Abstract
Background and Aims
The tribe Spermacoceae is essentially a herbaceous Rubiaceae lineage, except for some species that can be described as ‘woody’ herbs, small shrubs to treelets, or lianas. Its sister tribe Knoxieae contains a large number of herbaceous taxa, but the number of woody taxa is higher compared to Spermacoceae. The occurrence of herbaceous and woody species within the same group raises the question whether the woody taxa are derived from herbaceous taxa (i.e. secondary woodiness), or whether woodiness represents the ancestral state (i.e. primary woodiness). Microscopic observations of wood anatomy are combined with an independent molecular phylogeny to answer this question.
Methods
Observations of wood anatomy of 21 woody Spermacoceae and eight woody Knoxieae species, most of them included in a multi-gene molecular phylogeny, are carried out using light microscopy.
Key Results
Observations of wood anatomy in Spermacoceae support the molecular hypothesis that all the woody species examined are secondary derived. Well-known wood anatomical characters that demonstrate this shift from the herbaceous to the woody habit are the typically flat or decreasing length vs. age curves for vessel elements, the abundance of square and upright ray cells, or even the (near-) absence of rays. These so-called paedomorphic wood features are also present in the Knoxieae genera Otiophora, Otomeria, Pentas, Pentanisia and Phyllopentas. However, the wood structure of the other Knoxieae genera observed (Carphalea, Dirichletia and Triainolepis) is typical of primarily woody taxa.
Conclusions
In Spermacoceae, secondary woodiness has evolved numerous times in strikingly different habitats. In Knoxieae, there is a general trend from primary woodiness towards herbaceousness and back to (secondary) woodiness.
Key words: Knoxieae, LM, primary woodiness, Rubiaceae, Rubioideae, secondary woodiness, Spermacoceae, wood anatomy
INTRODUCTION
In its currently accepted circumscription, the tribe Spermacoceae is essentially a herbaceous lineage of the family Rubiaceae, representing about 61 genera and 1235 species (Kårehed et al., 2008; Groeninckx et al., 2009a). It includes the former tribes Spermacoceae sensu stricto and Manettieae, and the Hedyotis–Oldenlandia group of the former tribe Hedyotideae. Representatives are usually annuals or short-lived perennials found in grasslands or open forests throughout the (sub)tropics. A number of species are adapted to more extreme habitats, such as sand dunes, Kalahari sand plateaus (Fig. 1A), shores (Fig. 1B, C), montane scrublands or rocky areas (Table 1). Many species are entirely herbaceous, but several (short-lived) perennial species – especially those adapted to extreme habitats – produce a limited-to-considerable amount of wood (‘woody’ herbs to small shrubs, or occasionally treelets, Fig. 1D–F) while others develop secondary growth mainly in their underground organs (geoxylic herbs, Fig. 1A–C; Dessein et al., 2002, 2003). The occurrence of herbaceous and woody species within the same group raises the question whether the herbaceous taxa are derived from woody lineages, as is the case in the early-diverging lineages of the subfamily Rubioideae (e.g. in Coccocypselum and Cruckshanksia of the tribe Coussareeae sensu lato), or if herbaceousness represents the plesiomorphic state. From an evolutionary point of view, the second hypothesis refers to a secondary derived origin of the wood, meaning that these woody species are derived from herbaceous ancestors, which in turn have evolved from (primarily) woody species.
Fig. 1.
Examples of the variation in habitats and growth forms of woody Spermacoceae. (A, B) habitats plus growth form; (C–F) growth form. (A) Overview of Kalahari sand plateau (Mwinilunga, Zambia) where Spermacoce manikensis grows (geoxylic herb; inset, detail of habit). (B) Shore of Lake Bangweulu (Zambia) with S. bangweolensis (inset, detail of habit). (C) Gomphocalyx herniarioides, geoxylic herb (south-west Madagascar). (D) Lathraeocarpa acicularis, shrub (south-west Madagascar). (E) ‘Oldenlandia’ ambovombensis, shrub (south Madagascar). (F) ‘Oldenlandia’ humbertii, shrub (south-west Madagascar).
Table 1.
Habit and habitat details of the woody species studied
| Species | Habit | Habitat and distribution |
|---|---|---|
| Spermacoceae | ||
| Arcytophyllum lavarum | Subshrub | Volcanic mountains at high altitude; Costa Rica to Panama |
| Arcytophyllum setosum | Shrub | Grassland above timberline; western South America |
| Arcytophyllum thymifolium | Subshrub or shrub | Grassland above timberline; Colombia to Peru |
| Diodella sarmentosa | Straggling, scrambling or climbing, often perennial herb | Large ecological amplitude, including evergreen forest, riverine vegetation, bush land, rocky places; widespread in the tropics |
| Emmeorhiza umbellata | Climbing, perennial herb | Tropical forest; South America |
| Gomphocalyx herniarioides | Prostrate or decumbent herb with well-developed, often woody taproot | Sandy soils of thorn forest, dunes and beaches; Madagascar |
| Hedyotis flavescens | Shrub | Upper montane zone on open waterlogged sites; Sri Lanka |
| Hedyotis fruticosa | Shrub or small tree to 4 m | Open vegetation, rocky areas on mountain tops; southern India, Sri Lanka, Myanmar |
| Hedyotis lessertiana | Shrub or small tree to 4 m | Upper montane scrubland; Sri Lanka |
| ‘Hedyotis’ trichoglossa | Woody herb of suffrutex with slightly woody stems and taproot | Eastern humid forest; Madagascar |
| Kadua cordata | Subshrub or shrub | Mesic to wet forests; Hawaiian islands |
| Lathraeocarpa acicularis | Subshrub with woody stems and taproot | Sandy soils in dunes close to the sea; Madagascar |
| Mitracarpus frigidus | Subshrub or shrub | Scrubland, grassland, also in disturbed areas; tropical America |
| Nesohedyotis arborea | Tree up to 7 m | Tree fern thicket; St. Helena island |
| ‘Oldenlandia’ ambovombensis | Shrub | Dry spiny forest on limestone plateau; south-western Madagascar |
| ‘Oldenlandia’ humbertii | Subshrub | Dry spiny forest on limestone plateau; south-western Madagascar |
| Spermacoce bangweolensis | Prostrate or suberect subshrub with woody taproot | Sandy soil bordering Lake Bangweulu; Zambia |
| Spermacoce manikensis | Geoxylic herb with woody taproot and plant base | High plateaus on Kalahari sand; Zambia and D.R. Congo |
| Spermacoce macrocephala | Subshrub | Savanna areas on river banks; Colombia and Venezuela |
| Spermacoce occidentalis | Annual or more often perennial spreading or erect woody herb | Open woodland on sandy soil or dune vegetation close to sea; Australia |
| Spermacoce verticillata | Woody herb or (sub)shrub | Savanna, scrubland and disturbed areas; tropical and subtropical America, introduced elsewhere |
| Knoxieae | ||
| Carphalea kirondron | Shrub or tree up to 10 m | Mostly at edges of dry, deciduous forest on laterite or calcareous sand; Madagascar |
| Dirichletia virgata | Shrub | Forest and scrubland; Socotra, Republic of Yemen |
| Otiophora rupicola | Subshrub | Rocky grassland at high altitude; Burundi |
| Otomeria micrantha | Herb, often with woody base | Savanna, clearings in forest, along roadsides; Nigeria to west-central tropical Africa |
| Pentanisia schweinfurthii | Geoxylic herb with woody taproot and plant base | Submontane and lower grassland, also in clearings of woodland; tropical Africa |
| Pentas zanzibarica | Woody herb or shrub with a woody rootstock | Forest edge, scrubland and grassland; Uganda to Mozambique |
| Phyllopentas schimperiana | Woody herb or shrub | Forest edge and scrubland, at high altitudes; tropical Africa |
| Triainolepis polyneura | Shrub | Deciduous and semi-deciduous forest; Madagascar |
With respect to higher-level phylogenetic relationships of Spermacoceae, the tribe is considered as a derived taxon within Rubioideae and there is strong evidence for a sister relationship with Knoxieae (Bremer and Manen, 2000; Dessein, 2003; Robbrecht and Manen, 2006). The Knoxieae as currently accepted are a much smaller tribe than Spermacoceae: they include only 17 genera and 128 species, and unite Knoxieae s.s., Triainolepideae and members of the Pentas group of the former tribe Hedyotideae (Dessein, 2003; Kårehed and Bremer, 2007). Knoxieae generally differ from Spermacoceae in having predominantly 5-merous flowers instead of 4-merous flowers, but exceptions occur in both tribes. As in Spermacoceae, herbaceous and woody species are present in Knoxieae, although the percentage of woody representatives is clearly higher. For instance, the genera Carphalea, Dirichletia and Triainolepis are entirely woody and also usually taller than most woody Spermacoceae. In most remaining Knoxieae genera, some of the species produce a limited amount of wood and can be described as small (sub)shrubs, woody herbs or geoxylic herbs (cf. most woody Spermacoceae; Verdcourt, 1976; Table 1).
