Summary
Pathways in the B7:CD28 family regulate T cell activation and tolerance. B7 dependent responses in CD28/CTLA-4-/- T cells together with reports of stimulatory and inhibitory functions for PD-L1 and PD-L2 have suggested additional receptors for these B7 family members. We show that B7-1 and PD-L1 interact with an affinity intermediate to that of B7-1:CD28 and B7-1:CTLA-4. The PD-L1:B7-1 interface overlaps with the B7-1:CTLA-4 and PD-L1:PD-1 interfaces. We show that the interaction of B7-1 with PD-L1 inhibits T cell activation and cytokine production by comparing responses of T cells deficient in the well-described (CD28, CTLA4, and PD-1) and newly-characterized (B7-1 and PD-L1) receptors to either B7-1-Ig or PD-L1-Ig. The responses of PD-1-/- vs. PD-1/B7-1-/- T cells to PD-L1 and CD28/CTLA-4-/- vs. CD28/CTLA-4/PD-L1-/- T cells to B7-1 demonstrate that PD-L1 and B7-1 interact specifically to inhibit T cell activation. Our findings point to a significant bidirectional inhibitory interaction between B7-1 and PD-L1 and add an additional dimension to immunoregulatory functions of the B7:CD28 family.
Introduction
Pathways in the B7:CD28 family provide critical signals that regulate T cell activation and tolerance (Chen, 2004; Chikuma and Bluestone, 2003; Greenwald et al., 2005). The B7-1/B7-2:CD28/CTLA-4 pathway is the best characterized pathway, but is complex because of the dual specificity of the B7-1 (CD80) and B7-2 (CD86) costimulatory molecules for the CD28 and CTLA-4 (CD152) receptors. CD28 is constitutively expressed on T cells and its activation enhances T cell receptor (TCR) signaling. The higher affinity receptor CTLA-4 is rapidly induced in T cells upon TCR stimulation and its engagement diminishes TCR signaling. The B7-1 and B7-2 molecules differ in their expression on antigen presenting cells (APCs): B7-2 is constitutively expressed at low levels and rapidly upregulated, whereas B7-1 is inducibly expressed later than B7-2. B7-1 and B7-2 are not only expressed on APCs, but also are expressed on T cells. Although the function of B7-1 and B7-2 as costimulatory molecules on APCs is well established, their roles on T cells are less well understood. Recent studies have suggested that B7 on T cells may serve to down-regulate responses and deliver signals into T cells. B7-1 deficiency on T cells resulted in accelerated graft-versus-host disease in a model of allogeneic bone marrow transplantation (Taylor et al., 2004), and B7-1 expression on effector T cells was shown to be required for suppression by regulatory T cells in a colitis model (Paust et al., 2004).
Over the past several years the B7:CD28 family has grown. One of the newer pathways comprises the Programmed Death-1 (PD-1) receptor (CD279) and its two ligands, PD-L1 (B7-H1; CD274) (Dong et al., 1999; Freeman et al., 2000) and PD-L2 (B7-DC; CD273) (Latchman et al., 2001; Tseng et al., 2001). In contrast to CD28 and CTLA-4, PD-1 is inducibly expressed on T cells, B cells and monocytes upon activation (Okazaki et al., 2002). The two PD-1 ligands differ in their expression patterns: PD-L1 is constitutively expressed and upregulated to higher levels on murine hematopoietic cells (e.g., T cells, B cells, macrophages, dendritic cells (DCs), and bone marrow-derived mast cells) and non-hematopoietic cells (e.g., endothelial, epithelial, and muscle cells), while PD-L2 is only inducibly expressed on DCs, macrophages, and bone marrow-derived mast cells.
The roles that PD-L1 and PD-L2 play in T cell activation are only beginning to be understood. Although a number of studies have demonstrated inhibitory functions for PD-L1 and PD-L2, others reported that PD-L1 and PD-L2 can stimulate T cell proliferation and cytokine production (Dong et al., 2002; Dong et al., 1999). The reasons for the contradictory results of these functional studies are not yet clear. While a second stimulatory receptor for PD-Ls has been postulated (Dong and Chen, 2006; Liu et al., 2003; Shin et al., 2003), it has yet to be identified.
Our studies of T cells lacking CD28 and CTLA-4 provided evidence for an additional functional receptor for B7-1 or B7-2 on T cells. When stimulated with anti-CD3 in the presence of wild-type APCs, wild-type T cells proliferated more than CD28/CTLA4−/− T cells. However, the proliferation of both wild-type and CD28/CTLA4−/− T cells was reduced when CTLA-4-Ig was added to the cultures (Mandelbrot et al., 2001). These results motivated us to search for an additional receptor for B7-1 or B7-2.
Here we identify B7-1 and PD-L1 as binding partners. Expression cloning using a cDNA library prepared from CD28/CTLA4−/− T cells revealed PD-L1 as a binding partner for B7-1. We used biophysical techniques to demonstrate that B7-1 can specifically bind to PD-L1 and characterized the affinity of their interaction as being intermediate to that of B7-1 with CD28 and B7-1 with CTLA-4. Using protein cross-linking and mass spectrometry, we showed that the interaction site between these two proteins overlaps the B7-1:CTLA-4 interface and the PD-L1:PD-1 interface. Finally, functional studies indicated that the B7-1:PD-L1 interaction can inhibit T cell proliferation and cytokine production. These findings reveal an additional means by which B7-1 and PD-L1 can regulate T cell responses and lead to a revised view of the functions of pathways within the B7:CD28 family.
Results
Identification of PD-L1 as a ligand for B7-1
We sought other binding partners for B7-1 from a cDNA library made from mRNA of activated CD28/CTLA-4-/- CD4 T cells (Mandelbrot et al., 1999). The cDNA library was transfected into COS cells and panned on a plate coated with B7-1-Ig fusion protein. After three rounds of immunoselection, cDNA plasmids from adherent cells were sequenced. All of the plasmids (of 6 sequenced) revealed a cDNA encoding mPD-L1 (Supplemental Figure 1). The cDNA clones varied slightly in the length of the 5′ untranslated region, indicating that the PD-L1 gene was independently isolated multiple times. These findings suggested that PD-L1 transfected into COS cells bound immobilized B7-1.
