Summary
The ubiquitous application of selective oestrogen receptor modulators (SERMs) and aromatase inhibitors for the treatment and prevention of breast cancer has created a significant advance in patient care. However, the consequence of prolonged treatment with antihormonal therapy is the development of drug resistance. Nevertheless, the systematic description of models of drug resistance to SERMs and aromatase inhibitors has resulted in the discovery of a vulnerability in tumour homeostasis that can be exploited to improve patient care. Drug resistance to antihormones evolves, so that eventually the cells change to create novel signal transduction pathways for enhanced oestrogen (GPR30 + OER) sensitivity, a reduction in progesterone receptor production and an increased metastatic potential. Most importantly, antihormone resistant breast cancer cells adapt with an ability to undergo apoptosis with low concentrations of oestrogen. The oestrogen destroys antihormone resistant cells and reactivates sensitivity to prolonged antihormonal therapy. We have initiated a major collaborative program of genomics and proteomics to use our laboratory models to map the mechanism of subcellular survival and apoptosis in breast cancer. The laboratory program is integrated with a clinical program that seeks to determine the minimum dose of oestrogen necessary to create objective responses in patients who have succeeded and failed two consecutive antihormonal therapies. Once our program is complete, the new knowledge will be available to translate to clinical care for the long-term maintenance of patients on antihormone therapy.
Keywords: Aromatase inhibitors, tamoxifen, raloxifene, gene array analysis
Introduction
The translation and application of long-term antihormonal strategies, aimed at the tumour oestrogen receptor (OER), has significantly improved the prognosis of patients with breast cancer.1 Long-term adjuvant tamoxifen treatment not only enhances survival and disease-free survival in patients with OER positive tumours during treatment but also reduces mortality for at least 10 years after treatment has stopped.2, 3 Building on the success of long-term tamoxifen therapy, a number of aromatase inhibitors have been shown to improve prognosis and reduce side effects (blood clots and endometrial cancer) if given instead of tamoxifen4–6 or after tamoxifen treatment.7, 8 Thus, the original scientific strategy9 of long-term antihormonal adjuvant therapy targeted to patients with OER positive disease10, 11 has emerged as the standard of care for breast cancer patients worldwide.
The new dimension of chemoprevention has advanced significantly during the past decade.12 Preliminary studies were initiated in the 1980’s to explore the safety and suitability of administering tamoxifen to women only at risk for breast cancer.13–15 The rationale of these studies was based on the wide clinical experience using tamoxifen to treat all stages of breast cancer, the reduction of contralateral breast cancer noted in patients receiving adjuvant tamoxifen treatment16–18 and laboratory studies that repeatedly demonstrated that tamoxifen can prevent mammary cancer in animal models.19–22
The current status and results of the worldwide efforts to quantitate and evaluate the value of tamoxifen as a chemopreventive have been summarized recently23 but it is the P-1 trial completed by Fisher and the National Surgical Adjuvant Breast and Bowel Project (NSABP)24, 25 that is considered to be the landmark.26 The results can be summarized simply. Tamoxifen reduced the incidence of breast cancer by 50%24 in pre and postmenopausal women at high risk.27 Side effects noted were increases in early stage low grade endometrial cancer, blood clots, and cataracts24, 25 but only in postmenopausal women receiving long-term tamoxifen treatment. Tamoxifen is available in the United States for risk reduction in pre and postmenopausal women. However, the consensus today is that tamoxifen is better deployed as a chemopreventive for premenopausal women to reduce the risk of OER positive breast cancer.28–32 There are no increases in the side effects of endometrial cancer or blood clots but tamoxifen keeps preventing breast cancer long after treatment stops31 consistent with earlier treatment results.3
The concern that tamoxifen was going to be associated with the risk of endometrial cancer33 and the recognition that the drugs called nonsteroidal antioestrogens34 were in fact selective OER modulators (SERMs) led to a paradigm change for chemoprevention. SERMs were oestrogenic in ovariectomized rat bone35 but at the same time prevented mammary cancer.21 These data led to the evidence based hypothesis that SERMs could prevent breast cancer as a beneficial side effect during the treatment and prevention of osteoporosis.36, 37 Based on this laboratory based hypothesis, raloxifene was subsequently shown to reduce fractures in postmenopausal women with or at high risk for osteoporosis38 but at the same time caused a 75% reduction in the incidence of breast cancer.39 A follow-up trial P-2 by the NSABP40 established that raloxifene was equivalent to tamoxifen at preventing invasive breast cancer in high risk postmenopausal women but with significantly fewer side effects (hysterectomies, cataracts, overall thrombolic events). However, although lower numbers of endometrial cancer were noted in raloxifene treated women compared to tamoxifen treated women, this was not significant because of a higher hysterectomy rate.40 Nevertheless, a related trial called Raloxifene use for the Heart or RUTH, showed no increase in endometrial cancers during raloxifene treatment compared to placebo arm41.
Thus from this brief introduction, it can be appreciated that significant clinical advances have been made through the application of the principle of long-term antihormone therapy9, 36 for the treatment and prevention of breast cancer. All of the advances can now be applied in clinical practice to improve patient care. Nevertheless, despite these advances through the use of sustained administration of antihormonal drugs, there are consequences for the tumour with the eventual development of drug resistance. In the case of SERMs, the type of resistance is unique and is expressed as SERM stimulated growth.42 But, it is the consistent study of the process of drug resistance to antihormones that resulted in the discovery43 of a weakness in the mechanisms of antihormonal drug resistance that has potential for the future exploitation in clinical practice.
