Abstract
Background and Aims
The genus Cytinus is composed of rootless, stemless and leafless parasites whose flowers are only visible during the reproductive period when they arise from the host tissues. Most of the taxa occur in Madagascar and South Africa, where mammal pollination has been suggested for one species. There is only one species in the Mediterranean region, and its pollination system has been unknown. Here, a long-term field observation study is combined with experimental pollination treatments in order to assess the pollination biology and reproductive system in the Mediterranean species Cytinus hypocistis.
Methods
Field studies were carried out in six populations in southern Spain over 4 years. Temporal and spatial patterns of variation in the composition and behaviour of floral visitors were characterized. Pollen loads and pollen viability were observed, and exclusion and controlled-pollination treatments were also conducted.
Key Results
Cytinus hypocistis is a self-compatible monoecious species that relies on insects for seed production. Ants were the main visitors, accounting for 97·4 % of total floral visits, and exclusion experiments showed that they act as true pollinators. They consistently touched reproductive organs, carried large pollen loads and transported viable pollen, although the different ant species observed in the flowers differed in their pollination effectiveness. The abundance of flying visitors was surprisingly low, and only the fly Oplisa aterrima contributed to fruit production and cross-pollination.
Conclusions
Mutualistic services by ant are essential for the pollination of Cytinus hypocistis. Although this parasite does not exhibit typical features of the ‘ant-pollination syndrome’, many other characteristics indicate that it is evolving to a more specialized ant-pollination system. The striking interspecific differences in the pollination systems of Mediterranean Cytinus (ant-pollinated) and some South African Cytinus (mammal-pollinated) make this genus an excellent model to investigate the divergent evolution of pollination systems in broadly disjunct areas.
Key words: Ant, breeding system, Cytinus hypocistis, Cytinaceae, insects, flies, Mediterranean Basin, parasitic plant, pollination, Rafflesiaceae
INTRODUCTION
Biological oddities have traditionally attracted the attention of naturalists, either for understanding their particular way of life or for the study of their evolutionary history. In the plant kingdom one of the most intriguing groups of plants are the parasites, which depend partially or completely on their host for the supply of carbon, nutrients and water (Stewart and Press, 1990; Nickrent, 2002a; Shen et al., 2006). The parasitic mode of life in angiosperms has evolved independently at least 11 times (Barkman et al., 2007), and is found in approx. 4000 species in 18–22 dicot families (Nickrent et al., 1998; Nickrent, 2002b). Among them, the Rafflesiaceae s.l. has been considered as one of ‘the most extraordinary products of the plant kingdom’ (Kuijt, 1969, p. 104) due to the extreme parasitism found in the species and the unique floral features of some of their taxa.
All the species in Rafflesiaceae s.l. lack chlorophyll, and show extreme reduction in their morphological characters, being rootless, stemless and leafless parasites with a vegetative body reduced to an endophytic system that lives within the roots or stems of the host (Kuijt, 1969; Meijer, 1993; de Vega et al., 2007). The flowers are only visible during the reproductive period when they arise from the host tissues (Kuijt, 1969; de Vega, 2007). Because of the close similarity of their lifestyles, these endophytic parasites were long considered to constitute a monophyletic group, the family Rafflesiaceae s.l. However, data from recent molecular phylogenetic studies together with differences in the morphology of flowers, ovaries and seeds indicate that these endophytes are composed of four independent families, even belonging to different orders: (1) Apodanthaceae, with minute, solitary flowers (genera Apodanthes, Berlinianche and Pilostyles); (2) Cytinaceae, with flowers arranged in an inflorescence (Bdallophyton and Cytinus); (3) Mitrastemonaceae, with hypogynous, solitary flowers (Mitrastema); and (4) Rafflesiaceae s.s., with large, solitary flowers (Rafflesia, Rhizanthes and Sapria) (Bouman and Meijer, 1994; Barkman et al., 2004; Nickrent et al., 2004; Davis et al., 2007).
Despite the wide morphological variation exhibited by their flowers (e.g. size, shape, colour, floral scent), Rafflesiaceae s.l. has been traditionally associated with the pollination syndrome of sapromyiophily (i.e. pollination by carrion flies; van der Pijl, 1961; Molau, 1995). However, it is noteworthy that nearly all studies on the pollination biology of these plants have focused on species of the Rafflesiaceae s.s. (e.g. Beaman et al., 1988; Bänzinger, 1996; Hidayati et al., 2000; Bänzinger and Pape, 2004; but see García-Franco and Rico-Gray, 1997). Field observations on the pollination of species of the other newly recognized families suggest that, besides flies, flowers are visited by a great diversity of pollen vectors including bees (in Apodanthaceae; de Vattimo, 1971; Gómez, 1983) and birds (in Mitrastemonaceae; Matuda, 1947; Beehler, 1994), although it remains uncertain as to which the true pollinators are. Only recently, a distinct and novel pollination system carried out by two ground-dwelling mammal species has been confirmed in a South African species of Cytinus (Johnson et al., 2008; S.D. Johnson, University of Pietermaritzburg, South Africa, unpubl. res.), a genus whose reproductive biology and pollination ecology remain poorly known.
The genus Cytinus comprises some eight species (Mabberley, 1997) that occur as holoparasites on the roots of diverse plant families. Most of the species occur in South Africa and Madagascar, where the genus presents one centre of diversification. In this area the species are dioecious and parasitize roots of Asteraceae, Hamamelidaceae, Rhamnaceae, Rosaceae and Rutaceae (Visser, 1981; Burgoyne, 2006). The other centre of diversification in this genus occurs in the Mediterranean Basin, where the taxa are monoecious and parasitize exclusively roots of Cistaceae (Webb, 1964; de Vega et al., 2007). Recent molecular analyses indicate that only one species occurs in the Mediterranean, Cytinus hypocistis, which comprises several genetic races that infect different Cistaceae species (de Vega et al., 2008). Regarding the pollination biology in Cytinus, besides the small mammals previously mentioned, birds and ants have been proposed as possible pollinating agents of the South African species (Visser, 1981), while bees have been suggested as pollination vectors in the Mediterranean (Harms, 1935). Pollination by flies has not been documented for Cytinus, in contrast with the sapromyiophilous pollination described in Bdallophyton, the other genus of Cytinaceae, endemic to the American tropics (García-Franco and Rico-Gray, 1997). It is noteworthy that reliable studies on the pollination of Cytinaceae species have been restricted to tropical or subtropical areas, with Mediterranean taxa conspicuously absent.
In the present study, we combined a long-term field observation study conducted over 4 years with experimental pollination treatments at six study sites in order to assess the pollination biology and reproductive systems of three genetic races of the Mediterranean Cytinus. The goals were to characterize temporal and spatial patterns of variation in the composition and behaviour of their floral visitors, to evaluate the relative importance of different insect species in the reproductive success of the taxa, and to characterize the breeding systems of Mediterranean Cytinus. This information enables us to address whether the pollination systems of the Mediterranean taxa resembles those of Cytinaceae species from tropical regions, and represents a first step towards a comparative study of the pollination biology of the intercontinental disjunct genus Cytinus.
