Abstract
Background and Aims
Selection may favour a partial or complete loss of self-incompatibility (SI) if it increases the reproductive output of individuals in the presence of low mate availability. The reproductive output of individuals varying in their strength of SI may also be affected by population density via its affect on the spatial structuring and number of S-alleles in populations. Modifiers increasing levels of self-compatibility can be selected when self-compatible individuals receive reproductive compensation by, for example, increasing seed set and/or when they become associated with high fitness genotypes.
Methods
The effect of variation in the strength of SI and scrub density (low versus high) on seed set, seed germination and inbreeding depression in seed germination (δgerm) was investigated in the partially self-incompatible species Flourensia cernua by analysing data from self-, cross- and open-pollinated florets.
Key Results
Examination of 100 plants in both high and low scrub densities revealed that 51% of plants were strongly self-incompatible and 49 % varied from being self-incompatible to self-compatible. Seed set after hand cross-pollination was higher than after open-pollination for self-incompatible, partially self-incompatible and self-compatible plants but was uniformly low for strongly self-incompatible plants. Strongly self-incompatible and self-incompatible plants exhibited lower seed set, seed germination and multiplicative female fitness (floral display × seed set × seed germination) in open-pollinated florets compared with partially self-incompatible and self-compatible plants. Scrub density also had an effect on seed set and inbreeding depression: in low-density scrubs seed set was higher after open-pollination and δgerm was lower.
Conclusions
These data suggest that (a) plants suffered outcross pollen limitation, (b) female fitness in partially self-incompatible and self-compatible plants is enhanced by increased mate-compatibility and (c) plants in low-density scrubs received higher quality pollen via open-pollination than plants in high-density scrubs.
Key words: Flourensia cernua, population density, seed set, seed germination, female fitness, partial self-incompatibility, Mapimí Biosphere Reserve
INTRODUCTION
Genetic self-incompatibility (SI) is an important mechanism that prevents inbreeding in flowering plants (Barrett, 1998; de Nettancourt, 2001). By definition, self-incompatible species develop zero to very low numbers of fruits after self-pollination due to the action of the gene products of the S-locus which prevent pollen germination and/or growth whenever male (pollen) and female (pistil) tissues express the cognate S-alleles. Self-incompatible species often show relatively low fruit set (Sutherland and Delph, 1984; Sutherland, 1986; Larson and Barrett, 2000), a condition that depends upon factors such as the type of SI (gametophytic or sporophytic), the number of S-alleles in the population, the spatial distribution of S-phenotypes, and the availability of suitable pollinators. Nevertheless, some predominantly outcrossing, self-compatible species also exhibit low seed set. Studies have shown that a reduction in seed set in self-compatible but also highly outcrossing species is commonly caused by low pollinator availability, or high rates of fruit abortion in populations with high levels of genetic load (Charlesworth and Charlesworth, 1987; Wiens et al., 1987; Husband and Schemske, 1996; Wilcock and Neiland, 2002). However, a reduction in female fertility caused by SI is strictly a pre-zygotic mechanism (but see Sage et al., 1994), while one caused by inbreeding depression is post-zygotic (Charlesworth and Charlesworth, 1987).
Many members of the Asteraceae have sporophytically controlled SI in which the ability of two individuals to reproduce depends on whether they share one or more alleles at a multiallelic S-locus. Specifically, pollen will fail to hydrate and/or germinate on the stigmatic surface or the pollen tube may be unable to penetrate the stigma when the diploid genotype of the pollen-producing parent shares either S-allele with the recipient plant (de Nettancourt, 2001; Hiscock et al., 2002; Hiscock and McInnis, 2003; Hiscock and Tabah, 2003; Ferrer and Good-Avila, 2007) such that individuals may only mate with plants carrying both S-alleles distinct from their own unless the alleles show dominance in the pistil or pollen (de Nettancourt, 2001; Brennan et al., 2002, 2006; Hiscock and Tabah, 2003). Because this causes negative frequency-dependent selection of S-alleles, the mean reproductive success of an individual is expected to increase with the number of S-alleles in the population until the number of alleles is saturated (Wright, 1960; Schierup, 1998). The number of S-alleles in a population may decrease, however, as a consequence of drift processes, that are likely to occur when population size and density are small or when a population is founded by a few number of individuals (Byers and Meagher, 1992; Glemin et al., 2001).
Though many species are believed to be strongly self-incompatible, the evolutionary transition from SI to self-compatibility (SC hereafter) has occurred many times in flowering plants (Goodwillie, 1999; Takebayashi and Morrell, 2001; Stone, 2002; Igic et al., 2004, 2006, 2008; Ferrer and Good-Avila, 2007) and many species are partially self-incompatible (Levin, 1996; Stephenson et al., 2000). Partially self-incompatible species are those in which there is quantitative variation among plants in the strength of SI (e.g. plants vary from being partially self-incompatible to self-compatible). The increase in SC in species with functional SI can be caused by alterations in dominance relationships among S-alleles (Reinartz and Les, 1994; Brennan et al., 2002, 2003, 2006), unlinked modifier loci (Good-Avila et al., 2008b), differential rejection of self pollen in the presence of interspecific and allozygous mixtures (Desrochers and Rieseberg, 1998; Lipow and Wyatt, 2000), and a variety of environmental variables such as temperature, flower age, and the presence of developing fruits (Levin, 1996; Vogler et al., 1998; Stephenson et al., 2000; de Nettancourt, 2001; Good-Avila et al., 2008b). Such species are called partial or pseudo-self-compatible, partially self-compatible or pseudo self-fertile (Levin, 1996) and are relatively common in the Asteraceae (Stout, 1917; Anderson et al., 1988; Berry and Calvo, 1989; Cabrera and Dieringer, 1992; Giblin and Hamilton, 1999; Hiscock, 2000; Cheptou et al., 2002; Nielsen et al., 2003; Lafuma and Maurice, 2007) with approx. 10 % of the studied species in the family exhibiting partial SI (Ferrer and Good-Avila, 2007).
It is unclear whether species that are partially self-incompatible tend toward the evolutionary loss of SI or whether partial SI can be a stable mating system. A recent macrophylogenetic study of the role of partial SI in the Asteraceae indicates that partial SI is not a terminal state and can lead back to both SI and to SC (Ferrer and Good-Avila, 2007). The first genetic models on the conditions favouring the complete or partial breakdown of SI, found that mutations increasing rates of SC could not invade if background levels of inbreeding depression were greater than 0·5 (Charlesworth, 1980; Lande and Schemske, 1985). However, Porcher and Lande (2005) recently showed that the success of modifiers to either completely invade or find intermediate selfing rates in self-incompatible populations is influenced by whether the modifiers are linked or unlinked to the S-locus, the strength of S-linked and background genetic load, and the degree of pollen limitation. Similarly, two other theoretical models have found that intermediate selfing rates, i.e. partial SI, can be evolutionarily stable: Vallejo-Marín and Uyenoyama (2004) showed that partial SI can be a stable mating system when self-compatible plants receive reproductive compensation (e.g. higher seed set) for producing inbred offspring when seed set is pollen limited and Harder et al. (2008) found that mixed mating systems can be stable when reproductive compensation is given by increasing seed set through inbreeding if post-dispersal inbreeding depression remains low. In particular, when inbreeding depression is based on recessive and partially deleterious mutations, lineages practising higher rates of self-fertilization may purge genetic load and express lower inbreeding depression (Uyenoyama and Waller, 1991).
Seed set is often pollen limited in self-incompatible species (Larson and Barrett, 2000) because females are more likely to receive either an inadequate quantity or quality (compatible) of pollen. In animal-pollinated species, the strength of pollen limitation varies with the spatial distribution of plants, and plants inhabiting high-density stands usually have lower pollen limitation than those in low-density stands because of the increase in mate availability and pollinator attraction at high density (Kunin, 1993; Ghazoul, 2005). Lower pollen limitation in high-density stands has also been recorded in wind-pollinated species (Smith et al., 1988; Allison, 1990; Morgante et al., 1991; Arista and Talavera, 1996) in which an increase in cross- relative to self-pollen was observed in the pollen cloud of high-density stands (Allison, 1990). Therefore population density via its effect on pollen limitation may play an important role in the conditions favouring the complete or partial breakdown of SI.
