Abstract
Recent studies have demonstrated that CD28 provides critical costimulatory signals required for optimal CD8 T cell expansion and effector function in response to several viruses including, influenza, HSV, and vaccinia virus (VACV). CD28 has two ligands expressed largely on professional APC, named B7.1 (CD80) and B7.2 (CD86). Although some results suggest that these ligands are equivalent and both promote CD28 signaling, it is not clear whether they are equally important for priming of anti-viral T cells. Here we show that B7.2 is critical for early CD8 T cell responses to both dominant and subdominant VACV epitopes, correlating with its strong induction on CD8α+ dendritic cells. In contrast, B7.1 plays no significant role. Signals from an exogenously applied adjuvant can recruit B7.1 activity and lead to further enhanced priming of VACV-reactive CD8 T cells. However, during a natural infection, B7.1 is not functional, likely related to inefficient upregulation or active suppression by VACV. These studies provide evidence that B7.2 is the major ligand for the CD28 receptor on VACV-specific CD8 T cells, that B7.2 can promote efficient CD8 T cell priming without B7.1, and that B7.1 and B7.2 can be differentially utilized during anti-viral responses.
Keywords: CD28, B7-1/CD80, B7-2/CD86, Vaccinia Virus, Poxviruses, CD8 T cells
Introduction
Many viral infections elicit potent CD8 T cell responses that are instrumental in clearing virus from the tissues and in protecting from subsequent infection. Induction of an efficient T cell response requires several different signals including those brought about by the interaction of so-called T cell costimulatory receptors with their APC-expressed costimulatory ligands. Costimulatory ligands are generally present on professional APC (dendritic cells, B cells, macrophages), although they can be expressed on other cell types in conditions of strong inflammation. The costimulatory signals these ligands transmit through binding to their receptors on T cells appear to perform several functions, such as promoting cell cycle progression, inducing production of effector cytokines, and suppressing cell death (1–3).
The importance of membrane bound costimulatory receptors has been well documented by studies examining the requirement for CD28 signals for priming of naïve CD4 T cells (1–4). In the context of viral infections, CD4 T cell responses to LCMV (5, 6), VSV (5, 6), HSV-1 (7), and influenza (8) are substantially reduced in CD28-deficient (CD28−/−) mice. In contrast, the requirement for CD28 costimulation for priming of naïve CD8 T cells is less clear, and the results of different studies are in part contradictory. Based on experiments in vitro a number of years ago, several groups postulated that naive CD8 T cells are less dependent, or independent, of costimulation for proliferation and differentiation into cytotoxic effector cells (9–13). Negative data from in vivo studies targeting CD28 using gene-knockout or blocking strategies also supported this idea (14, 15), while other publications particularly in vitro have suggested that naïve CD8 T cells may be highly receptive to CD28 signals (16–20). In different viral infection models the dependency for CD28 signaling to generate virus-specific CD8 T cells also varies considerably. CD28−/− mice infected with LCMV generate a normal CD8 response (6, 21, 22) 5, 23). In contrast, primary CD8 T cell responses to VSV (5, 6, 20, 23), influenza (24, 25), HSV (7), and MHV-68 (26–28) are severely impaired. Thus, bypassing a requirement for CD28 signaling to elicit naive CD8 T cells is not a property of all viruses, and CD28-dependence likely reflects differences related to the rate of viral replication, antigenic load, cell tropism, and perhaps the specific cytokine milieu induced in response to each virus.
B7.1 (CD80) and B7.2 (CD86) are the ligands for CD28 that provide the major signal for initiating T cell responses, but these molecules also function as ligands for the inhibitory receptor CTLA-4 (29, 30). Although tremendous progress has been made over the past two decades in identifying the function of CD28 and CTLA-4, our understanding of the importance of the two alternative ligands has lagged behind. Initial thoughts were that B7.2 was the major ligand for CD28 and B7.1 for CTLA-4 based on differential expression and binding affinities. Because of the considerable complexity of these multiple receptor/ligand pairs, several issues have arisen. First, are both ligands required for every T cell response or does some degree of flexibility or redundancy exist. Second, do both molecules perform similar functions, or can separate functions be ascribed to individual ligands, and therefore is one ligand more important in distinct pathogenic situations.
Vaccinia virus (VACV) is a large DNA virus and is a member of the genus Orthopoxvirus, which includes variola, monkeypox, buffalopox, and cowpox. In humans and mice, VACV elicits a robust CD8 T cell response (31–33). At the peak of the effector phase, more than 20% of all CD8 T cells are directed against well-defined dominant and subdominant epitopes (34). Recently, CD28 signaling was shown to be required for optimal expansion of VACV-specific effector CD8 T cells directed against the immunodominant epitope of vaccinia, B8R (35, 36). Whether subdominant VACV-specific CD8 cells equally require CD28 and whether there is differential requirement for B7.1 vs. B7.2 in VACV-specific CD8 T cell responses is not clear. Using reagents that specifically block B7.1 and B7.2 interactions in combination with mice deficient in one or both ligands we clearly show that B7.2 dictates the absolute numbers of effector CD8 T cells that accumulate to VACV, whereas B7.1 plays little/no role. Using recombinant VACV expressing GFP we show that B7.2, but not B7.1, is upregulated on infected CD8α+ DC and this likely determines its availability and usage by VACV-specific CD8 T cells. These studies provide new insights into the role of B7.1 and B7.2 in acute viral infections.
Materials and Methods
Mice
The studies reported here conform to the animal Welfare Act and the NIH guidelines for the care and use of animals in biomedical research. All experiments were done in compliance with the regulations of the La Jolla Institute Animal care committee in accordance with the guidelines by the Association for assessment and Accreditation of laboratory Animal Care. 8–12 wk-old female and male C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). OT I TCR-transgenic mice were used as a source of Vβ5/Vα2 CD8+ T cells responsive to OVA-derived SIINFEKL peptide. CD28-deficient OT-I TCR transgenic mice were generated in house by crossing OT-I mice with CD28−/− mice. CD28−/−, B7.1−/−, B7.2−/−, and MHC class II−/− mice were all purchased from Jackson laboratory (Bar Harbor, ME). B7.1/B7.2 double deficient mice were kindly provided by Dr. A. Sharpe (Harvard Medical School).
Peptides and Tetramers
Vaccinia virus peptide epitopes used in this study were predicted and synthesized as described previously (34, 37). B8R (20–27; TSYKFESV), A3L (270–227; KSYNYMLL), A8R (189–196; ITYRFYLI), B2R (54–62; YSQVNKRYI), A23R (297–305; IGMFNLTFI). MHC/peptide tetramers for the VACV-WR epitope B8R (20–27; TSYKFESV)/H-2Kb, which were conjugated to allophycocyanin, were obtained from the National Institutes of Health Tetramer Core facility (Emory University, Atlanta, GA).
Viruses
The VACV Western Reserve (VACV-WR) strain was purchased from the American Type Culture Collection (Manassas, VA), grown in HeLa cells, and titered on VeroE6 cells (38).
Virus Infections
For most experiments, mice were infected intraperitonealy (i.p.) with 2 ×105 PFU of VACV-WR. Effector responses were analyzed between days 4 and 8 post-infection after restimulating in vitro with VACV peptides.
For adoptive transfer experiments, 5 × 104 naive wt or CD28 −/− OT-I CD8 T cells were transferred into wt non-transgenic B6 or CD28 −/−, B7.1 −/−, or B7.2 −/− mice. One day later, mice were infected i.p. with recombinant VACV expressing full-length OVA protein (VACV-OVA; 2 × 106 PFU/mouse) or PBS as indicated. OT-I expansion was detected by FACS staining of transgenic TCR α and β chains after gating on CD8 T cells and in some cases after restimulating in vitro with OVA (SINFEKL) peptide.
Peptide immunization
Groups of wild-type, CD28−/−, B7.1−/−, and B7.2−/− mice were immunized subcutaneously (s.c.) at the base of the tail once with 10 µg/mouse of B8R (20–27; TSYKFESV) peptide epitope emulsified in IFA. Seven days post-immunization spleens were harvested and stained with anti-CD8 (PerCp), anti-CD44 (FITC), and B8R-tetramer (APC). The percentages of B8R-specific CD8 T cells were determined by FACS.
In vivo antibody treatment
Hybridomas were cultured in Life Technologies Protein-Free Hybridoma Medium-II (Invitrogen, San Diego, CA), and mAbs were isolated by dialysis of supernatant. To deplete CD4+ T cells, groups of mice were given anti-CD4 (clone GK1.5; 200 µg/mouse) in one i.v injection 3 days prior to, and one i.p. injection 2 days after, infection with VACV-WR. CD4 depletion was confirmed by flow cytometry of spleens of treated mice using a non-competitive anti-CD4 (clone RM4-4). To block B7.1 and/or B7.2, 150 µg of anti-B7.1 (clone 16–10A1), anti-B7.2 (clone GL1) and/or control Ab was given i.p. at the indicated time points. In some experiments, groups of WT and B7.2−/− mice were treated with an agonistic anti-CD40 mAb (αCD40; 150-µg/mouse) at the time of VACV infection
DC culture
Spleen fragments were digested for 30 min at 37°C with collagenase/DNase (1 mg/ml collagenase D and 1 µg/ml grade II bovine pancreatic DNase I (Boehringer-Mannheim, Mannheim, Germany)) and then treated for 5 min with EDTA to disrupt T cell-DC complexes. After lysing red blood cells (RBCs), splenocytes were resuspended in RPMI-1640 medium (Gibco) supplemented with 10% FCS (Omega Scientific), 1% L-glutamine (Invitrogen), 100 µg/ml streptomycin and 100 U/ml penicillin. 1 × 106 cells were plated in 24-well plates in 2 ml with medium or recombinant VACV expressing GFP (VACV-GFP; MOI=10). After 24 hours, cells were stained with anti-CD11c, MHC-Class II, anti-CD8, and anti-CD4. DC subsets (CD8+, CD4+, CD4+CD8+, and CD8-CD4- double negative (DN)) were analyzed for GFP after gating on CD11chighMHC-classII+ cells by FACSCalibur™ flow cytometer using CellQuest (BD Biosciences) and FlowJo software (Tree Star, san Carlos, CA).
Avidity titration
Groups of wild-type and B7.2−/− mice were infected intraperitonealy (i.p.) with 2 ×105 PFU of VACV-WR. Eight days post-infection with VACV-WR, the avidity of B8R-specific CD8 T cells were titrated by IFN-γ and TNF intracellular staining after stimulation of splenocytes with graded concentrations of B8R peptide. Functional avidity, was measured as the concentration of the peptide yielding 50% of the maximal response (SD50).
