsis. The Plant Journal. 2003;35:33–43. doi: 10.1046/j.1365-313x.2003.01783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson TA. Epiphylly in angiosperms. The Botanical Review. 1978;44:181–232. [Google Scholar]
- Dhonukshe P, Klein-Vehn J, Friml J. Cell polarity, auxin transport, and cytoskeleton-mediated division planes: who come first? Protoplasma. 2005;226:67–73. doi: 10.1007/s00709-005-0104-8. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Mathur J, Hulskamp M, Gadella TW., Jr Microtubule plus-ends reveal essential links between intracellular polarization and localized modulation of endocytosis during division-plane establishment in plant cells. BMC Biology. 2005;3:11. doi: 10.1186/1741-7007-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Grigoriev I, Fischer R, et al. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proceedings of the National Academy of Sciences of the USA. 2008;105:4489–4494. doi: 10.1073/pnas.0711414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodeman VL, Ducreux G, Kreis M. Zygotic embryogenesis versus somatic embryogenesis. Journal of Experimental Botany. 1997;48:1493–1509. [Google Scholar]
- Fambrini M, Cionini G, Bianchi R, Pugliesi C. Epiphylly in a variant of Helianthus annuus × H. tuberosus induced by in vitro tissue culture. International Journal of Plant Science. 2000;161:13–22. doi: 10.1086/314235. [DOI] [PubMed] [Google Scholar]
- Fambrini M, Bonsignori E, Rapparini F, et al. stem fasciated, a recessive mutation in sunflower (Helianthus annuus L.), alters plant morphology and auxin level. Annals of Botany. 2006;98:715–730. doi: 10.1093/aob/mcl153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrini M, Durante C, Cionini G, et al. Characterization of LEAFY COTYLEDON1-LIKE gene in Helianthus annuus and its relationship with zygotic and somatic embryogenesis. Development Genes and Evolution. 2006;216:253–264. doi: 10.1007/s00427-005-0050-7. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, et al. Efflux-dependent auxin gradients establish apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Gaj MD, Zhang S, Harada JJ, Lemaux PG. Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta. 2005;222:977–988. doi: 10.1007/s00425-005-0041-y. [DOI] [PubMed] [Google Scholar]
- Gazzarrini S, Tsuchiya Y, Lumba S, Okamoto M, McCourt P. The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Developmental Cell. 2004;4:373–385. doi: 10.1016/j.devcel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof Y-D, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vescicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- Gordon SP, Heisler M, Reddy GV, Ohno C, Das P, Meyerowitz EM. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development. 2007;134:3539–3548. doi: 10.1242/dev.010298. [DOI] [PubMed] [Google Scholar]
- Hecht V, Vielle-Calzada JP, Hartog MV, et al. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiology. 2001;127:803–816. [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Umehara M, Kamada H. Embryogenesis-related genes; its expression and roles during somatic and zygotic embryogenesis in carrot and Arabidopsis. Plant Biotechnology. 2006;23:153–161. [Google Scholar]
- Jenik PD, Barton MK. Surge and destroy: the role of auxin in plant embryogenesis. Development. 2005;132:3577–3585. doi: 10.1242/dev.01952. [DOI] [PubMed] [Google Scholar]
- Jenik PD, Gilmor CS, Lukowitz W. Embryonic patterning in Arabidopsis thaliana. Annual Review of Cell and Developmental Biology. 2007;23:207–236. doi: 10.1146/annurev.cellbio.22.011105.102609. [DOI] [PubMed] [Google Scholar]
- Jones MPA, Cao J, O'Brien R, Murch SJ, Saxena PK. The mode of action of thidiazuron: auxins, indoleamines, and ion channels in the regeneration of Echinacea purpurea L. Plant Cell Reports. 2007;26:1481–1490. doi: 10.1007/s00299-007-0357-0. [DOI] [PubMed] [Google Scholar]
- Jürgens G. Apical-basal pattern formation in Arabidopsis embryogenesis. EMBO Journal. 2001;20:3609–3616. doi: 10.1093/emboj/20.14.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya Y, Toyoshima R, Okuda R, Usui H, Yamamoto A, Hattori T. LEAFY COTYLEDON1 controls seed storage protein genes through its regulation of FUSCA3 and ABSCISIC ACID INSENSITIVE3. Plant & Cell Physiology. 2005;46:399–406. doi: 10.1093/pcp/pci048. [DOI] [PubMed] [Google Scholar]
- Kerk NM, Feldman LJ. A biochemical model for the initiation and maintenance of the quiescent center: implications for organization of root meristems. Development. 1995;121:2825–2833. [Google Scholar]