Flourensia cernua (Asteraceae, Heliantheae) is a shrub characteristic of the Chihuahuan Desert (McMahon, 1999). Preliminary data suggested that F. cernua has an SI system, but that plants vary in their strength of SI, i.e. the species exhibits partial SI (Ferrer, 2004). Despite dichogamy, self-fertilization can occur in plants that are partially self-incompatible or self-compatible through geitonogamous pollination between synchronously open florets within and among flower heads. Indeed, an earlier study of the mating system of F. cernua using 11 isozyme loci found that the multilocus outcrossing rate means were 0·83 ± 0·27 s.d. and 1·19 ± 0·19 s.d. in the low- and high-density scrubs described in the next sentence. In northern Mexico, F. cernua is found in mosaics of plant communities distributed in bajadas (gentle sloping terrain connecting the foothill with the bottom closed basins – endorheic basins); populations at the base of foothills have lower density than those at the edge of the basin (Mauchamp, 1992). In populations from these mosaics, the species exhibits very low female fertility: 0–22 viable seeds per plant produced from approx. 5000 ovules (distributed in approx. 250 flower heads with 20 florets each) per reproductive season (Ferrer, 2004). Additionally, the proportion of seeds that germinate is low <10 % (Valencia-Díaz and Montaña, 2003; Ferrer, 2004). However, it is unknown whether the low levels of seed set and rates of seed germination observed in F. cernua are affected by variation in the strength of SI or scrub density.
In this study, the role of variation in the strength of SI and scrub density on outcross pollen limitation and the role of pollen source on the proportion of seeds that germinate (seed germination hereafter) are evaluated in F. cernua. Several hypotheses expected to be associated with the stability of partial SI were also evaluated. First, the association between inbreeding depression in seed germination with the strength of SI or scrub density was examined to test if historical variation in rates of self fertilization among lineages is associated with purging of genetic load (Uyenoyama et al., 1993). Secondly, the prediction by recent theoretical models favouring mixed mating systems that weak self-incompatible genotypes are associated with low postdispersal inbreeding depression was assessed (Harder et al., 2008). And finally, differences in female fitness among maternal plants were tested to evaluate if there is selection associated with either variation in the strength of SI and/or scrub density. To this end, the following were compared: (1) the mean seed set of progeny generated from self- and cross-pollination for each maternal plant to estimate their strength of SI (SI index); (2) the mean seed set of progeny generated from cross- and open- pollination to identify if seed set is outcross pollen limited; (3) the seed germination of progeny generated from three pollination treatments (self-, cross- and open-pollination) to identify the effect of pollen source on seed germination; (4) the relative success of seed germination in seeds sired from self- versus cross-pollination to estimate inbreeding depression in seed germination (δgerm); and (5) a multiplicative female fitness estimate (number of seeds produced after open-pollination per plant) in scrubs with contrasting population densities (low and high density) from which outcrossing rates were previously estimated (Ferrer et al., 2004).
MATERIALS AND METHODS
Species and study site
Flourensia cernua is considered to be an indicator plant species of the Chihuahuan Desert; it grows in arid or semi-arid habitats associated with small- to medium-sized shrubs such as Larrea, Opuntia, Agave and Acacia, and medium-sized trees such as Prosopis and Acacia (Rzedowski, 1988; McMahon, 1999). The species produces flowers at the end of autumn (October–November) and fruits, the single-seeded fruits – achenes – typical of the Asteraceae, during the winter (December–February) (Dillon, 1984). It has small flower heads (approx. 10 × 10 mm wide by long) with exclusively discoid florets (<2 mm long) which are arranged in terminal and lateral inflorescences along the tips of branches (Dillon, 1984). Morphological and ecological floral traits of F. cernua are associated with a wind-pollination syndrome (Culley et al., 2002) which has been confirmed in the species using exclusion experiments (Mauchamp, 1992). The size of the floral display varies significantly among plants: a survey in 1998 showed that the number of flower heads per plant varied from five to 500 with a median of 150 (n = 500 plants); while the number of discoid florets per flower head varied less among plants between 14 and 25 (rarely 30) with a median of 18 (n = 100 flower heads).
The current study was undertaken at the Mapimí Biosphere Reserve in two contrasting vegetation types: (1) low-density and (2) high-density scrubs located in the upper and lower bajadas, respectively. Plants are distributed along temporary streams running along the foothills and in terrains with slopes of approx. 3 % at the low-density sites while they occur within the vegetation arcs or ‘brousse tigrée’ described by (Montaña et al., 1990) in terrains with slopes <1·5 % in the high-density sites. Climatic and edaphic conditions are similar between the scrubs, except for slightly higher clay content in soils and higher water availability due to runoff rain-water redistribution in the high-density scrubs. Five permanent plots in each scrub type were randomly selected for a demographic long-term study (Ferrer, 2004). Each permanent plot covers an area of approx. 1·5 ha and of approx. 0·4 ha for the low- and high-density scrubs.
Scrub-density estimates
All of the reproductive plants of Flourensia cernua within the ten permanent plots (five low- and five high-density scrubs) were censused; the mean density of flowering plants in low- and high-density scrubs was 71·42 ± 44·46 s.d. and 3377 ± 1606 s.d. plants ha−1, respectively. To control for the effect of aggregation of plants, the distance from each maternal plant to the four nearest neighbours was measured (distance from neighbours hereafter), and the average of those values was used as a covariate in the analyses described below. Thus, the effect of the average distance among all plants within a scrub type was incorporated by analysing the differences in response variables between high- and low-density scrubs, while differences in the distances of the focal plants to the flowering plants in their neighbourhood was controlled by including the effect of the distance from neighbours.
Pollination experiment
In October 1998, ten reproductive adults were selected within each of the ten permanent plots (five in each scrub density). The focal plants were randomly chosen from a group of reproductive plants that had floral displays ranging from 100 to 300 flower heads and heights ranging from 1·5 m to 2 m. This selection criterion was used to control for differences in the availability of maternal resources which can affect fruit and seed abortion (Stephenson, 1981). The size of the experimental plants was estimated as the volume of the plant by measuring the plant height, and the length and width of the crown. The volume was then estimated by assuming that the shrub has the shape of an irregular cone using the following formula v = 1/3(hπlw), where v = volume, h = height, π = 3·1416, l = length and w = width. The plant volume was used as a covariate in the statistical analyses to remove variance in the size of the maternal plant as a possible factor limiting seed set and seed germination.
The 100 plants were fumigated with insecticide (Deltametrin; 12·5 mg L−1) twice a week from 15 October until the date of pollination (6–25 November) to prevent flower predation by flies and beetles. The insecticide remains active for approx. 2 d and applications were terminated approx. 3 d prior to anthesis to avoid interference with the pollination treatments. Fifteen branches were randomly chosen from each of the 100 maternal plants, and groups of five branches were randomly assigned to one of the three experimental treatments: (1) self-pollination – pollen from a different untreated branch on the same plant was applied to all the stigmas of all the flower heads on the experimental branch; (2) cross-pollination – pollen from one to five pollen donors was collected from synchronously blooming plants at least 250 m away and applied as in the self-pollination; and (3) open-pollination – an inflorescence was left uncovered during the entire flowering season, and bagged at flower senescence. Preceding and following the hand-pollination treatments, the top of the branches were wrapped in fine mesh bags to prevent contamination by exogenous pollen. Pollen for cross-pollinations was collected by tapping a flower head containing dehisced florets from the designated pollen donor onto the edge of a microcentrifuge tube. A variable number of pollen donors (one to five) was used to obtain the pollen mixtures used in the hand cross-pollination experiment because the availability of pollen donors varied across the flowering season (89 donors in total were used). A factorial analysis of variance (ANOVA) was performed to test if the number of pollen donors categorized as consisting of either (a) one or two or (b) three to five donors, or the interaction of this categorical factor with SI strength and scrub density affected seed set after cross pollination. The results of the ANOVA indicated that neither the number of pollen donors, nor the double and triple interactions of donor number with SI strength and scrub density were significant and this factor was not included in further analyses (Table S1 in Supplementary data, available online). The pollen mixtures were collected the same day they were used to pollinate the open florets and discarded after the pollination treatment. All of the hand-pollinations (self- and cross-pollination) were performed using a soft-bristle brush and maternal plants were re-visited every other day to perform the appropriate pollination treatments on newly opened florets. The number of florets per flower head and the number of flower heads per branch was recorded for all plants. After flowering, the experimental branches were bagged to prevent seed loss and predation.
Fitness components
The seeds from the experimental branches from each plant were collected in February 1999, transported to the laboratory, and filled fruits were stored in paper bags at ambient temperature. An achene was determined to contain a seed if it did not break after being squeezed gently with forceps and the embryo occupied at least three-quarters of the fruit. Preliminary assays (n = 500) demonstrated that seeds in the achenes that did not meet these criteria were not viable. Four female fitness components (floral display, seed set, seed germination and multiplicative female fitness) and the relative fitness of self- and cross-sired seeds (inbreeding depression) were estimated as follows.
