Summary
Mammalian Δ1-pyrroline-5-carboxylate synthase (P5CS) is a bifunctional ATP- and NAD(P)H-dependent mitochondrial enzyme that catalyzes the coupled phosphorylation and reduction-conversion of L-glutamate to P5C, a pivotal step in the biosynthesis of L-proline, L-ornithine and L-arginine. Previously, we reported cloning and characterization of two P5CS transcript variants generated by exon sliding that encode two protein isoforms differing only by a 2 amino acid-insert at the N-terminus of the γ-glutamyl kinase active site. The short form (P5CS.short) is highly expressed in the gut and is inhibited by ornithine. In contrast, the long form (P5CS.long) is expressed ubiquitously and is insensitive to ornithine. Interestingly, we found that all the established human cell lines we have studied expressed P5CS.long but not P5CS.short. In addition, expression of P5CS.long can be modulated by hormones: downregulation by hydrocortisone and dexamethasone and upregulation by estradiol, for example. Using a quantitative proteomic approach, we showed that P5CS.long is upregulated by p53 in p53-induced apoptosis in DLD-1 colorectal cancer cells. Functional genomic analysis confirmed that there are two p53-binding consensus sequences in the promoter region and in the intron 1 of the human P5CS gene. Interestingly, overexpression of P5CS by adenoviruses harboring P5CS.long or P5CS.short in various cell types has no effect on cell growth or survival. It would be of importance to investigate the role of P5CS as a p53 downstream effector and how P5CS.short expression is regulated by hormones and factors of alternative splicing in cells isolated from model animals.
Keywords: Alternative splicing, Apoptosis, Exon sliding, Hormones, P53, Proline, Quantitative proteomics, Δ1-pyrroline-5-carboxylate (P5C), P5C synthase (P5CS)
Introduction
Mammalian Δ1-pyrroline-5-carboxylate synthase (P5CS) is a bifunctional ATP- and NAD(P)H-dependent enzyme that catalyzes the coupled phosphorylation- and reduction-conversion of glutamate to P5C. It possesses two enzymatic domains, γ-glutamyl kinase (γ-GK) and γ-glutamyl phosphate (γ-GP) reductase (γ-GPR). The γ-GK domain phosphorylates glutamate to form γ-GP, a labile and reactive transitional intermediate. The γ-GPR domain then converts γ-GP to γ-glutamic semialdehyde (GSA), which is in tautomeric equilibrium with P5C. Importantly, GSA/P5C is the obligatory intermediate in the interconversions of proline, ornithine and glutamate (Adams and Frank, 1980; Phang et al., 2001, Hu et al., this issue). The P5CS reaction is unidirectional; another enzyme, P5C dehydrogenase (P5CDH), catalyzes the reverse reaction of P5CS that converts P5C to glutamate (Hu et al., 1996). P5CS localizes in mitochondrial inner membrane and is most active in the small intestine, with measurable activities in the colon, pancreas, thymus, and brain. It is worth mentioning that certain prokaryotes and unicellular eukaryotes utilize two separate enzymes encoded by two separate genes to perform the γ-GK and γ-GPR activity, yielding the same product, GSA/P5C (Phang et al., 2001).
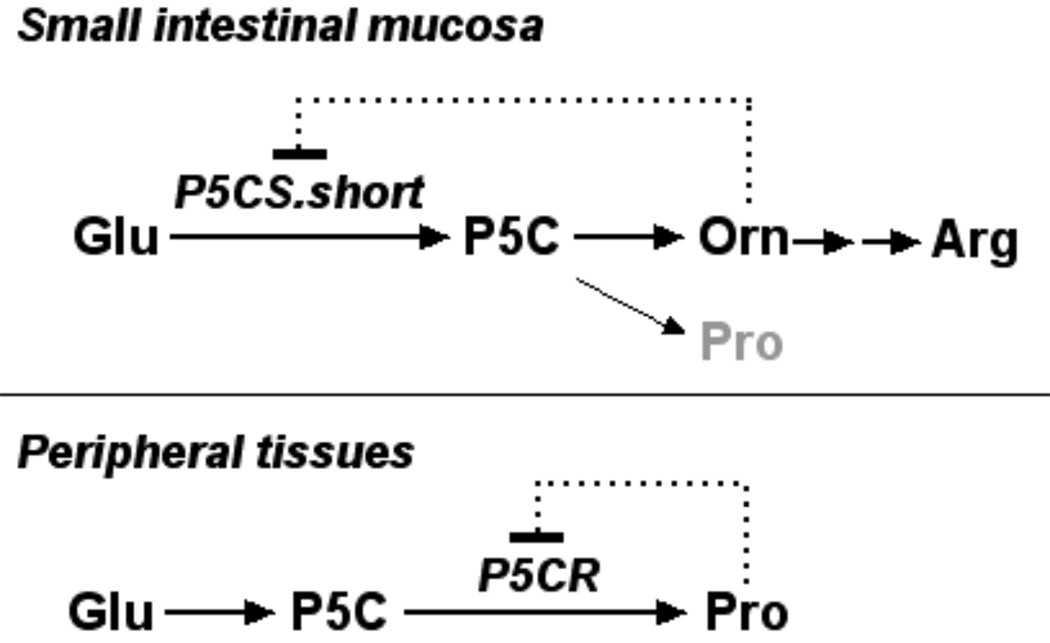
The full-length cDNA encoding P5CS in multicellular eukaryotes was first cloned and characterized in plant Vigna aconitifolia (Hu et al., 1992). Expression of plant P5CS has been shown to be upregulated by osmotic stress and downregulated by proline (Verbruggen and Hermans, this issue). Subsequently the full-length cDNA clones of Human P5CS were characterized (Aral et al., 1996; Hu et al., 1999). The human P5CS structural gene, also known as ALDH18A1 (aldehyde dehydrogenase family member 18A1; Vasiliou et al., 1999), is located on chromosome 10q24.3 and spans 15 kilobases. Detailed functional genomic analysis of human P5CS has been conducted (see Hu et al., this issue). Previously, we showed that human P5CS undergoes alternative splicing to generate two mature RNA transcripts which encode two isoforms/isozymes differing only by a 2 amino acid insert at the N-terminus of the γ-GK active site. The short isoform (P5CS.short) has high activity in the gut, where it participates in arginine biosynthesis and is subject to noncompetitive inhibition by ornithine. The long isoform (P5CS.long), expressed in multiple tissues, is necessary for the synthesis of proline from glutamate and is insensitive to ornithine (Figure 1). Both isoforms exhibit P5CS activity in yeast and CHO cells (Hu et al., 1999).
