Abstract
Background and Aims
Orobanche minor is a root-holoparasitic angiosperm that attacks a wide range of host species, including a number of commonly cultivated crops. The extent to which genetic divergence among natural populations of O. minor is influenced by host specificity has not been determined previously. Here, the host specificity of natural populations of O. minor is quantified for the first time, and evidence that this species may comprise distinct physiological races is provided.
Methods
A tripartite approach was used to examine the physiological basis for the divergence of populations occurring on different hosts: (1) host–parasite interactions were cultivated in rhizotron bioassays in order to quantify the early stages of the infection and establishment processes; (2) using reciprocal-infection experiments, parasite races were cultivated on their natural and alien hosts, and their fitness determined in terms of biomass; and (3) the anatomy of the host–parasite interface was investigated using histochemical techniques, with a view to comparing the infection process on different hosts.
Key Results
Races occurring naturally on red clover (Trifolium pratense) and sea carrot (Daucus carota ssp. gummifer) showed distinct patterns of host specificity: parasites cultivated in cross-infection studies showed a higher fitness on their natural hosts, suggesting that races show local adaptation to specific hosts. In addition, histological evidence suggests that clover and carrot roots vary in their responses to infection. Different root anatomy and responses to infection may underpin a physiological basis for host specificity.
Conclusions
It is speculated that host specificity may isolate races of Orobanche on different hosts, accelerating divergence and ultimately speciation in this genus. The rapid life cycle and broad host range of O. minor make this species an ideal model with which to study the interactions of parasitic plants with their host associates.
Key words: Parasitic plant, Orobanche, speciation, divergence, host-specificity, host-specific races
INTRODUCTION
Approximately 4000 species of angiosperm are parasitic and rely on other plants for water and solute acquisition (Kuijt, 1969). Parasitism is taxonomically and geographically widespread, occurring in 17 families in most major biomes (Barker et al., 1996; Press and Phoenix, 2005). Parasitic plants can be classified as hemiparasites, which are photosynthetic, or holoparasites, which are completely devoid of chlorophyll and thus obligately dependent on their host plants for their nutrition. Parasitic angiosperms have significant ecological effects on plant community structure by altering the competitive balance between host and non-host species (Press and Graves, 1995; Press and Phoenix, 2005). In spite of this, our understanding of the evolution of parasitic plants is rudimentary. This is largely because the evolutionary shift to heterotrophy has been associated with the degeneration of taxonomically useful morphological features and genome size, which has impeded efforts to resolve phylogenetic relationships among parasitic plants (Young et al., 1999; Nickrent et al., 2004; Davis et al., 2007). However, recent advances in molecular systematics have given new insights into the evolutionary relationships between highly reduced parasites and their photosynthetic ancestors. For example, Davis et al. (2007) used mitochondrial, nuclear and plastid gene sequence data to show that Rafflesia (Rafflesiaceae), which famously produces the largest flower on earth, has undergone an incredible 73-fold increase in flower diameter following divergence from its closest ancestor within the spurge family (Euphorbiaceae). Each of the 17 species of Rafflesia is specific to one or two of the 95 species of Tetrastigma vine (Vitaceae) in south-east Asia (Nais, 2001). Isolation of Rafflesia populations on host vines with differing ecologies and distributions, followed by genetic divergence, may have driven speciation in this genus. Analogous to this, host-associated habitat isolation has driven divergence among many insect species (Funk et al., 1995; Hawthorne and Via, 2001; Schwarz et al., 2005).
Similar studies of host-driven speciation in parasitic plants are, however, rare. Many parasitic plants have a broad potential host range (Press and Graves, 1995), but even host generalists show distinct patterns of host specificity at a local level (Thorogood and Hiscock, 2007; Thorogood et al., 2008). Therefore host specificity may have been an underestimated driver of genetic divergence, and ultimately speciation among parasitic plants.
The broomrape genus (Orobanche; Orobanchaceae) includes over 150 species of root-holoparasites with a centre of diversity in the Mediterranean Basin (Barker et al., 1996). A number of Orobanche species have undergone host shifts from their native hosts to crop species (Román et al., 2007), and are now a severe obstacle to food production in certain parts of the world (Parker and Riches, 1993). Current estimates suggest that approx. 1·2 % of the world's arable farmed land may be infested with Orobanche seed (Sauerborn, 1991). However, our understanding of the interactions between Orobanche and its hosts lags behind that of host–parasite associations of the related genus of hemiparasites, Striga, which are pernicious weeds that infect cereal crops in the semi-arid tropics of the Old World (Parker and Riches, 1993; Barker et al., 1996). A variety of physical, cultural, chemical and biological methods have been investigated as potential control approaches against parasitic weeds such as Orobanche and Striga, all with limited success (Fernández-Aparicio et al., 2008). Recently, a number of Orobanche spp. have been classified as emerging pests, most notably O. foetida on faba bean in Tunisia (Kharrat et al., 1992) and Morocco (Rubiales et al., 2005). In the light of this, an enhanced understanding of the evolution, cell biology and physiology of host specificity in this genus is currently crucial. As far as is known, no previous studies have investigated such aspects of the host specificity of natural populations of Orobanche, which may be a proxy for understanding the evolution of this genus from both an ecological and an agricultural standpoint.
Orobanche minor is known to infect hundreds of species in taxonomically diverse families from the Ranunculaceae to the Poaceae, but with a clear preference for the Fabaceae and Asteraceae (Rumsey and Jury, 1991). Despite the broad host range of O. minor, strains are known to only infect certain hosts (Musselman and Parker, 1982); however, the extent of race formation in this species has not been ascertained. Orobanche minor appears to be a largely self-pollinated species (Jones, 1989). Shifts to self fertility are characteristic of recently colonized populations (Antonovics, 1968; Larson and Barrett, 1998); therefore selfing may have acted as an isolating mechanism in this species, where gene flow between host-specific populations could potentially disrupt adaptive gene complexes. Thus, isolation by host specificity may drive patterns of genetic divergence among populations, reinforced by inbreeding. Previously, molecular markers were used to identify genetic divergence between two races of O. minor: O. minor var. minor, which grows preferentially on red clover (Trifolium pratense) as well on a range of native and alien exotic hosts, and O. minor ssp. maritima, which grows almost exclusively on sea carrot (Daucus carota ssp. gummifer) (Thorogood et al., 2008). Here, a three-way approach to quantify the host specificity of these races on their natural hosts, red clover and sea carrot, is described with a view to understanding the physiological basis for their divergence. Specifically, the following were examined: (a) the early stages of infection of these putative host-specific genetic races using ‘rhizotron’ bioassays, in which host and parasite are cultivated together in reciprocal ‘choice’ experiments; (b) the fitness of parasites cultivated on clovers and carrots as measured by biomass; and (c) the anatomy of the host–parasite interface using histochemical techniques. Due to the rapid life cycle of O. minor (approx. 16 weeks), and its broad host range, this species represents an excellent model with which to study host–parasite interactions using an experimentally tractable holoparasite.