The size of the floral display of maternal plants was estimated as the product of the number of branches bearing flower heads × the mean number of flower heads (over the five branches) × the mean number of florets per flower head (over the five branches). Seed set was estimated as the average number of seeds produced per floret by taking the sum of the number of seeds divided by the number of florets pollinated in all of the five experimental branches per treatment. Seed germination was estimated as the proportion of seeds that germinated from the seeds produced in each pollination treatment. To germinate the seeds, all filled fruits were placed in an environmental chamber and left to germinate on a moistened cotton substrate (12 h light at 26 °C:12 h darkness at 16 °C; 60 % relative humidity) during the months of July and August 1999. The number of seeds that germinated was counted after 25 d when most viable seeds will have germinated according to Valencia-Díaz and Montaña (2003). Finally, a multiplicative female fitness estimate was derived based only on the data for the open-pollination treatment. For this analysis, female fitness (ff) was calculated on a per plant basis using the following equation: ff = fdsssg, where fd = floral display, ss seed set and sg = seed germination.
Inbreeding depression in seed germination (δgerm hereafter) was calculated only for plants that produced seeds after both the self- and cross-pollination treatment (i.e. for self-incompatible, partially self-incompatible and self-compatible plants which comprised 49 out of the 100 maternal plants). For these plants inbreeding depression was calculated as: δ = 1 – (wi/wo), where δ = inbreeding depression, wi = the fitness of progeny obtained from self-pollination and wo = the fitness of progeny resulting from cross-pollination (Lande and Schemske, 1985).
Statistical analysis
Variation in the strength of SI
The SI index is a continuous variable that is defined by the equation SI index = ssi/sso, where ssi = is the mean seed set after self-pollination in a plant and sso = is the mean seed set after cross-pollination in the same plant. For the statistical analyses, plants were grouped by their SI index into the following four SI categories modified from Ruiz-Zapata and Arroyo (1978): (1) strongly self-incompatible (SI index = 0), (2) self-incompatible (0 > SI index < 0·149), (3) partially self-incompatible (0·15 ≤ SI index < 0·49) and (4) self-compatible (SI index ≥ 0·5). The frequency distributions of the number of plants in each SI category in each scrub type were compared using a χ2 test of independence (Sokal and Rolhf, 1995). This index is a relative measure of the seeds set after self- and cross-pollination; consequently some plants having low self-fertilization rates can be categorized as partially self-incompatible or self-compatible if the seeds set after self-pollination are 25–50 %, or >50 % of those set after cross-pollination, respectively. A χ2 test of independence was used to assess if the proportion of seeds set after hand self- and cross-pollination was independent of SI category (Sokal and Rolhf, 1995). For the test, there were four and seven categories for the proportion of seeds set following self- and cross-pollination, respectively, in which seed set values (the proportion of seeds set per pollinated floret) were grouped in increments of 0·09 units.
For the statistical analyses of outcross pollen limitation and female fitness, only plants that produced seeds in the open-pollination treatments (30 plants in the high-density scrub and 31 plants in the low-density scrub) were included so that comparisons between the cross- and open-pollination treatments could be made; while for the statistical analysis of inbreeding depression only the 49 plants that produced seeds in both self- and cross-pollination treatments were included because inbreeding depression is a relative measure of the performance of progeny produced via self- and cross-pollination.
Effect of strength of SI scrub density on outcross pollen limitation
Outcross pollen limitation was assessed by comparing seed set after cross- and open-pollination using a split-plot ANOVA to analyse the effect of variation in the strength of SI and scrub density on seed set; the data did not violate the assumption of sphericity required for this design (Von Ende, 2001). The split-plot ANOVA included the fixed factors SI category, scrub density (between subject factors) and pollination treatment (within subject factor), and the distance from nearest neighbours was included as a covariate. The mean seed set was subjected to an angular transformation prior to analysis using the equation sstransformed = arcsin (√ss), where sstransformed = transformed seed set data and ss = original seed set data to meet the ANOVA assumption of normally distributed residuals (Sokal and Rolhf, 1995).
Effect of strength of SI, scrub density and pollen source on seed germination
To analyse the effect of variation in the strength of SI and scrub density on seed germination a split-plot ANOVA (again the data did not violate the assumption of sphericity; Von Ende, 2001) was used to compare seed germination of progeny derived from three pollen sources: self-, cross- and open-pollination. The split-plot ANOVA included the fixed factors SI category, scrub density (between subject factors) and pollination treatment (within subject factor) and the distance from nearest neighbours was included as a covariate. When a pollination treatment did not produce seeds, the proportion of seeds that germinated (seed germination) was not included. The mean values for seed germination were subjected to an angular transformation prior to the analysis using the equation sgtransformed = arcsin (√sg), where sgtransformed = transformed seed germination data and sg = original seed germination data to meet the ANOVA assumption of normally distributed residuals (Sokal and Rolhf, 1995).
Effect of strength of SI and scrub density on inbreeding depression
To determine if δgerm was influenced by the strength of SI and/or scrub density, an ANOVA (Sokal and Rolhf, 1995) was performed on the response variable δgerm using SI category (three levels: self-incompatible, partially self-incompatible and self-compatible), scrub density and their interaction as fixed effects. Inbreeding depression in seed germination (δgerm) can be considered to be a post-dispersal estimate of inbreeding depression and, when weak, may become an important factor increasing the evolutionary stability of mixed mating systems (Harder et al., 2008).
Effect of strength of SI and scrub density on multiplicative female fitness
Variation in multiplicative female fitness for the open-pollination treatment was analysed using an ANOVA (Sokal and Rolhf, 1995) in which SI category, scrub density and their interaction were treated as fixed effects and the distance from the nearest neighbours and plant volume were used as covariates. Female fitness was subjected to a logarithmic transformation prior to analysis to meet the ANOVA assumption of normally distributed residuals (Sokal and Rolhf, 1995). All statistical analyses were performed with STATISTICA using the general linear model module because all the designs were unbalanced and type II sums of squares were consequently used to estimate the variance components (Statsoft, 1998).
RESULTS
Variation in the strength of SI
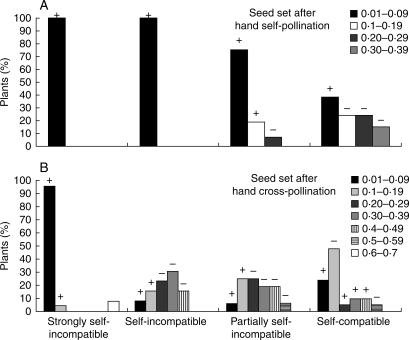
It was found that 51 % of the reproductive individuals in both scrub types had SI indexes <0·1, i.e. they were strongly self-incompatible (Fig. 1). However, it is noteworthy that most of these individuals produced very low seed set following both self- and cross-pollination (Fig. 2A, B). The remaining 49 % of the plants in the scrubs showed some degree of SC. In total, 16 % of plants were found to be self-incompatible (0 < SI index ≤ 0·149), 13 % partially self-incompatible (0·15 ≤ SI index ≤ 0·49) and 20 % self-compatible (SI index ≥ 0·50; Fig. 1). The frequency distribution of the four SI categories did not differ between scrub densities (χ2d.f. 5 = 4·77, P = 0·19; Fig. S1 in Supplementary data, available online). The frequency distribution of the number of seeds set after self-pollination differed among SI categories (χ2d.f. 9 = 47·5, P < 0·0001; Fig 2A). Seed set after self-pollination treatment was nil or lower than 0·1 in all of the strongly self-incompatible and self-incompatible plants and in 75 % and 38 % of the plants that were categorized as partially self-incompatible and self-compatible, respectively (Fig. 2A). Seed set after self-pollination varied between 0·1 and 0·36 in the remaining 25 % and 62 % of plants categorized as partially self-incompatible and self-compatible, respectively (Fig. 2A).
Fig. 1.
Frequency distribution of plants according to SI categories (strongly self-incompatible, SI index = 0; self-incompatible, 0 < SI index ≤ 0·149; partially self-incompatible, 0·15 ≤ SI index ≤ 0·49; self-compatible, SI index ≥ 0·5). Data gathered for Flourensia cernua in the Mapimí Biosphere Reserve, from a hand-pollination experiment (self- and cross-pollination) involving 50 plants from low-density scrubs and 50 plants from high-density scrubs. SI index was estimated as: (seed set after self-pollination)/(seed set after cross-pollination).
Fig. 2.
