Abstract
Background and Aims
Lateral root initiation is an essential and continuous process in the formation of root systems; therefore, its quantitative analysis is indispensable. In this study a new measure of lateral root initiation is proposed and analysed, namely the lateral root initiation index (ILRI), which defines how many lateral roots and/or primordia are formed along a parent-root portion corresponding to 100 cortical cells in a file.
Methods
For data collection, a commonly used root clearing procedure was employed, and a new simple root clearing procedure is also proposed. The ILRI was determined as 100dl, where d is the density of lateral root initiation events (number mm−1) and l is the average fully elongated cortical cell length (mm).
Key Results
Analyses of different Arabidopsis thaliana genotypes and of a crop plant, tomato (Solanum lycopersicum), showed that ILRI is a more precise parameter than others commonly used as it normalizes root growth for variations in cell length. Lateral root primordium density varied in the A. thaliana accessions Col, Ler, Ws, and C24; however, in all accessions except Ws, ILRI was similar under the same growth conditions. The nitrogen/carbon ratio in the growth medium did not change the lateral root primordium density but did affect ILRI. The ILRI was also modified in a number of auxin-related mutants, revealing new root branching phenotypes in some of these mutants. The rate of lateral root initiation increased with Arabidopsis seedling age; however, ILRI was not changed in plants between 8 and 14 d post-germination.
Conclusions
The ILRI allows for a more precise comparison of lateral root initiation under different growth conditions, treatments, genotypes and plant species than other comparable methods.
Key words: Arabidopsis thaliana, auxin, lateral root density, lateral root initiation index, mutant phenotype, pericycle, root architecture, root branching, root primordium, Solanum lycopersicum
INTRODUCTION
Lateral root initiation (LRI) occurs throughout the life-span of a plant and is a key determinant of the plant's ability to adapt to changing soil environments. In most eudicot plants, such as Arabidopsis thaliana and tomato (Solanum lycopersicum), an unbranched primary root emerges during germination that does not contain lateral root primordia. As roots mature, cells within the xylem-adjacent pericycle layer are activated to divide and initiate a lateral root primordium, the first event in the formation of a new lateral root. After emergence from the parent root, new lateral roots elongate and undergo further reiterative branching, allowing for exploitation of the soil resources and underground support of the plant. Initiation of new lateral roots is regulated by a plethora of signals (Malamy, 2005, 2009). Among plant hormones, auxin has the most prominent effect on activation of pericycle cells for LRI. Exogenously applied auxin can activate practically all xylem-adjacent pericycle cells, decreasing the spacing of lateral root primordia in a dose-dependent manner, and resulting in a massive increase of the LRI incidence at high concentrations (Blakely et al., 1982; Laskowski et al., 1995; Himanen, 2002; Benková et al., 2003). Similarly, A. thaliana plants with mutations that alter auxin signalling, including auxin transport genes, auxin response factor (ARF) genes, or auxin-inducible Aux/IAA genes, have defects in lateral root development or primordium initiation (Malamy, 2005; De Smet et al., 2006). Like other plant hormones, auxin can move from the shoot into the root through the phloem, together with photosynthates and other regulatory compounds, to synchronize root development with shoot development. Importantly, auxin is also transported on a cell-to-cell basis to regulate organogenesis of new plant organs (Friml, 2003; Teale et al., 2006; Vieten et al., 2007). In the xylem-adjacent pericycle of mature roots, auxin acts as the sufficient and necessary morphogenetic trigger that specifies some pericycle cells to become founder cells of new lateral roots (Dubrovsky et al., 2008). Other hormones, such as ethylene (Ivanchenko et al., 2008; Negi et al., 2008) and cytokinin (Li et al., 2006; Laplaze et al., 2007) interact with auxin to modify the activation of pericycle cells.
As summarized by Malamy (2005, 2009), many external factors, such as nutrient concentration and moisture availability, control lateral root initiation in addition to endogenous signals. Finally, two research groups have reported that gravitropic stimulation of the root generates a signal for lateral root initiation and might regulate the positioning of lateral roots along the root axis (De Smet et al., 2007; Lucas et al., 2008).
Regardless of these significant recent new advances, the regulation of LRI is still poorly understood due to its complexity and the absence of efficient methods for its precise quantitative analysis. Approaches commonly used in mutant phenotype and other plant analyses estimate the number of lateral roots per primary root (as in Xie et al., 2000; Rashotte et al., 2001; Magidin et al., 2003; Dharmasiri et al., 2005) or lateral root density, determined as number of lateral roots per mm (or cm) of the parent root (reviewed in Dubrovsky et al., 2006). Some studies have quantified emerged lateral roots in order to estimate LRI (for example, Zolman and Bartel, 2004, fig. 1B; Yamazoe et al., 2005, p. 783 and fig 4D; Wang et al., 2006, p. 94 and fig. 3D; De Smet et al., 2007, fig.2). New primordia are initiated at the tip of the primary root of A. thaliana within a relatively narrow developmental window and in a strictly acropetal pattern; however, primordia develop asynchronously with slowly developing or arrested primordia present among emerged lateral roots (Dubrovsky et al., 2006). Therefore, all initiation events, including primordia and emerged roots, should be calculated in the root portion of interest for a more reliable evaluation of the initiation process.
Fig. 1.
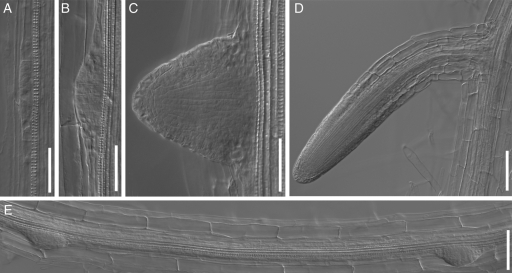
Roots cleared with the proposed NaI-based method, showing various stages of lateral root development in 13-d-old Arabidopsis plants. (A) Stage I primordium in Col-0. (B) Stage IV primordium in the max4-1 mutant. (C) Emerging primordium in the pin3 mutant. (D) Young lateral root in the max4-1 mutant. (E) Root portion between two successive primordia in the pin3 mutant; cortical cell end walls can be easily recognized. Scale bars: (A-C) = 50 µm; (D, E) = 100 µm
Fig. 3.
