Summary
Background
High affinity IgE receptor (FcεRI) expression on blood dendritic cells reportedly correlates with serum IgE levels. Our studies demonstrate that plasmacytoid dendritic cells (pDCs) secrete pro-inflammatory cytokines (IL-6, TNF-α) following FcεRI stimulation – a mode of activation that simultaneously reduces expression of Toll-like receptor 9 (TLR9). Whether or not TLR9 and/or FcεRI levels and their function on dendritic cells relate to allergic status is unknown.
Objective
The aim of this study is to compare the innate (TLR9-mediated) immune response of human plasmacytoid dendritic cells to TLR9 and FcεRIα receptor expression in allergic and nonallergic subjects.
Methods
Basophil depleted mononuclear cell fractions containing pDCs were prepared from peripheral blood of allergic and non-allergic subjects. Intracellular TLR9 and surface FcεRIα expression in BDCA-2 positive cells were determined by flow cytometry. Activating anti-IgE antibody, anti-FcεRIα antibody, and TLR9 agonist were used to stimulate cell suspensions, with cytokine levels determined by ELISA.
Results
No difference in the frequency of pDCs was detected among allergic (n = 9) versus nonallergic (n = 11) subjects (P = 0.261). While there was also no difference in baseline expression of TLR9, pDCs from allergic subjects produced 6-fold less IFN-α when stimulated with CpG (P = 0.002). Conversely, there was higher FcεRIα expression (P = 0.01) on the pDCs of allergic subjects.
Conclusions
Impaired TLR9-dependent immune responses in human plasmacytoid dendritic cells are associated with allergic status and inversely correlate with FcεRIα expression. This impaired innate immune response among dendritic cells of allergic subjects may lead to more targeted therapeutic approaches and could provide a better understanding of the mechanisms underlying conventional and CpG-based immunotherapy.
Keywords: allergy, dendritic cells, IFN-α, plasmacytoid, TLR9, CpG-DNA, cytokines
INTRODUCTION
Plasmacytoid dendritic cells (pDCs) are essential components of innate immunity and are increasingly recognized for their capacity to alter antigen driven T-cell responses. While the frequency of pDCs (designated CD123+, CD303+, CD11c−) are normally less than 0.4% of peripheral blood mononuclear cells (PBMC) they are the dominant source of type I interferons, particularly in response to TLR9 agonists such as CpG [1,2].
The ability and inability of pDCs to produce interferon has been linked with certain viral infections and possibly asthma, respectively [3–5]. An imbalance of interferon production from DCs has also been associated with autoimmune diseases such as systemic lupus erythematosus (SLE), atherosclerotic plaque complications and the proliferation of psoriasis [6–8]. In the case of allergic inflammation, however, the role of the pDC and its capacity to secrete interferon needs further clarification.
Studies have shown that pDCs express the high affinity IgE receptor (FcεRI) [9] but its function has not been fully established in this cell type. Our laboratory previously reported that in pDCs, TLR9 and FcεRI mediated responses oppose one another in vitro [10,11]. TLR9 responses down-regulate FcεRI receptor expression. Conversely, pretreatment of pDCs with IgE receptor stimulation results in a reduction in TLR9 expression and ultimately a reduced capacity to secrete IFN-α. These findings indicate that cross-regulatory mechanisms may exist between innate (TLR9) and adaptive (FcεRI) immune receptors co-expressed on pDCs. It has not yet been established whether there is a clinical correlate to these in vitro findings. For example, are pDCs from atopic subjects phenotypically and/or functionally different from those of non-atopic individuals?
In the present study we address this question by analyzing pDCs from atopic and nonatopic individuals. As anticipated, FcεRI levels on pDCs track well with allergic status and serum IgE levels. In contrast, pDCs from allergic individuals produce comparatively less IFN-α upon activation with CpG-DNA (a TLR9 specific ligand). Our data show that this impaired innate immune response is not a result of reduced pDC frequencies or to the absence of TLR9, but is inversely related to FcεRI expression.
While the relationship of FcεRI expression and TLR9 function in pDCs are indicative of allergic disease, they could more importantly help to further illuminate the central role that pDCs play in orchestrating innate versus adaptive immune responses. With the advent of targeted TLR9/CpG-based immunotherapy, understanding the mechanism of this axis becomes more crucial [12].
METHODS
Subjects
Twenty volunteer subjects aged 21 to 64 living in the Baltimore-Washington metropolitan area consented to participation in this study. A detailed allergic history identified ten individuals with symptoms of chronic seasonal and perennial rhinitis and in some cases additional food allergy related symptoms and/or asthma in recent months. An IRB approved protocol for venipuncture was then performed. Subjects with a history of allergic symptoms and a positive multi-allergen screen (described below) were defined as allergic.
Serum IgE measurement
Plasma was collected with EDTA and stored at −20°C. Total IgE and multi-allergen screen (Phadiatop) were measured by the Johns Hopkins University Dermatology Allergy and Clinical Immunology Reference Laboratory using a fluorescent-based enzyme immunoassay (FEIA) that was performed on the ImmunoCAP 250 (Phadia, Kalamazoo, MI). The multiallergen screen is a single analytical measurement which detects IgE antibody specific for any of ~15 common aeroallergens that are known to induce the majority of aeroallergen related allergic disease (a negative result was defined as <0.35kIU/L).