Frequency distribution of seed set (A) after hand self-pollination in four categories, and (B) after cross-pollination in seven categories. The values of seed set for each category are grouped into increments of seed of 0·09 units ranging from 0 to 0·39 for seeds sired from self-pollination and from 0 to 0·59 for seeds sired from cross-pollination. The percentage of plants observed in the different categories of seed set was plotted within each SI category (see Fig. 1 for definitions of categories). Data were gathered for Flourensia cernua in the Mapimí Biosphere Reserve from a hand-pollination experiment (self- and cross-pollination), involving 50 plants from low-density scrubs and 50 plants from high-density scrubs. Plus and minus signs are presented for the Haberman standardized residual analyses: + or – sign over a column indicates a significant excess or deficit of plants in that category, respectively.
The frequency distribution of the seed set values after cross-pollination differed among the SI categories (χ2d.f. 18 = 96·5, P < 0·0001). A large proportion of strongly self-incompatible plants (96 %) had seed set values of less than 0·1 after cross-pollination. Most of the self-incompatible plants, partially self-incompatible and self-compatible plants had seed set values between 0·1 and 0·29 after cross-pollination (38·5 %, 50 % and 52·4 %, respectively) and between 0·3 and 0·7 (53·8 %, 43·8 % and 23·8 %, respectively).
Effect of strength of SI and scrub density on outcross pollen limitation
Both SI category and pollination treatment had significant influences on seed set and explained 14 % and 23 %, respectively, of the variance in seed set in the ANOVA (Table 1). The covariate (distance from nearest neighbours) removed 1·8 % of the variance of the models from seed set. However, the interactions between SI category × pollination treatment and scrub density × pollination treatment were also significant and explained 7·1 % and 2·1 % of the variance (Table 1). Thus, the mean seed set after cross- and open-pollination varied with (a) SI strength and (b) scrub density.
Table 1.
Split-plot ANOVA results for the fitness components seed set and seed germination. Between-subjects effects are for SI category (strongly self-incompatible, self-incompatible, partially self-incompatible and self-compatible) growing in two scrubs types (high density and low density). The pollination treatments (within-subjects effects) that were included to evaluate the outcross pollen limitation were cross- and open-pollination, while the treatments included to evaluate the effect of pollen source in seed germination were self-, cross- and open-pollination
| Seed set |
Seed germination |
|||||||
|---|---|---|---|---|---|---|---|---|
| Source | d.f. | SS effect | SS error | F | d.f. | SS effect | SS error | F |
| Between subjects | ||||||||
| Self-incompatibility category | 3,52 | 0·917 | 1·819 | 8·74*** | 3,52 | 1·534 | 5·378 | 4·95** |
| Scrub density | 1,52 | 0·011 | 1·819 | 0·31 | 1,52 | 0·015 | 5·378 | 0·14 |
| Self-incompatibility category × scrub density | 3,52 | 0·073 | 1·819 | 0·69 | 3,52 | 0·121 | 5·378 | 0·39 |
| Within subjects | ||||||||
| Pollination treatment | 1,53 | 1·502 | 1·368 | 58·19*** | 2,106 | 1·387 | 6·335 | 11·60*** |
| Self-incompatibility category × pollination treatment | 3,53 | 0·455 | 1·368 | 5·87** | 6,106 | 0·241 | 6·335 | 0·67 |
| Scrub density × pollination treatment | 1,53 | 0·137 | 1·368 | 5·29* | 2,106 | 0·213 | 6·335 | 1·78 |
| Self-incompatibility category × scrub density × pollination treatment | 3,53 | 0·080 | 1·368 | 1·04 | 6,106 | 0·130 | 6·335 | 0·36 |
Average distance to the four nearest neighbours and plant volume from the 61 Flourensia cernua plants assessed were used as covariates and explained 1·8 % and 3·3 % of the variation for seed set and seed germination, respectively.
* P < 0·05; ** P < 0·005; *** P < 0·0001.
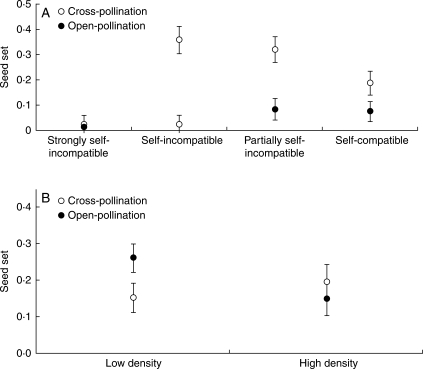
To understand the nature and direction of the interaction effects on seed set, box plots of the mean seed set by SI category and pollination treatment were constructed (Fig. 3A). A Tukey–Kramer test comparing the mean seed set by SI category and pollination treatment showed that (a) the mean seed set after open-pollination was the same for all SI categories, but (b) self-incompatible and partially self-incompatible plants had higher seed set after hand cross-pollination than self-compatible plants which again, had higher seed set than strongly self-incompatible plants (P ≤ 0·0001; Fig. 3A). The increase in seed set after hand cross-pollination relative to open-pollination for all plants except those that were found to be strongly self-incompatible suggests that outcross pollen limits seed set.
Fig. 3.
The mean (± s.e.) of seed set for 61 Flourensia cernua plants growing in the Mapimí Biosphere Reserve after cross-pollination and open-pollination grouped by (A) SI category and (B) scrub density. Seed set per plant was estimated as the mean proportion of seeds produced by florets in five experimental branches for each treatment. SI categories are defined in Fig. 1.
The interaction between pollination treatment × scrub density was primarily caused by the reverse response of plants to open- and cross-pollination in low- versus high-density scrubs: seed set was higher in low-density scrubs after open compared with hand cross-pollination (Fig. 3B).
Effect of strength of SI, scrub density and pollen source on seed germination
SI category and pollination treatment had significant influences on seed germination, explaining 10 % and 9 % of the variance, respectively, in the ANOVA analyses (Table 1). The covariate (distance from nearest neighbours) removed 3·3 % of the variance of the models from seed germination. Since there were no interaction effects for seed germination, mean values for the significant main effects of SI category and pollination treatment were compared using a Tukey–Kramer test. With respect to SI category, seed germination was: equal between partially self-incompatible and self-compatible plants (0·19 ± 0·030 s.e., n = 48, and 0·17 ± 0·028 s.e., n = 60, respectively, Tukey–Kramer test, P = 0·99) and both were significantly higher than that for strongly self-incompatible plants (Tukey–Kramer test, P = 0·049 and P = 0·038), while strongly self-incompatible and self-incompatible plants did not differ in seed germination (0·08 ± 0·021 s.e., n = 36 and 0·06 ± 0·025 n = 39 s.e., respectively; Tukey–Kramer test, P = 0·95). For pollination treatment, the Tukey–Kramer test indicates that the seeds sired following cross-pollination had approx. 2-fold higher seed germination than those sired from self-pollination (0·12 ± 0·022 s.e. and 0·06 ± 0·011 s.e., respectively, n = 61 Tukey–Kramer test, P = 0·004) while those sired from open-pollination had 1·8 times higher seed germination (0·23 ± 0·032 s.e., n = 61) than those sired from cross-pollination (Tukey–Kramer test, P ≤ 0·0001).
Effect of strength of SI and scrub density on inbreeding depression
Comparison of seed germination after self- and cross-pollination for all plants that were self-incompatible, partially self-incompatible or self-compatible (49 plants), indicated that scrub density had a significant effect on δgerm and explained 9 % of the variance in the ANOVA model (Table 2). Mean δgerm was six times lower in low- than in high-density scrubs (–0·05 ± 0·12 s.e., n = 26 and 0·30 ± 0·16 n = 22 s.e., respectively; Tukey–Kramer test, P ≤ 0·0001). The difference in mean δgerm was associated with significantly higher seed germination of seeds sired from self-pollination in low- compared with high-density scrubs (0·08 ± 0·02 s. e., n = 26 and 0·04 ± 0·02 n = 22 s.e., respectively; Tukey–Kramer test, P ≤ 0·0001) and a slightly but not significantly lower seed germination of seeds sired from cross-pollination (0·14 ± 0·03 s.e., n = 26 and 0·11 ± 0·03 n = 22 s.e., respectively; Tukey–Kramer test, P = 0·89). Levels of δgerm were highly variable and, in some cases, negative (Fig. 4), suggesting that purging of genetic load is taking place in some plants as indicated by values of δgerm ≤ 0 in 26 out of the 49 maternal plants (Fig. 4).
Table 2.