Surface plasmon resonance establishes B7-1:PD-L1 affinity
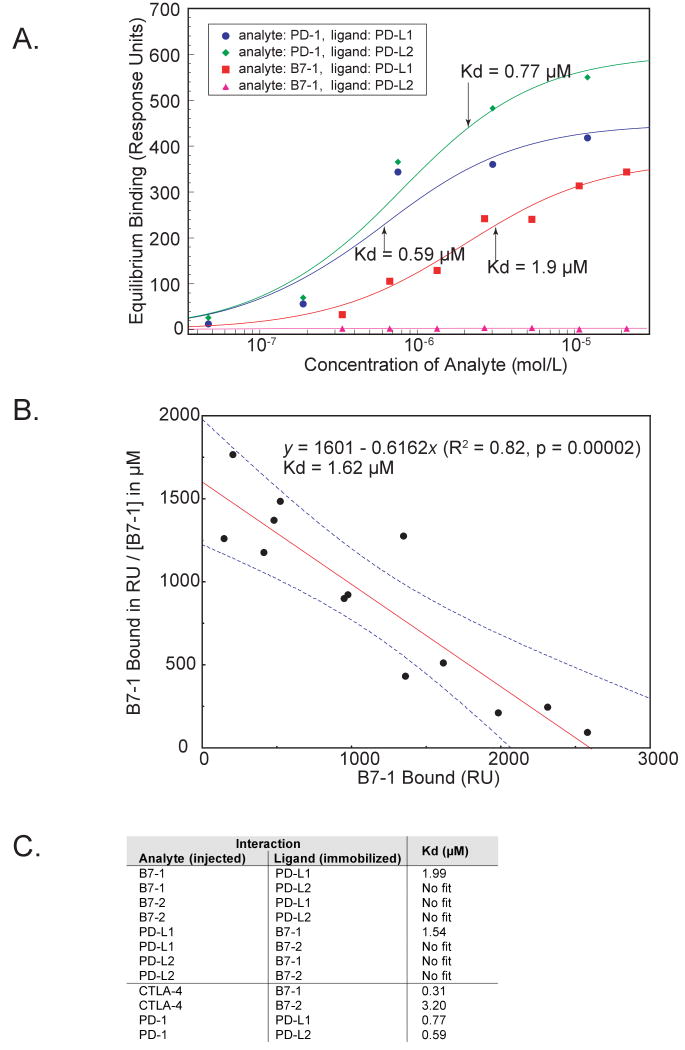
To investigate whether B7-1 can associate with PD-L1, we employed the technique of surface plasmon resonance and followed the interaction of PD-L1, B7-1, or control proteins. We immobilized PD-L1-Ig and PD-L2-Ig and injected B7-1-Ig and B7-2-Ig at various concentrations, and vice versa. We calculated affinities from equilibrium binding to eliminate considerations of mass-transport and to avoid the complexities of kinetic analyses of divalent ligands (kinetic data for B7-1 binding and dissociation to immobilized PD-L1 are shown in Supplemental Figure 2A). There was a specific interaction between B7-1 and PD-L1 with a dissociation constant (Kd) of 1.9 μM. In the reversed experiment where B7-1 was immobilized and PD-L1 injected, a Kd of 1.5 μM was determined (Figure 1). By comparison, the binding affinities of PD-1 to PD-L1 or PD-L2 were approximately three times stronger (Kd of 0.59 and 0.77 μM, respectively), and CTLA-4 for B7-1 was about seven times stronger (Kd of 0.31 μM; equilibrium binding data for B7-1:CTLA-4 shown in Supplemental Figure 2B). Our dissociation constants were similar to those published for PD-L1 and PD-L2 to PD-1 (0.52 μM and 0.26 μM, respectively) (Youngnak et al., 2003), and for B7-1 and B7-2 to CTLA-4 (0.2 μM and 2.6 μM, respectively) (Collins et al., 2002; van der Merwe et al., 1997). We did not observe any specific interaction between B7-1 and PD-L2, between B7-2 and PD-L1 or PD-L2, nor with control proteins. These results show a specific and unique interaction between PD-L1 and B7-1, with an affinity (∼1.7 μM) intermediate between the affinities of B7-1 for CD28 (4 μM) and CTLA-4 (0.2 μM), and PD-L1 for PD-1 (0.5 μM).
Figure 1. Equilibrium binding constants of B7-1 and PD-L1 interactions.
A) Equilibrium binding responses were measured using surface plasmon resonance of fusion proteins of B7-1, B7-2, and PD-1 to immobilized PD-L1 and PD-L2 fusion proteins. No binding response was seen for B7-2 to PD-L1 or PD-L2 (not shown). The Kds for the three significant interactions are shown (arrows).
B) Scatchard plot shows the dissociation constant for B7-1 to immobilized PD-L1 is 1.6 μM. The linear fit and its 90% confidence interval are shown.
C) Summary of dissociation constants obtained from Biacore equilibrium binding experiments.
To independently measure the dissociation constant between B7-1 and PD-L1 in solution, we performed analytical ultracentrifugation of the proteins individually and together at a concentration above their putative Kd. When B7-1 and PD-L1 were mixed, they shifted their concentration gradient at equilibrium, yielding a dissociation constant of 1.6 μM (Supplemental Figure 3). Additionally, dynamic light scattering with the B7-1, PD-L1, PD-L2, and CTLA-4 fusion proteins revealed a statistically significant increase in complex size for B7-1:PD-L1 and B7-1:CTLA-4. No increase in size was observed when PD-L1-Ig was incubated with a control Ig fusion protein (Supplemental Figure 4). These results confirm that a specific, high affinity interaction exists between B7-1 and PD-L1.
Chemical cross-linking reveals binding site for PD-L1 on B7-1
To determine which molecular surfaces of the B7-1 and PD-L1 molecules are involved in their binding, we performed cross-linking with the heterobifunctional cross-linker sulfo-SBED. B7-1-Ig was linked to sulfo-SBED, mixed with PD-L1-Ig, and cross-linked with UV light. The complex was separated by non-reducing SDS-PAGE and stained with Coomassie blue (Figure 2A). The band corresponding to a molecular weight equal to the sum of the B7-1-Ig and PD-L1-Ig was excised, washed, and subjected to proteolysis with chymotrypsin. The resulting fragments were analyzed by mass spectrometry, and a database search revealed that peptides from both B7-1 and PD-L1 were among these fragments. To determine if the fragments were physically linked, we subjected them to high-resolution quadrupole-TOF mass spectrometry and compiled a list of fragments containing a peptide from B7-1, a peptide from PD-L1, plus the cross-linker (Supplemental Figure 5). This list showed three principal areas of cross-linking between B7-1 and PD-L1 (Figure 2B) that comprised 84% of the annotated peptides.
Figure 2. Localization of the B7-1:PD-L1 binding site.
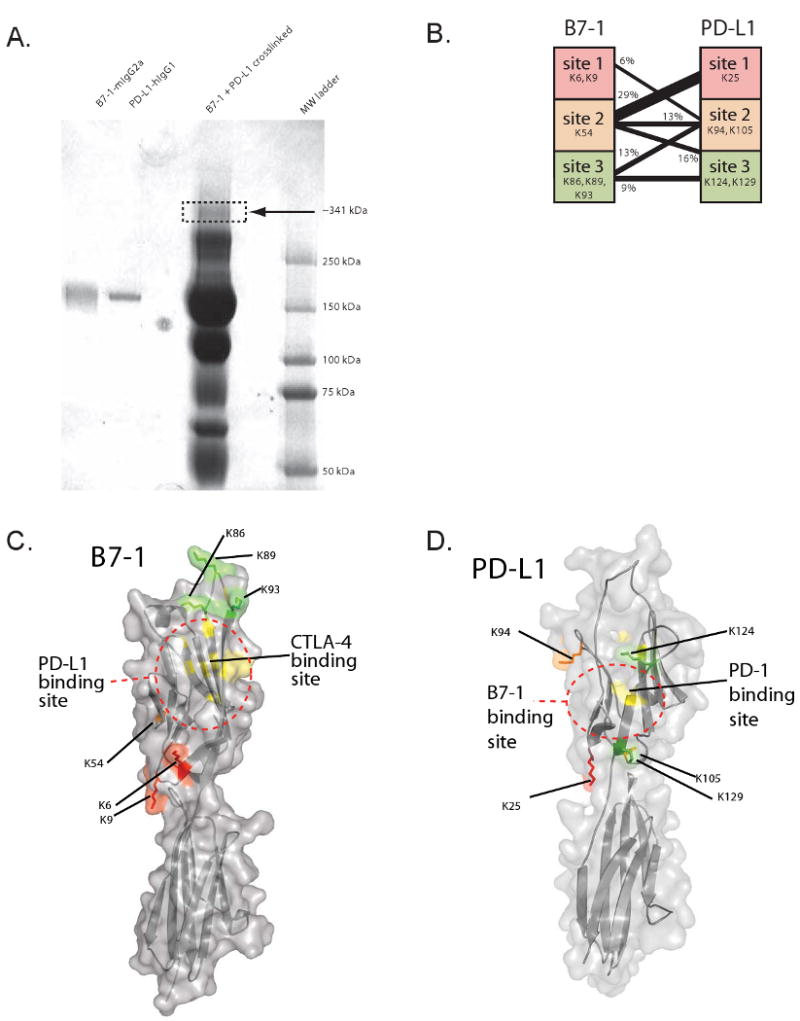
A) B7-1-Ig and PD-L1-Ig and the products of cross-linking with the heterobifunctional crosslinker sulfo-SBED are shown in an SDS-PAGE gel stained with Coomassie Blue. Arrow indicates band of gel that was cut out for mass spectrometric analysis.
B) Peptides were categorized into sites on PD-L1 and B7-1 and grouped according to their lysines. Amino acid numbers start from the position after signal cleavage. The number of distinct peptides containing the sites and the cross-linker were counted and the results are shown graphically.