- Koltunow AM, Grossniklaus U. Apomixis: a developmental perspective. Annual Review of Plant Biology and Plant Molecular Biology. 2003;54:547–574. doi: 10.1146/annurev.arplant.54.110901.160842. [DOI] [PubMed] [Google Scholar]
- Konar RN, Thomas E, Street HE. Origin and structure of embryoids arising from epidermal cells of the stem of Ranunculus sceleratus L. Journal of Cell Science. 1972;11:77–93. doi: 10.1242/jcs.11.1.77. [DOI] [PubMed] [Google Scholar]
- Kurczyńska EU, Gaj MD, Ujczak A, Mazur E. Histological analysis of direct somatic embryogenesis in Arabidopsis thaliana (L.) Heynh. Planta. 2007;226:619–628. doi: 10.1007/s00425-007-0510-6. [DOI] [PubMed] [Google Scholar]
- Kwong RM, Bui AQ, Lee H, et al. LEAFY COTYLEDON1-LIKE defines a class of regulators essential for embryo development. The Plant Cell. 2003;15:5–18. doi: 10.1105/tpc.006973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laublin G, Saini HS, Cappadocia M. In vitro plant regeneration via somatic embryogenesis from root culture of some rhizomatous irises. Plant Cell, Tissue and Organ Culture. 1991;27:15–22. [Google Scholar]
- Laurssen H, Kirik V, Herrmann P, Miséra S. FUSCA3 encodes a protein with a conserved VP1/ABI3-like B3 domain which is of functional importance for the regulation of seed maturation in Arabidopsis thaliana. The Plant Journal. 1998;15:755–764. doi: 10.1046/j.1365-313x.1998.00259.x. [DOI] [PubMed] [Google Scholar]
- Laux T, Würschum T, Breuninger H. Genetic regulation of embryonic pattern formation. The Plant Cell. 2004;16:S190–S202. doi: 10.1105/tpc.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan T, Ohto M-a, Yee KM, et al. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell. 1998;93:1195–1205. doi: 10.1016/s0092-8674(00)81463-4. [DOI] [PubMed] [Google Scholar]
- Meinke DW. A homoeotic mutant of Arabidopsis thaliana with leafy cotyledons. Science. 1992;258:1647–1650. doi: 10.1126/science.258.5088.1647. [DOI] [PubMed] [Google Scholar]
- Meinke DW, Franzmann LH, Nickle TC, Yeung EC. Leafy cotyledon mutants of Arabidopsis. The Plant Cell. 1994;6:1049–1064. doi: 10.1105/tpc.6.8.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moctezuma E. Changes in auxin patterns in developing gynophores of the peanut plant (Arachis hypogaea L.) Annals of Botany. 1999;83:235–242. doi: 10.1006/anbo.1998.0814. [DOI] [PubMed] [Google Scholar]
- Mordhorst AP, Toonen MAJ, de Vries SC. Plant embryogenesis. Critical Reviews in Plant Sciences. 1997;16:535–576. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Nissen P, Minocha SC. Inhibition by 2,4-D of somatic embryogenesis in carrot as explored by its reversal difluoromethylornithine. Physiologia Plantarum. 1993;89:673–680. [Google Scholar]
- Ogas J, Kaufmann S, Henderson J, Somerville C. PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proceedings of the National Academy of Sciences of the USA. 1999;96:13839–13844. doi: 10.1073/pnas.96.24.13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmiya A, Hayashi T, Kakiuchi N. Immuno-gold localization of indole-3-acetic acid in peach seedlings. Plant & Cell Physiology. 1990;31:711–715. [Google Scholar]
- Parcy F, Valon C, Kohara A, Miséra S, Giraudat J. The ABSCISIC ACID-INSENSITIVE3, FUSCA3, and LEAFY COTYLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. The Plant Cell. 1997;9:1265–1277. doi: 10.1105/tpc.9.8.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Harada JJ. Arabidopsis embryogenesis. Methods in Molecular Biology. 2008;427:3–16. doi: 10.1007/978-1-59745-273-1_1. [DOI] [PubMed] [Google Scholar]
- Petrášek J, Elckner M, Morris DA, Zažímalová E. Auxin efflux carrier activity and auxin accumulation regulate cell division and polarity in tobacco cells. Planta. 2002;216:302–308. doi: 10.1007/s00425-002-0845-y. [DOI] [PubMed] [Google Scholar]
- Rapparini F, Tam YY, Cohen JD, Slovin JP. Indole-3-acetic acid metabolism in Lemna gibba undergoes dynamic changes in response to growth temperature. Plant Physiology. 2002;128:1410–1416. doi: 10.1104/pp.011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzin SE. Plant microtechnique and microscopy. New York, NY: Oxford University Press; 1999. [Google Scholar]
- Santos Mendoza M, Dubreucq B, Miquel M, Caboche M, Lepiniec L. LEAFY COTYLEDON 2 activation is sufficient to trigger the accumulation of oil and seed specific mRNAs in Arabidopsis leaves. FEBS Letters. 2005;579:4666–4670. doi: 10.1016/j.febslet.2005.07.037. [DOI] [PubMed] [Google Scholar]