Flow cytometry
Cytokine production in T cells was assessed as previously described (39), with some modifications. Briefly, after lysing red blood cells (RBCs), splenocytes from infected mice were resuspended in RPMI-1640 medium (Gibco) supplemented with 10% FCS (Omega Scientific), 1% L-glutamine (Invitrogen), 100 µg/ml streptomycin, 100 U/ml penicillin and 50 µM 2-mercaptoethanol (Sigma). 1–2 × 106 cells were plated in round-bottomed 96-well microtiter plates in 200 µl with medium or the indicated VACV peptides at 1 µg/ml for 1 hr at 37°C. GolgiPlug (BD Biosciences) was then added to the cultures according to the manufacture’s instructions and the incubation continued for 7 hrs. Cells were stained with anti-CD8 (PerCP) and CD62L (PE), followed by fixation with cytofix-cytoperm (BD Biosciences) for 20 min at 4°C. Fixed cells were subjected to intracellular cytokine staining in BD Perm/Wash buffer for 30 min at 4°C. Anti-TNF (FITC) and IFN-γ (APC) were obtained from e-Biosience and used at a 1:100 dilution. Samples were analyzed for their proportion of cytoplasmic cytokines after gating on CD8+CD62Llow T cells by FACSCalibur™ flow cytometer using CellQuest (BD Biosciences) and FlowJo software (Tree Star, san Carlos, CA).
Statistics
Statistical significance was analyzed by Student’s t test. Unless otherwise indicated, data represent the mean ± SEM, with p < 0.05 considered statistically significant.
Results
CD28 costimulation is required for optimal effector CD8 T cell responses directed against both dominant and subdominant VACV epitopes
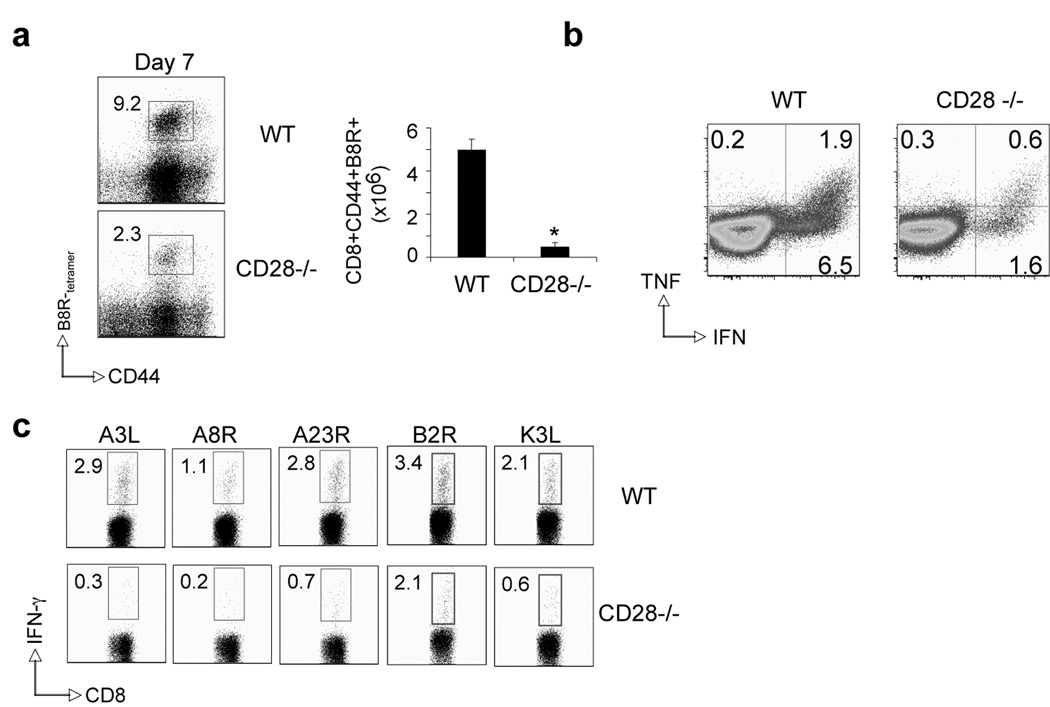
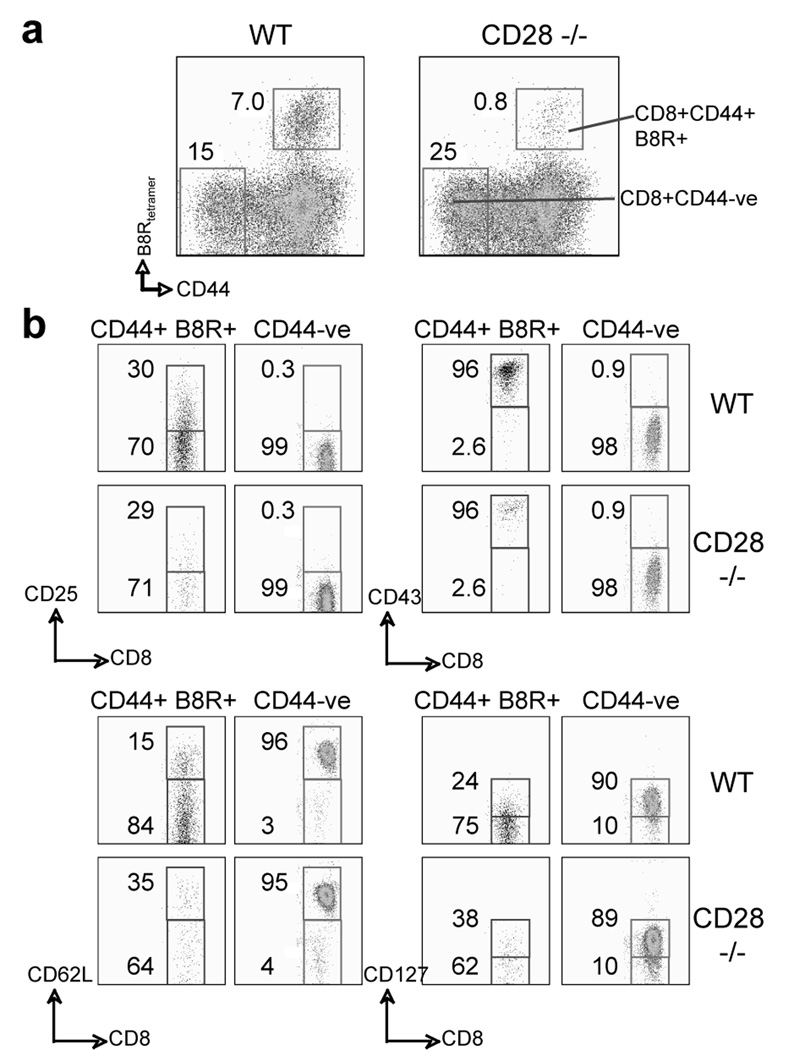
Recent studies have identified a role for CD28 in clonal expansion and functionality of VACV-specific CD8 T cells directed against the immunodominant B8R epitope (35, 36). Whether CD28 serves as the dominant driver for anti-viral CD8 T cells directed against subdominant VACV epitopes is not known. To examine this, wt and CD28−/− mice were infected with VACV-WR and generation of CD8 T cells specific for the dominant (B8R) and five subdominant (A3L, A8R, A23R, B2R, K3L) MHC Class I-restricted VACV epitopes were quantitated by tetramer and intracellular IFN-γ and TNF staining. The responses we chose account for up to 70% of the total VACV CD8 response (34). On day 7, at the peak of the primary response, 7–10% of total CD8 T cells were reactive with the B8R peptide in wt mice (Fig. 1a and 1b), whereas CD8 T cells reactive with other determinants were present at frequencies of 3% or less (Fig. 1c). VACV infection of CD28−/− mice resulted in impaired CD8 T cell responses not only against B8R, but also against all subdominant epitopes examined. To assess whether the differentiation state of VACV-specific CD8 T cells was impacted by CD28 signals the expression of CD44, CD25, CD43, CD62L, and CD127, were analyzed on CD8+ B8R-tetramer+ cells in the spleen six days postinfection with VACV-WR (Fig. 2). CD44 (Fig. 2a), CD25 and CD43 (Fig. 2b) were similarly elevated on the few B8R-specific effector CD8 cells in the spleen of CD28−/− mice as compared with wt mice. In contrast, down regulation of CD62L and CD127 were impaired in the absence of CD28 signaling (Fig. 2b). Thus, the differentiation status as well as expansion/accumulation of VACV-specific effector CD8 T cells is significantly impaired in the absence of CD28 signaling.
Figure 1. Priming of CD8 T cells is defective in the absence of CD28 after infection with VACV-WR.
(a) Wt or CD28-deficient (CD28−/−) mice were infected i.p with VACV-WR (2 × 105 PFU/mouse). Seven days post-infection splenocytes were harvested and stained for CD8, CD44, B8R-tetramer. Left: Representative plots of tetramer staining, gating on CD8+ cells. Percentages of activated B8R-tetramer positive CD8 T cells (CD8+CD44+B8R+) are indicated. Right: Total numbers of B8R-tetramer positive CD8+CD44+ T cells per spleen. (b) At day 7, splenocytes were stimulated with B8R peptide for intracellular IFN-γ and TNF staining. Representative plots for cytokine-staining, gating on CD8+CD62Llow cells. Percentages that stained positive for IFN-γ alone, or TNF and IFN-γ/TNF are indicated. Quadrant settings were based on controls, using infected splenocytes that were not stimulated with peptide, and uninfected splenocytes stimulated with each peptide (data not shown). (c) At day 7, splenocytes were stimulated with A3L, A3R, A23R, B2R, and K3L peptides for intracellular IFN-γ staining as described in (b). Data are representative plots of IFN-γ staining in gated CD8+CD62Llow T cells, with percent positive indicated. *, p < 0.05 (WT vs CD28−/−). Similar results were obtained in 3 separate experiments.
Figure 2. Effector VACV-specific CD8 T cells in CD28−/− mice exhibit a partially differentiated phenotype.
Mice were infected as in Fig. 1. At day 6, CD8 T cell differentiation was assessed with up-regulation of CD25, CD43, and down-regulation of CD62L and CD127 on B8R-tetramer positive CD44-high cells. Naï (CD44-low B8R-tetramer negative) CD8 T cells were used as controls. (a) Representative gating on individual populations. (b) Percentages that stained positive for each marker are indicated. Similar results were obtained in 3 separate experiments.