C and D) Molecular model of (C) B7-1 and (D) PD-L1 showing sites of interaction identified by cross-linking. On each structure, lysines are colored: site 1 lysines are colored red, site 2 lysine(s) are colored orange, and site 3 lysine(s) are colored green. The binding site for CTLA-4 (Stamper et al., 2001) is shown in yellow on the B7-1 model, and the binding site for PD-1 (Wang et al., 2003) is shown in yellow on the PD-L1 model. Dotted lines are shown to summarize the putative B7-1:PD-L1 binding site. There is partial overlap among the B7-1:CTLA-4, B7-1:PD-L1, and PD-L1:PD-1 interfaces.
Because sulfo-SBED has flexible arms, the lysine residues from these cross-linked peptides provide a boundary to the interface of interaction for B7-1 and PD-L1. To identify the B7-1:PD-L1 interaction site, we mapped these lysines onto the crystal structure of B7-1 and onto a molecular model of PD-L1 that we generated from the structure of B7-1 (Ikemizu et al., 2000). We also superimposed the putative binding sites for CTLA-4 (Stamper et al., 2001) and PD-1 (Wang et al., 2003) onto the models (Figure 2C and 2D). These mappings show that the B7-1 and PD-L1 interaction occurs on the GFCC′C″ face of B7-1, suggesting a partial overlap with that of B7-1:CTLA-4. Likewise, the PD-L1 side of the B7-1:PD-L1 overlaps at least partially with the putative GFCC′C″ interface of PD-L1:PD-1 in that they share two lysines (K124 and K129). The partial overlap of the B7-1:PD-L1 interface with those of B7-1:CTLA-4 and PD-L1:PD-1 suggests that CTLA-4, CD28, and PD-L1 could compete for binding to B7-1. Similarly, B7-1 and PD-1 may compete for binding to PD-L1.
Cell adhesion assay shows that cell surface B7-1:PD-L1 can interact
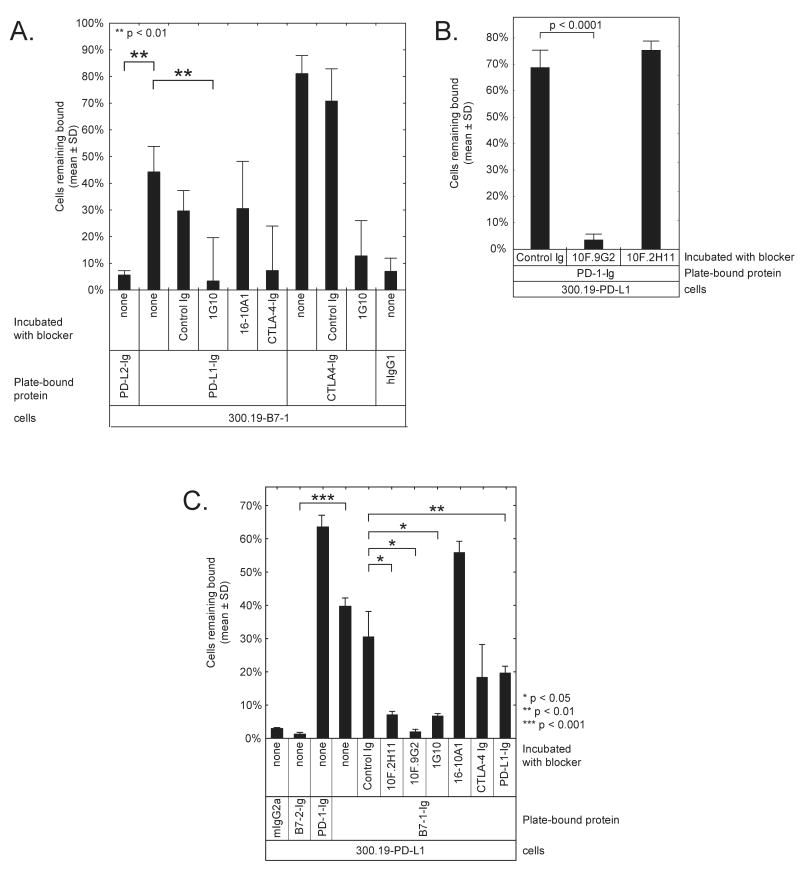
To verify the interaction between B7-1 and PD-L1 when these proteins are expressed on living cells and to further characterize the B7-1:PD-L1 interaction, we used an avidity-based cell adhesion assay. We found that mB7-1 transfectants of 300.19 cells adhered to CTLA-4-Ig-coated wells (∼80% of cells bound) and PD-L1-Ig (∼40% binding) but not to PD-L2-Ig or hIgG1-Fc coated wells (<5%) (Figure 3A). Untransfected 300.19 cells did not adhere to any of the wells (<1%, not shown). To demonstrate that the binding to PD-L1 was specifically due to B7-1, we pre-incubated the cells with anti-B7-1 mAbs (clones 16-10A1 (Razi-Wolf et al., 1992) or 1G10 (Nabavi et al., 1992)), CTLA-4-Ig, or a control IgG1. Both anti-B7-1 mAbs could bind to B7-1 on the transfected 300.19 cells, but did not bind to control 300.19 cells (data not shown). The 1G10 antibody and CTLA-4-Ig blocked the interaction between B7-1 on the 300.19-B7-1 cells and PD-L1 on the plate (p < 0.01), confirming that the adhesion of the cells to the plate-bound PD-L1 was due to B7-1. The other anti-B7-1 antibody (16-10A1) and the rat control antibody did not block adhesion to PD-L1 (Figure 3A). These results suggest that 1G10 and 16-10A1 engage different epitopes on B7-1, and that only the 1G10 epitope overlaps with that of the B7-1:PD-L1 interface. The partial blocking by CTLA-4-Ig confirms an overlap of the B7-1:PD-L1 binding interface with that of the CTLA-4 binding site on B7-1.
Figure 3. Cell adhesion assay shows specific binding of B7-1 to PD-L1.
A) Adhesion assay with 300.19-B7-1 cells shows specific binding to PD-L1. The mouse pre-B cell line 300.19 was stably transfected with mB7-1 (300.19-B7-1) and labeled with BCECF. Some cells were pre-incubated with 40 μg/mL of anti-B7-1 mAb or CTLA4-Ig, as indicated. Wells were coated with PD-L1-Ig, PD-L2-Ig, CTLA-4-Ig, or hIgG1-Fc and blocked. Fluorescent 300.19-B7-1 cells were introduced into the wells. Fluorescence was measured before and after washing. Untransfected 300.19 cells showed less than 1% binding to similarly coated wells (not shown).
B. Adhesion assay with 300.19 cells stably transfected with mPD-L1 (300.19-PD-L1) demonstrates the differential ability of two anti-PD-L1 mAbs to block the interaction between PD-L1 and PD-1.
C. Adhesion assay with 300.19-PD-L1 cells shows specific binding to B7-1. Wells were coated with B7-1-Ig, B7-2-Ig, PD-1-Ig, or hIgG1-Fc and blocked, then some cells were incubated with 20 μg/mL of the indicated mAbs. 300.19-PD-L1 cells were labeled with BCECF, introduced into the wells, and fluorescence was measured before and after washing. Untransfected 300.19 cells showed less than 4% binding (not shown).
To independently characterize the B7-1:PD-L1 interaction, we repeated the adhesion assay using 300.19 cells transfected with mPD-L1 and allowed them to engage plate-bound B7-1-Ig. We used the following mAbs to characterize the specificity of the interaction: two anti-PD-L1 antibodies, one of which blocks the PD-L1:PD-1 interaction (10F.9G2 (Rodig et al., 2003)), and one that does not (10F.2H11), (Figure 3B), as well as the two anti-B7-1 antibodies used above. 300.19-PD-L1 cells bound B7-1 (36% binding) but not B7-2 (1% binding) (Figure 3C). Both anti-PD-L1 antibodies and the 1G10 anti-B7-1 antibody were able to block the PD-L1:B7-1 interaction. CTLA-4-Ig, PD-L1-Ig, and PD-1-Ig showed intermediate ability to block the PD-L1:B7-1 interaction, which corroborates their overlapping interfaces. These results together confirm that B7-1 and PD-L1 expressed on cell surfaces can interact, and that this interaction can be specifically blocked.
