Abstract
Background and Aims
The evolution of selfing from outcrossing is characterized by a series of morphological changes to flowers culminating in the selfing syndrome. However, which morphological traits initiate increased self-pollination and which are accumulated after self-fertilization establishes is poorly understood. Because the expression of floral traits may depend on the conditions experienced by an individual during flower development, investigation of changes in mating system should also account for environmental and developmental factors. Here, early stages in the evolution of self-pollination are investigated by comparing floral traits among Brazilian populations of Eichhornia paniculata (Pontederiaceae), an annual aquatic that displays variation in selfing rates associated with the breakdown of tristyly to semi-homostyly.
Methods
Thirty-one Brazilian populations under uniform glasshouse conditions were compared to investigate genetic and environmental influences on flower size and stigma–anther separation (herkogamy), two traits that commonly vary in association with transitions to selfing. Within-plant variation in herkogamy was also examined and plants grown under contrasting environmental conditions were compared to examine to what extent this trait exhibits phenotypic plasticity.
Key Results
In E. paniculata a reduction in herkogamy is the principal modification initiating the evolution of selfing. Significantly, reduced herkogamy was restricted to the mid-styled morph and occurred independently of flower size. Significant genetic variation for herkogamy was detected among populations and families, including genotypes exhibiting developmental instability of stamen position with bimodal distributions of herkogamy values. Cloned genets exposed to contrasting growth conditions demonstrated environmental control of herkogamy and genotypic differences in plasticity of this trait.
Conclusions
The ability to modify herkogamy independently of other floral traits, genetic variation in the environmental sensitivity of herkogamy, and the production of modified and unmodified flowers within some individuals, reveal the potential for dynamic control of the mating system in a species that commonly confronts heterogeneous aquatic environments.
Key words: Eichhornia paniculata, expressivity, flower morphology, herkogamy, phenotypic plasticity, pleiotropy, population variation, self-fertilization, stigma–anther separation, outcrossing, tristyly
INTRODUCTION
The evolutionary transition from outcrossing to predominant self-fertilization in flowering plants is commonly associated with modifications to a range of floral traits (table 1 in Ornduff, 1969). Compared with their outcrossing relatives, selfing species generally have smaller less-conspicuous flowers, reduced physical separation between anthers and stigmas – hereafter herkogamy – and lower pollen:ovule ratios [e.g. Leavenworthia (Lloyd, 1965), Mimulus (Ritland and Ritland, 1989) and Collinsia (Armbruster et al., 2002)]. However, determining the sequence of floral modifications that culminate in the ‘selfing syndrome’ has remained a challenge for evolutionary biologists. Although the evolution of selfing is the most frequent mating-system transition in plants (Stebbins, 1974), it is not clear which changes initiate increased rates of selfing and which accumulate after a species has become largely selfing. Plant species that display wide intra-specific variation in mating system provide opportunities to investigate early stages in the transition towards self-fertilization.
Table 1.
Phenotypic (above diagonal) and maternal family (below diagonal) Spearman-rank correlations between flower number, plant size, days to flowering and flower morphology in Eichhornia paniculata from north-east Brazil (trimorphic populations only)
| Days to 1st flower | Plant size (PC1) | Flower no. | Perianth size | Nectar-guide size | Stigma–anther separation | |
|---|---|---|---|---|---|---|
| Days to 1st flower | — | 0·034 (0·398) | −0·211 (<0·0001) | 0·009 (0·828) | −0·217 (<0·0001) | −0·047 (0·246) |
| Plant size (PC1) | 0·189 (0·028) | — | 0·551 (<0·0001) | 0·156 (<0·0001) | −0·046 (0·254) | 0·111 (0·006) |
| Flower no. | −0·060 (0·496) | 0·539 (<0·0001) | — | 0·209 (<0·0001) | 0·115 (0·004) | 0·130 (0·001) |
| Perianth size | −0·027 (0·754) | 0·180 (0·037) | 0·206 (0·017) | — | 0·467 (<0·0001) | 0·217 (<0·0001) |
| Nectar guide size | −0·210 (0·015) | −0·096 (0·271) | 0·070 (0·425) | 0·522 (<0·0001) | — | 0·171 (<0·0001) |
| Stigma–anther separation | 0·097 (0·262) | 0·007 (0·934) | 0·038 (0·663) | 0·251 (0·003) | 0·145 (0·094) | — |
Nominal statistical significance is shown in parenthesis. Bold face indicates significance after a sequential Bonferroni correction. PC1 = first eigenvectors of the principal component analysis of the correlation matrix of vegetative (plant size) traits. Sample size for phenotypic correlations n = 607–620 plants, mean 3·61 flowers per individual, range 1–18; genetic correlations n = 132–134 families, mean 4·62 plants per family, range 1–12.
In self-compatible species, herkogamy has long been considered a major determinant of mating patterns in plant populations (Darwin, 1862; Müller, 1883; Webb and Lloyd, 1986). Reduced herkogamy correlates positively with fruit set in both the presence (Rick et al., 1978) and absence of pollinators (Ennos, 1981; Carr and Fenster, 1994; Elle and Hare, 2002), self-pollen deposition (Thomson and Stratton, 1985) and marker-based estimates of selfing (Barrett and Shore, 1987; Holtsford and Ellstrand, 1992; Motten and Antonovics, 1992; Brunet and Eckert, 1998; Takebayashi et al., 2006). Furthermore, studies that have statistically controlled for the effect of other traits potentially associated with herkogamy, such as flower size, have clearly shown that herkogamy can be a primary determinant of autonomous seed set and rates of self-fertilization (Brunet and Eckert, 1998; Elle and Hare, 2002), although some exceptions do occur (Medrano et al., 2005).
Variation in herkogamy is heritable in diverse animal-pollinated species (Ennos, 1981; Shore and Barrett, 1990; Herlihy and Eckert, 2007; Kulbaba and Worley, 2008) and has been shown to respond to artificial selection (Chang and Rausher, 1998). Less is known about the role of environmental factors in modifying the expression of herkogamy (but see Brock and Weinig, 2007) and to what extent this trait exhibits phenotypic plasticity – the ability of a genotype to express alternative phenotypes under different environmental conditions (Bradshaw, 1965). Plasticity in herkogamy could allow genotypes to adopt different mating strategies depending on local environmental conditions. For example, in plants where outcrossing is generally favoured, stressful conditions, e.g. due to seasonal droughts or limited pollinator service, may favour self-pollination as a mechanism of reproductive assurance (Stebbins, 1957; Levin, 1972; Moeller and Geber, 2005). According to this scenario phenotypic plasticity of herkogamy expression could be adaptive and represent an evolutionary response to heterogeneous environments (Via et al., 1995).
The annual tristylous aquatic plant Eichhornia paniculata provides an opportunity to investigate modifications to flower architecture during the early stages in the evolution of self-fertilization. In the central part of its distribution in north-east Brazil, populations display a wide range of mating patterns (Barrett and Husband, 1990). Tristylous populations contain long-, mid- and short-styled morphs (hereafter L-, M- and S-morphs) and are largely outcrossing, whereas dimorphic (L- and M-morphs) and monomorphic (M-morph only) populations exhibit variation in selfing rates, depending on the presence of self-pollinating variants of the M-morph with reduced herkogamy (Barrett et al., 1989). As a result, the selfing rate of populations is correlated with among-population variation in the degree of herkogamy in the M-morph (fig. 3b in Barrett and Husband, 1990). In Brazilian populations of E. paniculata, reduction in herkogamy in the M-morph is largely achieved through elongation of a single short-level stamen (Seburn et al., 1990; Richards and Barrett, 1992). Predominantly selfing populations of E. paniculata on the Caribbean Islands of Jamaica and Cuba are composed primarily of the M-morph and have much smaller and less-showy flowers, lower pollen:ovule ratios and up to three elongated stamens in the mid-level position (Barrett, 1985; Morgan and Barrett, 1989, S. C. H. Barrett, unpubl. res.). These highly autogamous variants are described as semi-homostylous (Ornduff, 1972). Hence, E. paniculata displays the full spectrum of floral modifications associated with the evolution of the selfing syndrome.