MATERIALS AND METHODS
Plant material
Plants of red clover (Trifolium pratense) were raised from seeds collected from a population infested with Orobanche minor var. minor in Somerset (ST5471) on calcareous grassland. Sea carrot (Daucus carota ssp. gummifer) plants were raised from seeds collected from a population in Dorset (SY8179) infested with O. minor ssp. maritima on south-facing sea cliffs. For each host species, seeds from ten individuals were pooled and sown in trays, then repotted after 6 weeks for use in rhizotron and pot experiments. Seeds from O. minor var. minor and O. minor ssp. maritima, identified to be genetic races (Thorogood et al., 2008), were also collected from populations at the sites described above. For each race, seeds from ten individuals were pooled prior to inoculation. Both host and parasite seeds were stored at 4 °C until use.
Rhizotron bioassay study of early infection
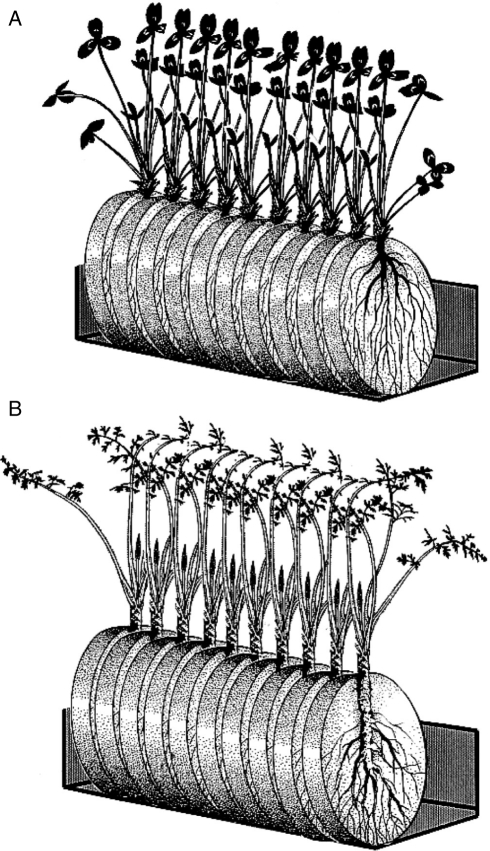
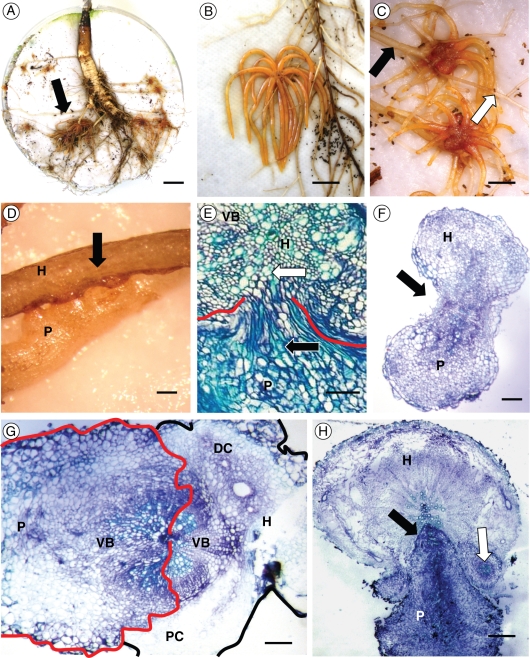
Early stages of parasite infection were observed by cultivating host plants with their parasite associates in rhizotron bioassays. Rhizotrons consisted of Petri dishes (10 cm diameter) filled with sand in which a hole was cut at each end, one to allow the host plant to grow though, and the other for drainage (Fig. 1). Host plant root systems were spread out on two layers of glass-fibre filter paper (Sigma-Aldrich), and the rhizotrons then sealed with light-excluding polyethene to prevent root greening (Keith et al., 2004). Rhizotrons were maintained for 3 weeks under a 16-h day-length regime in a controlled environment growth cabinet, operating with a photon flux density of 900 µmol m−2 s−1 at 21 °C, to allow the root systems to develop. Rhizotrons were then inoculated with parasite seed in a reciprocal experimental design in which ten clovers and ten carrots were each inoculated with seed from both their ‘alien’ and ‘natural’ parasite races, respectively. Orobanche seed was pre-conditioned for 2 weeks at 20 °C in the dark, and then surface-sterilized with 70 % ethanol for 2 min followed by 10 % commercial bleach and 0·1 % Tween 20 for 5 min, followed by six rinses with double-distilled water (ddH2O). Orobanche seed was immersed in distilled water and 100 mg (dry seed weight) inoculated on the filter paper of the rhizotron by pipette. Inoculated rhizotrons were then transferred to a controlled environment growth cabinet (conditions as described above) and watered weekly. Thirty days after inoculation, rhizotrons were observed under a Nikon binocular microscope, and the following stages of infection recorded: (a) number of Orobanche seeds germinated; (b) number of germinated seeds attached to host roots; (c) number of parasite tubercles present (Fig. 2A). Due to the difficulties associated with counting large numbers of tiny parasite seeds, a 5 × 5 mm grid marked on a transparent acetate sheet was placed on each rhizotron and germination and attachment counts carried out in three randomly chosen grid-squares for each replicate, in which both host root and parasite seeds were present. Attempts to artificially germinate seeds to manipulate on host roots were unsuccessful, due to the lack of responsiveness of O. minor ssp. maritima to the germination inducer GR-24, which to date is the most universal germination stimulant for parasitic plant seeds.
Fig. 1.
Rhizotron bioassays in which host plants were inoculated with alien and natural O. minor races. Using a reciprocal infection design, ten clover plants (A) and ten sea carrot plants (B) were each inoculated with O. minor var. minor and O. minor ssp. maritima.
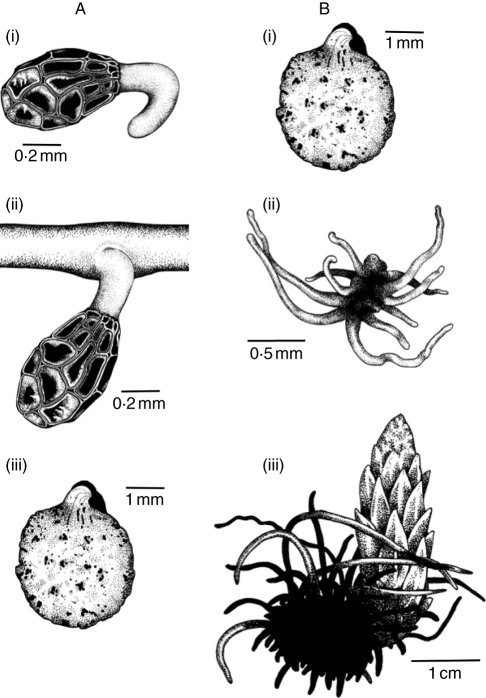
Fig. 2.