- Schellenbaum P, Jacques A, Maillot P, et al. Characterization of VvSERK1, VvSERK2, VvSERK3 and VvL1L genes and their expression during somatic embryogenesis of grapevine (Vitis vinifera L.) Plant Cell Reports. 2008;27:1799–1809. doi: 10.1007/s00299-008-0588-8. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Millam S, Hedley PE, McNicol , Bryan GJ. Molecular regulation of somatic embryogenesis in potato: an auxin led perspective. Plant Molecular Biology. 2008;68:185–201. doi: 10.1007/s11103-008-9360-2. [DOI] [PubMed] [Google Scholar]
- Stone SL, Kwong LW, Yee KM, et al. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proceedings of the National Academy of Sciences of the USA. 2001;98:11806–11811. doi: 10.1073/pnas.201413498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Braybrook SA, Paula SL, et al. Arabidopsis LEAFY COTYLEDON2 induces maturation traits and auxin activity: implications for somatic embryogenesis. Proceedings of the National Academy of Sciences of the USA. 2008;105:3151–3156. doi: 10.1073/pnas.0712364105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Wang HH-Y, McCarty DR. Repression of the LEAFY COTYLEDON 1/B3 regulatory network in plant embryo development by VP1/ABSCISIC ACID INSENSITIVE 3-LIKE B3 genes. Plant Physiology. 2007;143:902–911. doi: 10.1104/pp.106.092320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Dhonukshe P, Brewer PB, Friml J. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cellular and Molecular Life Sciences. 2006;63:2738–2754. doi: 10.1007/s00018-006-6116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Bronner R, Molinier J, Prinsen E, van Onckelen H, Hanhe G. Immuno-cytochemical localization of indole-3-acetic acid during induction of somatic embryogenesis in cultured sunflower embryos. Planta. 2002;215:577–583. doi: 10.1007/s00425-002-0791-8. [DOI] [PubMed] [Google Scholar]
- To A, Valon C, Savino G, et al. A network of local and redundant gene regulation governs Arabidopsis seed maturation. The Plant Cell. 2006;18:1642–1651. doi: 10.1105/tpc.105.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MR, Araujo A-CG, Paech NA, et al. Sexual and apomictic reproduction in Hieracium subgenus Pilosella are closely interrelated developmental pathways. The Plant Cell. 2003;15:1524–1537. doi: 10.1105/tpc.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Niu Q-W, Teng C, Li C, Mu J, Chua N-M, Zuo J. Overexpression of PGA37/MYB118 and MYB115 promotes vegetative-to-embryonic transition in Arabidopsis. Cell Research. 2008 doi: 10.1038/cr.2008.276. doi: 10.1038/cr.2008.276. [DOI] [PubMed] [Google Scholar]
- West M, Yee KM, Danao J, et al. LEAFY COTYLEDON1 is an essential regulator of late embryogenesis and cotyledon identity in Arabidopsis. The Plant Cell. 1994;6:1731–1745. doi: 10.1105/tpc.6.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MA, Harada JJ. Embryogenesis in higher plants: an overview. The Plant Cell. 1993;5:1361–1369. doi: 10.1105/tpc.5.10.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Annals of Botany. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Hofhuis H, Heidstra R, Sauer M, Friml J, Scheres B. A molecular framework for plant regeneration. Science. 2006;311:385–388. doi: 10.1126/science.1121790. [DOI] [PubMed] [Google Scholar]
- Yazawa K, Takahata K, Kamada H. Isolation of the gene encoding carrot leafy cotyledon1 and expression analysis during somatic and zygotic embryogenesis. Plant Physiology and Biochemistry. 2004;42:215–223. doi: 10.1016/j.plaphy.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wong L, Meng L, Lemaux PG. Similarity of expression patterns of knotted1 and ZmLEC1 during somatic and zygotic embryogenesis in maize (Zea mays L.) Planta. 2002;215:191–194. doi: 10.1007/s00425-002-0735-3. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Su YH, Cheng ZC, Zhang XS. Cell fate switch during in vitro plant organogenesis. Journal of Integrative Plant Biology. 2008;50:816–824. doi: 10.1111/j.1744-7909.2008.00701.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y. The role of local biosynthesis of auxin and cytokinin in plant development. Current Opinion in Plant Biology. 2008;11:16–22. doi: 10.1016/j.pbi.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Zimmerman JL. Somatic embryogenesis: a model for early development in higher plants. The Plant Cell. 1993;5:1411–1423. doi: 10.1105/tpc.5.10.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]