Classification of SERM resistance
During the past twenty years we have focused our laboratory research program on developing models of SERM resistance in vivo to replicate events that could potentially occur clinically. The models were initially developed in vivo to avoid problems with cell culture where cells that become resistant to short term SERM treatment do not develop the essential requirements for angiogenesis that are necessary to survive and grow in patients. We now have a range of models that have been evaluated for growth in vivo (athymic mice) and that have been passaged in vivo for more than 5–10 years to replicate the long-term antihormonal therapy routinely used to treat patients (Table 1).
Table 1.
The available SERM resistant OER positive tumours used to investigate drug resistance in our laboratory. Phase I resistance refers to tumours that can be stimulated to grow into oestrogen or a SERM whereas Phase II resistance refers to tumours stimulated to grow only with a SERM. Oestrogen causes Phase II tumors to undergo apoptosis and regress42
Initial studies of resistance to tamoxifen treatment demonstrated the unique feature of SERM stimulated growth. Resistant tumours that develop in athymic mice from both OER positive breast and endometrial cells grow in response to either a SERM or estradiol.33, 44 This is why an aromatase inhibitor or the pure antioestrogen fulvestrant (that binds to OER and facilitates the rapid destruction of the complex)45 are successful second line therapies.46, 47 This form of resistance is referred to as Phase I resistance.42
However, these models represent only a few years of SERM treatment which is inconsistent with clinical experience of 5 years of adjuvant tamoxifen or possibly 10 years or more of raloxifene treatment to maintain bone density. The discovery that long-term SERM treatment exposes a vulnerability in the cancer cell that could have potential therapeutic applications was first reported at the St. Gallen meeting in the early 1990’s.43 Simply stated, long-term SERM treatment creates an absolute dependency on the SERM for tumour growth but small physiologic doses of oestradiol cause tumour cell death. Small tumours respond more readily to the apoptotic action of oestrogen but when tumours regrow during continuous oestrogen therapy, the tumours again respond to the SERM or no treatment48 (equivalent to treatment with an aromatase inhibitor for patients). This form of resistance is referred to as Phase II resistance.42 The models for SERM resistance are summarized in Table 1. Thus, it is plausible to consider a clinical strategy whereby limited duration, low dose oestrogen treatment could be used to purge and destroy Phase II resistant breast cancer cells but then patients could be treated again with antihormonal therapy to control tumour growth. However, a case could be made that the ubiquitous use of tamoxifen is declining and over the next decade the standard of care will be long-term treatment with one of several aromatase inhibitors. The question we have addressed in the laboratory is whether long-term oestrogen deprivation of breast cancer cells will expose the vulnerability to the apoptotic actions of oestrogen.
Resistance of breast cancer to oestrogen deprivation
There are two laboratory approaches to developing models of drug resistance to aromatase inhibitors. The traditional model is to study the impact of oestrogen withdrawal on the growth of OER positive breast cancer cells. In contrast, there is a model in vivo employing athymic mice transplanted with MCF-7 cells stably transfected with the aromatase enzyme. Without oestrogen tumours do not grow but when animals are treated with the enzyme substrate androstenedione to make oestrogen, tumour growth occurs. Simultaneous treatment with a number of aromatase inhibitors results in initial control of oestrogen-stimulated tumour growth but then the inhibitors fail and tumour growth occurs despite continuing treatment. This approach has been most instructive about strategies for antihormonal sequencing and the rationale of avoiding a combination of a SERM and an aromatase inhibitor for breast cancer therapy.49, 50
The traditional approach of oestrogen withdrawal using breast cancer cells not engineered in any way, was not possible until Berthois and coworkers51 discovered that cell culture media contained significant quantities of oestrogen found to increase the growth rate of MCF-7 cells. In other words, despite the fact that investigators were adding charcoal stripped serum to remove endogenous oestrogen, the media already contained oestrogenic chemical contaminants from the phenol red pH indicator.
Initial studies of the short and long-term effects of oestrogen deprivation of MCF-752, 53 and T47D54 breast cancer cells noted some interesting differences based on the regulation of OER in the different cell types.55 The MCF-7 cells that are obtained following long-term oestrogen deprivation remain OER positive (Table 2) whereas the T47D lose the OER.56 The levels of OER increase in the oestrogen deprived MCF-7 cells (Table 2) and also there are increases in GPR3057 noted in our gene array data. Thus, the oestrogen-deprived cells have an enhanced signal transduction pathway to support survival. Since breast cancers seem to rarely lose the OER efforts to study antihormonal drug resistance have focused on the MCF-7 line.
Table 2.
The basic characteristics of the MCF-7 cell lines developed from long-term oestrogen deprivation. Results are replicate (5) data from the affymetrix U-133 gene arrays relative to wild type MCF-7 cells. However, the biology of responses to antioestrogens are based on cell growth experiments where no effect is − and 100% response is ++++.
| Cell Line |
||
|---|---|---|
| MCF-7:2A | MCF-7:5C | |
| OER | ++ | ++ |
| Oestrogen induced PgR | ++ | − |
| GPR30 | ++++ | ++++ |
| Growth Inhibitory response to SERMs | ++ | − |
| Growth Inhibitory response to fulvestrant | +++ | ++ |
| Invasion proteins | ++ | ++++ |
Our program to develop MCF-7 cell lines resistant to oestrogen withdrawal successfully described two clones of cells: the MCF-7:5C and the MCF-7:2A line. The MCF-7:5C line58 is OER positive but progesterone receptor (PgR) negative and unresponsive to both oestrogen and SERM treatment. In contrast, the MCF-7:2A cell line59 did respond to SERM therapy with a reduction in growth rate but oestrogen did not affect the growth rate, except at high concentrations.