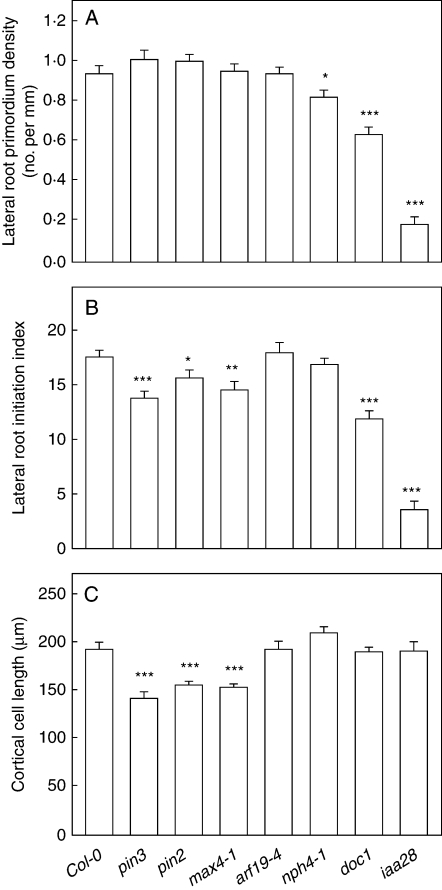
Lateral root primordium density, lateral root initiation index and length of fully elongated cortical cells in Col-0 and mutants; with the exception of iaa 28 (Ws), all in the Col-0 background, in 13-d-old plants. (A) Lateral root primordium density; (B) lateral root initiation index; and (C) length of fully elongated cortical cells. Statistical analysis was performed by pairwise comparisons of each parameter in the mutant versus the same parameter in Col-0 using an independent Student's t test or alternatively (when normality test or equal variance tests failed) by a Mann–Whitney rank sum test: *, P < 0·05; **, P < 0·01; ***, P < 0·001. Data are mean ± s.e.; n = 14–38, combined data of two-to-four independent experiments.
Fig. 2.
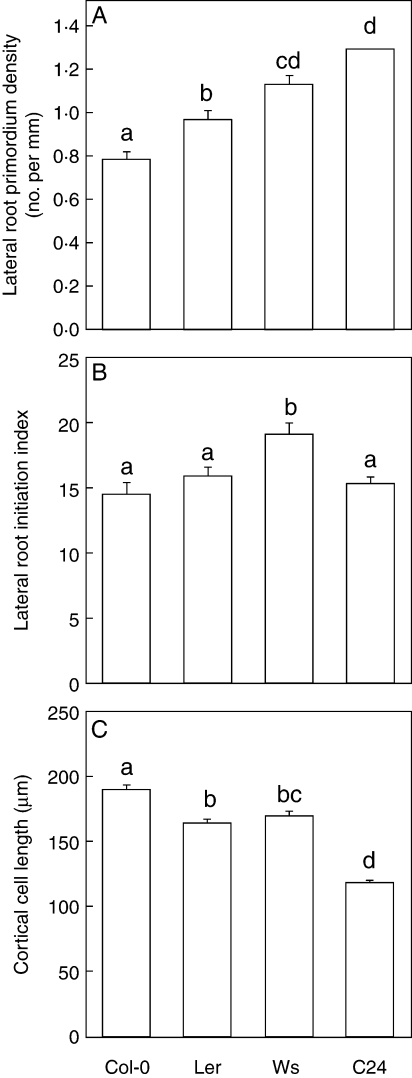
(A) Lateral root primordium density, (B) lateral root initiation index, (C) and fully elongated cortical cell length in four accessions of Arabidopsis thaliana. 10-d-old (Col-0, Ler, and Ws) and 8-d-old (C24) plants were analysed. Different letters indicate a statistically significant difference when analysed by one-way ANOVA and a multiple comparison using Tukey's test at P < 0·05. Combined data of two to three independent experiments are shown. Data are mean ± s.e.; n = 33 (Col-0), 22 (Ler), 26 (Ws), and 19 (C24).
The density of emerged laterals is often determined for the entire length of the parent root (De Smet et al., 2008; Swarup et al., 2008; Uehara et al., 2008). However, as the root grows, the length of the mature portion that contains emerged roots increases in length whereas the distal portion that contains only primordia remains of relatively invariable length. Thus, if estimated for the entire length of the parent root, the lateral root density will show an artificial increase with time simply because the parent roots will have relatively longer portions with emerged laterals. Therefore, when lateral root density is calculated, it is essential that this is done strictly for the parent root portion that contains emerged roots, i.e. between the root base and the most-distal emerged lateral. For example, studies that considered the entire length of the primary root concluded that LRI increases in A. thaliana with seedling age (Marchant et al., 2002; Uehara et al., 2008), whereas researchers who considered only the root portion containing emerged laterals concluded that density is age-independent in other plant species (Blakely et al., 1982; Lloret et al., 1988; Lloret and Pulgarín, 1992). The latter conclusion is supported by a report of MacLeod and Thompson (1979) who determined that the total number of lateral roots and primordia increases linearly with root length in four different species, indicating that the lateral root density changes neither with plant age nor along the root.
Taking the above considerations into account, we have proposed to estimate density of primordia instead of emerged lateral roots, using the lateral-root-formation zone of the parent root portion for these calculations, which is the root portion from the most-distal initiated primordium to the most-distal emerged lateral root (Dubrovsky et al., 2006). Primordia counts in this zone have given a better comparison of LRI between different accessions, genetic backgrounds and experimental conditions compared with counts along the entire length of the primary root, or counts considering only the number of emerged lateral roots. Hereafter, by ‘lateral root primordium density’ we mean the number of primordia within the parent root portion between the most-distal primordium and the most-distal lateral root, per mm of the same root portion.
We have recently encountered yet another difficulty when trying to estimate changes in LRI resulting from elevated ethylene levels in roots of A. thaliana. The ethylene precursor, ACC, affected the number of lateral root initiation events in a complex concentration-dependent manner, and at the same time caused dose-dependent variations in the primary root length, cell length and cell production rates (Ivanchenko et al., 2008). Calculation of density per unit root length (mm or cm) was not informative in this case, as unit root length was represented by different numbers of cells with each ACC concentration. We estimated how many initiation events, including both emerged lateral roots and primordia, were found per primary root length corresponding on average to 100 cortical cells in a file. This approach demonstrated that a very low ACC level could be inductive for LRI whereas higher doses that were still within the physiological range were strongly inhibitory for LRI (Ivanchenko et al., 2008). At the same time, the lateral root density first increased at the lowest ACC concentrations, but then became similar to that in the controls over a 25-fold ACC concentration range. This study suggested that calculation of LRI on a cellular basis allows for a more precise and sensitive evaluation of LRI. We have referred to this way of evaluation as lateral root frequency (Ivanchenko et al., 2008) as opposed to lateral root density, which is measured based on the root's physical length. However, sometimes the term ‘lateral root frequency’ is used as a synonym for ‘lateral root density’ (for example, Berta et al., 1990; Kerk et al., 2000; Lynch and Brown, 2001). In order to avoid confusion, we propose here the term ‘lateral root initiation index’ (ILRI) to describe how many LRI events have taken place along a root portion corresponding to 100 cortical cells in a file.
In the present study, we describe the method and provide further evidence that estimation of ILRI on a cellular basis allows for a more precise evaluation of LRI compared to estimation of lateral root density or number of lateral roots per primary root. The ILRI does not reveal the actual number of cells between any two given successive lateral root initiation events along the primary root, which is a strongly variable parameter. However, ILRI is an averaged parameter that integrates the lateral root initiation density of the parent root with the average number of fully elongated cells between successive initiation events. This integration allows a normalization of the parent root length for changes that might have resulted from alterations in cell elongation within the tissues of the parent root, rather than from changes in the number of cells that were produced and have exited the meristematic zone of the parent root.