Impaired primary VACV-specific CD8 T cell responses in the absence of B7.2 but not B7.1
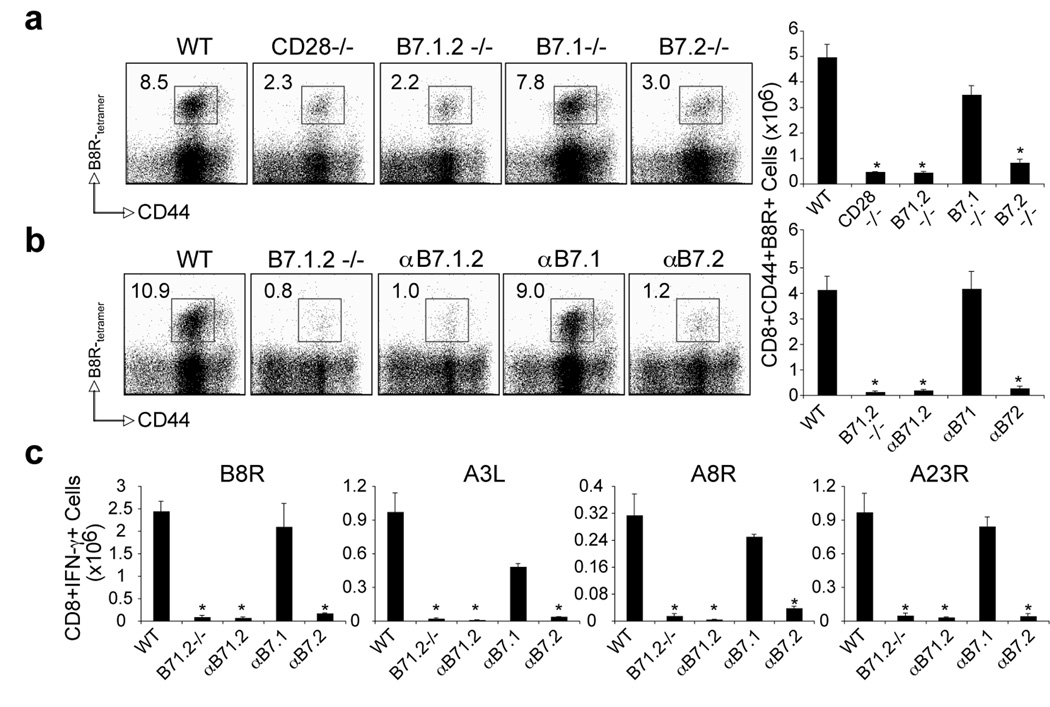
To determine whether B7.1 and B7.2 play distinct or overlapping roles in priming VACV-specific CD8 T cells, wt, B7.1−/−, B7.2−/−, and B7.1/B7.2 double-deficient mice were analyzed. Seven days post-infection, irrespective of epitope specificity, the accumulation of VACV-specific effector CD8 T cells in B7.2−/−, and B7.1/B7.2 double-deficient mice was reduced by 80 to 90%, equivalent to that seen in CD28−/− mice (Fig. 3a for B8R, and not shown for other epitopes). Strikingly, VACV infection of B7.1−/− mice resulted in almost comparable CD8 T cell responses to wt mice, not only against B8R, but also against all subdominant epitopes examined. Similarly, treatment with a non-depleting anti-B7.1 blocking antibody did not impair VACV-specific CD8 T cells, whereas treatment with anti-B7.2 (or a combination of anti-B7.1 and anti-B7.2) abrogated generation of CD8 T cells to both dominant and subdominant epitopes (Fig. 3b and 3c). In some cases, reduced responses were apparent when blocking B7.1, but found to be not statistically significant. Thus, B7.2 plays a dominant role compared to B7.1 in the generation of VACV-specific effector CD8 T cells.
Figure 3. Differential requirement for B7.1 and B7.2 in primary VACV-specific CD8 T cell responses to both dominant and subdominant VACV epitopes.
(a) WT, CD28−/−, B7.1/B7.2-double deficient (B7.1.2−/−), B7.1−/−, or B7.2−/− mice were infected i.p with VACV-WR (2 × 105 PFU/mouse). Seven days post-infection splenocytes were harvested and stained for CD8, CD44, and B8R-tetramer. Left: Representative plots of tetramer staining, gating on CD8 cells. Percentages of activated B8R-tetramer positive CD8 T cells (CD8+CD44+B8R+) are indicated. Right: Total numbers of B8R-tetramer positive CD8+CD44+ T cells per spleen. (b). WT mice were infected as (a). Groups of mice were injected i.p on days 0, 1, 2, and 3, with 150 µg of either control IgG or non-depleting anti-B7.1, anti-B7.2, or anti-B7.1 plus B7.2 blocking antibodies in PBS. Groups of B7.1.2−/− mice were used as positive control. At day 7 post-infection splenocytes were harvested and stained for CD8, CD44, B8R-tetramer. Left: Representative plots of B8R-tetramer staining, gating on CD8 T cells. Right: Total number of CD8+CD44+B8R+ T cells per spleen is shown. (c) At day 7, splenocytes from wt and CD28−/− infected mice were stimulated with B8R, A3L, A3R, and A23R peptides for intracellular IFN-γ staining. Total numbers ± SEM of CD8+IFN-γ+ T cells per spleen from four individual mice are shown. *, p < 0.05 (WT vs knockout or blocking Ab treated groups). Similar results were obtained in 3 separate experiments.
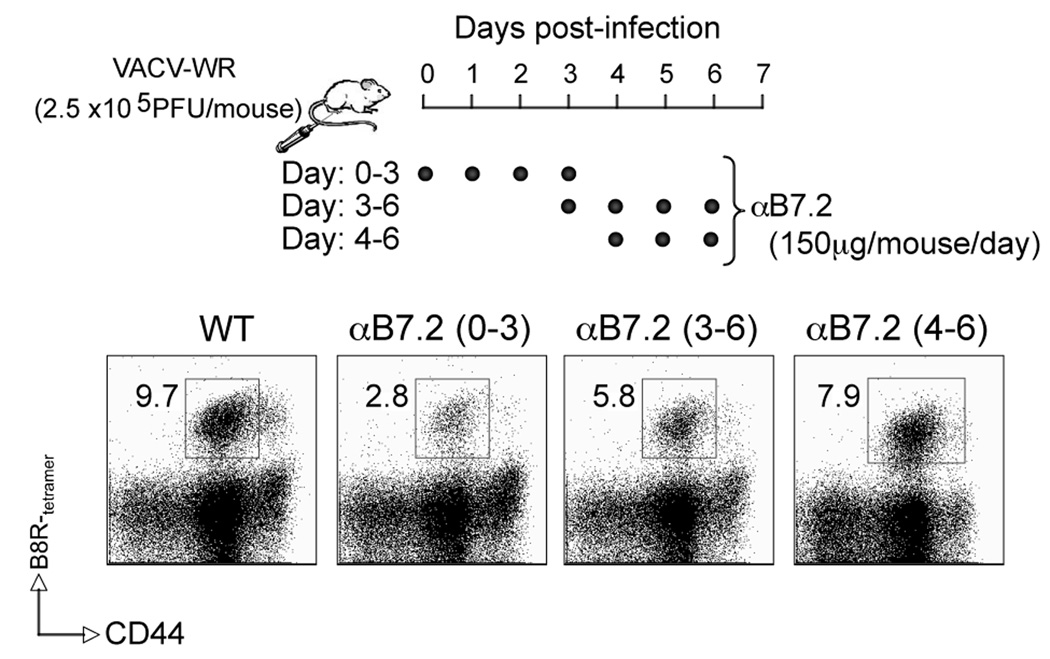
Next we performed kinetic blocking experiments where mice received anti-B7.2 blocking antibody on four consecutive days starting on day 0, 3 or 4 days after the initial infection (Fig. 4). Although delaying anti-B7.2 treatment by 3 or 4 days reduced the response by 40% and 20% respectively, the most dramatic effect was seen when CD28/B7.2 interactions were inhibited at the time of initial encounter with antigen, suggesting that B7.2 largely plays an early role during the primary CD8 T cell response to VACV.
Figure 4. B7.2 plays an early role during the primary CD8 T cell response to VACV.
Wild type mice were infected as in figure 3. Groups of mice were injected i.p on days (0–3), (3–6), or (4–6), with 150 µg of either control IgG or anti-B7.2 blocking antibody in PBS as indicated. At day 7 post-infection splenocytes were harvested and stained for CD8, CD44, B8R-tetramer. Representative plots of B8R-tetramer staining, gating on CD8 T cells is shown. Percentages of B8R-tetramer positive CD8 T cells (CD8+CD44+B8R+) are indicated. Similar results were obtained in 2 separate experiments.
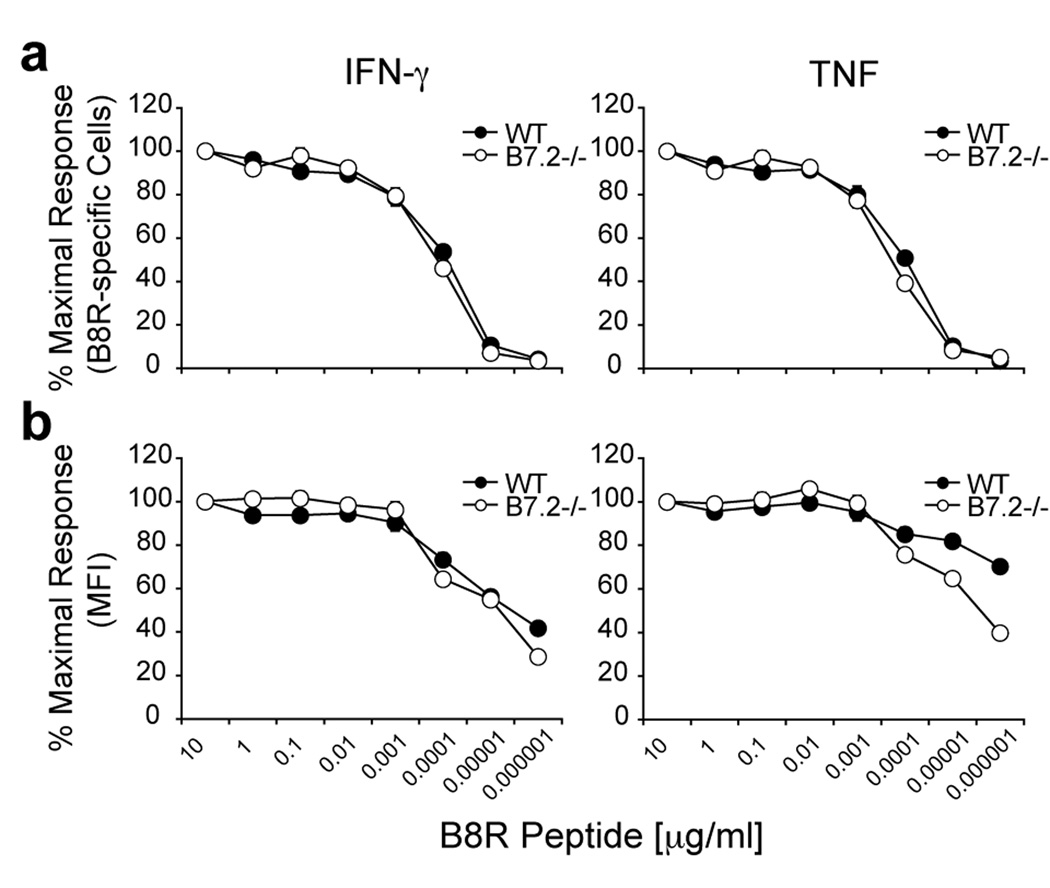
T cell avidity was also assessed by intracellular IFN-γ and TNF staining of freshly isolated splenocytes from VACV-WR infected wild-type and B7.2−/− mice. Eight days post-infection, CD8 T cells were stimulated for 6 hrs with graded concentrations of B8R peptide, and cytokine producing cells were analyzed by intracellular cytokine staining. As shown in Fig. 5a the percentages of IFN-γ or TNF producing B8R-specific cells were almost identical between wt and B7.2−/− over a wide range of peptide concentrations. However, when the amount of TNF production on a per cell basis was analyzed (as measured by mean fluorescent intensity; MFI) CD8 T cells from wt mice were much more (1.5-fold) sensitive to a lower concentration of peptide than CD8 T cells from B7.2 −/− mice (Fig. 5b).