Special Reagents and Buffers
The following reagents were purchased: crystallized human serum albumin (Calbiochem-Behring Corp, La Jolla, CA); PIPES, fetal calf serum (FCS), and crystallized BSA (Sigma Chemical Co, St Louis, MO); gentamicin, IMDM and nonessential amino acids (Life Technologies, Inc, Grand Island, NY); Percoll (Pharmacia Biotec, Inc, Piscataway, NJ). Phycoerytherin (PE)-labeled antibodies to human TLR9 (eBioscience, San Diego, CA), antihuman FcεRIα monoclonal antibody (eBioscience) conjugated with allophycocyanin (APC) or PE and relevant rat IgG1 isotype controls were used for flow cytometry and cell activation (eBioscience, San Diego, CA). Use of polyclonal goat anti-human IgE for stimulation and preparation of PIPES-albumin-glucose (PAG) and isotonic percoll has been described elsewhere [10]. Conditioned medium (C-IMDM) consisted of IMDM supplemented with 5% heat-inactivated (56°C for 30 minutes) FCS, 1x nonessential amino acids, and 10 μg/mL gentamicin. Western blot buffers were prepared according to manufacturer recommendations (Invitrogen, Carlsbad, CA).
Cell preparation
Blood specimens were processed as previously described [13]. Briefly, whole blood in 10mM EDTA underwent centrifugation at 500xg to isolate a buffy coat. Buffy coats were diluted 1:1 (vol/vol) with PAG-EDTA and then subjected to double-Percoll (1.075/1.081 g/mL) density centrifugation. This produced both a basophil-depleted cell (BDC) suspension (containing mostly mononuclear cells and platelets) and a basophil-enriched cell (BEC) suspension. Platelets were removed from the BDC suspension using four low-speed (100xg) centrifugations. In some circumstances (see Western blot section) pDCs were purified from the BDC suspensions using blood dendritic cell antigen (BDCA)-4 positive selection (Miltenyi Corp, Auburn, CA). An analysis of pDC suspensions prepared in this manner indicated >95% purity, as determined by BDCA-2 staining.
Culture conditions
To prevent any potential excessive manipulation associated with purifying pDCs from a limited volume of blood, BDC fractions were used for culture rather than purified pDC suspensions. These BDC fractions were cultured at 5×106/ml in C-IMDM containing 5% FCS. Specifically, 1.25×106 BDCs were added to 96-well plates (0.125 mL per well). After incubating for 15 minutes to equilibrate to 37°C and 5% CO2, an equal volume of stimulus or C-IMDM was added to duplicate cultures. The following stimuli were used for separate culture conditions in each subject, with optimal concentrations having been pre-determined: anti-FcεRI at 1:167 (75ng/mL), goat polyclonal anti-human IgE (3μg/mL), CpG ODN-2216 of the type A class (100nM), IL-3 (1ng/mL)), or CpG plus IL-3 (100nM and 1ng/mL respectively). After a 16-hour incubation period, supernatants were isolated and frozen at −70°C and ultimately assayed for cytokines by ELISA.
Cytokine measurement
TNF-α, and IL-6 protein levels in culture supernatants were determined using commercial ELISA kits (eBioscience, San, Diego, CA). IFN-α measurements were performed with an in-house assay [10], using mouse monoclonal anti-human IFN-α (clone MMHA-2 at 2.5 μg/mL) as the capture antibody and rabbit polyclonal anti-human IFN-α (250 ng/mL) as the detection antibody (PBL Biomedical Laboratories, Piscataway, NJ). Detection of the rabbit anti- IFN-α was achieved using goat anti-rabbit/HRP-conjugate at a 1:1000 dilution (R&D, Minneapolis, MN). Human recombinant IFN-α (Biosource) was used for assay standardization.
Flow cytometric analysis
A portion of each BDC suspension (5×106 cells) was fixed in 4% buffered paraformaldehyde and stored at −70°C in 10% DMSO for flow cytometry. pDCs staining positive for BDCA-2+ (CD303) were further analyzed for FcεRIα and intracellular TLR9 (CD289) with APC- or PE-conjugated antibodies and appropriate isotype controls using methods previously described [10]. Flow cytometry was performed using a FACSCalibur instrument. Histograms were prepared using FlowJo software (Tree Star Inc., Ashland, OR).
Western blot
Whole cell lysates were prepared using 5×105 purified pDCs. Samples were immediately boiled for five minutes in 45μL Tris-Glycine SDS buffer (Invitrogen, Carlsbad, CA) and stored at −70°C for future analysis. Protein from 2–5×105 pDCs or 15μL SeeBlue ladder (Invitrogen) was electrophoresed on a 10% Tris-Glycine gel (Invitrogen, Carlsbad, CA). After transfer to a nitrocellulose membrane (Trans-Blot, Biorad) the blots were incubated with PE conjugated monoclonal anti-human TLR9 (eBioscience) at 0.2μg/mL in 3% NFM overnight at −4°C. Blots were then incubated with a goat anti-rat IgG HRP antibody (Amersham Bioscience, Buckinghamshire, UK) for 45 minutes before developing. Membranes were re-probed for the p85 subunit of phosphatidylinositol 3-kinase for normalization of protein loading.
Statistics
Data are presented as mean ±SEM unless otherwise indicated. Statistical analysis was performed with InStat software (GraphPad, San Diego, CA) by a two-tailed nonparametric Mann-Whitney test unless otherwise stated. A linear regression or Spearman Rank formula was used to calculate correlations as indicated. P values ≤ 0.05 were considered significant.