Fig. 3.
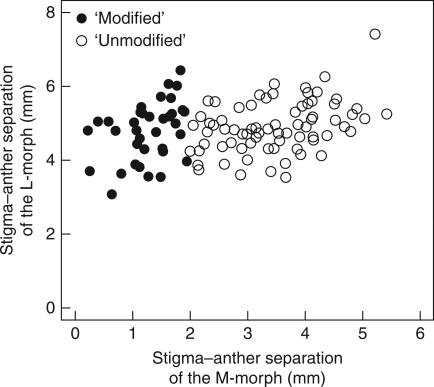
Mean stigma–anther separation for families segregating both long-styled (L-morph) and mid-styled (M-morph) plants. Closed symbols represent families containing ‘modified’ M-morph plants (average stigma–anther separation <2 mm), and open symbols those with ‘unmodified’ M-morph plants (average stigma–anther separation >2 mm). n = 111, 683 and 2374 for families, plants and flowers, respectively.
In addition to significant population-level differentiation in floral traits, plants of E. paniculata also display remarkable patterns of within-individual variation. The variation can be expressed in several ways, including developmental instability in perianth shape resulting from differences in tepal number, and variation in the position of stamens within a flower as a result of differences in filament elongation (Barrett, 1985, 1996; Richard and Barrett, 1992). Developmental instability in stamen position is of particular functional significance because it can influence the facility for autonomous self-pollination of flowers and hence mating patterns. Plants in dimorphic and monomorphic populations commonly produce a mixture of flowers, some with well-developed herkogamy and others in which herkogamy is weakly developed or absent resulting in autonomous self-pollination (Seburn et al., 1990; Barrett and Harder, 1992). These two flower types have been referred to as unmodified and modified flowers, respectively, and are particularly characteristic of plants of the M-morph. The occurrence in E. paniculata of developmental instability in herkogamy provides an opportunity to investigate the genetic and environmental control of within-individual variation in a trait of obvious functional significance to mating patterns.
Here, using comparisons of populations grown under uniform glasshouse conditions and experimental manipulation of growing conditions, the morphological changes associated with the evolution of selfing in E. paniculata are investigated, focusing in particular on populations from north-east Brazil because in this region the early stages in the transition from outcrossing to selfing are evident (Barrett et al., 1989). Therefore plants have not been included from other geographic areas (e.g. Caribbean and Central America) where populations are small flowered and highly selfing (Barrett, 1985; Barrett and Husband, 1990, S. C. H. Barrett, unpubl. res.). In the present study, particular attention is paid to variation in herkogamy, because previous work has demonstrated that it is functionally associated with variation in the mating system of E. paniculata. The following specific questions were addressed in this study: (a) Is within- and among-population variation in herkogamy associated with other floral and vegetative traits, especially flower size? (b) To what extent is variation in herkogamy limited to the M-morph, especially in segregating families containing self-pollinating variants and individuals of the L-morph? (c) What are the relative roles of genetic and environmental variation in the expression of herkogamy, and how phenotypically plastic is this trait? (d) Based on measurements of within-plant variation, what are the patterns of developmental instability in herkogamy among individuals? It is proposed that the regulation of herkogamy may provide populations of E. paniculata with the potential for dynamic control of mating in the face of unpredictable environmental conditions.
MATERIALS AND METHODS
Study populations and glasshouse culture
Thirty-one Brazilian populations of Eichhornia paniculata (Spreng.) Solms. (Pontederiaceae), collected as open-pollinated progenies from maternal parents in May–June 2005, were investigated. The localities and morph structure of populations [i.e. whether populations are trimorphic (L-, M-, S-morph), dimorphic (L-, M-morph) or monomorphic (M-morph only)] are provided in Table S1 in Supplementary Data, available online). The sample included 18 trimorphic, 11 dimorphic and two monomorphic populations. In the glasshouse, seeds from up to ten maternal families per population were sown on 11 August 2006 onto wet soil. Pots were randomly placed in plastic water-filled trays (30 pots per tray) and maintained at approx. 34°C to stimulate germination. After germinated seedlings reached the two-to-three-leaf stage, pots were transferred to a cooler glasshouse (25°C), and each tray was fertilized with 150 mL of a 300 ppm solution of 20:20:20 fertilizer (Plant Products Co. Ltd, Brampton, Ontario, Canada). Beginning on 21 September, up to 12 seedlings from each maternal family were transplanted into 5·7-cm pots and randomly assigned to water-filled trays on a glasshouse bench. Plants received sporadic fungicide treatment (No-Damp fungicide; Plant Products Co.) and a weekly dose of 150 mL of liquid fertilizer. Fertilizer concentration was doubled once flowering began to maintain plant growth.
Measurements of floral and vegetative traits
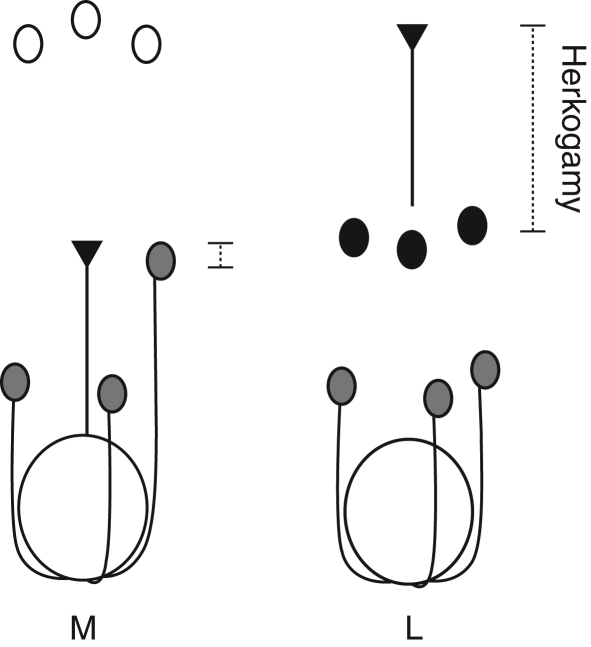
Date to first flower and floral morph identity (L-, M- or S-morph) for each plant were recorded between 27 October 2006 and 8 January 2007, when the vast majority of plants had flowered. For the first two inflorescences produced by each plant, four randomly chosen flowers (two per inflorescence) were measured for the following characters: (a) vertical and horizontal width of the perianth; (b) width and length of one of the two nectar guides; and (c) stigma–anther separation (herkogamy), measured from the stigma surface to the midpoint of the nearest anther, which corresponded to one of the short-level anthers in the M-morph, and a mid-level anther in the L-morph (see Fig. 1). In addition, the following were measured: (d) the size of the leaf (bract) subtending the first inflorescence (width, length to petiole, and length to base); (e) plant height from the soil surface; and (f) flower number per inflorescence. All measurements were made with digital calipers to the nearest 0·01 mm, except plant height which was measured with a ruler to the nearest centimetre.
Fig. 1.
Diagram illustrating the relative positions of sexual organs in flowers of the M- and L-morph of Eichhornia paniculata. Large circle = ovary; line ending in an inverted triangle = style and stigma. Short-level anthers are shown in grey, mid-level anthers in black, and long-level anthers in white. The filaments for mid- and long-level anthers, and the perianth have been omitted for clarity. Herkogamy (dashed lines) is the distance between the stigmatic surface and the midpoint of the nearest anther (a short-level anther for the M-morph, and a mid-level anther for the L-morph).
Correlations among traits
To determine the extent to which floral traits co-varied among themselves, as well as with plant size and flowering time, phenotypic and genetic (maternal family) correlations were estimated using Spearman rank-correlations. Genetic correlations were approximated by calculating correlations of family means (Falconer and Mackay, 1996). Because only the correlation among traits within populations in which selfing adaptations arise was of interest, this analysis was restricted to trimorphic populations only. Perianth and nectar guide size were calculated as the product of length × breadth. This measurement of perianth size has been used in previous studies of E. paniculata and correlates well with floral dry weight (Worley and Barrett, 2001). The four measured vegetative traits were summarized using principal component analysis of the correlation matrix, and the first principal component – which explained 67·12% of the variance and was positively correlated with all vegetative traits (data not shown) – was used for this analysis. All analyses were conducted using the statistical program R ver. 2·6·2 (R-Development Core Team, 2008).