Figure 5. Differential reactivity of B8R-specific CD8 T cells in VACV-WR infected B7.2-deficient and wild-type mice.
Groups of C57BL/6 wild type or B7.2-deficient (B7.2 −/−) mice were infected i.p with VACV-WR (2 × 105 PFU/mouse). Eight days post-infection splenocytes were harvested and stimulated for 6 h with graded concentrations of B8R peptide as indicated. (a) CD8 T cell reactivity was assessed by intracellular IFN-γ and TNF staining or (b) mean fluorescent intensity (MFI) on a per cell basis. The percentages or MFI of cytokine positive B8R-specific CD8 T cells were plotted against the peptide concentration used to stimulate the cells. Results are mean number ± SEM (n=4 mice/group) from one experiment. *, p < 0.05 (wt mice vs knockout) as determined by Student’s t test.
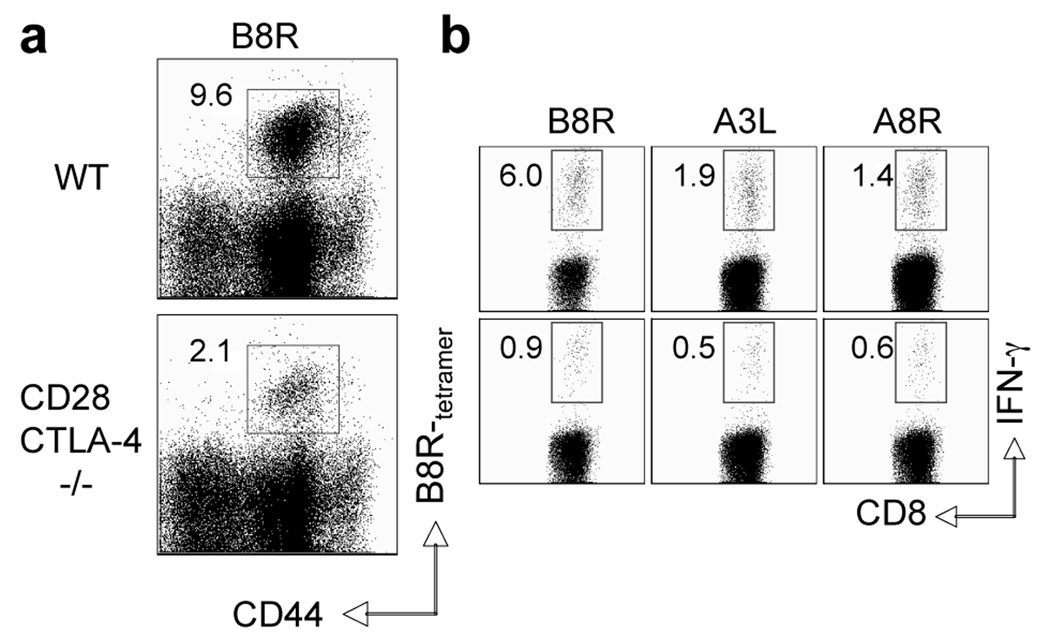
Following activation, T cells upregulate surface expression of CTLA-4, a homologue of CD28 that binds the same B7 ligands and serves as a negative regulator of T cell activation (3). CTLA-4 may function either by direct competition with CD28 for B7.1/B7.2 binding or through intracellular signaling (3, 30). Therefore, one possibility is that in the absence of B7.2, B7.1-CTLA-4 interactions predominate leading to impaired CD8 T cell responses caused not by lack of costimulation but enhanced coinhibition. In this respect B7.1 has superior affinity and avidity for CTLA-4, whereas B7.2 has relatively weak affinity (30). To address, wt and CD28/CTLA-4 double-deficient mice were infected with VACV-WR and CD8 T cell priming assessed (Fig. 6). CD28/CTLA-4−/− mice showed a pronounced defect in expansion and cytokine production of VACV-specific CD8 T cells, directed against the immunodominant (B8R) and subdominant epitopes, similar to CD28−/− mice.
Figure 6. Impaired generation of effector CD8 T cells to both dominant and subdominant VACV epitopes in CD28/CTLA-4-deficient mice.
Groups of C57BL/6 wild type or CD28/CTLA-4-deficient (CD28/CTLA-4−/−) mice were infected i.p with VACV-WR (2 × 105 PFU/mouse). Seven days post-infection splenocytes were harvested and stained for CD8, CD44, B8R-tetramer. Representative plots of tetramer staining, gating on CD8 cells. Percentages of activated B8R-tetramer positive CD8 T cells (CD8+CD44+B8R+) are indicated. (b) At day 7, splenocytes were stimulated with VACV peptides as indicated and CD8 T cell priming assessed by intracellular IFN-γ staining. Representative plots of IFN-γ staining in gated CD8 T cells. Percent positive indicated. Results are mean number ± SEM (n=4 mice/group) from one experiment. *, p < 0.05 (wt mice vs knockout) as determined by Student’s t test. Similar results were obtained in 2 separate experiments.
B7.2 drives VACV-specific CD8 T cell responses independent of CD4 T cell help
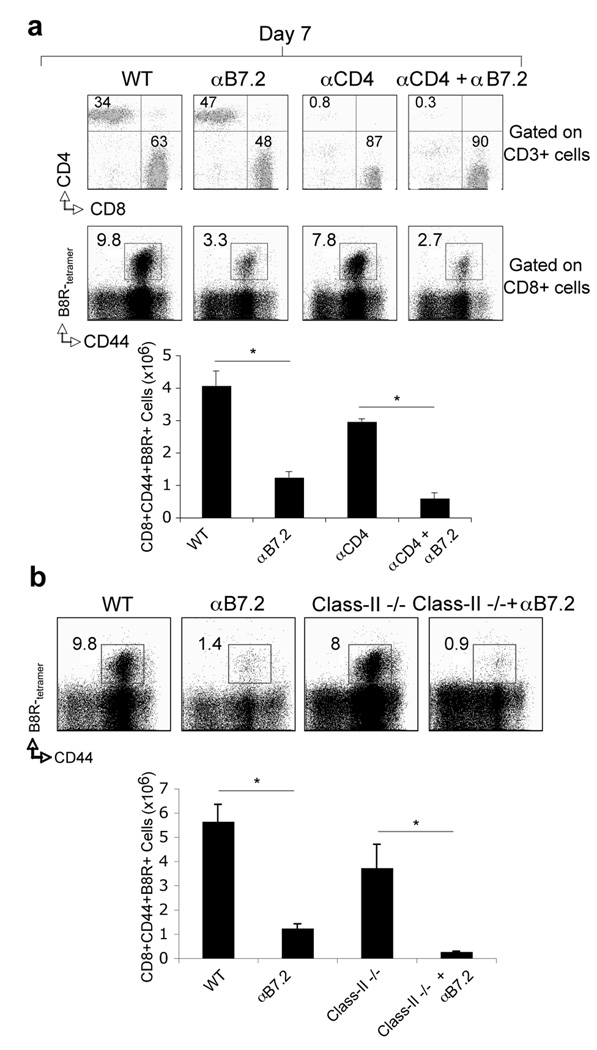
The experiments described thus far indicated an important role for B7.2 costimulation in the generation of VACV-specific effector CD8 T cells. However, this requirement could have been due to regulating CD4 T cells. Earlier work has shown that MHC class II-deficient mice, which are devoid of CD4 T cell responses, can mount strong primary CD8 T cell responses to VACV (33). To determine the role of B7.2 in the CD4 T cell-independent generation of CD8 T cells, wt mice depleted of CD4+ cells (Fig. 7a) or MHC class II-deficient mice (Fig. 7b) were treated with anti-B7.2 and infected with VACV-WR. In both hosts, treatment with anti-B7.2 abrogated VACV-specific CD8 T cell responses. These results suggest that B7.2 did not act indirectly through CD4 T cells and most likely B7.2 had a direct effect in ligating CD28 on CD8 T cells.
Figure 7. B7.2 controls VACV-specific CD8 T cell responses independent of CD4 T cells.
(a) Wt or (b) MHC-Class II deficient (Class-II−/−) mice were infected i.p with VACV-WR (2 × 105 PFU/mouse). (a) As indicated, groups of WT mice were depleted of CD4 (αCD4) T cells prior to VACV infection. Wild-type (a and b), CD4-depleted (a) or Class-II−/− (b) mice were injected i.p on days 0, 1, 2, and 3, with 150 µg of either control IgG or anti-B7.2 in PBS. Seven days post-infection splenocytes were harvested and stained for CD3, CD4, CD8 (a; top panel) or CD8, CD44, B8R-tetramer (a; bottom panel and b). (a) Top: Representative plots of CD4 and CD8 staining, gating on CD3 cells. Percentages of CD3+CD4+ and CD3+CD8+ T cells are indicated. Bottom: Representative plots of tetramer staining, gating on CD8 cells. Percentages of activated B8R-tetramer positive CD8 T cells (CD8+CD44+B8R+) are indicated. Total numbers of B8R-tetramer positive CD8+CD44+ T cells per spleen are shown. Results are mean number ± SEM (n=4 mice/group) from one experiment. *, p < 0.05 (wt mice vs knockout or anti-B7.2 treated) as determined by Student’s t test. Similar results were obtained in 2 separate experiments.