We have known for nearly 20 years that activation of growth factor receptor pathways can create intrinsic SERM resistance60, 61 and a down regulation of PgR induction.62 These data would be consistent with the finding for the MCF-7:5C cells (Table 2). The laboratory observation that deactivation of the OER signal transduction pathway with fulvestrant is consistent with clinical observation that fulvestrant produces reasonable control of aromatase resistant breast cancer.63 However, the models of oestrogen deprivation we developed in the early 1990’s were to take centre stage once the SERM resistant models were found to be reproducible48 and worthy of further development (Table 1). The key to the value of the two MCF-7 clones (5C, 2A) were that they could be studied in vitro to understand the mechanism of oestrogen-induced apoptosis using genomics.
The new biology of estrogen action
A re-examination of MCF-7 clones 5C and 2A occurred at the time when clinical investigators were re-examining the value of high dose oestrogen therapy in those patients who had been treated exhaustively with successive antihormonal therapies.64 The clinical studies demonstrated that high dose oestrogen therapy could cause tumour regression or stasis (30%) in patients treated exhaustively with antihormones.64 Additionally, high concentrations of oestrogen could induce apoptosis in long-term oestrogen deprived cells in culture.65 In contrast, we pursued our original hypothesis that the apoptotic supersensitization of breast cancer cells by long-term antihormonal therapy could occur with physiologic or a very low concentration of oestrogen treatment.43, 48
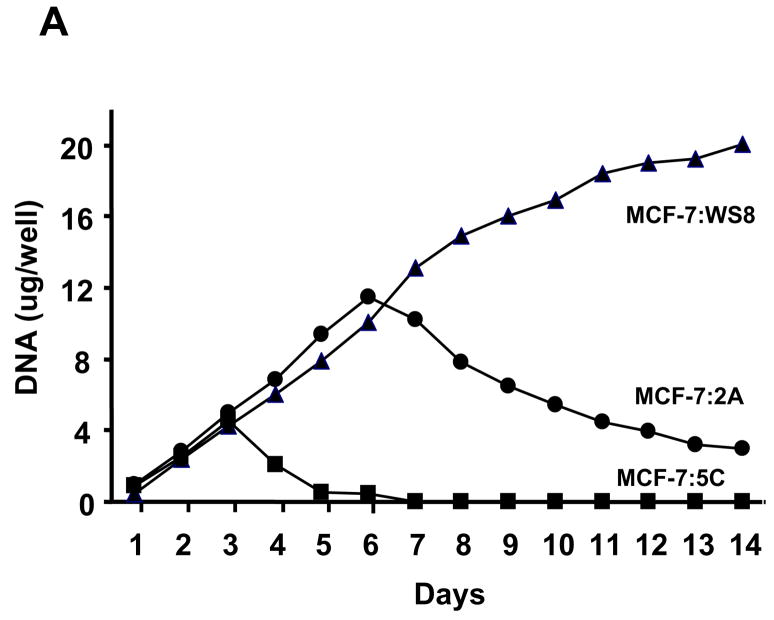
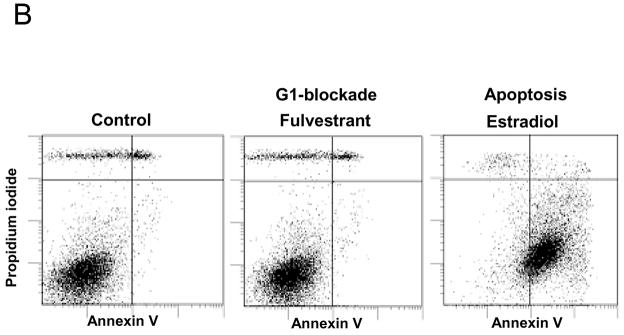
Two important observations, that were made during the re-evaluation of the MCF-7:5C and 2A cells, reinforced the view that oestrogen-induced apoptosis could be applied to reverse resistance to aromatase inhibitors. The first observation occurred by changing the charcoal stripped serum from the original 5% charcoal stripped calf serum58 to 10% developed stripped fetal bovine serum.66 This caused a dramatic increase in the growth rate of the 5C cells to be comparable to the MCF-7:2A cells (Figure 2). Remarkably, physiologic oestradiol (lnM) now caused a massive apoptotic response in the MCF-7:5C cells (Figure 3a, b). The MCF-7:2A cells had previously59 been found to be responsive to antioestrogens by inhibiting growth and oestrogen by inducing progesterone receptor synthesis. The 2A cells, however, only weakly responded to the growth inhibitory effects of high concentrations 1μM oestradiol. This original assumption is not true if the time course is extended (Figure 3a). The 2A cells appear to have a survival mechanism that is able to protect them initially from the apoptotic actions of oestradiol. Nevertheless, this survival mechanism eventually fails. Overall, our models now create an interesting opportunity to interrogate the time courses with genomics and proteomics to find the precise oestrogen-induced mechanisms for protecting the cell from apoptosis.