Comparisons among floral morphs
Floral traits among morphs were compared using non-parametric Wilcoxon signed-rank tests. Non-parametric tests were chosen for these analyses because the bimodal distribution of herkogamy in some individuals of the M-morph (see below) could not be easily transformed to normality. Because calculated correlations used combined data across populations the estimates include the contribution of both genetic variation within populations and differentiation among populations (Armbruster, 1991). Both trimorphic and dimorphic populations were included in this analysis, but they were analysed separately.
Patterns of herkogamy variation in the floral morphs
Previous work (Seburn et al., 1990; Barrett and Harder, 1992) and preliminary observations of the populations suggested that in some plants of the M-morph the distance separating stigmas and anthers was bimodally distributed. To characterize this pattern, a mixed-distribution analysis was conducted using the module mixdist (MacDonald and Du, 2008). The module mixdist uses maximum likelihood to estimate the parameters of a mixed distribution formed by the combination of k unimodal distributions. For the mixed-distribution analysis, a combination of two normal distributions was fitted to the observed frequency of stigma–anther distance and this was compared with the expected distributions using a χ2-test. The maximum likelihood estimates of the mean, variance and relative frequency (mixing proportion) of the predicted combination of the two normal curves were also obtained.
To determine if herkogamy variation was largely confined to the M-morph, a correlation analysis was used to investigate the extent to which stigma–anther separation values among maternal families of the M-morph segregating both L- and M-individuals (plants of genotype ssMm; see Barrett et al., 1989) from trimorphic and dimorphic populations were associated. If modification of herkogamy is not morph specific, a correlation between values for stigma–anther distances in plants of both the L- and M-morph from the same maternal family would be expected. In contrast, if the genetic modifications to herkogamy are morph-specific there should be no correlation between stigma–anther separation in segregating families. Stigma–anther separation for 2384 flowers from 685 individuals from 111 segregating families was measured. The mean stigma–anther distance was calculated for each morph within each family and these values used for calculating Spearman rank-order correlations.
To analyse the bimodal expression of herkogamy across different levels of organization (population morph structure, population, family and individual plant) stigma–anther distance was transformed into a binary trait. Flowers were classified into either modified (herkogamy <2 mm) or unmodified (herkogamy ≥2 mm). The threshold of 2 mm was chosen a priori following a preliminary examination of flowers, which indicated that this value corresponded to the lowest point between the two modes in the frequency distribution of stigma–anther distance (Fig. 2). A mixed-effects model with a binomial error and a logit link function was then fitted to the transformed data using the module lme4 (Pinheiro and Bates, 2000). The use of mixed effects models allowed the structure inherent to the data set to be incorporated. The unit of observation was the flower, which was nested within plant, family and population (random effects). Population morph structure was treated as a fixed effect and flower size (perianth area) as a covariate. For the response variable (stigma–anther distance), modified flowers were coded as (1) and unmodified flowers (0). Statistical significance of individual terms was assessed via likelihood ratio tests (LRT) of the full model versus a model excluding the term of interest.
Fig. 2.
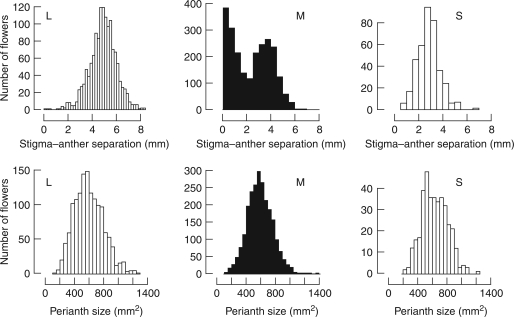
Frequency distribution of stigma–anther separation (top) and perianth length (bottom) for long- (L), mid- (M) and short-styled flowers (S) from 31 populations of Eichhornia paniculata from north-east Brazil grown under uniform glasshouse conditions. n = 1374, 2365 and 378 flowers from n = 400, 685 and 107 plants of the long-, mid- and short-styled morph, respectively.
Phenotypic plasticity in stigma–anther separation
To determine the extent to which herkogamy is influenced by growing conditions during development, flower and vegetative traits of a sample of genotypes were compared in two contrasting environments. Only individuals of the M-morph were used in the experiment because this morph displays considerable variation in herkogamy (see Results). A sample of ten genotypes representing the range of variation in herkogamy observed in the 31 populations sampled above was chosen. The genotypes originated from one trimorphic (two genotypes), four dimorphic (six genotypes) and one monomorphic (two genotypes) populations (for details see Table S2 in the Supplementary Data, available online). To encourage clone production via axillary shoots for each of these genotypes, plants were transplanted on 23 May 2007 to 10·2-cm pots with a soil mix of 1:1 soil:sand in 5-L plastic buckets. Water levels were kept above the soil level and plants were fertilized weekly with 250 mL of 900 ppm 20:20:20 fertilizer. On 8 August axillary shoots produced by all genotypes were excised from maternal plants and transplanted to separate pots. Population origin and the number of ramets (clones) per genotype (genet) are given in Table S2.
To impose different growth conditions to ramets of the same genotype, the cloned axillary shoots from each genotype were randomly assigned to each of two treatments that differed in pot size, water level and nutrient availability. The two contrasting growth conditions are referred to as ‘low stress’ and ‘high stress’ for convenience. Ramets in the low-stress environment were grown in 10·2-cm pots, the water level was maintained above the soil, and plants were fertilized weekly with 250 mL of a 900 ppm solution of 20:20:20 liquid fertilizer. In contrast, ramets in the high-stress treatment were grown in smaller pots (7·6 cm), water levels were much reduced and plants were fertilized weekly with 50 mL of fertilizer. At the start of the experimental treatments, leaf size and height of the main stem were measured, and a single measure of plant size obtained using principal component analysis of the correlation matrix as described above. There was no significant difference among ramets transplanted to the two treatments (t = −0·606, d.f. = 69·34, P = 0·546), indicating that the sample of ramets in each treatment was of equivalent size. Starting on 28 August, traits were measured on ramets in the two growth treatments. For the first two inflorescences produced by each plant, leaf size and leaf height were measured as described above. For the first five flowers produced by each ramet, perianth and nectar guide length and width were measured and for 25 flowers per inflorescence (up to 55 per ramet) stigma–anther separation was measured.
Data from this experiment were analysed using logistic regression (Barrett and Harder, 1992). For each ramet, the number of modified (stigma–anther distance <2 mm) and unmodified flowers was counted and pooled across inflorescences within genets. The response variable for this analysis was a matrix of the number of modified and unmodified flowers for each plant (n = 72 ramets; n = 3548 flowers). Perianth size was calculated as the mean area of the five flowers measured per ramet. Because the ten genotypes included in this experiment were chosen to represent the range of herkogamy variation, genotype was treated as a fixed effect. Treatment was also treated as a fixed effect and mean perianth size was used as a covariate. The model was fitted using the module glm with a binomial error and a logit link function and statistical significance was assessed via LRT of the full model versus a model excluding the term of interest. In common with other studies of phenotypic plasticity (Schlichting, 1986), statistical significance of the genotype term (G) was interpreted as evidence of genetic variation in the trait of interest (in the present case herkogamy), significance of the treatment effect (E) as indication of environmental effects, and its interaction (G × E) as evidence of genetic variation for plasticity.
RESULTS
Trait correlations and comparisons among the floral morphs
In the sample of 18 trimorphic Brazilian populations, the correlation structure of floral and vegetative characters indicated that several traits were significantly associated at both the phenotypic and genetic (maternal family) levels (Table 1). For example, large flowers were associated with large plant size, more flowers per inflorescence and large nectar guides at the phenotypic level, and, with the exception of plant size, these correlations were also evident at the family level. Nectar-guide size varied positively with flower number at both the phenotypic and genetic level, and with perianth size at the phenotypic level only. Greater stigma–anther separation was correlated with larger perianth size at both phenotypic and genetic levels, but this trait was only associated at the phenotypic level with larger plants, more flowers per inflorescence and larger nectar guides.