In order to validate this cellular approach, as well as to review and evaluate other methods for quantitative estimation of LRI, comparisons are made here of four A. thaliana accessions grown under the same conditions, plants of the Columbia-0 accession grown on two media compositions, and seedlings of different age. Using the same approach, selected A. thaliana mutants and a crop plant, tomato, were also analysed. It is shown that LRI has a similar average number of primary root cells between the successive initiation events in A. thaliana accessions and in seedlings of different age in the Columbia background, and that LRI is very sensitive to alterations in the growth media. In addition, differences are shown in the establishment of the root system between tomato and A. thaliana, and new root phenotypes in some well-known A. thaliana mutants are revealed. As these analyses were performed microscopically on cleared roots, we also propose a simple and efficient one-step, whole-mount clearing method that allows non-laborious data collection using unstained plant roots.
MATERIALS AND METHODS
Plant material and growth conditions
The Arabidopsis C24 accession (CS906) was obtained from the Arabidopsis Biological Resource Center, Ohio State University. Arabidopsis thaliana mutants pin2, pin3, max4-1, arf19-4, nph4-1 and doc1 were the Columbia-0 background, and iaa 28 was the Ws background. Arabidopsis thaliana DR5::GUS line (Ulmasov et al., 1997) and tomato DR5::GUS line (Dubrovsky et al., 2008) have been described previously. Seeds were surface-sterilized in folded Whatman filter paper submerged in 20 % commercial bleach with 0·08 % triton X-100 at room temperature for 10 min, followed by four 10-min washings with sterile water. Sterilized seeds were incubated at 4 °C for 2 d to ensure even germination, and then plated on medium containing 0·2× MS salts (pH 5·8), 1 % sucrose, and 0·8 % agar, unless otherwise indicated. Plants were grown on vertically orientated Petri dishes in a growth chamber at 21 °C under a 16/8 h photoperiod and a light intensity of 105 µmol m−2 s−1.
Preparation and observation of cleared roots
Root samples were cleared by the method of Malamy and Benfey (1997) with a few modifications, as described in Dubrovsky et al. (2006). Alternatively, a new one-step clearing procedure, which is simpler, faster and preserves the cytological details very well was developed and employed as follows. Roots of A. thaliana were fixed in 4 % formaldehyde prepared in 0·025 m phosphate buffer (pH 7·2) for at least 4 h at room temperature, or overnight at 4 °C. The fixative was replaced with 30 % (aq. v/v) glycerol containing 2 % (v/v) DMSO and left for at least 30 min at room temperature. Roots were then mounted in clearing solution and observed at least 1 h after the sample preparation. The clearing solution was composed of 4·2 m NaI and 8 mm Na2S2O3 prepared in 65 % (aq. v/v) glycerol supplemented with 2 % (v/v) DMSO. This proposed method allows for sample storage before analysis and significantly reduces the labour for tissue preparation. Primordium and root cell-length analysis were performed under a Zeiss Axiovert 200M microscope (Zeiss, Oberkochen, Germany) equipped with DIC optics. Photographs were taken using a Photometrics CoolSNAPcf Color Camera (Valley International Corporation, Austin, TX, USA).
Estimation of lateral root initiation index
We define the ILRI as the number of lateral root primordia (p) initiated along a parent root portion that corresponds to the length (l, mm) of 100 fully elongated cortical cells in a single file determined in the same parent root. The number of primordia was determined in cleared roots on microscopic slides within the lateral-root-formation zone, or between the most-distal initiated primordium and the most-distal emerged lateral root (Dubrovsky et al., 2006). In Tables 2 and 3, the root portions corresponding to 3 and 4 d of growth, respectively, were used for analysis. In the iaa28 and doc1 A. thaliana mutants, the entire primary root was used for analysis. The length of cortical cells was measured in the same cleared roots using an ocular micrometer: starting from the position in the primary root where the most-distal primordium was found and moving up in a proximal direction, the length of ten cortical cells was measured in the same file and average cell length was estimated.
Table 2.
Primary root growth and lateral root initiation in Arabidopsis DR5::GUS line seedlings of different ages
| Seedling age (d) | Root growth increment (mm) | Number of lateral root initiation events | Density of LR and LRP (mm−1) | Lateral root initiation index (no. per 100 cells) | Cell length (μm) |
|---|---|---|---|---|---|
| 8 | 14·3 ± 0·6a | 10·7 ± 0·6a | 0·77 ± 0·04a | 14·3 ± 0·7a | 187 ± 2a |
| 11 | 23·1 ± 0·8b | 15·7 ± 0·7b | 0·68 ± 0·02a | 13·2 ± 0·5a | 192 ± 2b |
| 14 | 26·5 ± 1·1b | 19·4 ± 1·0b | 0·75 ± 0·04a | 14·5 ± 0·8a | 192 ± 2b |
| P < 0·001 | P < 0·001 | P = 0·486 | P = 0·496 | P = 0·043 |
Growth parameters were determined for the root portion corresponding to the last 3 d of seedling growth. The length of this root portion, except for the fragment from the earliest (most-distal) primordium to the primary root tip, was used for calculation of lateral root (LR) and lateral root primordium (LRP) density.
Values are mean ± s.e., n =33–39, for at least two independent experiments. Data evaluation for each parameter was done using Kruskal–Wallis one-way ANOVA on ranks and P is indicated; pair-wise multiple data comparison was carried out using Dunn's test; different letters indicate statistical differences; identical letters indicate the absence of significant differences (P >0·05).
Table 3.
Comparison of lateral root initiation in tomato DR5::GUS seedlings and Arabidopsis DR5::GUS seedlings
| Species | Root growth increment (mm) | Number of LR initiation events | Density of LR and LRP (mm−1) | Lateral root initiation index (no. per 100 cells) | Cell length (μm) |
|---|---|---|---|---|---|
| Arabidopsis† | 18·6 ± 1·9 | 13·6 ± 1·5 | 0·8 ± 0·1 | 13·0 ± 1·6 | 176 ± 5 |
| Tomato‡ | 42·5 ± 1·3 | 12·8 ± 0·8 | 0·3 ± 0·02 | 4·1 ± 0·3 | 136 ± 3 |
| n/a | P = 0·3 | P < 0·001 | P < 0·001 | P < 0·001 |
† Growth parameters were determined for the root portion corresponding to the last 3 d of seedling growth.
‡ Growth parameters were determined for the root portion corresponding to the last 4 d of seedling growth.