RESULTS
Subject characteristics
To evaluate the innate immune response of pDCs in atopic disease eleven non-allergic and nine allergic subjects were analyzed with baseline characteristics shown in Table I. The multi-allergen IgE antibody assay (P < 0.001) and serum IgE levels (P = 0.012) were statistically elevated in the atopic group. One subject had a convincing atopic history but was reassigned to the non-allergic group as a result of a negative multi-allergen screen. No subjects with a negative history tested positive on multi-allergen screen. Seven of nine subjects defined as allergic and one of the non-allergic subjects commented on using antihistamines and/or topical nasal steroids routinely in the past year. There was no significant difference in age or gender in the two groups (P = 0.224 and 0.331 respectively).
Table I.
Participant baseline characteristics
| Characteristic | Non-Allergic (n = 11) | Allergic (n = 9) | P value |
|---|---|---|---|
| Age† | 36.5 (±3.5) | 30.9 (±1.7) | 0.224 |
| Male:Female | 2:9 | 4:5 | 0.331 |
| Allergic symptoms | 1/11 | 9/9 | < 0.001* |
| Serum IgE (kU/ml) † | 61.4 (±26.3) | 223 (±71.3) | 0.012* |
| Multi-allergen screen† | 0.36 (±0.01) | 25.1 (±9.5) | < 0.001* |
| Medication use | 1/11 | 7/9 | 0.010* |
considered significant
mean results ±SEM
FcεRIα expression on pDCs correlates with serum IgE
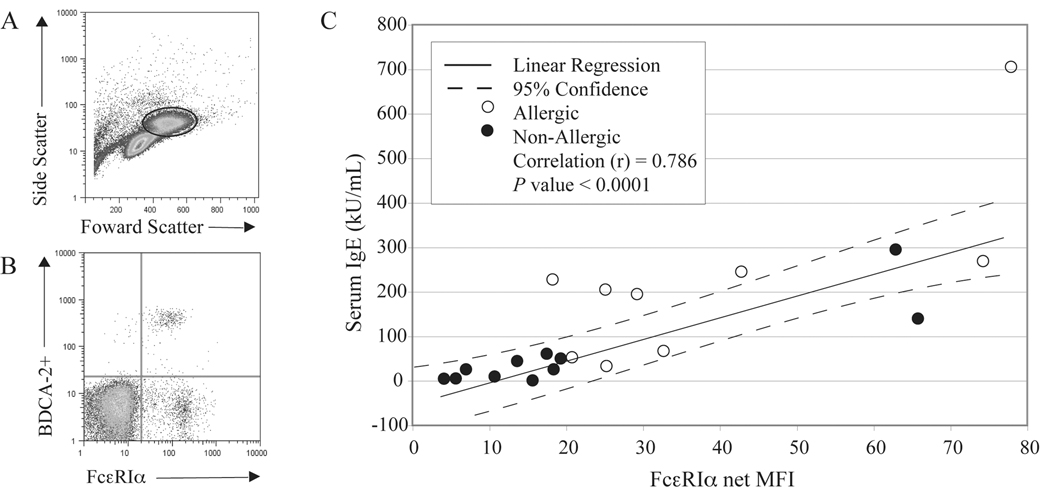
We first used flow cytometry to assess the frequency of pDCs within the basophildepleted mononuclear cell suspension and to determine the relative expression of FcεRIα found on this DC subtype. Figure 1A shows a representative plot from an allergic individual highlighting the side and forward scatter properties for the area of cells gated for BDCA-2+ pDCs. Overall, there were no significant differences in the frequency of pDCs detected among the BDC suspensions prepared from allergic (0.46±.05) vs. non-allergic (0.37±.07) subjects (P = 0.261). Figure 1B shows a representative scatter plot demonstrating the staining for FcεRIα co-expressed on these BDCA-2+ pDCs. In agreement with Foster et al. (9), we too observed that FcεRIα expression significantly correlates with serum IgE levels (P < 0.0001) (Figure 1C). Repeating the linear regression analysis for the independent non-atopic and allergic groups, the correlation values remain significant (P = 0.0003 and 0.0176 respectively), although the latter generally expressed more FcεRIα. The net median florescence intensity (net MFI) of FcεRIα on pDCs among the non-allergic subjects averaged 21.8±6.5 (n = 11), while that for the allergic group was 38.4±7.5 (n = 9, P = 0.009). While staining for alcian-blue cells indicated that these BDC suspensions were devoid of basophils (data not shown), a BDCA2−FcεRIα+ population was detected among these mixed leukocytes (Fig. 1B). Although not formally investigated, this population most likely represents a subset of monocytes and/or mDC, both of which are reported to express FcεRI.
FIG 1.
A, Representative flow cytometric data showing side and forward scatter of BDC suspensions and the area gated for analysis of pDCs. B, Representative staining of FcεRIα co-expressed on BDCA−2+ cells (i.e. pDCs) from an atopic individual. C, Serum IgE levels expressed in kU/mL combined from non-allergic (n = 11) and allergic (n = 9) subjects correlate with net median fluorescence intensity (net MFI) of FcεRIα. A linear regression (solid line) is plotted with 95% confidence intervals (dotted line). The correlation coefficient (r) for the plot is 0.786 and is highly statistically significant (P < 0.0001).