Defective priming of CD28-deficient CD8 T cells to VACV
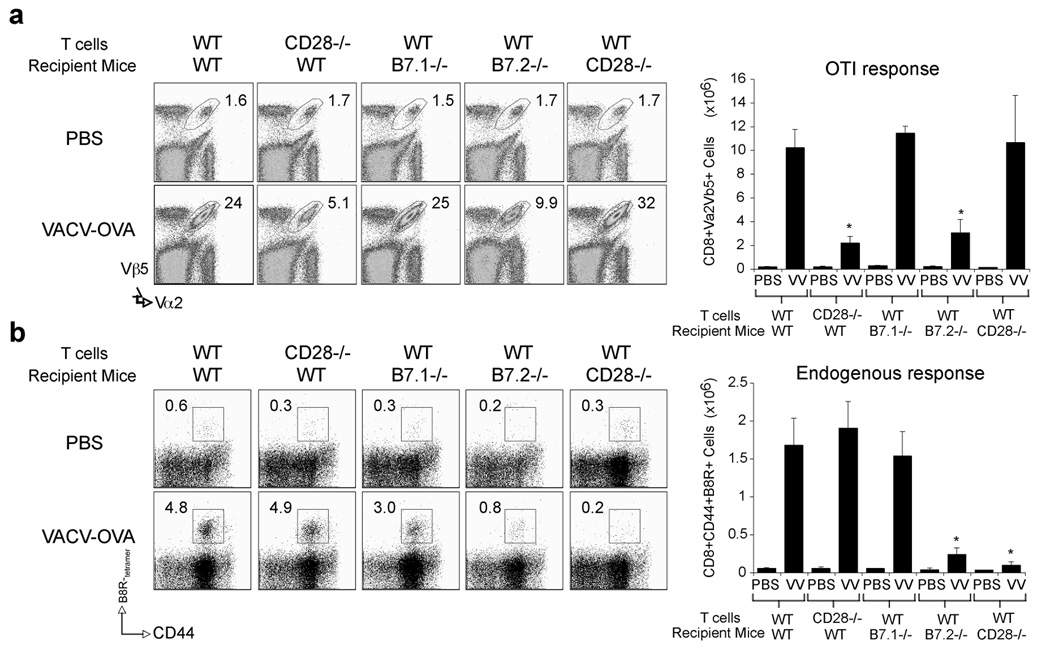
Next we sought to show whether CD8 cells responding to VACV infection directly require CD28/B7.2 interactions. OVA-specific wt and CD28-deficient CD8 T cells from OT-I and CD28-deficient OT-I TCR transgenic mice were transferred into naïve wt recipients subsequently infected with recombinant VACV expressing the full length OVA protein (VACV-OVA). Strong expansion of wt OVA-specific CD8 T cells was observed, similar to endogenous VACV-specific CD8 cells. In contrast, CD28-deficient CD8 T cells poorly expanded to VACV-OVA (Fig. 8a). This closely mimicked the data with VACV-specific CD8 T cell responses in CD28−/− mice infected with VACV-WR (Fig. 1). Similarly, expansion of wt OVA-specific CD8 cells was severely impaired in B7.2−/− but not B7.1−/− mice infected with VACV-OVA. To exclude that CD28 expressed on a non-T cell population contributed to the defect observed in CD28−/− mice, we performed the reverse experiment. VACV-OVA induced strong expansion of wt OVA-reactive CD8 T cells regardless of whether they were transferred into CD28−/− or wt mice (Fig. 8a). Consistent with these data, analysis of endogenous B8R-specific CD8 T cells revealed an intact response when wt or CD28−/− CD8 T cells were adoptively transferred to wt or B7.1−/− mice whereas responses in B7.2−/− and CD28−/− mice where significantly reduced (Fig. 8b). Thus, interaction of CD28 expressed on a CD8 T cell with B7.2 is required for formation of large populations of effector CD8 T cells during infection with VACV.
Figure 8. CD28 is required directly by CD8 T cells responding to VACV.
Naive WT or CD28−/− OT-I CD8 T cells were adoptively transferred into naïve WT B6, or B7.1−/−, B7.2−/−, or CD28−/− mice. One day later, mice were infected i.p. with recombinant VACV expressing full-length OVA (VACV-OVA; 2 × 106 PFU/mouse) or PBS as indicated. (a) After 8 days, OT-I CD8 T cell expansion was analyzed by tracking the transgenic TCR. Dot plots: Representative co-staining for Vα2 and Vβ5 after gating on CD8 cells. Percent positive indicated. Right: Total numbers of CD8+Vα2+Vβ5+ cells per spleen. (b) Endogenous B8R-specific CD8 response after OT-I cell transfer. Naive WT or CD28−/− OT-I CD8 T cells were adoptively transferred into naïve WT B6, or B7.1−/−, B7.2−/−, or CD28−/− mice and infected with VACV-OVA as in (a). Seven days post-infection splenocytes were harvested and stained for CD8, CD44, B8R-tetramer. Left: Representative plots of tetramer staining, gating on CD8 cells. Percentages of activated B8R-tetramer positive CD8 T cells (CD8+CD44+B8R+) are indicated. Right: Total numbers of B8R-tetramer positive CD8+CD44+ T cells per spleen. Results are mean number ± SEM (n=4 mice/group) from one experiment. *, p < 0.05 (wt mice vs knockout) as determined by Student’s t test. Similar results were obtained in 1 additional experiment.
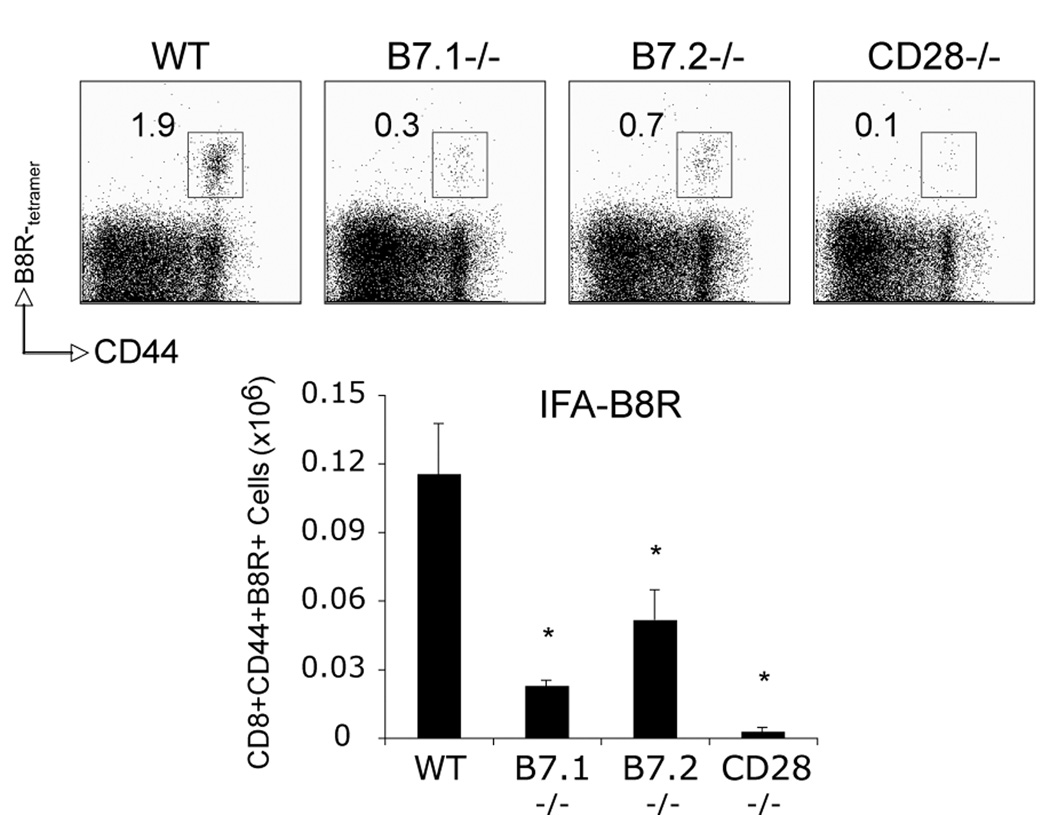
B7.1 and B7.2 are both required for B8R-specific CD8 T cell responses after peptide immunization in IFA
To assess whether the differential requirement for B7.2 vs. B7.1 was related to VACV-WR infection or a more general phenomenon, groups of wt, CD28−/−, B7.1−/−, and B7.2−/− mice were immunized with B8R-peptide in IFA. Consistent with VACV-WR infection, B8R-specific CD8 T cell responses were severely impaired in the absence of CD28 (Fig. 9). However, both B7.1 and B7.2 were now required for optimal expansion of B8R-specific CD8 T cells. This implied that the differential usage of B7.2 vs. B7.1 during VACV infection might be due to differences in the availability of these two molecules, which could be a direct or indirect consequence of virulence mechanisms possessed by VACV-WR.
Figure 9. B7.1 and B7.2 are both required for B8R-specific CD8 T cell responses after peptide immunization in IFA.
WT, CD28−/−, B7.1−/−, or B7.2−/− mice were immunized s.c. with B8R-peptide (10 µg/mouse) in IFA. On day seven splenocytes were harvested and stained for CD8, CD44, B8R-tetramer. Top: Representative plots of tetramer staining, gating on CD8 cells. Percentages of activated B8R-tetramer positive CD8 T cells (CD8+CD44+B8R+) are indicated. Bottom: Total numbers of B8R-tetramer positive CD8+CD44+ T cells per spleen. Results are mean number ± SEM (n=4 mice/group) from one experiment. *, p < 0.05 (wt mice vs knockout) as determined by Student’s t test. Similar results were obtained in 1 additional experiment.