MATERIAL AND METHODS
Study area
The study was carried out over 4 years (2002–2005) in six populations of Cytinus hypocistis L. on three Cistaceae host species: two populations parasitizing Cistus ladanifer (hereafter referred to as Cl1 and Cl2), two populations on Cistus salviifolius (Cs1 and Cs2) and two populations on Halimium halimifolium (Hh1 and Hh2). The populations were separated by 0·5–2·5 km and were located at 80–90 m a.s.l in Huelva province, south-west Spain. The climate is typically Mediterranean, and climatic conditions were similar in the six populations. During the study period, annual mean temperatures ranged from 16·6 to 17·6 °C and annual precipitation was 910·5 mm in 2001–2002 (September–August), 661·5 mm in 2002–2003 and 970·3 mm in 2003–2004; in 2004–2005 total precipitation was markedly lower, being only 226 mm.
Study species
Cytinus hypocistis is a monoecious perennial endophyte that shows a marked reduction of morphological characters, with a vegetative body reduced to an endophytic system that grows exclusively inside its host (de Vega et al., 2007). Only in the blooming period (March–May) are the plants visible, with inflorescences bursting through the host root tissues. Inflorescences appear at ground level in clusters of 1–22 on the same host root, and belong to the same or different, genetically distinct individuals (de Vega, 2007). The inflorescence is a simple short spike with 5·6 ± 0·1 (mean ± s.e.) female flowers (5·3–14·9 mm corolla width) at basal positions and 6·2 ± 0·1 male flowers (4·1–16·1 mm corolla width) at distal ones (de Vega, 2007). Female and male flowers produce similar amounts of nectar, approx. 1 µL d−1, which is accumulated in four nectariferous cavities, with higher nectar sugar concentration in female than in male flowers (32 % and 29 % sucrose equivalents, respectively; de Vega, 2007). Each flower lasts approx. 6 d and within inflorescences female flowers open 1–2 d earlier than males. The fruit is a berry that ripens from May to July and contains thousands of dust-like seeds (de Vega and de Oliveira, 2007).
Pollen vectors
In order to check the possibility of anemophily in C. hypocistis, airborne pollen was sampled in populations Cl1, Cs1 and Hh1. In each population three pollen traps were placed at ground level at 0·5–1 m around each of ten randomly chosen C. hypocistis plants. Each pollen trap comprised a plastic tube surrounded by a cigarette paper coated with a thin layer of petroleum jelly (see Arista and Talavera, 1994). Pollen traps were collected after a 48-h exposure time and the pollen content was determined under a microscope.
The composition and abundance of floral visitors was determined in the six populations over 4 years (2002–2005). In each population, clusters of C. hypocistis inflorescences on 15–22 different host individuals were studied. Diurnal censuses were always made by observers, situated ∼1·5 m from focal flower clusters, who recorded the number and activity of insects foraging in flowers during 15-min-long watching periods (‘censuses’ hereafter) separated by 15-min intervals throughout daytime (0800–2200). Given that many floral visitors were small ants, we made four additional censuses of 5 min each hour at 0·5 m from the plants in order to register their activity more accurately. Data for insect composition and abundance are shown pooled across years in the Results section. We recorded insect identity, number of visitors per flower (n = 4764 visits; throughout the text insect abundance are expressed as individuals per 5 min ), time spent in flowers from arrival to departure (n = 2248 foraging times) and number of flowers visited. The number of inter- and intra-plant movements (n = 656 foraging sequences) and type of reward collected were also recorded in every instance. A total of 262·65 h of diurnal censuses were made, distributed as follows: 64·5 h in Cl1, 29·4 h in Cl2, 45·75 h in Cs1, 28·25 h in Cs2, 44·5 h in Hh1 and 50·25 h in Hh2. In addition, a total of 16·5 h of nocturnal censuses (2200–0300) were made in populations Cl1, Cs1, Hh1 and Hh2 using red light to minimize the effect of illumination on flower visitors (Peitsch et al., 1992). Voucher specimens of all species recorded visiting flowers were collected, identified and measured under a binocular microscope.
Ant pollen loads
The size of pollen loads carried by individual ants was estimated for 15 individuals of each of the three most abundant species. Pollen was removed from all parts of the insects by dabbing them with small cubes of stained glycerine jelly under a binocular microscope. The jelly was then transferred to a slide, melted, and all pollen grains were counted. Ants were always collected while on C. hypocistis female flowers, to avoid the likely pollen contamination that would have ensued from collections in male flowers.
Pollen viability after contacting ant bodies
Most authors assume that ants are ineffective pollinators, mainly due to the ‘antibiotic hypothesis’ according to which ants produce antibiotic secretions, mainly from metapleural glands, that reduced pollen viability and germination percentage (Beattie et al., 1984, 1985; Hull and Beattie, 1988). To determine the possible effect of ant secretions on pollen germinability, experiments were carried out using the three most abundant diurnal ant species and the nocturnal one (see Results). Male flowers of C. hypocistis were collected, and in the laboratory their pollen was mixed and distributed between vials. One ant was placed in each vial and left in contact with the pollen for 10 or 30 min. Ten individual ants per species were used for each exposure period. In other vials, control pollen was left for the same time but with no ant present. Pollen from the vials was then transferred to culture slides with Brewbaker–Kwack medium (200 g L−1 sacarose, 0·1 g L−1 boric acid, 0·3 g L−1 calcium nitrate, 0·2 g L−1 magnesium sulphate and 0·1 g L−1 potassium nitrate) for an incubation period of 48 h, and germination rate was then assessed under a microscope (n = 11 476 ant-treated pollen grains and 11 270 control pollen grains counted).
Breeding system and reproductive success
The breeding system of C. hypocistis was studied by hand-pollinations in plants from five populations (Cl1, Cs1, Cs2, Hh1 and Hh2) using within-plant self-pollination (n = 212 flowers pollinated), cross-pollination (n = 173), total exclusion (to test for possible apomixis, n = 65), and controls (open pollination, n = 6071; fruit set after open pollination was recorded in all populations from 2002 to 2005). Before pollination, each female bud was covered using a plastic tube with cotton on the top to exclude pollinators. A subsample of the hand-pollinated female flowers was collected 1 week after the pollinations, softened with KOH, stained with aniline blue, and observed under a fluorescence microscope to detect pollen tubes in the style and ovules (Martin, 1959). The remaining pollinations were left in the field until fruit maturation, and the percentage of flowers that set fruit was recorded. The number of seeds per fruit was counted in 15 fruit per treatment by means of a particle counter (Coulter Multisizer III, Beckman Coulter, UK). Germination tests are critical for assessing seed viability, but unfortunately for Cytinus in particular, and for all species traditionally included in Rafflesiaceae s.l. in general, such studies are extremely difficult due to the special requirements for germination. So, we used an alternative technique described by de Vega and de Oliveira (2007) to examine the viability of seeds. Briefly, seeds were treated successively with Franklin's and with Jeffrey's softening fluids, and then with Herr's clearing fluid. The combined action of the two softening fluids caused sufficient seed coat rupture to allow Herr's fluid to clear the seed tissues, permitting effective observation of the embryo and hence assessment of viability.
Exclusion experiments
In order to assess the contribution of flying visitors to pollination we tried to exclude ants from C. hypocistis plants applying Tanglefoot® around the base of 35 inflorescences. However, ant exclusion was ineffective since inflorescences arise at ground level and ants forage primarily on plants under which they nest. To asses the effect of ants as pollinators, flying visitors were excluded from flowers. In populations Cl1, Cs1 and Hh2, 10–14 plants were randomly selected and were covered prior to blooming with a small net tent (15 cm tall, 0·25 mm2 mesh) so that only small, crawling insects could visit the enclosed inflorescences. In each population we used inflorescences freely exposed to all insects as ‘controls’. Pollen tubes in the style and ovules were examined in a subsample of flowers, as described above, and seed number was counted in 16 fruit in each population.