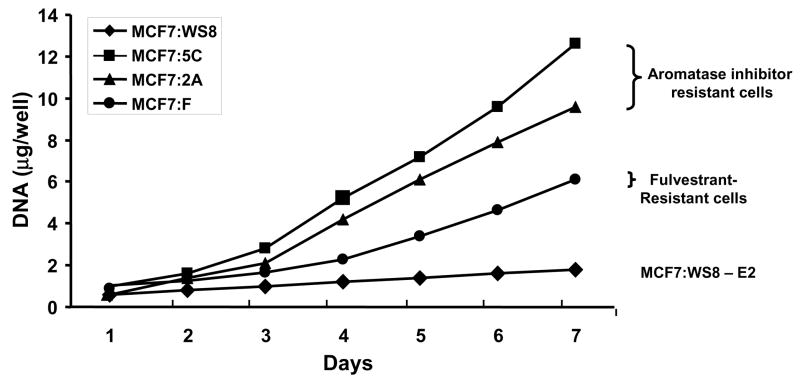
Figure 2.
The growth of wild type MCF-7 cells (WS8) and various antihormonally resistant sublines in an oestrogen-free environment. The cells MCF-7:5C and 2A grow spontaneously and could be considered to represent aromatase inhibitor resistant cells. These remain OER positive. In contract, MCF-7F are fulvestrant resistant (MCF-7 cells grown for over a year in an oestrogen-deprived environment containing fulvestrant). These cells grow spontaneously but have no OER.
Figure 3.
The action of oestradiol (1nM) on the growth of wild type MCF-7 cells (WS8) or long-term oestrogen-deprived MCF-7 cells (5C and 2A). In Panel A the MCF-7:5C cells undergo rapid apoptosis during the first few days of oestradiol exposure whereas the MCF-7:2A cells slowly initiate apoptosis during the days after 6 of oestradiol treatment. In panel B MCF07:5C cells respond to fulvestrant (1μM) with a G1 blockade at 72 hour whereas oestradiol (1nM) causes massive and complete apoptosis. These results were obtained using flow cytometry.
Analysis of apoptotic pathways
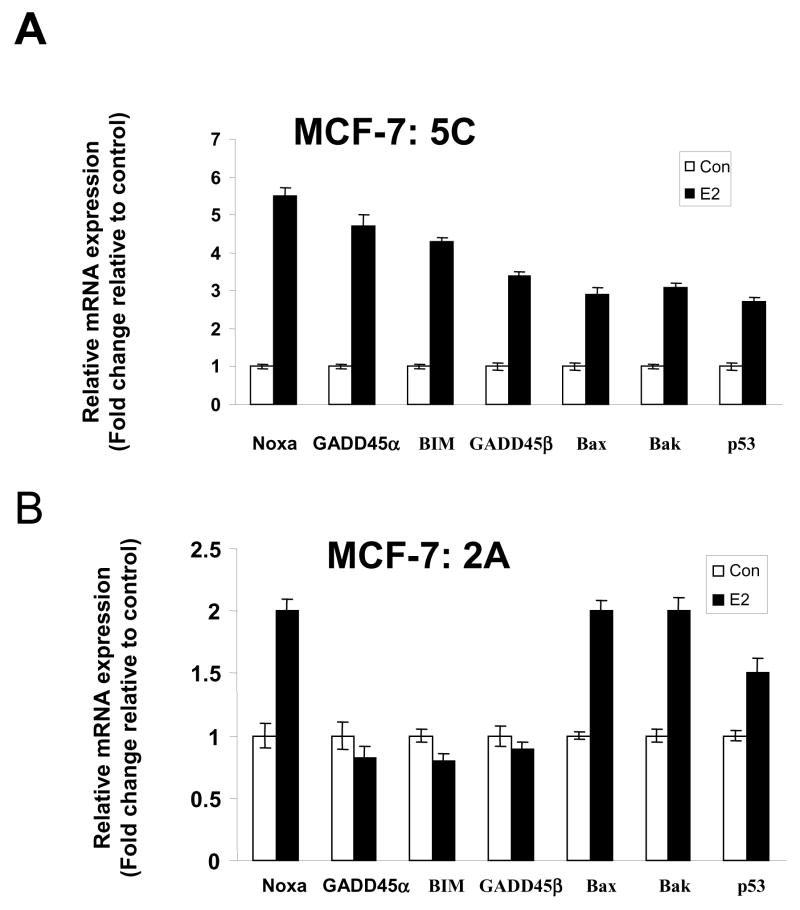
A number of U-133 Affymetrix gene arrays were completed using the MCF-7, MCF-7:5C and 2A cell lines to define the early events of oestrogen action. A 48 hour time point was used in our preliminary studies and 5 replicates were analyzed to ensure statistical veracity. All gene array analyses were completed at Translational Genomics, AZ. Results illustrated in Figure 4 show the 48 hour increase in proapoptotic genes that are activated by oestrogen in the MCF-7:5C cells. This is consistent with the time course for the apoptotic death response of the MCF-7:5C cells noted in Figure 3. In contrast, oestrogen had not yet activated the full apoptotic response in MCF-7:2A cells that become apoptotic over a much longer time course (Figure 3).
Figure 4.
Oestrogenic regulation of apoptotic genes in long term estrogen-deprived MCF-7:5C and MCF-7:2A breast cancer cells as determined by Affymetrix gene microarrays. For experiment, cells were treated with 1nM oestradiol for 48 hours and total RNA was prepared using the Qiagen Rneasy Mini kit. cRNA was generated, labeled, and hybridized to the Affymetrix Human Genome U133 plus 2l0 arrays containing 54,300 probe sets. Chips were then scanned and analyzed using the Affymetrix Microarray Analysis Suite version 5.0. Assessment of data quality was conducted following default guidelines in the Affymetrix’s GeneChip® Expression Analysis Data Analysis Fundamentals Training Manual. Global scaling for average signal intensity for all arrays was set to 500. Four biological replicates from each of the two cell lines were arrayed to determine consistent and reproducible patterns of gene expression. The above figure shows that oestradiol treatment caused 3- to 6-fold induction of the proapoptotic genes NOXA, GADD45α, GADD45β, BIM, BAX, BAK and p53 in (A) MCF-7:5C cells but only a 2-fold induction of NOXA, BAX, and BAK in (B) MCF-7:2A cells.