To determine the length of the lateral-root-formation zone (L, mm), the positions of the most-distal primordium and that of the most-distal emerged lateral root were marked with a felt-tip marker on the microscope slide. The most-distal (youngest) emerged root was considered as any root observed under a microscope that protruded through the epidermis of the parent root. Slides were scanned, and the length of the root portion was measured between the marks using Image J software (http://rsb.info.nih.gov/ij/). The number of primordia (p) was determined in this root portion. From these parameters, lateral root primordium density (d) was calculated for each individual primary root as d = pL−1 (number of primordia per mm in the lateral-root-formation zone), and ILRI was determined as 100dl, where l is the average cortical cell length in mm for each individual primary root.
At least two independent experiments were performed, each with 7–20 plants, unless otherwise stated, and statistical analysis was performed on the combined data using Microsoft Excel or Sigma Stat (Systat Software, Inc, Point Richmond, CA, USA), as indicated in the tables or the figure legends.
RESULTS
Theoretical background for the lateral root initiation index (ILRI)
The LRI index (ILRI) is a measure of LRI that integrates the number of lateral root primordia produced by the parent root with the number of cells produced in the tissue layers of the same parent root during the same growth period. From the lateral root primordium density, d (number of primordia per mm of the parent root portion from the most-distal initiated primordium to the most-distal emerged lateral root), we can find the average physical distance that separates one primordium at the beginning of its initiation from a previously initiated primordium, or more simply the average physical distance between successive primordia in the parent root. This distance is equal to d−1 (distance in mm). To integrate this physical distance with the number of fully elongated cells of the parent root that it represents, we divide it by the averaged cortical cell length, l (expressed in mm), in the same parent root portion, which yields d−1l−1. Thus, a primordium is initiated when, on average, d−1l−1 cells separate it from a previously formed primordium, and ILRI by definition is the number of initiated primordia per 100 cells in the cortical cell file. ILRI is then determined as follows. If one primordium is separated on average by d−1l−1 cells, then the number of primordia corresponding to 100 cortical cells will be 100(d−1l−1)−1, or 100dl.
In most cases in this study, the number of primordia was determined in the lateral-root-formation zone of the primary root, a zone between the most-distal initiated primordium and the most-distal emerged lateral root (Dubrovsky et al., 2006). This zone is sufficiently long in 8-d and older plants. For example, in 10-d-old Col-0, Ler and Ws and 8-d-old C24 plants, the length of the zone was on average 12·3, 14·3, 9·9 and 7·3 mm, respectively, and contained on average 10, 14, 11, and 9 primordia (n = 30, 22, 26 and 19, respectively). Interestingly, the length of the lateral-root-formation zone was statistically similar in Col-0 and Ler, but was significantly shorter in Ws and C24 when compared with Col-0 (Kruskal–Wallis ANOVA on Ranks, Dunn's test, P < 0·05,). In younger plants, or in mutants with decreased LRI where this zone is relatively short and contains five or less primordia, estimation of ILRI is more practical in the root portion from the youngest most-distal primordium to the root base, which results in statistically more consistent data. In this case, it is important to verify whether the length of the fully elongated cortical cells is similar along the entire root. We found that in most cases the cell length was similar. For example, in 10-d-old Col-0 plants the cortical cell length was 168 ± 3 µm between the 8th and 12th mm from the hypocotyl, and 171 ± 4 µm between 6th and 10th mm from the root tip (mean ± s.e., n = 8 roots with ten cells measured per individual root portion; P = 0·505, Student's t-test). If a length difference exists, it is advisable to determine ILRI separately for root portions that differ in average cortical cell length.
As LRI takes place in the xylem-adjacent pericycle, ideally ILRI should be measured based on the xylem-adjacent pericycle cell length. In practice this would be a very difficult task because the xylem-adjacent pericycle cells are very narrow and their end walls are difficult to recognize in whole-mount preparations (Dubrovsky et al., 2006). However, the length of xylem-adjacent pericycle cells changes proportionally with that of the fully elongated cortical cells in A. thaliana roots treated with the ethylene precursor, ACC (Ivanchenko et al., 2008), suggesting that cortical cell length can be used instead of xylem-adjacent pericycle cells to estimate the LRI on a cellular basis. Estimates of ILRI presented in this study are based on measurements of fully elongated cortical cells.
For accurate estimation of both lateral root primordium density and ILRI, it is essential to detect and score all lateral root primordia within the fragment of the parent root analysed, including very early-stage primordia. The proposed new method for root clearing (see Materials and Methods) permited easy recognition of different stages of primordium formation, starting from stage-I (classification of Malamy and Benfey, 1997), throughout primordium development and lateral root emergence in all the A. thaliana lines tested (Fig. 1). It also provided an accurate identification of cortical cell walls and measurement of cortical cell length.
Lateral root initiation index in four A. thaliana accessions
The LRI in A. thaliana accessions was estimated based on both lateral root primordium density and ILRI. The data presented in Fig. 2A indicate significant differences among A. thaliana accessions in density of primordia, with the highest density (found in C24) being 65 % greater than the lowest density (Col-0). ILRI showed less variation among accessions, with significant differences only between Ws and other accessions (Fig. 2B). Moreover, the difference between the ILRI values for Ws and Col-0 was only 32 %, approximately half the difference estimated based on primordium density. Analysis of the length of fully elongated cortical cells in the accessions revealed clearly that the disagreement between the lateral root density and the ILRI values came from variations in the primary root cortical cell length (Fig. 2C). For example, the C24 accession had the shortest average cortex cell length, giving the impression that LRI occurred with a higher incidence in this accession than in the other three accessions when LRI was estimated based on the lateral root density, or in other words on the physical distances between initiation events. The use of ILRI eliminated the effect of cell length variations between the accessions.
ILRI helps to better understand the effect of nutrient concentration in the growth medium on lateral root initiation
The nitrogen-to-sucrose ratio in the growth medium is a significant factor in lateral root initiation (Malamy and Ryan, 2001); however, many laboratories use different media formulas, ranging from full- to 0·2-strength of Murashige and Skoog (MS) salts with sucrose concentrations ranging from 1 to 2 %. We analysed the effect of medium dilution on LRI, comparing ILRI in 10-d-old A. thaliana plants grown on either full-strength MS medium (60 mm of N; Malamy and Ryan, 2001) or 0·2× MS (12 mm of N), supplemented with 1 % sucrose in both cases. The lateral root primordium density showed no difference between plants grown in the two conditions (Table 1), although the daily root growth increment was greater in seedlings grown on the 0·2× MS medium (data not shown). However, estimation of ILRI revealed that the incidence of LRI was increased by 30 % on the 0·2 MS medium. The failure of the primordium density estimations to reveal this increase in LRI apparently resulted from a change in the elongated cell length. Because cells were 32 % longer in seedlings grown on 0·2× MS, this obscured the fact that, on average, primordia were separated by fewer cells along the primary root on this medium. The data indicate that ILRI is a more sensitive parameter when analysing the effect of nutrient concentration on LRI than the primordium density. This also shows the importance of consistency in media composition and root-volume-to-medium-volume ratio when analysing LRI.