Statistical analysis
To analyse whether flower choice made by ants was statistically independent of the sex Wilcoxon's tests were used, and when comparing the time spent in female and male flowers we used generalized linear models (GLZs) with a Poisson distribution and a log link-function (for statistical analysis the GLZs were used when the response variables followed distributions other than the normal). The number of flowers visited was compared among insect species and between type of visitors (flying and ants) by means of a GLZ with a Poisson distribution and a log link-function.
Pollen loads on insects were compared among species by means of a one-way ANOVA, and differences in pollen germinability between control and ant-treated pollen were compared using a GLZ with a Poisson distribution and a log link-function.
Differences in fruit set after hand-pollination treatments were compared using a GLZ with a binomial distribution and a logit link-function, and the same method was used to compare fruit set after exclusion experiments vs. control. Mean numbers of seeds per fruit between pollination treatments and populations were compared by means of a factorial ANOVA, and differences among seeds produced after exclusion and control experiments were compared by a one-way ANOVA. All statistical analyses were performed in Statistica 6·0 (StatSoft, 2001). Throughout the text, all means are shown ± s.e.
RESULTS
Floral visitor assemblage
Pollen traps showed a low presence of C. hypocistis pollen grains (0·9 ± 0·23 grains per trap; n = 90) indicating that wind-pollination is unlikely in this species.
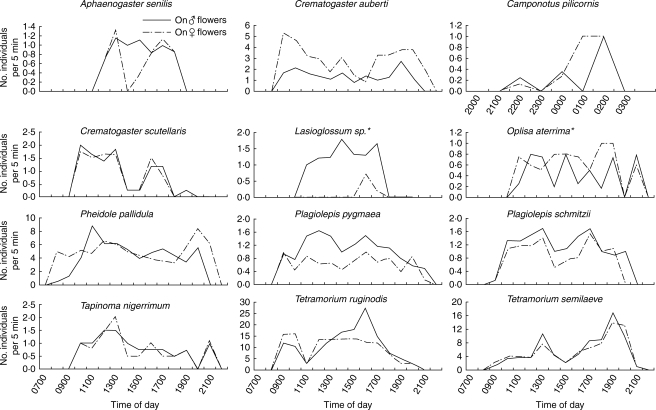
The flowers of C. hypocistis were visited by 17 insect species belonging to six families, with ants being much more abundant than flying visitors and accounting for 97·4 % of total floral visits (n = 4638; Table 1, Fig. 1). The most abundant daytime ant species were Pheidole pallidula (35·3 % of total floral visits), Plagiolepis pygmaea (19·4 %) and Crematogaster auberti (13·4 %). During the night only the ant Camponotus pilicornis visited the flowers of C. hypocistis, appearing in 50 % of the nocturnal censuses, but only accounting for 0·3 % of total visits. These four ant species and Plagiolepis schmitzii foraged on C. hypocistis flowers in all the years of the study, their abundance being consistent across years, but none of them appeared in all the populations studied (Table 1). The other ant species recorded only appeared in some years (Aphaenogaster senilis and Crematogaster scutellaris: 2002, 2003 and 2005; Tapinoma nigerrimum: 2002, 2004; Tetramorium ruginode: 2002; and Tetramorium semilaeve: 2002–2004).
Table 1.
Characteristics and behaviour of all Cytinus hypocistis pollinators that accounted for at least 0·2 % of total floral visits (pooled across all populations and years).
| Taxa | Body length (mm) | Metapleural glands1 | Pollen load2 | Percent of total floral visits | Reward3 | Populations |
|---|---|---|---|---|---|---|
| Hymenoptera | ||||||
| Formicidae | ||||||
| Aphaenogaster senilis | 6·89 ± 0·25 | Yes | – | 1·53 | N | Cs1, Cs2, Hh2 |
| Camponotus pilicornis | 7·76 ± 0·24 | No | – | 0·29 | N | Cl1, Cs1 |
| Crematogaster auberti | 3·69 ± 0·09 | Yes | 123·33 ± 15·25 (53–237) | 13·43 | N | Cl1, Cs1 Cs2, Hh2 |
| Crematogaster scutellaris | 4·79 ± 0·23 | Yes | – | 2·69 | N | Cl1, Cs1, Hh2 |
| Pheidole pallidula | 2·11 ± 0·10 | Yes | 122·53 ± 17·64 (40–268) | 35·26 | N | Cl1, Cl2, Cs1, Hh1, Hh2 |
| Plagiolepis pygmaea | 1·63 ± 0·06 | 278·33 ± 47·47 (66–669) | 19·37 | N | Cl1, Cl2, Cs1, Cs2 | |
| Plagiolepis schmitzii | 2·16 ± 0·19 | Yes | – | 5·71 | N | Hh1, Hh2 |
| Tapinoma nigerrimum | 3·82 ± 0·17 | Yes | – | 1·36 | N | Cs1, Hh2 |
| Tetramorium ruginode | 3·05 ± 0·07 | Yes | – | 9·05 | N | Hh2 |
| Tetramorium semilaeve | 2·32 ± 0·10 | Yes | – | 8·67 | N | Cl1, Cs2, Hh2 |
| Halictidae | ||||||
| Lasioglossum sp. | 6·95 ± 0·21 | – | – | 1·09 | P, N | All |
| Diptera: Rinophoridae | ||||||
| Oplisa aterrima | 3·25 ± 0·38 | – | – | 1·34 | N, TS | All |
1 Presence or absence of metapleural glands.
2 Pollen loads are given for the three most abundant species. Values are means ± s.e. (range in parentheses).
3 Reward collected: P, pollen; N, nectar; TS, tepal secretions.
Insects that accounted for less than 0·2 % of total floral visits were Apis mellifera (Apidae, 0·02 % of total floral visits), Bombus terrestris (Apidae, 0·04 %), an unidentified Braconidae (0·04 %), an unidentified Camilidae (0·02 %), and an unidentified Rinophoridae (0·06 %).
Fig. 1.
Flowers and pollinators of Cytinus hypocistis: (A) Cytinus hypocistis, (B) Aphaenogaster senilis, (C) Crematogaster auberti, (D) Pheidole pallidula, (E) Tetramorium semilaeve and (F) Oplisa aterrima. Scale bars = 5 mm.
The number of individuals foraging on female and male flowers of C. hypocistis varied greatly among ant species, ranging from 0·18 (C. pilicornis) to 12·05 individuals per 5 min (T. ruginode, Table 2). It is noteworthy that, although the two Tetramorium species appeared in a low number of censuses, when they did appeared they were very abundant (Fig. 2, Table 2).
Table 2.
