Abstract
Background and Aims
Evolutionary change in response to natural selection will occur only if a trait confers a selective advantage and there is heritable variation. Positive connections between pollen traits and fitness have been found, but few studies of heritability have been conducted, and they have yielded conflicting results. To understand better the evolutionary significance of pollen competition and its potential role in sexual selection, the heritability of pollen tube-growth rate and the relationship between this trait and sporophytic offspring fitness were investigated in Collinsia heterophylla.
Methods
Because the question being asked was if female function benefited from obtaining genetically superior fathers by enhancing pollen competition, one-donor (per flower) crosses were used in order to exclude confounding effects of post-fertilization competition/allocation caused by multiple paternity. Each recipient plant was crossed with an average of five pollen donors. Pollen-tube growth rate and sporophytic traits were measured in both generations.
Key Results
Pollen-tube growth rate in vitro differed among donors, and the differences were correlated with in vivo growth rate averaged over two to four maternal plants. Pollen-tube growth rate showed significant narrow-sense heritability and evolvability in a father–offspring regression. However, this pollen trait did not correlate significantly with sporophytic-offspring fitness.
Conclusions
These results suggest that pollen-tube growth rate can respond to selection via male function. The data presented here do not provide any support for the hypothesis that intense pollen competition enhances maternal plant fitness through increased paternity by higher-quality sporophytic fathers, although this advantage cannot be ruled out. These data are, however, consistent with the hypothesis that pollen competition is itself selectively advantageous, through both male and female function, by reducing the genetic load among successful gametophytic fathers (pollen), and reducing inbreeding depression associated with self–pollination in plants with mix-mating systems.
Key words: Collinsia heterophylla, evolvability, female fitness, good genes, heritability, male fitness, mixed-mating system, Plantaginaceae, pollen competition, sexual selection
INTRODUCTION
Over 80 % of flowering plants are hermaphrodites, meaning that both male and female reproductive functions may contribute to individual fitness (Richards, 1997). Despite this fact, selection pressures on the two reproductive functions can differ (Willson, 1979; Bell, 1985; Campbell, 1989; Maad and Alexandersson, 2004; Maad and Nilsson, 2004), potentially leading to sexual selection of floral traits (Skogsmyr and Lankinen, 2002; Delph and Ashman, 2006). Divergent selection pressures are likely to be particularly important during pollen competition in the pistil, because pollen has the opportunity to represent the sole ‘interests’ of male reproductive function. Although pollen performance directly reflects the male reproductive function, selection should favour female (pistil) reproductive traits that promote pollen competition, or otherwise screen pollen, since this may increase the fitness of her own offspring (Skogsmyr and Lankinen, 2002; Bernasconi et al., 2004).
Several studies have indeed shown that high pollen competitive ability, particularly pollen-tube growth rate, is associated with high siring ability (e.g. Snow and Spira, 1991, 1996; Marshall, 1998; Skogsmyr and Lankinen, 1999; Aronen et al., 2002). This indicates that selection on this trait may have evolutionary effects on the male function, provided that there is heritable variation for this trait (Fisher, 1958; Charlesworth and Charlesworth, 1992; Walsh and Charlesworth, 1992). Despite the great importance of heritability, there is only limited information available on levels of heritability of traits related to pollen competitive ability, and this only for a handful of species. Experiments with cultivated species suggest the presence of a genetic component of pollen competitive ability (e.g. Ottaviano et al., 1988; Schlichting et al., 1990; Sarkissian and Harder, 2001), but the few heritability studies in wild species have yielded contradictory results (see Snow and Mazer, 1988; Havens, 1994; Skogsmyr and Lankinen, 2000; Lamborn et al., 2005).
Since Mulcahy (1979) first suggested that pollen competition may be an important way to enhance offspring fitness by the screening of haploid pollen to eliminate malfunctioning genomes, numerous studies have investigated if enhanced pollen competition can improve offspring fitness. Assuming overlapping gene expression in gametophytes and sporophytes (Mulcahy et al., 1992, 1996; Walsh and Charlesworth, 1992; Hormaza and Herrero, 1994), older studies often found increased offspring fitness following higher levels of pollen competition (e.g. Mulcahy and Mulcahy, 1975; Winsor et al., 1987; Bertin, 1990; Quesada et al., 1993). These studies have, however, been criticized for not separating out the influences of male versus female reproductive functions (Charlesworth et al., 1987; Snow, 1994). More recent research has attempted to test mechanisms of how enhanced pollen competition and/or larger pollen loads can lead to higher offspring quality. They include (a) ovules being fertilized by highly competitive pollen produced by donors of superior genetic quality, (cf. ‘good genes’ of sexual selection theory; Zahavi, 1975; e.g. Skogsmyr and Lankinen, 2000; Pasonen et al., 2001), (b) larger pollen loads leading to increased sampling of superior, more-compatible, or genetically more variable pollen (e.g. Paschke et al., 2002; Bernasconi et al., 2003; Kron and Husband, 2006), and (c) maternal plants allocating more resources to ovules fertilized first or to ovules in pistils pollinated by more pollen (e.g. Delph et al., 1998). Other mechanisms include reduction of inbreeding depression by (d) self pollen performing worse than outcross pollen (cryptic self-incompatibility; e.g. Bertin and Sullivan, 1988; Kruszewski and Galloway, 2006) and (e) self pollen of low quality being avoided (Armbruster and Rogers, 2004; Lankinen and Armbruster, 2007).
The mechanism involving sexual selection (a above) in particular needs more research. For example, the relationship between offspring fitness and pollen traits of donors has been tested only in a few studies (see Mulcahy, 1971; Skogsmyr and Lankinen, 2000; Pasonen et al., 2001). These studies mainly used two-donor crosses, which indeed gives a direct test of pollen competitive ability. On the other hand, this approach may reduce the possibility of detecting paternal genetic effects on offspring quality because other factors can not be eliminated, such as the ‘sampling effect’ (Bernasconi et al., 2003) or differential allocation of maternal resources (Walsh and Charlesworth, 1992). When evaluating the relationship between offspring fitness and pollen competitive ability, it is also important to assess offspring fitness beyond the earliest offspring traits (cf. discussion on animals; Kokko et al., 2003). This is because traits expressed very early in the life-cycle of plants are known often to be strongly influenced by maternal effects (Roach and Wulff, 1987).
Previously it had been found that intense pollen competition in the self-compatible annual Collinsia heterophylla can reduce inbreeding depression following one-donor crosses of unequal pollen load size (Lankinen and Armbruster, 2007), suggesting a benefit of avoiding fertilization by self pollen of low quality. In order to understand better the possible selective advantages accruing from pollen competition, examined here are (a) the heritability and evolvability of pollen competitive ability, and (b) the relationship between pollen competitive ability and sporophytic fitness of donors and offspring at different stages of the life-cycle. To achieve this goal, one-donor crosses were performed on several recipients per donor in the greenhouse, in order to control for sampling effects and post-fertilization competition, as noted above. Because the aim was to study sexual selection of pollen competitive ability, rather than, for example, effects of genetic self-incompatibility, the focus was on evaluating the average performance of a donor across several recipients rather than estimating donor-by-recipient interactions (cf. Marshall, 1998). Even when donor-by-recipient interactions influence the success of pollen donors, selection will still favour a donor that is on average more successful than others. To assess pollen competitive ability, differences in pollen-tube growth rate were examined among the donors used in the crosses both in vitro (without any female influence) and in vivo. Also the performance of outcross and self pollen (in single pollinations) was compared in order to get an indirect test of whether more intense pollen competition would be advantageous as a means to avoid self-fertilization (cf. cryptic incompatibility, e.g. Bertin and Sullivan, 1988). It should be noted, however, that the test here is incomplete, as such differences are often only expressed when self and outcross pollen are growing together in the pistil (Aizen et al., 1990; Nemeth and Smith-Huerta, 2002; Kruszewski and Galloway, 2006).