The only consistent difference in floral traits among morphs within trimorphic and dimorphic populations was stigma–anther separation. In trimorphic populations, flowers of the L-morph had the largest mean herkogamy value; 4·90 mm ± 0·06 (mean ± s.e.) compared with 3·24 ± 0·07 and 2·81 ± 0·07 for the M- and S-morph (P < 0·0001 for all morph comparisons, n = 220, 293 and 105 individuals for the L-, M- and S-morph, respectively). In dimorphic populations, herkogamy in the L-morph had a similar value (4·82 ± 0·07, n = 174) to the L-morph in tristylous population. However, the M-morph had a significantly lower stigma–anther separation (1·85 ± 0·07, n = 307), which was significantly different from the L-morph (P < 0·001).
Perianth size (mm2) was not significantly different among morphs in either trimorphic (L-morph, 727·28 ± 14·46; M-morph, 699·03 ± 10·93; S-morph, 744·88 ± 16·17) or dimorphic (L-morph, 650·70 ± 12·45; M-morph, 660·30 ± 7·87; P = 0·33) populations. In trimorphic populations nectar guides were significantly larger in the M-morph than the S-morph (P = 0·041), but not different from the L-morph (L-morph, 7·42 ± 0·16; M-morph, 6·93 ± 0·11; S-morph, 7·57 ± 0·22). Both L- and M-morphs had similar-sized nectar guides in dimorphic populations (L-morph, 5·72 ± 0·17; M-morph, 5·55 ± 0·13; P = 0·5).
Patterns of herkogamy in the M-morph
An unusual feature of variation in herkogamy in E. paniculata is the bimodal distribution of stigma–anther separation in the M-morph. The top part of Fig. 2 illustrates the frequency distribution of this measurement among the three morphs. Bimodality is evident in the M-morph but not the remaining morphs. No morph exhibits bimodality for perianth size (Fig. 2, bottom part). Further examination of the bimodal nature of herkogamy in the M-morph, using the mixed-distribution (n = 2365 flowers), revealed that the observed and predicted distributions did not differ significantly from each other (χ2 = 16·275, d.f. = 10, P = 0·092). This indicates that a combination of two normal curves provides a reasonable approximation to bimodality. Based on the mixed-distribution analysis, herkogamy in the M-morph can be conveniently divided into two classes: flowers with a mean herkogamy of 3·57 mm (s.d. = 0·90), and those with a mean herkogamy of 0·68 mm (s.d. = 0·63). The relative frequency of these two classes in the entire sample was 58·3% and 41·7%, respectively. As in previous studies (e.g. Barrett and Harder 1992), flowers with a herkogamy closer to the lower mean (i.e. near zero stigma–anther separation) are referred to as modified, and those with greater distances (stigma–anther separation near 3·57) as unmodified.
Relationship between herkogamy of the L- and M-morph in segregating families
The present analysis of segregating families indicated a small but significant correlation between herkogamy in flowers of the L- and M-morph (ρ = 0·239, P = 0·011, n = 111 families). To distinguish the association between herkogamy in the L- and M- morphs from that between herkogamy and flower size, a partial Spearman-rank correlation analysis was conducted (Kim and Yi, 2007). This analysis indicated that the coefficients of herkogamy in the L- and M-morphs are not significantly correlated after accounting for correlations with perianth size (ρ = 0·176, P = 0·062, n = 111). Moreover, Figs 2 and 3 illustrate that flowers of the M-morph commonly exhibit herkogamy values near zero, a pattern not observed in the L-morph. Indeed, families that produce M-morph flowers with small stigma–anther separation (i.e. <2 mm, ‘modified’ flowers) have the same herkogamy in the L-morph as families that produce M-morph flowers with large stigma–anther separation (i.e. >2 mm, ‘unmodified’ flowers) (W = 1459, n = 111, P = 0·575; Fig. 3). These results indicate that the modification in stigma–anther separation in E. paniculata is largely morph specific.
Patterns of variation in herkogamy among populations, families and individuals in the M-morph
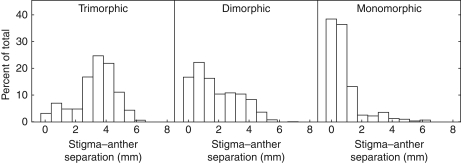
The occurrence of modified flowers of the M-morph, here treated as a binary representation of herkogamy variation, was significantly different among the three population morph structures (χ2 = 29·29, d.f. = 2, P < 0·0001; Fig. 4). Back transformation of the logistic regression parameters allowed the expected probability of modified flowers in each population morph structure to be calculated (Table 2A). The predicted probability of producing modified flowers in trimorphic, dimorphic and monomorphic was 2·6%, 67·3% and 99·3%, respectively. Mean observed values were 16·60%, 59·32% and 89·80%, respectively. The difference between observed and predicted values is likely to be due to the significant contribution of population identity, family and individuals within families to variation in herkogamy (Table 2B). Segregation of different genes within families and micro-environmental conditions in the glassshouse probably contribute to the expression of herkogamy. In contrast, flower size contributed only marginally to the probability of herkogamy modification (χ2 = 3·46, d.f. =1, P = 0·062) and its contribution was relatively small (Table 2A).
Fig. 4.
Frequency distribution of stigma–anther separation in the mid-styled morph in trimorphic, dimorphic and monomorphic populations of Eichhornia paniculata from north-east Brazil. n = 1048, 1013 and 304 flowers and n = 294, 305 and 86 plants for 16 trimorphic, 11 dimorphic and two monomorphic populations, respectively. Two trimorphic populations in which no mid-styled plants flowered in the greenhouse are not included in this analysis.
Table 2.
Analysis of the occurrence of modified flowers in the M-morph (stigma–anther separation <2 mm) in Brazilian populations of Eichhornia paniculata
| (A) Estimates of the effect of population morph structure and perianth size (fixed-effects) on the production of modified flowers (the regression estimates are back transformed to the proportion of modified flowers for comparison) | |||||
|---|---|---|---|---|---|
| Population type | Proportion of modified flowers | Regression estimate | s.e. | z-value | P-value |
| Trimorphic | 0·026 | −3·590 | 0·678 | −5·294 | <0·0001 |
| Dimorphic | 0·673 | 0·725 | 0·809 | 5·333 | <0·0001 |
| Monomorphic | 0·993 | 4·959 | 1·475 | 5·794 | <0·0001 |
| Perianth size (mm2) | −0·001 | 0·0006 | −1·924 | 0·054 | |
| (B) Effect of population identity, family and individual (all random effects) on the production of modified flowers (fit of the full model and sub-models deleting the indicated random effect are shown in each row) | ||||
|---|---|---|---|---|
| Effects | Log likelihood | χ2 | d.f. | P-value |
| Full model | −952·75 | |||
| Population | −968·00 | 30·50 | 1 | <0·0001 |
| Family | −971·67 | 37·84 | 1 | <0·0001 |
| Individual | −1035·73 | 165·96 | 1 | <0·0001 |
Sample sizes: populations n = 29, families n = 186, plants n = 685, flowers n = 2365. Models were fitted using logistic mixed-effects regression.