Behaviour of visitors while foraging on Cytinus hypocistis flowers. All species are ants except those marked with an asterisk
| No. of individuals per 5 min |
Time spent in flowers (s) |
|||||
|---|---|---|---|---|---|---|
| Species | Female flowers | Male flowers | Female flowers | Male flowers | No flowers visited | Within-plant movements (%) |
| Aphaenogaster senilis | 0·48 ± 0·12 | 0·55 ± 0·14 | 20·90 ± 3·91 | 74·85 ± 24·84 | 3·65 ± 0·50 | 76·19 |
| Camponotus pilicornis | 0·18 ± 0·07 | 0·24 ± 0·08 | 9·00 ± 3·76 | 8·67 ± 5·17 | 3·00 ± 0·52 | 40·00 |
| Crematogaster auberti | 2·88 ± 0·34 | 1·51 ± 0·166 | 70·05 ± 11·70 | 60·71 ± 8·59 | 2·97 ± 0·11 | 95·87 |
| Crematogaster scutellaris | 1·03 ± 0·18 | 1·03 ± 0·17 | 69·00 ± 35·13 | 24·33 ± 17·84 | 2·64 ± 0·17 | 92·13 |
| Lasioglossum sp.* | 0·15 ± 0·11 | 1·18 ± 0·18 | 15·48 ± 4·86 | 13·71 ± 2·11 | 2·62 ± 0·29 | 58·62 |
| Oplisa aterrima* | 0·69 ± 0·09 | 0·49 ± 0·09 | 27·09 ± 6·15 | 19·41 ± 8·43 | 2·77 ± 0·22 | 61·29 |
| Pheidole pallidula | 4·94 ± 0·65 | 4·94 ± 0·53 | 69·81 ± 6·24 | 80·80 ± 8·39 | 2·62 ± 0·07 | 87·57 |
| Plagiolepis pygmaea | 0·60 ± 0·06 | 1·00 ± 0·07 | 41·01 ± 4·38 | 43·43 ± 3·99 | 2·83 ± 0·09 | 98·61 |
| Plagiolepis schmitzii | 0·93 ± 0·12 | 1·17 ± 0·12 | 145·96 ± 23·13 | 61·18 ± 15·73 | 2·65 ± 0·14 | 92·50 |
| Tapinoma nigerrimum | 0·76 ± 0·18 | 0·78 ± 0·17 | 37·05 ± 17·52 | 16·34 ± 3·86 | 2·82 ± 0·18 | 96·97 |
| Tetramorium ruginode | 10·74 ± 1·19 | 12·05 ± 1·73 | 75·14 ± 26·74 | 86·11 ± 26·71 | 3·01 ± 0·52 | 91·94 |
| Tetramorium semilaeve | 5·26 ± 0·74 | 5·78 ± 0·88 | 27·40 ± 8·13 | 38·47 ± 7·38 | 3·32 ± 0·31 | 89·47 |
Values are means ± s.e.
Fig. 2.
Insects visiting the flowers of Cytinus hypocistis during the day. Note that for each species the y-axis differs, and the x-axis is also different for the nocturnal Camponotus pilicornis. Differences in the number of male and female flowers visited were observed for C. auberti (Z = 3·96; P < 0·0001), O. aterrima (Z = 1·91; P = 0·055) Lasioglossum (Z = 3·69; P = 0·0002), P. pygmaea (Z = 7·71; P = <0·0001) and P. schmitzii (Z = 1·97; P = 0·049). All species are ants except those marked with an asterisk.
Flying visitors mainly foraged in C. hypocistis inflorescences without ants; however, aggressive interactions between ants and flying insects were not seen. The fly Oplisa aterrima (1·3 % of overall floral visits, and 51·2 % of visits of flying insects; Fig. 1F) and solitary female bees of Lasioglossum sp. (1·1 % and 41·6 %, respectively) were the most abundant flying species (Table 1). Oplisa aterrima was observed in all the populations and years studied, with its abundance being consistent across years, whereas Lasioglossum appeared in all populations but only during 2005 (Table 1).
Floral visitor foraging behaviour
Ants and flying insects visited both male and female flowers and made contact with the plant reproductive organs when foraging for floral resources, acting as potential pollinators (Fig. 1). Ants foraged for nectar, Lasioglossum foraged both for pollen and nectar, and Oplisa aterrima for nectar and secretions from tepal glandular trichomes; none of them destroyed floral organs. Some of the visitors foraged more frequently and spent more time in flowers of a given sex (Figs 2 and 3; Table 2). For each species, the number of visits and mean time spent in a flower was highly variable throughout the day (Fig. 3).
Fig. 3.
Time spent by different pollinators on female and male flowers of Cytinus hypocistis during the day. Note that for each species the y-axis differs, and the x-axis is also different for the nocturnal Camponotus pilicornis. Aphaenogaster senilis spent more time on male flowers (χ2 = 11·83; P = 0·0006), whereas P. schmitzii (χ2 = 22·01; P < 0·0001) and T. nigerrima (χ2 = 22·01; P < 0·0001) spent more time on female flowers. In the other species no differences were found. All species are ants except those marked with an asterisk.
The mean number of flowers foraged per plant by each individual insect ranged from 2·62 to 3·65, the differences being significant among species (χ2 = 20·574, d.f. = 9, P = 0·015). The mean number of flowers per plant foraged by ants (2·83 ± 0·05; all species combined) was similar to that for winged pollinating insects (2·68 ± 0·20, χ2 = 1·075, d.f. = 1, P = 0·299). Between 76·19–96·97 % of movements made by ants in the daytime were between flowers of the same plant; however, such movements were much less for the nocturnal C. pilicornis (40 %; Table 2). Oplisa aterrima and Lasioglossum made approx. 60 % of flights within plants (Table 2).
Pollen loads and germination ability
All ants consistently touched the anthers with their bodies and carried pollen loads that were significantly different among species (χ2 = 20·79, d.f. = 2, P < 0·0001). Pollen loads were not related to body size, since the smallest ant, P. pygmaea, carried the highest pollen loads (Table 1). In all ant species pollen grains were attached to the head, thorax and gaster. There was no detrimental effect to pollen germination after contact with the integument of C. auberti and P. pallidula, either in the treatment of 10 min or 30 min (P > 0·25 in all cases; Table 3). In contrast, P. pygmaea and C. pilicornis significantly reduced pollen germination (Table 3); however, it is noteworthy that, even in the latter two species, a considerable fraction of pollen remained viable after contact.
Table 3.
Percentage germination of Cytinus hypocistis pollen without contacting ants (control) or after contact with ants of different species (ant-treated pollen) for either 10 or 30 min. Sample sizes (total number of pollen grains examined) are shown in parentheses
| Contact time and ant species | Germination (%) |
F | P | |
|---|---|---|---|---|
| Control | Ant-treated | |||
| 10 min | ||||
| Crematogaster auberti | 64·05 ± 7·94 (2305) | 58·45 ± 5·75 (2358) | 0·327 | 0·574 |
| Pheidole pallidula | 65·52 ± 7·65 (2207) | 53·56 ± 7·19 (2226) | 1·298 | 0·269 |
| 30 min | ||||
| Crematogaster auberti | 57·92 ± 6·50 (2203) | 47·85 ± 3·98 (2128) | 1·744 | 0·203 |
| Pheidole pallidula | 60·75 ± 7·19 (2204) | 51·15 ± 6·37 (2181) | 0·999 | 0·331 |
| Plagiolepis pygmaea | 63·39 ± 9·94 (1088) | 26·71 ± 5·29 (1319) | 11·725 | 0·008 |
| Camponotus pilicornis | 63·28 ± 4·48 (1263) | 14·76 ± 9·27 (1264) | 22·192 | 0·001 |
Pollen loads were not measured on winged insects. However, during the censuses it was observed that the fly Oplisa aterrima and the bee Lasioglossum carried pollen loads on the legs and body.