MATERIALS AND METHODS
Study species
Collinsia heterophylla (Plantaginaceae) is a self-compatible, diploid annual endemic to the California Floristic Province (Newsom, 1929; Neese, 1993). It is widely distributed and grows in shady places and on dry slopes (<1000 m). Flowering occurs between March and June depending on latitude and elevation. A variety of native bees serve as pollinators (Armbruster et al., 2002; W. S. Armbruster, unpubl. res.). Estimates of mean population outcrossing rates range from 0·32 to 0·64, based on allozyme markers (Charlesworth and Mayer, 1995), and up to 0·94 ± 0·27 (s.d.) based on morphological markers (Weil and Allard, 1964). Flowers are arranged in whorls on spikes. The zygomorphic flowers have five-lobed corollas comprising one upper and one lower lip. Corolla colour is generally white to pale purple on the upper lip and dark purple on the lower lip, although some populations can be pale purple or off-white on both lips. Flowers have four epipetalous stamens and one pistil. Newly opened flowers have undehisced anthers and non-receptive stigmas. As flowers develop, the anthers dehisce usually one at a time over the course of 3–4 d. During this period, the stigma becomes receptive and the style elongates. This eventually places the stigma in contact with the dehisced anthers, and self pollination can occur (for a more detailed description, see Armbruster et al., 2002; see also Kalisz et al., 1999). Four days after flower opening, the length of the style (including the stigma) is 13·2 ± 0·96 mm (± s.d.; n = 19; Å. Lankinen and W. S. Armbruster, unpubl. res.). Pollen is binucleate (Schrock and Palser, 1967). Ovaries develop into dry, dehiscent seed capsules.
A rough estimation indicates that at least 50 pollen grains can be in direct contact with the stigmatic surface at the same time (Å. Lankinen and W. S. Armbruster, unpubl. res), and the stigma can hold more pollen in additional layers. Because ovaries contain on average 11·2 ovules (max = 16 ovules; coastal populations; Armbruster et al., 2002; J. Maad and W. S. Armbruster, unpubl. res.), it is probable that the number of pollen grains on the stigma often greatly exceeds the number of ovules, suggesting that pollen competition can occur.
The approx. 40 maternal families used in this study had pale-purple flowers and originated from a population in Sisar Canyon, Ventura County, California (Population 4 in Armbruster et al., 2002). Plants were raised from seeds and grown in an insect-free greenhouse in winter/spring in a greenhouse at the Norwegian University of Science & Technology, Trondheim, during two seasons (parental and offspring generation). Because seeds from the different maternal families were not kept separate, the possibility cannot be excluded that some of the plants may have been full or half siblings, but this likely mimics roughly the structure of a field population in this self-compatible, mixed-mating species.
Generation of offspring for estimates of heritability and relationships between pollen tube growth rate and fitness
In order to evaluate heritability of pollen-tube growth rate and the relationship between pollen-tube growth rate and offspring fitness, a series of one-donor crosses was conducted. In total, 12 donors and 11 recipient plants were used. Some individuals were utilized as both donors and recipients. Pollen donors were combined in six pairs depending on their pollen-tube growth rate in a germination medium (see next section). Each pair consisted of one fast-growing and one slow-growing donor (the fast donors differed significantly from the slow ones, P < 0·0001). The reason for using this design was that we wanted to maximize the difference in pollen performance between the two donors crossed with each recipient plant. Seven recipient plants received pollen from two donor pairs, and four recipients received pollen from four donor pairs. Each donor was crossed with four to six recipients (mean = 5). All crossing combinations were repeated once. The total sample size was: 6 donor pairs × 2 donors per donor pair × 5 recipients/donor × 2 crosses per recipient = 120 crosses. Crosses involving the two donors in a pair were performed on different flowers of the same recipient plant only a few minutes apart on the same day. Crossing order of a donor pair in relation to other pairs was changed between recipients so that crossing order was as random as possible.
Crosses were performed on emasculated, fully receptive flowers (4 d after flower opening). Pollen from two flowers from the same donor plant was mixed on a microscopic slide. Pollen was added to the stigma directly from the slide until the stigma was completely covered with pollen. Crosses were repeated if the cross failed. This happened in only 3·8 % of all crosses. A few days before seed capsules were ripe, they were bagged to avoid losing any seeds.
Collected seeds were counted, weighed and eventually sown. Seeds from a cross were sown together (five seeds or less per pot) in ordinary potting compost. The pots were placed in a refrigerator (4 °C) for approx. 1 week to break seed dormancy. Pots were then placed in the greenhouse. Supplemental lights were on for 16 h a day. At the age of 4 weeks, six randomly chosen offspring per crossing combination (three per replicate cross) were repotted in one pot each and saved for assessment of later fitness components.
Measurements of pollen-tube growth rate in germination medium and in pistil
To investigate if pollen donors used in the crosses differed in pollen tube growth rate and to compare this trait in parents and their offspring, pollen was germinated in Hoekstra medium (Hoekstra and Bruinsma, 1975) in a dark chamber at a constant temperature of 20–21 °C. Pollen from two flowers per individual was sprinkled sparsely onto a drop of medium. In order to estimate in vitro pollen-tube growth rate, pollen tubes were allowed to grow in the medium for 1·5 h and the lengths of ten pollen tubes per sample measured under a light microscope (pollen tubes can be seen clearly without stain). Tubes of recently germinated pollen (shorter than 0·15 mm, i.e. less than approx. 25 % of average length) were not measured in order to reduce the influence of germination time on the metric. The average of the ten tubes was used in subsequent statistical analyses.
In vitro measurements can sometimes be unreliable, but an advantage is that pollen performance can be evaluated without influence of the maternal tissue. This is particularly important in a species with delayed stigma receptivity, where other options of assessing pollen performance excluding maternal influence are more difficult (e.g. bud pollination, Cruzan, 1993; see also Lyons et al., 1989). Because pollen tubes that were allowed to grow over night continued growing and became longer than the length of the pistil (Å. Lankinen and W. S. Armbruster, pers. obs.), it is assumed that pollen of C. heterophylla utilized both stored and exogenous resources (comparable to uptake of nutrients from the pistil after initial germination and growth; Herrero and Hormaza, 1996). To evaluate further the accuracy of the in vitro measurements using the parental generation, (a) repeatability was tested at the same greenhouse temperature, and (b) in vitro growth compared to pollen-tube growth rate in receptive pistils (see below). In the parental generation, pollen from all donors was germinated at the same time to control for temperature differences (see below; conducted twice, the average of the two measurements being used in correlation tests).