Baseline TLR9 receptor expression is similar among allergic and non-allergic subjects
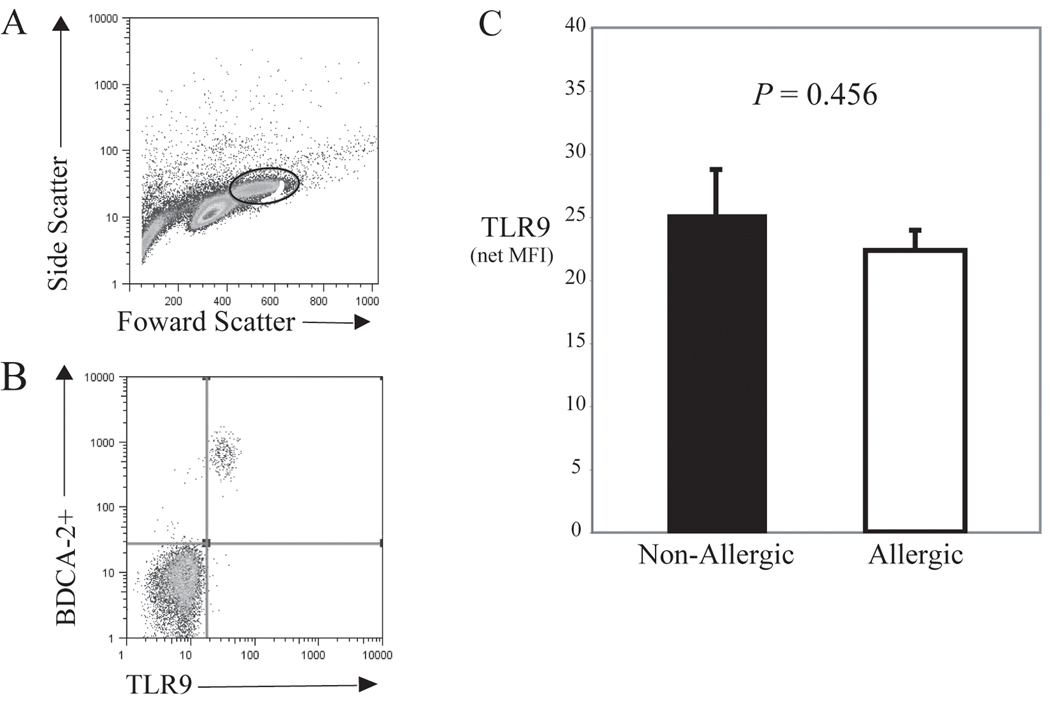
Of the cells that expressed BDCA-2+, >99% also stained for TLR9, as indicated by the representative scatter plot from an allergic individual shown in Fig. 2B. However, there was no significant difference in the overall intracellular TLR9 receptor expression detected between the two groups, as shown in Fig. 2C. The net MFI for TLR9 expression was 25.2±3.4 for nonallergics and 22.3±1.6 for allergics (P = 0.456). Similarly, there was no difference in the frequency of cells staining for TLR9 among groups (P = 0.175, see Table II). It is important to note that virtually no staining of TLR9 was detected in the BDCA-2− cell population gated for analysis (Fig 2B). A summary of the TLR9 and FcεRIα receptor expression data along with the frequency of BDCA-2+ cells is presented in Table II.
FIG 2.
A, Representative flow cytometric data from BDC suspensions are shown in a scatter plot with the indicated area gated for analysis of intracellular TLR9 expression in pDCs. B, A plot from a representative atopic individual indicating that virtually all BDCA−2+ cells (pDC) coexpress intracellular TLR9. C, Bar graph showing the averaged net MFI ±SEM for TLR9 expressed in non-allergic (n=11) vs. allergic (n=9) subjects, with no significant differences detected (P=0.456).
Table II.
Flow cytometric analysis of FcεRI and TLR9 expression in BDCA-2+ dendritic cells†
| Measurement | Non-Allergic (n = 11) | Allergic (n = 9) | P value |
|---|---|---|---|
| BDCA-2+ frequency | 0.371(±0.07) | 0.46 (±0.05) | 0.261 |
| TLR9 frequency | 0.35 (±0.07) | 0.48 (±0.08) | 0.175 |
| TLR9 ΔMFI† | 25.26 (±3.4) | 22.32 (±1.6) | 0.456 |
| FcεRIα ΔMFI† | 21.8 (±6.5) | 38.4 (±7.5) | 0.009* |
considered significant
average of median values of net MFI ±SEM
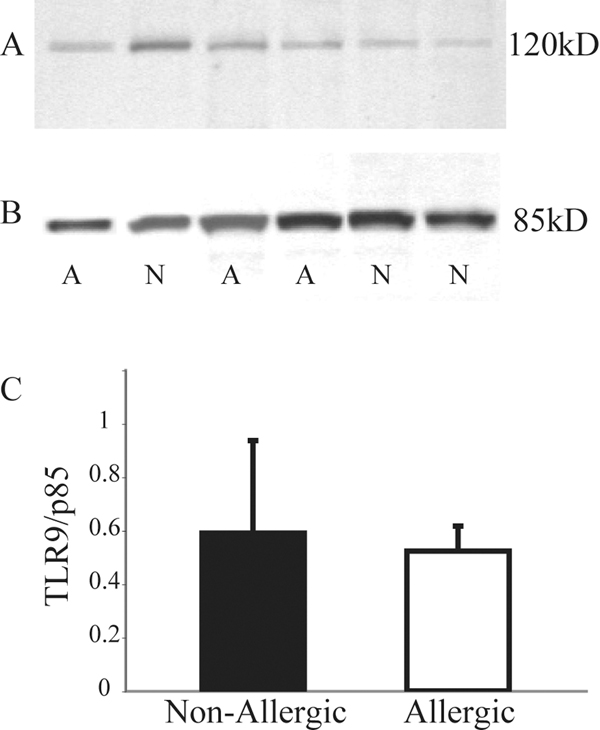
To help confirm the flow cytometry data as a method of detecting intracellular TLR9, Western blot analysis for TLR9 expression was performed using purified pDCs isolated from 6 of the 20 subjects (3 atopic and 3 non-atopic) with the data presented in Figure 3. A representative blot shows the presence of a single band (~120kd) that was identified using whole cell lysates of pure pDC suspensions (Fig 3A). These bands were each compared to the corresponding band for p85 (i.e. subunit of PI3 Kinase) to normalize for protein loading (Fig 3B). Although not powered for statistical analysis, densitometry measurements indicated no apparent differences in TLR9 expression levels among the 3 atopic and 3 non-atopic subjects (Fig 3C). This is consistent with the flow cytometry results.