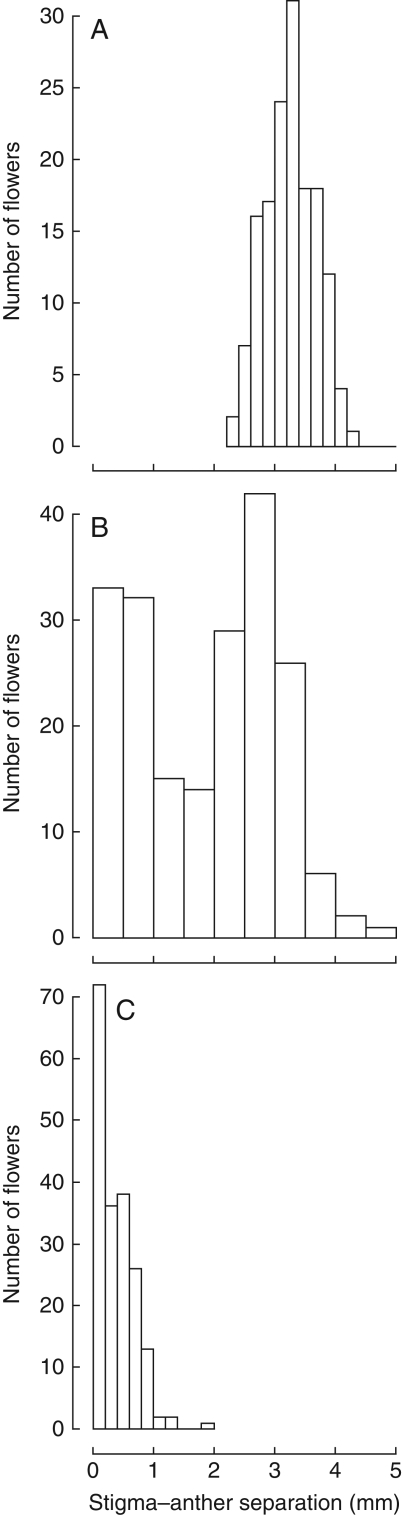
Among the 685 plants of the M-morph included in the total sample, 404 (58·97%) produced at least one modified flower. Among these plants, 231 produced only modified flowers with the remainder (173) exhibiting developmental instability producing both modified and unmodified flowers. However, these specific values should be interpreted with caution due to the small number of flowers measured per individual (mean = 3·45). However, developmental instability was also evident among genotypes used in the phenotypic plasticity experiment described below. Because of the much larger number of flowers measured on each genotype it was possible to construct frequency histograms to illustrate the contrasting patterns of herkogamy variation (Fig. 5). Three genotypes are contrasted, each of which illustrates a distinct pattern of herkogamy variation. Genotypes (B191-3-5) and (B182-9-1) were developmentally stable, producing flowers with large versus small herkogamy values, respectively. In contrast, genotype (B189-7-1) exhibited marked developmental instability producing a bimodal distribution of herkogamy values.
Fig. 5.
Representative patterns of variation in herkogamy in three genotypes of Eichhornia paniculata grown under the same environmental conditions in a glasshouse (low stress, see Materials and Methods for details). Genotypes B191-5-1 and B182-9-1 (A and C, respectively) illustrate unimodal distributions of herkogamy values whereas B189-7-1 (B) exhibits developmental instability in herkogamy resulting in a bimodality of stigma–anther values.
Phenotypic plasticity in the expression of herkogamy
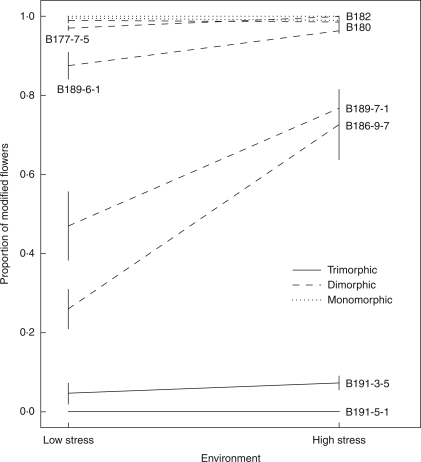
Plants of the M-morph grown in smaller pots with lower fertilizer dosage were smaller (t = 16·72, d.f. = 63·89, P < 0·0001) and produced more modified flowers than those that were grown with higher resource supply (LRT of treatment effect; χ2 = 8·45, d.f. = 1, P < 0·01; proportion of modified flowers = 0·721 ± 0·002 and 0·561 ± 0·003, for high- and low-stress environments, respectively) demonstrating plasticity in the expression of herkogamy. Genotypes differed significantly in their relative production of modified flowers, as expected based on the population survey (χ2 = 1164·32, d.f. = 9, P < 0·001). Genotypes from a trimorphic population produced mostly unmodified flowers, whereas genotypes from a monomorphic population produced largely modified flowers. Genotypes from dimorphic populations displayed a wide range of floral modification (0·260–0·995 and 0·726–1, in the low- and high-stress environments, respectively; Fig. 6). The significant genotype × environment interaction (treatment × genotype effect; χ2 = 20·62, d.f. = 9, P = 0·014) indicates genetic variation for phenotypic plasticity in stigma–anther separation among the ten genotypes. Consistent with the results of the population survey, perianth size was not a significant determinant of the expression of modified flowers (χ2 = 0·44, d.f. = 1, P = 0·507).
Fig. 6.
Norm of reaction between two environments for the mean proportion of modified flowers (stigma–anther separation <2 mm) in ten genotypes of the M-morph in Eichhornia paniculata originating from trimorphic (B191-3-5, B191-5-1; solid lines), dimorphic (B189-6-1, B189-7-1, B180-8-4, B180-8-7, B186-9-7, B177-7-5; dashed lines) and monomorphic (B182-5-6, B182-9-1; dotted lines) populations. In the figure, the labels B180 and B182 represent two genotypes each that lay too close to be labelled separately. The two environments involve different growing conditions and are referred to as low and high stress. Vertical bars represent standard errors of the mean.
DISCUSSION
The most significant alteration to floral architecture during the early stages of the evolution of selfing in Brazilian populations of Eichhornia paniculata is a reduction in herkogamy in the M-morph (Fig. 2). The results indicate that changes in herkogamy are largely independent of modifications to other floral traits, including flower size. An analysis of stigma–anther separation in segregating families of the M-morph confirmed that modifications to herkogamy are morph-specific. It was also found that the production of modified flowers was influenced by environmental conditions in some genotypes, with higher proportions produced under more stressful conditions. The ability to alter herkogamy independently of other floral characters and the plasticity of this trait has implications for the regulation of mating patterns in a species adapted to ephemeral environments (Barrett and Husband, 1997). Below are discussed the mechanisms influencing the expression of selfing modifications in E. paniculata and the ecological context in which changes to mating patterns occur.
Reduction in herkogamy is largely independent of flower size
Flowers in animal-pollinated plants are made up of correlated traits that function in an integrated manner to promote pollination and mating (Berg, 1960; Armbruster et al., 2004; Harder and Barrett, 2006). An important issue associated with the transition from outcrossing to selfing is to what extent individual traits can be modified independently of other traits during floral evolution (Fishman et al., 2002). The results indicate that in all style morphs of E. paniculata, perianth size, nectar guide size, flower number and herkogamy are positively correlated among each other at the phenotypic level, although at the family level only the correlations between perianth size, herkogamy and nectar guide size are significant (Table 1). Phenotypic and genetic correlations among floral traits are generally expected to occur because of shared developmental pathways (Krizek and Fletcher, 2005) and strong stabilizing selection for floral integration (Armbruster et al., 2004). Functional integration would be especially likely in a heterostylous species, such as E. paniculata, where reciprocity of sex-organ position promotes disassortative pollination among the style morphs by long-tongued bees.
The positive correlation between stigma–anther separation and perianth size in trimorphic populations of E. paniculata might suggest that genetic modifications to stigma–anther separation would depend, in part, on changes to flower size. Indeed, this association commonly occurs in other groups where reductions in herkogamy are often associated with the evolution of smaller flowers (Fishman et al., 2002). However, the present results indicate that during the early stages of the establishment of selfing this association does not occur and that stigma–anther separation can be altered independently of changes to flower size (Table 2). Earlier, Fenster and Barrett (1994) reported weak correlations (Pearson's correlation coefficients <0·25) between perianth width and the length of stamen filaments. Since herkogamy modification is mostly due to elongation of short-level stamen filaments (Seburn et al., 1990; Richards and Barrett, 1992) weak associations between perianth characteristics and herkogamy are not unexpected. Accordingly, the present results show that variation in herkogamy among the floral morphs is not paralleled by changes in perianth size (Fig. 2), and the analysis of the production of modified flowers in the M-morph demonstrate that whether a flower is modified or not is not influenced by perianth size (Table 2). Collectively these results support the conclusion that early stages in the evolution of the selfing syndrome in E. paniculata are initiated by relatively simple changes to stamen position that occur independently of modifications to other floral traits. However, once selfing is firmly established, selection for reduced flower size may often proceed, as seems likely to have occurred in the highly autogamous populations of E. paniculata in Jamaica and Cuba, which possess much smaller flowers than occur in north-east Brazil. These changes to flower size in selfing populations are likely to be a response to selection for a reduction in the organs of attraction and a more economical use of floral resources.