Fig1.
Model for the regulation of mammalian P5CS and P5CR by ornithine and proline in arginine and proline biosynthesis, respectively. The pathway for arginine (Arg) biosynthesis in the small intestinal mucosa is shown above. In this tissue, particularly in the neonates, the short isoform of P5C synthase (P5CS.short), which converts glutamate (Glu) to Δ1-pyrroline-5-carboxylate (P5C), predominates and is subject to feedback inhibition by ornithine (Orn). In peripheral tissues the pathway serves to synthesize proline (Pro). In these tissues, the long isoform of P5C synthase predominates and is insensitive to Orn. The pathway appears to be regulated by Pro inhibition of P5C reductase (P5CR).
Several functional domains were found in human P5CS. The N-terminal ~64 amino acids possess a putative mitochondrial targeting sequence. Protein sequence analysis showed 36 percent identity when comparing the N-terminal half of P5CS to the yeast (S. cerevisiae) γ-GK. In addition, a highly conserved motif corresponding to an aspartokinase active-site was found within the same region. The C-terminal half of P5CS showed 48 percent sequence identity with yeast γ-GPR and possessed an eleven-codon NAD(P)H binding motif (Hu et al., 1999).
It has been shown that in the neonatal gut, most of the glutamine-derived P5C is converted into ornithine rather than proline and ornithine is subsequently utilized for efficient synthesis of citrulline and arginine (Wu and Knabe, 1995). Studies in animal models have demonstrated the necessity of enzymatic activity in the intestinal epithelial cells for the purposes of arginine synthesis. For example, ornithine δ-aminotransferase (OAT), which catalyzes the reversible conversion of P5C to ornithine, is pivotal in the synthesis of arginine. Targeted disruption of OAT in mice is observed to be lethal during the neonatal period. A nonfunctioning urea cycle coupled with low levels of citrulline and arginine caused lethality in OAT−/− mice within 48 hours unless sustained on arginine injections until dietary arginine is sufficient for growth (Wang et al., 1996, Valle and Simell, 2001). P5CS, upstream from OAT when using glutamate as a substrate for P5C and subsequently for ornithine, citrulline and arginine synthesis, is also required. Evidently, the secondary role of the small intestine as an organ for arginine synthesis, especially in neonates, agrees with the observation of a predominant amount of P5CS.short in the tissue, as the ornithine-sensitive P5CS will self-sufficiently regulate ornithine and, consequently, arginine synthesis. When not expressed, as is the case in animal models such as cats, the animal’s diet must contain high amounts of arginine (Phang et al., 2001). In addition, it is known that arginine is remarkably deficient in the milk of most mammals (including human, pig, rat and mouse), endogenous biosynthesis of arginine from proline and glutamine via the intestinal-renal axis plays a crucial role in maintaining arginine homeostasis in neonates (Wu and Morris, 1998).