Overall, we have confirmed our novel observations that breast cancer and endometrial cancer cells (unpublished observation) become resistant to long-term antihormonal interventions by reconfiguring the oestrogen signal transduction pathway to induce an apoptotic response rather than enhancing survival and further growth. These data plus the emerging anecdotal results of clinical case reports (James Ingle, MD and Mr. Michael Dixon personal communications) prompted us to develop a multicentre program to explore our unique model systems systematically so that we can describe the mechanisms of oestrogen-induced survival and apoptosis in breast cancer. Completion of these studies would then provide an invaluable database to translate to patient care. The goal would be to determine the lowest dose of oestrogen necessary to cause apoptosis in a significant number of women whose tumours no longer respond to antihormonal therapy. This would reverse antihormone resistance in a significant proportion of patients.
Translation of laboratory results to patient care
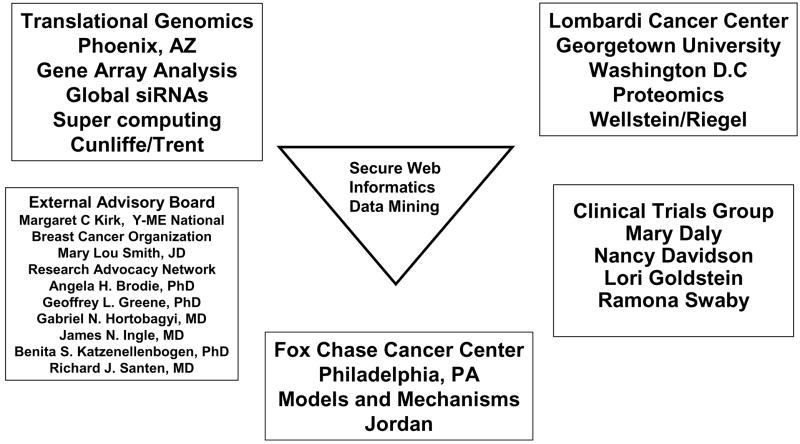
We have established a multi-centre collaborative translational research grant with headquarters at the Fox Chase Cancer Center (FCCC) (Figure 5). The five year program is sponsored by the U.S. Department of Defense Breast Cancer Program BC050277 entitled “A New Therapeutic Paradigm for Breast Cancer Exploiting Low-Dose Estrogen-Induced Apoptosis.”
Figure 5.
The organization of our Department of Defence Center of Excellence Grant entitled “A New Therapeutic Paradigm for Breast Cancer Exploiting Low-Dose Estrogen-Induced Apoptosis.” The model systems to study the survival and apoptosis induced with oestrogen are used for time course experiments at the Fox Chase Cancer Center. The materials are distributed to Translational Genomics for siRNA analysis or gene array and the Vincent T. Lombardi Cancer Center is involved to conduct proteomics. All results are uploaded into a shared secure web for data processing and target identification by our informatics and biostatistical group. Each laboratory is able to validate emerging pathways and study individual genes of interest. Our program is integrated with a clinical trials program that provides patient samples for validation of apoptotic or survival pathways. We are grateful to our external advisory board of Patient Advocates and professional colleagues for their continuing advice and support.
Our goal is to create maps of the survival and apoptotic responses to oestrogen noted in our models in vivo and in vitro. Biological samples from our time course experiments using our models at the FCCC are being distributed to Translational Genomics in Arizona for Agilent gene array analysis, CGH and CpG methylation arrays. Total human genome siRNA analysis is also being completed on our cell lines. Additionally, samples for proteomics are being dispatched to Georgetown University (Vincent T. Lombardi Cancer Center, PIs Anton Wellstein and Anna T. Riegel). All processed data is then being uploaded into a secure website for data mining and target identification, so that verification and validation studies can occur at each of the collaborating sites. A clinical program is exploring the clinical applications of our laboratory observation with two successive protocols:
A Single Arm Phase II Study of Pharmacologic Dose Estrogen in Postmenopausal Women with Hormone Receptor-Positive Metastatic Breast Cancer After Failure of Sequential Endocrine Therapies; and,
Reversal of Anti-Estrogen Resistance with Sequential Dose De-escalation of Pharmacologic Estrogen in a Single Arm Phase II Study of Postmenopausal Women with Hormone Receptor-Positive Metastatic Breast Cancer After Failure of Sequential Endocrine Therapies.
Our clinical studies are in place 1) to confirm the clinical finding64 that high dose oestrogen treatment following exhaustive antihormonal treatment of OER positive breast cancer will give a 30% response rate and 2) to determine the lowest dose of oestrogen that will induce an equivalent tumour regression as high dose oestrogen (30 mg. oestradiol daily). All patients will be monitored weekly using the Apoptosense® serum assay to detect apoptotic markers in responding and non-responding patients. Additionally, where possible, patients will have biopsies of accessible tumour tissue before and after 12 weeks of oestrogen therapy (or shorter if patients rapidly progress). Responding patients will be retreated with 1 mg. anastrozole daily until progression.