FIG 3.
A, Representative Western blot using whole cell lysates of pure pDCs from three allergic (indicated by the letter A) and three non-allergic subjects (N), with each revealing a distinct band at 120kD after staining with monoclonal antibody to human TLR9. B, Membranes were reprobed with p85 for normalization of protein loading and subsequent TLR9 density calculations. C, Mean protein densitometry expressed as TLR9/p85 for allergic (n = 3) and non-allergic (n = 3) subjects reveals no apparent difference in protein expression.
Impaired innate immune function of dendritic cells in allergic subjects
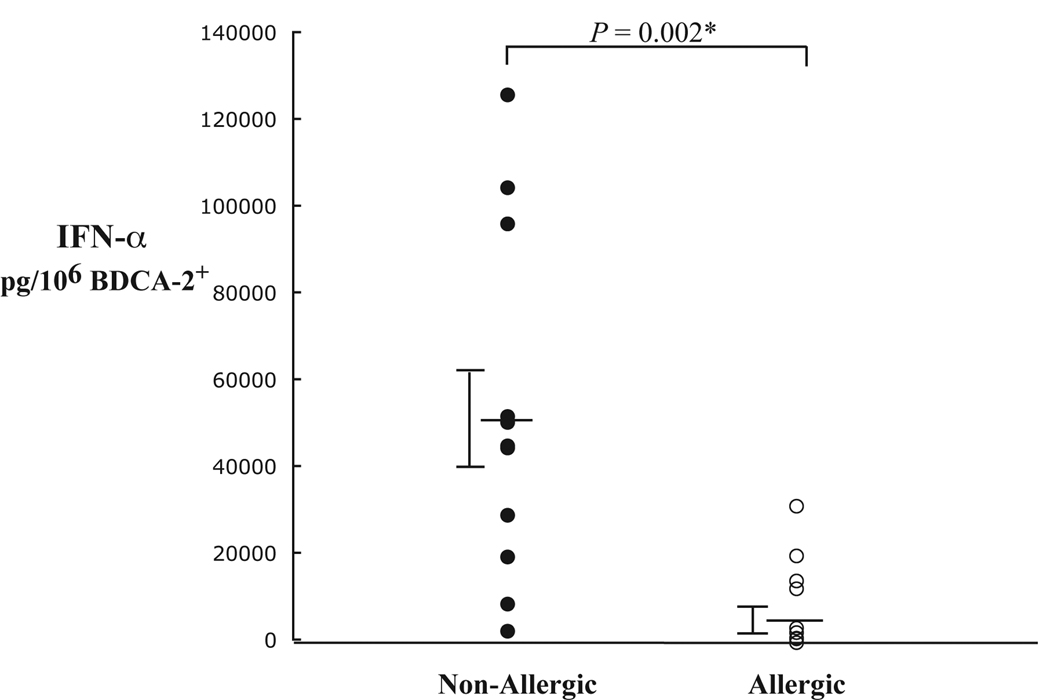
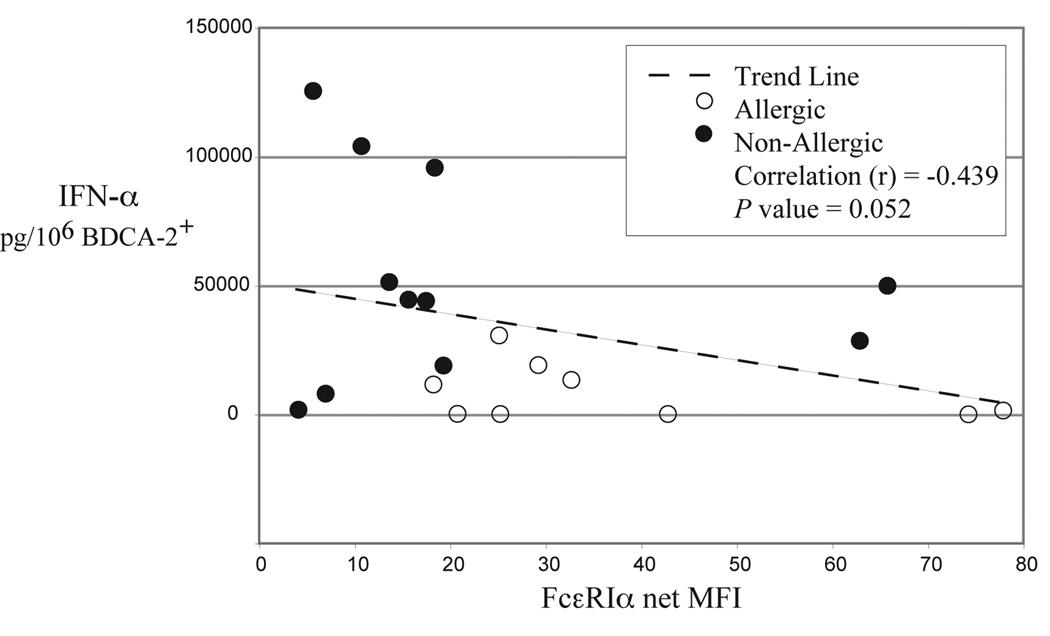
CpG-induced IFN-α production is a pDC specific response. Furthermore, the type A 2216 CpG oligo used for stimulation is a particularly potent stimulus for this cell. Therefore, impaired IFN-α production from these cells would indicate a significant derangement in the typical pDC innate response. As shown in Fig. 4, we found that IFN-α production from pDCs stimulated with the 2216 CpG oligo was 6-fold higher in non-allergic subjects as determined by ELISA (P = 0.002). The IFN-α results are normalized to the frequency of pDCs in these BDC suspensions, but were also significantly different between the two groups when comparing the actual levels measured (P = 0.03). As noted above, there was no difference in the expression of TLR9 or BDCA-2+ that could possibly account for this finding (Table II). There was, however, an inverse correlation (P = 0.052) of IFN-α production and FcεRI receptor expression on pDCs combined from both allergic and non-allergic individuals (Fig 5). This trend might be expected given our previously reported data that correlates IFN-α production with FcεRI down-regulation in vitro [10]. Baseline levels of IFN-α measured from pDCs that were not stimulated with CpG were undetectable in both groups (data not shown).