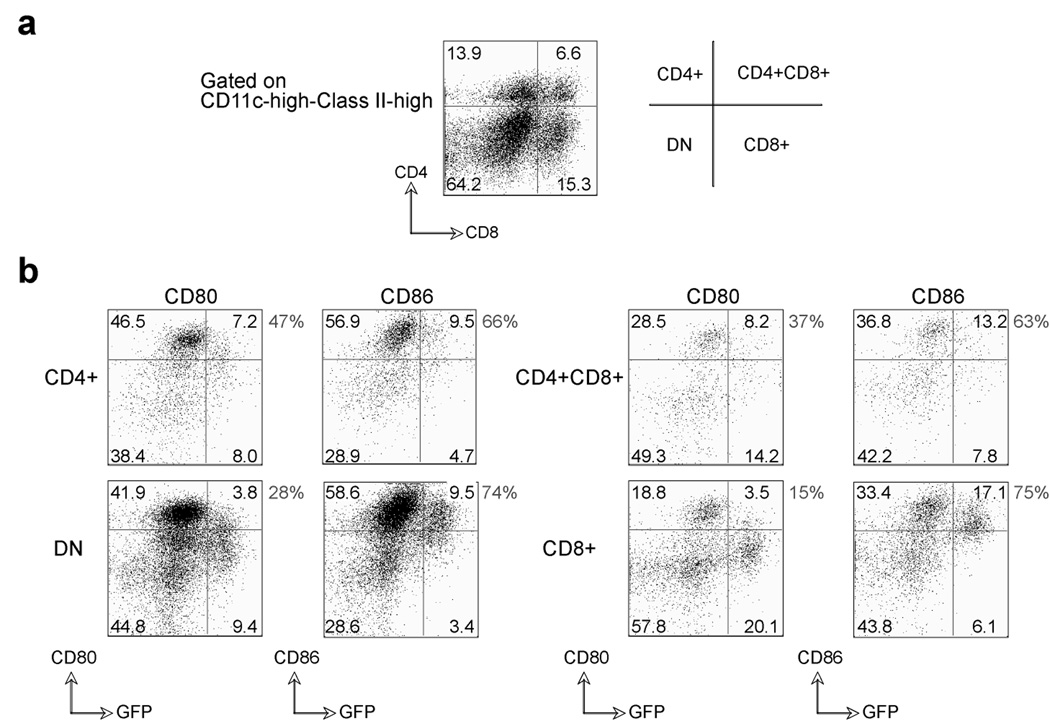
VACV-WR infection results in selective up-regulation of B7.2 on infected CD8+ DC subsets
From in vivo studies, CD8α+ dendritic cells are the major subset involved in priming of VACV-specific CD8 T cells (40). Recombinant VACV-WR expressing GFP was used to infect in vitro a DC population containing various subsets isolated from the spleen (Fig. 10a). The expression of B7.1 and B7.2 was subsequently assessed on infected (GFP+) and uninfected (GFP-) cells (Fig. 10b). Unfortunately, we could not perform this experiment in vivo due to the instability of GFP, and/or some active process occurring in vivo that results in loss of GFP expression within 9–12 hrs. Both B7.1 and B7.2 were upregulated on all uninfected DC subsets in response to VACV. CD4+GFP- and CD4-CD8α-GFP- (double negative; DN) DC were the major subsets that were positive for both ligands. In contrast, B7.2 was predominantly expressed by CD8α+GFP+ DC, while B7.1 was minimally induced on this population after VACV infection. These results indicate that VACV selectively leads to upregulation of B7.2 expression on infected CD8α+ DC, which likely accounts for the primary use of B7.2 to costimulate CD28 and to promote optimal generation of VACV-specific effector CD8 T cells.
Figure 10. Differential expression of B7.1 and B7.2 on VACV-infected dendritic cell subsets.
Total splenocytes from BL6 mice were infected in vitro with VACV-GFP (MOI=10) for 24 hrs. (a) Representative plots of CD4 and CD8 staining, gating on CD11c-high/MHC Class-II-high cells. Percentages of DC subsets (CD4+, CD8α+, CD4+CD8α+, and double negative (DN)) in the spleen are indicated. (b) Upregulation of B7.1 and B7.2 was assessed on GFP positive and GFP negative DC subsets (CD11c-high, MHC Class-II-high). Percentages of DC subsets (CD4+, CD8α+, CD4+CD8α+, and double negative (DN)) positive for B7.1 and B7.2 are indicated within the plots, and percentages of GFP+ DC expressing B7.1 vs. B7.2 are shown outside the plots.
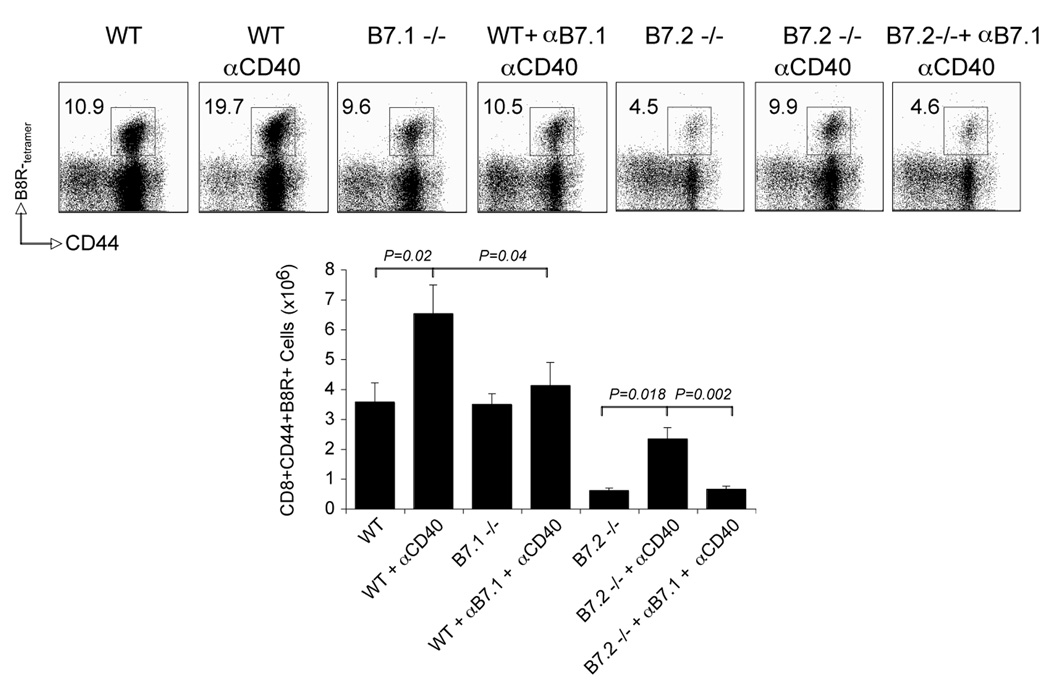
Lastly, we assessed whether B7.1 could become relevant to further act as an additional CD28 ligand to promote effector generation during VACV infection, and if the absence of B7.1 might limit the VACV T cell response. Wt and B7−/− mice were infected with VACV-WR and injected with an agonist antibody to CD40 that has previously been shown to induce upregulation of B7.1 on DC (Fig. 11). Anti-CD40 enhanced CD8 priming to VACV-WR with 150–200% more B8R-specific cells detected, which was fully dependent on B7.1. Interestingly, the defective expansion of B8R-reactive cells in B7.2−/− mice was largely restored to wt levels by anti-CD40 treatment, which was again completely dependent on B7.1. These results demonstrate that B7.1 can be active, and hence expressed, in the context of a VACV infection.
Figure 11. B7.1 contributes to anti-CD40 induced VACV-specific CD8 T cell responses.
(a) WT, B7.1−/−, and B7.2−/− mice were infected i.p with VACV-WR (2 × 105 PFU/mouse). Seven days post-infection splenocytes were harvested and stained for CD8, CD44, B8R-tetramer. As indicated, groups of WT and B7.2−/− mice were treated with agonistic CD40 mAb (αCD40) at the time of VACV infection and were injected i.p on days 0, 1, 2, and 3, with 150 µg of either control IgG or blocking anti-B7.1 in PBS. Top: Representative plots of tetramer staining, gating on CD8+ cells. Percentages of activated B8R-tetramer positive CD8 T cells (CD8+CD44+B8R+) are indicated. Bottom: Total numbers of B8R-tetramer positive CD8+CD44+ T cells per spleen are shown. Results are mean number ± SEM (n=4 mice/group) from one experiment. Statistical significance was determined by Student’s t test. Similar results were obtained in 2 separate experiments.
Discussion
A major gap in our understanding of the CD28/CTLA-4 system in anti-viral immunity has been the role played by their natural ligands, B7.1 and B7.2. With VACV, our results highlight a previously unappreciated dominant role for B7.2 in initial anti-viral CD8 T cell responses. We show that B7.2 is selectively induced on virus-infected CD8α+ DCs and can strongly influence the response of CD8 T cells to VACV.
The effect of a CD28 deficiency on CD8 T cell responses to VACV infection has been studied recently by two groups. CD28-deficient mice infected i.p. (35) or i.n. (36) generated a weak effector CD8 T cell response directed against the immunodominant B8R epitope, while the frequency of memory CD8 T cells was not affected. Our studies extend these previous reports by showing that the generation of primary VACV-specific CD8 T cell responses against subdominant epitopes also requires CD28 signaling. Several early studies showed that primary anti-LCMV CD8 T cell responses are unchanged in the absence of CD28, while primary anti-VSV, HSV, and influenza responses are profoundly decreased. The prolonged and systemic replication of LCMV, compared to the brief duration of VSV infection and localized infections with HSV and influenza virus, could lower the dependency on a CD28 costimulatory signal, perhaps by promoting a stronger and/or more sustained signal through the TCR. Alternatively, the CD28 independence to LCMV might be related to excess production of an inflammatory cytokine that mimicked and hence replaced the need for the CD28 signal. Based on these and other data (5, 23), it was proposed that the requirement for CD28 costimulation by anti-viral CD8 T cells correlates inversely with viral replication and the strength of TCR signaling. Unlike VSV and influenza virus, VACV-WR replicates to high titers in vivo and can be found readily for extended periods of time in multiple tissues after either i.p or i.n. infection. Therefore, large amounts of antigen should be available for sustained activation of CD8 T cells. However, our own experiments and those of others (35, 36) have clearly shown impaired CD8 T cell responses in the absence of CD28, indicating that factors other than viral abundance and availability of antigen might influence the dependence on CD28. Consistent with the VACV data, CD28 costimulation was recently shown to be required for CD8 T cell mediated resistance to disease and death caused by ectromelia virus, the agent of mouse pox, that can also replicate widely and cause strong inflammation (35). Together, these results argue against the idea that anti-viral CD8 T cell responses may require CD28 signals only for those viruses that replicate abortively or induce localized infections in the host. Importantly, they demonstrate that a CD28-dependence by anti-viral CD8 T cells may be a more general phenomenon that applies to a variety of viruses and that the relatively CD28-independent activation of LCMV-specific CD8 T cells may represent an atypical situation.