Morph-specific expression of reduced herkogamy
The reduction in herkogamy associated with the transition to self-fertilization in Brazilian populations of E. paniculata is largely restricted to individuals of the M-morph (Fig. 2). Stigma–anther separation in long-styled individuals was not influenced by whether their mid-styled siblings have modified or unmodified flowers. This lack of correspondence demonstrates morph-limited expression of selfing adaptations in E. paniculata. Morph-specific expression in this species may arise because different anther levels are involved in determining any potential reduction in herkogamy in the M- and L-morphs (see Fig. 1). It is likely that in this heterostylous species mutations causing filament elongation are specific to particular stamen levels and are simply not expressed in alternate morphs. In the present study, the position of short-level anthers in flowers of the L-morph was not measured. Instead attention was paid to the distance separating the closest mid-level anther and stigma of long styles. However, a previous investigation failed to find evidence of elongation of short-level anthers in flowers of the L-morph among families containing selfing variants of the M-morph (Fenster and Barrett, 1994). This supports the interpretation that the mutations modifying short-level stamens in the M-morph are not expressed in either of the two stamen levels of the L-morph. Field observations are also consistent with morph-specific expression of herkogamy variation. In dimorphic populations in north-east Brazil, Jamaica and Cuba individuals of the M-morph commonly (Brazil) or exclusively (Caribbean), produce modified flowers whereas those of the L-morph maintain significant stigma–anther separation.
Small-flowered disjunct populations of E. paniculata in Mexico and Nicaragua are composed exclusively of semi-homostylous long-styled plants. This demonstrates that in other parts of the geographical range the evolution of selfing is not restricted to the M-morph (Barrett, 1996; S. C. H. Barrett, unpubl. res.). Preliminary genetic studies of these semi-homostylous variants of the L-morph suggest that floral modifications promoting selfing result from quantitative inheritance, unlike the self-pollinating forms of the M-morph from Brazil in which stamen elongation is simply inherited (Fenster and Barrett, 1994; M. Vallejo-Marín and S. C. H. Barrett, unpubl. res.). Why selfing modifications are restricted to the M-morph in Brazilian and Caribbean populations is unclear. It has been suggested that the likelihood of selfing evolving in the L-morph may be contingent on whether the M-morph is present in populations because of the speed in which selfing can evolve under polygenic versus major gene control (Barrett, 1996). Ongoing work is investigating the genetic architecture and evolutionary dynamics of selfing in the L- and M-morphs. In particular, it will be important to determine the role of genetic and developmental factors in causing morph-limited expression of short-stamen modification in the M-morph.
Variation in the incidence of self-pollinating flowers
The incidence of reduced herkogamy in the M-morph of E. paniculata varies among populations with different morph structures. Modified flowers are less common in tristylous populations, abundant in dimorphic populations, and largely fixed in monomorphic populations (Fig. 4 and Table 2B). This pattern is associated with the evolutionary breakdown of tristyly and the transition from outcrossing to selfing. However, appreciable frequencies of self-pollinating flowers of the M-morph (mean 16·60%; range 0–36·8%) were recorded among the 18 tristylous populations that were examined under uniform glasshouse conditions. Elsewhere it has been proposed that the joint action of stochastic forces and natural selection can explain the loss of morph diversity and spread and fixation of self-pollinating variants in dimorphic and monomorphic populations of E. paniculata, respectively (Barrett et al., 1989; Husband and Barrett, 1992). The glasshouse comparisons demonstrate that selfing modifiers are represented among the standing genetic variation of largely outcrossing tristylous populations. The facility for self-pollination would enable modified plants of the M-morph to found colonies following dispersal events from trimorphic populations. Founders that are heterozygous at the M-locus would produce L- and M-styled plants and this may account for the origin of some dimorphic populations.
The glasshouse comparisons were not specifically designed to measure the heritability of modified flower production in M-styled plants. In addition, the experiments did not explicitly account for potential maternal effects. However, variation in the incidence of modified flower production among families grown under uniform conditions (Table 2B) provides evidence that this trait has a genetic basis. Together with the significant variation among genotypes detected in the plasticity experiment (Fig. 6), and the analysis of segregation patterns in controlled crosses between modified and unmodified plants (Fenster and Barrett, 1994, M. Vallejo-Marín and S .C. H. Barrett, unpubl. res.), there seems little doubt that herkogamy variation in E. paniculata has a genetic component. The variation detected within segregating families of the M-morph indicates that flowers produced by siblings are often more different than flowers produced within individuals (Table 2B). This variation among siblings could be due to subtle microenvironmental variation in the glasshouse and/or segregation of different alleles within families. Controlled crosses suggest that herkogamy modification is due to a small number of recessive factors (Fenster and Barrett, 1994). The segregation of stamen-modification alleles in heterozygous families may be responsible for some of the variation observed among siblings.
Phenotypic plasticity and developmental instability of floral traits
Floral traits commonly display relatively uniform morphological expression resulting from developmental homeostasis (reviewed in Fenster and Galloway, 1997) and stabilizing selection (Armbruster et al., 2004). A consistent correspondence between sexual organs and pollinator positioning is necessary to ensure effective pollen transfer, especially in heterostylous species (Lloyd and Webb, 1992). In this context, plasticity and/or developmental instability of floral characters is most often considered maladaptive and few studies have considered the potential benefits of these forms of intra-plant variation in reproductive traits (but see Lloyd, 1984). Although the present sample of genotypes was relatively small (n =10), variation among genotypes was detected in the plasticity of herkogamy (Fig. 6). Variation ranged from strong canalization with almost identical proportions of modified flowers between the two growing conditions (e.g. B191-3-5, B191-5-1, B182-5-6, B182-9-1, B177-7-5 and B180-8-4), to an increase in the proportion of modified flowers in B186-9-7 and B189-7-1 from 26% to 76% and 57% to 78%, respectively, from low- to high-stress environments.
Several plants investigated produced bimodal distributions of stigma–anther separation values (Fig. 5; see Seburn et al., 1990; Barrett and Harder, 1992). This pattern results from the production of two classes of flowers by a single individual: modified flowers with herkogamy values near zero and unmodified flowers with higher herkogamy values. The bimodality is mainly due to differences in filament elongation, which occur through changes in cell elongation 24 h prior to anthesis (Richards and Barrett, 1992). No other example is know in the literature in which a species produces this pattern of herkogamy variation. It can be viewed as a form of developmental instability, but also as an expression of phenotypic plasticity because environmental conditions modify the frequencies of modified and unmodified flowers.
Developmental instability can be influenced by a variety of internal and external factors including homozygosity, mutation, breakdown of adapted gene complexes, and environmental stress (for a recent review of developmental instability, see Polack, 2003). In E. paniculata, developmental instability of stamen position in the M-morph of E. paniculata is not easily explained by architectural (position) effects (Diggle, 2003), as the probability of producing a modified flower depends on a complex interaction between plant identity, floral position, inflorescence identity, age of the plant and environmental conditions (Barrett and Harder, 1992). Developmental instability could result from the increased selfing that is characteristic of selfing variants in dimorphic populations (Barrett et al., 1989). Inbreeding is thought to reduce the ability of genetic systems to buffer against environmental and developmental noise (Lerner, 1954; Jinks and Mather, 1955; Rendel, 1959; Levin, 1970; Fenster and Galloway, 1997). Another possibility is that developmental instability results from incomplete expression of mutations modifying stigma–anther separation (i.e. partial expressivity; Suzuki et al., 1986, p. 71; Richards and Barrett, 1992). Novel mutations may display variable expression when they first arise because their interactions with the rest of the genome have not been canalized by selection (see Waddington, 1942; Schmalhausen, 1949; Fenster and Galloway, 1997; De Visser et al., 2003). The fate of incomplete expressivity is often determined by selection acting on additional modifier genes that regulate trait expression resulting in either full or partial expressivity (Levin, 1970). This hypothesis predicts that developmental instability should be highest in populations where herkogamy mutations have recently spread (e.g. dimorphic populations) compared with those in which herkogamy mutations have been refined by selection (monomorphic populations). Field observations and glasshouse comparisons of genotypes from dimorphic and monomorphic populations generally support this hypothesis (S. C. H. Barrett, unpubl. res.).