In vitro proliferation of CD28/CTLA4-/- T cells is inhibited by B7-1-coated beads
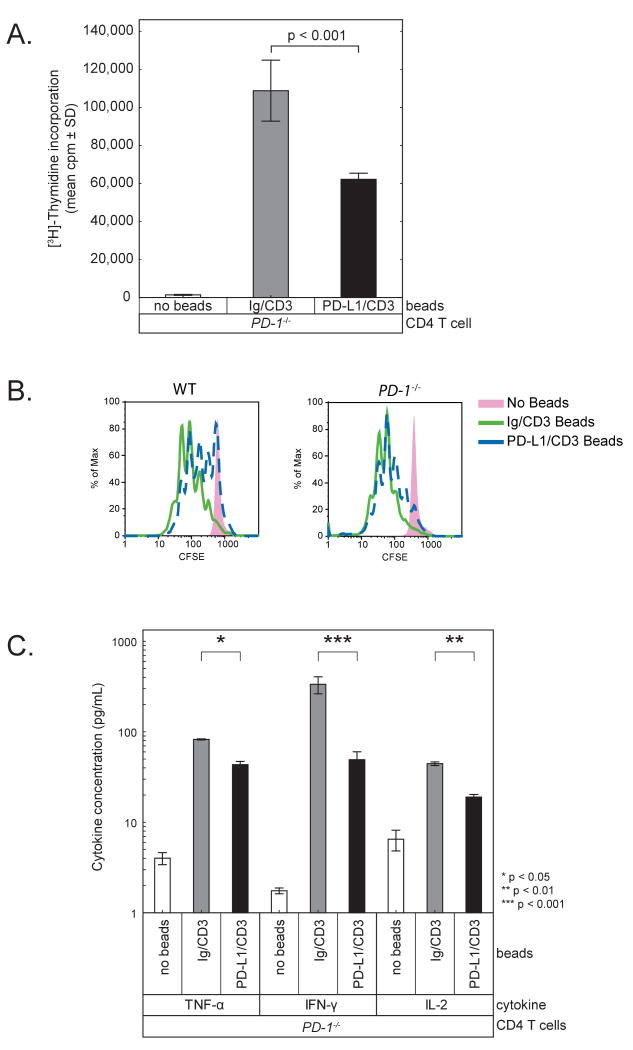
To investigate the functional significance of B7-1:PD-L1 interactions we utilized T cells from mice lacking the well-characterized receptors for B7-1 or PD-L1. We compared responses of CD28/CTLA4-/-, PD-1-/-, or wild-type C57BL/6 (WT) CD4 T cells to beads covalently coupled with stimulatory anti-CD3 antibody (clone 2C11) along with B7-1-Ig or PD-L1-Ig (termed B7-1/CD3 or PD-L1/CD3), or an Ig fusion control (termed Ig/CD3).
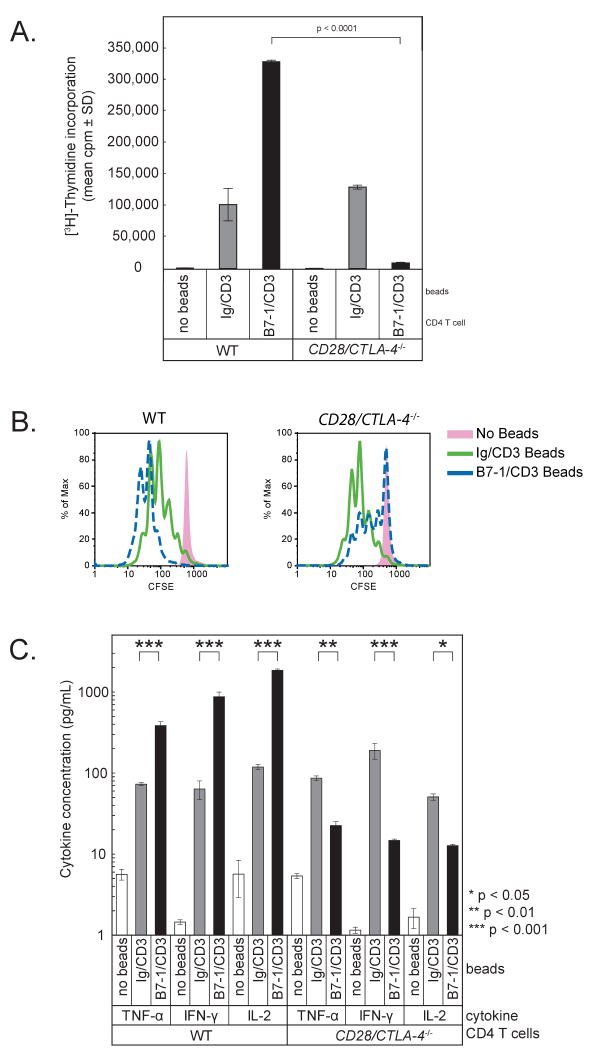
We first examined the effect of B7-1/CD3 beads on responses of CD28/CTLA4-/- versus WT CD4 T cells. WT T cells demonstrated a predictable increase in proliferation when cultured with B7-1/CD3 beads compared to Ig/CD3 beads. In contrast, CD28/CTLA4-/- T cells proliferated less when cultured with B7-1/CD3 beads as compared to Ig/CD3 beads (p < 0.0001) (Figure 4A). To further elucidate T cell responses we measured T cell expansion by 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) dilution. Analysis of CFSE dilution at 64 hours of culture with the same beads as above recapitulated the results of thymidine incorporation. Compared to Ig/CD3 beads, B7-1/CD3 beads increased expansion of WT T cells but inhibited CD28/CTLA-4-/- T cells (Figure 4B). B7-2/CD3 beads could costimulate WT T cells, but did not inhibit proliferation of CD28/CTLA-4-/- T cells, providing further evidence of the specificity of the B7-1:PD-L1 interaction (data not shown). CD28/CTLA4-/- T cells produced markedly less (∼60%) IFN-γ, TNF-α, and IL-2 when cultured with B7-1/CD3 beads as compared to Ig/CD3 beads (p = 0.05) (Figure 4C). IL-4 and IL-5 were low but also reduced in cultures of CD28/CTLA4-/- T cells with B7-1/CD3 beads compared to WT T cells (not shown).
Figure 4. Proliferation of CD28/CTLA-4-/- T cells is reduced by B7-1.
CD28/CTLA4-/- or WT CD4 T cells were stimulated with beads coated with anti-CD3 plus either B7-1-Ig (“B7-1”) or hIgG1Fc (“Ig”) control.
A. T cell proliferation was measured by 3H-thymidine incorporation for the last 16 hours of a 64 hour culture. These results are representative of at least 5 independent experiments.
B. T cell expansion was measured by flow cytometric analysis of CFSE dilution at 64 hours. These results are representative of 3 independent experiments.
C. Cytokines were measured by BD Cytokine Bead Array in supernatants obtained at 48 hrs.
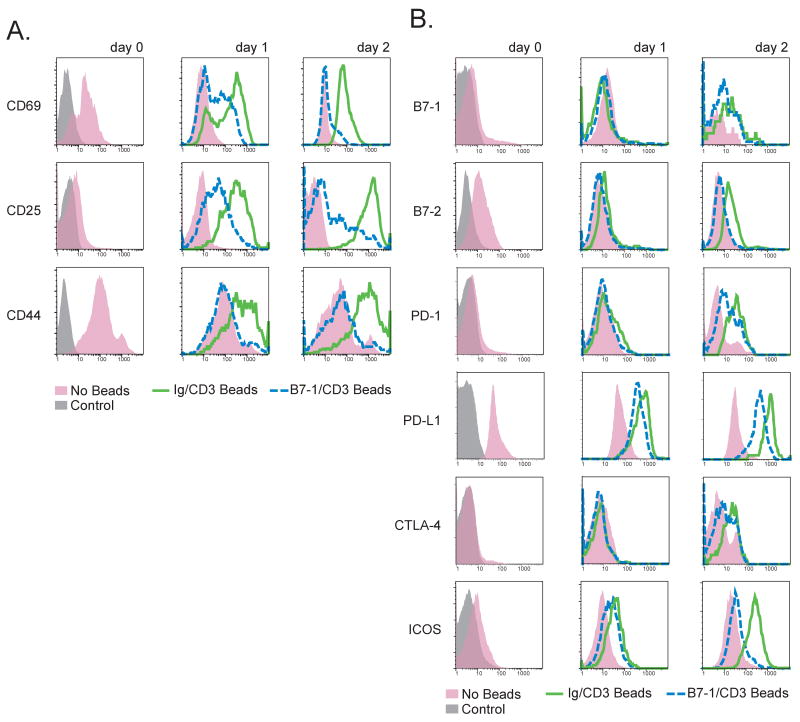
To further examine the effect of B7-1 signaling on T cell activation, we evaluated cell surface markers of activation and the expression of costimulatory molecules on T cells by flow cytometry. When CD28/CTLA4-/- T cells were cultured with B7-1/CD3 beads, they showed decreased expression of CD69, CD25, and CD44 as early as day 1 and also on day 2 (Figure 5A) as compared to Ig/CD3 beads. B7-2, PD-L1, and ICOS were also upregulated less on CD28/CTLA4-/- T cells cultured with B7-1/CD3 beads (Figure 5B), whereas B7-1, PD-1, CTLA-4 (Figure 5B), and IL7R-α (not shown) showed scant differences between groups. These studies demonstrate that an inhibitory signal can be delivered by B7-1 to CD28/CTLA4-/- T cells resulting in decreased proliferation, cytokine production, and expression of activation markers.