ANOVA for the inbreeding depression in germination (δgerm) for 49 Flourensia cernua plants growing in the Mapimi Biosphere Reserve
| Source | d.f. | SS effect | SS error | F | P | R2 |
|---|---|---|---|---|---|---|
| Self-incompatibility category | 2,42 | 0·798 | 21·461 | 0·78 | 0·465 | 0·035 |
| Scrub density | 1,42 | 2·188 | 21·461 | 4·28 | 0·045 | 0·095 |
| Self-incompatibility category × scrub density | 2,42 | 0·161 | 21·461 | 0·16 | 0·855 | 0·007 |
Sources of variation were: SI category (strongly self-incompatible, self-incompatible, partially self-incompatible and self-compatible) and scrub density (low and high).
Fig. 4.
Values of inbreeding depression associated with seed germination (δgerm) for 49 Flourensia cernua plants growing in the Mapimí Biosphere Reserve by SI category. δgerm was estimated as 1 – [(seed germination after self-pollination)/(seed germination after cross-pollination)]. SI categories are defined in Fig. 1.
Effect of strength of SI and scrub density on multiplicative female fitness
Using only the results from open-pollinated florets, SI category was found to have a significant effect on multiplicative female fitness and explained 15·5 % of the variance in the ANOVA model (Table 3). The covariates distance from nearest neighbours and plant volume removed 2·6 % and 2·3 % of the variance from the model. The differences in multiplicative female fitness were found to be associated with variation in seed set and seed germination across SI categories but not with the variation in floral display which was similar for all plants across SI categories and between high- and low-density scrub (Tables S2 and S3 in Supplementary data, available online). Partially self-incompatible and self-compatible plants had 1·61 and 2·14 times higher female fitness after open-pollination (193 ± 57 s.e. n = 16 and 256 ± 77 n = 20 s.e., respectively), than strongly self-incompatible plants (12 ± 10 s.e. n = 12; Tukey–Kramer test, P = 0·03 and P = 0·02, respectively). Self-incompatible plants had, on average, similar female fitness as partially self-incompatible and self-compatible plants (160 ± 67 s.e., n = 13; Tukey–Kramer test, P = 0·65 and P = 0·55, respectively) and as strongly self-incompatible plants (Tukey–Kramer test, P = 0·31).
Table 3.
ANOVA for the multiplicative female fitness estimates (floral display × seed set × seed germination after open-pollination) and floral display of 61 Flourensia cernua plants growing in the Mapimi Biosphere Reserve
| Source | d.f. | SS effect | SS error | F | P | R2 |
|---|---|---|---|---|---|---|
| Self-incompatibility category | 3,51 | 44·540 | 263·486 | 2·87 | 0·0452 | 0·140 |
| Scrub density | 1,51 | 2·093 | 263·486 | 0·41 | 0·5273 | 0·007 |
| Scrub density × self-incompatibility category | 3,51 | 6·914 | 263·486 | 0·45 | 0·7211 | 0·022 |
Sources of variation were SI category (strongly self-incompatible, self-incompatible, partially self-incompatible and self-compatible) and scrub density (low and high).
Distance from neighbours (average distance to the four nearest neighbours of the maternal plants) and plant volume were used as covariates and explained 2·6 % and 2·3 % of the variance, respectively.
DISCUSSION
Variation in the strength of SI
In this study, considerable variation was found in the strength of SI among maternal plants, as indicated by the SI index (seed set after self-pollination/seed set after cross-pollination): 51 % of the individuals in natural populations of F. cernua were found to be strongly self-incompatible, while the remaining 49 % varied in the degree to which they were capable of self-fertilization. While conclusive evidence was found that there is variation in the strength of SI in F. cernua, no difference was found in the frequency of SI categories among plants in high- and low-density scrubs.
A previous study showed that F. cernua is a highly outcrossing species based on molecular estimates of the outcrossing rate of plants in both high- and low-density scrubs (Ferrer et al., 2004). Here it is found that a large proportion of the plants in the population (83 %) are not capable of setting seed, or set very few seeds, following hand self-pollination (Fig. 2A). This suggests that F. cernua possesses an SI system which effectively prevents self-fertilization, as found in many other members of the Asteraceae (Ferrer and Good-Avila, 2007). However, up to 17 % of the population is capable of setting selfed seed and the species should be considered partially self-incompatible. Interestingly, in several other partially self-incompatible members of the Asteraceae, Euryibia furcatus (synonymous with Aster furcatus; Reinartz and Les, 1994), Arnica montana (Luijten et al., 1996). Senecio squalidus (Brennan et al., 2005) and S. inaequidens (Lafuma and Maurice, 2007) detailed pollination studies have also found that the majority of plants in a population are self-incompatible (95 %, 78 %, 97 % and 86 % of the individuals were strongly SI in each species, respectively), while 3–22 % of the population expressed variable levels of SC as found here. This suggests that partial SI, at least in these members of the Asteraceae, does not allow intermediate selfing rates, but it may allow low levels of self-fertilization and/or biparental inbreeding which would not occur in strictly self-incompatible taxa.
Almost all of the 51 strongly self-incompatible individuals found in this study produced very few fruits following either self- or cross-fertilization. This could be caused by (a) sterility, (b) selective abortion, and/or (c) pollen limitation due to few S-alleles segregating in the population. Although detailed studies assessing the viability of female and male gametes are needed to rule out sterility as a factor limiting seed set, there is no evidence to suggest that strongly self-incompatible plants are sterile because both stamens and pollen, and pistils and ovules appeared identical across all plants. Additionally strongly self-incompatible plants set seed following both cross- and open-pollination suggesting that female sterility was not a limiting factor. However, male sterility cannot be ruled out and some strongly self-incompatible plants may produce inviable pollen. Secondly, although many studies have shown that plants will selectively abort seeds when resources are low (Stephenson, 1981), three results of this study suggest that pollen origin (i.e. quality) was more important than resource availability as an explanation for the low seed set observed particularly in strongly self-incompatible plants: (a) self-incompatible, partially self-incompatible and self-compatible plants showed pollen limitation following open-pollination, but did benefit from pollen supplementation, suggesting that mate availability rather than resources limited seed set; (b) seed set was higher after open-pollination in low- compared with high-density scrubs, although water availability is higher in high-density scrubs; and (c) although the multiplicative female fitness varied significantly with plant volume (an indirect indicator of maternal resources availability), including plant size as a covariate removed 2·8 % of the variance while SI category explained 14 % of the variance.
If pollen is not limiting, cross-pollination in species presenting sporophytic SI should result in either no (cross-incompatible) or full (cross-compatible) seed set. However, low reproductive output can be caused by pollen limitation if populations exhibit low S-allele diversity, non-isoplethic S-alleles frequencies and or spatial clustering of similar S-phenotypes because of limited pollen and seed dispersal (Byers and Meagher, 1992; Schierup, 1998). To determine the number of cross-incompatible and cross-compatible pollinations in the study, a separate analysis was performed in which a cross was considered to be cross-incompatible if the seed set value was <0·1 (since self-pollination typically results in seed set values between 0 and 0·09), whereas a cross-pollination was considered to be cross-compatible if the seed set value was >0·1 (Fig. S2 in Supplementary data, available online). Analysing the crossing data in this way revealed that 96 % of the strongly self-incompatible plants, 7·7 % of the self-incompatible, 6·3 % of the partially self-incompatible and 23·8 % of the self-compatible plants were cross-incompatible with their pollen donor(s); this suggests that only strongly self-incompatible individuals were predominantly cross-incompatible with their pollen donors even though seed set was pollen limited in all the other SI categories (i.e. full seed set was not achieved) (Fig. S2 in Supplementary data). Consequently, we propose that plants in the strongly self-incompatible category shared S-alleles with their plant donors and probably harbour S-alleles that are common in the population. On the other hand, we propose that in partially self-incompatible and self-compatible plants, the partial breakdown of SI has evolved to compensate reproduction in F. cernua because compatible mates are limiting.
Without further breeding work, it is difficult to elucidate the relative roles of sterility versus low S-allele diversity as factors limiting seed set and to say whether partial SI in F. cernua is caused by the effect of dominance relationships among S-alleles or modifier loci. Detailed reciprocal crosses among individuals differing in their expression of SI such as those performed in Senecio squalidus (Brennan et al., 2002, 2006) or Campanula rapunculoides (Good-Avila and Stephenson, 2003; Good-Avila et al., 2008a) would be required to understand the genetic basis of partial SI.