Variation in herkogamy resulting from either phenotypic plasticity and/or developmental instability could allow individuals to modify rates of self- and cross-fertilization to match current environmental conditions. An increase in self-fertilization may be favoured in more stressful environments where either biotic (e.g. pollinator abundance) or abiotic conditions (e.g. water availability causing reduced density) limit cross-pollination (Stebbins, 1957; Elle and Hare, 2002; Elle, 2004; Moeller and Geber, 2005). Populations of E. paniculata in north-east Brazil grow in ephemeral pools and inundated areas where growing conditions change rapidly (Barrett and Husband, 1997). Increased selfing may be favoured in stressful environments experiencing drought. Under these conditions small plant stature and small population sizes could make it difficult to attract pollinators to ensure seed set. Plastic increases in autonomous self-pollination could then confer reproductive assurance in such populations. In contrast, plants growing in more favourable (e.g. wetter) environments are generally larger in size with more showy floral displays and may be able to attract sufficient pollinators to outcross successfully. To the extent that heterogeneous environments favour different mating strategies, within-plant variation of herkogamy in E. paniculata may represent an adaptive strategy in which individuals produce more than one functional class of reproductive organ, i.e. a multiple strategy sensu Lloyd (1984). Similar variation is evident in the production of self-fertilizing (cleistogamous) and open-pollinated (chasmogamous) flowers in many taxa (reviewed in Oakley et al., 2007). Elsewhere, discrete morphological variation in reproductive organs within individuals, e.g. somatic polymorphisms such as andromonoecy (male and hermaphrodite flowers), gynomonoecy (female and hermaphrodite flowers) and heteranthery (two kinds of anthers in the same flower) – has evolved repeatedly in the evolutionary history of angiosperms. Future field studies are required to investigate whether within-plant variation of herkogamy in E. paniculata has any adaptive value.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank R. Ness and W. Cole for seed collections and for valuable discussions, I. Dworkin for statistical advice, and A. Simonsen and J. Brett for assistance with data collection. M.V.-M. was supported by a post-doctoral fellowship from the Canada Research Chair's program to S.C.H.B. Funding for this work was provided by an NSERC Discovery Grant to S.C.H.B.
LITERATURE CITED
- Armbruster WS. Multilevel analysis of morphometric data from natural plant populations: insights into ontogenetic, genetic, and selective correlations in Dalechampia scandens. Evolution. 1991;45:1229–1244. doi: 10.1111/j.1558-5646.1991.tb04389.x. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Mulder CPH, Baldwin BG, Kalisz S, Wessa B, Nute H. Comparative analysis of late floral development and mating-system evolution in Tribe Collinsieae (Scrophulariaceae sl) American Journal of Botany. 2002;89:37–49. doi: 10.3732/ajb.89.1.37. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Pelabon C, Hansen TF, Mulder CPH. Floral integration, modularity and accuracy: distinguishing complex adaptations from genetic constraints. In: Pigliucci M, Preston K, editors. Phenotypic integration: studying the ecology and evolution of complex phenotypes. Oxford: Oxford University Press; 2004. [Google Scholar]
- Barrett SCH. Floral trimorphism and monomorphism in continental and island populations of Eichhornia paniculata (Spreng.) Solms. (Pontederiaceae) Biological Journal of the Linnean Society. 1985;25:41–60. [Google Scholar]
- Barrett SCH. The reproductive biology and genetics of island plants. Philosophical Transactions of the Royal Society of London Series B. Biological Sciences. 1996;351:725–733. [Google Scholar]
- Barrett SCH, Harder LD. Floral variation In Eichhornia paniculata (Spreng.) Solms (Pontederiaceae). II. Effects of development and environment on the formation of selfing flowers. Journal of Evolutionary Biology. 1992;5:83–107. [Google Scholar]
- Barrett SCH, Husband BC. Variation in outcrossing rates in Eichhornia paniculata: the role of demographic and reproductive factors. Plant Species Biology. 1990;5:41–55. [Google Scholar]
- Barrett SCH, Husband BC. Ecology and genetics of ephemeral plant populations: Eichhornia paniculata (Pontederiaceae) in northeast Brazil. Journal of Heredity. 1997;88:277–284. [Google Scholar]
- Barrett SCH, Shore JS. Variation and evolution of breeding systems in the Turnera ulmifolia L complex (Turneraceae) Evolution. 1987;41:340–354. doi: 10.1111/j.1558-5646.1987.tb05802.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Morgan MT, Husband BC. The dissolution of a complex genetic polymorphism: the evolution of self-fertilization in tristylous Eichhornia paniculata (Pontederiaceae) Evolution. 1989;43:1398–1416. doi: 10.1111/j.1558-5646.1989.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Berg RL. The ecological significance of correlation pleiades. Evolution. 1960;14:171–180. [Google Scholar]
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics. 1965;13:115–155. [Google Scholar]
- Brock MT, Weinig C. Plasticity and environment-specific covariances: an investigation of floral-vegetative and within flower correlations. Evolution. 2007;61:2913–2924. doi: 10.1111/j.1558-5646.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- Brunet J, Eckert CG. Effects of floral morphology and display on outcrossing in blue columbine, Aquilegia caerulea (Ranunculaceae) Functional Ecology. 1998;12:596–606. [Google Scholar]
- Carr DE, Fenster CB. Levels of genetic variation and covariation for Mimulus (Scrophulariaceae) floral traits. Heredity. 1994;72:606–618. [Google Scholar]
- Chang SM, Rausher MD. Frequency-dependent pollen discounting contributes to maintenance of a mixed mating system in the common morning glory Ipomoea purpurea. American Naturalist. 1998;152:671–683. doi: 10.1086/286198. [DOI] [PubMed] [Google Scholar]
- Darwin C. The various contrivances by which British and foreign orchids are fertilized by insects. London: Murray; 1862. [PMC free article] [PubMed] [Google Scholar]
- De Visser JAGM, Hermisson J, Wagner GP, et al. Evolution and detection of genetic robustness. Evolution. 2003;57:1959–1972. doi: 10.1111/j.0014-3820.2003.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Diggle PK. Architectural effects on floral form and function: a review. In: Stuessy TF, Mayer V, Hörandl E, editors. Deep morphology: toward a renaissance of morphology in plant systematics. Ruggell, Liechtenstein: Gantner Verlag; 2003. [Google Scholar]
- Elle E. Floral adaptations and biotic and abiotic selection pressures. In: Cronk QCB, Whitton J, Ree RH, Taylor IEP, editors. Plant adaptation: molecular genetics and ecology; Proceedings of an International Workshop held 11–13 December 2002 in Vancouver, British Columbia; Canada. Ottawa, Ontario: NRC Research Press; 2004. pp. 111–118. [Google Scholar]
- Elle E, Hare JD. Environmentally induced variation in floral traits affects the mating system in Datura wrightii. Functional Ecology. 2002;16:79–88. [Google Scholar]
- Ennos RA. Quantitative studies of the mating system in two sympatric species of Ipomoea (Convolvulaceae) Genetica. 1981;57:93–98. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4th edn. Essex: Longman; 1996. [Google Scholar]
- Fenster CB, Barrett SCH. Inheritance of mating system modifier genes in Eichhornia paniculata (Pontederiaceae) Heredity. 1994;72:433–445. [Google Scholar]