The intratribal relationships of Spermacoceae have been the subject of controversy. Continuous changes in the delimitation of genera have resulted in a very complex taxonomic history of the tribe. Recent molecular studies (Kårehed et al., 2008; Groeninckx et al., 2009a) have shed new light on the phylogeny of Spermacoceae and relationships between genera are starting to crystallize. The genera of the Spermacoceae s.s. form a monophyletic clade deeply nested among taxa of the Hedyotis–Oldenlandia group, and are closely related to Bouvardia and Manettia. Oldenlandia is polyphyletic and should be restricted to include only close relatives of its type species O. corymbosa. Hedyotis is possibly to be restricted to Asia. Many small-to-medium-sized genera (e.g. Arcytophyllum, Conostomium, Hedythyrsus, Houstonia, Stenotis, etc), often reduced to the synonymy of either Hedyotis or Oldenlandia, should be recognized at generic level. With respect to the Knoxieae, generic rearrangements have been proposed by a molecular study of Kårehed and Bremer (2007). For instance, Pentas was shown to be polyphyletic and three new genera were recognized to accommodate its species, Placopoda and African members of the genus Carphalea should be placed into Dirichletia, and the generic circumscription of Pentanisia and Triainolepis must be enlarged. The current molecular framework of Spermacoceae (sensu Kårehed et al., 2008; Groeninckx et al., 2009a) and Knoxieae (Kårehed and Bremer, 2007) forms a good starting point to assess the origin of woodiness within both tribes.
In order to evaluate secondary woodiness of a particular species, independent methods should be applied. If a molecular phylogeny is present, the first and most obvious way is to trace evolutionary shifts from herbaceous to woody taxa (e.g. Böhle et al., 1996; Thiv et al., 1999; Lee et al., 2005). Another option might be to make woody mutants from herbaceous wild-types (Groover, 2005; Melzer et al., 2008). If molecular data are insufficient or unavailable, evidence in the microscopic wood structure – the so-called paedomorphic features – can be used to identify secondary derived shrubs and trees (Carlquist, 1962). Paedomorphic features typically remain in a juvenile ontogenetic state, which can be demonstrated in the wood structure by (juvenile) characters of the primary xylem that are transferred into the (mature) secondary xylem (= wood). Examples are the continuous decrease of vessel element length from the pith towards the cambium, the presence of elongated scalariform intervessel pits in wood (or even wide, gaping intervessel pits resembling helical tracheids in the primary xylem; e.g. fig. 7 in Lens et al., 2005), and the absence of rays and/or the presence of rays with an abundance of square-to-upright ray cells.
Fig. 7.
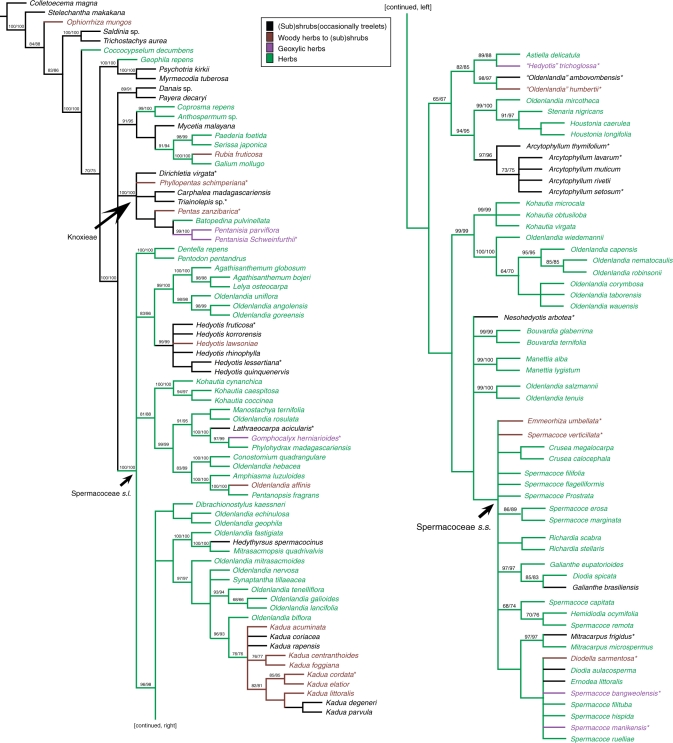
Strict consensus tree based on maximum-parsimony analysis of atpB-rbcL, rps16, petD and trnL-trnF showing bootstrap/jackknife support values above the branches. The colour of the species' names indicates their habit: black = (sub)shrubs (or occasionally treelets); brown = woody herbs to (sub)shrubs; purple = geoxylic herbs; green = herbs. Asterisks indicate the species of woody Spermacoceae and Knoxieae taxa that were investigated in this study. This tree supports our wood anatomical hypothesis: the common ancestor of Knoxieae is primarily woody, while the common ancestor of Spermacoceae is herbaceous.
In at least some (derived) Rubioideae lineages with mixed herbaceous and woody species, such as Paederieae and Rubieae, earlier publications of wood anatomy favour the secondary woodiness option for at least some species (Koek-Noorman, 1976; Koek-Noorman and Puff, 1983; Carlquist, 1992). Consequently, it would be interesting to investigate if this is also valid in other derived Rubioideae clades, such as Spermacoceae and its sister tribe Knoxieae. In order to do this, the wood anatomical variation of Spermacoceae and Knoxieae should be studied using a broader sampling than is currently available. The poor knowledge of wood anatomy of both tribes can be illustrated by two large-scale wood anatomical review papers in Rubiaceae: the first one covering the subfamily Rubioideae (Jansen et al., 2001) studied only two young twigs of Triainolepis (Knoxieae), while the subsequent wood anatomical survey concerning the entire family (Jansen et al., 2002) dealt with mainly juvenile material of only six Spermacoceae species and six Knoxieae species.
The objectives of this study were to present a detailed overview of the wood anatomy of woody representatives of the tribe Spermacoceae and Knoxieae, and to compare this overview with a multi-gene molecular phylogeny in order to assess the origin of woodiness in the two tribes. In addition, a comparison is made of the wood structure between Spermacoceae and its sister tribe Knoxieae.
MATERIALS AND METHODS
Plant material
Wood samples from 21 Spermacoceae species and eight Knoxieae species were collected from the xylaria in Kew (Kw), Tervuren (Tw), Utrecht (Uw) and from the National Botanic Garden of Belgium (BR; see Table 2 and Appendix). The sampling covers all major Spermacoceae and Knoxieae clades as identified in the molecular phylogenies (Kårehed and Bremer, 2007; Kårehed et al., 2008; Groeninckx et al., 2009a).
Table 2.
Overview of selected wood anatomical characters within Spermacoceae and Knoxieae (Rubiaceae). Species are arranged alphabetically according to the tribal studies of Groeninckx et al. (2009a) and Kårehed and Bremer (2007). Numbers between hyphens are mean values flanked by minimum and maximum values. For specimens of the same taxon, superscript numbers after the species name refer to the order of the specimens as followed in the species list (see Appendix). Species names in bold represent underground woody organs, and juvenile wood samples are marked with an asterisk behind the species names
| Species studied | Primarily or secondarily woody | Length vs. age curve | Tendency to (semi-)ringporosity | Solitary vessels | Radial vessel multiples | Tangential vessel multiples | Vessel clusters | Vessel diameter (μm) | Vessel density (mm−1) | Vessel element length (μm) | Tracheid length (μm) | Tracheids thick-walled | Occasional septate libriform fibres | Dif axial parenchyma | Dia axial parenchyma | Banded axial parenchyma | Scanty axial parenchyma | Rays absent | Rays exclusively uniseriate | Multiseriate ray width (no. cells) | Multiseriate ray height (μm) | Ray cells mostly or all square-to-upright |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spermacoceae | ||||||||||||||||||||||
| Arcytophyllum lavarum* | S | Flat | − | + | + | − | − | 15–20–30 | 100–125–160 | 150–195–250 | 250–300–350 | − | − | ± | − | − | ± | − | + | / | / | + |
| Arcytophyllum setosum | S | Decr | − | + | + | − | − | 15–20–25 | 80–100–140 | 150–290–400 | 300–370–450 | − | − | − | − | − | − | + | C | / | / | / |
| Arcytophyllum thymifolium* | S | Flat | − | + | + | − | − | 10–16–20 | 480–540–640 | 100–160–250 | 150–200–250 | − | − | + | − | − | + | − | + | / | / | + |
| Diodella sarmentosa | S | Flat | − | ± | ± | + | ± | 25–60–120 | 36–48–66 | 150–300–550 | 350–465–550 | − | − | − | ± | − | + | − | − | 2–14 | 200–2130–8000 | + |
| Emmeorhiza umbellata* | S | Decr | − | + | − | − | − | 15–55–120 | 60–81–85 | 200–350–500 | 500–610–700 | − | + | − | ± | − | + | − | + | / | / | + |
| Gomphocalyx herniarioides | S | Decr | − | + | ± | − | − | 15–28–40 | 135–150–180 | 100–160–250 | 250–340–400 | − | − | − | ± | − | ± | − | − | 2–3 | 300–840–1500 | + |
| Hedyotis flavescens* | S | Decr | − | + | − | − | − | 10–23–30 | 90–110–140 | 300–650–850 | 750–910–1150 | − | + | ± | − | − | ± | − | + | / | / | + |
| Hedyotis fruticosa* | S | Decr | − | + | ± | − | − | 25–36–50 | 80–95–120 | 250–480–700 | 600–765–900 | − | + | ± | − | − | ± | − | + | / | / | + |
| Hedyotis lessertiana* | S | Flat | − | + | ± | − | − | 15–25–30 | 75–45–120 | 300–660–1000 | 850–1050–1300 | − | + | − | − | − | − | − | + | / | / | + |
| ‘Hedyotis’trichoglossa | S | Decr | − | + | ± | − | − | 15–21–30 | 100–120–145 | 300–540–700 | 500–665–800 | − | + | − | − | − | − | − | + | / | / | + |
| Kadua cordata | S | Flat | − | + | ± | − | − | 20–29–50 | 140–170–230 | 350–450–700 | 500–630–800 | − | + | ± | − | − | ± | − | + | / | / | + |