Simultaneous germination of pollen was not possible in the offspring generation, however, because of the large number of offspring analysed. Instead, pollen was germinated in 13 different batches, each consisting of 25 samples (representing 25 individual plants). Three of the 25 individuals per batch were germinated in two additional germination batches. Using the mean of the repeatedly germinated donors made it possible to standardize values in order to make all 13 batches comparable to each other. This standardization worked well because donors did not vary markedly in their response to temperature (ANOVA interaction term not significant; performance at 25 °C versus approx. 20 °C was 0·505 versus 0·333 mm 1·5 h−1, respectively; two-way ANOVA (mixed model); temperature: F1,11·7 = 41·8, P < 0·001; pollen donor (random factor): F17,6·55 = 5·08, P = 0·021; interaction: F11,16 = 1,00, P = 0·485; Å. Lankinen and W. S. Armbruster, unpubl. res.). Pollen germination in the offspring generation was conducted over a period of 2 weeks. Individuals were assessed once in the order in which they started flowering (approx. 1–2 weeks after they started flowering).
To assess pollen performance in the pistil, pollen traits were evaluated in fully receptive pistils (i.e. 4 d after flower opening) across 20 pollen donors in the parental generation (including the 12 donors used in the crosses). Pistils were collected after 1·5 and 2 h, respectively, because it takes about 1–1·5 h or longer for pollen to germinate on the stigma (Å. Lankinen, pers. obs.). Because it is crucial to assess performance averaged over several pistils to be able to tell if in vitro measurements reflect performance in receptive pistils, pollen from each of the 20 donors was placed on stigmas of emasculated flowers on three (or more) of the 13 recipient plants. To compare pollen-tube growth rate of self and outcross pollen, self pollen was placed on one stigma of each pollen donor. Crosses were distributed fairly evenly across the recipients receiving outcross pollen (between two and nine donors per recipient). In a few cases the same crossing combination was replicated, and the average was used. Minimum sample size = 20 pollen donors × 4 recipients per donor = 80 crosses for each of the two collection times. Some data were missing for pollen-tube growth rate, as a result of excluding data points when there was only one of the two measurements of pollen tube length (after 1·5 and 2 h) and when both measurements were zero. Sample size for pollen tube growth rate was reduced to 20 pollen donors × 3·35 recipients per donor = 67 crosses.
Collected pistils were immediately stored in ethanol (70 %). To stain pollen tubes, pistils were rinsed with distilled water and tissues were softened in 1 m NaOH for 2–3 h. Pistils were then thoroughly rinsed under distilled water and stained in a dye solution of aniline blue in aqueous K3PO4 for at least 3 h.
The length of the longest pollen tube was measured under UV epifluorescence light. The maximum rather than the mean pollen-tube length per sample was used in this experiment, because it was only possible to distinguish tissue with or without pollen tubes (though it was fairly easy to determine the tip of the longest pollen tube). The in vivo value that was compared with the in vitro measurement was thus the mean value of the longest tube seen in each of several recipients. Even though in vitro and in vivo measurements are slightly different, a positive correlation between the two measurements would still indicate that tube growth rate in the artificial medium reflects how far down the style pollen-tubes will have grown. Pollen-tube growth rate was estimated as the difference in pollen tube length between 2 and 1·5 h.
Measurements of sporophytic traits
Sporophytic traits were measured in donors involved in one-donor crosses and in the resulting offspring. For the parental generation, seedling height was measured at 9 weeks, day of first flowering, and final plant height. In the offspring generation, seeds were counted and weighed to get an estimate of mean seed weight of the seeds that became the offspring generation. Total germination percentage was recorded, and seedling size measured at both 4 and 7 weeks. Early seedling size was estimated by measuring the width of the entire seedling (most seedlings only consisted of two pairs of leaves). Later seedling size was measured as the plant height times the total number of branches. In the year of the offspring generation, plant growth rate was much faster (probably because plants were grown later in the spring, hence experienced longer days). Seedling size at 9 weeks in the parents thus equalled the size somewhere between week 4 and week 7 in the offspring. The date of first flowering was noted. Number of flowers in the first inflorescence (main shoot) was measured as an indication of plant size. Seed production was investigated by counting seeds in three capsules per individual. Even though all seeds produced by an individual were not counted, it is likely that the two traits assessed, number of flowers on the main shoot and seeds per capsule, strongly influence life-time seed production.
Statistics
When testing the relationship between in vitro and in vivo pollen-tube growth rate, data were pooled from related and unrelated pistils in order to get as large a sample size as possible (because of the large measurement error in the pistil). No differences were found between pollen types; see Results. Even though the distribution of in vivo pollen-tube growth rate did not deviate from normal, the distribution of the error terms did. However, because the distribution of error terms was not skewed, this deviation should not be a serious problem (Sokal and Rohlf, 1995). It was not possible to improve the distribution by transformation, nor was an appropriate non-parametric test found.
Narrow-sense heritability (h2) of pollen-tube growth rate was estimated in vitro in a father–offspring regression (Falconer and McKay, 1996), using a hierarchical analysis [a nested ANOVA that takes differences in sample size (of paternal offspring groups) into account; Sokal and Rohlf, 1995]. The level of replication used to test the regression is not different from that in a more ordinary test involving the unweighted mean of each paternal offspring group. Because heritability may be an inaccurate assessment of the ability of a trait to evolve (for a detailed discussion see, for example, Houle, 1992; Hansen et al., 2003), evolvability (IA) was also estimated as the additive genetic variance (VA) scaled by the square of the trait mean (Z): IA = 100 × VA/Z2 = 100 × h2 × σ2T/Z2 (Hansen et al., 2003), where σ2T represents the total phenotypic variance.
When analysing relationships between pollen-tube growth rate of donors and quality of donors or offspring, we used pollen-tube growth rate in vivo rather than in vitro. The relationship between in vivo pollen-tube growth rate of donors and sporophytic quality of their offspring was evaluated using the same kind of hierarchical analysis as for the heritability estimates of pollen-tube growth rate (see above).
Differences were analysed between self- and outcross-pollen performance after 1·5 and 2 h with a mixed model, using pollination type (self or outcross) as a fixed factor and pollen donor and the interaction as random factors. Because the error terms deviated significantly from normality, the difference between self- and outcross-pollen performance was also tested with a non-parametric test (Wilcoxon signed rank test). This test showed the same result as the parametric test (P > 0·20 for both traits).
Most analyses were performed with SPSS (2004), specifically the GLM procedure for the ANOVAs. The GLM procedure uses weighted least squares to estimate model parameters and can deal with an unbalanced design. The Satterthwaite method was used to calculate the degrees of freedom in the mixed models. Type-III sums of squares were used in all ANOVAs.
RESULTS
Difference in pollen-donor performance in germination medium and pistils
Pollen started germinating within 15 min in the germination medium. Germination ability was generally high (>90 %). Pollen donors differed in pollen-tube growth rate in the medium (one-way ANOVA; F11,23 = 6·63, P = 0·0014). The donor with fastest growing pollen was on average 2·14 faster than the slowest donor. The repeatability of pollen-tube growth rate was high (strong correlation) in the same greenhouse temperature (Pearson correlation; r = 0·853, d.f. = 9, P = 0·008), suggesting high consistency of in vitro measurements.