FIG 4.
Individual IFN-α cytokine measurements in BDC suspensions stimulated with CpG-DNA from non-allergic (n = 11) and allergic (n = 9) subjects as determined by ELISA. IFN-α values are plotted as pg/106 BDCA-2+ cells (pDC), with pDC frequency determined by flow cytometry. Averaged results show that IFN-α levels are 6-fold higher in non-allergic subjects compared to allergics (P = 0.002). * Indicates significance.
FIG 5.
IFN-α production from pDCs of allergic (n = 9) and non-allergic (n = 11) subjects is plotted against FcεRI receptor expression on these cells. A strong trend (P = 0.052) is noted indicating an inverse correlation using data from the combined groups.
IgE receptor mediated responses in unfractionated cells
It is worth noting that the FcεRI-dependent responses among the unfractionated BDC suspensions were increased in cells from the atopic group, and that this adaptive immune response inversely correlated with pDC specific IFN-α production. Thus, as innate immune function decreased in these cells, the adaptive immune response among unfractionated cells became more robust. Although not the primary focus of this report, we initially examined FceRI responses (IL-6 and TNF-α) in these cells as a positive control to ensure that adaptive immune function remained intact despite impaired innate immune function.
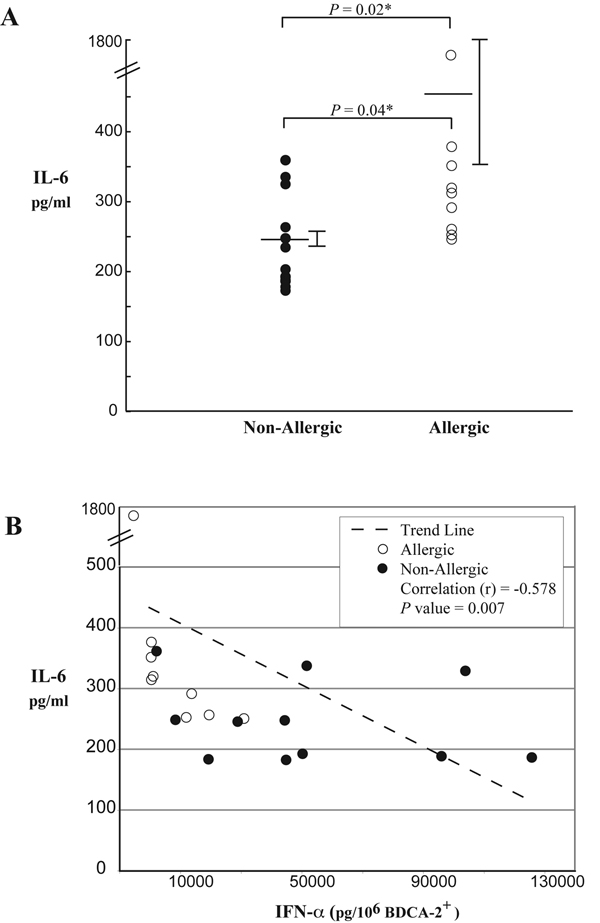
As shown in Figure 6A, IL-6 responses were significantly greater in atopic individuals (P = 0.02). Intracellular cytokine analysis by flow cytometry revealed that the source of IL-6 came from pDCs but also from cells other than pDCs such as monocytes and mDCs (data not shown). Figure 6B demonstrates an inverse correlation between IL-6 and IFN-α production from all subjects (P = 0.007). As expected IgE crosslinking did not produce detectable levels of IFN-α.Stimulation with CpG produced IL-6 levels 2-fold higher than baseline in both allergic and nonallergic subjects (P = 0.033) but was substantially less (~10 fold) when compared to FcεRI dependent activation.
FIG 6.