| Lathraeocarpa acicularis | S | Decr | + | + | − | ± | ± | 10–21–40 | 200–255–290 | 100–220–300 | 400–470–600 | ± | − | − | ± | − | + | − | + | / | / | + |
| Mitracarpus frigidus | S | Flat | ± | + | ± | ± | − | 15–25–45 | 140–170–200 | 250–375–550 | 550–690–850 | − | + | ± | − | − | ± | − | − | 2 | 150–345–650 | + |
| Nesohedyotis arborea1 | S | Flat | − | + | + | − | − | 20–50–80 | 16–20–25 | 200–340–500 | 600–750–900 | − | − | − | + | − | ± | − | − | 4–6 | 300–550–900 | ± |
| ‘Oldenlandia’ ambovombensis1* | S | Flat | + | + | − | − | − | 10–19–40 | 240–265–300 | 150–300–450 | 350–485–600 | ± | − | ± | − | − | + | − | + | / | / | + |
| ‘Oldenlandia’ ambovombensis2 | S | Flat | + | + | − | − | − | 10–20–40 | 260–285–320 | 150–275–350 | 400–460–550 | ± | − | ± | − | − | + | − | + | / | / | + |
| ‘Oldenlandia’ humbertii* | S | Flat | ± | + | − | − | − | 10–19–30 | 290–325–380 | 100–200–300 | 300–405–500 | + | − | − | − | − | + | − | + | / | / | + |
| Spermacoceae bangweolensis | S | Flat | − | + | + | − | − | 20–30–45 | 110–125–160 | 150–320–400 | 350–500–650 | − | − | − | ± | − | ± | − | − | 2 | 200–300–550 | + |
| Spermacoce macrocephala | S | Flat | − | + | − | ± | − | 15–26–35 | 110–140–160 | 450–690–950 | 650–840–950 | − | − | ± | − | − | ± | − | − | 2 | 600–950–1800 | + |
| Spermacoce manikensis | S | Flat | − | + | ± | ± | ± | 15–23–30 | 100–115–130 | 100–300–500 | 500–605–800 | − | + | − | ± | − | ± | − | + | / | / | + |
| Spermacoce occidentalis | S | Flat | − | + | ± | − | − | 20–35–50 | 120–140–160 | 200–350–450 | 350–460–550 | − | − | − | ± | − | + | − | − | 2–3 | 200–400–700 | + |
| Spermacoce verticillata1 | S | Decr | − | + | ± | − | ± | 15–22–30 | 120–160–190 | 300–415–550 | 500–640–800 | − | + | ± | − | − | ± | − | + | / | / | + |
| Spermacoce verticillata2 | S | Decr | ± | + | ± | − | ± | 15–30–45 | 130–145–170 | 150–240–500 | 400–495–600 | − | + | ± | − | − | ± | − | − | 2 | 300–440–600 | + |
| Knoxieae | ||||||||||||||||||||||
| Carphalea kirondron* | P | Norm | ± | + | ± | − | ± | 25–55–100 | 60–72–85 | 300–410–550 | 600–720–850 | − | − | − | + | ± | + | − | − | 2–3 | 300–565–1000 | − |
| Dirichletia virgata | P | Norm | ± | − | ± | − | + | 20–40–70 | 60–75–85 | 150–330–500 | 850–1045–1200 | − | − | − | + | − | + | − | − | 3–4 | 250–410–600 | − |
| Otiophora rupicola* | S | Flat | − | + | ± | − | − | 15–20–30 | 140–160–180 | 300–450–600 | 400–575–700 | − | − | ± | − | − | ± | − | − | 2–3 | 100–520–1100 | + |
| Otomeria micrantha | S | Flat | − | + | + | − | − | 15–22–30 | 210–240–280 | 450–680–1100 | 700–840–950 | − | − | − | − | − | − | − | + | / | / | + |
| Pentanisia schweinfurtii1 | S | Decr | − | + | + | − | − | 20–29–50 | 200–215–240 | 150–285–400 | 400–525–650 | − | − | − | + | + | − | − | − | 2–3 | 400–760–1850 | + |
| Pentanisia schweinfurtii2* | S | Decr | − | + | + | − | − | 15–35–75 | 100–135–160 | 150–270–350 | 300–380–450 | − | − | − | + | + | − | − | − | 2–8 | 600–2060–4500 | + |
| Pentas zanzibarica | S | Flat | ± | + | + | − | − | 15–30–50 | 140–180–185 | 200–390–600 | 600–725–800 | − | − | ± | − | − | ± | − | − | 2–4 | 200–720–1600 | + |
| Phyllopentas schimperiana* | S | Decr | ± | + | − | − | − | 20–30–50 | 90–105–120 | 250–450–650 | 550–680–800 | − | − | ± | − | − | ± | − | − | 2–3 | 600–760–1500 | + |
| Triainolepis polyneura | P | Norm | ± | − | + | − | + | 25–65–130 | 25–32–38 | 300–490–650 | 800–1020–1200 | − | − | − | ± | + | − | − | − | 2–3 | 200–400–600 | − |
Dif, diffuse; Dia, diffuse-in-aggregates; P, primarily woody; S, secondarily woody; Decr, decreasing length vs. age curve; Flat, flat length vs. age curve; Norm, normal length vs. age curve; +, present; ± , sometimes present; −, absent; / indicates not applicable.
We tried to investigate as many mature wood samples as possible, but due to the limited wood production in some species, some samples need to be considered as juvenile twigs, for example Arcytophyllum lavarum, A. thymifolium, Emmeorhiza umbellata, Hedyotis flavescens, H. fruticosa, H. lessertiana and ‘Oldenlandia’ humbertii (all Spermacoceae). The Knoxieae species Carphalea kirondron, Otiophora rupicola and Phyllopentas schimperiana also represent juvenile wood material.
Geoxylic Spermacoceae species with woody underground organs are observed in Gomphocalyx herniarioides and Spermacoce manikensis; of these, G. herniarioides has primary xylem in the centre, while the underground parts of S. manikensis has a stem anatomy. Species with a similar geoxylic habit in Knoxieae are Otiophora rupicola and Pentanisia schweinfurthii. Spermacoce bangweolensis and S. occidentalis have woody underground parts combined with more elaborate woody parts above ground.
‘Oldenlandia’ humbertii and ‘Oldenlandia’ ambovombensis represent two undescribed species from Madagascar: both will be described in a new genus (I. Groeninckx, unpubl. data). The Malagasy species ‘Hedyotis’ trichoglossa also needs to be transferred to a new genus. Lathraeocarpa acicularis, a small (sub)shrub also endemic to Madagascar, was previously placed within the monogeneric tribe Lathraeocarpeae (Bremekamp, 1957); however, a recent molecular–morphological study by Groeninckx et al. (2009b) strongly supports the inclusion of this Malagasy endemic genus within the tribe Spermacoceae, sister to Phylohydrax and Gomphocalyx.
Description of wood anatomy and microtechniques
The methodology of wood sectioning and the subsequent steps are described in Lens et al. (2005). The wood anatomical terminology follows IAWA list of microscopic features for hardwood identification (IAWA Committee, 1989), except for the concept of imperforate tracheary elements with distinctly bordered pits (i.e. fibre-tracheids and tracheids). Because the distinction between these two cell types is very hard to make in Spermacoceae due to continuous overlap in pit border size and pit density, we prefer to distinguish between fibre-tracheids and tracheids based on the degree of vessel grouping (Carlquist, 1984). In general, species with a low vessel grouping that grow in mesic conditions possess non-conducting fibre-tracheids (or libriform fibres) in the ground tissue, but when these species occur in dry conditions the imperforate cells are hypothesized to be functionally water-conducting tracheids that take over the water transport from drought-induced embolized vessels (although we acknowledge that more functional work remains to be done to assess the difference in water-conducting capacity between fibre-tracheids and tracheids).
For length vs. age curves, measurements for vessel elements were made using radial sections from the pith towards the cambium. In addition, the length of vessel elements and fibres from wood splinters taken at various distances between the pith and cambium region – usually 1 mm between each splinter – were measured using maceration slides. In Table 2, the length of vessel elements and fibres were always based on the outer part of the wood sample.
Phylogenetic analysis
The phylogenetic analysis is based on an existing data matrix of atpB-rbcL, rps16, petD and trnL-trnF sequences, which includes most of the Spermacoceae species studied (Groeninckx et al., 2009a). The matrix was enlarged with own sequences of Anthospermum sp., Coccocypselum decumbens, Colletecoema magna, Coprosma repens, Danais sp., Galium mollugo, Geophila repens, Mycetia malayana, Myrmecodia tuberosa, Ophiorrhiza mungos, Paederia foetida, Payera decaryi, Psychotria kirkii, Rubia fruticosa, Saldinia sp., Serissa japonica, Spermacoce bangweolensis, S. manikensis, Stelechantha makakana, Triainolepis polyneura and Trichostachys aurea. Additional rps16 and trnL-trnF sequences from GenBank were incorporated for Dirichletia virgata (accession numbers AM266894, AM266980), Pentanisia schweinfurthii (AM266861, AM266949), Pentas zanzibarica (AM266892, AM266978) and Phyllopentas schimperiana (AM266887, AM266973). No sequences were obtained for the woody Spermacoceae Hedyotis flavescens, Spermacoce macrocephala and S. occidentalis, and the woody Knoxieae species Otomeria micrantha and Otiophora rupicola. The combined matrix includes 133 taxa, of which six basal Rubioideae were designated as the outgroup (Coccocypselum decumbens, Colletecoema magna, Ophiorrhiza mungos, Saldinia sp., Stelechantha makakana and Trichostachys aurea), eight Knoxieae species, 107 representatives of Spermacoceae and 12 species from additional rubioid lineages. The dataset comprised 6045 characters, of which 1021 were parsimony informative.