Pollen from all donors had initiated pollen-tube growth in a majority of the pistils (79 %) after 1·5 h [mean tube-length 2·2 ± 0·86 (s.d.) mm]. At 2 h the number of pistils with pollen tubes present had not increased, but pollen tubes were significantly longer [mean tube-length 2·8 ± 1·6 (s.d.) mm; paired t-test; n = 20, P = 0·02]. Pollen-tube growth rate (difference in tube-length between 2 h and 1·5 h) showed large variation [mean 0·7 ± 2·1 (s.d.) mm 0·5 h−1], probably partly as a consequence of a large measurement error caused by the fact that different pistils were collected at 1·5 h and 2 h and hence were independent. The difference in tube-length between 2 h and 1·5 h ranged from negative to positive values, which means that the mean value may be unreliable (probably an underestimate). Growth rate of the fastest donor was 3·35 mm 0·5 h−1, but it was not possible to get a reliable estimate of the absolute performance of the slowest donor as the slowest donor could not have had a negative value. The fastest pollen donor should grow to the base of the style (13 mm) in 3–3·5 h (including 1–1·5 h of germination), assuming growth rate is constant through the style. Preliminary data indicated that 46 % of pistils had pollen tubes at the base of the style 3 h after pollination and 93 % had pollen tubes at the base after 4 h (Å. Lankinen, W. S. Armbruster, unpubl. res.). It is thus probable that tube growth rate is slightly higher nearer the base of the style.
Despite the large variation in the in vivo estimates, the pollen-tube growth rates in germination medium and in pistils were correlated (Pearson correlation; r = 0·448, d.f. = 18, P = 0·048), suggesting that growth rate in medium reflects the average growth rate in the 3·35 recipient plants per donor. This result also indicates probable in vivo differences among donors. There was an even stronger relationship between pollen-tube growth rates of the fastest tube in the medium and in vivo measurements (Pearson correlation; r = 0·575, d.f. = 18, P = 0·008), presumably reflecting that this in vitro measurement was more similar to what was actually measured in the pistil. In the medium, measurements of the longest tube and the average of ten tubes were strongly correlated (Pearson correlation; r = 0·931, d.f. = 18, P << 0·0001). Pollen-tube growth rate was on average 3·37 times faster in pistils than in medium (paired t-test; n = 20, P = 0·11).
Heritability and evolvability of pollen tube growth rate in vitro
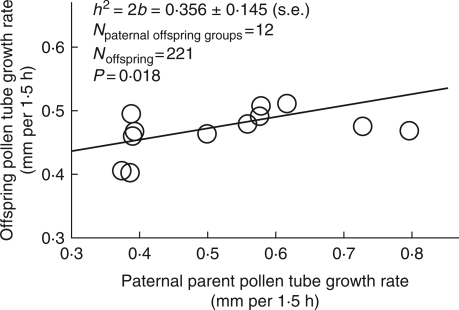
The father–offspring regression coefficient for pollen-tube growth rate in vitro was significant (Fig. 1). This indicates that there is additive genetic variation for this trait (narrow-sense h2 (± s.e.) = 0·356 ± 0·145; P = 0·018; n = 12 fathers, 221 offspring).
Fig. 1.
Father–offspring regression calculated with a hierarchical analysis indicated narrow-sense heritability (h2) of in vitro pollen tube growth rate in Collinsia heterophylla. b = regression coefficient.
Evolvability, IA, of pollen-tube growth rate was calculated to be 3·8 % (IA = 100 × VA /Z2 = 100 × h2 × σ2T/Z2 = 100 × 0·356 × 0·02372/0·4712). The regression between mothers and offspring did not, however, have a significant slope [hierarchical analysis; h2 = 2 × b = 0·161 ± 0·234 (s.e.), P = 0·50; nmaternal offspring groups = 11, noffspring = 221], suggesting maternal effects cancelled out or swamped additive genetic effects.
Quality of donors and offspring in relation to pollen-tube growth rate
No significant relationships between pollen-tube growth rate in pistils and sporophytic traits were detected in pollen donors of the parental generation (Table 1). Similarly, no significant correlations were detected between pollen-tube growth rate in vitro and sporophytic traits of individuals in the offspring generation (Table 2).
Table 1.
Correlations between pollen tube growth rate* (mm 0·5 h−1) in vivo and sporophytic traits of pollen donors of parental generation in Collinsia heterophylla
| Correlated trait | r | d.f. | P |
|---|---|---|---|
| Seedling height, 9 weeks (cm) | 0·431 | 18 | 0·058 |
| Day of first flowering | −0·234 | 18 | 0·32 |
| Final plant height (cm) | −0·266 | 18 | 0·26 |
| No. of seeds per capsule | 0·378 | 9 | 0·25 |
* Estimated as the difference in pollen tube length between 2 and 1·5 h.
Table 2.
Relationships between pollen-tube growth rate in vitro and sporophytic traits in plants of offspring generation in Collinsia heterophylla, calculated with partial correlations controlling for relatedness of siblings
| Correlated trait | rp | nsibling groups (noffspring) | P |
|---|---|---|---|
| Mean seed weight (mg) | 0·029 | 47 (47) | 0·85 |
| Proportion of seeds germinated* | 0·105 | 47 (47) | 0·48 |
| Seedling size, 4 weeks (cm) | −0·070 | 47 (219) | 0·30 |
| Seedling size, 7 weeks (cm) | −0·104 | 47 (218) | 0·125 |
| Day of first flowering | 0·129 | 47 (218) | 0·057 |
| No. of flowers (1st inflorescence) | −0·090 | 47 (217) | 0·19 |
| No. of seeds per capsule | 0·0033 | 45 (121) | 0·97 |
rp = partial correlation coefficient.
* Arcsine transformed.
Between generations, pollen-tube growth rate was significantly correlated with only one offspring trait: there was a negative correlation with the earliest measurement of seedling size (Table 3). This correlation was not significant, however, after correcting for multiple comparisons.
Table 3.
Sporophytic offspring traits regressed on pollen-tube growth rate in vivo of their fathers calculated with hierarchical analysis in Collinsia heterophylla
| Pollen tube growth rate (mm 0·5 h−1)* |
|||
|---|---|---|---|
| Offspring trait | npaternal offspring groups (noffspring) | b | P |
| Mean seed weight (mg) | 12 (128) | 0·00001 | 0·90 |
| Proportion of seeds germinated† | 12 (128) | 0·038 | 0·44 |
| Seedling size, 4 weeks (cm) | 12 (292) | − 2·21 | 0·0078 |
| Seedling size, 7 weeks (cm) | 12 (292) | 3·82 | 0·25 |
| Day of first flowering | 12 (287) | −0·076 | 0·90 |
| No. of flowers (1st inflorescence) | 12 (289) | −0·223 | 0·62 |
| No. of seeds per capsule | 12 (162) | 0·305 | 0·36 |
Variation among paternal offspring groups was significant for all investigated traits (test not shown). b = regression coefficient; significant (P < 0·05) correlation coefficients are in bold (no significant P values were found when correcting for multiple comparisons using Bonferroni).
* Estimated as the difference in pollen tube length between 2 and 1·5 h.
† Arcsine transformed.