Figure 5. Markers of activation are diminished in CD28/CTLA-4-/- T cells stimulated with anti-CD3 plus B7-1.
CD28/CTLA4-/- CD4 T cells were cultured with beads coated with anti-CD3 plus either B7-1-Ig (“B7-1”) or hIgG1Fc (“Ig”). T cells were then stained and analyzed by FACS after 24 or 48 hours for A. activation markers and B. costimulatory receptors and ligands. Shaded histogram indicates control cells.
In vitro proliferation of PD-1-/- T cells is inhibited by PD-L1-coated beads
To determine if the B7-1:PD-L1 interaction could operate bidirectionally, and thus whether PD-L1 could deliver a signal via B7-1 on the T cell surface, we reversed the experimental setup. To eliminate the known receptor of PD-L1, we used PD-1-/- T cells and examined the effect of PD-L1/CD3 beads as compared to Ig/CD3 beads. PD-1-/- T cells proliferated less and produced less IL-2 and IFN-γ following incubation with PD-L1/CD3 beads compared to Ig/CD3 beads (p < 0.0001) (Figure 6A and C). IL-4 or IL-5 production was low but similarly reduced by PD-L1/CD3 beads, but not to as great an extent as with CD28/CTLA-4-/- T cells simulated by B7-1/CD3. Compared to Ig/CD3 beads, PD-L1/CD3 beads inhibited the expansion of PD-1-/- T cells as well as WT T cells (Figure 6B). In contrast, PD-L2/CD3 beads did not inhibit PD-1-/- T cells but did inhibit expansion of WT T cells (data not shown). These results indicate that there is an inhibitory counter-receptor for PD-L1 but not PD-L2 on PD-1-/- T cells, consistent with the capacity of B7-1 to bind to PD-L1 but not PD-L2.
Figure 6. Proliferation of PD-1-/- T cells is reduced by PD-L1.
PD-1-/- or WT T cells were stimulated with beads coated with anti-CD3 plus either PD-L1-Ig (“PD-L1”) or hIgG1Fc (“Ig”) control.
A. T cell proliferation was measured by 3H-thymidine incorporation for the last 16 hours of a 64 hour culture. These results are representative of at least 3 independent experiments.
B. T cell expansion was measured by flow cytometric analysis of CFSE dilution at 64 hours. These results are representative of 3 independent experiments.
C. Cytokines were measured by BD Cytokine Bead Array in supernatants obtained at 48 hrs.
In vitro confirmation of B7-1:PD-L1 specificity
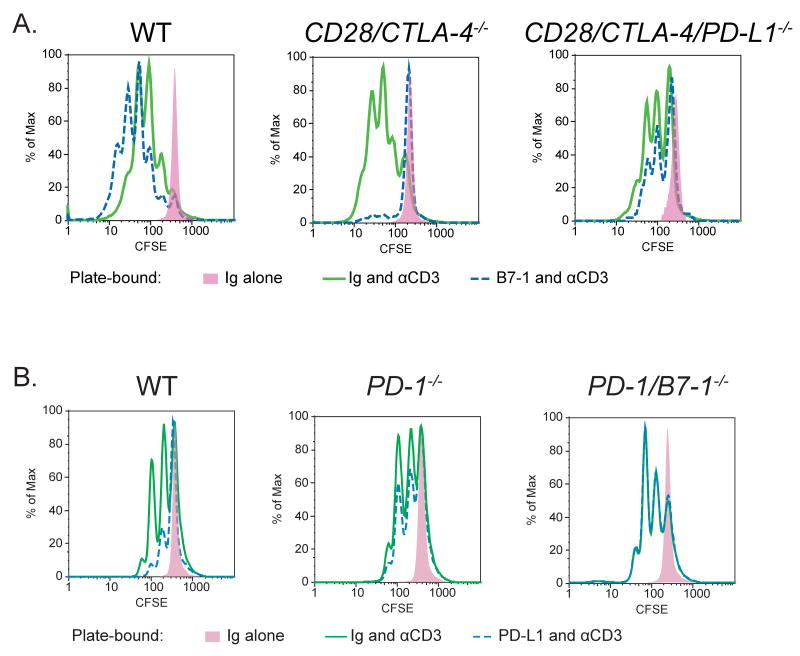
To further evaluate the specificity of the interaction between PD-L1 and B7-1, we generated PD-1/B7-1-/- mice and CD28/CTLA4/PD-L1-/- mouse strains, i.e., mice deleted of all receptors for PD-L1 or B7-1. To test whether B7-1 acts specifically through PD-L1 to inhibit T cell proliferation, we compared the responses of CD28/CTLA4-/- and CD28/CTLA4/PD-L1-/- T cells to B7-1-Ig and anti-CD3. B7-1-Ig inhibited the expansion of CD28/CTLA4-/- T cells in response to anti-CD3 (p = 0.05). However, B7-1-Ig failed to inhibit the expansion of CD28/CTLA4/PD-L1-/- T cells in response to anti-CD3 (p = 0.38) (Figure 7A). These results show that B7-1 acts specifically through PD-L1 to inhibit T cell proliferation in the absence of CD28 and CTLA-4. When PD-L1 is further eliminated from CD28/CTLA-4-/- cells, B7-1-Ig cannot exert its inhibitory effects.
Figure 7. Specificity of B7-1:PD-L1 interactions.
A. WT, CD28/CTLA-4-/-, or CD28/CTLA-4/PD-L1-/- CD4 T cells were cultured in wells coated with anti-CD3 antibody (10 μg/mL) plus either PD-L1-Ig (10 μg/mL) or control Ig (10 μg/mL). CFSE dilution was examined by flow cytometry at 48 hours.
B. WT, PD-1-/-, or PD-1/B7-1-/- CD4 T cells were cultured in wells coated with anti-CD3 antibody (5 μg/mL) plus either PD-L1-Ig (10 μg/mL) or control Ig (10 μg/mL). CFSE dilution was examined by flow cytometry at 48 hours.
In complementary studies, we evaluated whether PD-L1 acts specifically through B7-1 on T cells to inhibit T cell proliferation by comparing the responses of PD-1-/- and PD-1/B7-1-/- T cells to PD-L1-Ig and anti-CD3. PD-L1-Ig decreased the proliferation of WT (p = 0.01) and PD-1-/- (p = 0.02) T cells in response to anti-CD3 to a statistically significant extent, as seen in six replicate experiments. PD-L1-Ig slightly reduced proliferation of B7-1/PD-1-/- T cells, but this was not statistically significant (p = 0.29) (Figure 7B). Thus, these studies show that PD-L1 can exert an inhibitory effect on T cells either through B7-1 or PD-1. Taken together, the results demonstrate a specific and significant bidirectional interaction between B7-1 and PD-L1 that inhibits T cell responses.