Overall, the map of survival and apoptotic pathways we create from our laboratory models will be invaluable to guide our selection of target genes in biopsies using real time RTPCR. This will provide clues as to our future strategy of improving response rates with agents that selectively block survival pathways which can then be used in combination with our apoptotic oestrogen purge. It is our long term goal to improve oestrogen-induced response rates in patients refractory to antihormonal therapies. In so doing, select patients with metastatic breast cancer can anticipate longer disease control before chemotherapy is necessary. Most importantly, the new knowledge will provide an in silico platform to identify the apoptotic target so effectively located by the OER.
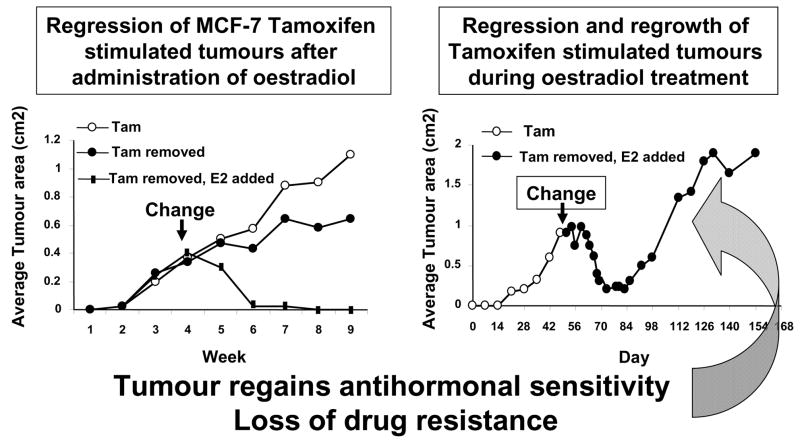
Figure 1.
Diagrammatic representation of the actions of physiologic oestradiol (E2) on the growth of small phase II MCF-7 tamoxifen resistant tumors in ovariectomized athymic mice. A larger tumour will regress with oestrdiol treatment but will eventually display oestrogen-stimulated growth. If tumours are retransplanted into a new generation of ovariectomized athymic mice and treated with oestradiol, tamoxifen will block oestrogen-stimulated tumour growth.48 First presented in St. Gallen, 1993.43
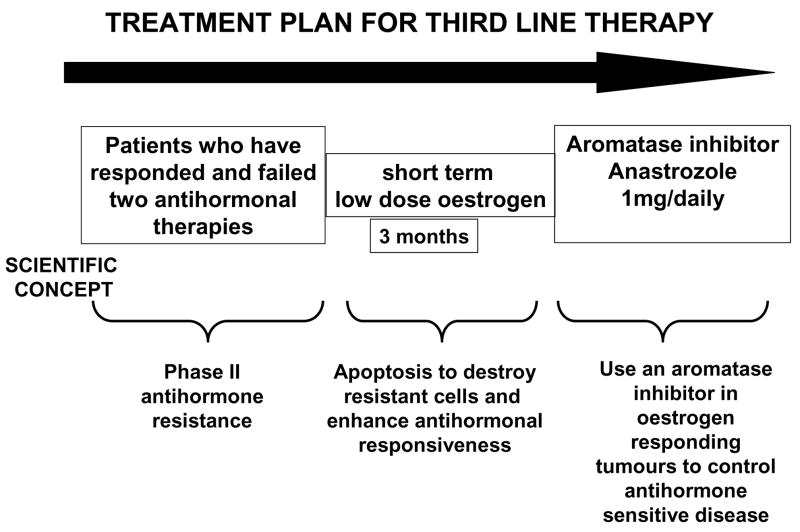
Figure 6.
An anticipated treatment plan for third line endocrine therapy. Patients must have responded and failed two successive antihormonal therapies to be eligible for a course of low dose oestradiol therapy for 3 months. The anticipated response rate is 30%64 and responding patients will be treated with anastrozole until relapse. Validation of the treatment plan via the Center of Excellence grant (Figure 5) will establish a platform to enhance response rates with apoptotic oestrogen by integrating known inhibitors of tumour survival pathways into the 3 month debulking treatment plan. The overall goal is to increase response rates and maintain patients for longer on antihormonal strategies before chemotherapy is required.