Pollen performance of self and outcross pollen in vivo
Pollen tubes were present more often in pistils pollinated with self pollen than pistils pollinated with outcross pollen (Wilcoxon signed ranks test: self, 25 out of 26; outcross, 99 out of 134; T+ = 46, n = 10, P = 0·032). Likewise, pollen was better at germinating on pistils of the donor individual (‘self pistils’) than on unrelated pistils (Wilcoxon signed ranks test: self, 42 out of 46; outcross, 108 out of 144; T+ = 115·5, n = 17, P = 0·010). Pollen-tube growth rate did not differ significantly between pollination types (self vs. cross): mean unrelated (± s.d.) = 0·78 (± 1·7); mean self (± s.d.) = 0·72 (± 2·0) mm (Table 4).
Table 4.
Mixed model ANOVA for pollen-tube growth rate in vivo (mm 0·5 h−1)* when pollen from a donor (random effect) was used in crosses with itself or with up to three unrelated plants (pollination type, fixed effect) in Collinsia heterophylla
| Source of variation | d.f. | F | P |
|---|---|---|---|
| Pollination type | 1 | 0·007 | 0·94 |
| Donor | 16 | 0·635 | 0·81 |
| Pollination type × donor | 16 | 1·42 | 0·21 |
| Error | 25 |
* Estimated as the difference in pollen tube length between 2 h and 1·5 h.
DISCUSSION
Pollen-tube growth rate in vitro differed among pollen donors in Collinsia heterophylla. This gametophytic (pollen) trait also correlated with average in vivo growth rates on the two to four flowers receiving the pollen. Narrow-sense heritability and evolvability of pollen-tube growth rate in vitro were significant, as calculated by father–offspring regression. However, no positive relationships between pollen-tube growth rate and sporophytic fitness was found in either the parental or offspring generation.
Differences among pollen donors in pollen competitive ability
Pollen traits can vary among pollen donors, and such variation can generate differences in siring success (e.g. Snow and Spira, 1996; Skogsmyr and Lankinen, 1999). In some species, however, recipient plants influence pollen competitive ability so that the rank order of donors is inconsistent across recipients (sire–dam interaction); this is often connected to self-incompatibility, inbreeding-depression or outbreeding depression (e.g. Fenster and Sork, 1988; Cruzan, 1990; Johnston, 1993; but see Marshall, 1998). A pollen trait can, however, be selected for even when there is female influence, provided the trait confers a high average pollen competitive ability. In this study of C. heterophylla, measurement error of pollen-tube growth rate in the pistil was large. This was presumably at least partly caused by the large variation in pollen-germination time in the pistil in combination with the use of two different pistils (collected at two time intervals) to assess pollen tube growth rate. Nevertheless, in vitro pollen-tube growth rate of donors correlated with average in vivo growth rate across multiple recipients. This suggests that the variation in pollen tube growth rate among donors was on average consistent across maternal plants.
Finding large variation in pollen-germination time may indicate that the ability to germinate fast may be more important for siring success than inherent differences in pollen tube growth rate. A numerical comparison of the average length of fully receptive stigmas and styles with the maximum difference in pollen tube growth rate between donors in C. heterophylla (fast/slow = 2·14, provided the in vitro ratio was similar to the in vivo ratio), indicated that the fastest donor will reach the base of the style 2·2 h sooner than the slowest donor [2 h (fast)–4·2 h (slow)]. Thus the variation among donors in when pollen tubes reach ovules should be considerably greater than the variation in germination time (maximum is approx. 0·5 h). It should thus be possible for fast-growing donors to overtake slow-growing ones, at least when donors are forced to start germinating on the stigma at a similar time. Because stigma receptivity is delayed in C. heterophylla and an unreceptive stigma can collect pollen for several days (Armbruster et al., 2002; Lankinen et al., 2007), it is likely that arrival time of pollen has little effect on reproductive success.
Heritability and evolvability of pollen tube growth rate
Heritable variation of pollen performance was not detected in Raphanus raphanistrum (Snow and Mazer, 1988). Similarly in Oenothera organensis, only a small portion (9 %) of the variation in pollen tube growth rate was explained by the genetic component (Havens, 1994). More recent studies have, however, detected higher heritabilities of pollen traits likely associated with performance: pollen-tube growth rate (49 % in Viola tricolor; Skogsmyr and Lankinen, 2000), pollen-grain size (19–40 % across three populations of Mimulus guttatus; Lamborn et al., 2005), and (probably) pollen-germination rate (36 % in intra- and inter-population crosses across six populations of Silene latifolia; Jolivet and Bernasconi, 2007). It is, of course, not certain that the last two traits are related to pollen competitive ability. The present estimate in C. heterophylla indicates that narrow-sense heritability of pollen tube growth rate was approx. 36 %, a value within the above range. The present estimate of evolvability indicates that the trait will change 3·8 % per generation in response to a standardized selection gradient of 1 (i.e. selection as strong as selection on fitness itself; see Hansen et al., 2003). In comparison, evolvabilities ranged between 0·01 % and 1·71 % for 24 floral traits in Dalechampia (Hansen et al., 2003) and between 0·16 % and 2·24 % for 11 floral characters in Campanula rotundifolia (J. Maad and W. S. Armbruster, unpubl. res.). The present value was surprisingly high, given that pollen-tube growth rate has been expected to have little evolutionary potential (e.g. Charlesworth and Charlesworth, 1992), in comparison to floral morphological traits.
Because temperature affects pollen-tube growth rate in C. heterophylla, and because only one (standard) temperature was considered in the present study, the estimate of heritability might be higher (or lower) than under field conditions. It seems unlikely, however, that the evolvability is overestimated for this reason, because this estimate of evolvability is expected to be largely unaffected by environmental variance (Houle, 1992; Hansen et al., 2003). In future studies, it will be important to confirm that measurements of pollen-tube growth rate are related to siring ability in this species, as in some others (e.g. Snow and Spira, 1991, 1996; Marshall, 1998; Skogsmyr and Lankinen, 1999; Aronen et al., 2002), because selection only acts on a heritable trait if it confers higher survival or reproductive success.
Potential benefits of more intense pollen competition: reduced inbreeding depression or higher-quality fathers?
Delayed stigma receptivity may enhance pollen competition in C. heterophylla (Armbruster et al., 2002; Lankinen et al., 2007; see Galen et al., 1986). This raises the question of what potential benefits may accrue from more intense pollen competition. A previous study showed that a possible advantage of pollen competition in this self-compatible species is reduced inbreeding depression (Lankinen and Armbruster, 2007; see also Armbruster and Rogers, 2004). The current study was designed to test the potential benefit of higher offspring fitness when fertilization is by pollen from sporophytes with more competitive pollen (see Mulcahy, 1979). If it is advantageous to be fertilized by pollen that is more competitive, relationships between pollen competitive ability and vigour, both within and between generations, would be expected (cf. good-genes hypothesis; Zahavi, 1975; see reviews by Andersson, 1994; Skogsmyr and Lankinen, 2002). In inbred lines of Zea mays, Mulcahy (1971) found a positive correlation between relative pollen-tube growth rate of two competitors and relative seed weight in the next generation. Because one-donor crosses did not result in the same correlation and because seed weight is not usually influenced by paternal genotype, competitive interactions between developing seeds probably caused the positive correlation. Such effects may or may not be connected with higher offspring fitness. Following one-donor crosses in Betula pendula, donors with higher pollen-tube growth rate sired offspring with heavier seeds, but not larger seedlings (Pasonen et al., 2001). In Viola tricolor, on the other hand, more competitive pollen gave rise to offspring that themselves produced more seeds (Skogsmyr and Lankinen, 2000). No support was found in C. heterophylla for the predictions that donors with faster-growing pollen have greater sporophytic vigour or that their sporophytic offspring show higher fitness in any part of the life-cycle. The power (sample size) could, however, have been too low to detect real relationships, or it is possible that differences in offspring quality were not expressed in greenhouse conditions (see Kalla and Ashman, 2002). Maternal effects (Roach and Wulff, 1987) or effects of maternal condition could also have masked relationships between generations. In Raphanus sativus, for example, pollen donors with relatively higher pollen-competitive ability on young maternal plants produced earlier-maturing offspring, but this relationship was not seen on older maternal plants (Marshall et al., 2007).