Discussion
In this report, we identify a previously unsuspected, specific interaction between two B7 family members, B7-1 and PD-L1, and demonstrate that this interaction is functionally significant. Signals through B7-1 or PD-L1 are inhibitory, resulting in diminished expression of cell-surface activation markers, decreased T cell proliferation, and reduced cytokine production. The direct interaction between these two molecules adds a new dimension to the immunoregulatory interactions within the B7:CD28 family and compels a revised view of the interactions among molecules within the B7:CD28 family in regulating T cell activation and tolerance. Our work also gives increased significance to B7-1 and PD-L1 on T cells.
The B7-1:PD-L1 interaction has a dissociation constant of ∼1.7 μM, which is relatively strong compared to that of B7-1:CD28 (4 μM) (van der Merwe et al., 1997). This finding implies that B7-1 on an APC might preferentially ligate PD-L1 on a resting T cell rather than CD28, which would result in some degree of inhibition. Indeed, PD-L1-/- T cells were shown to have augmented cytokine production compared to wild-type T cells in vitro and to regulate pathogenic effector T cells in vivo (Latchman et al., 2004). These findings take on greater significance in light of the B7-1:PD-L1 interaction.
Both PD-L1 and B7-1 are induced on T cells upon activation, but detailed kinetics of their relative expression on T cells during an immune response and the stimuli that regulate their expression on T cells are not clear. In our in vitro studies comparing responses of T cells lacking the well-described receptors for B7-1 (CD28/CTLA4-/-) with T cells also lacking the newly-identified receptor for B7-1 (CD28/CTLA-4/PD-L1-/-), loss of PD-L1 removed a strong inhibitory signal (Figure 7A). When the responses of T cells lacking the previously-known receptor for PD-L1 (PD-1-/-) were compared with T cells also lacking the new receptor for PD-L1 (PD-1/B7-1-/-), there was also a reduction in inhibitory signals (Figure 7B), but the effects were less robust than those observed when PD-L1 was eliminated from T cells. We repeatedly observed higher levels of expression of PD-L1 relative to B7-1 on T cells in our in vitro cultures (Figure 5). The inhibitory effects seen in this reductionist system may reflect these expression differences. An inhibitory role of B7-1 on T cells is also supported by the findings that B7-1-/- CD4 T cells proliferated more than WT T cells when stimulated with specific antigen (Schweitzer and Sharpe, 1999) or anti-CD3 in vitro and accelerated graft versus host disease in a mouse model of bone marrow transplantation (Taylor et al., 2004). In addition, studies have pointed to an inhibitory role for B7-1 on effector T cells in suppression by regulatory T cells in a colitis model (Paust et al., 2004). Our results suggest that these inhibitory effects of B7-1 on T cells may be due to interaction with PD-L1 on DCs, or PD-L1 or CTLA-4 on regulatory T cells.
The molecular interface of the B7-1:PD-L1 interaction and those of B7-1:CTLA-4 or PD-L1:PD-1 overlap, providing a biophysical explanation for distinct functional outcomes of blockade of PD-L1, PD-L2, and PD-1 by mAbs in mouse models of colitis (Kanai et al., 2003), contact hypersensitivity (Tsushima et al., 2003), asthma (Matsumoto et al., 2004), and allogeneic heart transplantation (Ito et al., 2005). In general, these studies found a greater effect of anti-PD-L1 blockade as compared with anti-PD-1 or anti-PD-L2 blockade, which has been attributed to differences in the expression of PD-L1 and PD-L2 or to the half-life or affinities of the antibodies. We have found dual-specific anti-PD-L1 antibodies that can block two inhibitory interactions, PD-L1:PD-1 and PD-L1:B7-1. In contrast, blockade of PD-1 or PD-L2 would only affect one pathway. Anti-PD-L1 antibodies that block solely PD-L1:PD-1 or PD-L1:B7-1 interactions should have reduced effects on T cell activation as compared to the dual-specific anti-PD-L1 mAb.
The PD-L1:B7-1 interaction may be relevant to recent studies showing that “exhausted” T cells in chronic viral infections can be rejuvenated by anti-PD-L1 antibody (Day et al., 2006; Freeman et al., 2006; Trautmann et al., 2006; Urbani et al., 2006). Notably, anti-PD-L1 mAb had greater effects than anti-PD-1 mAb during chronic LCMV infection (Barber et al., 2006), and the anti-PD-L1 mAb used in this study (10F.9G2) blocked both PD-L1:PD-1 and PD-L1:B7-1 interactions. Further studies are needed to examine whether an anti-PD-L1 mAb that blocks PD-L1:B7-1 but not PD-L1:PD-1 (e.g., 10F.2H11) can similarly rejuvenate exhausted T cells and to investigate the effect of anti-B7-1 antibodies.
While the role of B7-1 in controlling T cell activation is well established, recent work has shown that B7-1 can also play an intrinsic role in podocytes and DCs. B7-1 on podocytes in the kidney has been shown to influence cytoskeletal distribution and affect kidney function, regulating the development of proteinuria in a mouse model (Reiser et al., 2004). B7-1 may also influence immune responses by signaling into DCs. DCs cultured with CTLA-4-Ig or cross-linking antibodies to B7-1 or B7-2 develop a tolerogenic phenotype (Baban et al., 2005; Grohmann et al., 2002; Mellor et al., 2004; Munn et al., 2004), whereas DCs cultured with CD28-Ig develop a stimulatory phenotype (Orabona et al., 2004). Similarly, intrinsic signals that activate DCs via the PD-1 ligands have been reported (Radhakrishnan et al., 2005). Further studies are needed to determine the effects of PD-L1 engagement of B7-1 on DCs, and vice versa.
PD-L1 and B7-1 are both expressed on T cells, B cells, DCs, and macrophages, suggesting the potential for bidirectional interactions between B7-1 and PD-L1 on these cell types. Such heterotypic binding has been seen in another immunoglobulin subfamily, the junctional adhesion molecules (Bazzoni, 2003), but our report is the first demonstrating cross-talk within the B7 family. In addition, PD-L1 on non-hematopoietic cells may interact with B7-1 as well as PD-1 on T cells to protect tissues from autoimmunity (Keir et al., 2006). Further work is needed to comprehend how T cells integrate the competing influences of B7-1 binding to CD28, CTLA-4 and PD-L1.
Our reductionist approach revealed a net inhibitory signal delivered by B7-1:PD-L1 interactions in vitro. The exact balance of interactions among these B7 family members and their receptors is complex and reflects differences in temporal and spatial expression of these molecules and their affinities for one another. Our identification of this new interaction necessitates a reassessment of the roles of B7-1 and PD-L1 in regulating the activation and inhibition of immune responses, as well as their therapeutic manipulation.
Experimental Procedures
Proteins
Extracellular domains of murine B7-1, B7-2, PD-L1, PD-L2, CTLA-4, and PD-1 joined to the Fc portion of either the human IgG1 protein (R&D Systems, Minneapolis, MN) or mouse IgG2a (Chimerigen, Alston, MA or kindly provided by Dr. Mary Collins, Wyeth Pharmaceuticals) were used.
Expression Cloning
A cDNA library in the pAXEF mammalian expression vector was prepared from CD4 T cells from CD28/CTLA4-/- 129 SvS4Jae mice that were activated with anti-CD3 mAb plus APCs (T-depleted CD28/CTLA4-/- 129 SvS4Jae splenocytes stimulated overnight with 5 μg/mL of anti-CD40 mAb then treated with mitomycin C (50 μg/mL for 40 minutes at 37 °C). RNA was prepared and pooled after 16, 24, and 40 hr.
COS cells were transfected with cDNA library DNA and plasmids encoding B7-1 binding proteins were isolated by three rounds of immunoselection on plates coated with murine B7-1-IgG2a/goat anti-mouse IgG2a antibody as described (Freeman et al., 1989). Individual plasmid DNAs were prepared and sequenced.