A newly recognized inborn error due to deficiency of P5CS was first reported by Rabier et al (1992) and subsequently characterized by Kamoun et al (1998). The two sibs were born to consanguineously related parents and the proband was presenting at birth with hypotonia, dysmorphic signs, pes planus and clonic seizures. Both developed progressive neurodegeneration and peripheral neuropathy, joint laxity, skin hyperelasticity and bilateral subcapsular cataracts. Their metabolic phenotype includes mild hyperammonemia, hypo-ornithinemia, hypocitrullinemia, hypo-argininemia and hypoprolinemia. When the children were in the fed state, urea cycle intermediates derived from dietary protein allowed for urea cycle function. However, hyperammonemia was observed upon mild fasting, due to impaired urea cycle function from the reduced concentration of urea cycle intermediates. Baumgartner et al (2000) reported that both patients were homozygous for a G-to-A transition at position 251 of the P5CS gene, resulting in an arg84-to-gln (R84Q) substitution. The R84Q mutation alters a conserved residue in the P5CS γ-GK domain and dramatically reduces the activity of both P5CS isoforms when expressed in mammalian cells. Additionally, R84Q appears to destabilize the long isoform. A recent study showed that when used 3H-glutamate as the source, the incorporation of 3H-proline into protein was deficient in fibroblasts established from the P5CS-deficient patients (Baumgartner et al., 2005). Though the frequency of this disorder is unknown, the metabolic phenotype of P5CS deficiency is easily missed. The combination of low levels of ornithine, citrulline, arginine and proline plus a tendency to hyperammonemia or one of the above together with a clinical phenotype of neurodegeneration (e.g., peripheral neuropathy, cataracts and connective tissue manifestations) should suggest this disorder. Early recognition would allow a therapeutic trial with citrulline and proline (Baumgartner et al., 2005).
Recent research has given P5CS more of a role in maintaining cell homeostasis than previously recognized. Redox mechanisms, central to cell survival, signaling and death, are becoming more recognized to proline metabolism. A few of the proline metabolic enzymes have been shown to be upregulated by p53, a transactivating factor and a tumor suppressor. For example, expression of proline oxidase (POX) has been observed in p53-induced apoptosis in cancer cells. POX, a mitochondrial inner membrane protein, catalyzes the rate-limiting oxidation of proline to pyrroline-5-carboxylate using cytochrome c and FAD as electron and hydrogen acceptors. It has been shown that POX is one of the p53 downstream effectors that induces ROS- and mitochondria-mediated apoptosis in cancer cells. In addition, POX initiates both intrinsic and extrinsic apoptotic pathways, possibly through NFAT and MEK/ERK signaling (Liu et al., Hu et al., 2007; Phang et al., this issue). The redox coupling of proline and P5C to NADPH and NADP+, formerly understood only as a way to transfer redox potential between cellular compartments and tissues, is now being recognized for its more significant role in apoptosis (Hagedorn and Phang, 1983; Phang 1985; Phang et al., 2001). This study is aimed at a better understanding how human P5CS is regulated by growth factors and p53.
Materials and Methods
Cell Lines, chemicals and culture media
DLD-1.vector and DLD-1.p53 cells, “Tet-Off” TP53 inducible DLD-1 cells, were cultured in D.20 medium, comprised of α-MEM supplemented with 10% FBS, 1X antibacterial antimycotic solution, 400 µg/ml G418, 250 ng/ml hygromycin B and 20 ng/ml Doxycycline (Dox; D.20) as previously described (Gu et al., 2004, Liu et al., 2007). HEK 293, human control fibroblast cell lines, HepG2, prostate cancer cell lines, LNCaP, PC3 and DU145, and human intestine CCL-6 cell line were purchased from ATCC and cultured in DMEM (Gibco Life Technologies, Grand Island, NY, USA) supplemented with 10% regular or dialyzed FBS (Hyclone, CA, USA) and antibiotic/antimycotic solution as described previously (Hu et al., 2007, Liu et al., 2007). DOX, dimethyl sulphoxide (DMSO), hydrogen peroxide, DAPI, hydrocortisone-21-acetate (HYC), dexamethasone (DEX) and 17β-estradiol (17β-E) were purchased from Sigma (St. Louis, MO, USA). Hygromycin B was purchased from Invivogen (La Jolla, CA, USA). HYC, DEX and 17β-E were dissolved in DMSO and kept as 1 mM stock at −20°C. The deuterium (D)-labeled amino acid [4,4,5,5-D4] lysine (LysD4) was purchased from Cambridge Isotope (Andover, MA, USA) as previously described (Gu et al., 2004).
Treatment of human intestine CCL-6 cells with hormones
Human CCL-6 cells were cultured in DMEM supplemented with 10% regular FBS and antibiotics. At 60% confluence, cells were rinsed in PBS and re-fed with the complete medium containing 1 µM HYC, 1µM DEX, 1 µM, 5 µM or 20 µM 17β-E for 24 hrs and were subjected to RNA isolation.
Semi-quantitative RT-PCR
Total RNA was isolated from cells using a Purescript kit (Gentra Systems, USA). RT was conducted using P5CS specific primer (DV1537) 5’-TCCACAATGTCTGTGATGACGTGC-3’ and PCR was conducted using two other P5CS gene specific primers and a standard protocol as described (Hu et al., 1999). The primers used for PCR were these: reverse, (DV2374) 5’-CAGTCGGGCAGCCAGGCTATCATTATC-3’ and forward, (DV1475) 5’-CCAGCTGAGCCCAACAGTGACC-3’. α-tubulin was used as a loading control.