Parsimony analyses were conducted with NONA (Goloboff, 1999) using WinClada ver. 1·0000 as interface (Nixon, 2002). A heuristic search was carried out with 1000 random addition replicates, tree-bisection and reconnection (TBR) branch-swapping holding ten trees per replicate, followed by TBR branch-swapping on all trees resulting from the 1000 replicates. In order to evaluate the relative support of the clades, jackknife (JS) and bootstrap (BS) analyses were executed using 1000 replicates with 100 initial trees holding one tree per random addition, performing TBR to hold 1000 trees and calculating a consensus on each repetition. Frequency values (>65%) were plotted onto the consensus of the most-parsimonious trees.
The evolution of woodiness was investigated by deltran optimization by adding four habit types [type 1 = (sub)shrubs (or occasionally treelets); type 2 = woody herbs to (sub)shrubs; type 3 = geoxylic herbs; type 4 = herbs] onto the strict consensus tree from the parsimony analysis using WinClada ver. 1·0000 (Nixon, 2002). We chose deltran optimization to overcome the problem of restricted sampling in several Rubioideae clades (such as, for example, Knoxieae and Psychotrieae): the result was an optimization pattern that agreed with what was expected based on the much more elaborate phylogenetic studies within these clades (Kårehed and Bremer, 2007; Razafimandimbison et al., 2008).
RESULTS
Wood description
The Spermacoceae studied are described according to the recent tribal delimitation of Groeninckx et al. (2009a) and Kårehed et al. (2008; Figs 2–4 and 6A–D). For each genus examined, the numerator presented in the text represents the number of species studied and the denominator includes the total number of species. Numbers without parentheses are ranges of means, while numbers between parentheses represent minimum or maximum values. Descriptions of continuous characters are based on mature wood samples. A summary of selected wood features is shown in Table 2. Because the number of mature wood samples within Knoxieae is rather limited, we decided not to provide a tribal wood description. As an alternative, we have summarized our anatomical observations of Knoxieae in Table 2, added with illustrations (Figs 5 and 6E, F). The species of Spermacoceae that were studied and their descriptions are as follows.
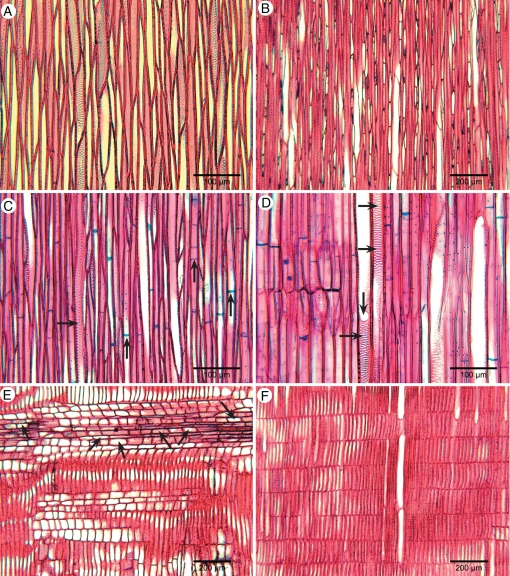
Fig. 2.
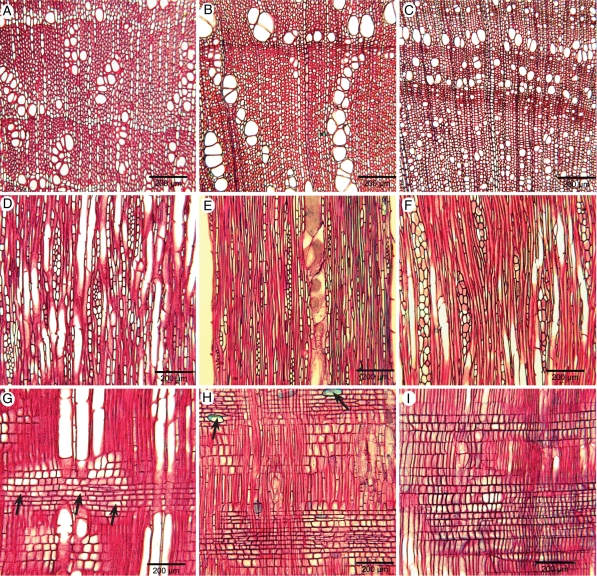
Transverse light-microscope sections of woody Spermacoceae showing variation in vessel distribution, thickness of tracheids and scarcity of axial parenchyma. (A) Arcytophyllum setosum. (B) Spermacoce macrocephala. (C) Lathraeocarpa acicularis, thick-walled tracheids and vessels in the ground tissue. (D) Diodella sarmentosa, climbing species, wood cylinder partly broken up by large unlignified rays. (E) Nesohedyotis arborea, larger mean vessel diameter and lower vessel density than in most other woody Spermacoceae, axial parenchyma more common (arrows).
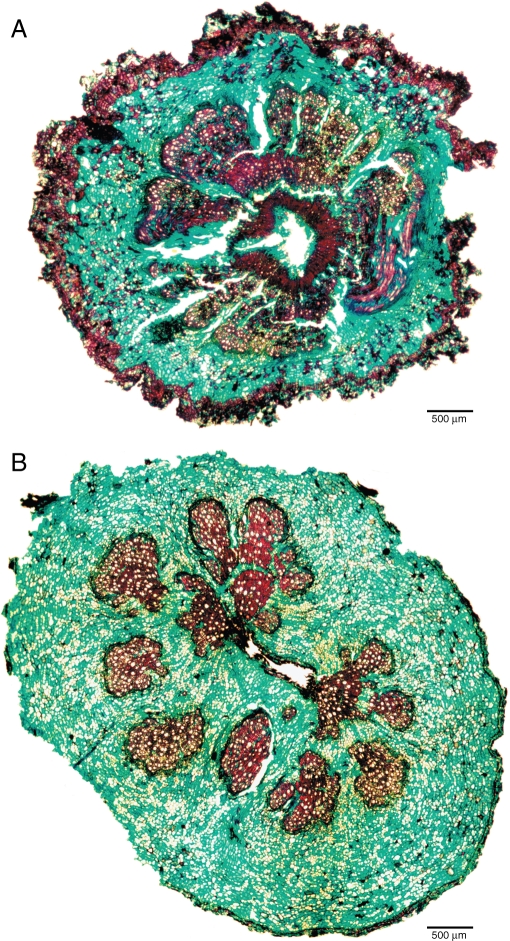
Fig. 3.
Transverse light-microscope sections of the underground stem of Spermacoce manikensis showing an initial wood cylinder that is more dramatically dispersed by intense divisions of the surrounding parenchyma tissue with age. (A) Part of young stem, and (B) part of older stem.
Fig. 4.
Tangential and radial longitudinal light-microscope sections of woody Spermacoceae showing intervessel pits (C, D) and ray characters. (A–C) Tangential sections; (D–F) radial sections. (A) Arcytophyllum setosum, rayless wood. (B) Spermacoce macrocephala, abundance of uniseriate rays with upright cells. (C) ‘Hedyotis’ trichoglossa, intervessel pits alternate (horizontal arrow), ground tissue mostly consisting of septate libriform fibres (vertical arrows), intervessel pitting alternate. (D) ‘Hedyotis’ trichoglossa, simple perforation (vertical arrow), wide scalariform intervessel pitting (horizontal arrows). (E) Nesohedyotis arborea, upper ray with many procumbent body ray cells (arrows). (F) Kadua cordata, ray consisting of upright cells.
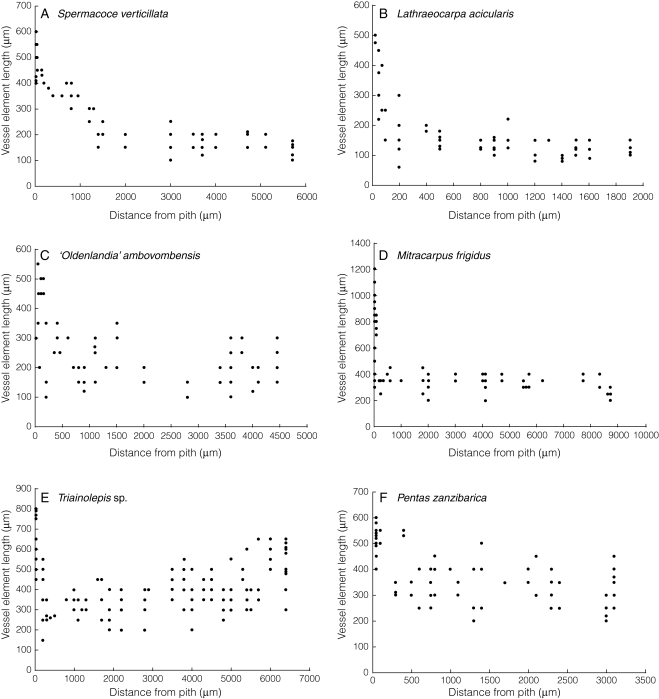
Fig. 6.
Length vs. age curves for vessel elements in Spermacoceae (A–D) and Knoxieae (E–F). Vessel element length strongly decreases in the primary xylem and the first-formed wood (close to the pith), followed by a subsequent slight decrease (decreasing curve, A, B) or subsequent stabilization (flat curve, C, D, F) or slight increase (normal curve, E) towards the cambium. (A–D, F) Secondarily woody species; (E) primarily woody species.