An important observation in our study is that CD28 interactions with B7.2 and not B7.1 are essential for early CD8 T cell responses following VACV infection. Some published studies in simple model systems have suggested that B7.1 can provide a stronger costimulus than B7.2 for T cell activation (41–44), as measured by proliferation, cytokine production, and cytolytic activity, while others have reported that these molecules appear to be functionally equivalent and play overlapping roles in many experimental situations (45–50). In the context of viral infection, deficiency in either B7.1 or B7.2 alone does not inhibit any parameters associated with the CD8 immune response to VSV (51), HSV (7), MHV-68 (26, 27), and MCMV (Arens and Schoenberger, unpublished), whereas responses in mice lacking both B7 ligands are impaired. These studies demonstrate that B7.1 and B7.2 have critical, overlapping functions in CD8 T cell responses to these viruses. Similarly, treatment with either anti-B7.1 or anti-B7.2 alone has been shown to not significantly inhibit CTL response to exogenous OVA, while a combination of both antibodies completely abolished the response (52). With VACV, our results highlight a quite novel dominant role for B7.2, which contrasts significantly with these prior studies. Reagents that specifically block B7.1 and B7.2, and adoptive transfer of wt and CD28-deficient CD8 TCR transgenic T cells responding to antigen in B7-deficient backgrounds, fully supported a direct action of B7.2 in ligating CD28 expressed by CD8 T cells. These findings show that the use and requirement for B7.1 and B7.2 during anti-viral CD8 T cell responses can vary with different viruses.
Multiple factors might contribute to the differential requirement for B7.1 and B7.2. One possibility was that B7.1 might bind to PD-L1 or CTLA-4 and transmit inhibitory signals, and therefore it would not be available to positively interact with CD28. However, we found CTLA-4 was not involved in the response, and it is highly unlikely that the interaction between PD-L1 and CD80 can explain the impaired CD8 T cell responses seen in CD28 or B7.2-deficient mice. CD8 T cells in B7.1-deficient mice infected with VACV did not show any signs of hyperactivation or enhanced proliferation that would correlate with an inhibitory interaction, and wt CD8 T cells adoptively transferred to B7.1−/− mice expanded similarly to T cells transferred to wt mice. Lastly, treatment with anti-CD40 promoted enhanced CD8 T cell responses that could be completely blocked with anti-B7.1 blocking antibody. Thus, we believe that the relative expression of B7.1 vs. B7.2 dictated their use, rather than differential binding to alternate ligands. Indeed, B7.1 and B7.2 have been reported to be differentially regulated (29, 30). B7.2 is constitutively expressed by APC and can be further up-regulated after activation, whereas B7.1 is expressed only after activation of these cells. Conceivably, the relative expression of either molecule might be dependent on the viral load and/or the inflammatory environment induced by the virus. Alternatively, viruses like VACV-WR might have evolved strategies to actively down-modulate or inhibit the up-regulation of the B7.1 molecule. Potentially in line with some of these ideas is the finding that both B7.1 and B7.2 were required for optimal expansion of B8R-specific CD8 T cells in response to peptide immunization in IFA, an adjuvant known to strongly upregulate both.
Collectively, this supports the notion that the preferential usage of B7.2 vs. B7.1 by VACV-specific CD8 T cells is most likely due to differences in the availability of the two molecules. Consistent with this, we showed that while either ligand were expressed on uninfected DC subsets after in vitro VACV exposure, B7.2 was selectively up-regulated on CD8α+ DC, the main subset involved in priming of VACV-specific CD8 T cells in vivo (40). Whether virulence mechanisms possessed by VACV directly or indirectly modulate B7.1 expression on DC requires further investigation. Our results with injection of anti-CD40 demonstrate that B7.1 can be active, and hence expressed, in the context of a VACV infection. But they do not address whether the normal lack of use of B7.1 is simply due to the absence of a factor that is required for its expression, or due to active viral modulation suppressing this molecule that was simply overcome by the unphysiological level of CD40 signals provided by the antibody. There is, however, precedence for viral control of B7 family members on APC. Measles virus inhibits CD40L-dependent maturation of DC, thereby inhibiting the up-regulation of B7.1 and B7.2 (53). Impaired expression of B7.1 and B7.2 has been demonstrated upon infection with Kaposi’s sarcoma-associated herpesvirus (KSHV) (54), EBV (55), LCMV (56), VSV (57), CMV (58, 59), and HIV-1 (60, 61). Therefore, given that VACV-WR possesses many immune evasion genes, it is highly plausible that it possesses genes that prevent B7.1 from being expressed efficiently. As shown by the experiment where CD8 T cell accumulation was enhanced by anti-CD40 in a manner fully dependent on B7.1, VACV targeting of B7.1 could be a mechanism to try to limit the magnitude of the T cell response.
In conclusion, our observations show that B7.2 is the primary CD28 ligand involved in the induction of virus-specific CD8 T cell responses to VACV-WR. In this regard, it is becoming increasing apparent that no two viruses are identical, and their regulation and molecular control might vary strongly, dictated by TLR usage, inflammatory cytokine induction, as well as virulence mechanisms and immune evasion strategies that can differ significantly even within members of the same genus. To date, a number of VACV strains have been isolated, including Lister, and the New York City Board of Heath strain (NYCBOH) used as a smallpox vaccine (produced by Wyeth as Dryvax). VACV-WR encodes a number of genes that allow enhanced viral replication over time, including B18R, an IFN-I-binding protein. In contrast, VACV-Lister does not possess this gene, and VACV-NYCBOH/Wyeth produces a truncated B18R gene product that is inactive. Other differences include but are not limited to an activity targeting the IL-1β-converting enzyme encoded by B13R that is found in WR, but produced as a truncated protein in NYCBOH/Wyeth, and not present in Lister. Although, it is well established that deletion of these genes can alter pathogenesis and the period of viral replication, direct comparisons between VACV variants have not been extensive with regard to how they elicit adaptive immunity. If alternate molecular control mechanisms are evident, this is important in terms of vaccination strategies where effective immunization might depend on efficiently promoting multiple arms of the immune response. Furthermore, given this type of potential variation within any one virus strain, this additionally highlights the importance of not assuming that all viruses will behave identically, both in terms of the magnitude of the adaptive immune response as well as the molecular control of virus-specific CD8 T cells. Thus, future studies should address whether virus-specific CD8 T cells show a similar dependence on B7.2 during infection with other VACV strains that display differential virulence; whether the CD8 response to other poxviruses that express multiple immune evasion genes are also controlled by B7.2; and if the extent of plasticity and redundancy in use of costimulatory receptors is influenced by the virus strain and range of viral immune evasion strategies.
Acknowledgments
This work was supported by NIH grants AI77079 to S.S.-A, AI67341 to M.C., CA081261 and AI076972 to S.S., and a Veni grant (916.56.155) from The Netherlands Organization for Scientific Research to R.A. This is publication #1056 from the La Jolla Institute for Allergy and Immunology.
Contributor Information
Shahram Salek-Ardakani, La Jolla Institute for Allergy and Immunology, Divisions of Molecular Immunology, La Jolla, CA 92037.
Ramon Arens, Developmental Immunology, La Jolla, CA 92037.
Rachel Flynn, La Jolla Institute for Allergy and Immunology, Divisions of Molecular Immunology, La Jolla, CA 92037.
Alessandro Sette, Vaccine Discovery, La Jolla, CA 92037.
Stephen P. Schoenberger, Developmental Immunology, La Jolla, CA 92037.
Michael Croft, La Jolla Institute for Allergy and Immunology, Divisions of Molecular Immunology, La Jolla, CA 92037.