Quantitative proteomic analysis of regulated proteins in p53-induced apoptosis
A newly developed methodology that combined incorporation of deuterium-labeled amino acid into the proteome and tandem mass spectrometry was used to profile and quantitate regulated proteins in apoptosis was used as previously described (Gu et al., 2004, Liu et al., 2005b). Briefly, stably transfected, “Tet-Off” inducible DLD-1.p53 and DLD-1.vector cells were first maintained in D.20 medium supplemented with 10% dialyzed fetal bovine serum, then the induced cells were cultured in the Amino Acid-Code Tagging (AACT) medium which contained the LysD4. At about 60% confluence, cells were rinsed in PBS and re-fed with the induction medium for the induction of p53 and apoptosis for 22 h. Equal numbers of the induced AACT-labeled DLD-1.p53 and the unlabeled DLD-1.vector cells were mixed and lysed in 1× SDS-PAGE loading buffer. Proteomic liquid chromatography and QSTAR tandem mass spectroscopy (LCQ MS/MS) analysis were carried out as previously described. We compared the peak areas of unlabeled and labeled peptides. Peptides that showed altered ratios in the paired unlabeled and labeled peaks were subjected to a sequence search as described (Gu et al., 2004).
Immunoblot analysis
Total cellular extracts were isolated and used for immunoblot analysis from indicated cells as previously described (Liu et al., 2005a; Hu et al., 2007). Proteins were separated using 10 % SDS-PAGE (20 µg protein/lane) and transferred to nitrocellulose membranes, which were then incubated with polyclonal anti-P5CS antibody as described (Baumgartner et al., 2000). Subsequently, the blots were incubated with HRP-labeled secondary antibody (Bio-Rad, Hercules, CA, USA). β-actin was used as loading control. Proteins were detected using ECL+plus western blotting detection system (Amersham Biosciences, Piscataway, NJ, USA).
P5CS Adenovirus (AD-P5CS) construction, infection and cell death assay
Construction, amplification and assaying for titers of AD-P53, AD-P5CS.long and AD-P5CS.short, and viral infection were performed as previously described (Liu et al., 2005b; Liu et al., 2007). DLD-1 Cells were plated on P100 plates and grown to 70–80% confluence, rinsed with PBS and refed with virus-containing medium with a range of virus concentrations from 0.01 to 5 multiplicity of infection (MOI) (or pfu/cell, which was estimated to be 0.2–100 virus particles/cell) for 36 hrs. After incubation, attached cells were harvested and counted using a hemocytometer. To confirm that cell death was due to apoptosis, total cells, including floating cells, were collected by centrifugation and stained with Hoechst 33258 as previously described (Liu et al., 2005a). Shrunk, rounded up and multi-nucleated cells observed under the fluorescence microscope were considered to be apoptotic cells.
Results and Discussion
P5CS.long, no P5CS.short, is expressed in established human cell lines
To investigate patterns of expression of P5CS.long and P5CS.short in established human cell lines and to search for cellular model(s) to explore the molecular regulators that dictate the exon sliding selection in P5CS expression, we performed RT-PCR analysis of total RNA isolated from various human cell lines, including fibroblasts, small intestine cell line CCL-6, HEK 293, HepG2 and prostate cancer cell lines, LNCaP, PC3 and DU145. The PCR primers were designed to differentiate the long and short products differing in length by 6 bps (Hu et al., 1999). We showed that only the P5CS.long transcripts were detected in established human cell lines. In other words, expression of the short P5CS transcript was lost in established cell lines including those that were originated from the gut (Fig 2 A&B). We previously showed that at the tissue level the P5CS.short is predominantly expressed in human small intestine whereas the P5CS.long predominates in all other tissues. However, the P5CS.short transcript is always detectable in human tissues (Fig 1; Hu et al., 1999). The mechanism of complete loss of expression of P5CS.short in established cell lines is currently unknown.
Fig 2.
A Expression of P5CS.long in established human cell lines. RT-PCR analysis showed that P5CS.long, no P5CS.short, is expressed in established human cell lines. B Regulation of P5CS.long expression by hormones. Semi-quantitative RT-PCR was conducted using total RNA isolated from human small intestine CCL-6 cells treated with indicated compounds for 24 hrs.
Regulation of P5CS expression by hormones
Metabolism of proline, arginine and some other amino acids has been shown to be modulated by insulin, glucocorticoids, estrogen cAMP in a variety of animal and cellular models. For example, Guoyao Wu’s group has shown that glucocorticoids play a crucial role in the weaning maturation of intestinal enzymes and stimulate the catabolism of several amino acids, including glutamine, proline and arginine (Flynn and Wu, 2003; Wu et al., 2000). They showed that expression of P5CS, OAT, ornithine decarboxylase and arginase were upregulated by glucocorticoid surge in postnatal pigs. In addition, other groups showed that levels of the urea cycle enzymes in vivo undergo coordinate, adaptive changes in response to alterations in dietary protein intake or catabolic state, which are mediated largely by hormones such as insulin, glucagon (via cAMP), and glucocorticoids (Morris 2002; Ulbright et al., 1993). Furthermore, effects of dexamethasone (DEX), a potent synthetic member of the glucocorticoids, on amino acid metabolism in rat liver have been investigated. Importantly, glucocorticoids and estrogen have been shown to regulate RNA splicing of genes encoding urea cycle enzymes in rat (Norgren et al., 1994; Morris et al., 1987). To investigate whether expression and alternative splicing of human P5CS can be regulated by glucocorticoids and estrogen, we treated human small intestine CCL-6 cells with hydrocortisone (HYC), DEX and 17β estradiol (17β-E) and conducted semi-quantitative RT-PCR analysis. Interestingly, we showed that expression of P5CS.long was downregulated by glucocorticoids, HYD and DEX, but upregulated by estrogen, 17β-E (Fig 2B). In addition, we did not observe expression of P5CS.short in CCL-6 cells after any of the hormone treatments, suggesting that there are factors dictating the selection of exon sliding exclusively for generation of P5CS.long. It would be of importance to investigate how P5CS.short expression is regulated by other hormones and/or alternative splicing factors in tissues/cells isolated from model animals (e.g., pig and rat).