Table 1.
Lateral root primordium (LRP) density, lateral root initiation index (ILRI) and fully elongated cell length in 10-d-old Arabidopsis (Col-0) seedlings grown on full MS (1 × ) medium and 0·2× MS, both supplemented with 1 % sucrose
| Medium | LRP density (no. of LRP mm−1 of primary root) | ILRI (no. of LRP per 100 cortical cells) | Length of fully elongated cortical cells (μm) |
|---|---|---|---|
| 0·2× MS | 0·78 ± 0·04 | 14·8 ± 0·8 | 190 ± 3 |
| 1× MS | 0·83 ± 0·03 | 11·8 ± 0·4 | 144 ± 2 |
| P = 0·426 | P < 0·001 | P = 0·008 |
Statistical differences were estimated using a Mann–Whitney rank sum test; values are mean ± s.e.; n = 33 (0·2× MS) and 21 (1× MS) of at least two independent experiments.
Relationship between primary root growth and lateral root initiation in A. thaliana seedlings of different age
In A. thaliana, the first lateral root primordium is normally initiated at day 2 post-germination (Beeckman et al., 2001). This raises the question of how seedling age affects LRI and how LRI is related to the exponential increase of primary root growth, shown to take place during the first 2 weeks after germination (Beemster and Baskin, 1998). In order to relate LRI, primary root growth and seedling age, we analysed the last 72-h primary root growth increments in 8-d-, 11-d- and 14-d-old A. thaliana seedlings, excluding the root portion from the tip of the root to the earliest most-distal primordium. These root portions contained both primordia and emerged roots, so all lateral root initiation events were analysed (Table 2). The DR5::GUS line (Ulmasov et al., 1997) commonly used to analyse lateral root formation in A. thaliana (for example, see Benková et al., 2003) was used in this analysis. The number of lateral root initiation events increased in the root portions analysed between the 8-d- and 11-d-old plants but remained statistically similar between the 11-d- and 14-d-old plants. An overall increase in primary root growth was also observed between the 8-d- and 11-d-old plants, indicating that there was a correlation between seedling age, primary root growth rate and lateral root primordium initiation rate. The lateral root average density was 0·7 initiation events per mm of primary root with no statistically significant difference among the age groups, and the ILRI remained, on average, 14 initiation events per 100 cortical cells for all three age groups. Only a minor difference was found for the root cortical cell length between 8-d- and 11-d-old plants, suggesting that the increase in root growth was related mainly to an increase in cell production and not to cell elongation (Table 2), similar to previously reported findings (Beemster and Baskin, 1998). Emergence of lateral root primordia also increased with seedling age, from virtually none in the 8-d-old plants to more than three emerged lateral roots per root portion in the 11-d- and 14-d-old plants (results not shown). Overall, these data show that the rate of LRI is synchronized with the rate of root growth, and that lateral root density and ILRI are steady parameters between 8-d- and 14-d-old plants for the background and conditions tested. Arabidopsis thaliana seedlings of the DR5::GUS line showed a slight reduction in lateral root primordium density and ILRI compared with the wild-type Columbia seedlings of a similar age (compare Fig. 2 and Tables 1 and 2).
Lateral root initiation in tomato
In order to evaluate LRI in tomato, 6-d-old seedlings were transferred from Magenta boxes to vertically orientated 150 × 150 mm Petri dishes and allowed to grow for 4 more days. It was essential that during this period primary roots did not reach the bottom of the Petri dish where moisture condensation could affect root growth. Plants of the tomato DR5::GUS line (Dubrovsky et al., 2008) were compared to the A. thaliana DR5::GUS line (Ulmasov et al., 1997) in these experiments. As shown in Table 3, tomato primary roots grew almost twice as fast as roots of A. thaliana. However, tomato required 4 d of growth to accumulate a comparable number of lateral root initiation events (13 on average) to those generated in only 3 d in seedlings of A. thaliana of similar age and under similar growth conditions; the primary root growth increment was 2·6 greater than that in A. thaliana. The difference between the species was obvious from an estimation of the lateral root density, which was on average 2·6 times lower in tomato, but became more pronounced when expressed as ILRI, which was 13 ± 1·6 for A. thaliana seedlings and only 4·1 ± 0·3 for tomato (means ± s.e., Table 2). Thus, although tomato apparently has very robust primary root growth, it initiates lateral root branches at a lower incidence when compared to A. thaliana, at least in the AC background and uner the conditions studied here.
ILRI helps to estimate more precisely lateral root initiation in A. thaliana mutants
Lateral root primordium density and ILRI were analysed in selected A. thaliana mutants and were compared to the respective values in wild-type plants. Plants were grown under uniform conditions of 0·2× MS supplemented with 1 % sucrose, and were analysed 13 d after germination.
The pin3 and pin2 A. thaliana auxin efflux mutants showed no difference in lateral root primordium density under our growth conditions compared to the wild type (Fig. 3A); however, the ILRI in the mutants decreased and was 78 % of the wild type in pin3 and 89 % in pin2 (Fig. 3B). Fully elongated cortical cells in both mutants were shorter than in the wild type (Fig. 3C; in agreement with Blilou et al., 2005), which could account, at least in part, for the observed discrepancy between the ILRI and the primordium density estimations. Thus, analysis of ILRI revealed defects in LRI in pin3 and pin2 whilst estimation of the primordium density failed to reveal the phenotype. The conclusion that pin3 and pin2 have reduced LRI is in agreement with Benková et al. (2003): under their growth conditions, pin3 was found to have a density of lateral root primordia that was 54 % of the wild type, whilst for pin 2 the number of initiated lateral roots was 93 % of the wild type.
The max4-1 (more axillary branching) mutant (Stirnberg et al., 2002; Sorefan et al., 2003; Bennett et al., 2006) has increased shoot branching due to a deficiency in strigolactone production (Gomez-Roldan et al., 2008; Umehara et al., 2008) and shows an increased capacity for auxin transport in the shoot (Bennett et al., 2006). Although it is known as a shoot-specific mutant, we were interested in max4-1 because of the MAX4 gene expression in the tip of the root and distribution of promoter activity into the root differentiation zone upon treatment with exogenous auxin (Sorefan et al., 2003), suggesting a function in root auxin responses and root development. Calculation of the lateral root primordium density did not reveal a difference between the max4-1 and Col-0 wild-type plants (Fig. 3A); however, the ILRI was decreased significantly (Fig. 3B), demonstrating a decreased incidence of LRI in max4-1. The max4-1 mutant has shorter primary root cells, explaining why the root branching effect was obvious only from the ILRI estimations (Fig. 3C). These findings suggest that the MAX4 pathway is involved not only in shoot branching but also in root branching, although the effects are apparently opposite in aerial and underground tissues.