References
- 1.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 3.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 4.Salek-Ardakani S, Croft M. Regulation of CD4 T cell memory by OX40 (CD134) Vaccine. 2006;24:872–883. doi: 10.1016/j.vaccine.2005.07.108. [DOI] [PubMed] [Google Scholar]
- 5.Christensen JE, Christensen JP, Kristensen NN, Hansen NJ, Stryhn A, Thomsen AR. Role of CD28 co-stimulation in generation and maintenance of virus-specific T cells. Int Immunol. 2002;14:701–711. doi: 10.1093/intimm/dxf037. [DOI] [PubMed] [Google Scholar]
- 6.Andreasen SO, Christensen JE, Marker O, Thomsen AR. Role of CD40 ligand and CD28 in induction and maintenance of antiviral CD8+ effector T cell responses. J Immunol. 2000;164:3689–3697. doi: 10.4049/jimmunol.164.7.3689. [DOI] [PubMed] [Google Scholar]
- 7.Edelmann KH, Wilson CB. Role of CD28/CD80–86 and CD40/CD154 costimulatory interactions in host defense to primary herpes simplex virus infection. J Virol. 2001;75:612–621. doi: 10.1128/JVI.75.2.612-621.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertram EM, Tafuri A, Shahinian A, Chan VS, Hunziker L, Recher M, Ohashi PS, Mak TW, Watts TH. Role of ICOS versus CD28 in antiviral immunity. Eur J Immunol. 2002;32:3376–3385. doi: 10.1002/1521-4141(200212)32:12<3376::AID-IMMU3376>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, Maile R, Greenwood R, Collins EJ, Frelinger JA. Naive CD8+ T cells do not require costimulation for proliferation and differentiation into cytotoxic effector cells. J Immunol. 2000;164:1216–1222. doi: 10.4049/jimmunol.164.3.1216. [DOI] [PubMed] [Google Scholar]
- 10.Pardigon N, Bercovici N, Calbo S, Santos-Lima EC, Liblau R, Kourilsky P, Abastado JP. Role of co-stimulation in CD8+ T cell activation. Int Immunol. 1998;10:619–630. doi: 10.1093/intimm/10.5.619. [DOI] [PubMed] [Google Scholar]
- 11.Motta I, Lone YC, Kourilsky P. In vitro induction of naive cytotoxic T lymphocytes with complexes of peptide and recombinant MHC class I molecules coated onto beads: role of TCR/ligand density. Eur J Immunol. 1998;28:3685–3695. doi: 10.1002/(SICI)1521-4141(199811)28:11<3685::AID-IMMU3685>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein JS, Chen T, Brunswick M, Mostowsky H, Kozlowski S. Purified MHC class I and peptide complexes activate naive CD8+ T cells independently of the CD28/B7 and LFA-1/ICAM-1 costimulatory interactions. J Immunol. 1998;160:3180–3187. [PubMed] [Google Scholar]
- 13.Sepulveda H, Cerwenka A, Morgan T, Dutton RW. CD28, IL-2-independent costimulatory pathways for CD8 T lymphocyte activation. J Immunol. 1999;163:1133–1142. [PubMed] [Google Scholar]
- 14.Wen T, Kono K, Shahinian A, Kiessling R, Mak TW, Klein G. CD28 is not required for rejection of unmanipulated syngeneic and autologous tumors. Eur J Immunol. 1997;27:1988–1993. doi: 10.1002/eji.1830270824. [DOI] [PubMed] [Google Scholar]
- 15.Kawai K, Shahinian A, Mak TW, Ohashi PS. Skin allograft rejection in CD28-deficient mice. Transplantation. 1996;61:352–355. doi: 10.1097/00007890-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 16.Cai Z, Sprent J. Influence of antigen dose and costimulation on the primary response of CD8+ T cells in vitro. J Exp Med. 1996;183:2247–2257. doi: 10.1084/jem.183.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krummel MF, Heath WR, Allison J. Differential coupling of second signals for cytotoxicity and proliferation in CD8+ T cell effectors: amplification of the lytic potential by B7. J Immunol. 1999;163:2999–3006. [PubMed] [Google Scholar]
- 18.Chai JG, Vendetti S, Bartok I, Schoendorf D, Takacs K, Elliott J, Lechler R, Dyson J. Critical role of costimulation in the activation of naive antigen-specific TCR transgenic CD8+ T cells in vitro. J Immunol. 1999;163:1298–1305. [PubMed] [Google Scholar]
- 19.Guerder S, Carding SR, Flavell RA. B7 costimulation is necessary for the activation of the lytic function in cytotoxic T lymphocyte precursors. J Immunol. 1995;155:5167–5174. [PubMed] [Google Scholar]
- 20.McAdam AJ, Gewurz BE, Farkash EA, Sharpe AH. Either B7 costimulation or IL-2 can elicit generation of primary alloreactive CTL. J Immunol. 2000;165:3088–3093. doi: 10.4049/jimmunol.165.6.3088. [DOI] [PubMed] [Google Scholar]
- 21.Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- 22.Bachmann MF, McKall-Faienza K, Schmits R, Bouchard D, Beach J, Speiser DE, Mak TW, Ohashi PS. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 23.Kundig TM, Shahinian A, Kawai K, Mittrucker HW, Sebzda E, Bachmann MF, Mak TW, Ohashi PS. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Wenger RH, Zhao M, Nielsen PJ. Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes. J Exp Med. 1997;185:251–262. doi: 10.1084/jem.185.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyon AB, Sarawar SR. Differential requirement for CD28 and CD80/86 pathways of costimulation in the long-term control of murine gammaherpesvirus-68. Virology. 2006;356:50–56. doi: 10.1016/j.virol.2006.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuse S, Obar JJ, Bellfy S, Leung EK, Zhang W, Usherwood EJ. CD80 and CD86 control antiviral CD8+ T-cell function and immune surveillance of murine gammaherpesvirus 68. J Virol. 2006;80:9159–9170. doi: 10.1128/JVI.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BJ, Reiter SK, Anderson M, Sarawar SR. CD28(−/−) mice show defects in cellular and humoral immunity but are able to control infection with murine gammaherpesvirus 68. J Virol. 2002;76:3049–3053. doi: 10.1128/JVI.76.6.3049-3053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 30.Sansom DM, Manzotti CN, Zheng Y. What's the difference between CD80 and CD86? Trends Immunol. 2003;24:314–319. doi: 10.1016/s1471-4906(03)00111-x. [DOI] [PubMed] [Google Scholar]
- 31.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 32.Harrington LE, Most Rv R, Whitton JL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 34.Moutaftsi M, Peters B, Pasquetto V, Tscharke DC, Sidney J, Bui HH, Grey H, Sette A. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol. 2006;24:817–819. doi: 10.1038/nbt1215. [DOI] [PubMed] [Google Scholar]
- 35.Fang M, Sigal LJ. Direct CD28 costimulation is required for CD8+ T cell-mediated resistance to an acute viral disease in a natural host. J Immunol. 2006;177:8027–8036. doi: 10.4049/jimmunol.177.11.8027. [DOI] [PubMed] [Google Scholar]
- 36.Fuse S, Zhang W, Usherwood EJ. Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response. J Immunol. 2008;180:1148–1157. doi: 10.4049/jimmunol.180.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, Williams S, Sidney J, Sette A, Bennink JR, Yewdell JW. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, Hirst S, Villarreal L, Felgner PL, Crotty S. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol. 2005;79:11724–11733. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Differential regulation of Th2 and Th1 lung inflammatory responses by protein kinase C theta. J Immunol. 2004;173:6440–6447. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- 40.Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 41.Matulonis U, Dosiou C, Freeman G, Lamont C, Mauch P, Nadler LM, Griffin JD. B7-1 is superior to B7-2 costimulation in the induction and maintenance of T cell-mediated antileukemia immunity. Further evidence that B7-1 and B7-2 are functionally distinct. J Immunol. 1996;156:1126–1131. [PubMed] [Google Scholar]
- 42.Gajewski TF. B7-1 but not B7-2 efficiently costimulates CD8+ T lymphocytes in the P815 tumor system in vitro. J Immunol. 1996;156:465–472. [PubMed] [Google Scholar]
- 43.Gajewski TF, Fallarino F, Uyttenhove C, Boon T. Tumor rejection requires a CTLA4 ligand provided by the host or expressed on the tumor: superiority of B7-1 over B7-2 for active tumor immunization. J Immunol. 1996;156:2909–2917. [PubMed] [Google Scholar]
- 44.Fields PE, Finch RJ, Gray GS, Zollner R, Thomas JL, Sturmhoefel K, Lee K, Wolf S, Gajewski TF, Fitch FW. B7.1 is a quantitatively stronger costimulus than B7.2 in the activation of naive CD8+ TCR-transgenic T cells. J Immunol. 1998;161:5268–5275. [PubMed] [Google Scholar]
- 45.Lanier LL, O'Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K, Ito D, Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 46.Natesan M, Razi-Wolf Z, Reiser H. Costimulation of IL-4 production by murine B7-1 and B7-2 molecules. J Immunol. 1996;156:2783–2791. [PubMed] [Google Scholar]
- 47.Mark DA, Donovan CE, De Sanctis GT, He HZ, Cernadas M, Kobzik L, Perkins DL, Sharpe A, Finn PW. B7-1 (CD80) and B7-2 (CD86) have complementary roles in mediating allergic pulmonary inflammation and airway hyperresponsiveness. Am J Respir Cell Mol Biol. 2000;22:265–271. doi: 10.1165/ajrcmb.22.3.3747. [DOI] [PubMed] [Google Scholar]
- 48.Harris NL, Prout M, Peach RJ, Fazekas de St Groth B, Ronchese F. CD80 costimulation is required for Th2 cell cytokine production but not for antigen-specific accumulation and migration into the lung. J Immunol. 2001;166:4908–4914. doi: 10.4049/jimmunol.166.8.4908. [DOI] [PubMed] [Google Scholar]
- 49.Greenwald RJ, Lu P, Halvorson MJ, Zhou X, Chen S, Madden KB, Perrin PJ, Morris SC, Finkelman FD, Peach R, Linsley PS, Urban JF, Jr, Gause WC. Effects of blocking B7-1 and B7-2 interactions during a type 2 in vivo immune response. J Immunol. 1997;158:4088–4096. [PubMed] [Google Scholar]
- 50.Greenwald RJ, Urban JF, Ekkens MJ, Chen S, Nguyen D, Fang H, Finkelman FD, Sharpe AH, Gause WC. B7-2 is required for the progression but not the initiation of the type 2 immune response to a gastrointestinal nematode parasite. J Immunol. 1999;162:4133–4139. [PubMed] [Google Scholar]
- 51.McAdam AJ, Farkash EA, Gewurz BE, Sharpe AH. B7 costimulation is critical for antibody class switching and CD8(+) cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J Virol. 2000;74:203–208. doi: 10.1128/jvi.74.1.203-208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sigal LJ, Reiser H, Rock KL. The role of B7-1 and B7-2 costimulation for the generation of CTL responses in vivo. J Immunol. 1998;161:2740–2745. [PubMed] [Google Scholar]
- 53.Servet-Delprat C, Vidalain PO, Bausinger H, Manie S, Le Deist F, Azocar O, Hanau D, Fischer A, Rabourdin-Combe C. Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J Immunol. 2000;164:1753–1760. doi: 10.4049/jimmunol.164.4.1753. [DOI] [PubMed] [Google Scholar]
- 54.Coscoy L, Ganem D. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J Clin Invest. 2001;107:1599–1606. doi: 10.1172/JCI12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salek-Ardakani S, Arrand JR, Mackett M. Epstein-Barr virus encoded interleukin-10 inhibits HLA-class I, ICAM-1, and B7 expression on human monocytes: implications for immune evasion by EBV. Virology. 2002;304:342–351. doi: 10.1006/viro.2002.1716. [DOI] [PubMed] [Google Scholar]
- 56.Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrow G, Slobedman B, Cunningham AL, Abendroth A. Varicella-zoster virus productively infects mature dendritic cells and alters their immune function. J Virol. 2003;77:4950–4959. doi: 10.1128/JVI.77.8.4950-4959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mintern JD, Klemm EJ, Wagner M, Paquet ME, Napier MD, Kim YM, Koszinowski UH, Ploegh HL. Viral interference with B7-1 costimulation: a new role for murine cytomegalovirus fc receptor-1. J Immunol. 2006;177:8422–8431. doi: 10.4049/jimmunol.177.12.8422. [DOI] [PubMed] [Google Scholar]
- 59.Loewendorf A, Kruger C, Borst EM, Wagner M, Just U, Messerle M. Identification of a mouse cytomegalovirus gene selectively targeting CD86 expression on antigen-presenting cells. J Virol. 2004;78:13062–13071. doi: 10.1128/JVI.78.23.13062-13071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaudhry A, Das SR, Hussain A, Mayor S, George A, Bal V, Jameel S, Rath S. The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J Immunol. 2005;175:4566–4574. doi: 10.4049/jimmunol.175.7.4566. [DOI] [PubMed] [Google Scholar]
- 61.Majumder B, Janket ML, Schafer EA, Schaubert K, Huang XL, Kan-Mitchell J, Rinaldo CR, Jr, Ayyavoo V. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J Virol. 2005;79:7990–8003. doi: 10.1128/JVI.79.13.7990-8003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]