Stages of O. minor development. (A) Stages recorded from rhizotron bioassays: (i) seed germination, (ii) seedling attachment to host root, (iii) tubercle. (B) Stages recorded from pot-cultivated host–parasite interactions: (i) tubercle, (ii) ‘spider’, (iii) floral shoot.
Pot study of parasite and host growth and development
Fifty seedlings each of red clover and sea carrot cultivated previously for 6 weeks in seed trays were transplanted into 8 × 8 cm pots and maintained in controlled-environment growth cabinets (conditions as described above) for a further 6 weeks to develop. After this period, weaker individuals were discarded, and ten of the remaining plants selected at random to infect with Orobanche seed for each treatment. Using a reciprocal infection design, ten clovers and ten carrots were each inoculated with alien and natural parasite races, respectively. Ten clovers and ten carrots were also kept as uninfected controls. To infect host plants, 12 × 12 cm pots were filled to a depth of 10 cm with a substrate of 40 : 40 : 20 sharp sand, compost and garden lime (to simulate the calcareous substrates on which both host species naturally occur), and 100 mg of dry Orobanche seed added. A single host plant was then added to each pot, and the remainder of the pot filled with the substrate. Pots were then arranged in a randomized design, and watered weekly. Every 2 weeks, plants were watered additionally with Vitafeed 111 (Vitax Ltd) at 0·5 g L−1 (half the recommended dose). After 16 weeks, the first parasites formed floral buds, and plants were harvested: host shoots (including stems, leaves and flowers) were separated from root balls, which were carefully washed under running water over a 2-mm-meshed sieve (Barker et al., 1996). Parasites were then dissected from the host roots and classified into three developmental stages: (a) tubercles; (b) ‘spiders’ and (c) floral shoots (Fig. 2B); attempts to classify developmental stages further appeared to be subjective, and were therefore abandoned. Host and parasite material were then dried to constant weight at 70 °C for 7 d prior to weight determination.
Data analyses
The data were subjected to Student's t-test or analysis of variance (ANOVA) as appropriate using SPSS statistical software for Windows. Differences between individual means were tested for significance using the post-hoc Tukey's multiple comparison test. Proportion data from the rhizotron studies were transformed using arcsine square root transformation of the raw data (y = arcsine√x/100, where x = percentage germination) prior to statistical analysis (Fenández-Aparicio et al., 2007).
Histological studies
Orobanche tubercles attached to the corresponding host root were dissected from rhizotrons 50 d after inoculation. Five samples per treatment were fixed in FAA (50 % ethanol + 5 % formaldehyde + 10 % glacial acetic acid in ddH2O) for 48 h at 4 °C (Pérez-de-Luque et al., 2005). Fixed samples were dehydrated through an ethanol series (50 %, 75 % overnight, 80 %, 100 %, 100 %; 2 h each). Samples were then taken through a Histo-Clear™ (Raymond A. Lamb) ethanol series (30 %, 50 %, 80 %, 100 %, 100 %; 0·5 h each) and then saturated in paraffin (VWR International) in a vacuum oven (Hearson, UK). Thin sections (10 µm) were cut with a rotary microtome (Leica) and mounted on microscope slides in a water bath at 60 °C. Paraffin was removed from sample material with Histo-Clear™ (5 min twice) and rehydrated through an ethanol-water series (100 %, 100 %, 80 %, 70 %, 50 %, 3 0 %, ddH2O; 5 min each). Toluidine blue was used as a general stain for parasite and host tissue; sections were immersed in 0·5 % toluidine blue in 0·05 m phosphate buffer, pH 7·2 for 60 s (Hiscock et al., 2002). Sections were then dehydrated through an ethanol–water series, immersed in Histo-Clear™ twice and mounted with DPX (Fisher, UK). Sections were observed using a light microscope (Leica DMLB) under bright field illumination (magnification ×100 to ×400) and photographed using an SLR camera (RICOH photo micro).
RESULTS
Early stages of infection on sea carrots and red clovers
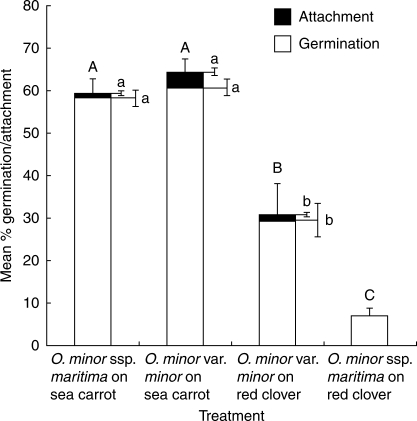
Orobanche minor races showed evidence of host preference even during the initial stages of infection (Table 1 and Fig. 3). Seeds of both O. minor var. minor and O. minor ssp. maritima showed high levels of germination on sea carrots (60·56 % ± 3·50 and 59·30 % ± 3·43, respectively), which were not significantly different on this host (P = 0·99). Levels of germination on clovers were significantly lower than on carrots for both O. minor var. minor and O. minor ssp. maritima (28·10 % ± 7·23 and 4·80 % ± 1·99, respectively; P < 0·01). Attachments (Fig. 2A) were observed in all treatments other than O. minor ssp. maritima on clover roots though this may be a direct consequence of poor germination on this host, which was significantly lower than for all other treatments (P < 0·01). However, levels of attachment among the parasite races were not equal on all hosts; the highest level of attachment was observed in O. minor var. minor infecting sea carrot (3·29 % ± 1·05 of germinated seedlings; Table 1). Indeed, this race showed a higher level of attachment on sea carrot than on red clover (the natural host for this race) which may be a result of the higher level of germination on the sea carrots. Tubercles (Fig. 2A) were only produced by O. minor var. minor infecting sea carrot roots at the time of data collection (0·22 % ± 0·16 of germinated seedlings), suggesting a higher rate of infection on this host compared with other treatments.
Table 1.
Relative proportions of the three classified stages of parasite development observed in rhizotron bioassays
| Treatment (n = 10) | % seeds germinated (± s.e.) | % seedlings attached (± s.e.) | % tubercles (± s.e.) |
|---|---|---|---|
| O. minor ssp. maritima on sea carrot | 59·35 ± 3·43 | 0·87 ± 0·30 | 0 |
| O. minor var. minor on sea carrot | 60·56 ± 3·50 | 3·29 ± 1·05 | 0·22 ± 0·16 |
| O. minor ssp. maritima on red clover | 4·80 ± 1·99 | 0 | 0 |
| O. minor var. minor on red clover | 28·10 ± 7·23 | 1·17 ± 0·43 | 0 |
Values are expressed as a percentage of the total number of seeds counted.