Breeding system
None of the permanently covered female flowers produced fruit, indicating that pollinators are required to set seeds. Microscopic observations revealed many pollen tubes on the style and also penetrating the ovules of all flowers examined in both geitonogamous and cross-pollination, giving evidence of self-compatibility in this species. Cytinus hypocistis showed high values of fruit set after self-pollination (89·85–98·11 %), similar to those observed after cross-pollination and open-pollination (Table 4). Significant differences were not found either among treatments (χ2 = 2·59, d.f. = 2, P = 0·274) or populations (χ2 = 9·35, d.f. = 4, P = 0·063). Differences among years for each population in the percentage of fruit set after open-pollination were not significant (in all cases P > 0·6).
Table 4.
Percentage fruit set of Cytinus hypocistis after self-pollination, cross-pollination, open-pollination, ant-only pollination or total exclusion of flower visitors (sample sizes are shown in parentheses)
| Cl1 | Cs1 | Cs2 | Hh1 | Hh2 | |
|---|---|---|---|---|---|
| Self-pollination | 97·9 (53) | 95·4 (41) | 90·4 (69) | 100 (15) | 100 (34) |
| Cross-pollination | 94·1 (36) | 96·1 (23) | 90·4 (68) | 98·0 (11) | 99·1 (35) |
| Open-pollination | 97·3 (1555) | 98·9 (871) | 96·2 (707) | 96·9 (1258) | 98·5 (1680) |
| Ant-only pollination | 80·2 (55) | 86·3 (42) | – | – | 88 (31) |
| Total exclusion | 0 (15) | 0 (10) | 0 (15) | 0 (10) | 0 (15) |
The mean number of seeds per fruit was 19 305 ± 2171 in self-pollinated flowers, 19 200 ± 2333 in cross-pollinated flowers, and 24 163 ± 3847 in open-pollinated flowers. Mean number of seeds per fruit did not vary among treatments (F = 0·96, d.f. = 2, P = 0·394) or among populations (F = 2·68, d.f. = 4, P = 0·083).
Exclusion experiments
Flying-visitor exclusion experiments showed that ants act as true pollinators. In the three populations studied, plants exposed only to ants showed a fruit set of 80–87 %, and in all flowers analysed pollen tubes were observed on the styles and ovules.
The fruit set of plants of the ‘ant-only’ treatment was statistically lower than that of control flowers (χ2 = 43·07, d.f. = 1, P < 0·0001), suggesting that flying insects also contribute significantly to fruit production in C. hypocistis despite the low quantitative importance of their visitation rates. Although differences among populations were statistically significant (χ2 = 7·53, d.f. = 2, P = 0·023), fruit set after the ant-only treatment was similarly reduced relative to control plants in all populations (population-by-treatment interaction not significant: χ2 = 2·33, d.f. = 2, P = 0·311). However, the mean number of seeds per fruit in plants exposed only to ants (19 418 ± 2776) was not significantly different to those produced after open-pollination (F = 1·02, d.f. = 1, P = 0·321).
Ripe seeds from ant-, cross-, open- and self-pollination seemed to be viable. All of them presented a small embryo composed of about ten cells surrounded by an endosperm composed of larger cells, as is typical of C. hypocistis seeds (see de Vega and de Oliveira, 2007).
DISCUSSION
This study has shown that Cytinus hypocistis is a self-compatible species consistently exhibiting a high fruit set and extensive seed production in natural conditions (i.e. open-pollination). The species relies on insects for seed production since neither wind pollination nor agamospermy were recorded. The long-term field observations and exclusion experiments strongly suggest that ants are the main pollinators in C. hypocistis, accounting for most flower visits in all populations and years, and yielding a fruit set close to 80 % when other potential visitors were excluded. Accordingly, this study joins a growing body of evidence highlighting the prominent role that ants can play in some plant–pollinator systems (Wyatt, 1981; Peakall, 1989; Peakall and Beattie, 1989; Gómez and Zamora, 1992, 1999; García et al., 1995; Ramsey, 1995; Gómez et al., 1996; Puterbaugh, 1998; Gómez, 2000; Schürch et al., 2000; Ashman and King, 2005; Sugiura et al., 2006). However, contrary to most previous studies in which single ant species were involved in pollination (but see Gómez et al., 1996; Ashman and King, 2005), in C. hypocistis as many as ten species can be considered as true pollinators.
The effectiveness of a given pollinator not only depends on their abundance and frequency of flower visitation but also on the efficiency with which they remove and deposit pollen (quantity and quality components of the plant–pollinator interaction, respectively; Herrera, 1987, 1989). Ants were the most abundant pollinators during both the day and night, accounting for 97·36 % of floral visits, and while foraging for nectar they frequently touched the anthers (each male flower may produce more than one million pollen grains; de Vega, 2007) and the wide stigmatic surface (4·2 × 2·5 mm in size). In addition, we have demonstrated that ants carried large pollen loads and that they spent a long time foraging at each flower, which increases the probability for them to contact the reproductive organs of C. hypocistis, and thus promoting pollination. Furthermore, pollen germination experiments showed that the ants' metapleural secretions did not play a major role in inhibiting pollen viability, in contrast to the hypothesis stating that these antimicrobial secretions mostly prevent the transfer of viable pollen (Beattie et al., 1984, 1985; Hull and Beattie, 1988; but see Peakall and Beattie, 1989; Gómez and Zamora, 1992; García et al., 1995; Gómez et al., 1996). It may be possible that the high pollen production could ‘neutralize’ the effects of ant metapleural secretions, as has been suggested by Hull and Beattie (1988). Two of the most abundant ant species, P. pallidula and C. auberti, have metapleural glands, but they did not significantly reduce pollen viability. It is noteworthy that although C. pilicornis reduced pollen germinability (the same trend has been observed for other Camponotus species; Beattie et al., 1985; Hull and Beattie, 1988), this ant species has no metapleural glands, a common feature in the genus (Hölldobler and Engel-Siegel, 1984). Perhaps antibiotic substances are distributed throughout the cuticle or may be secreted from a different gland (Beattie et al., 1985; Hull and Beattie, 1988). Our findings have shown that when different ant species are pollinating a given plant species they can differ in their pollination effectiveness, as is well known to occur in other pollinator guilds (Herrera, 1987; Larsson, 2005; Fumero-Cabán and Meléndez-Ackerman, 2007). Consequently, an ant can be either an excellent or a poor pollinator, the same as for a bee or a butterfly, and so generalizations about the ability and quality of ants as pollinators should be carefully considered.
Even though C. hypocistis flowers offer abundant nectar and pollen, the diversity and abundance of flying insects foraging in this parasite is surprisingly low, in spite of many bees, flies, butterflies, moths and beetles being observed foraging on the flowers of other plant species nearby. Similar observations were done in more than 50 populations elsewhere in Spain and Morocco (C. de Vega, CSIC, Sevilla, Spain, unpubl. res.). Since winged-visitors were only observed in inflorescences with few or no ants, it is likely that the presence of ants deterred flying pollinators from visiting the flowers, as has been suggested in other species (McDade and Kinsman, 1980; Schürch et al., 2000; Philpott et al., 2005). Among the flying insects, only the fly O. aterrima was a predictable visitor of C. hypocistis, appearing in all populations and years, whereas other winged insects only appeared during the drought year of 2005, when the dramatically low availability of flowers of other species in the populations probably forced them to search for alternative feeding resources. Temporal variation in the identity and importance of pollinators has been observed in other plant species (Herrera, 1988; Gómez and Zamora, 1999; Thompson, 2001; Ivey et al., 2003); nonetheless, for C. hypocistis, despite the differences found in pollinator composition between years, the pollinator assemblage that accounted for most visits remained remarkably constant.