Effect of strength of SI and scrub density on outcross pollen limitation
This study supports the hypothesis that strongly self-incompatible individuals suffer from outcross pollen limitation as found in many other self-incompatible species (Larson and Barrett, 2000) but, additionally, evidence is found that individuals presenting a partial or complete breakdown of SI show reproductive compensation sensu Vallejo-Marín and Uyenoyama (2004). It is found that both self-incompatible and partially self-incompatible plants and all plants presenting a partial or complete breakdown of SI showed a ≥2-fold increase in seed set following both cross- and open-pollination compared with strongly self-incompatible plants (Fig. 3A). Interestingly, studies on the partially self-incompatible species Campanula rapunculoides (Good-Avila and Stephenson, 2003) and in Eurybia furcatus (Reinartz and Les, 1994) also observed higher outcrossed seed set in weak compared with strongly SI individuals, suggesting that partial SI may not only be a transient occurrence in SI species, but may be under selection.
Seed set from open-pollinated florets in low-density scrubs was higher than seed set in the remaining three treatments. This result seems to contradict the idea that outcross pollen limitation is an important factor leading to partial SI in this species. However, we suggest that the pollen donors used for the hand cross-pollination treatments were more likely to share the same S-phenotype with the recipient plants in the low-density scrubs than occurred for the pollen arriving on the open-pollinated branches. In particular, the pollen used for the hand cross-pollinations was selected from plants located at least 250 m away from the recipient plants but from plants located both up- and downstream in the same plot. Since low-density populations are established from secondary seed dispersal from surface water runoff, low-density sites may exhibit stronger clustering of similar S-phenotypes. On the other hand, the pollen arriving on the open-pollinated branches more likely originated from adjacent plots (e.g. ones at higher altitudes) since in the low-density scrubs, the wind-dispersed pollen can travel longer distances because the vegetation is open (Ackerman, 2000).
Effect of strength of SI, scrub density and pollen source on seed germination
Approximately 90 % of the seeds set in F. cernua were found to be inviable (Ferrer, 2004) and the species exhibits low seed germination compared with other members of the Asteraceae growing in arid, tropical or temperate zones (Valencia-Díaz and Montaña, 2003). In this study, it was found that (a) seed germination was lower for strongly self-incompatible and self-incompatible plants compared with that found in partially self-incompatible and self-compatible plants, and (b) seed germination was 1·7 times lower for seeds sired following self-pollination compared with those sired by cross-pollination. The first result suggests that progeny of plants presenting a greater breakdown in SI had higher fitness following all pollination types. This suggests that these plants harbour lower levels of genetic load caused by deleterious recessive or partially recessive mutations, perhaps because of prior exposure to purifying selection in lineages capable of inbreeding. On the other hand, the low seed set and seed germination of strongly self-incompatible plants may be caused by factors other than SI such as environmental variation in temperatures causing impaired development of the seeds (Valencia-Díaz and Montaña, 2005). Higher rates of seed germination in self-compatible compared with self-incompatible lineages were also observed in Leavenworthia alabamica (Busch, 2004).
Effect of strength of SI and scrub density on inbreeding depression
Inbreeding depression in seed germination (δgerm) decreased fitness in the plants of F. cernua that were capable of self-fertilization and this effect was stronger in high- than in low-density scrubs. In the low-density scrub, seeds sired from self-pollination had similar seed germination to those sired from cross-pollination in both scrub types, and higher seed germination than those sired from self-pollination in high-density scrubs. This suggests that the low value of δgerm in low-density scrubs is associated with a reduction of genetic load in this early fitness component. Cumulative levels of inbreeding depression in another partially SI species, Campanula rapunculoides, were found to be lower in weak compared with strong SI lines, but in all cases were >0·5 (Vogler et al., 1999; Good-Avila et al., 2003), while in Senecio squalidus they were <0·5 under greenhouse conditions for both partially self-compatible and self-incompatible plants (Brennan et al., 2005).
Although cumulative levels of inbreeding depression across multiple fitness characters would certainly be higher than those recorded only for seed germination, cumulatively, the data presented here suggest that a mixed mating system, with low rates of self-fertilization, may be possible in F. cernua because levels of δgerm were frequently found to be lower than 0·5 (30 plants) and lower or equal to 0 (26 plants) (Fig. 4). Low levels of self-fertilization may be maintained in populations when reproductive compensation occurs in the presence of pollen limitation (Vallejo-Marín and Uyenoyama, 2004; Porcher and Lande, 2005). Even when inbred progeny are competing with outbred progeny for maternal resources, reproductive compensation is an important factor leading to the stability of mixed mating systems if the cumulative inbreeding depression after the dispersal of seeds is low (Harder et al., 2008). However, the complete loss of SI and selection for increased rates of self-fertilization would be constrained in the species, as shown by the presence of a large number of plants, 83 %, that set very few seeds after self-fertilization.
Effect of strength of SI and population density on multiplicative female fitness
Theoretical work has shown that if modifiers increasing rates of SC and/or self-fertilization become associated with high-fitness genotypes, selection for SC can occur (Holsinger, 1988). There is some evidence that this could be occurring in F. cernua because (a) more than half of the self-incompatible to self-compatible plants showed low or negative δgerm (Fig. 4) and (b) partially self-incompatible and self-compatible plants had higher overall female fitness. Thus collectively the present data on pollen limitation, variation in the strength of SI and inbreeding depression suggest that the partial breakdown of SI may be selected because (a) it helps to ensure reproduction in this pollen-limited species, (b) individuals expressing a breakdown in SI achieve higher outcrossed reproductive success and (c) modifiers increasing rates of self-fertility have become weakly associated with lineages expressing reduced genetic load.
The possibility that low levels of self-fertility are favoured in low- and high-density scrubs is consistent with the possibility that SC could be favoured during periods of colonization in F. cernua (Montaña et al., 1990). Secondary dispersal of F. cernua seeds along ephemeral, surface water runoff routes may occur from low- to high-density scrubs as the former are located upstream of high-density scrubs (Montaña, 1992). Thus, population establishment in high-density scrubs could be facilitated by the establishment of seeds from partially self-incompatible and self-compatible plants, while SI could be restored when seeds from strongly self-incompatible and self-incompatible plants establish after S-allele diversity has increased. Partial SI, characterized by variation in the strength of SI, increased SC and/or low self-fertilization rates, may be a common feature of colonizing and invasive plants, and has been documented for Crepis sancta, another colonizing member of the Asteraceae (Cheptou et al., 2002) and for the successful invader species Senecio squalidus (Brennan et al., 2005), S. inaequidens (Lafuma and Maurice, 2007) and Campanula rapunculoides (Good-Avila et al., 2008a).
Conclusions
In conclusion, F. cernua shows variation in the strength of SI: while approx. 83 % of the plants did not set seeds after self-pollination, the remaining 17 % presented a partial or complete breakdown in SI. Low female fertility in F. cernua appears to be caused by poor availability of compatible mates that limits seed set, particularly in strongly self-incompatible plants. Seed germination in the species is generally low, however, approximately one-half of the self-compatible plants showed low or negative δgerm, indicating that modifiers increasing rates of SC may have become associated with high-fitness genotypes. Finally, all plants exhibiting either a partial or complete breakdown of SI had significantly higher outcrossed seed set than self-incompatible plants, suggesting that reproductive compensation as well as pollen limitation is occurring in this species. Cumulatively these results suggest that partial SI may be selected in F. cernua; even though the species maintains low levels of inbreeding it may be sufficient to allow populations to increase reproductive output and establish new populations during periods of colonization.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank E. Vega, K. Herrera, A. Valera, S. Valencia-Díaz, J. Flores, R. Avila, A. Herrera, M. Ortega, A. Ortega and J. P. Ramírez for help in field and laboratory work and to the staff of the Laboratorio del Desierto of the Instituto de Ecología, A. C. at the Mapimí Biosphere Reserve for logistical support, and Dr Simon Hiscock, two anonymous referees and Jorge A. González-Astorga for helpful comments on the manuscript. This project was funded by grants from Consejo Nacional de Ciencia y Tecnología (4126P-N9608 to C.M.), Dirección de Estudios de Posgrado, Universidad Nacional Autónoma de México (Graduate Studies Scholarship to M.M.F.), the Organization of American States (2004-1384 to M.M.F.) and the National Sciences and Engineering Research Council (to S.V.G.-A.).