Single arf 19 and arf 7 mutants with defects in primary auxin response proteins (ARFs) essential for lateral root formation were analysed. Lack of the lateral root phenotype in single mutants could result from protein redundancy, but the combined (arf 19-1 nph4-1) mutant has a strong auxin-related phenotype that includes complete absence of lateral roots (Okushima et al., 2005, 2007; Wilmoth et al., 2005). We detected no primordia of any stage in the double arf 19-1 nph4-1 mutant (n = 60). In contrast, the single arf 19-1 mutant had completely normal lateral root primordium density (0·93 ± 0·03, mean ± s.e., n = 14) and nph4-1 had slightly decreased primordium density (0·82 ± 0·04, n = 14), whereas the ILRI and cortical cell lengths in both single mutants were indistinguishable from those in the Col-0 parent line (Fig. 3). Overall, these data confirm the lateral root phenotype reported by Okushima et al. (2005, 2007) and show that in cases where cell length in the parent root does not change between the lines studied, estimations of ILRI better match estimations of primordium density.
doc1 [dark over-expression of CAB (chlorophyll a/b-binding proteins of photosystem II)] is allelic to tir3 (transport inhibitor response)/big/lpr1 (low phosphate-resistant) and has defects in the BIG protein involved in auxin transport (Li et al., 1994; Gil et al., 2001; López-Bucio et al., 2005). Significant inhibition of lateral root development has been known both in the tir3-1 mutant allele (Ruegger et al., 1997) and in the lrp1 mutant allele (López-Bucio et al., 2005), and involvement of the BIG protein in pericycle cell activation during the LRI process has been demonstrated (López-Bucio et al., 2005). Fig. 3 shows that both lateral root primordium density and ILRI are decreased in the doc1 allele, confirming reported defects in LRI. The length of the fully elongated cortical cells was the same as in wild type plants (Fig. 3C), and a reduction of root growth was caused primarily by a decrease in cell production in the root apical meristem. As no change in root cell length took place, the decrease in ILRI was proportional to the primordium density decrease in doc1 (Fig. 3B).
A gain-of-function mutation in a gene of the Aux/IAA auxin-response family, iaa28-1, severely inhibits LRI (Rogg et al., 2001). We found that in this mutant both lateral root primordium density and ILRI were significantly reduced, but the fully elongated cortical cell length was not changed in comparison to wild type plants (compare Ws in Fig. 2 and iaa28 in Fig. 3).
DISCUSSION
The lateral root initiation index is a general developmental parameter
Estimation of ILRI provides an idea of the average number of lateral root initiation events per 100 elongated cells. This parameter gives only average values, as LRI is a rather plastic process responding to growth conditions. Lucas et al. (2008) have shown that LRI can be induced experimentally by controlled gravi-stimulations with various time intervals between the stimulations, resulting in variable physical spacing between successive initiation events. Nevertheless, it was also noted in the same study that there seemed to be an internal mechanism that does not permit successive primordia initiations to be separated by too short or too long time intervals. Consistencies in the average physical spacing of primordia, as well as in the average cellular spacing between successive initiation events that we report here, could be a reflection of such a control mechanism. However, the variability in distances between successive primordia and/or lateral roots along the primary root irrespective of the protoxylem strands is relatively high. In the conditions used here, the average distance in 10-d-old Col-0 plants was 1·531 ± 0·046 mm (mean ± s.e., for a total of 235 individual measurements in nine roots), while the minimal and maximal distances were 0·146 mm and 3·977 mm, respectively, and the coefficient of variation estimated as the ratio between the s.d. and mean values was 46 %. Such variation still allows (with a sufficient number of plants analysed) for comparison of LRI in different genetic backgrounds. For instance, while the lateral root primordium density was greater in C24 than in Col-0, ILRI was the same in the two accessions (Fig. 2).
We would like to stress that both ILRI and lateral root density are important and complementary parameters in understanding the establishment of root systems. For example, the data on the physical spatial distribution of lateral roots could be informative for understanding root ecology or soil exploration. However, when different genotypes, growth conditions or treatments are compared, and when different species are investigated for common or different features in root development, the ILRI is the parameter of choice. As this parameter normalizes data on root (and/or primordia) density for cell length variations, it allows for a more complete understanding of whether LRI actually increases, decreases or remains the same in each specific experimental design. Therefore, from a developmental perspective, ILRI is a more precise parameter.
The importance of ILRI for understanding root development can be illustrated by the following observations made with cactus roots (Dubrovsky and Gómez-Lomelí, 2003). In cactus primary roots developing under severe water stress conditions, the density of lateral roots and primordia was found to increase on average by a factor of 1·6, which would suggest that water stress increases LRI. However, the length of fully elongated epidermal cells in the same water stressed roots also decreased on average by a factor of 1·6 compared to non-stressed conditions, demonstrating that the average cellular distance between roots or primordia was maintained and the change in lateral root density did not indicate an increase in LRI. This exemplifies that only when all three parameters are known – lateral root density, ILRI and fully elongated cell length – can a more complete analysis of the root formation process can be performed, whereas data only on density could be insufficient or even misleading, especially when both cell production and cell length change simultaneously. Cellular spacing between successive lateral root initiation events could reflect how photosynthates and regulatory signals are allocated at the cellular level, and also reflect their input into either formation of new roots or other developmental processes. This is a complex problem not yet understood, but of vital importance for the growth and shaping of the root system.
It is interesting that not only the rate of root growth (Beemster and Baskin, 1998) but also the rate of root branching is age-dependent, and the two parameters are tightly interconnected in A. thaliana. As seen in Table 2, when an age-dependent increase in root growth takes place, LRI is also increased but ILRI remains unchanged. Earlier studies of MacLeod and Thompson (1979) showed that in Vicia faba, Pisum sativum, Phaseolus vulgaris and Zea mays the number of lateral roots initiated per day was in correlation with the root growth rate, so that the lateral root or primordium density was the same for plants between 5 and 12 d after germination, and was species-specific. The findings in the current study determine that age-dependent changes in rate of lateral root formation do take place in A. thaliana, whereas the ILRI is maintained stable.
In the plants studied here, lateral root initiation takes place in the primary root differentiation zone and this explains why the cellular spacing between successive LRI events is independent of the rate of root growth. The latter is determined in part by the length of fully elongated cells and in part by the rate of cell production in the primary root meristem. However, when LRI starts, xylem-adjacent pericycle cells have already left the meristem and completed their elongation. In a number of plant species, lateral root initiation takes place within the root apical meristem (reviewed in Dubrovsky and Rost, 2003). In such cases cellular spacing between LRI events may depend on the rate of cell production and cell-cycle time in the parent root meristem. This interesting question requires further study.