When pollinator assemblages are taxonomically heterogeneous, determination of the behaviour of each species is important for understanding their differential contribution to plant reproduction (Herrera, 1987; Giménez-Benavides et al., 2007; Li et al., 2008). Our findings suggest that despite their low proportional frequency, the fly O. aterrima might play a key role in producing outcrossed progeny in C. hypocistis, since it tends to visit few flowers per individual and move more often among different plants. In contrast, ants exhibited a restricted foraging area and repeatedly visited individual flowers and inflorescences, thus potentially enhancing the occurrence of geitonogamous selfing, as has been observed in other ant-pollination systems (Svensson, 1985; Peakall and Beattie, 1991; Gómez and Zamora, 1992). Although selfing often negatively affects plant reproductive success, self-compatibility may be advantageous in species with small population sizes, subjected to strong stochastic demographic fluctuations (Stebbins, 1957; Schemske and Lande, 1985; Pannell and Barrett, 1998) as it occurs in C. hypocistis (de Vega, 2007; de Vega et al., 2008). In C. hypocistis pollination exclusively by ants resulted in lower fruit set than open-, cross- and geitonogamous pollinations; however, it is unlike that this decrease is due to the self-pollen carried by ants, because geitonogamous hand-pollinations result in similar levels of fruit set to cross-pollinations. Our field observations suggest that ant behaviour could account for this reduction in fruit set, given that ants frequently avoided some particular flowers without contacting them. However, it could also be plausible that some female flowers did not receive enough visits (and enough pollen) to set fruit. Since there was no decrease in the seed production of ant-pollinated flowers compared to that obtained after hand- and open-pollinations, our findings indicate that when ants continuously visit flowers they act as effective pollinators.
Selfing and different types of crosses can affect the proportion of viable seeds (Wallace, 2003; Jersáková and Johnson, 2006), such that self-pollinated plants sometimes produce fruits with empty seeds (Ferdy et al., 2001; Smithson, 2006). However, in C. hypocistis seeds produced after open-, cross-, geitonogamous and ant-pollination shared similar features, containing a small embryo and endosperm Although it has been not possible so far to experimentally induce the germination of C. hypocistis, a recent genetic study based on AFLP markers has suggested that geitonogamous seeds produced naturally do germinate and the resulting plants become established in the host populations (de Vega, 2007). Moreover, the low levels of genetic diversity recorded in all populations (de Vega, 2007; de Vega et al., 2008) were similar, or even lower, than those characteristically found in species with high selfing rates (Nybom, 2004).
To summarize, our results indicate that mutualistic services by ants are essential for the pollination of the Mediterranean root holoparasite C. hypocistis. As in other reported cases of ant pollination (Peakall et al., 1987; Peakall and Beattie, 1991), C. hypocistis does not exhibit some features of the purported ‘ant-pollination syndrome’ (Hickman, 1974), such as small, open flowers with a small amount of pollen, readily accessible nectaries with very little nectar production, and a low number of ovules. However, many other features exhibited by this parasite –self-compatibility, the large amount of pollen produced per flower, the big size of reproductive organs, the long floral life span and the low stature of plants – seem to play a crucial role in enhancing the effectiveness of ants as efficacious pollen carriers. Although it seems unlikely that these features of C. hypocistis have evolved to facilitate pollination by ants, the fact that they contribute to successful pollination, together with our recent findings suggesting that floral scents attract ants (C. de Vega, CSIC, Sevilla, Spain, unpubl. res.), may indicate that this species could be evolving to a more specialized pollination system. The sweet scent emitted by C. hypocistis flowers is clearly different to the yeasty and unpleasant scent produced by the dark flowers of Bdallophyton (also Cytinaceae) to attract their pollinators (Sarcophagidae flies; García-Franco and Rico-Gray, 1997). Perhaps some minor floral morphological changes and altered scent production could be playing key roles in a shift between those pollination systems. The fact that striking interspecific differences exist between the pollination systems of Mediterranean Cytinus (ant-pollinated) and South African Cytinus (mammal-pollinated; Johnson et al., 2008; S. D. Johnson, University of Pietermaritzburg, South Africa, unpubl. res.) make this genus an excellent model to investigate the divergent evolution of pollination systems in broadly disjunct areas. Floral morphology, nectar characteristics and floral scents could be playing crucial roles in establishing mutualistic pollination interactions in Cytinus, and may be critical for understanding the evolution of pollination systems in this poorly known genus.
ACKNOWLEDGMENTS
We thank R. Vega-Durán, E. Durán, and J. L. García-Castaño for help with the field work, R. G. Albaladejo for taking some of the pictures of ants, and A. Tinaut (ants), F. J. Ortiz (bees) and A. Sánchez (flies) for identification of insects. Special thanks to R. G. Albaladejo and S. D. Johnson for helpful comments on the manuscript. This work was supported by a Ph.D. grant from the Spanish Ministerio de Educación y Ciencia to C. de V. and by funds from the Ministerio de Educación y Ciencia and Fondo Europeo de Desarrollo Regional (REN2002–04354-C02-02, CGL 2005–01951 to M.A.) and Consejería de Innovación, Ciencia y Empresa, Junta de Andalucía (EXC/2005/RNM-204 to S.T., P06-RNM-01627 to C.M.H.).