LITERATURE CITED
- Ackerman JD. Abiotic pollen and pollination: ecological, functional, and evolutionary perspectives. Plant Systematics and Evolution. 2000;222:167–185. [Google Scholar]
- Allison TD. Pollen production and plant density affect pollination and seed production in Taxus canadensis. Ecology. 1990;71:516–522. [Google Scholar]
- Anderson NO, Liedl BE, Ascher PD, Widmer RE, Desborough SL. Evaluating self-incompatibility in Chrysanthemum: the influence of ovule number. Sexual Plant Reproduction. 1988;1:173–181. [Google Scholar]
- Arista M, Talavera S. Density effect on the fruit-set, seed crop viability and seedling vigour of Abies pinsapo. Annals of Botany. 1996;77:187–192. [Google Scholar]
- Barrett SCH. The evolution of mating strategies in flowering plants. Trends in Plant Science. 1998;3:335–341. [Google Scholar]
- Berry PE, Calvo RN. Wind pollination, self-incompatibility, and altitudinal shifts in pollination systems in the high Andean genus Espeletia (Asteraceae) American Journal of Botany. 1989;76:1602–1614. [Google Scholar]
- Brennan AC, Harris SA, Taba DA, Hiscock SJ. The population genetics of sporophytic self-incompatibility in Senecio squalidus L. (Asteraceae). I. S allele diversity in a natural population. Heredity. 2002;89:430–438. doi: 10.1038/sj.hdy.6800159. [DOI] [PubMed] [Google Scholar]
- Brennan AC, Harris SA, Hiscock SJ. The population genetics of sporophytic self-incompatibility in Senecio squalidus L. (Asteraceae): avoidance of mating constraints imposed by low S-allele number. Philosophical Transactions of the Royal Society of London. 2003;358:1047–1050. doi: 10.1098/rstb.2003.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AC, Harris SA, Hiscock SJ. Modes and rates of selfing and associated inbreeding depression in the self-incompatible plant Senecio squalidus (Asteraceae): a successful colonizing species in the British Isles. New Phytologist. 2005;168:475–486. doi: 10.1111/j.1469-8137.2005.01517.x. [DOI] [PubMed] [Google Scholar]
- Brennan AC, Harris SA, Hiscock SJ. The population genetics of sporophytic self-incompatibility in Senecio squalidus L. (Asteraceae): the number, frequency, and dominance interactions of S alleles across its British range. Evolution. 2006;60:213–224. [PubMed] [Google Scholar]
- Busch JW. Inbreeding depression in self-incompatible and self-compatible populations of Leavenworthia alabamica. Heredity. 2004;94:159–165. doi: 10.1038/sj.hdy.6800584. [DOI] [PubMed] [Google Scholar]
- Byers D, Meagher T. Mate availability in small populations of plant species with homomorphic sporophytic self-incompatibility. Heredity. 1992;68:353. [Google Scholar]
- Cabrera LR, Dieringer G. Reproductive biology of a population of Acourtia runcinata (Asteraceae: Mutisieae: Mutisieae) at the northeastern limit of its range. American Midland Naturalist. 1992;128:83–88. [Google Scholar]
- Charlesworth B. The cost of sex in relation to mating system. Journal of Theoretical Biology. 1980;84:655–671. doi: 10.1016/s0022-5193(80)80026-9. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Cheptou P-O, Lepart J, Escarre J. Mating system variation along a successional gradient in the allogamous and colonizing plant Crepis sancta (Asteraceae) Journal of Evolutionary Biology. 2002;15:753–762. [Google Scholar]
- Culley TM, Weller SG, Sakai AK. The evolution of wind pollination in angiosperms. Trends in Ecology and Evolution. 2002;17:361. [Google Scholar]
- Desrochers AM, Rieseberg LH. Mentor effects in wild species of Helianthus (Asteraceae) American Journal of Botany. 1998;85:770–775. [PubMed] [Google Scholar]
- Dillon MO. A systematic study of Flourensia (Asteraceae, Heliantheae) Fieldiana, Botany New Series. 1984;16:1–66. [Google Scholar]
- Ferrer MM. D. F., México: Doctoral, Universidad Nacional Autónoma de México, México; 2004. Efecto de la densidad poblacional en la dinámica demográfica, ecología reproductiva y estructura genética de Flourensia cernua DC. [Google Scholar]
- Ferrer MM, Good-Avila SV. Macrophylogenetic analyses of the gain and loss of self-incompatibility in the Asteraceae. New Phytologist. 2007;173:401–414. doi: 10.1111/j.1469-8137.2006.01905.x. [DOI] [PubMed] [Google Scholar]
- Ferrer MM, Eguiarte LE, Montaña C. Genetic structure and outcrossing rates in Flourensia cernua (Asteraceae) growing at different densities in the south-western Chihuahuan desert. Annals of Botany. 2004;94:419–426. doi: 10.1093/aob/mch159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazoul J. Pollen and seed dispersal among dispersed plants. Biological Reviews. 2005;80:413–443. doi: 10.1017/s1464793105006731. [DOI] [PubMed] [Google Scholar]
- Giblin DE, Hamilton CW. The relationship of reproductive biology to the rarity of endemic Aster curtus (Asteraceae) Canadian Journal of Botany. 1999;77:140. [Google Scholar]
- Glemin S, Bataillon T, Ronfort J, Mignot A, Olivieri I. Inbreeding depression in small populations of self-incompatible plants. Genetics. 2001;159:1217–1229. doi: 10.1093/genetics/159.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good-Avila SV, Stephenson AG. Parental effects in a partially self-incompatible herb: influence of variation in the strength of self-incompatibility on seed set and progeny performance. The American Naturalist. 2003;161:615–630. doi: 10.1086/368290. [DOI] [PubMed] [Google Scholar]
- Good-Avila SV, Nagel T, Vogler DW, Stephenson AG. Effects of inbreeding on male function and self-fertility in the partially self-incompatible herb Campanula rapunculoides (Campanulaceae) American Journal of Botany. 2003;90:1736–1745. doi: 10.3732/ajb.90.12.1736. [DOI] [PubMed] [Google Scholar]
- Good-Avila SV, Majumder D, Amos H, Stephenson AG. Characterisation of self-incompatibility in Campanula rapunculoides (Campanulaceae) through genetic analyses and microscopy. Canadian Journal of Botany. 2008;a 86:1–13. [Google Scholar]
- Good-Avila SV, Mena-Ali JI, Stephenson AG. Genetic and environmental causes and evolutionary consequences of variations in self-fertility in self incompatible species. In: Franklin-Tong V, editor. Self-incompatibility in flowering plants: evolution, diversity, and mechanisms. b. Berlin: Springer-Verlag; 2008. pp. 33–47. [Google Scholar]
- Goodwillie C. Multiple origins of self-compatibility in Linanthus section leptosiphon (Polemoniaceae): phylogenetic evidence from internal-transcribed-spacer sequence data. Evolution. 1999;53:1387–1395. doi: 10.1111/j.1558-5646.1999.tb05403.x. [DOI] [PubMed] [Google Scholar]
- Harder LD, Richards SA, Routley MB. Effects of reproductive compensation, gamete discounting and reproductive assurance on mating-system diversity in hermaphrodites. Evolution. 2008;62:157–172. doi: 10.1111/j.1558-5646.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- Hiscock SJ. Self-incompatibility in Senecio squalidus L. (Asteraceae) Annals of Botany. 2000;85:181–190. doi: 10.1093/aob/mcr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock SJ, McInnis SM. Pollen recognition and rejection during the sporophytic self-incompatibility response: Brassica and beyond. Trends in Plant Science. 2003;12:606. doi: 10.1016/j.tplants.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Hiscock SJ, Tabah DA. The different methods of sporophytic self-incompatibility. Philosophical Transactions of the Royal Society of London. 2003;358:1037–1045. doi: 10.1098/rstb.2003.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock SJ, Hoedemaekers K, Friedman WE, Dickinson HG. The stigma surface and pollen-stigma interactions in Senecio squalidus L. (Asteraceae) following cross (compatible) and self (incompatible) pollinations. International Journal of Plant Science. 2002;163:1–16. [Google Scholar]
- Holsinger KE. Inbreeding depression doesn't matter: the genetic basis of mating-system evolution. Evolution. 1988;42:1235–1244. doi: 10.1111/j.1558-5646.1988.tb04183.x. [DOI] [PubMed] [Google Scholar]
- Husband BC, Schemske DW. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution. 1996;50:54–70. doi: 10.1111/j.1558-5646.1996.tb04472.x. [DOI] [PubMed] [Google Scholar]
- Igic B, Bohs L, Kohn JR. Historical inferences from the self-incompatibility locus. New Phytologist. 2004;161:97–105. [Google Scholar]