The only apparent benefit of being fathered by donors with high pollen-tube growth rates detected in C. heterophylla is that the offspring themselves also have high pollen-tube growth rates. It is worth noting, as has been pointed out recently, that a negative correlation between reproductive success and viability may result if ‘male attractiveness’ is increased at the expense of viability (Kokko, 2001). In this case a negative correlation between reproductive success and viability may still be connected to higher total offspring fitness (including offspring fitness through male reproductive success). Interestingly, in another study on C. heterophylla, pollen donor identity affected how early during floral development seeds could be sired (Lankinen and Kiboi, 2007). Pollen donors that were able to fertilize seeds early had higher germination speed, possibly indicating that competitive ability of donors differ depending on how receptive the stigma is. In addition to investigating how pollen traits are related to siring success on fully receptive pistils, in the future it would also be interesting to study what determines pollen competitive ability at earlier stages of floral development.
A further partial test of an additional potential benefit of enhanced pollen competition was made: cryptic self-incompatibility, leading to a higher proportion of out-crossed offspring (see Bertin and Sullivan, 1988). However, the present results suggested that cross-pollen does not perform better than self-pollen in the pistil. (Indeed self-pollen can sometimes perform better than outcross pollen, based on germination percentage on fully receptive stigmas.) On the other hand, the limitations of the present in vivo measurements (it was only possible to measure the longest pollen tube) made it hard to compare average pollen tube lengths of the two pollen types. It is also important to note that only the outcome for the two pollen types in separate pistils was investigated. It remains a distinct possibility that outcross pollen performs better only when both pollen types are growing together in the pistil (Aizen et al., 1990; Nemeth and Smith-Huerta, 2002; Kruszewski and Galloway, 2006). Because C. heterophylla has a delayed selfing mechanism (Armbruster et al., 2002), it is possible that selfing mostly occurs when pollinators failed to visit, i.e. selfing will mainly be beneficial in terms of reproductive assurance. This could potentially lead to weak selection for pistil mechanisms that reduce the performance of self pollen.
Concluding remarks
Provided that pollen-tube growth rate influences pollen competitive ability in Collinsia heterophylla as in other plants, the degree of phenotypic and heritable variation reported here indicates that this trait can respond to sexual selection on the male reproductive function. The present data do not, however, suggest an advantage for the female reproductive function of favouring sporophytic fathers with fast-growing tubes, apart from the gametophytic benefit in the next generation (i.e. offspring that themselves produce pollen with faster-growing tubes). The present data, though incomplete, suggest an absence of cryptic self-incompatibility in this species and that the benefit of promoting pollen competition is unrelated to the ability to favour outcross pollen over self pollen. However, studies comparing self and outcross pollen growing simultaneously in the same pistils are necessary before this possible benefit can be ruled out. It is important to note that the present results do not exclude the possibility that pollen competition generates an advantage to female function in terms of higher offspring quality. If the pollen load is small, ovules are likely to be fertilized by lower-quality pollen (independent of pollen donor quality). Thus features that increase the size of the pollen load should increase female reproductive fitness, as has been shown in numerous previous studies (e.g. Johannsson and Stephenson, 1997; Quesada et al., 2001; Davis, 2004). The fitness advantage of larger pollen loads may be even stronger when the pollen load only consists of self pollen (Armbruster and Rogers, 2004). Indeed, the only detected advantage of enhanced pollen competition in C. heterophylla thus far, is the ability to reduce inbreeding depression (by increasing the relative quality of selfed offspring), as revealed by varying the size and composition of the pollen load (Lankinen and Armbruster, 2007). In future studies, it will be important to assess whether the main advantage of large pollen loads is a larger sample of superior donors and/or genetically more diverse pollen (Bernasconi et al., 2003; Kron and Husband, 2006) rather than more intense pollen competition per se (but see Armbruster and Rogers, 2004).
ACKNOWLEDGEMENTS
We wish to thank A. Stephenson and an anonymous reviewer for useful comments on the text. This work was supported by European Science Foundation (Plant Adaptation programme); the Knut and Alice Wallenberg Foundation; Marie Curie Individual Fellowship; the Norwegian Research Council; the US National Science Foundation (grant number DEB-0324808), the Royal Swedish Academy of Sciences and the Swedish Research Council.
LITERATURE CITED
- Aizen MA, Searcy KB, Mulcahy DL. Among-flower and within-flower comparisons of pollen-tube growth following self-pollinations and cross-pollinations in Dianthus chinensis (Caryophyllaceae) American Journal of Botany. 1990;77:671–676. [Google Scholar]
- Andersson M. Sexual selection. Princeton, NJ: Princeton University Press; 1994. [Google Scholar]
- Armbruster WS, Rogers DG. Does pollen competition reduce the cost of inbreeding? American Journal of Botany. 2004;91:1939–1943. doi: 10.3732/ajb.91.11.1939. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Mulder CPH, Baldwin BG, Kalisz S, Wessa B, Nute H. Comparative analysis of late floral development and mating-system evolution in tribe Collinsiae (Scrophulariaceae s.l.) American Journal of Botany. 2002;89:37–49. doi: 10.3732/ajb.89.1.37. [DOI] [PubMed] [Google Scholar]
- Aronen T, Nikkanen T, Harju A, Tiimonen H, Häggman H. Pollen competition and seed-siring success in Picea abies. Theoretical and Applied Genetics. 2002;104:638–642. doi: 10.1007/s00122-001-0789-9. [DOI] [PubMed] [Google Scholar]
- Bell G. On the function of flowers. Proceedings of the Royal Society of London Series B: Biological Sciences. 1985;224:223–265. [Google Scholar]
- Bernasconi G, Paschke M, Schmid B. Diversity effects in reproductive biology. Oikos. 2003;102:217–220. [Google Scholar]
- Bernasconi G, Ashman T-L, Birkhead TR, et al. Evolutionary ecology of the pre-zygotic stage. Science. 2004;303:971–974. doi: 10.1126/science.1092180. [DOI] [PubMed] [Google Scholar]