Expression of human P5CS is upregulated by p53 as revealed by quantitative proteomics, RT-PCR and immunoblot analyses
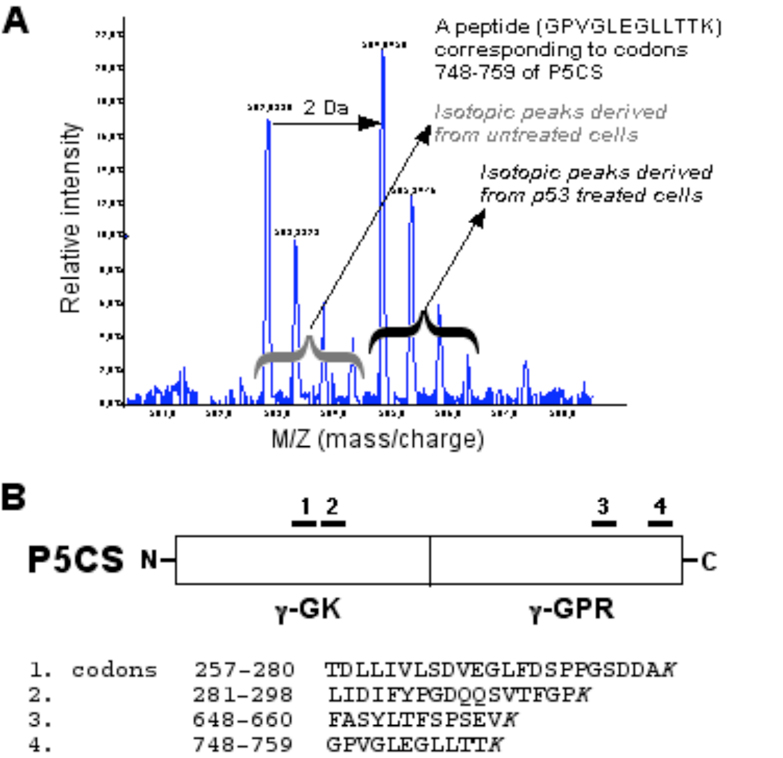
Two proline metabolic enzymes, POX (Polyak et al., 1997) and P5CDH (Yoon et al., 2004) have been shown to be upregulated by p53, a tumor suppressor protein, which plays a pivotal role in regulating cell cycle arrest, angiogenesis, differentiation and apoptosis (Vogelstein et al., 2000; Vousden and Lu, 2002; Vousden and Prives, 2005). In apoptosis, the elevated expression of p53 leads to mitochondrial-mediated pathway, in part, through the overproduction of reactive oxygen species (ROS) (Johnson et al., 1996). Genes that are directly involved in the generation of ROS are induced in p53-mediated apoptotic pathway (Vogelstein et al., 2000). For example, POX, also known as PIG6, is one of the p53 inducible genes (PIGs), when over-expressed in cells, triggers ROS generation and apoptosis (Polyak, et al., 1997; Phang et al., this issue). Importantly, it has been shown that expression of P5CS is induced in apoptotic LNCaP cells that were treated with epigallocatechin-3-gallate, the major polyphenolic constituent in green tea (Adhami et al., 2003). Using MALDI-TOF tandem mass spectrometry in profiling the regulated proteins by p53, we previously identified and characterized proteins that are upregulated at the execution stage of the p53-mediated apoptosis. Approximately 70 differentially regulated proteins, for example, 14-3-3 δ, cyclophilin A, cyclophilin B, peroxiredoxins, annexin IV, GAPDH, pyruvate kinases, and adenine nucleotide translocator (ANT), were identified in the late stage of p53-induced apoptosis. Based on functions of those upregulated proteins, the p53-induced apoptosis involves the systematic activation of multiple pathways that are glycolysis-relevant, energy-dependent, oxidative stress-mediated, and possibly mediated through inter-organelle crosstalk (Gu et al., 2004). In the same experiment, we also found that P5CS was upregulated (by 47%) in induced DLD-1.p53 cells. Four peptides derived from P5CS were identified in the analysis and all shown to be upregulated by p53 (Fig. 3). For example, spectrum analysis of a peptide corresponding to codons 748 to 759 of P5CS showed significantly increased intensity in the labeled isotopic peaks, indicating upregulation of the corresponding protein by p53 (Fig. 3A).
Fig 3.