- Igic B, Bohs L, Kohn JR. Ancient polymorphism reveals unidirectional breeding system shifts. Proceedings of the National Academy of Sciences of the USA. 2006;103:1359–1363. doi: 10.1073/pnas.0506283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igic B, Lande R, Kohn J. Loss of self-incompatibility and its evolutionary consequences. International Journal of Plant Sciences. 2008;169:93–104. [Google Scholar]
- Kunin WE. Sex and the single mustard: population-density and pollinator behavior effects on seed-set. Ecology. 1993;74:2145. [Google Scholar]
- Lafuma L, Maurice S. Increase in mate availability without loss of self-incompatibility in the invasive species Senecio inaequidens (Asteraceae) Oikos. 2007;116:201–208. [Google Scholar]
- Lande R, Schemske DW. The evolution of self-fertilization and inbreeding depression in plants. 1. Genetic models. Evolution. 1985;39:24–40. doi: 10.1111/j.1558-5646.1985.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Larson BMH, Barrett SCH. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society. 2000;69:503–520. [Google Scholar]
- Levin DA. The evolutionary significance of pseudo self-fertility. The American Naturalist. 1996;148:321–332. [Google Scholar]
- Lipow SR, Wyatt R. Towards an understanding of the mixed breeding system of swamp milkweed (Asclepias incarnata) Journal of the Torrey Botanical Society. 2000;127:193–199. [Google Scholar]
- Luijten SH, Oostermeijer JGB, Van Leeuwen Nico C, Den Nijs Hans CM. Reproductive success and clonal genetic structure of the rare Arnica montana (Compositae) in The Netherlands. Plant Systematics and Evolution. 1996;201:15–30. [Google Scholar]
- McMahon JA. Warm deserts. In: Barbour MG, Billings WD, editors. North American terrestrial vegetation. New York, NY: Cambridge University Press; 1999. [Google Scholar]
- Mauchamp A. L'hetérogénéite spatiale, sa dynamique et ses implications dans une mosaïque de végétation en zone aride. Montpellier, France: Doctorale, Academie de Montpellier, Universite Montpellier II; 1992. [Google Scholar]
- Montaña C. The colonization of bare areas in two-phase mosaics of an arid ecosystem. Journal of Ecology. 1992;80:315–327. [Google Scholar]
- Montaña C, López-Portillo J, Mauchamp A. The response of two woody species to the conditions created by a shifting ecotone in an arid ecosystem. Journal of Ecology. 1990;78:789–798. [Google Scholar]
- Morgante M, Vendramin GG, Rossi P. Effects of stand density on outcrossing rate in two Norway spruce (Picea abies) populations. Canadian Journal of Botany. 1991;12:2704–2708. [Google Scholar]
- de Nettancourt D. Incompatibility and incongruity in wild and cultivated plants. 2nd edn. Berlin: Springer-Verlag; 2001. [Google Scholar]
- Nielsen LR, Siegismund HR, Philipp M. Partial self-incompatibility in the polyploid endemic species Scalesia affinis (Asteraceae) from the Galápagos: remnants of a self-incompatibility system? Botanical Journal of the Linnean Society. 2003;142:93–101. [Google Scholar]
- Porcher E, Lande R. Loss of gametophytic self-incompatibility with evolution of inbreeding depression. Evolution. 2005;59:46–60. [PubMed] [Google Scholar]
- Reinartz JA, Les DH. Bottleneck-induced dissolution of self-incompatibility and breeding system consequences in Aster furcatus (Asteraceae) American Journal of Botany. 1994;81:446–455. [Google Scholar]
- Ruiz-Zapata T, Arroyo MTK. Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica. 1978;10:221–230. [Google Scholar]
- Rzedowski J. Vegetación de México. México: Editorial Limusa; 1988. [Google Scholar]
- Sage TL, Bertin RI, Williams EG. Ovarian and other late acting SI systems. In: Williams EG, Clarke AE, Knox RB, editors. Genetic control of self-incompatibility and reproductive development in flowering plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 116–140. [Google Scholar]
- Schierup MH. The number of self-incompatibility alleles in a finite, subdivided population. Genetics. 1998;149:1153–1162. doi: 10.1093/genetics/149.2.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Hamrick JL, Kramer CL. The effect of stand density on frequency of filled seed and fecundity in Lodgepole pine (Pinus conforta Dougl.) Canadian Journal of Botany. 1988;18:453–460. [Google Scholar]
- Sokal RR, Rolhf FJ. Biometry. 3rd edn. New York, NY: W.H. Freedman and Co; 1995. [Google Scholar]
- Statsoft I. Statistica for windows 98. Tulsa, OK: Statsoft, Inc; 1998. [Google Scholar]
- Stephenson AG. Flower and fruit abortion: proximate causes and ultimate functions. Annual Review of Ecology and Systematics. 1981;12:253–279. [Google Scholar]
- Stephenson AG, Good SV, Vogler DW. Interrelationships among inbreeding depression, plasticity in the self-incompatibility system, and the breeding system of Campanula rapunculoides L. (Campanulaceae) Annals of Botany. 2000;85:211–219. [Google Scholar]
- Stone JL. Molecular mechanisms underlying the breakdown of gametophytic self-incompatibility. The Quarterly Review of Biology. 2002;77:17–32. doi: 10.1086/339200. [DOI] [PubMed] [Google Scholar]
- Stout AB. Fertility in Cichorium intibus: the sporadic ocurrence of self-fertile plants among the progeny of self-sterile plants. American Journal of Botany. 1917;4:375–395. [Google Scholar]
- Sutherland S. Patterns of fruit-set: what controls fruit-flower ratios in plants? Evolution. 1986;40:117–128. doi: 10.1111/j.1558-5646.1986.tb05723.x. [DOI] [PubMed] [Google Scholar]
- Sutherland S, Delph LF. On the importance of male fitness in plants: patterns of fruit-set. Ecology. 1984;65:1093–1104. [Google Scholar]
- Takebayashi N, Morrell PL. Is self-fertilization an evolutionary dead end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. American Journal of Botany. 2001;88:1143–1150. [PubMed] [Google Scholar]
- Uyenoyama MK, Waller DM. Coevolution of self-fertilization and inbreeding depression. III. Homozygous lethal mutations at multiple loci. Theoretical Population Biology. 1991;40:173–210. doi: 10.1016/0040-5809(91)90052-h. [DOI] [PubMed] [Google Scholar]
- Uyenoyama MK, Holsinger KE, Waller DM. Ecological and genetic factors directing the evolution of self-fertilization. Oxford Surveys in Evolutionary Biology. 1993;9:327–381. [Google Scholar]
- Valencia-Díaz S, Montaña C. Effects of seed age, germination substrate, gibberelic acid, light, and temperature on seed germination in Flourensia cernua (Asteraceae), a Chihuahuan desert shrub. The Southwestern Naturalist. 2003;48:1–13. [Google Scholar]
- Valencia-Díaz S, Montaña C. Temporal variability in the maternal environment and its effect on seed size and seed quality in Flourensia cernua DC. (Asteraceae) Journal of Arid Environments. 2005;63:686–695. [Google Scholar]
- Vallejo-Marín M, Uyenoyama MK. On the evolutionary cost of self-incompatibility: incomplete reproductive compensation due to pollen limitation. Evolution: International Journal of Organic Evolution. 2004;58:1924–1935. doi: 10.1111/j.0014-3820.2004.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Vogler DW, Das C, Stephenson AG. Phenotypic plasticity in the expression of self-incompatibility in Campanula rapunculoides. Heredity. 1998;81:546–555. [Google Scholar]
- Vogler DW, Filmore K, Stephenson AG. Inbreeding depression in Campanula rapunculoides L. I. A comparison of inbreeding depression in plants derived from strong and weak self-incompatibility phenotypes. Journal of Evolutionary Biology. 1999;12:483–494. [Google Scholar]
- Von Ende CN. Repeated-measures analysis: growth and other time-dependent measures. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. New York, NY: Chapman & Hall; 2001. pp. 113–137. [Google Scholar]
- Wiens D, Calvin CL, Wilson CA, Davern CI, Frank D, Seavey SR. Reproductive success, spontaneous embryo abortion, and genetic load in flowering plants. Oecologia. 1987;71:501–509. doi: 10.1007/BF00379288. [DOI] [PubMed] [Google Scholar]
- Wilcock C, Neiland R. Pollination failure in plants: why it happens and when it matters. Trends in Plant Science. 2002;7:270–277. doi: 10.1016/s1360-1385(02)02258-6. [DOI] [PubMed] [Google Scholar]
- Wright S. On the number of self-incompatibility alleles maintained in equilibrium by a given mutation rate in a population of given size: re-examination. Biometrics. 1960:61–85. [Google Scholar]