Fig. 5.
Transverse (A–C), tangential (D–F) and radial (G–I) light-microscope sections of woody Knoxieae showing vessel distribution (A–C), ray width (D–F) and ray composition (G–I) of primarily woody representatives (A, B, D, E, G, H) and secondarily woody members (C, F, I). (A, D, G) Dirichletia virgata; (B, E, H) Triainolepis polyneura; (C, F, I) Pentas zanzibarica. (A) Tendency to dendritic vessel pattern; (B) pronounced radial vessel mutiples ranging into dendritic pattern; (C) semi-ring porous wood, vessels mainly solitary and in short radial multiples; (D) body ray cells small and rounded; (E) body ray cells small and rounded; (F) rays consisting of mainly upright ray cells; (G) body ray cells procumbent (arrows); (H) body ray cells procumbent, enlarged ray cells containing raphides (arrows); and (I) multiseriate ray with mainly upright cells.
Arcytophyllum 3/17, Diodella 1/ ∼ 10, Emmeorhiza 1/1, Gomphocalyx 1/1, Hedyotis 4/ ∼ 115, Kadua 2/28, Lathraeocarpa 1/2, Mitracarpus 1/ ∼ 50, Nesohedyotis 1/1, Oldenlandia 2/ ∼ 240, and Spermacoce 5/ ∼ 275 (Figs 2–4 and 6A–D). Growth ring boundaries usually distinct. Diffuse-porous, but tendency to (semi-)ring-porosity in Lathraeocarpa acicularis (Fig. 2C), Mitracarpus frigidus, ‘Oldenlandia’ ambovombensis, ‘Oldenlandia’ humbertii and Spermacoce verticillata. Vessels (16)–20–540–(640) mm−2, mostly solitary and in radial multiples of 2–3 (Fig. 2A, B, E), few additional vessel clusters in species of Diodella (Fig. 2D), Lathraeocarpa (Fig. 2C) and Spermacoce; vessel outline mostly slightly angular, sometimes rounded; perforation plates exclusively simple (Fig. 4D), sometimes a small percentage of scalariform perforations in Hedyotis flavescens (10%; 2–5 bars) and H. fruticosa (20%; 4–10 bars), or a high percentage of reticulate and irregular perforations in Spermacoce bangweolensis (>50%). Intervessel pits alternate (Fig. 4C), pits 3–5–(6) μm in horizontal diameter, vestured; additional (pseudo-)scalariform vestured intervessel pits also present in ‘Hedyotis’ trichoglossa (Fig. 4D), 10–15 µm in horizontal diameter. Vessel-ray pits similar to intervessel pits in shape and size. Wall sculpturing absent. Tyloses sometimes present in Gomphocalyx. Tangential diameter of vessels (10)–16–60–(120) μm, vessel elements (100)–160–690–(1000) μm long. Tracheids generally present, generally thin- or thin-to-thick-walled but thick-walled in Lathraeocarpa and Oldenlandia; pit borders generally distinctly bordered, (3)–4–5 µm in diameter concentrated in tangential and radial walls; tracheid length (150)–200–1050–(1300) μm. Few septate fibres with simple-to-minutely bordered pits (pit borders 2–3 µm in diameter and concentrated in radial walls) found in Emmeorhiza, Hedyotis, Kadua, Mitracarpus and Spermacoce; in ‘Hedyotis’ trichoglossa much more septate fibres are present (Fig. 4C, D). Axial parenchyma often scarce to apparently absent (Fig. 2B), usually a combination of diffuse or diffuse-in-aggregates apotracheal parenchyma with scanty paratracheal parenchyma (Fig. 2C), parenchyma not observed in Arcytophyllum setosum (Fig. 2A), Hedyotis lessertiana and ‘Hedyotis’ trichoglossa; 2–3–(4) cells per parenchyma strand. Rays present, except in Arcytophyllum setosum (Fig. 4A). Exclusively uniseriate rays present in species of Arcytophyllum, Emmeorhiza, Hedyotis, Kadua, Oldenlandia and Spermacoce (Fig. 4B); 0–25 rays mm−1, (50)–120–1950–(3300) μm long, consisting of upright cells (Fig. 4F). If present, multiseriate rays generally 2–3-seriate, 4–6-seriate in Nesohedyotis arborea and 2–14-seriate in Diodella sarmentosa, (150)–300–2130–(8000) μm high, (0)–2–5 rays mm−1, consisting of mainly upright or square ray cells (Fig. 4F), but an even distribution of procumbent, square and upright ray cells in Nesohedyotis arborea (Fig. 4E); sheath cells present in N. arborea. No dark amorphous contents in ray cells. No crystals or silica bodies observed.
Phylogenetic analysis
The phylogenetic analysis of the molecular data resulted in 8519 most-parsimonious trees with length 3412 (CI = 0·49; RI = 0·75). The strict consensus tree with length 3511 (CI = 0·47; RI = 0·74) is shown in Figure 7. The tribe Spermacoceae s.l. was well supported, but the sister relationship with Knoxieae was not found.
Within Spermacoceae s.l., a polytomy representing four major lineages was present: (1) the herbaceous genera Dentella–Pentodon; (2) a predominantly herbaceous lineage comprising Kohautia and the Pentanopsis clade (including the woody Lathraeocarpa and Gomphocalyx); (3) a lineage including herbaceous Agathisanthemum species that are sister to the woody herbs and (sub)shrubs of the Hedyotis s.s. clade (including the type species H. fruticosa and H. lessertiana); and (4) a large clade representing the remaining herbaceous and woody species of Spermacoceae. Within this fourth clade, relationships were not fully resolved, but it is clear that the woody species are scattered over different clades, such as the Kadua clade, the Arcytophyllum–Houstonia clade and the Spermacoceae s.s. clade. The relationships within Knoxieae cannot be discussed in detail because of the limited sampling. Nevertheless, some results of the Kärehed and Bremer (2007) study were also found in our analysis: Carphalea sister to Triainolepis, and the relationship between the genera Pentas, Batopedina and Pentanisia.
DISCUSSION
Diversity of wood anatomy within Spermacoceae
A typical feature in Spermacoceae wood is the flat or decreasing length vs. age curve for vessel elements (Fig. 6A–D). Although we are aware that the maximal stem diameter in some of our samples might not be wide enough to present definitive length vs. age curves (e.g. Fig. 6B), we feel confident that the results are representative for the species. This is predominantly based on the ideas of Bailey (1920), who demonstrated that the vessel element length remains almost constant with age in species having so-called ‘derived’ vessel elements, i.e. very short vessel elements (<350 µm) combined with exclusively simple perforations. Furthermore, Carlquist (1962) demonstrated that nearly all secondarily woody species with very short vessel elements had a flat length vs. age curve. Based on the maceration slides observed, length vs. age curves for fibre-tracheids/tracheids are similar but show less variation in length than vessel elements. For example, average length values of fibre-tracheids/tracheids from the pith towards the cambium range from 560–495 µm in Spermacoce verticillata (cf. Fig. 6A), from 500–460 µm in ‘Oldenlandia’ ambovombensis (cf. Fig. 6C) and from 925–690 µm in Mitracarpus frigidus (cf. Fig. 6D).
Most Spermacoceae species observed had a similar wood anatomy (Figs 2 and 4; Table 2). Besides the paedomorphic length vs. age curves, the tribe could also be identified by the following features: exclusively simple perforations, alternate and vestured intervessel pits, mainly solitary vessels in combination with short radial multiples, narrow vessels (usually 20–40 µm in tangential width), high vessel densities (often more than 100 mm−2), relatively short vessel elements and imperforate tracheary elements with distinctly bordered pits (often between 150–500 µm and 350–800 µm, respectively), scarce apo- and paratracheal axial parenchyma, and a predominance of uniseriate rays including nearly exclusively square-to-upright cells. Furthermore, about half of the species studied had few septate libriform fibres, which most likely act as a rapidly increased photosynthetic storage capacity in woods with few (or nearly no) axial parenchyma cells (so-called tracheid dimorphism, sensu Carlquist, 1988). The evolution of fibre-tracheids towards septate libriform fibres was most pronounced in the underground wood sample of ‘Hedyotis’ trichoglossa, in which many tracheids in the ground tissue have been replaced during evolution by septate libriform fibres (Fig. 4C, D).
The variation in wood anatomy described above corresponds to the rubiaceous wood type I (Koek-Noorman, 1977; Jansen et al., 2002), in which the type of imperforate element in the ground tissue is the main characterizing feature (I, tracheids/fibre-tracheids with distinctly bordered pits vs. II, libriform fibres with simple-to-minutely bordered pits). Furthermore, the fibre character in Rubiaceae was found to be highly correlated with other wood characters, such as vessel grouping (I, mainly solitary vs. II, mostly in short multiples), axial parenchyma distribution (I, diffuse, diffuse-in-aggregates or banded vs. II, absent or scanty paratracheal) and ray width (I, narrow with long upright uniseriate margins vs. II, wider rays with short uniseriate margins). With regard to Spermacoceae, the combination of type I fibres/tracheids with the axial parenchyma distribution (scarce apotracheal and paratracheal parenchyma) and ray structure (no difference between body and marginal ray cells) is unusual within Rubiaceae, and can be at least partly attributed to the secondary origin of the wood structure (see following sections, below).