A, IL-6 production from basophil depleted cell suspensions (BDC) obtained from allergic (n = 9) and non-allergic subjects (n = 11) stimulated with anti-human FcεRI antibody. Allergic subjects produce approximately 2 fold higher levels of IL-6 (P = 0.02). B, IL-6 production is compared to IFN-α from BDC suspensions obtained from allergic and non-allergic subjects. An inverse correlation is noted (P = 0.007) indicating that impaired innate immune function is associated with a robust adaptive immune response in these cells.
DISCUSSION
Our data are consistent with the mounting evidence that plasmacytoid dendritic cells lie at an important immune axis orchestrating IFN-α associated Th1-like responses via innate immune receptors (TLR9) as well as FcεRI adaptive immune receptor operation and expression on these cells [14,15]. We also observed an inverse relationship of IFN-α production to FcεRI-dependent mononuclear cell production of IL-6. When viewed in the context of our previously reported in vitro data [10], these inverse relationships portend a link between innate and adaptive immunity in the pDC. The impaired IFN-α production seen in allergic subjects may signify a critical dysregulation of this immune axis.
Bufe and Gehlhar et al. have previously shown that virally stimulated PBMC from asthmatic adults and children produce lower levels of IFN-α compared to non-asthmatics [4,16]. Given what is known regarding this mode of stimulation with virus, these data suggest potential deficiencies in pDC numbers and/or reduced TLR9-dependent signaling. However, neither possibility was explored in these asthmatic subjects. Our data obtained from subjects with allergic rhinitis are consistent with these findings. More importantly, our study further illustrates that the observed decrease in IFN-α production is not due to reduced TLR9 expression, nor is it a result of reduced pDC frequencies among the allergic subjects.
IFN-α is a cytokine predominantly produced by pDCs, particularly in response to CpG (and viral RNA) and therefore observed levels are considered specific for this cell type [17]. However, the basophil-depleted cell fractions (BDC) used for cultures also contain mDCs (and other mononuclear cells) both of which produce IL-6 and TNF-α in response to FcεRI stimulation in a manner similar to pDCs (JTS, unpublished data). We chose to use fresh unfractionated cell suspensions for our cultures rather than purified pDCs to limit potential manipulation associated with the purification process. While binding of blood dendritic cell antigen (BDCA)-4 or BDCA-2 is often utilized for purification, it has been suggested that BDCA-2 and perhaps BDCA-4 ligation may in fact suppress induction of interferon production from pDCs [18]. While our approach was appropriate for IFN-α measurements, it did not allow for determination of the relative contribution of TNF-α and IL-6 secretion by either DC subtype. We therefore, accepted the likelihood that both pDC and mDC subtypes contribute to the production of IL-6 and TNF-α. Intracellular flow cytometry performed on stimulated BDC suspensions indicate that this is indeed the case. Nonetheless, the secreted IL-6 response observed in the unfractionated cell suspensions does serve as a surrogate marker of intact FcεRI-dependent adaptive immune responses in the general mononuclear cell suspension that includes pDCs. This increased adaptive immune response in allergic subjects may not be unique to pDCs. Noga et al. have recently reported a similar phenomenon in monocyte derived DCs whereby dendritic cells from allergic subjects stimulated with neurotrophins secrete more IL-6 and less IL-10 as compared to non-allergics [19].
We found that FcεRI receptor expression on pDCs correlates with serum IgE levels confirming the work of Foster et al [9]. We additionally found that FcεRI receptor expression inversely correlates with IFN-α production. It seems plausible that FcεRI density and/or interactions mediated through this adaptive immune receptor ultimately play a role in regulating the TLR9 mediated response. This concept was first suggested by experiments showing that IgE cross-linking inhibits TLR9-dependent IFN-α generation [10,20]. More recent data have substantiated these observations, with evidence that an innate immune receptor found on pDCs, ILT7, interacts with the γ subunit of FcεRI to further inhibit TLR mediated IFN-α production [21]. Assuming the hypothesis that IgE/FcεRI interactions suppress specific innate immune functions in pDCs is correct, it will be important to observe whether depleting IgE (perhaps using omalizumab) will correct such deficiencies.
The finding that IFN-α secretion induced by CpG did not correlate with TLR9 expression is particularly intriguing. Based on our previous in vitro observations that FcεRI cross-linking reduces TLR9 expression, we considered that pDCs from allergic subjects might be deficient in TLR9 [10]. However, this was not the case. This finding suggests that in our allergic cohort the level of IgE cross-linking that theoretically occurs from ambient exposure of allergens found in the environment is not sufficient to down regulate TLR9 in circulating pDCs. Instead, the phenomenon could be a transient event. Perhaps if pDCs were to be procured shortly after subjects are exposed to allergen (e.g. during peak ragweed season or following experimental challenge) or recovered from an allergic reaction site, a decrease in TLR9 would be evident in these cells. Alternatively, recent data indicate that downstream signaling elements such as IRF-7, osteopontin, and HMGB1 may all be required for TLR9 mediated IFN-α gene activation in pDCs [22–25]. A deficiency in any one of these components could theoretically impair IFN-α production in circulating pDCs of allergic subjects independently of TLR9 expression. Future studies are needed to address these hypotheses.
Recently, Creticos et al. presented a novel approach to immunotherapy using a vaccine consisting of the primary allergen in ragweed (Amb a 1) linked to a CpG-DNA motif [12]. Thus, pDCs must be viewed as a primary cell target for this vaccine by the mere fact that they express TLR9. While pDCs from the non-allergic subjects in our current study generated prodigious amounts of IFN-α via CpG activation, pDCs from allergic subjects demonstrated impaired production of IFN-α. It will be important to investigate whether allergic individuals treated with this CpG-conjugated vaccine become better producers of IFN-α. More work is needed to elucidate the mechanism of CpG-based immunotherapy but it is clear that pDCs should be the focus of these investigations.