ILRI can be applied to a variety of experimental conditions
Based on the results presented here, we conclude that ILRI can help to quantitatively evaluate LRI in different accessions, plant species, mutants or plants under different growth conditions. In most of the experiments discussed here, ILRI was measured in the lateral root formation zone, i.e. the zone between the most-distal primordium and the most-distal lateral root. In the conditions used, this zone comprises a root portion formed within 1·5–3 d of growth depending on the the genetic background and seedling age. Primary root length comprising 100 cortical cells in a file corresponds to approx. 19 mm, and is formed in 11-d-old plants within 2·5 d of growth (estimates made from data in Table 2). Between the 8th and 14th day of growth, the average length of elongated cells did not change significantly in absolute terms (even though there was a statistical difference in this parameter between the 8- and 11-d-old plants, Table 2). This indicates that the lateral root formation zone in this example (DR5::GUS line of A. thaliana) does indeed comprise approx. 100 cortex cells in a file, and estimations of ILRI performed here were based on actual counts of primordia over a root length comprising on average 100 cortical cells in a file. Exceptions are data in Table 2 and 3, which were collected over controlled 3- or 4-d growth periods. It is important to note that calculation of ILRI is not restricted to the lateral root formation zone of a plant and can be estimated, for example, using the entire root, or any root portion that comprises a number of lateral root initiation events that is sufficient for statistical analysis. ILRI can then be either calculated for 100 cells, or any chosen number of cells, although the absolute values in the different cases will not always be the same. When root initiation is significantly diminished as in some mutants, for example the iaa28 mutant studied here, it is practical to estimate ILRI along the entire root.
In this study, we analysed A. thaliana and tomato plants, which both have a diarch vascular structure with two xylem strands in the vascular cylinder. However, ILRI can be estimated in plants with any vascular pattern, from diarch to polyarch. In this case, one has to consider comparing roots with the same number of vascular strands. Alternatively, a normalization of ILRI for only one xylem (or phloem, or intermediate between xylem and phloem) strand, depending on the type of primordium positioning, would be required. In Leguminosae, a nodule formation index could be also estimated on a cellular base, as the number of root nodules along a root portion comprising a defined number of cortical or any other type of cells.
ILRI reveals new phenotypes in mutants
This study demonstrates that analysis of ILRI in mutants provides a way to identify new LRI phenotypes, as was the case with the max4-1 mutant described. It could also potentially help to better understand some of the known LRI phenotypes. We suggest that the effect of a gene mutation on root branching can be estimated more precisely when both lateral root primordium density and ILRI are taken into account and compared. In the seven mutants analysed here (Fig. 3), we can recognize four different scenarios: (1) neither lateral root primordium density nor ILRI is affected (arf19-4), indicating no root branching phenotype is present; (2) lateral root primordium density is decreased, but the ILRI is the same as in the wild type (nph4-1), indicating a weak root branching phenotype; (3) lateral root primordium density is not affected compared to wild-type; however, ILRI is significantly decreased (pin2, pin 3, and max4-1), indicating a moderate root branching phenotype; and (4) both lateral root primordium density and ILRI are significantly affected (doc1 and iaa28), indicating a strong root branching phenotype. In A. thaliana, there have been 22 described mutants with reduced and 14 mutants with an increased number of lateral roots (reviewed in De Smet et al., 2006) and this list is growing continuously. The approach described here should help to better understand some of the mutant phenotypes, and/or to expand this list in the future.
In conclusion, ILRI represents a new quantitative parameter for description of lateral root formation in plants. As such it can provide a better understanding of developmental aspects of root architecture, or it can reveal new phenotypes in mutants not previously known as being affected in root development. In comparison with lateral root density and lateral root primordium density, ILRI is more sensitive to changes in initiation of lateral roots caused by genetic or environmental factors. ILRI can be also successfully used for comparison of lateral root formation between plant species.
ACKNOWLEDGMENTS
We thank J. M. Hurtado-Ramirez, S. Ainsworth, and N. Doktor for excellent technical help, A. Hernández-Barrera for help in data collection, M. Miller for critical reading of the manuscript, and J. Fowler for lab space provided for M.G.I. We are grateful for materials provided by J. Friml (pin3), L. Herrera-Estrella (eir1/pin2/wav6), O. Leyser (max4-1), J. Reed (arf19-4, nph4-1 single and double mutants), J. Chory (doc1), B. Bartel (iaa28) and the Arabidopsis Biological Resource Center, Ohio State University (wild type A. thaliana). We are very grateful to V. B. Ivanov for his encouragement to investigate the corresponding author's idea of the lateral root initiation index. This work was supported by The US Department of Agriculture National Research Initiative Competitive Grants Program [2006–03434 to M.G.I.]; Postdoctoral Fellowship of the Dirección General de Asuntos del Personal Académico de la Universidad Nacional Autóma de México [to A.S.]; Czech Ministry of Youth, Education and Sports [LC06034 to A.S. ]; Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica de la Universidad Nacional Autóma de México [IN225906 to J.G.D.]; Consejo Nacional de Ciencia y Tecnología of Mexico (CONACyT) [49267 to J.G.D.], and CONACyT-Secretaría de Medio Ambiente y Recursos Naturales of Mexico [2004-C01-80 to J.G.D.].