Some Spermacoceae species deserve special attention because of their distinctive anatomy. The first one is Diodella sarmentosa, the only straggling-to-climbing species with mature wood in our study, which can be distinguished by its wide and mainly unlignified rays (2–14-seriate) that partly break up the wood cylinder (Fig. 2D). This division of the wood cylinder is much more prominent in the underground wood material of the geoxylic herb Spermacoce manikensis (Fig. 3). Although the wood cylinder is more-or-less intact in the youngest part of the underground structure (Fig. 3A), it dramatically breaks down into several small ‘lignified islands’ that float in a sea of dividing parenchyma cells (Fig. 3B; see divided xylem cylinder according to the cambial variant types of Carlquist, 2001). A similar, but less pronounced cambial variant is encountered in the underground structure of S. occidentalis, in which larger wood portions are starting to get dispersed by parenchyma divisions. The abundant presence of parenchymatous tissue in these two species is probably related to an increased water-storage capacity in order to cope with long periods of drought (Table 1).
Variation in wood anatomy within Knoxieae
From a wood-anatomy point of view, the variation within Knoxieae is more complex than in Spermacoceae (Figs 5 and 6E, F). Primarily based on differences in the length vs. age curve and the cellular composition of rays, the anatomical diversity of wood in Knoxieae can be divided into two groups: (1) Otomeria, Pentanisia, Pentas (Fig. 5C, F, I), and probably Otiophora and Phyllopentas; and (2) Carphalea, Dirichletia (Fig. 5A, D, G) and Triainolepis (Fig. 5B, E, H). The first group of genera is nearly identical to the Spermacoceae species studied: flat or decreasing length vs. age curves for vessel elements (Fig. 6F, and to a lesser extent also for fibre-tracheids/tracheids), exclusively simple perforations, alternate and vestured intervessel pits, mainly solitary vessels in combination with radial vessel multiples, narrow vessels (usually 20–50 µm in tangential width), high vessel densities (often >100 mm−2), relatively short vessel elements and imperforate tracheary elements with distinctly bordered pits (often between 200–600 µm and 450–800 µm, respectively), and a predominance of upright-to-square ray cells in uni- and multiseriate rays (Fig. 5F, I). More mature material of Otiophora and Phyllopentas needs to be carefully studied in order to investigate specific characters more in detail, such as length vs. age curves and cellular ray composition, before we can more confidently place these two genera in the first group.
The second group of species can be distinguished from the first group by the presence of normal length vs. age curves (Fig. 6E) and the predominance of procumbent body ray cells (Fig. 5D, E, G, H). Among the three species of the second group, Dirichletia virgata and Triainolepis polyneura are remarkable in having prominent radial vessel multiples and/or large vessel clusters (even a strong tendency to a dendritic vessel pattern in D. virgata; Fig. 5A), wider vessels (up to 130 µm in tangential width), and much longer fibres (800–1200 µm; Table 2). In addition, the juvenile sample of Carphalea kirondron also showed a tendency to produce more pronounced vessel multiples and wider vessels. The difference in vessel width and fibre length between both groups can be related to differences in habit: species of the second group are all shrubs to trees, which are taller than the woody members of the first group (smaller shrubs to woody herbs or geoxylic herbs; Verdcourt, 1976). In addition, the pronounced vessel multiples in Dirichletia and Triainolepis provide support for their adaptation to relatively dry (semi-)deciduous forests (Carlquist, 1984; Table 1).
When the diversity of wood anatomy of Knoxieae is compared with the remaining Rubiaceae, it is evident that the anatomical characters of the three species of the second group (i.e. Triainolepis polyneura, Dirichletia virgata and Carphalea kirondron) fit better within the rubiaceous wood type I due to their narrow rays with long uniseriate ends (Fig. 5D, E). However, the pronounced vessel multiples, especially in Triainolepis and Dirichletia (Fig. 5A-B), strikingly contradict the general occurrence of solitary vessels in type I Rubiaceae (Jansen et al., 2002).
Are Spermacoceae and Knoxieae primarily woody or secondarily woody?
With respect to the wood anatomy of Spermacoceae, the presence of flat or decreasing length vs. age curves for vessel elements (and to a lesser extent also fibre-tracheids/tracheids) in all the species observed (Fig. 6A–D) and the occurrence of mainly square-to-upright ray cells in nearly all the species observed [Fig. 4B–D, F; rays absent in Arcytophyllum setosum (Figs 2A and 4A); deviating ray cell morphology in Nesohedyotis arborea] provide strong arguments for secondary woodiness (Carlquist, 1962, 1992). Our study also provides new evidence that these typical paedomorphic features are also valid in underground woody organs of geoxylic herbs. In addition, the presence of wide scalariform intervessel pits in combination with the normal alternate vessel pits in ‘Hedyotis’ trichoglossa provides further support for the general validity of paedomorphic features in underground woody structures (Fig. 4C, D). Nevertheless, there are some wood features that deviate from the typical paedomorphic wood pattern. For instance, rays with a significant percentage of procumbent body ray cells were observed throughout the large wood sample of the only arborescent member within the tribe Spermacoceae that we investigated, Nesohedyotis arborea (trees up to 7 m tall). Apparently, the expanded ontogeny of wood cells in this species has changed many body ray cells into the matured procumbent shape (Fig. 4E). Nevertheless, according to the overview study of Carlquist (1962), it is the gradual decrease in vessel element length towards the outer stem parts that is the decisive factor to infer secondary woodiness in a given species [although the pith region was not included in our large sample (70 mm in diameter), we did measure, on average, a 150-μm decrease in vessel element length between the youngest part and the cambium zone]. Other wood features in Spermacoceae that are unusual in paedomorphic species were the low level of parenchymatization throughout the tribe (Fig. 2A–C; except in the underground structure of Spermacoce manikensis, Fig. 3) and the presence of thick-walled fibres/tracheids in the genera Oldenlandia and Lathraeocarpa (Carlquist 1962, Fig. 2C).
According to our observations of wood anatomy, the origin of woodiness in Knoxieae is more complex. All taxa observed of the first group (Otiophora, Otomeria, Pentanisia, Pentas and Phyllopentas) are hypothesized to be secondarily woody based on the presence of paedomorphic length vs. age curves for vessel elements (and to a lesser extent also fibre-tracheids/tracheids) and the predominance of square-to-upright ray cells (Figs 5C, F, I and 6F). In contrast, the three genera observed in the second group (Carphalea, Dirichletia and Triainolepis) can be distinguished from the first group by wood features that are typical of ‘normal’ primarily woody dicots: (1) length vs. age curves indicate a strong initial decrease of vessel element length in the primary xylem and in the first-formed wood, followed by an increase and subsequent stabilization (Fig. 6E), and (2) rays are composed of procumbent body ray cells and square-to-upright rows of marginal ray cells (Fig. 5D, E, G, H).
Figure 7 demonstrates that the general assumption of a primarily woody Rubioideae ancestor is plausible: early-diverging Rubioideae clades include many woody shrubs or (small) trees having non-paedomorphic rays with procumbent body ray cells and upright marginal ray cells, such as for example Stelechantha (Urophylleae; Jansen et al., 2001), Lasianthus (Lasiantheae, represented by Saldinia and Trichostachys in Fig. 7; Jansen et al., 2001) and Psychotria (Psychotrieae; Jansen et al., 1997). Moreover, our study in Knoxieae reveals that at least some primarily woody species remain present in deeply nested Rubioideae lineages. With respect to Knoxieae, the molecular phylogeny of Kårehed and Bremer (2007) shows that our three primarily woody species are all placed in early-diverging branches within the tribe, which would indicate that the ancestor of Knoxieae was probably primarily woody (Fig. 7). This is further corroborated by the fact that all species of Carphalea, Dirichletia and Triainolepis are clearly woody (shrubs to small trees), although the earliest diverging Knoxieae lineage, Chamaepentas, consists of species that are considered to be woody herbs (Verdcourt, 1976). In contrast, all the secondarily woody species studied belong to later-diverging Knoxieae branches (Kårehed and Bremer, 2007), in which entirely herbaceous or herbaceous-like shrubby species are common (Verdcourt, 1976). Within Spermacoceae s.l., Fig. 7 demonstrates that woodiness has developed multiple times in all major essentially herbaceous lineages, and therefore current molecular evidence suggests that the ancestor of Spermacoceae was herbaceous. As a result, woody members are best interpreted as secondarily woody.
Consequently, the present molecular phylogenies seem to support our wood anatomical hypothesis that points to primary as well as secondary woodiness in Knoxieae on the one hand, and to exclusively secondary woodiness within Spermacoceae on the other. However, it should be kept in mind that several important nodes within the molecular phylogeny of Rubioideae as a whole and Spermacoceae–Knoxieae in particular are not fully resolved.
Ecology of secondarily woody species
Why do some species that belong to essentially herbaceous clades develop a woody habit? First of all it should be noted that probably a majority of Spermacoceae species are not annuals but short-lived perennials. In addition, some essentially annual species become short-lived perennials when growth conditions (ideal temperature and water availability) allow the species to grow longer. The wood production in most of these species, however, is limited. Geoxylic herbs, sometimes developing considerable amounts of secondary derived wood (Fig. 1A, detail), probably evolved to withstand longer periods of drought. This could, for example, be true for Spermacoce manikensis growing on the nutrient poor Kalahari sands, which are inundated during the rainy season but are completely dry for the rest of the year. In this species there is also evidence that the massive amount of parenchyma tissue in the underground organs plays a role in water storage (Fig. 3B). Another evolutionary advantage of geoxylic herbs is their ability to survive under periodic fire conditions, which has been clearly demonstrated for example in some Otiophora species (Knoxieae; Puff, 1981). Other secondarily woody Spermacoceae–Knoxieae can be generally described as woody herbs, subshrubs or shrubs, and some grow in habitats that are dry for at least part of the year (Table 1); in particular, the woody Spermacoceae growing in Madagascar undergo severe drought stress (Table 1). Consequently, it appears that – at least for some Spermacoceae and Knoxieae species – recurring water stress goes hand-in-hand with wood production.