Acknowledgments
Supported (VCJ) by the Department of Defense Breast Program under award number BC050277 Center of Excellence (Views and opinions of, and endorsements by the author(s) do not reflect those of the US Army or the Department of Defense), SPORE in Breast Cancer CA 89018, R01 GM067156, FCCC Core Grant NIH P30 CA006927, the Avon Foundation and the Weg Fund of Fox Chase Cancer Center. Ramona Swaby, MD is the recipient of the clinical trials grant from AstraZeneca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jordan VC, Brodie AMH. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EBCTCG. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;354:1451–67. [PubMed] [Google Scholar]
- 3.EBCTCG. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 5.Thurlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–57. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 6.Coates AS, Keshaviah A, Thurlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25(5):486–92. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 7.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350(11):1081–92. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 8.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA. 17. J Natl Cancer Inst. 2005;97:1262–71. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 9.Jordan VC, Allen KE. Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer. 1980;16:239–51. doi: 10.1016/0014-2964(80)90156-5. [DOI] [PubMed] [Google Scholar]
- 10.Jordan VC, Koerner S. Tamoxifen (ICI 46,474) and the human carcinoma 8S oestrogen receptor. Eur J Cancer. 1975;11:205–06. doi: 10.1016/0014-2964(75)90119-x. [DOI] [PubMed] [Google Scholar]
- 11.Jordan VC, Dix CJ, Allen KE. The effectiveness of long term tamoxifen treatment in a laboratory model for adjuvant hormone therapy of breast cancer. Adjuvant Therapy of Cancer. 1979;2:19–26. [Google Scholar]
- 12.Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nature Reviews Cancer. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]
- 13.Powles TJ, Hardy JR, Ashley SE, et al. A pilot trial to evaluate the acute toxicity and feasibility of tamoxifen for prevention of breast cancer. Br J Cancer. 1989;60(1):126–31. doi: 10.1038/bjc.1989.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326(13):852–56. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 15.Love RR, Wiebe DA, Newcomb PA, et al. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. Ann Intern Med. 1991;115(11):860–4. doi: 10.7326/0003-4819-115-11-860. [DOI] [PubMed] [Google Scholar]
- 16.Cuzick J, Baum M. Tamoxifen and contralateral breast cancer [letter] Lancet. 1985;2(8449):282. doi: 10.1016/s0140-6736(85)90338-1. [DOI] [PubMed] [Google Scholar]
- 17.Fornander T, Rutqvist LE, Cedermark B, et al. Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet. 1989;1(8630):117–20. doi: 10.1016/s0140-6736(89)91141-0. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320(8):479–84. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 19.Jordan VC. Antitumour activity of the antioestrogen ICI 46,474 (tamoxifen) in the dimethylbenzanthracene (DMBA)-induced rat mammary carcinoma model. J Steroid Biochem. 1974;5:354. [Google Scholar]
- 20.Jordan VC. Effect of tamoxifen (ICI 46,474) on initiation and growth of DMBA- induced rat mammary carcinoma. Eur J Cancer. 1976;12:419–24. doi: 10.1016/0014-2964(76)90030-x. [DOI] [PubMed] [Google Scholar]
- 21.Gottardis MM, Jordan VC. Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea- induced rat mammary carcinoma model. Cancer Res. 1987;47(15):4020–24. [PubMed] [Google Scholar]
- 22.Jordan VC, Lababidi MK, Langan-Fahey S. Suppression of mouse mammary tumorigenesis by long-term tamoxifen therapy. J Natl Cancer Inst. 1991;83(7):492–6. doi: 10.1093/jnci/83.7.492. [DOI] [PubMed] [Google Scholar]
- 23.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 25.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 26.http://www.nature.com/milestones/milecancer/full/milecancer05.html.
- 27.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 28.Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91(21):1829–46. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 29.Jordan VC. Optimising endocrine approaches for the chemoprevention of breast cancer. Beyond the Study of Tamoxifen and Raloxifene (STAR) Trial. European Journal of Cancer. 2006;42:2909–13. doi: 10.1016/j.ejca.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Veronesi U, Maisonneuve P, Decensi A. Tamoxifen: an enduring star. J Natl Cancer Inst. 2007;99(4):258–60. doi: 10.1093/jnci/djk072. [DOI] [PubMed] [Google Scholar]
- 31.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99(4):272–82. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 32.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99(4):283–90. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 33.Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988;48:812–15. [PubMed] [Google Scholar]
- 34.Jordan VC. Biochemical pharmacology of antiestrogen action. Pharmacol Rev. 1984;36(4):245–76. [PubMed] [Google Scholar]
- 35.Jordan VC, Phelps E, Lindgren JU. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat. 1987;10(1):31–35. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- 36.Jordan VC. Chemosuppression of breast cancer with tamoxifen: laboratory evidence and future clinical investigations. Cancer Invest. 1988;6(5):589–95. doi: 10.3109/07357908809082124. [DOI] [PubMed] [Google Scholar]
- 37.Lerner LJ, Jordan VC. The development of antiestrogens for the treatment of breast cancer: Eighth Cain Memorial Award Lecture. Cancer Res. 1990;50:4177–89. [PubMed] [Google Scholar]
- 38.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators [see comments] [published erratum appears in JAMA 1999 Dec 8;282(22):2124] JAMA. 1999;282(7):637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 39.Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–97. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 40.Vogel VG, Costantino JP, Wickerham DL, et al. The Study of Tamoxifen and Raloxifene (STAR): Report of the National Surgical Adjuvant Breast and Bowel Project P-2 Trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 41.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355(2):125–37. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 42.Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5:207–13. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 43.Wolf DM, Jordan VC. Recent Results in Cancer Research. Vol. 127. Heidelberg: Springer-Verlag; 1993. A laboratory model to explain the survival advantage observed in patients taking adjuvent tamoxifen therapy; pp. 23–33. [DOI] [PubMed] [Google Scholar]