Measurements were taken 30 d after inoculation.
Fig. 3.
Percentage germination and attachment of O. minor var. minor and O. minor ssp. maritima seedlings, reciprocally cultivated on sea carrots and clovers in rhizotrons. Percentages calculated from data pooled from ten rhizotrons per treatment; results presented as means ± s.e. Columns sharing the same letter are not significantly different (ANOVA, P > 0·01): upper-case letters are the column totals; lower-case letters refer to the components within each column.
O. minor infectivity on pot-grown sea carrots and red clovers
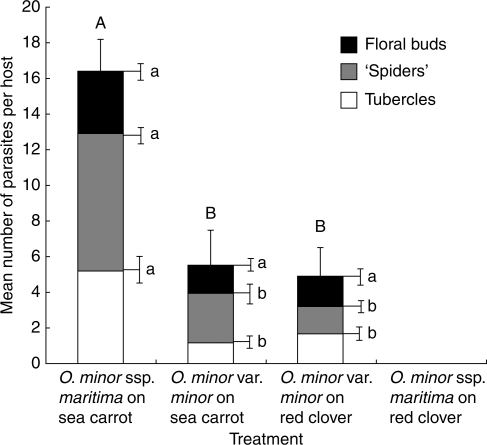
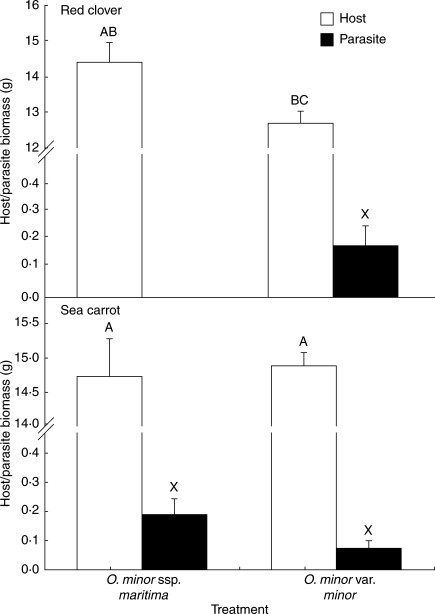
Orobanche minor races showed quantitative differences in infectivity on pot-grown hosts (Fig. 4). The largest mean number of plants per host was observed in sea carrots infected with O. minor ssp. maritima (P < 0·01). Sea carrots and red clovers infected with O. minor var. minor yielded similar numbers of plants (P = 0·97), whereas clovers inoculated with O. minor ssp. maritima showed no evidence of infection. In all treatments where infection was observed, mature Orobanche plants produced floral buds, indicating completion of the parasite life cycle on that host. Orobanche minor races also showed quantitative differences in biomass accumulation on different hosts, irrespective of the total number of plants attached (Table 2 and Fig. 5). The highest accumulation of biomass was observed for O. minor races cultivated on their natural hosts (O. minor var. minor on red clover and O. minor ssp. maritima on sea carrot), which did not differ significantly (177·3 and 203·1 mg host−1; P = 0·39). The lowest accumulation of parasite biomass was observed for O. minor var. minor on sea carrot – the alien host for this race, though this was not significantly lower than the other treatments (65·5 mg host−1; P = 0·24). Qualitative differences were also observed among the different treatments; ‘spiders’ (Fig. 2B) attached to sea carrots induced a significant distension of the host root, such that the diameter of the host root above the point of attachment was approximately four times the diameter of the corresponding root below the point of attachment (Fig. 7C). Such tissue hyperplasia was absent or less pronounced in clover roots in response to infection. In addition, ‘spiders’ frequently produced secondary haustoria on carrot roots (Fig. 7D) that were never observed on clover roots, suggesting differences may occur in the modes of nutrition and perhaps the life history of races infecting different hosts.
Fig. 4.
Mean number of O. minor plants per host cultivated in pots, classified as tubercles, ‘spiders’ and floral buds. Results presented as means ± s.e. (n = 10). Columns sharing the same letter are not significantly different (ANOVA, P > 0·05): upper-case letters are the column totals; lower-case letters refer to the components within each column.
Table 2.
Biomass values obtained from pot-cultivated host plants and O. minor races in dry weight, showing the relative abundance of three developmental stages of parasite infection (A) and the partitioning of biomass between host shoots and roots (B)
| (A) Mean parasite biomass | |||
|---|---|---|---|
| Treatment (n = 10) | Tubercles (mg host−1; ± s.e.) | ‘Spiders’ (mg host−1; ± s.e.) | Floral buds (host−1; ± s.e.) |
| O. minor ssp. maritima on sea carrot | 16·50 ± 0·002 | 60·00 ± 0·002 | 126·6 ± 0·012 |
| O. minor var. minor on sea carrot | 0·40 ± 0·001 | 27·70 ± 0·003 | 37·0 ± 0·006 |
| O. minor ssp. maritima on red clover | – | – | – |
| O. minor var. minor on red clover | 6·40 ± 0·002 | 21·10 ± 0·004 | 149·8 ± 0·030 |
| (B) Mean host biomass | |||
| Treatment (n = 10) | Shoot (g; ± s.e.) | Roots (g; ± s.e.) | Shoot : root |
| Sea carrot infected with O. minor ssp. maritima | 8·39 ± 0·40 | 6·32 ± 0·18 | 1·33 |
| Sea carrot infected with O. minor var. minor | 8·55 ± 0·17 | 6·31 ± 0·13 | 1·35 |
| Uninfected sea carrots | 8·69 ± 0·27 | 6·44 ± 0·26 | 1·35 |
| Red clover infected with O. minor ssp. maritima | – | – | – |
| Red clover infected with O. minor var. minor | 6·88 ± 0·26 | 5·80 ± 0·10 | 1·19 |
| Uninfected red clover | 7·96 ± 0·39 | 6·22 ± 0·19 | 1·28 |
Biomass was harvested 16 weeks after inoculation.
Fig. 5.
Host and parasite biomass harvested from pot-cultivated red clovers and sea carrots. Note the split scale; results presented as means ± s.e. (n = 10). Bars sharing the same letter (ABC = hosts; X = parasites) are not significantly different (ANOVA, P > 0·01).
Fig. 7.