From the point of view of wood anatomy, there are some well-known features that can be correlated with dry habitats, such as exclusively simple vessel perforations, vestured intervessel pits, high vessel densities (often more than 100 mm−2), narrow vessels (often between 20–40 µm), short vessel elements (often below 350 µm, although this is also induced by the secondary woodiness), and the presence of water-conducting tracheids in the ground tissue that acts as a subsidiary water transport system in case the vessels become embolized (Carlquist, 1966, 1984; Baas et al., 1983; Carlquist and Hoekman, 1985; Baas, 1986; Choat et al., 2004). The species observed that have the most pronounced xeromorphic wood features were Arcytophyllum thymifolium, Lathraeocarpa acicularis, ‘Oldenlandia’ ambovombensis and ‘Oldenlandia’ humbertii. Furthermore, the latter three species were the only ones having thick-walled (narrow) vessels and thick-walled fibre-tracheids/tracheids in their ground tissue, which were linked in order to support against vessel implosion by negative pressures in the xylem during drought (Hacke et al., 2001; Jacobsen et al., 2005). Not surprisingly, these three species are found in the markedly dry south-western part of Madagascar, characterized by a short rain season and a long dry season (Jury, 2003; Table 1).
Although the link between drought resistance and wood production is also observed in other angiosperms, such as some additional Rubiaceae (Rubia and Crucianella; Koek-Noorman, 1976), Brassicaceae (Carlquist, 1971), and some Asteraceae (Argyroxiphium and Dubautia; Carlquist, 2003), it is certainly not the most typical habitat for secondarily woody species to occur. Indeed, Table 1 shows that most non-Malagasy Spermacoceae/Knoxieae species studied have developed a secondarily woody habitat in areas that do not experience severe drought stress (cf. Carlquist, 1974). Examples are oceanic islands such as Hawaii (Kadua cordata) and St. Helena (Nesohedyotis arborea), and various high-elevation tropical montane areas (Arcytophyllum, Hedyotis). Likewise, when we look for a worldwide distribution of secondarily woody species throughout angiosperms, it is evident that environments with uniform annual temperatures and rainfall (for instance, on oceanic islands and tropical mountains) seem perfectly suitable for secondary woodiness to occur (Carlquist, 1974).
The degree of woodiness within most species studied (woody herbs or shrubs) appears to be related to the temperature and amount of rainfall. For example, the particular environmental conditions on St. Helena (15–20°C mean annual temperature, approx. 1000 mm annual precipitation) favour the arborescent habit of Nesohedyotis arborescens, and might explain why this species is the only truly arborescent Spermacoceae in our study. This aberrant habit type also accounts for the wider and fewer vessels of N. arborescens (Fig. 2E) compared to the other smaller woody species studied, although vessel element length is comparable (Table 2).
The numerous independent evolutionary shifts from herbaceousness to secondary woodiness in various flowering plant families and in diverse habitats must go hand-in-hand with a flexible genetic background mechanism that allows these frequent shifts. Preliminary evidence for this plastic genetic basis is provided by Melzer et al. (2008), who demonstrated that the inactivation of only two genes in the herbaceous Arabidopsis thaliana resulted in a perennial-like, shrubby mutant phenotype.
ACKNOWLEDGEMENTS
We thank Dr Sherwin Carlquist and Dr Jesper Kårehed for their valuable discussions, and the director of the BR herbarium and the curators of the xylaria of Kew (Kw), Tervuren (Tw) and Utrecht (Uw) for their supply of wood samples. We also thank Nathalie Geerts for technical assistance. This work was supported by research grants of the K.U. Leuven (grant number OT/05/35) and the Fund for Scientific Research – Flanders (Belgium) (F.W.O. – Vlaanderen) (grant number G.0250·05). F.L. is postdoctoral fellow of the Fund for Scientific Research – Flanders (Belgium) (F.W.O. – Vlaanderen). I.G. holds a PhD grant from the F.W.O. – Vlaanderen.
APPENDIX
List of taxa investigated in this study with reference to their locality, vouchers and stem diameter. Nomenclature of species' names follow Govaerts et al. (2006) and Kårehed and Bremer (2007). Institutional wood collections used in this study are abbreviated according to Index Xylariorum (Stern, 1988). Species names in bold refer to underground wood material.
SPERMACOCEAE: Arcytophyllum lavarum K.Schum: Costa Rica, J. Jangoux 1394 (BR), 3 mm; Arcytophyllum setosum (Ruiz & Pav.) Schltdl.: Ecuador, P. M. Jørgensen et al. 2378 (BR), 6 mm; Arcytophyllum thymifolium (Ruiz & Pav.) Standl.: Ecuador, F. Billiet and B. Jadin 6580 (BR), 3 mm; Diodella sarmentosa (Sw.) Bacigalupo & E.L.Cabral ex Borhidi: Cameroon, S. Dessein & B. Sonké 1423 (BR), 8 mm; Emmeorhiza umbellata (Spreng.) K.Schum: origin and collector unknown (BR-S.P.802 367), 3·5 mm; Gomphocalyxherniarioides Baker: Madagascar, P. De Block 2217 (BR), 4 mm; Hedyotis flavescens Thwaites: Sri Lanka, Fosberg 58004 (BR), 5 mm; Hedyotis fruticosa L.: Sri Lanka, D. D. Tirvangadum 663 (BR), 4 mm; Hedyotis lessertiana Arn.: Sri Lanka, P. L. Comanor 959 (BR), 4 mm; ‘Hedyotis’ trichoglossa Baker (unplaced taxon): Madagascar, H. Humbert 17722 (BR), 7 mm; Kadua cordata Cham. & Schltdl.: Hawaii Islands (USA), Stern, W.L. 2996 (Uw 18607), 7 mm; Lathraeocarpa acicularis Bremek.: Madagascar, P. De Block 2316 (BR), 6 mm; Mitracarpus frigidus (Willd. ex Roem. & Schult.) K.Schum.: National Botanic Garden of Belgium, F. Van Caekenberghe 2 (BR living collection), 19 mm; Nesohedyotis arborea (Roxb.) Bremek.: UK (St. Helena), J. C. Melliss s.n. (Kw 11160), mature (about 70 mm); ‘Oldenlandia’ ambovombensis sp. nov. ined.: Madagascar, P. De Block 2328 (BR), 4 mm, 11 mm; ‘Oldenlandia’ humbertii sp. nov. ined.: Madagascar, P. De Block 2294 (BR), 3 mm; Spermacoce bangweolensis (R.E.Fr.) Verdc.: Zambia, Dessein et al. 550 (BR), 20 mm; Spermacoce manikensis Dessein: Zambia, Dessein et al. 976 (BR), 16 mm; Spermacoce macrocephala (Standl. & Steyerm.) Govaerts: Venezuela, B. Maguire et al. 41668 (Tw 36238), 7 mm; Spermacoce occidentalis Hardwood: Australia, Harwood 1541 (BR), 10 mm; Spermacoce verticillata L.: South America, Cavalcanti et al. 358 (Uw 33690), 10 mm; Spermacoce verticillata L: Cultivated in the National Botanic Garden of Belgium, F. Van Caekenberghe 104 (BR), 11 mm;
KNOXIEAE: Carphalea kirondron Baill. subsp. geayi (Homolle) Puff: Madagascar, A. M. Homolle 1415 (BR), 6 mm; Dirichletia virgata (Balf.f.) Kårehed & B.Bremer: Yemen (Socotra Archipelago), Schweinfurth 43 (Uw 25955), mature; Otiophorarupicola Verdc., Burundi, M. Reekmans 178 (BR), 7 mm; Otomeria micrantha K.Schum.: DR Congo, J. Louis 3389 (BR), 5 mm; Pentanisia schweinfurthii Hiern: DR Congo, G. F. de Witte 07197 (BR), 7 mm; Pentanisia schweinfurthii Hiern: DR Congo, G. F. de Witte 02767 (BR), 5 mm; Pentas zanzibarica Vatke var. rubra Verdc.: DR Congo, Lebrun 3691 (BR), 8 mm; Phyllopentas schimperiana (Vatke) Kårehed & B.Bremer: Ethiopia, C. Puff 810920–1/2 (Uw 27081), 8 mm; Triainolepis polyneura Bremek.: Madagascar, P. De Block 593 (BR), 16 mm.