LITERATURE CITED
- Arista M, Talavera S. Pollen dispersal capacity and pollen viability of Abies pinsapo Boiss. Silvae Genetica. 1994;43:155–158. [Google Scholar]
- Ashman TL, King EA. Are flower-visiting ants mutualists or antagonists? A study in a gynodioecious wild strawberry. American Journal of Botany. 2005;92:891–895. doi: 10.3732/ajb.92.5.891. [DOI] [PubMed] [Google Scholar]
- Bänzinger H. Pollination of a flowering oddity: Rhizanthes zippelii (Blume) Spach (Rafflesiaceae) Natural History Bulletin of the Siam Society. 1996;44:113–142. [Google Scholar]
- Bänzinger H, Pape T. Flowers, faeces and cadavers: natural feeding and laying habits of flesh flies in Thailand (Diptera: Sarcophagidae, Sarcophaga spp.) Journal of Natural History. 2004;38:1677–1694. [Google Scholar]
- Barkman TJ, Lim SH, Salleh M, Nais J. Mitochondrial DNA sequences reveal the photosynthetic relatives of Rafflesia, the world's largest flower. Proceedings of the National Academic of Sciences of the USA. 2004;101:787–792. doi: 10.1073/pnas.0305562101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkman TJ, McNeal JR, Lim SH, et al. Mitochondrial DNA suggests at least 11 origins of parasitism in angiosperms and reveals genomic chimerism in parasitic plants. BMC Evolutionary Biology. 2007;7:248. doi: 10.1186/1471-2148-7-248. doi:10.1186/1471-2148-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman RS, Decker PJ, Beaman JH. Pollination of Rafflesia (Rafflesiaceae) American Journal of Botany. 1988;75:1148–1162. [Google Scholar]
- Beattie AJ, Turnbull C, Knox RB, Williams EG. Ant inhibition of pollen function: a possible reason why ant pollination is rare. American Journal of Botany. 1984;71:421–426. [Google Scholar]
- Beattie AJ, Turnbull C, Hough T, Jobson S, Knox RB. The vulnerability of pollen and fungal spores to ant secretions: evidence and some evolutionary implications. American Journal of Botany. 1985;72:606–614. [Google Scholar]
- Beehler BM. Canopy-dwelling honeyeater aggressively defends terrestrial nectar resource. Biotropica. 1994;26:459–461. [Google Scholar]
- Bouman F, Meijer W. Comparative structure of ovules and seeds in Rafflesiaceae. Plant Systematics and Evolution. 1994;193:187–212. [Google Scholar]
- Burgoyne PM. A new species of Cytinus (Cytinaceae) from South Africa and Swaziland, with a key to the Southern African species. Novon. 2006;16:315–319. [Google Scholar]
- Davis CC, Latvis M, Nickrent DL, Wurdack KJ, Baum DA. Floral gigantism in Rafflesiaceae. Science. 2007;315:1812. doi: 10.1126/science.1135260. [DOI] [PubMed] [Google Scholar]
- Ferdy JB, Loriot S, Sandmeier M, Lefranc M, Raquin C. Inbreeding depression in a rare deceptive orchid. Canadian Journal of Botany. 2001;79:1181–1188. [Google Scholar]
- Fumero Cabán J, Meléndez-Ackerman E. Relative pollination effectiveness of floral visitors of Pitcairnia angustifolia (Bromeliaceae) American Journal of Botany. 2007;94:419–424. doi: 10.3732/ajb.94.3.419. [DOI] [PubMed] [Google Scholar]
- García MB, Antor RJ, Espadaler X. Ant pollination of the paleoendemic dioecious Borderea pyrenaica (Dioscoreaceae) Plant Systematics and Evolution. 1995;198:17–27. [Google Scholar]
- García-Franco JG, Rico-Gray V. Reproductive biology of the holoparasitic endophyte Bdallophyton bambusarum (Rafflesiaceae) Botanical Journal of the Linnean Society. 1997;123:237–247. [Google Scholar]
- Giménez-Benavides L, Dötterl S, Jürgens A, Escudero A, Iriondo JM. Generalist diurnal pollination provides greater fitness in a plant with nocturnal pollination syndrome: assessing the effects of a Silene–Hadena interaction. Oikos. 2007;116:1461–1472. [Google Scholar]
- Gómez JM. Effectiveness of ants as pollinators of Lobularia maritima: effects on main sequential fitness components of the host plant. Oecologia. 2000;122:90–97. doi: 10.1007/PL00008840. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Zamora R. Pollination by ants: consequences of the quantitative effects on a mutualistic system. Oecologia. 1992;91:410–418. doi: 10.1007/BF00317631. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Zamora R. Generalization vs. specialization in the pollination system of Hermantophylla spinosa (Crucifera) Ecology. 1999;80:796–805. [Google Scholar]
- Gómez JM, Zamora R, Hódar JA, García D. Experimental study of pollination by ants in Mediterranean high mountain and arid habitats. Oecologia. 1996;105:236–242. doi: 10.1007/BF00328552. [DOI] [PubMed] [Google Scholar]
- Gómez LD. Flora Costaricensis: Rafflesiaceae. Fieldiana Botany. 1983;13:89–93. [Google Scholar]
- Harms H. Rafflesiaceae, Tribe IV. Cytineae. In: Engler A, Harms H, editors. Die Natürlichen Planzenfamilien. Leipzig: Wilhelm Engelman; 1935. pp. 276–281. [Google Scholar]
- Herrera CM. Components of pollinator ‘quality’: comparative analysis of a diverse insect assemblage. Oikos. 1987;50:79–90. [Google Scholar]
- Herrera CM. Variation in mutualism: the spatiotemporal mosaic of a pollinator assemblage. Biological Journal of the Linnean Society. 1988;35:95–125. [Google Scholar]
- Herrera CM. Pollinator abundance, morphology, and flower visitation rate: analysis of the ‘quantity’ component in a plant–pollinator system. Oecologia. 1989;80:241–248. doi: 10.1007/BF00380158. [DOI] [PubMed] [Google Scholar]
- Hickman JC. Pollination by ants: a low-energy system. Science. 1974;184:1290–1292. doi: 10.1126/science.184.4143.1290. [DOI] [PubMed] [Google Scholar]
- Hidayati SN, Meijer W, Baskin JM, Walck JL. A contribution to the life history of the rare Indonesian holoparasite Rafflesia patma (Rafflesiaceae) Biotropica. 2000;32:408–414. [Google Scholar]
- Hölldobler B, Engel-Siegel H. On the metapleural gland of ants. Psyche. 1984;91:201–224. [Google Scholar]
- Hull DA, Beattie AJ. Adverse effects on pollen exposed to Atta texana and other North American ants: implications for ant pollination. Oecologia. 1988;75:153–155. doi: 10.1007/BF00378829. [DOI] [PubMed] [Google Scholar]
- Ivey CT, Martinez P, Wyatt R. Variation in pollinator effectiveness in swamp milkweed (Asclepias incarnata) American Journal of Botany. 2003;90:212–223. doi: 10.3732/ajb.90.2.214. [DOI] [PubMed] [Google Scholar]
- Jersáková J, Johnson SD. Lack of floral nectar reduces self-pollination in a fly-pollinated orchid. Oecologia. 2006;147:60–68. doi: 10.1007/s00442-005-0254-6. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Burgoyne PM, Harder LD, Dötterl S. Evidence for pollination by small mammals in the parasitic plant Cytinus visseri (Cytinaceae) South African Journal of Botany. 2008;74:369. [SAAB Annual Meeting Abstracts] [Google Scholar]
- Kuijt J. The biology of parasitic flowering plants. Berkeley, CA: University of California Press; 1969. [Google Scholar]
- Larsson M. Higher pollinator effectiveness by specialist than generalist flower-visitors of unspecialized Knautia arvensis (Dipsacaceae) Oecologia. 2005;146:394–403. doi: 10.1007/s00442-005-0217-y. [DOI] [PubMed] [Google Scholar]
- Li P, Luo Y, Bernhardt P, Kou Y, Perner H. Pollination of Cypripedium plectrochilum (Orchidaceae) by Lasioglossum spp. (Halictidae): the roles of generalist attractants versus restrictive floral architecture. Plant Biology. 2008;10:220–230. doi: 10.1111/j.1438-8677.2007.00020.x. [DOI] [PubMed] [Google Scholar]