(A) Rhizotron-propagated root system of a sea carrot infected with O. minor ‘spiders’ (arrow); (B) ‘spider’ of O. minor var. minor on clover root; (C) ‘spiders’ of O. minor ssp. maritima on carrot roots (note the host root is distended before the point of parasite attachment, indicated by a black arrow, relative to post-attachment); (D) secondary haustoria of O. minor subsp. maritima parasite (P) attached to host root (H); (E) haustorial interface of O. minor on clover root with host (H) xylem elements (white arrow) and a concentration of parasite (P) xylem elements (black arrow) resulting in vascular connectivity (red lines mark the host–parasite interface); (F) haustorium of O. minor var. minor (P) attached to clover root (H) with vascular connectivity (arrow) at host–parasite interface; (G) O. minor ssp. maritima haustorium (P) attached to carrot root (H) showing connectivity of vascular bundles (VB) and massive distension of distal host cortical parenchyma (DC) relative to proximal host cortical cells (PC). Parasite tissue is marked in red, host tissue is marked in black; (H) O. minor var. minor haustorium (P) attached to host carrot root (H) with parasitic endophyte (black arrow) and secondary parasite vascular tissue differentiation. Scale bars: (A) 1 cm; (B) 4 mm; (C) 3 mm; (D) 0·5 mm; (E) 50 µm; (F) 200 µm; (G) 200 µm; (H) 200 µm.
Host biomass
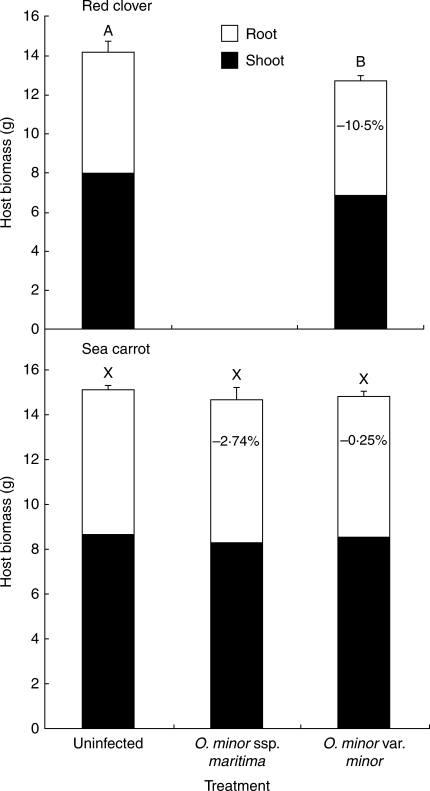
Host plants generally showed little reduction in total dry weight in response to O. minor infection (Table 2 and Fig. 6). Red clovers suffered a significant decrease in biomass (−10·5 %; P < 0·05) in response to infection with O. minor var. minor. This reduction in host biomass was only partially compensated for by Orobanche biomass (1·32 g unaccounted for). However, sea carrot showed only a small reduction in biomass in response to infection with either O. minor var. minor or O. minor ssp. maritima that was not significantly different from uninfected controls (−0·25 and −2·74 %, respectively; P = 0·859). The allometric relationship between host shoot and root dry weight was not affected by parasitism in clovers or carrots (Table 2).
Fig. 6.
Mean host biomass from pot-cultivated red clovers and sea carrots, separated into shoot and root tissue. Numbers in columns represent percentage reduction in total host biomass compared with controls. Results presented as means ± s.e. (n = 10). Columns sharing the same letter are not significantly different (A + B: t-test, P > 0·05; X: ANOVA, P > 0·05).
Histological observations of haustorial morphology
Orobanche minor ssp. maritima produced haustoria on sea carrot roots, and O. minor var. minor produced haustoria on both sea carrot and red clover roots, and no evidence of host resistance was observed. Haustoria on both host species were large and highly differentiated, producing interfacial parenchyma and a concentration of haustorial xylem strands at the host–parasite interface, suggesting vascular conduits were present (Fig. 7E). Morphological differences were also observed; penetration of the haustorium through the clover root cortex resulted in little disruption of host cortical cells, or distension of the host root. The endophyte (internal haustorial cells) penetrated the vascular core of the host root (Fig. 7F). Penetration of carrot roots by haustoria produced by both O. minor var. minor and O. minor ssp. maritima also resulted in vascular continuity between host and parasite tissues, but produced significant distortion of host tissues. The large endophyte engulfed the host root cortex, resulting in significant destruction of cortical cells proximal to the haustorium, and a distal hyperplasia of the root cortex (Fig. 7G). In addition, infection of carrot roots resulted in extensive differentiation of secondary xylem at the host–parasite interface (Fig. 7H).
DISCUSSION
Orobanche minor sensu lato is not a strict host generalist, and genetic races appear to show physiological adaptations to their local host associates. Patterns of host specificity may have isolated populations of O. minor on hosts with particular ecological niches, and driven their genetic divergence, as suggested by a recent study by Thorogood et al. (2008). The current data show that O. minor var. minor can infect both red clover and sea carrot, but with a degree of preference towards clover, the commonest host for this taxon. However O. minor ssp. maritima showed a high degree of host specificity towards sea carrot, and did not develop on red clover beyond the stage of germination. Germination of O. minor ssp. maritima seed on clover was significantly lower than for O. minor var. minor, suggesting that low induction of germination may be an important component of the host specificity of this race. Germination in response to host-derived chemical cues, followed by rapid attachment is believed to be a cause of host selection (Press and Phoenix, 2005; Phoenix and Press, 2005). In addition, attempts to artificially induce germination of seeds using the strigol analogue GR-24 during the course of these experiments were successful for O. minor var. minor, yet seeds of O. minor ssp. maritima were unresponsive (data not shown), indicating distinct biochemical differences between these taxa in their mechanisms of host detection. Chemical features of host phenotypes have previously been shown to affect parasite specificity, and potentially facilitate genetic differentiation of host-specific races in the dwarf mistletoe (Arceuthobium vaginatum; Snyder et al., 1996). Differences in infection rates of O. minor races on red clover and sea carrots may also be an indirect result of differences in host root-mass, as well as biochemistry. Spread, size and growth rate of root systems vary between species, and potential hosts with spreading and fast-growing roots may be more likely to encounter parasite seeds (Keith et al., 2004). The root systems of pot-cultivated sea carrots and red clovers yielded similar values in biomass; however, in rhizotrons, carrot roots had a greater spread. Higher germination of both races on sea carrot may therefore have been a consequence of increased root availability, and of a subsequently greater production of germination stimulants in this host.