- Bertin RI. Effects of pollination intensity in Campsis radicans. American Journal of Botany. 1990;77:178–187. doi: 10.1002/j.1537-2197.1990.tb13544.x. [DOI] [PubMed] [Google Scholar]
- Bertin RI, Sullivan M. Pollen interference and cryptic self-fertility in Campsis radicans. American Journal of Botany. 1988;75:1140–1147. [Google Scholar]
- Campbell DR. Measurements of selection in a hermaphroditic plant: variation in male and female pollination success. Evolution. 1989;43:318–334. doi: 10.1111/j.1558-5646.1989.tb04230.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. The effects of selection in the gametophyte stage on mutational load. Evolution. 1992;46:703–720. doi: 10.1111/j.1558-5646.1992.tb02077.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Mayer SS. Genetic variability of plant characters in the partial inbreeder Collinsia heterophylla (Scrophulariaceae) American Journal of Botany. 1995;82:112–120. [Google Scholar]
- Charlesworth D, Schemske DW, Sork VL. The evolution of plant reproductive characters; sexual versus natural selection. In: Bradbury JW, Andersson MB, editors. Sexual selection: testing the alternatives. New York, NY: Wiley; 1987. pp. 317–335. [DOI] [PubMed] [Google Scholar]
- Cruzan MB. Pollen-pollen and pollen-style interactions during pollen tube growth in Erythronium grandiflorum (Liliaceae) American Journal of Botany. 1990;77:116–122. [Google Scholar]
- Cruzan MB. Analysis of pollen-style interactions in Petunia hybrida – the determination of variance in male reproductive success. Sexual Plant Reproduction. 1993;6:275–281. [Google Scholar]
- Davis SL. Natural levels of pollination intensity and effects of pollen loads on offspring quality in females of Thalictrum pubescens (Ranunculaceae) Plant Systematics and Evolution. 2004;244:45–54. [Google Scholar]
- Delph LF, Ashman T-L. Trait selection in flowering plants: how does sexual selection contribute? Integrative and Comparative Biology. 2006;46:465–472. doi: 10.1093/icb/icj038. [DOI] [PubMed] [Google Scholar]
- Delph LF, Weinig C, Sullivan K. Why fast-growing pollen tubes give rise to vigorous progeny: the test of a new mechanism. Proceedings of the Royal Society of London Series B: Biological Sciences. 1998;265:935–939. [Google Scholar]
- Falconer DS, MacKay TFC. Introduction to quantiative genetics. Harlow: Longman; 1996. [Google Scholar]
- Fenster CB, Sork VL. Effect of crossing distance and male parent on in vivo pollen tube growth in Chamaecrista fasciculata. American Journal of Botany. 1988;75:1898–1903. [Google Scholar]
- Fisher RA. The genetical theory of natural selection. Oxford: Clarendon Press; 1958. [Google Scholar]
- Galen C, Shykoff JA, Plowright RC. Consequences of stigma receptivity schedules for sexual selection in flowering plants. American Nauralist. 1986;127:462–476. [Google Scholar]
- Hansen TF, Pelabon C, Armbruster WS, Carlson ML. Evolvability and genetic constraint in Dalechampia blossoms: components of variance and measures of evolvability. Journal of Evoutionary Biology. 2003;16:754–766. doi: 10.1046/j.1420-9101.2003.00556.x. [DOI] [PubMed] [Google Scholar]
- Havens K. Clonal repeatability of in vitro pollen tube growth rates in Oenothera organensis (Onagraceae) American Journal of Botany. 1994;81:161–165. [Google Scholar]
- Herrero H, Hormaza JI. Pistil strategies controlling pollen tube growth. Sexual Plant Reproduction. 1996;9:343–347. [Google Scholar]
- Hoekstra FA, Bruinsma J. Respiration and vitality of binucleate and trinucleate pollen. Physiologia Plantarum. 1975;34:221–225. [Google Scholar]
- Hormaza JI, Herrero M. Gametophytic competition and selection. In: Williams EG, Clarke AE, Knox RB, editors. Genetic control of self-incompatibility and reproductive development in flowering plants. Dordrecht: Kluwer; 1994. pp. 372–400. [Google Scholar]
- Houle D. Comparing evolvability and variability of quantitative traits. Genetics. 1992;130:195–204. doi: 10.1093/genetics/130.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsson MH, Stephenson AG. Effects of pollination intensity on the vigor of the sporophytic and gametophytic generation of Curcubita texana. Sexual Plant Reproduction. 1997;10:236–240. [Google Scholar]
- Johnston MO. Tests of two hypotheses concerning pollen competition in a self-compatible, long-styled species (Lobelia cardinalis: Lobeliaceae) American Journal of Botany. 1993;80:1400–1406. [Google Scholar]
- Jolivet C, Bernasconi G. Within/between population crosses reveal genetic basis for siring success in Silene latifolia (Caryophyllaceae) Journal of Evolutionary Biology. 2007;20:1361–1374. doi: 10.1111/j.1420-9101.2007.01344.x. [DOI] [PubMed] [Google Scholar]
- Kalisz S, Vogler D, Fails B, et al. The mechanism of delayed selfing in Collinsia verna (Scrophulariaceae) American Journal of Botany. 1999;86:1239–1247. [PubMed] [Google Scholar]
- Kalla SE, Ashman T-L. The effects of pollen competition on progeny vigor in Fragaria virginiana (Rosaceae) depend on progeny growth environment. International Journal of Plant Sciences. 2002;163:335–340. [Google Scholar]
- Kokko H. Fisherian and ‘good genes’ benefits of mate choice: how (not) to distinguish between them. Ecology Letters. 2001;4:322–326. [Google Scholar]
- Kokko H, Brooks R, Jennions MD, Morley J. The evolution of mate choice and mating biases. Proceedings of the Royal Society of London Series B: Biological Sciences. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron P, Husband BC. The effects of pollen diversity on plant reproduction: insights from apple. Sexual Plant Reproduction. 2006;19:125–131. [Google Scholar]
- Kruszewski LJ, Galloway LF. Explaining outcrossing rate in Campanulastrum americanum (Campanulaceae): geitonogamy and cryptic self-incompatibility. International Journal of Plant Sciences. 2006;167:455–461. [Google Scholar]
- Lamborn E, Cresswell JE, Macnair MR. The potential for adaptive evolution of pollen grain size in Mimulus guttatus. New Phytologist. 2005;167:289–296. doi: 10.1111/j.1469-8137.2005.01403.x. [DOI] [PubMed] [Google Scholar]
- Lankinen Å, Armbruster WS. Pollen competition reduces inbreeding depression in Collinsia heterophylla (Plantaginaceae) Journal of Evoutionary Biology. 2007;20:737–749. doi: 10.1111/j.1420-9101.2006.01233.x. [DOI] [PubMed] [Google Scholar]
- Lankinen Å, Kiboi S. Pollen-donor identity affects timing of stigma receptivity in Collinsia heterophylla (Plantaginaceae): a sexual conflict during pollen competition? American Naturalist. 2007;170:854–863. doi: 10.1086/522839. [DOI] [PubMed] [Google Scholar]
- Lankinen Å, Armbruster WS, Antonsen L. Delayed stigma receptivity in Collinsia heterophylla (Plantaginaceae): genetic variation and adaptive significance in relation to pollen competition, delayed self-pollination, and mating-system evolution. American Journal of Botany. 2007;94:1183–1192. doi: 10.3732/ajb.94.7.1183. [DOI] [PubMed] [Google Scholar]