- Mabberley D. The plant book. 2nd edn. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Martin FW. Staining and observing pollen tubes in the styles by means of fluorescence. Stain Technology. 1959;34:125–128. doi: 10.3109/10520295909114663. [DOI] [PubMed] [Google Scholar]
- Matuda E. On the genus Mitrastemon. Bulletin of the Torrey Botanical Club. 1947;74:133–141. [Google Scholar]
- McDade LA, Kinsman S. The impact of floral parasitism in two neotropical hummingbird-pollinated plant species. Evolution. 1980;34:944–958. doi: 10.1111/j.1558-5646.1980.tb04033.x. [DOI] [PubMed] [Google Scholar]
- Meijer W. Rafflesiaceae. In: Kubitzki K, Rohwer JG, Bittrich V, editors. The families and genera of vascular plants, vol. II. Berlin: Sringer-Verlag; 1993. pp. 557–563. [Google Scholar]
- Molau U. Reproductive ecology and biology. In: Press MC, Graves JD, editors. Parasitic plants. London: Chapman and Hall; 1995. pp. 141–177. [Google Scholar]
- Nickrent DL. Plantas parásitas en el mundo. In: López-Sáez JA, Catalán P, Sáez L, editors. Plantas Parásitas de la Península Ibérica e Islas Baleares. a. Madrid: Mundi-Prensa Libros S.A; 2002. pp. 7–28. [Google Scholar]
- Nickrent DL. Origen filogenético de las plantas parásitas. In: López-Sáez JA, Catalán P, Sáez L, editors. Plantas Parásitas de la Península Ibérica e Islas Baleares. b. Madrid: Mundi-Prensa Libros S.A; 2002. pp. 29–56. [Google Scholar]
- Nickrent DL, Duff RJ, Colwell AE, et al. Molecular phylogenetic and evolutionary studies of parasitic plants. In: Soltis DE, Soltis PS, Doyle JJ, editors. Plant molecular systematics II. Boston, MA: Kluwer; 1998. pp. 211–241. [Google Scholar]
- Nickrent DL, Blarer A, Qiu YL, Vidal-Russell R, Anderson FE. Phylogenetic inference in Rafflesiales: the influence of rate heterogeneity and horizontal gene transfer. BMC Evolutionary Biology. 2004;4:40. doi: 10.1186/1471-2148-4-40. doi:10.1186/1471-2148-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybom H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Molecular Ecology. 2004;13:1143–1155. doi: 10.1111/j.1365-294X.2004.02141.x. [DOI] [PubMed] [Google Scholar]
- Pannell JR, Barrett SCH. Baker's law revised: reproductive assurance in a metapopulation. Evolution. 1998;52:657–668. doi: 10.1111/j.1558-5646.1998.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Peakall R. The unique pollination of Leporella fimbriata (Orchidaceae): Pollination by pseudocopulating male ants (Myrmecia urens, Formicidae) Plant Systematics and Evolution. 1989;167:137–148. [Google Scholar]
- Peakall R, Beattie AJ. Pollination of the orchid Microtis parviflora R. Br. by flightless worker ants. Functional Ecology. 1989;3:515–522. [Google Scholar]
- Peakall R, Beattie AJ. The genetic consequences of worker ant pollination in a self-compatible, clonal orchid. Evolution. 1991;45:1837–1848. doi: 10.1111/j.1558-5646.1991.tb02691.x. [DOI] [PubMed] [Google Scholar]
- Peakall R, Beattie AJ, James SH. Pseudocopulation of an orchid by male ants: a test of two hypotheses accounting for the rarity of ant pollination. Oecologia. 1987;73:522–524. doi: 10.1007/BF00379410. [DOI] [PubMed] [Google Scholar]
- Peitsch D, Fietz A, Hertel H, Desouza J, Ventura DF, Menzel R. The spectral input systems of hymenopteran insects and their receptor-based color-vision. Journal of Comparative Physiology A. 1992;170:23–40. doi: 10.1007/BF00190398. [DOI] [PubMed] [Google Scholar]
- Philpott SM, Greenberg R, Bichier P. The Influence of ants on the foraging behavior of birds in an agroforest. Biotropica. 2005;37:468–471. [Google Scholar]
- van der Pijl L. Ecological aspects of flower evolution. II. Zoophilous flower classes. Evolution. 1961;15:44–59. [Google Scholar]
- Puterbaugh MN. The roles of ants as flowers visitors: experimental analysis in three alpine plant species. Oikos. 1998;83:36–46. [Google Scholar]
- Ramsey M. Ant pollination of the perennial herb Blandfordia grandiflora (Liliaceae) Oikos. 1995;74:265–272. doi: 10.1007/BF00328430. [DOI] [PubMed] [Google Scholar]
- Schürch S, Pfunder M, Roy BA. Effects of ant on the reproductive success of Euphorbia cyparissias and associated pathogenic rust fungi. Oikos. 2000;88:6–12. [Google Scholar]
- Schemske DW, Lande R. The evolution of self-fertilization and inbreeding depression in plants. II. Empirical observations. Evolution. 1985;39:41–52. doi: 10.1111/j.1558-5646.1985.tb04078.x. [DOI] [PubMed] [Google Scholar]
- Shen H, Ye W, Hong L, et al. Progress in parasitic plant biology: host selection and nutrient transfer. Plant Biology. 2006;8:175–185. doi: 10.1055/s-2006-923796. [DOI] [PubMed] [Google Scholar]
- Sugiura N, Miyazaki S, Nagaishi S. A supplementary contribution of ants in the pollination of an orchid, Epipactis thunbergii, usually pollinated by hover flies. Plant Systematics and Evolution. 2006;258:17–26. [Google Scholar]
- Smithson A. Pollinator limitation and inbreeding depression in orchid species with and without nectar rewards. New Phytologist. 2006;169:419–430. doi: 10.1111/j.1469-8137.2005.01592.x. [DOI] [PubMed] [Google Scholar]
- StatSoft Inc. STATISTICA (data analysis software), version 6. 2001. http://www.statsoft.com .
- Stebbins GL. Self-fertilization and population variability in the higher plants. American Naturalist. 1957;91:337–354. [Google Scholar]
- Stewart GR, Press MC. The physiology and biochemistry of parasitic angiosperms. Annual Review of Plant Physiology and Plant Molecular Biology. 1990;41:127–151. [Google Scholar]
- Svensson L. An estimate of pollen carryover by ants in a natural population of Scleranthus perennis L. (Caryophyllaceae) Oecologia. 1985;66:373–377. doi: 10.1007/BF00378301. [DOI] [PubMed] [Google Scholar]
- Thompson JD. How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia. 2001;126:386–394. doi: 10.1007/s004420000531. [DOI] [PubMed] [Google Scholar]
- de Vattimo I. Contribuição ao conhecimento da tribu Apodantheae R. Br. Parte 1 Conspecto das espécies (Rafflesiaceae) Rodriguésia. 1971;26:37–62. [Google Scholar]
- de Vega C. Spain: University of Seville; 2007. Reproductive biology of Cytinus hypocistis (L.) L.: host-parasite interactions. Ph.D. Thesis. [Google Scholar]
- de Vega C, de Oliveira RC. A new procedure for making observations of embryo morphology in dust-like seeds with rigid coats. Seed Science Research. 2007;17:63–67. [Google Scholar]
- de Vega C, Ortiz PL, Arista M, Talavera S. The endophytic system of Mediterranean Cytinus (Cytinaceae) developing on five host Cistaceae species. Annals of Botany. 2007;100:1209–1217. doi: 10.1093/aob/mcm217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vega C, Berjano R, Arista M, Ortiz PL, Talavera S, Stuessy TF. Genetic races associated with the genera and sections of host species in the holoparasitic plant Cytinus (Cytinaceae) in the Western Mediterranean basin. New Phytologist. 2008;178:875–887. doi: 10.1111/j.1469-8137.2008.02423.x. [DOI] [PubMed] [Google Scholar]
- Visser J. South African parasitic flowering plants. Capetown: Juta & Co; 1981. [Google Scholar]
- Wallace LE. The cost of inbreeding in Platanthera leucophaea (Orchidaceae) American Journal of Botany. 2003;90:235–242. doi: 10.3732/ajb.90.2.235. [DOI] [PubMed] [Google Scholar]
- Webb DA. In: Cytinus. Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA, editors. Cambridge: Cambridge University Press; 1964. p. 75. Flora Europaea. [Google Scholar]
- Wyatt R. Ant-pollination of the granite outcrop endemic Diamorpha smallii (Crassulaceae) American Journal of Botany. 1981;68:1212–1217. [Google Scholar]