Data from pot-cultivated hosts were largely congruent with the results from the rhizotron bioassay study. Interestingly, sea carrots infected with O. minor ssp. maritima yielded a significantly higher density of established plants than other treatments, yet the biomass accumulated by the parasites was not significantly different from that of red clovers infected with O. minor var. minor, suggesting that a finite biomass is available to the parasite, regardless of their abundance. Similar results were obtained from experiments in which tobacco plants inoculated with different densities of O. cernua seed yielded the same parasite biomass (Hibberd et al., 1998). The largest biomass accumulation was observed in treatments in which host plants were infected with the races of O. minor with which they naturally co-occur. This reinforces evidence from the rhizotron bioassay study, which suggests that parasite races show patterns of adaptation to their local hosts. Similarly, O. minor ssp. maritima showed no evidence of infecting red clover, indicating that this race shows little or no development on this species beyond the stage of germination. The treatment with the largest number of infections in the pot-growth experiment was O. minor ssp. maritima on sea carrot; however, in the rhizotron bioassay experiment, O. minor var. minor showed a greater infectivity on this host. This disparity may suggest a time lag in infectivity by O. minor ssp. maritima on its natural host, or that pots provide a more accurate simulation of natural host–parasite interactions. Plants used for rhizotron bioassays were also younger and smaller than pot-grown plants. Evidence suggests that variation in source–sink relationships in host plants is age-dependent, and may affect the infectivity of parasitic plants; older hosts have a greater source strength but also have a larger sink strength, obstructing the parasite's access to resources (Cameron et al., 2005). This should be a minor limitation of the present study since rhizotrons were only used to measure the early stages of infection. Interestingly, total host biomass, and biomass partitioning between shoot and root showed very little reduction or change in response to infection for each treatment, in contrast to previous studies, in which the biomass of host plants was reduced by up to 50 % due to infection by parasitic plants (Barker et al., 1996; Hibberd et al., 1998; Labrousse et al., 2001; Vasey et al., 2005). However, it is known that the relationship between parasitic angiosperms and their hosts is variable, and infection may result in negligible effects in terms of host growth and physiology (Cameron et al., 2005). Indeed the effect of Orobanche parasitism on its host plants in particular is known to vary (Labrousse et al., 2001).
It has been suggested previously that parasitic plants have a higher fitness on leguminous hosts, probably as a consequence of their high nitrogen content (Seel and Press, 1993; Radomiljac et al., 1998, 1999). In the present study, O. minor ssp. maritima was unable to infect red clover, and O. minor races performed better on the hosts on which they naturally occur. Therefore populations of O. minor sensu lato probably show patterns of host specificity regardless of the nitrogen content of potential hosts. Similarly, Jiang et al. (2008) have established that nitrogen fixation does not influence the quality of leguminous hosts for the root hemiparasite Rhinanthus minor, and that haustorial anatomy and host resistance may in fact underpin host preference. Parasites may also show a preference towards deep-rooted hosts with improved access to water (Pate et al., 1990); sea carrot occurs on sun-baked sea cliffs and produces tap roots, which may confer advantages over co-occurring potential host species. Most species of Orobanche parasitize a narrow range of perennial hosts and are potentially perennial themselves, while a few have shifted to annual hosts in anthropogenically disturbed ecosystems. Therefore host-specialization may be associated with predictable resources (perennial hosts) and host-generalism with unpredictable resources (annual hosts), a pattern often found in animal parasites (Schneeweiss, 2007). Orobanche minor ssp. maritima parasitizes sea carrot, which is perennial, and is therefore potentially perennial itself. Furthermore, secondary haustoria were observed arising from this parasite on sea carrot, which could potentially give rise to multiple foci of infection, and confer a perennial life history to this race of O. minor. Secondary haustoria have recently been observed arising from the roots of O. aegyptiaca on the tubers of Solanum tuberosum, and are believed to serve the parasite in acquiring additional nutrients from neighbouring roots (Joel, 2007).
Orobanche minor var. minor often parasitizes red clover which is probably short-lived, and occurs on disturbed grassland. In the present study, O. minor var. minor showed no evidence of producing secondary haustoria on red clover, indicating the life history of O. minor varies on different hosts. Host root anatomy may also contribute to the evolution of physiological races of parasite; for instance, thicker carrot roots may contain larger vascular bundles for efficient host–parasite solute transfer, but also require significant energy expenditure to penetrate. Parasites attached to carrot roots induced conspicuous distention on the host root. Previous studies have also identified host root distension at the point of Orobanche attachment (Dale and Press, 1998). While such a response was also observed on clover roots, the degree of swelling at the point of infection was substantially less. Observations of the host–parasite interface suggest that the significant swelling response to infection seen in carrot roots may be a result of a proliferation of host root cortical cells causing a hyperplasia of root tissue. The unusually large differentiation of the haustorial cells of the parasite endophyte may cause disruption to the host root cortex, since cells appeared to be crushed in response to haustorial penetration. Deformation of the host root cortex has also been observed in sunflower roots infected by O. cumana (Labrousse et al., 2001). Similarly, Cameron et al. (2006) report parasite-induced host root deformation, and cellular resistance to haustoria in the root hemi-parasite Rhinanthus minor. In susceptible hosts, haustoria encompassed the host root, crushed the cortex, and formed a penetration peg containing differentiated secondary xylem. In contrast, haustoria on resistant hosts showed poor development, and failed to encompass the host root. Variation in root anatomy and host susceptibility to infection may lead to differential parasite fitness on particular hosts, which is likely to have played a major role in the evolution of physiological races of parasitic plants, and coevolution with their hosts.
The present data support previous research which suggested that O. minor may comprise host-specific strains (Musselman and Parker, 1982). Characterization of host-specific races among natural populations of Orobanche will be a useful tool in understanding patterns of speciation in parasitic plants. It has been well documented that host specificity can drive the synchronous speciation of parasites with their host associates, a relationship referred to as Fahrenholz's rule (Fahrenholz, 1913; Hafner and Nadler, 1988; Hafner and Page, 1995). Divergence followed by isolation on hosts with differing ecologies has been established as a probable route to speciation in insects (Drès and Mallet, 2002), e.g. leaf beetles (Ophraella spp.; Funk et al., 1995), aphids (Acyrthosiphon spp.; Hawthorne and Via, 2001), stick insects (Timema spp.; Nosil et al., 2002) and tephritid fruitflies (Rhagoletis spp.; Schwarz et al., 2005). It has also been hypothesized that host-driven divergence may have played an important role in the speciation of parasitic plants (Zuber and Widmer, 2000; Thorogood et al., 2008; de Vega et al., 2008). For instance, host specificity may have preceded genetic divergence among hemiparasitic mistletoes (Viscum album): Zuber and Widmer (2000) used chloroplast DNA and nuclear DNA internal transcribed spacer markers to elucidate relationships among morphologically cryptic subspecies of mistletoe parasitizing different hosts. More recently, the root holoparasite Cytinus hypocistis (Cytinaceae) which shows distinct patterns of host specificity at a local level, has been shown to form regional patterns of divergence on different lineages of host in the Cistaceae (Thorogood and Hiscock, 2007; de Vega et al., 2008). While studies on speciation in parasitic angiosperms are scarce, it is likely that host-driven divergence may be an underestimated driving force in the evolution of this poorly understood group of plants.