- 44.Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48:5183–8187. [PubMed] [Google Scholar]
- 45.Wijayaratne AL, McDonnell DP. The human estrogen receptor-alpha is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J Biol Chem. 2001;276:35684–92. doi: 10.1074/jbc.M101097200. [DOI] [PubMed] [Google Scholar]
- 46.Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20(16):3386–95. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 47.Howell A, Robertson JFR, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 48.Yao K, Lee ES, Bentrem DJ, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6(5):2028–36. [PubMed] [Google Scholar]
- 49.Jelovac D, Macedo L, Goloubeva OG, Handratta V, Brodie AMH. Additive antitumor effect of aromatase inhibitor letrozole and antiestrogen fulvestrant in a postmenopausal breast cancer model. Cancer Res. 2005;65:5439–44. doi: 10.1158/0008-5472.CAN-04-2782. [DOI] [PubMed] [Google Scholar]
- 50.Long BJ, Jelovac D, Handratta V, et al. Therapeutic strategies using the aromatase inhibitor letrozole and tamoxifen in a breast cancer model. JNCI. 2004;96:456–65. doi: 10.1093/jnci/djh076. [DOI] [PubMed] [Google Scholar]
- 51.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83(8):2496–500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katzenellenbogen BS, Kendra KL, Norman MJ, Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Res. 1987;47:4355–60. [PubMed] [Google Scholar]
- 53.Welshons WV, Jordan VC. Adaptation of estrogen-dependent MCF-7 cells to low estrogen (phenol red-free) culture. Eur J Cancer. 1987;232:1935–39. doi: 10.1016/0277-5379(87)90062-9. [DOI] [PubMed] [Google Scholar]
- 54.Murphy CS, Meisner LF, Wu SQ, Jordan VC. Short- and long-term estrogen deprivation of T47D human breast cancer cells in culture. Eur J Cancer Clin Oncol. 1989;25(12):1777–88. doi: 10.1016/0277-5379(89)90348-9. [DOI] [PubMed] [Google Scholar]
- 55.Pink JJ, Jordan VC. Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res. 1996;56(10):2321–30. [PubMed] [Google Scholar]
- 56.Pink JJ, Bilimoria MM, Assikis J, Jordan VC. Irreversible loss of the oestrogen receptor in T47D breast cancer cells following prolonged oestrogen deprivation. Br J Cancer. 1996;74(8):1227–36. doi: 10.1038/bjc.1996.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bologa CG, Revankar CM, Young SM, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2(4):207–12. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 58.Jiang SY, Wolf DM, Yingling JM, Chang C, Jordan VC. An estrogen receptor positive MCF-7 clone that is resistant to antiestrogens and estradiol. Mol and Cell Endo. 1992;90:77–80. doi: 10.1016/0303-7207(92)90104-e. [DOI] [PubMed] [Google Scholar]
- 59.Pink JJ, Jiang SY, Fritsch M, Jordan VC. An estrogen-independent MCF-7 breast cancer cell line which contains a novel 80-kilodalton estrogen receptor-related protein. Cancer Res. 1995;55:2583–90. [PubMed] [Google Scholar]
- 60.Cormier EM, Jordan VC. Contrasting ability of antiestrogens to inhibit MCF-7 growth stimulated by estradiol or epidermal growth factor. Eur J Cancer Clin Oncol. 1989;25(1):57–63. doi: 10.1016/0277-5379(89)90051-5. [DOI] [PubMed] [Google Scholar]
- 61.Robinson SP, Jordan VC. The paracrine stimulation of MCF-7 cells by MDA-MB-231 cells: possible role in antiestrogen failure. Eur J Cancer Clin Oncol. 1989;25:493–7. doi: 10.1016/0277-5379(89)90262-9. [DOI] [PubMed] [Google Scholar]
- 62.Cormier EM, Wolf MF, Jordan VC. Decrease in estradiol-stimulated progesterone receptor production in MCF-7 cells by epidermal growth factor and possible clinical implication for paracrine-regulated breast cancer growth. Cancer Res. 1989;49:576–80. [PubMed] [Google Scholar]
- 63.Ingle JN, Suman VJ, Rowland KM, et al. Fulvestrant in women with advanced breast cancer after progression on prior aromatase inhibitor therapy: North Central Cancer Treatment Group Trial N0032. J Clin Oncol. 2006;24(7):1052–6. doi: 10.1200/JCO.2005.04.1053. [DOI] [PubMed] [Google Scholar]
- 64.Lonning PE, Taylor PD, Anker G, et al. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67:111–16. doi: 10.1023/a:1010619225209. [DOI] [PubMed] [Google Scholar]
- 65.Song RX, Mor G, Naftolin F, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst. 2001;93:1714–23. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 66.Lewis JS, Osipo C, Meeke K, Jordan VC. Estradiol induced apoptosis in a breast cancer cell line resistant to estrogen deprivation. J Steroid Biochem. 2005;94/1–3:131–41. doi: 10.1016/j.jsbmb.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 67.Gottardis MM, Jiang SY, Jeng MH, Jordan VC. Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res. 1989;49(15):4090–3. [PubMed] [Google Scholar]
- 68.Wolf DM, Langan-Fahey SM, Parker CJ, McCague R, Jordan VC. Investigation of the mechanism of tamoxifen-stimulated breast tumor growth with nonisomerizable analogues of tamoxifen and metabolites. J Natl Cancer Inst. 1993;85(10):806–12. doi: 10.1093/jnci/85.10.806. [DOI] [PubMed] [Google Scholar]
- 69.MacGregor-Schafer JI, Lee E-S, O’Regan RM, Yao K, Jordan VC. Rapid development of tamoxifen stimulated mutant p53 breast tumors (T47D) in athymic mice. Clin Cancer Res. 2000;6:4373–7380. [PubMed] [Google Scholar]
- 70.Osipo C, Meeke K, Liu H, et al. Trastuzumab therapy for tamoxifen-stimulated endometrial cancer. Cancer Res. 2005;65(18):8504–13. doi: 10.1158/0008-5472.CAN-04-4107. [DOI] [PubMed] [Google Scholar]
- 71.Osipo C, Gajdos C, Liu H, Chen B, Jordan VC. Paradoxical action of fulvestrant on estradiol-induced regression of tamoxifen-stimulated breast cancer. J Natl Cancer Inst. 2003;95:1597–607. doi: 10.1093/jnci/djg079. [DOI] [PubMed] [Google Scholar]
- 72.Liu H, Lee ES, Gajdos C, et al. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95(21):1586–97. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]