Expression of human P5CS is upregulated by p53 as revealed by quantitative proteomic analysis. MALDI-TOF tandem mass spectrometry was used to profile the regulated proteins by p53. A An example of spectrum analysis of a peptide showed significant signal increase and 2-Da shift in the labeled isotopic peaks, indicating upregulation of the corresponding protein by p53. This peptide corresponded to codons 748–759 of human P5CS. B Four peptides derived from P5CS were identified and shown to be upregulated by p53. The identified peptides were located on a schematically presented P5CS protein, and corresponding codons and sequence of each peptide were as indicated.
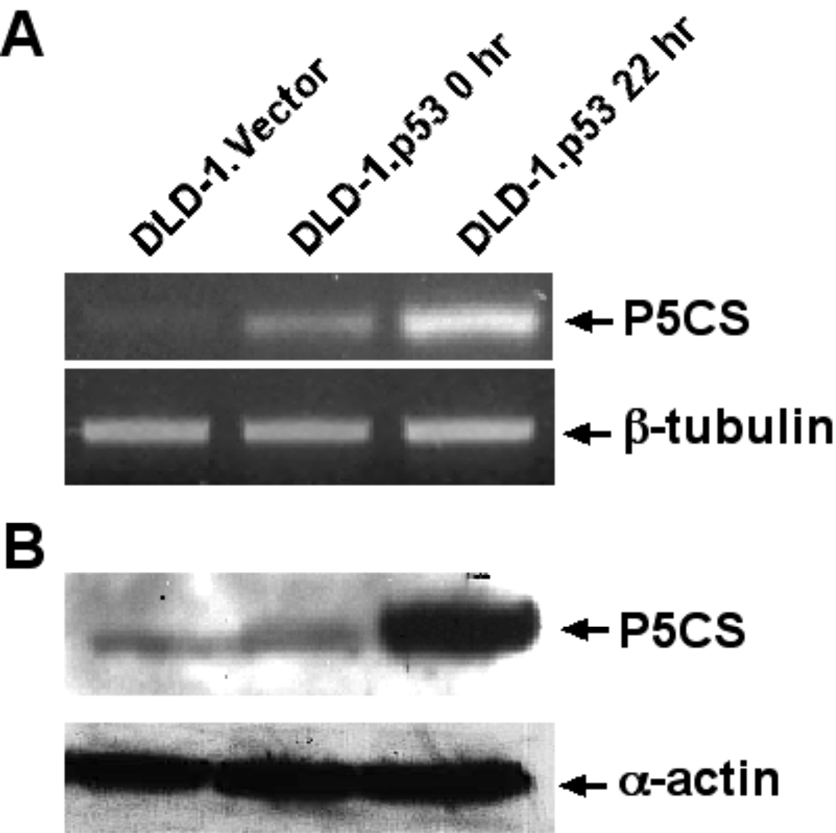
To further confirm p53 induces overexpression of human P5CS, we assayed expression of P5CS by semi-quantitative RT-PCR and immunoblot analyses. We showed significant upregulation of P5CS at both RNA and protein levels 22 hrs after p53 overexpression in DLD-1.p53 cells (Fig 4 A&B). Sequencing analysis of the RT-PCR product confirmed that it is P5CS.long, not P5CS.short, which was overproduced in apoptotic DLD-1.p53 cells. In addition, functional genomic analysis confirmed that there are two p53-binding consensus sequences in the promoter region and the intron 1 of the human P5CS gene (See Hu et al., this issue).
Fig 4.
P53 induces overexpression of human P5CS. A Semi-quantitative RT-PCR and B Immunoblot analysis confirmed that expression of P5CS is inducible by p53 at both RNA and protein levels 22 hrs after p53 overexpression in DLD-1.p53 cells. β-tubulin and α–actin served as loading control for RT-PCR and immunoblot analysis, respectively.
To determine whether P5CS.long or P5CS.short was able to induce cell death, adenoviruses harboring full-length P5CS.long (AD- P5CS.long) or P5CS.short (AD- P5CS.short) were constructed and used to infect a variety of cancer cells (e.g., DLD-1, MCF7 (breast), and Hec50co (endometrial) cells). We showed that AD-TP53, as a positive control, induced ~40% apoptotic cell death 36 hr after infection. By contrast, cells infected with AD-P5CS.long or AD-P5CS.short were healthy and showed no sign of death or cell cycle arrest (Figure 5). Consistent with this observation, we previously showed that P5CS.long or P5CS.short stably transfected CHO cells, which expressed high levels of P5CS activity, were healthy (Hu, 1999; Baumgartner, 2000). Taken together, our results showed that overexpression of P5CS.long or P5CS.short does not induce cell death or growth arrest in human cells. It is logical to speculate that ROS overproduction and oxidative stress induced by p53 may induce P5CS expression. Our preliminary data indicated it is indeed the case: expression of P5CS is inducible by H2O2 in HepG2 and DLD-1 cells (Khalil and Hu, unpublished observation).
Fig 5.
Overexpression of human P5CS failed to induce cell death or growth arrest in human cells. DLD-1 cells were infected with AD-p53, AD-P5CS.long or AD-P5CS.short. Attached cells were counted 36 hrs after infection. AD-p53 clearly induced apoptosis whereas P5CS.long and P5CS.short did not.