CONCLUDING REMARKS
Orobanche minor is a host generalist, attacking hosts from taxonomically disparate families (Rumsey and Jury, 1991). Using reciprocal-infection experiments, further incremental evidence that genetic divergence among populations of O. minor could be a consequence of host specificity (Thorogood et al., 2008) has been provided by showing that O. minor comprises physiological races. Races of O. minor are morphologically cryptic, yet plants raised on sea carrot and red clover showed distinct host preferences and a higher fitness, measured in biomass, on their natural host associates. These races showed host specificity even at the level of germination, and histological observations using sea carrot and red clover suggest that host responses to infection vary between species. Nevertheless, the present study only investigated the interaction between host and parasite over a small time scale. Further work will determine the differential fitness of parasitic races in terms of reproductive output, and extend this investigation to quantify host specificity of multiple populations, because O. minor var. minor occurs on numerous other host species besides red clover. Valuable information will be obtained from investigating the differences in solute flux of natural Orobanche strains on different hosts, which has to date, only been carried out using weedy species. In conclusion, we propose that physiological shifts in Orobanche taxa may underpin the host specificity of different cryptic races, and precede genetic divergence and ultimately speciation in this genus, and that this mode of speciation may be a phenomenon common to parasitic plants.
ACKNOWLEDGEMENTS
This research was supported by a University of Bristol Post-graduate scholarship to C. J. Thorogood, along with additional funding from the Lady Emily Smythe Agricultural Research Station (LESARS) of the University of Bristol, and the Botanical Society of the British Isles (BSBI).
LITERATURE CITED
- Antonovics J. Evolution in closely adjacent plant populations. V. Evolution of self-fertility. Heredity. 1968;23:219–238. doi: 10.1038/sj.hdy.6800835. [DOI] [PubMed] [Google Scholar]
- Barker ER, Press MC, Scholes JD, Quick WP. Interactions between the parasitic angiosperm Orobanche aegyptiaca and its tomato host: growth and biomass allocation. New Phytologist. 1996;133:637–642. [Google Scholar]
- Cameron DD, Hwangbo JK, Keith AM, et al. Interactions between the hemiparasitic angiosperm Rhinanthus minor and its hosts: from the cell to the ecosystem. Folia geobotanica. 2005;40:217–229. [Google Scholar]
- Cameron DD, Coats AM, Seel WE. Differential resistance among host and non-host species underlies the variable success of the hemi-parasitic plant Rhinanthus minor. Annals of Botany. 2006;98:1289–1299. doi: 10.1093/aob/mcl218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale H, Press MC. Elevated atmospheric CO2 influences the interaction between the parasitic angiosperm Orobanche minor and its host Trifolium repens. New Phytologist. 1998;140:65–73. [Google Scholar]
- Davis CC, Latvis M, Nickrent DL, Wurdack KJ, Baum DA. Floral gigantism in Rafflesiaceae. Science. 2007;315:1812. doi: 10.1126/science.1135260. [DOI] [PubMed] [Google Scholar]
- Drès M, Mallet J. Host races in plant-feeding insects and their importance in sympatric speciation. Philosophical Transactions of the Royal Society of London B. 2002;357:471–492. doi: 10.1098/rstb.2002.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenholz H. Ectoparasiten und abstammungslehre. Zoologischer Anzeiger. 1913;41:371–374. [Google Scholar]
- Fenández-Aparicio M, Sillero JC, Rubiales D. Intercropping with cereals reduces infection by Orobanche crenata in legumes. Crop Protection. 2007;26:1166–1172. [Google Scholar]
- Fernández-Aparicio M, Emeran AA, Rubiales D. Control of Orobanche crenata in legumes intercropped with fenugreek (Trigonella foenum-graecum) Crop Protection. 2008;27:653–659. [Google Scholar]
- Funk DJ, Futuyma DJ, Orti G, Meyer A. A history of host associations and evolutionary diversification for Ophraella (Coleoptera: Chrysomelidae): new evidence from mitochondrial DNA. Evolution. 1995;49:1008–1017. doi: 10.1111/j.1558-5646.1995.tb02335.x. [DOI] [PubMed] [Google Scholar]
- Hafner MS, Nadler SA. Phylogenetic trees support the coevolution of parasites and their hosts. Nature. 1988;332:258–259. doi: 10.1038/332258a0. [DOI] [PubMed] [Google Scholar]
- Hafner MS, Page RDM. Molecular phylogenies and host–parasite cospeciation: gophers and lice as a model system. Philosophical Transactions of the Royal Society B. 1995;349:77–83. doi: 10.1098/rstb.1995.0093. [DOI] [PubMed] [Google Scholar]
- Hawthorne DJ, Via S. Genetic linkage of ecological specialization and reproductive isolation in pea aphids. Nature. 2001;412:904–907. doi: 10.1038/35091062. [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Quick WP, Press MC, Scholes JD. Can source–sink relations explain responses of tobacco to infection by the root holoparasitic angiosperm Orobanche cernua? Plant, Cell & Environment. 1998;21:333–340. [Google Scholar]
- Hiscock SJ, Hoedemaekers K, Friedman WE, Dickinson HG. The stigma surface and pollen–stigma interactions in Senecio squalidus L. (Asteraceae) following cross (compatible) and self (incompatible) pollinations. International Journal of Plant Science. 2002;163:1–16. [Google Scholar]
- Jiang F, Jeschke WD, Hartung W, Cameron DD. Does legume nitrogen fixation underpin host quality for the hemiparasitic plant Rhinanthus minor? Journal of Experimental Botany. 2008;59:917–925. doi: 10.1093/jxb/ern015. [DOI] [PubMed] [Google Scholar]
- Joel DM. Direct infection of potato tubers by the root parasite Orobanche aegyptiaca. Weed Research. 2007;47:276–279. [Google Scholar]
- Jones M. Progress in Orobanche Research Proceedings. Tubingen: 1989. Studies into the pollination of Orobanche species in the British Isles; pp. 6–17. [Google Scholar]
- Keith AM, Cameron DD, Seel WE. Spatial interactions between the hemiparasitic angiosperm Rhinanthus minor and its host are species-specific. Functional Ecology. 2004;18:435–442. [Google Scholar]