- Lyons EE, Waser NM, Price MV, Antonovics J, Motten AF. Sources of variation in plant reproductive success and implications for concepts of sexual selection. American Naturalist. 1989;134:409–433. [Google Scholar]
- Maad J, Alexandersson R. Variable selection in Platanthera bifolia (Orchidaceae): phenotypic selection differed between sex functions in a drought year. Journal of Evolutionary Biology. 2004;17:642–650. doi: 10.1111/j.1420-9101.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- Maad J, Nilsson LA. On the mechanism of floral shifts in speciation: gained pollination efficiency from tongue- to eye-attachment of pollinia in Platanthera (Orchidaceae) Biological Journal of the Linnean Society. 2004;83:481–495. [Google Scholar]
- Marshall DL. Pollen donor performance can be consistent across maternal plants in wild radish (Raphanus sativus, Brassicaceae): a necessary condition for the action of sexual selection. American Journal of Botany. 1998;85:1389–1397. [PubMed] [Google Scholar]
- Marshall DL, Reynolds J, Abrahamson NJ, et al. Do differences in plant and flower age change mating patterns and alter offspring fitness in Raphanus sativus (Brassicaceae)? American Journal of Botany. 2007;94:409–418. doi: 10.3732/ajb.94.3.409. [DOI] [PubMed] [Google Scholar]
- Mulcahy DL. A correlation between gametophytic characters in Zea mays L. Science. 1971;171:1155–1156. doi: 10.1126/science.171.3976.1155. [DOI] [PubMed] [Google Scholar]
- Mulcahy DL. The rise of the angiosperms: a genecological factor. Science. 1979;206:20–23. doi: 10.1126/science.206.4414.20. [DOI] [PubMed] [Google Scholar]
- Mulcahy DL, Mulcahy GB. The influence of gametophytic competition on sporophytic fitness in Diantus chinensis. Theoretical and Applied Genetics. 1975;46:277–280. doi: 10.1007/BF00281149. [DOI] [PubMed] [Google Scholar]
- Mulcahy DL, Mulcahy GB, Searcy KB. Evolutionary genetics of pollen competition. In: Wyatt R, editor. Ecology and evolution of plant reproduction. New York: Chapman and Hall; 1992. pp. 25–36. [Google Scholar]
- Mulcahy DL, Sari-Gorla M, Bergamini Mulcahy GB. Pollen selection – past, present and future. Sexual Plant Reproduction. 1996;9:353–356. [Google Scholar]
- Neese EC. Collinsia. In: Hickman JC, editor. The Jepson manual: higher plants of California. Berkeley, CA: University of California Press; 1993. pp. 1024–1027. [Google Scholar]
- Nemeth MB, Smith-Huerta NL. Effects of pollen load composition and deposition pattern on pollen performance in Clarkia unguiculata (Onagraceae) International Journal of Plant Sciences. 2002;163:795–802. [Google Scholar]
- Newsom VM. A revision of the genus Collinsia. Botanical Gazette. 1929;87:260–231. [Google Scholar]
- Ottaviano E, Sari-Gorla M, Villa M. Pollen competitive ability in maize: within population variability and response to selection. Theoretical and Applied Genetics. 1988;76:601–608. doi: 10.1007/BF00260915. [DOI] [PubMed] [Google Scholar]
- Paschke M, Abs C, Schmid B. Effects of population size and pollen diversity on reproductive success and offspring size in the narrow endemic Cochlearia bavarica (Brassicaceae) American Journal of Botany. 2002;89:1250–1259. doi: 10.3732/ajb.89.8.1250. [DOI] [PubMed] [Google Scholar]
- Pasonen HL, Pulkkinen P, Käpylä M. Do pollen donors with fastest-growing pollen tubes sire the best offspring in an anemophilous tree, Betula pendula (Betulaceae)? American Journal of Botany. 2001;88:854–860. [PubMed] [Google Scholar]
- Quesada M, Winsor JA, Stephenson AG. Effects of pollen competition on progeny performance in a heterozygous cucurbit. American Naturalist. 1993;142:694–706. doi: 10.1086/285564. [DOI] [PubMed] [Google Scholar]
- Quesada M, Fuchs EJ, Lobo JA. Pollen load size, reproductive success, and progeny kinship of naturally pollinated flowers of the tropical dry forest tree Pachira quinata (Bombacaceae) American Journal of Botany. 2001;88:2113–2118. [PubMed] [Google Scholar]
- Richards AJ. Plant breeding systems. London: Chapman and Hall; 1997. [Google Scholar]
- Roach DA, Wulff RD. Maternal effects in plants. Annual Review of Ecology and Systematics. 1987;18:209–235. [Google Scholar]
- Sarkissian TS, Harder LD. Direct and indirect responses to selection on pollen size in Brassica rapa L. Journal of Evolutionary Biology. 2001;14:456–468. [Google Scholar]
- Schlichting CD, Stephenson AG, Small LE. Pollen loads and progeny vigor in Cucurbita pepo: the next generation. Evolution. 1990;44:1358–1372. doi: 10.1111/j.1558-5646.1990.tb05238.x. [DOI] [PubMed] [Google Scholar]
- Schrock GF, Palser BF. Floral development, anatomy, and embryology of Collinsia heterophylla with some notes on ten other species of Collinsia and on Tonella tenella. Botanical Gazette. 1967;128:83–104. [Google Scholar]
- Skogsmyr I, Lankinen Å. Selection on pollen competitive ability in relation to stochastic factors influencing pollen deposition. Evolutionary Ecology Research. 1999;1:971–985. [Google Scholar]
- Skogsmyr I, Lankinen Å. Potential selection for female choice in Viola tricolor. Evolutionary Ecology Research. 2000;2:965–979. [Google Scholar]
- Skogsmyr I, Lankinen Å. Sexual selection: an evolutionary force in plants? Biological Review. 2002;77:537–562. doi: 10.1017/s1464793102005973. [DOI] [PubMed] [Google Scholar]
- Snow AA. Postpollination selection and male fitness in plants. American Naturalist. 1994;144(suppl.):69–83. [Google Scholar]
- Snow AA, Mazer SJ. Gametophytic selection in Raphanus raphanistrum: a test for heritable variation in pollen competitive ability. Evolution. 1988;42:1065–1075. doi: 10.1111/j.1558-5646.1988.tb02524.x. [DOI] [PubMed] [Google Scholar]
- Snow AA, Spira TP. Pollen vigour and the potential for sexual selection in plants. Nature. 1991;352:796–797. [Google Scholar]
- Snow AA, Spira TP. Pollen-tube competition and male fitness in Hibiscus moescheutos. Evolution. 1996;50:1866–1870. doi: 10.1111/j.1558-5646.1996.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3rd edn. New York, NY: W. H. Freeman and Co; 1995. [Google Scholar]
- SPSS. SPSS for Windows. Chicago, IL: SPSS; 2004. release 13.0. [Google Scholar]
- Walsh NE, Charlesworth D. Evolutionary interpretations of differences in pollen tube growth rates. Quarterly Review of Biology. 1992;67:19–37. [Google Scholar]
- Weil J, Allard RW. The mating system and genetic variability in natural populations of Collinsia heterophylla. Evolution. 1964;18:515–525. [Google Scholar]
- Willson MF. Sexual selection in plants. American Naturalist. 1979;133:777–790. [Google Scholar]
- Winsor JA, Davis LE, Stephenson AG. The relationship between pollen load and fruit maturation and the effect of pollen load on offspring vigor in Cucurbita pepo. American Naturalist. 1987;129:643–656. [Google Scholar]
- Zahavi A. Mate selection – a selection for a handicap. Journal of Theoretical Biology. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]