There are several questions that deserve further investigation. First, P5CS appears to be induced by p53 in p53-induced apoptosis, however, overexpression of P5CS.long or P5CS.short is not sufficient to field cell death or growth arrest. The question is whether P5CS truly has a role in apoptosis or does it need other molecule(s) to initiate cell death? Second, how alternative splicing of P5CS expression is regulated in mammals? Evidently the established human cell lines are not the appropriate system to answer this question since expression of P5CS.short is lost in established cultured cell lines. One should consider use animal models, such as pig and rodents, to dissect the molecular basis of exon sliding in P5CS expression. Nevertheless, the diverse roles P5CS plays in different biochemical functions in mammalian cells and links to an important but easy-to-miss inborn error make it an enzyme worthy of in-depth investigations.
Acknowledgements
This work is supported by NM-INBRE grant (2 P20 RR016480-04), DOD PCRP (#W81XWH-05-1-0357) and NCI-RO1 (5RO1 CA106644) (to Hu CAA) and Howard Hughes Medical Institute (to Valle D).
References
- Adhani VM, Ahmad N, Kukhtar H. Molecular targets for green tea in prostate cancer prevention. J Nutr. 2003;133:2417S–2424S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- Adams E, Frank L. Metabolism of proline and the hydroxyproline. Annu Rev Biochem. 1980;49:1005–1061. doi: 10.1146/annurev.bi.49.070180.005041. [DOI] [PubMed] [Google Scholar]
- Aral B, Schlenzig JS, Liu G, Kamoun P. Database cloning human delta 1-pyrroline-5-carboxylate synthetase (P5CS) cDNA: a bifunctional enzyme catalyzing the first 2 steps in proline biosynthesis. C R Acad Sci III. 1996;319:171–178. [PubMed] [Google Scholar]
- Baumgartner MR, Aral B, Rabier D, Kamoun P, Valle D, Saudubray J-M. Hyperammonemia with reduced ornithine, citrulline, arginine and proline: a new inborn error caused by a mutation in the gene encoding delta-1-pyrroline-5-carboxylate synthase. Eur J Pediatr. 2005;164:31–36. doi: 10.1093/hmg/9.19.2853. [DOI] [PubMed] [Google Scholar]
- Baumgartner MR, Hu CAA, Almashanu S, Steel G, Obie C, Aral B, Rabier D, Kamoun P, Saudubray J-M, Valle D. Hyperammonemia with reduced ornithine, citrulline, arginine and proline: a new inborn error caused by a mutation in the gene encoding delta-1-pyrroline-5-carboxylate synthase. Hum Mol Gen. 2000;9:2853–2858. doi: 10.1093/hmg/9.19.2853. [DOI] [PubMed] [Google Scholar]
- Flynn NE, Wu G. Regulation of intestinal amino acid metabolism by glucocorticoids. Recent Research and Development in Physiology. 2003;1:169–178. [Google Scholar]
- Gu S, Liu Z, Pan S, Jiang Z, Lu H, Amit O, Bradbury EM, Hu CAA, Chen X. Global investigation of p53-induced apoptosis through quantitative proteomic profiling using comparative amino acid-coded tagging. Mol Cell Proteomics. 2004;3:998–1008. doi: 10.1074/mcp.M400033-MCP200. [DOI] [PubMed] [Google Scholar]
- Hagedorn CH, Phang JM. Transfer of reducing equivalents into mitochondria by the interconversions of proline and Δ1-pyrroline-5-carboxylate. Arch Biochem Biophys. 1983;225:95–101. doi: 10.1016/0003-9861(83)90010-3. [DOI] [PubMed] [Google Scholar]
- Hu CAA, Delauney A, Verma DPS. A bifunctional enzyme (Δ1-pyrroline-5-carboxylate synthetase) catalyzes the first two steps in proline biosynthesis. Proc Natl Acad Sci USA. 1992;89:9354–9358. doi: 10.1073/pnas.89.19.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CAA, Lin WW, Valle D. Cloning, characterization and expression of cDNAs encoding human Δ1-pyrroline-5-carboxylate dehydrogenase. J Biol Chem. 1996;271:9795–9800. doi: 10.1074/jbc.271.16.9795. [DOI] [PubMed] [Google Scholar]
- Hu CAA, Lin WW, Obie C, Valle D. Molecular enzymology of mammalian delta1-pyrroline-5-carboxylate synthase. Alternative splice donor utilization generates isoforms with different sensitivity to ornithine inhibition. J Biol Chem. 1999;274:6754–6762. doi: 10.1074/jbc.274.10.6754. [DOI] [PubMed] [Google Scholar]
- Hu CAA, Donald SP, Yu J, Lin WW, Liu Z, Steel G, Valle D, Phang JM. Overexpression of proline oxidase induces proline-dependent and mitochondria-mediated apoptosis. Mol Cell Biochem. 2007;295:85–92. doi: 10.1007/s11010-006-9276-6. [DOI] [PubMed] [Google Scholar]
- Johnson TM, Yu ZX, Ferrans VJ, Lowenstein RA, Finkel T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci USA. 1996;93:1848–1852. doi: 10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun P, Aral B, Saudubray JM. A new inherited metabolic disease: delta-1-pyrroline 5-carboxylate synthetase deficiency. Bull Nat Acad Med. 1998;182:131–139. [PubMed] [Google Scholar]