- Kharrat M, Halila MH, Linke KH, Haddar T. First report of Orobanche foetida Poiret on faba bean in Tunisia. FABIS Newsletter. 1992;30:46–47. [Google Scholar]
- Kuijt J. The biology of parasitic flowering plants. Berkeley, CA: University of California Press; 1969. [Google Scholar]
- Labrousse P, Arnaud MC, Serieys H, Bervillé A, Thalouarn P. Several mechanisms are involved in resistance of Helianthus to Orobanche Cumana Wallr. Annals of Botany. 2001;88:859–868. [Google Scholar]
- Larson BMH, Barrett SCH. Reproductive biology of island and mainland populations of Primula mistassinica (Primulaceae) on Lake Huron shorelines. Canadian Journal of Botany. 1998;76:1819–1827. [Google Scholar]
- Musselman LJ, Parker C. Preliminary host ranges of some strains of economically important broomrapes (Orobanche) Economic Botany. 1982;36:270–273. [Google Scholar]
- Nais J. Kota Kinabalu: Sabah Parks; 2001. Rafflesia of the world. [Google Scholar]
- Nickrent DL, Blarer A, Qiu Y-L, Vidal-Russell R, Anderson FE. Phylogenetic inference in Rafflesiales: the influence of rate heterogeneity and horizontal gene transfer. BMC Evolutionary Biology. 2004;4:40. doi: 10.1186/1471-2148-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosil P, Crespi BJ, Sandoval CP. Host–plant adaptation drives the parallel evolution of reproductive isolation. Nature. 2002;417:440–443. doi: 10.1038/417440a. [DOI] [PubMed] [Google Scholar]
- Parker C, Riches CR. Parasitic weeds of the world: biology and control. Wallingford: CAB International; 1993. [Google Scholar]
- Pate JS, Davidson NJ, Kuo J, Milburn JA. Water relations of the root hemiparasite Olax phyllanthi (Labill) R.Br. (Oleaceae) and its multiple hosts. Oecologia. 1990;84:186–193. doi: 10.1007/BF00318270. [DOI] [PubMed] [Google Scholar]
- Pérez-de-Luque A, Rubiales D, Cubero JI, et al. Interaction between Orobanche crenata and its host legumes: unsuccessful haustorial penetration and necrosis of the developing parasite. Annals of Botany. 2005;95:935–942. doi: 10.1093/aob/mci105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix GK, Press MC. Linking physiological traits to impacts on community structure and function: the role of root hemiparasitic Orobanchaceae (ex-Scrophulariaceae) Journal of Ecology. 2005;93:67–78. [Google Scholar]
- Press MC, Graves JD. Parasitic plants. London: Chapman and Hall; 1995. [Google Scholar]
- Press MC, Phoenix GK. Impacts of parasitic plants on natural communities. New Phytologist. 2005;166:737–751. doi: 10.1111/j.1469-8137.2005.01358.x. [DOI] [PubMed] [Google Scholar]
- Radomiljac AM, McComb JA, Pate JS, Tennakoon KU. Xylem transfer of organic solutes in Santalum album L. (Indian sandalwood) in association with legume and non-legume hosts. Annals of Botany. 1998;82:675–682. [Google Scholar]
- Radomiljac AM, McComb JA, Pate JS. Gas exchange and water relations of the root hemi-parasite Santalum album L. in association with legume and non-legume hosts. Annals of Botany. 1999;83:215–224. [Google Scholar]
- Román B, Satovic Z, Alfaro C, et al. Host differentiation in Orobanche foetida Poir. Flora. 2007;202:201–208. [Google Scholar]
- Rubiales D, Sadiki M, Román B. First report of Orobanche foetida on common vetch (Vicia sativa) in Morocco. Plant Disease. 2005;89:528. doi: 10.1094/PD-89-0528A. [DOI] [PubMed] [Google Scholar]
- Rumsey FJ, Jury SL. An account of Orobanche L. in Britain and Ireland. Watsonia. 1991;18:257–295. [Google Scholar]
- Sauerborn J. The economic importance of the phytoparasites Orobanche and Striga. In: Ransom JK, Musselman LJ, Worsham AD, Parker C, editors. Proceedings of the 5th International Symposium of Parasitic Weeds. Nairobi, Kenya: 1991. pp. 137–143. [Google Scholar]
- Schneeweiss GM. Correlated evolution of life history and host range in the nonphotosynthetic parasitic flowering plants Orobanche and Phelipanche (Orobanchaceae) Journal of Evolutionary Biology. 2007;20:471–478. doi: 10.1111/j.1420-9101.2006.01273.x. [DOI] [PubMed] [Google Scholar]
- Schwarz D, Matta BM, Shakir-Botteri NL, McPheron BA. Host shift to an invasive plant triggers rapid animal hybrid speciation. Nature. 2005;436:546–549. doi: 10.1038/nature03800. [DOI] [PubMed] [Google Scholar]
- Seel WE, Press MC. Influence of the host on three sub-Arctic annual facultative root hemiparasites. 1. Growth, mineral accumulation and aboveground dry-matter partitioning. New Phytologist. 1993;125:131–138. doi: 10.1111/j.1469-8137.1993.tb03871.x. [DOI] [PubMed] [Google Scholar]
- Snyder MA, Fineschi B, Linhart YB, Smith RH. Multivariate discrimination of host use by dwarf mistletoe Arceuthobium vaginatum subsp. cryptopodum: inter- and intraspecific comparisons. Journal of Chemical Ecology. 1996;22:295–305. doi: 10.1007/BF02055100. [DOI] [PubMed] [Google Scholar]
- Thorogood CJ, Hiscock SJ. Host specificity in the parasitic plant Cytinus hypocistis. Research Letters in Ecology. 2007 article 84234, doi: 10·1155/2007/84234. [Google Scholar]
- Thorogood CJ, Rumsey FJ, Harris SA, Hiscock SJ. Host-driven divergence in the parasitic plant O. minor Sm. (Orobanchaceae) Molecular Ecology. 2008;17:4289–4303. doi: 10.1111/j.1365-294x.2008.03915.x. [DOI] [PubMed] [Google Scholar]
- Vasey RA, Scholes JD, Press MC. Wheat (Triticum aestivum) is susceptible to the parasitic angiosperm Striga hermonthica, a major cereal pathogen in Africa. Genetics and Resistance. 2005;95:1294–1300. doi: 10.1094/PHYTO-95-1294. [DOI] [PubMed] [Google Scholar]
- de Vega C, Berjano R, Arista M, Ortiz PL, Talavera S, Stuessy TF. Genetic races with the genera and sections of host species in the holoparasitic plant Cytinus (Cytinaceae) in the Western Mediterranean basin. New Phytologist. 2008;178:875–887. doi: 10.1111/j.1469-8137.2008.02423.x. [DOI] [PubMed] [Google Scholar]
- Young ND, Steiner KE, dePamphilis CW. The evolution of parasitism in Scrophulariaceae/Orobanchaceae: plastid gene sequences refute evolutionary transition series. Annals of the Missouri Botanical Garden. 1999;86:876–893. [Google Scholar]
- Zuber D, Widmer A. Genetic evidence for host specificity in the hemi-parasitic Viscum album L. (Viscaceae) Molecular Ecology. 2000;9:1069–1073. doi: 10.1046/j.1365-294x.2000.00963.x. [DOI] [PubMed] [Google Scholar]