Biacore
Protein-protein interactions were measured with a BIAcore 3000 surface plasmon resonance instrument (BIAcore AB, Uppsala, Sweden). Proteins were covalently coupled to the sensor chip as follows: 0.1 M N-hydroxysuccinimide (NHS) and 0.4 M N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) were mixed 1:1 and injected at 10 μL/min for 10 min over the flow cells. The protein to be coupled was diluted in 0.1 M sodium acetate buffer pH 5.5 to a concentration of 50 μg/mL and subsequently injected at 10 μL/min for 10 min. One flow cell per chip did not receive any protein at this step and served as a reference channel. To quench any remaining NHS molecules, 1 M ethanolamine was injected at 10 μL/min for 10 min into all flow cells. The channels were subsequently washed with PBS until a stable baseline was observed. Subtracting this baseline from the Resonance Units (RU) obtained prior to adding protein gave a measure of protein immobilized in each flow cell. The four flow cells revealed capture of ∼2,000 RU of B7-1-mIgG2a, B7-2-mIgG2a, and control mIgG2a-Fc. The reverse experiment, where PD-L1 and PD-L2 were immobilized, revealed capture of 5,000-8,000 RU of PD-L1-hIgG1, PD-L2-hIgG1, and control hIgG1-Fc.
All Biacore experiments were performed at 25 °C. Analyte samples were injected in randomized order in triplicate. To prevent extreme sample consumption, a flow rate of 5 or 10 μL/min was used throughout the binding and dissociation experiments. To provide a double reference, we injected buffer blanks intermittently throughout the course of an experiment – at least 40 such blanks were averaged to provide a reference. Samples or blanks were injected using the KINJECT command for 6 min and then dissociation was followed for 6 min. Equilibrium binding data were analyzed using Scrubber software (Univ. of Utah Protein Interactions Group) (Roden and Myszka, 1996) and graphs were made using Statistica (Statsoft) and Adobe Illustrator.
Protein Cross-linking and Mass Spectrometry
B7-1-mIgG2a was dialyzed against 20 mM HEPES buffer pH 7.0, concentrated to 3 μM, then mixed with ∼60-fold molar excess sulfo-SBED (sulfosuccinimidyl-2-[6-(biotin-amido)-2-(p-azidobenzamido)-hexanoamido]-ethyl-1,3′-dithiopropionate) (Pierce). and incubated at room temperature for one hour in the dark. Non-reacted sulfo-SBED was removed by spin filter (Microcon YM-10). PD-L1-hIgG1 was dialyzed against 20 mM HEPES buffer (pH 7.0) and concentrated such that when added to the mixture above, it was in molar equivalence to the B7-1. The complex was irradiated at 0 °C with 302 nm UV light using a gel box lamp (15W, Alpha Innotech) at a distance of 5 cm for 40 min, and subsequently run on a non-reducing SDS-PAGE gel. The disulfide bond in the sulfo-SBED linker was not cleaved in these experiments.
We identified a band of interest on the gel with a molecular weight roughly equal to the MW of B7-1-Ig plus PD-L1-Ig. The band was excised, rinsed with 50% acetonitrile twice, and subjected to complete in-gel proteolysis with chymotrypsin. The resulting peptides were sampled by microcapillary liquid chromatography tandem mass spectrometry (LC/MS/MS) using a Thermoelectron LTQ linear ion trap mass spectrometer. The chymotryptic fragments were interrogated in a high-resolution Applied Biosystems/MDS Sciex QSTAR quadrupole-TOF mass spectrometer. Multiply charged mass spectral data was obtained in the range of 390 to 2000 m/z and deconvoluted using AnalystQS 1.1 software (Applied Biosystems) to produce a peak list of uncharged monoisotopic peptide masses (M+0H+). To identify peptides from both proteins that also shared a cross-linker, we used MS-Bridge version 4.0.7 (http://prospector.ucsf.edu/prospector/4.0.7/html/msbridge.htm) (Clauser et al., 1999) and manually annotated the resulting file.
Molecular model of PD-L1
We developed a structurally-biased sequence alignment of mB7-1, mB7-2 and mPD-L1, using published crystal structures as a guide (Davis et al., 2001; Ikemizu et al., 2000; Schwartz et al., 2001; Stamper et al., 2001). The AUTOMODEL script of the software package Modeller (Sali and Blundell, 1993) version 8.2 (obtained from http://salilab.org/modeller/) was used to generate a molecular model of PD-L1. Figures of the B7-1 and PD-L1 models were generated using Pymol (Delano, 2006).
Preparation of antibody-coupled beads
Combinations of anti-CD3 (clone 2C11), B7-1-hIgG1 or PD-L1-hIgG1 were covalently attached to Dynabeads M450 glycidyl ether beads following the manufacturer's directions (Invitrogen). We ensured equal loading of proteins during preparation by keeping constant the molar ratios of the proteins using hamster IgG or hIgG1-Fc antibodies as filler, as appropriate (Broeren et al., 2000; Riley et al., 2002). In general, for each 107 beads, 1 μg of anti-CD3 (20% of total protein) and 4 μg of fusion protein (80%) or control Ig were used.
Adhesion Assay
Mouse pre-B cell line 300.19 was transfected with mB7-1 or mPD-L1 cDNA in the pAXEF expression vector and a plasmid encoding puromycin resistance. Cells were selected in media containing 5 μg/mL puromycin, sorted, and subcloned. Cell-surface expression of B7-1 or PD-L1 (as appropriate) was verified by FACS (not shown). The cells were loaded with BCECF (2′, 7′-(bis-2-carboxyethy1)-5-(and-6)-carboxyfluorescein) fluorescent dye (Invitrogen) by incubating cells in a 10 μM solution as per the manufacturer's directions. A 96 well Maxisorp plate (Nunc) was coated with PD-L1-hIgG1Fc, PD-L2-hIgG1Fc, CTLA-4-hIgG1Fc, or control hIgG1Fc overnight at 4 °C, then washed with PBS, and blocked with 2% BSA/PBS for 2 hr at 37 °C. Antibodies that bound PD-L1 were introduced into wells (0.2 mL) as indicated at 40 μg/mL to test the effect on cell adhesion. Cells were suspended in 0.1% BSA/PBS and loaded into the wells (5 × 104 / well), centrifuged briefly at 700 rpm (80 × g), and incubated for 30 min at 37 °C. The fluorescence of each well, indicating the number of cells, was read in a plate reader (Victor-3, PerkinElmer) exciting at 488 nm and reading at 520 nm. The plate was then washed by gentle inversion in a 2 L bath of 0.1% BSA+PBS for 30 minutes at room temperature, allowing non-adherent cells to fall at 1 g, after which the plate was read again. Experiments were conducted in triplicate wells. Percentage bound was calculated by dividing the fluorescence before and after the “wash”. Results are reported +/- SEM by Statistica (StatSoft), and statistical significance was determined by two-tailed, paired Student's t-test across samples (n = 9 for each group).
Mice
C57BL/6 mice were purchased from The Jackson Laboratory. CD28/CTLA4-/- (Mandelbrot et al., 2001) and PD-1-/- (Keir ME, et al., manuscript in preparation) mice were generated in our lab. We interbred PD-L1-/- mice with CD28/CTLA4-/- mice and confirmed genotypes using PCR (Keir et al., 2006; Mandelbrot et al., 2001). Likewise, we interbred PD-1-/- mice with B7-1-/- mice, and verified genotypes using PCR. To test for PD-1, we used the following deletion-specific primers: 5′ACA ACA CAG GGT AGG CAT GTA GCA-3′, 5′-TCC TGC CAA ACC TTG TAG TCA-3′, and 5′-GCT AGC CAA CCA GAA GTC TAA-3′. PCRs were run at 94 °C for 2 minutes, then 35 cycles of 94 °C for 1 minute, 60 °C for 1 minute, and 72 °C for 90 seconds, then 72 °C for 5 minutes yielding a KO band of 325 bp and a WT band of 234 bp. Harvard Medical School is accredited by the American Association of Accreditation of Laboratory Animal Care. Mice were maintained and used according to institutional and National Institutes of Health guidelines in a pathogen-free facility. Mice were sacrificed between 6 and 10 wk of age.