LITERATURE CITED
- Beeckman T, Burssens S, Inzé D. The peri-cell-cycle in Arabidopsis. Journal of Experimental Botany. 2001;52:403–411. doi: 10.1093/jexbot/52.suppl_1.403. [DOI] [PubMed] [Google Scholar]
- Beemster GTS, Baskin TI. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiology. 1998;116:1515–1526. doi: 10.1104/pp.116.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berta G, Fusconi A, Trotta A, Scannerini S. Morphogenetic modifications induced by the mycorrhizal fungus Glomus strain E3 in the root system of Allium porrum L. New Phytologist. 1990;114:207–215. [Google Scholar]
- Benková E, Michniewicz MSM, Teichmann T, Seifertova D, Jürgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Current Biology. 2006;16:553–563. doi: 10.1016/j.cub.2006.01.058. [DOI] [PubMed] [Google Scholar]
- Blakely LM, Durham M, Evans TA, Blakely RM. Experimental studies on lateral root formation in radish seedling roots. I. General methods, developmental stages, and spontaneous formation of laterals. Botanical Gazette. 1982;143:341–352. [Google Scholar]
- Blilou I, Xu J, Wildwater M, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- De Smet I, Vanneste S, Inzé D, Beeckman T. Lateral root initiation or the birth of a new meristem. Plant Molecular Biology. 2006;60:871–887. doi: 10.1007/s11103-005-4547-2. [DOI] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development. 2007;134:681–690. doi: 10.1242/dev.02753. [DOI] [PubMed] [Google Scholar]
- De Smet I, Vassileva V, De Rybel B, et al. Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science. 2008;322:594–597. doi: 10.1126/science.1160158. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, et al. Plant development is regulated by a family of auxin receptor F box proteins. Developmental Cell. 2005;9:109–119. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Gómez-Lomelí LF. Water deficit accelerates determinate developmental program of the primary root and does not affect lateral root initiation in a Sonoran Desert cactus (Pachycereus pringlei, Cactaceae) American Journal of Botany. 2003;90:823–831. doi: 10.3732/ajb.90.6.823. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Rost TL. Plant growth and development: lateral root initiation. In: Thomas B, Murphy DJ, Murray BG, editors. Encyclopedia of applied plant sciences. Oxford: Elsevier Academic Press; 2003. pp. 1101–1107. [Google Scholar]
- Dubrovsky JG, Gambetta GA, Hernandez-Barrera A, Shishkova S, Gonzalez I. Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density and predictability. Annals of Botany. 2006;97:903–915. doi: 10.1093/aob/mcj604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proceedings of the National Academy of Sciences of the USA. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J. Auxin transport – shaping the plant. Current Opinion in Plant Biology. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, et al. BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes and Development. 2001;15:1985–1997. doi: 10.1101/gad.905201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, Almeida Engler J, Inzé D, Beeckman T. Auxin-mediated cell cycle activation during early lateral root initiation. The Plant Cell. 2002;14:2339–2351. doi: 10.1105/tpc.004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanchenko MG, Muday GK, Dubrovsky JG. Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. The Plant Journal. 2008;55:335–347. doi: 10.1111/j.1365-313X.2008.03528.x. [DOI] [PubMed] [Google Scholar]
- Kerk NM, Jiang K, Feldman LJ. Auxin metabolism in the root apical meristem. Plant Physiology. 2000;122:925–932. doi: 10.1104/pp.122.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaze L, Benková E, Casimiro I, et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation. The Plant Cell. 2007;19:3889–3900. doi: 10.1105/tpc.107.055863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski MJ, Williams ME, Nusbaum HC, Sussex IM. Formation of lateral root meristems is a two-stage process. Development. 1995;121:3303–3310. doi: 10.1242/dev.121.10.3303. [DOI] [PubMed] [Google Scholar]
- Li HM, Altschmied L, Chory J. Arabidopsis mutants define downstream branches in the phototransduction pathway. Genes and Development. 1994;8:339–349. doi: 10.1101/gad.8.3.339. [DOI] [PubMed] [Google Scholar]
- Li X, Mo X, Shou H, Wu P. Cytokinin-mediated cell cycling arrest of pericycle founder cells in lateral root initiation of Arabidopsis. Plant and Cell Physiology. 2006;47:1112–1123. doi: 10.1093/pcp/pcj082. [DOI] [PubMed] [Google Scholar]
- Lloret PG, Pulgarín A. Effect of naphthaleneacetic acid of the formation of lateral roots in the adventitious root of Allium cepa: number and arrangement of laterals along the parent root. Canadian Journal of Botany. 1992;70:1891–1896. [Google Scholar]
- Lloret PG, Casero PJ, Navascues J, Pulgarín A. The effects of removal of the root tip on lateral root distribution in adventitious roots of onion. New Phytologist. 1988;110:143–149. [Google Scholar]
- López -Bucio J, Hernandez-Abreu E, Sánchez-Calderón L, et al. An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiology. 2005;137:681–691. doi: 10.1104/pp.104.049577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Godin C, Jay-Allemand C, Laplaze L. Auxin fluxes in the root apex co-regulate gravitropism and lateral root initiation. Journal of Experimental Botany. 2008;59:55–66. doi: 10.1093/jxb/erm171. [DOI] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant and Soil. 2001;237:225–237. [Google Scholar]
- MacLeod RD, Thompson A. Development of lateral root primordia in Vicia faba, Pisum sativum, Zea mays and Phaseolus vulgaris: rates of primordium formation and cell doubling times. Annals of Botany. 1979;44:435–449. [Google Scholar]
- Magidin M, Pittman JK, Hirschi KD, Bartel B. ILR2, a novel gene regulating IAA conjugate sensitivity and metal transport in Arabidopsis thaliana. The Plant Journal. 2003;35:523–534. doi: 10.1046/j.1365-313x.2003.01826.x. [DOI] [PubMed] [Google Scholar]
- Malamy JE. Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment. 2005;28:67–77. doi: 10.1111/j.1365-3040.2005.01306.x. [DOI] [PubMed] [Google Scholar]
- Malamy JE. Lateral root development. In: Beeckman T, editor. Root development. Oxford, UK: Blackwell Publishing Limited; 2009. in press. [Google Scholar]
- Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Ryan KS. Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiology. 2001;127:899–909. [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, et al. AUX1 promotes lateral root formation by facilitating Indole-3-Acetic acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant Cell. 2002;14:589–597. doi: 10.1105/tpc.010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. The Plant Journal. 2008;55:175–187. doi: 10.1111/j.1365-313X.2008.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Overvoorde PJ, Arima K, et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. The Plant Cell. 2005;17:444–463. doi: 10.1105/tpc.104.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. The Plant Cell. 2007;19:118–130. doi: 10.1105/tpc.106.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK. Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. The Plant Cell. 2001;13:1683–1697. doi: 10.1105/TPC.010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogg LE, Lasswell J, Bartel B. A gain-of-function mutation in IAA28 suppresses lateral root development. The Plant Cell. 2001;13:465–480. doi: 10.1105/tpc.13.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, et al. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. The Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogne K, et al. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes and Development. 2003;17:1469–1474. doi: 10.1101/gad.256603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, van de Sande K, Leyser HMO. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development. 2002;129:1131–1141. doi: 10.1242/dev.129.5.1131. [DOI] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nature Cell Biology. 2008;10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nature Reviews Molecular Cell Biology. 2006;7:847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- Uehara T, Okushima Y, Mimura T, Tasaka M, Fukaki H. Domain II mutations in CRANE/IAA18 suppress lateral root formation and affect shoot development in Arabidopsis thaliana. Plant and Cell Physiology. 2008;49:1025–1038. doi: 10.1093/pcp/pcn079. [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends in Plant Science. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu Y, Han Y, et al. Overexpression of RAN1 in rice and Arabidopsis alters primordial meristem, mitotic progress, and sensitivity to auxin. Plant Physiology. 2006;140:91–101. doi: 10.1104/pp.105.071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmoth JC, Wang S, Tiwari SB, et al. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. The Plant Journal. 2005;43:118–130. doi: 10.1111/j.1365-313X.2005.02432.x. [DOI] [PubMed] [Google Scholar]
- Xie Q, Frugis G, Colgan D, Chua NH. Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development. Genes and Development. 2000;14:3024–3036. doi: 10.1101/gad.852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazoe A, Hayashi K, Kepinski S, Leyser O, Nozaki H. Characterization of terfestatin A, a new specific inhibitor for auxin signaling. Plant Physiology. 2005;139:779–789. doi: 10.1104/pp.105.068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Bartel B. An Arabidopsis indole-3-butyric acid-response mutant defective in PEROXIN6, an apparent ATPase implicated in peroxisomal function. Proceedings of the National Academy of Sciences of the USA. 2004;101:1786–1791. doi: 10.1073/pnas.0304368101. [DOI] [PMC free article] [PubMed] [Google Scholar]