- Fenster CB, Galloway LF. Developmental homeostasis and floral form: evolutionary consequences and genetic basis. International Journal of Plant Sciences. 1997;158:S121–S130. [Google Scholar]
- Fishman L, Kelly AJ, Willis JH. Minor quantitative trait loci underlie floral traits associated with mating system divergence in Mimulus. Evolution. 2002;56:2138–2155. doi: 10.1111/j.0014-3820.2002.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Harder LD, Barrett SCH. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. [Google Scholar]
- Herlihy CR, Eckert CG. Evolutionary analysis of a key floral trait in Aquilegia canadensis (Ranunculaceae): genetic variation in herkogamy and its effect on the mating system. Evolution. 2007;61:1661–1674. doi: 10.1111/j.1558-5646.2007.00137.x. [DOI] [PubMed] [Google Scholar]
- Holtsford TP, Ellstrand NC. Genetic and environmental variation in floral traits affecting outcrossing rate in Clarkia temblorensis (Onagraceae) Evolution. 1992;46:216–225. doi: 10.1111/j.1558-5646.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- Husband BC, Barrett SCH. Genetic drift and the maintenance of the style length polymorphism in tristylous populations of Eichhornia paniculata (Pontederiaceae) Heredity. 1992;69:440–449. [Google Scholar]
- Jinks JL, Mather K. Stability in development of homozygotes and heterozygotes. Proceedings of the Royal Society B. Biological Sciences. 1955;143:561–578. doi: 10.1098/rspb.1955.0029. [DOI] [PubMed] [Google Scholar]
- Kim SH, Yi SV. Understanding relationship between sequence and functional evolution in yeast proteins. Genetica. 2007;131:151–156. doi: 10.1007/s10709-006-9125-2. [DOI] [PubMed] [Google Scholar]
- Krizek BA, Fletcher JC. Molecular mechanisms of flower development: an armchair guide. Nature Reviews Genetics. 2005;6:688–698. doi: 10.1038/nrg1675. [DOI] [PubMed] [Google Scholar]
- Kulbaba MW, Worley AC. Floral design in Polemonium brandegei (Polemoniaceae): genetic and phenotypic variation under hawkmoth and hummingbird pollination. International Journal of Plant Sciences. 2008;169:509–522. [Google Scholar]
- Lerner IM. Genetic homeostasis. London: Oliver and Boyd; 1954. [Google Scholar]
- Levin DA. Developmental instability and evolution in peripheral isolates. American Naturalist. 1970;104:343–353. [Google Scholar]
- Levin DA. Plant density, cleistogamy, and self-fertilization in natural populations of Lithospermum caroliniense. American Journal of Botany. 1972;59:71–77. [Google Scholar]
- Lloyd DG. Evolution of self-compatibility and racial differentiation in Leavenworthia (Cruciferae) Contributions from the Gray Herbarium of Harvard University. 1965;195:3–134. [Google Scholar]
- Lloyd DG. Variation strategies of plants in heterogeneous environments. Biological Journal of the Linnean Society. 1984;21:357–385. [Google Scholar]
- Lloyd DG, Webb CJ. The selection of heterostyly. In: Barret SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. [Google Scholar]
- MacDonald P, Du J. mixdist: finite mixture distribution models. 2008. http://www.r-project.org .
- Medrano M, Herrera CM, Barrett SCH. Herkogamy and mating patterns in the self-compatible daffodil Narcissus longispathus. Annals of Botany. 2005;95:1105–1111. doi: 10.1093/aob/mci129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller DA, Geber MA. Ecological context of the evolution of self-pollination in Clarkia xantiana: population size, plant communitites, and reproductive assurance. Evolution. 2005;59:786–799. doi: 10.1554/04-656. [DOI] [PubMed] [Google Scholar]
- Morgan MT, Barrett SCH. Reproductive correlates of mating system variation in Eichhornia paniculata (Spreng.) Solms (Pontederiaceae) Journal of Evolutionary Biology. 1989;2:183–203. [Google Scholar]
- Motten AF, Antonovics J. Determinants of outcrossing rate in a predominantly self-fertilizing weed, Datura stramonium (Solanaceae) American Journal of Botany. 1992;79:419–427. [PubMed] [Google Scholar]
- Müller H. The fertilisation of flowers. London: Macmillan; 1883. [Google Scholar]
- Oakley CG, Moriuchi KS, Winn AA. The maintenance of outcrossing in predominantly selfing species: ideas and evidence from cleistogamous species. Annual Review of Ecology Evolution and Systematics. 2007;38:437–457. [Google Scholar]
- Ornduff R. Reproductive biology in relation to systematics. Taxon. 1969;18:121–133. [Google Scholar]
- Ornduff R. The breakdown of trimorphic incompatibility in Oxalis section Corniculatae. Evolution. 1972;26:52–65. doi: 10.1111/j.1558-5646.1972.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed effects models in S and S-Plus. New York, NY: Springer; 2000. [Google Scholar]
- Polack M. Developmental instability: causes and consequences. Oxford: Oxford University Press; 2003. [Google Scholar]
- R-Development Core Team. R: a language and environment for statistical computing. Vienna: Foundation for Statistical Computing; 2008. [Google Scholar]
- Rendel JM. Canalization of the scute phenotype in Drosophila. Evolution. 1959;13:425–439. [Google Scholar]
- Richards JH, Barrett SCH. The development of heterostyly. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. [Google Scholar]
- Rick CM, Holle M, Thorp RW. Rates of cross-pollination in Lycopersicon pimpinellifolium: impact of genetic variation in floral characters. Plant Systematics and Evolution. 1978;129:31–44. [Google Scholar]
- Ritland C, Ritland K. Variation of sex allocation among eight taxa of the Mimulus guttatus species complex (Scrophulariaceae) American Journal of Botany. 1989;76:1731–1739. [Google Scholar]
- Schlichting CD. The evolution of phenotypic plasticity in plants. Annual Review of Ecology and Systematics. 1986;17:667–693. [Google Scholar]
- Schmalhausen II. Factors of evolution: the theory of stabilizing selection. Chicago, IL: University of Chicago Press; 1949. [Google Scholar]
- Seburn CNL, Dickinson TA, Barrett SCH. Floral variation in Eichhornia paniculata (Spreng) Solms (Pontederiaceae). 1. Instability of stamen position in genotypes from Northeast Brazil. Journal of Evolutionary Biology. 1990;3:103–123. [Google Scholar]
- Shore JS, Barrett SCH. Quantitative genetics of floral characters in homostylous Turnera ulmifolia var. angustifolia Willd (Turneraceae) Heredity. 1990;64:105–112. [Google Scholar]
- Stebbins GL. Self fertilization and population variability in higher plants. The American Naturalist. 1957;91:337–354. [Google Scholar]
- Stebbins GL. Flowering plants: evolution above the species level. Cambridge, MA: Harvard University Press; 1974. [Google Scholar]
- Suzuki DT, Griffiths AJT, Miller JH, Lewontin RC. An introduction to genetic analysis. 3rd edn. New York, NY: Freeman and Co; 1986. [Google Scholar]
- Takebayashi N, Wolf DE, Delph LF. Effect of variation in herkogamy on outcrossing within a population of Gilia achilleifolia. Heredity. 2006;96:159–165. doi: 10.1038/sj.hdy.6800780. [DOI] [PubMed] [Google Scholar]
- Thomson JD, Stratton DA. Floral morphology and cross-pollination in Erythronium grandiflorum (Liliaceace) American Journal of Botany. 1985;72:433–437. [Google Scholar]
- Via S, Gomulkiewicz R, De Jong G, Scheiner SM, Schlichting CD, Van Tienderen PH. Adaptive phenotypic plasticity: consensus and controversy. Trends in Ecology & Evolution. 1995;10:212–217. doi: 10.1016/s0169-5347(00)89061-8. [DOI] [PubMed] [Google Scholar]
- Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–565. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- Webb CJ, Lloyd DG. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkogamy. New Zealand Journal of Botany. 1986;24:163–178. [Google Scholar]
- Worley AC, Barrett SCH. Evolution of floral display in Eichhornia paniculata (Pontederiaceae): genetic correlations between flower size and number. Journal of Evolutionary Biology. 2001;14:469–481. [Google Scholar]