In vitro T cell experiments
All cell culture was performed using RPMI 1640 (Invitrogen) supplemented with 10% FBS (Sigma), 2 mM L-glutamine (Invitrogen), 10 mM HEPES (Invitrogen), penicillin-streptomycin-amphotericin B (Invitrogen), and 50 μM β-mercaptoethanol (Sigma). CD4 T cells were purified from spleens using magnetic bead isolation (Miltenyi Biotec) with purity greater than 95% measured by flow cytometry and plated (105/well in 0.2 mL) in U-bottom tissue culture wells along with beads at a 10:1 (bead:T cell) ratio. Cultures were incubated at 37 °C for 2 days, then 100 μL supernatant was removed for cytokine analysis and 1 μCi 3H-thymidine was added for 16 hr. Cells were harvested, and radioactivity was counted as described previously (Latchman et al., 2004). For CFSE dilution experiments, 5 × 105 cells were labeled with 1 μM CFSE for 13 minutes, washed, and plated either as above with beads (106 beads and 105 T cells/well in 96-well U-bottom tissue culture plates) or with plate-bound Ig fusion proteins as in the Figure Legends (105 T cells/well in 96-well flat-bottom tissue culture plates). CFSE-labeled cells were harvested, stained with anti-CD4 and 7-amino Actinomycin D (7AAD), and analyzed by 4-color flow cytometry. Data shown were gated on CD4 positive, 7AAD negative cells. Statistical comparisons of proliferative responses were done by two-tailed paired T-tests on 3H-thymidine counts (n = 3 for each group). Statistical comparisons for CFSE data were two-tailed T-tests performed on the Percent Divided cells as calculated by FlowJo (TreeStar). Cytokines were measured using the BD Cytokine Bead Array kit as per the manufacturer's directions, and compared statistically using a two-tailed T-test (n = 3 for each group).
Supplementary Material
Acknowledgments
We thank Başar Bilgiçier for assistance with analytical ultracentrifugation experiments and dynamic light scattering, Tatyana Chernova for assistance with expression cloning, and John Asara for assistance with mass spectrometry. We are grateful to the BIDMC/NRB Mass Spectrometry and Proteomics Core, the Verdine Lab at Harvard University for use of dynamic light scattering equipment, the Bauer Center for Genomics Research for use of the Biacore 3000, and Dr. Krishna Kumar's Lab at Tufts University for use of the Beckman analytical ultracentrifuge (NIH/NCRR S10RR017948-01A1).
Funding for this project came from the NIH/NIAID (R01 38310 and R01 46414 to AHS and P01 AI56299 to AHS and GJF) and from the GlaxoSmithKline Allergy Fellow award (to MJB). TP was a summer student in the Harvard Medical School Pathology Proctor Program.
Footnotes
The authors disclose no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baban B, Hansen AM, Chandler PR, Manlapat A, Bingaman A, Kahler DJ, Munn DH, Mellor AL. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int Immunol. 2005;17:909–919. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Bazzoni G. The JAM family of junctional adhesion molecules. Curr Opin Cell Biol. 2003;15:525–530. doi: 10.1016/s0955-0674(03)00104-2. [DOI] [PubMed] [Google Scholar]
- Broeren CP, Gray GS, Carreno BM, June CH. Costimulation light: activation of CD4+ T cells with CD80 or CD86 rather than anti-CD28 leads to a Th2 cytokine profile. J Immunol. 2000;165:6908–6914. doi: 10.4049/jimmunol.165.12.6908. [DOI] [PubMed] [Google Scholar]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- Chikuma S, Bluestone JA. CTLA-4 and tolerance: the biochemical point of view. Immunol Res. 2003;28:241–253. doi: 10.1385/IR:28:3:241. [DOI] [PubMed] [Google Scholar]
- Clauser KR, Baker P, Burlingame AL. Role of accurate mass measurement (+/- 10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal Chem. 1999;71:2871–2882. doi: 10.1021/ac9810516. [DOI] [PubMed] [Google Scholar]
- Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, van der Merwe PA, Davis SJ. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- Davis SJ, Ikemizu S, Collins AV, Fennelly JA, Harlos K, Jones EY, Stuart DI. Crystallization and functional analysis of a soluble deglycosylated form of the human costimulatory molecule B7-1. Acta Crystallogr D Biol Crystallogr. 2001;57:605–608. doi: 10.1107/s0907444901001895. [DOI] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Delano W. The PyMOL Molecular Graphics System 2006 [Google Scholar]
- Dong H, Chen X. Immunoregulatory role of B7-H1 in chronicity of inflammatory responses. Cell Mol Immunol. 2006;3:179–187. [PMC free article] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- Freeman GJ, Freedman AS, Segil JM, Lee G, Whitman JF, Nadler LM. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989;143:2714–2722. [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- Ikemizu S, Gilbert RJ, Fennelly JA, Collins AV, Harlos K, Jones EY, Stuart DI, Davis SJ. Structure and dimerization of a soluble form of B7-1. Immunity. 2000;12:51–60. doi: 10.1016/s1074-7613(00)80158-2. [DOI] [PubMed] [Google Scholar]
- Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, Azuma M, Sharpe AH, Auchincloss H, Jr, Sayegh MH, Najafian N. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- Kanai T, Totsuka T, Uraushihara K, Makita S, Nakamura T, Koganei K, Fukushima T, Akiba H, Yagita H, Okumura K, et al. Blockade of B7-H1 suppresses the development of chronic intestinal inflammation. J Immunol. 2003;171:4156–4163. doi: 10.4049/jimmunol.171.8.4156. [DOI] [PubMed] [Google Scholar]
- Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, Gajewski TF, Fu YX, Zheng P, Liu Y. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med. 2003;197:1721–1730. doi: 10.1084/jem.20022089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbrot DA, McAdam AJ, Sharpe AH. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) J Exp Med. 1999;189:435–440. doi: 10.1084/jem.189.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbrot DA, Oosterwegel MA, Shimizu K, Yamada A, Freeman GJ, Mitchell RN, Sayegh MH, Sharpe AH. B7-dependent T-cell costimulation in mice lacking CD28 and CTLA4. J Clin Invest. 2001;107:881–887. doi: 10.1172/JCI11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, Tsushima F, Hoshino T, Aizawa H, Akiba H, et al. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J Immunol. 2004;172:2530–2541. doi: 10.4049/jimmunol.172.4.2530. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- Nabavi N, Freeman GJ, Gault A, Godfrey D, Nadler LM, Glimcher LH. Signalling through the MHC class II cytoplasmic domain is required for antigen presentation and induces B7 expression. Nature. 1992;360:266–268. doi: 10.1038/360266a0. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, et al. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5:1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan S, Iijima K, Kobayashi T, Kita H, Pease LR. Dendritic cells activated by cross-linking B7-DC (PD-L2) block inflammatory airway disease. J Allergy Clin Immunol. 2005;116:668–674. doi: 10.1016/j.jaci.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Razi-Wolf Z, Freeman GJ, Galvin F, Benacerraf B, Nadler L, Reiser H. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by peritoneal exudate cells. Proc Natl Acad Sci U S A. 1992;89:4210–4214. doi: 10.1073/pnas.89.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson BP, June CH, Linsley PS. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden LD, Myszka DG. Global analysis of a macromolecular interaction measured on BIAcore. Biochem Biophys Res Commun. 1996;225:1073–1077. doi: 10.1006/bbrc.1996.1297. [DOI] [PubMed] [Google Scholar]
- Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Schuck P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal Biochem. 2003;320:104–124. doi: 10.1016/s0003-2697(03)00289-6. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410:604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- Schweitzer AN, Sharpe AH. Mutual regulation between B7-1 (CD80) expressed on T cells and IL-4. J Immunol. 1999;163:4819–4825. [PubMed] [Google Scholar]
- Shin T, Kennedy G, Gorski K, Tsuchiya H, Koseki H, Azuma M, Yagita H, Chen L, Powell J, Pardoll D, Housseau F. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J Exp Med. 2003;198:31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, Stahl ML, Seehra J, Somers WS, Mosyak L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions. J Immunol. 2004;172:34–39. doi: 10.4049/jimmunol.172.1.34. corrections. [DOI] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima F, Iwai H, Otsuki N, Abe M, Hirose S, Yamazaki T, Akiba H, Yagita H, Takahashi Y, Omura K, et al. Preferential contribution of B7-H1 to programmed death-1-mediated regulation of hapten-specific allergic inflammatory responses. Eur J Immunol. 2003;33:2773–2782. doi: 10.1002/eji.200324084. [DOI] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngnak P, Kozono Y, Kozono H, Iwai H, Otsuki N, Jin H, Omura K, Yagita H, Pardoll DM, Chen L, Azuma M. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307:672–677. doi: 10.1016/s0006-291x(03)01257-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.