Our data demonstrate that the impaired innate immune response of plasmacytoid dendritic cells stimulated with CpG correlates with allergic status and inversely correlates with adaptive immune receptor expression on these cells. These relationships may highlight key signals governing the Th1/Th2 axis associated with immune tolerance. The pDC is central to this immune axis as is further illustrated by the fact that clinically effective CpG-conjugated immunotherapy exploits the ability of the pDC to simultaneously detect FcεRI and TLR9 dependent stimuli.
Acknowledgments
We thank Professor Donald MacGlashan, Jr., Professor Susan MacDonald and Jackie Langdon for providing reagents, expert discussion, and constructive criticism.
Funding: Supported by an unrestricted education grant from Altana Pharma U.S., Florham Park, N.J., the Asthma and Allergic Diseases Cooperative Research Centers grant AI070345–01, and National Institute of Health grant R01A42221 (JTS).
Footnotes
The authors of this study have no conflicts of interest to declare.
REFERENCES
- 1.Schmidt B, Fujimura SH, Martin JN, Levy JA. Variations in plasmacytoid dendritic cell (PDC) and myeloid dendritic cell (MDC) levels in HIV-infected subjects on and off antiretroviral therapy. J Clin Immunol. 2006;26:55–64. doi: 10.1007/s10875-006-8401-3. [DOI] [PubMed] [Google Scholar]
- 2.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 3.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 4.Gehlhar K, Bilitewski C, Reinitz-Rademacher K, Rohde G, Bufe A. Impaired virus-induced interferon-alpha2 release in adult asthmatic patients. Clin Exp Allergy. 2006;36:331–337. doi: 10.1111/j.1365-2222.2006.02450.x. [DOI] [PubMed] [Google Scholar]
- 5.Schroder NW, Maurer M. The role of innate immunity in asthma: leads and lessons from mouse models. Allergy. 2007;62:579–590. doi: 10.1111/j.1398-9995.2007.01386.x. [DOI] [PubMed] [Google Scholar]
- 6.Meyers JA, Mangini AJ, Nagai T, Roff CF, Sehy D, van Seventer GA, van Seventer JM. Blockade of TLR9 agonist-induced type I interferons promotes inflammatory cytokine IFN-gamma and IL-17 secretion by activated human PBMC. Cytokine. 2006;35:235–246. doi: 10.1016/j.cyto.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogensensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006;114:2482–2489. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 8.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster B, Metcalfe DD, Prussin C. Human dendritic cell 1 and dendritic cell 2 subsets express FcepsilonRI: correlation with serum IgE and allergic asthmaHuman dendritic cell 1 and dendritic cell 2 subsets express FcepsilonRI: correlation with serum IgE and allergic asthma. J Allergy Clin Immunol. 2003;112:1132–1138. doi: 10.1016/j.jaci.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder JT, Bieneman AP, Xiao H, Chichester KL, Vasagar K, Saini S, Liu MC. TLR9- and FcepsilonRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol. 2005;175:5724–5731. doi: 10.4049/jimmunol.175.9.5724. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder JT, Chichester KL, Bieneman AP. Toll-like receptor 9 suppression in plasmacytoid dendritic cells after IgE-dependent activation is mediated by autocrine TNF-alpha. J Allergy Clin Immunol. 2007 doi: 10.1016/j.jaci.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 12.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, Li H, Coffman R, Seyfert V, Eiden JJ, Broide D. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder JT, MacGlashan DW, Jr, Kagey-Sobotka A, White JM, Lichtenstein LM. IgE-dependent IL-4 secretion by human basophils. The relationship between cytokine production and histamine release in mixed leukocyte cultures. J Immunol. 1994;153:1808–1817. [PubMed] [Google Scholar]
- 14.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 15.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, Zal T, Mellman I, Schroder JM, Liu YJ, Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 16.Bufe A, Gehlhar K, Grage-Griebenow E, Ernst M. Atopic phenotype in children is associated with decreased virus-induced interferon-alpha release. Int Arch Allergy Immunol. 2002;127:82–88. doi: 10.1159/000048173. [DOI] [PubMed] [Google Scholar]
- 17.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 18.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noga O, Peiser M, Altenahr M, Knieling H, Wanner R, Hanf G, Grosse R, Suttorp N. Differential activation of dendritic cells by nerve growth factor and brain-derived neurotrophic factor. Clin Exp Allergy. 2007 doi: 10.1111/j.1365-2222.2007.02832.x. [DOI] [PubMed] [Google Scholar]
- 20.Novak N, Kraft S, Bieber T. Unraveling the mission of FcepsilonRI on antigen-presenting cells. J Allergy Clin Immunol. 2003;111:38–44. doi: 10.1067/mai.2003.2. [DOI] [PubMed] [Google Scholar]
- 21.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 23.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, Hua J, An LL, Audoly L, La Rosa G, Bierhaus A, Naworth P, Marshak-Rothstein A, Crow MK, Fitzgerald KA, Latz E, Kiener PA, Coyle AJ. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 25.Ivanov S, Dragoi AM, Wang X, Dallacosta C, Louten J, Musco G, Sitia G, Yap GS, Wan Y, Biron CA, Bianchi ME, Wang H, Chu WM. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007 doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]