LITERATURE CITED
- Baas P. Ecological patterns in xylem anatomy. In: Givnish TJ, editor. On the economy of plant form and function. Cambridge: Cambridge University Press; 1986. pp. 327–352. [Google Scholar]
- Baas P, Werker E, Fahn A. Some ecological trends in vessel characters. International Association of Wood Anatomists Bulletin, new series. 1983;4:141–159. [Google Scholar]
- Bailey IW. The cambium and its derivative tissues. II. Size variations of cambial initials in gymnosperms and angiosperms. American Journal of Botany. 1920;7:355–367. [Google Scholar]
- Böhle UR, Hilger HH, Martin WF. Island colonization and evolution of the insular woody habit in Echium L. (Boraginaceae) Proceedings of the National Academy of Sciences of the USA. 1996;93:11740–11745. doi: 10.1073/pnas.93.21.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremekamp CEB. Les Lathraeocarpées. Tribu nouvelle des Rubioïdées (Rubiacées) Bulletin du Jardin Botanique de l'Etat a Bruxelles. 1957;27:159–166. [Google Scholar]
- Bremer B, Manen J-F. Phylogeny and classification of the subfamily Rubioideae (Rubiaceae) Plant Systematics and Evolution. 2000;225:43–72. [Google Scholar]
- Carlquist S. A theory of paedomorphosis in dicotyledonous woods. Phytomorphology. 1962;12:30–45. [Google Scholar]
- Carlquist S. Wood anatomy of Compositae: a summary, with comments on factors controlling wood evolution. Aliso. 1966;6:25–44. [Google Scholar]
- Carlquist S. Wood anatomy of Macaronesian and other Brassicaceae. Aliso. 1971;7:365–384. [Google Scholar]
- Carlquist S. Island biology. New York: Columbia University Press; 1974. pp. 350–428. (chapter 10, ‘Insular woodiness’) [Google Scholar]
- Carlquist S. Vessel grouping in dicotyledon wood: significance and relationship to imperforate tracheary elements. Aliso. 1984;10:505–525. [Google Scholar]
- Carlquist S. Tracheid dimorphism: a new pathway in evolution of imperforate tracheary elements. Aliso. 1988;12:103–118. [Google Scholar]
- Carlquist S. Wood anatomy of sympetalous dicotyledon families: a summary, with comments on systematic relationships and evolution of the woody habit. Annals of the Missouri Botanic Garden. 1992;79:303–332. [Google Scholar]
- Carlquist S. Comparative wood anatomy. 2nd edn. Berlin, Heidelberg: Springer-Verlag; 2001. [Google Scholar]
- Carlquist S. Wood anatomy of Madiinae in relation to ecological diversification. In: Carlquist S, Baldwin B, Carr G, editors. Tarweeds & silverswords: evolution of the Madiinae (Asteraceae) St Louis, MO: Missouri Botanical Garden Press; 2003. pp. 129–144. [Google Scholar]
- Carlquist S, Hoekman DA. Ecological wood anatomy of the woody southern Californian flora. International Association of Wood Anatomists Bulletin, new series. 1985;6:319–347. [Google Scholar]
- Choat B, Jansen S, Zwieniecki MA, Smets E, Holbrook NM. Changes in pit membrane porosity due to deflection and stretching; the role of vestured pits. Journal of Experimental Botany. 2004;55:1569–1575. doi: 10.1093/jxb/erh173. [DOI] [PubMed] [Google Scholar]
- Dessein S. Systematic studies in the Spermacoceae (Rubiaceae) Leuven, Belgium: Katholieke Universiteit Leuven; 2003. Ph.D. thesis. [Google Scholar]
- Dessein S, Jansen S, Robbrecht E, Smets E. A new species of Spermacoceae (Rubiaceae) from the Manika high plateau (Katanga; R.D. Congo) Nordic Journal of Botany. 2002;22:513–523. [Google Scholar]
- Dessein S, Ntore S, Robbrecht E, Smets E. Pollen and seeds reveal that Spermacoceae thymoidea s.l. (African Rubiaceae, Spermacoceae) represents three endemic or disjunct species from the Zambezian High Plateaus. Systematic Botany. 2003;28:130–144. [Google Scholar]
- Goloboff PA. NONA ver. 2.0. Tucumán, Argentina: published by the author; 1999. [Google Scholar]
- Govaerts R, Ruhsam M., Andersson L, et al. World checklist of Rubiaceae. Kew, London: The Board of Trustees of the Royal Botanic Gardens, Kew; 2006. Published online: http://apps.kew.org/wcsp/prepareChecklist.do?checklist=rubiaceae%40%40320161120060825802 . [Google Scholar]
- Groeninckx I, Dessein S, Ochoterena H, et al. Phylogeny of the herbaceous tribe Spermacoceae (Rubiaceae) based on plastid DNA data. Annals of the Missouri Botanical Garden. 2009;a In press. [Google Scholar]
- Groeninckx I, Dessein S, Ochoterena H, et al. Rediscovery of Malagassy Lathraeocarpa allows determination of its taxonomic position within Rubiaceae. Taxon. 2009;b In press. [Google Scholar]
- Groover AT. What genes make a tree a tree? Trends in Plant Science. 2005;10:210–214. doi: 10.1016/j.tplants.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia. 2001;126:457–461. doi: 10.1007/s004420100628. [DOI] [PubMed] [Google Scholar]
- IAWA Committee. IAWA list of microscopic features for hardwood identification. International Association of Wood Anatomists Bulletin, new series. 1989;10:219–332. [Google Scholar]
- Jacobsen AL, Ewers FW, Pratt RB, Paddock WA, III, Davis SD. Do xylem fibers affect vessel cavitation resistance? Plant Physiology. 2005;139:546–556. doi: 10.1104/pp.104.058404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S, Robrecht E, Beeckman H, Smets E. Wood anatomy of the predominantly African representatives of the tribe Psychotrieae (Rubiaceae–Rubioideae) International Association of Wood Anatomists Journal. 1997;18:169–196. [Google Scholar]
- Jansen S, Lens F, Ntore S, Piesschaert F, Robbrecht E, Smets E. Contributions to the wood anatomy of the Rubioideae (Rubiaceae) Journal of Plant Research. 2001;114:269–289. [Google Scholar]
- Jansen S, Robrecht E, Beeckman H, Smets E. A survey of the systematic wood anatomy of the Rubiaceae. International Association of Wood Anatomists Journal. 2002;23:1–67. [Google Scholar]
- Jury MR. Climate. In: Goodman SM, Benstead JP., editors. The natural history of Madagascar. Chicago and London: The University of Chicago Press; 2003. pp. 75–87. [Google Scholar]
- Kårehed J, Bremer B. The systematics of Knoxieae (Rubiaceae)—molecular data and their taxonomic consequences. Taxon. 2007;56:1051–1076. [Google Scholar]
- Kårehed J, Groeninckx I, Dessein S, Motley TJ, Bremer B. The phylogenetic utility of chloroplast and nuclear DNA markers and the phylogeny of the Rubiaceae tribe Spermacoceae. Molecular Phylogenetics and Evolution. 2008;49:843–866. doi: 10.1016/j.ympev.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Koek-Noorman J. Juvenile chracters in the wood of certain Rubiaceae with special emphasis to Rubia fruticosa Ait. International Association of Wood Anatomists Bulletin. 1976;3:38–42. [Google Scholar]
- Koek-Noorman J. Systematische Holzanatomie einiger Rubiaceen. Berichte der Deutschen Botanischen Gesellschaft. 1977;90:183–190. [Google Scholar]
- Koek-Noorman J, Puff C. The wood anatomy of Rubiaceae tribes Anthospermeae and Paederieae. Plant Systematics and Evolution. 1983;143:17–45. [Google Scholar]
- Lee C, Kim S-C, Lundy K, Santos-Guerra A. Chloroplast DNA phylogeny of the woody Sonchus alliance (Asteraceae: Sonchinae) in the Macaronesian islands. American Journal of Botany. 2005;92:2072–2085. doi: 10.3732/ajb.92.12.2072. [DOI] [PubMed] [Google Scholar]
- Lens F, Dressler S, Jansen S, Van Evelghem L, Smets E. Relationships within balsaminoid Ericales: a wood anatomical approach. American Journal of Botany. 2005;92:941–953. doi: 10.3732/ajb.92.6.941. [DOI] [PubMed] [Google Scholar]
- Melzer S, Lens F, Gennen J, Vanneste S, Rhode A, Beeckman T. Flowering time genes modulate meristem determinacy and growth form in. Arabidopsis. Nature Genetics. 2008;40:1489–1492. doi: 10.1038/ng.253. [DOI] [PubMed] [Google Scholar]
- Nixon KC. WinClada ver. 1.0000. Ithaca, NY: Published by the author; 2002. [Google Scholar]
- Puff C. Studies in Otiophora Zucc. (Rubiaceae): 2. A new classification of the southern African taxa. Journal of South African Botany. 1981;47:297–329. [Google Scholar]
- Razafimandimbison SG, Rydin C, Bremer B. Evolution and trends in the Psychotrieae alliance (Rubiaceae). A rarely reported evolutionary change of many-seeded carpels from one-seeded carpels. Molecular Phylogenetics and Evolution. 2008;48:207–223. doi: 10.1016/j.ympev.2008.03.034. [DOI] [PubMed] [Google Scholar]
- Robbrecht E, Manen J-F. The major evolutionary lineages of the coffee family (Rubiaceae, angiosperms). Combined analysis (nDNA and cpDNA) to infer the position of Coptosapelta and Luculia, and supertree construction based on rbcL, rps16, trnL-trnF and atpB-rbcL data. A new classification in two families, Cinchonoideae and Rubioideae. Systematics and Geography of Plants. 2006;76:85–146. [Google Scholar]
- Stern WL. Index xylariorum – 3. Institutional wood collections of the world. International Association of Wood Anatomists Bulletin, new series. 1988;9:203–252. [Google Scholar]
- Thiv M, Struwe L, Kadereit JW. The phylogenetic relationships and evolution of the Canarian laurel forest endemic Ixanthus viscosus (Aiton) Griseb. (Gentianaceae): evidence from matK and ITS sequences, and floral morphology and anatomy. Plant Systematics and Evolution. 1999;218:299–317. [Google Scholar]
- Verdcourt B. Rubiaceae (part 1) In: Polhill RM, editor. Flora of tropical East Africa. London: Crown Agents for Oversea Governments and Administrations; 1976. pp. 1–414. [Google Scholar]