- Liu Y, Borchert GL, Surazynski A, Hu CA, Phang JM. Proline oxidase activates both intrinsic and extrinsic pathways for apoptosis: the role of ROS/superoxides, NFAT and MEK/ERK signaling. Oncogene. 2006;25:5640–5647. doi: 10.1038/sj.onc.1209564. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lu H, Jiang Z, Pastuszyn A, Hu CAA. Apolipoprotein L6, a novel proapoptotic Bcl-2 homology 3-only protein, induces mitochondria-mediated apoptosis in cancer cells. Mol Cancer Res. 2005a;3:21–31. [PubMed] [Google Scholar]
- Liu Z, Lu H, Shi H, Du Y, Yu J, Gu S, Chen X, Hu CAA. PUMA overexpression induces reactive oxygen species generation and proteasome-mediated stathmin degradation in colorectal cancer cells. Cancer Res. 2005b;65:1647–1654. doi: 10.1158/0008-5472.CAN-04-1754. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wan G, Heaphy C, Bisoffi M, Griffith JK, Hu CA. A novel loss-of-function mutation in TP53 in an endometrial cancer cell line and uterine papillary serous carcinoma model. Mol Cell Biochem. 2007;297:179–187. doi: 10.1007/s11010-006-9345-x. [DOI] [PubMed] [Google Scholar]
- Morris SM. Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- Morris SM, Moncman CL, Rand KD, Dizikes GJ, Cederbaum SD, O'Brien WE. Regulation of mRNA levels for five urea cycle enzymes in rat liver by diet, cyclic AMP, and glucocorticoids. Arch Biochem Biophys. 1987;256:343–353. doi: 10.1016/0003-9861(87)90455-3. [DOI] [PubMed] [Google Scholar]
- Norgen S, Li L-S, Luthman H. Regulation of human insulin receptor RNA splicing in HepG2 cells: Effects of glucocorticoid and low glucose concentration. Biochem Biophy Res Comm. 1984;199:277–284. doi: 10.1006/bbrc.1994.1225. [DOI] [PubMed] [Google Scholar]
- Phang JM. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr Topics Cell Regul. 1985;25:91–132. doi: 10.1016/b978-0-12-152825-6.50008-4. [DOI] [PubMed] [Google Scholar]
- Phang JM, Hu CA, Valle D. Disorders of proline and hydroxyproline metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Basis of Inherited Disease. New York: McGraw Hill Press; 2001. pp. 1821–1838. [Google Scholar]
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- Rabier D, Nuttin C, Poggi F, Padovani JP, Abdo K, Bardet J, Parvy P, Kamoun P, Saudubray JM. Familial joint hyperlaxity, skin hyperelasticity, cataract and mental retardation with hyperammonemia and low citrulline, ornithine and proline: a new disorder of collagen metabolism?. Abstracts of Free Communications, 30th Annual Symposium Leuven; Sept. 8-11, 1992; Leuven: The Society for the Study of Inborn Errors of Metabolism (SSIEM); 1992. Note: P. 61. [Google Scholar]
- Ulbright C, Snodgrass PJ. Coordinate induction of the urea cycle enzymes by glucagon and dexamethasone is accomplished by three different mechanisms. Arch Biochem Biophys. 1993;301:237–243. doi: 10.1006/abbi.1993.1139. [DOI] [PubMed] [Google Scholar]
- Valle D, Simell O. Hyperornithinemias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Basis of Inherited Disease. New York: McGraw Hill Press; 2001. pp. 1857–1895. [Google Scholar]
- Vasiliou V, Bairoch A, Tipton KF, Nebert DW. Eukaryotic aldehyde dehydrogenase (ALDH) genes: human polymorphisms, and recommended nomenclature based on divergent evolution and chromosomal mapping. Pharmacogenetics. 1999;9:421–434. [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Prives C. P53 and prognosis: new insights and further complexity. Cell. 2005;120:7–10. doi: 10.1016/j.cell.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Wang T, Milam AH, Steel G, Valle D. A mouse model of gyrate atrophy of the choroids and retina. Early retinal pigment epithelium damage and progressive retinal degeneration. J Clin Invest. 1996;97:2753–2762. doi: 10.1172/JCI118730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Knabe DA. Arginine synthesis in enterocytes of neonatal pigs. Comp Physiol. 1995;269:R621–R629. doi: 10.1152/ajpregu.1995.269.3.R621. [DOI] [PubMed] [Google Scholar]
- Wu G, Meininger CJ, Kelly K, Watford M, Morris SM., Jr A cortisol surge mediates the enhanced expression of pig intestinal pyrrolinee-5-carboxylate synthesis during weaning. J Nutri. 2000;130:1914–1919. doi: 10.1093/jn/130.8.1914. [DOI] [PubMed] [Google Scholar]
- Wu G, Morris SM. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K-A, Nakamura Y, Arakama H. Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. J Hum Genet. 2004;49:134–140. doi: 10.1007/s10038-003-0122-3. [DOI] [PubMed] [Google Scholar]