Abstract
Background and purpose:
Bladder contractility is regulated by intrinsic myogenic mechanisms interacting with autonomic nerves. In this study, we have investigated the physiological role of spontaneous release of acetylcholine in guinea pig and rat bladders.
Experimental approach:
Conventional isotonic or pressure transducers were used to record contractile activity of guinea pig and rat bladders.
Key results:
Hyoscine (3 µmol·L−1), but not tetrodotoxin (TTX, 1 µmol·L−1), reduced basal tension, distension-evoked contractile activity and physostigmine (1 µmol·L−1)-evoked contractions of the whole guinea pig bladder and muscle strips in vitro. ω-Conotoxin GVIA (0.3 µmol·L−1) did not affect physostigmine-induced contractions when given either alone or in combination with ω-agatoxin IVA (0.1 µmol·L−1) and SNX 482 (0.3 µmol·L−1). After 5 days in organotypic culture, when extrinsic nerves had significantly degenerated, the ability of physostigmine to induce contractions was reduced in the dorso-medial strips, but not in lateral strips (which have around 15 times more intramural neurones). Most muscle strips from adult rats lacked intramural neurones. After 5 days in culture, physostigmine-induced or electrical field stimulation-induced contractions of the rat bladder strips were greatly reduced. In anaesthetized rats, topical application of physostigmine (5–500 nmol) on the bladder produced a TTX-resistant tonic contraction that was abolished by atropine (4.4 µmol·kg−1 i.v.).
Conclusions and implications:
The data indicate that there is spontaneous TTX-resistant release of acetylcholine from autonomic cholinergic extrinsic and intrinsic nerves, which significantly affects bladder contractility. This release is resistant to blockade of N, P/Q and R type Ca2+ channels.
British Journal of Pharmacology (2009) 157, 607–619; doi:10.1111/j.1476-5381.2009.00166.x; published online 3 April 2009
Keywords: cholinergic autonomic nerves, bladder, contractile activity, neuronal Ca2+ channels
Introduction
Acetylcholine plays a crucial role in the excitation of smooth muscle in the coordinated contraction of the bladder body during micturition (Abrams et al., 2006). Spontaneous, localized, small contractions, called micromotions, also occur in vivo and in vitro (Coolsaet et al., 1993). In the guinea pig bladder in vitro, these micromotions are largely of myogenic origin as tetrodotoxin (TTX) has no effect (Drake et al., 2003). Specialized smooth muscle cells or interstitial cells are likely to be responsible for these micromotions (McCloskey and Gurney, 2002; Hashitani et al., 2004; Ikeda et al., 2007), and acetylcholine and muscarinic agonists significantly enhance their induction (Drake et al., 2003; de Jongh et al., 2007; Kanai et al., 2007). Different subtypes of muscarinic receptors are expressed throughout the bladder, by smooth muscle, myofibroblasts, afferent and efferent nerves (Abrams et al., 2006).
In most visceral organs, there is ongoing activity of intramural neurones, even under resting conditions, leading to spontaneous release of neurotransmitters, including acetylcholine (Furness, 2006). In the bladders of most animal species, including humans, there is a well-developed network of intramural neurones whose precise function is still unclear (Gabella, 1990; Hanani and Maudlej, 1995). These neurones may contribute to intramural neural circuits rather than just simply acting as relay neurones (Gillespie et al., 2006b). Maggi et al. (1984) reported that the acetylcholine esterase inhibitor, physostigmine, induced contractions of rat bladder strips, apparently by a mechanism involving the spontaneous release of acetylcholine. Recently, it has been established that there are two main sources of acetylcholine in the bladder: a neuronal source, which is sensitive to TTX, and a TTX-resistant non-neuronal source (Yoshida et al., 2004; 2008). It is becoming increasingly evident that spontaneous release of acetylcholine from neuronal and non-neuronal sources may play an important role in the pathophysiology of bladder function. It has been hypothesized that spontaneous release of acetylcholine could be involved in initiating an overactive bladder, with increased local contractile activity leading, in turn, to overstimulation of primary afferent neurones (Andersson, 2004). It has also been suggested that in the elderly, the increased spontaneous release of acetylcholine from the urothelium contributes to bladder wall tone and, possibly via activation of muscarinic receptors on sub-urothelial afferent nerves, leads to lower urinary tract symptoms (Yoshida et al., 2004; 2008; Kim et al., 2005).
Given the importance of acetylcholine in bladder function, the aim of this study was to investigate the mechanism of the spontaneous release of acetylcholine from autonomic nerves. We have found that the continuous, TTX-resistant, spontaneous release of acetylcholine, from both extrinsic and intrinsic cholinergic nerve terminals in the bladder wall, affected the contractility of the detrusor smooth muscle. This release was resistant to blockade of N, P/Q and R type Ca2+ channels.
Methods
In vitro studies
All animal care and experimental protocols were approved by the Animal Welfare Committee of the Flinders University. Adult male guinea pigs (outbred, Duncan Hartley IMVS, total N= 79), weighing between 270 and 320 g, were killed humanely by stunning and exsanguination. Mature adult (8–10 months old, N= 8) male rats (Sprague Dawley) weighing 700–800 g were also used.
Whole bladder in vitro
The bladder was removed from the freshly killed animal, both ureters were ligated and a 2 mm O.D. stainless steel cannula was inserted into the urethra and tied securely in place. The bladder was flushed gently two to three times with Krebs solution and placed in an 150 mL organ bath containing Krebs solution of following composition (in mmol·L−1: NaCl, 118; KCl, 4.75; NaH2PO4, 1.0; NaHCO3, 25; MgSO4, 1.2; CaCl2, 2.5; glucose, 11; bubbled with 95% O2–5% CO2) and maintained at 34–35°C. The cannula was then attached to T-piece adaptor, the left arm of which was connected to a syringe pump (Sage Model 341B, Orion Research Inc., Boston, MA, USA) to allow the slow infusion of Krebs solution. The right arm of the T-piece was connected to a pressure transducer (Viggo-Spectramed model P23XL, Oxnard, CA, USA). Intravesical pressure was recorded on a Maclab/8s data acquisition system with Chart 5 software (ADInstruments, Castle Hill, NSW, Australia) using a Macintosh G4 computer. A residual volume of 0.75 mL was maintained throughout the entire experiment and the bladder was allowed to equilibrate for at least 30 min. Infusions were then performed at 0.5 mL·min−1 for 6 min, followed by manual emptying via a syringe of the 3 mL of infusate, with a 15 min interval before the next infusion. Usually, no more than six infusions were performed on each bladder. Phasic contractile activity, measured as integrated tension (area under the curve of each individual contractile peak) in units of hPa, was calculated. During the first two to four infusions in a preparation, a small decay in the contractile activity typically occurred (see Figure 2C). For this reason, the effects of drugs (TTX and hyoscine) were studied between the 4th and 5th infusions, which evoked a similar contractile activity. In order to study the effect of drugs on the contractile activity evoked by physostigmine, integrated tension was measured for a 5 min period in physostigmine alone and 25 min after addition of either hyoscine or TTX.
Figure 2.
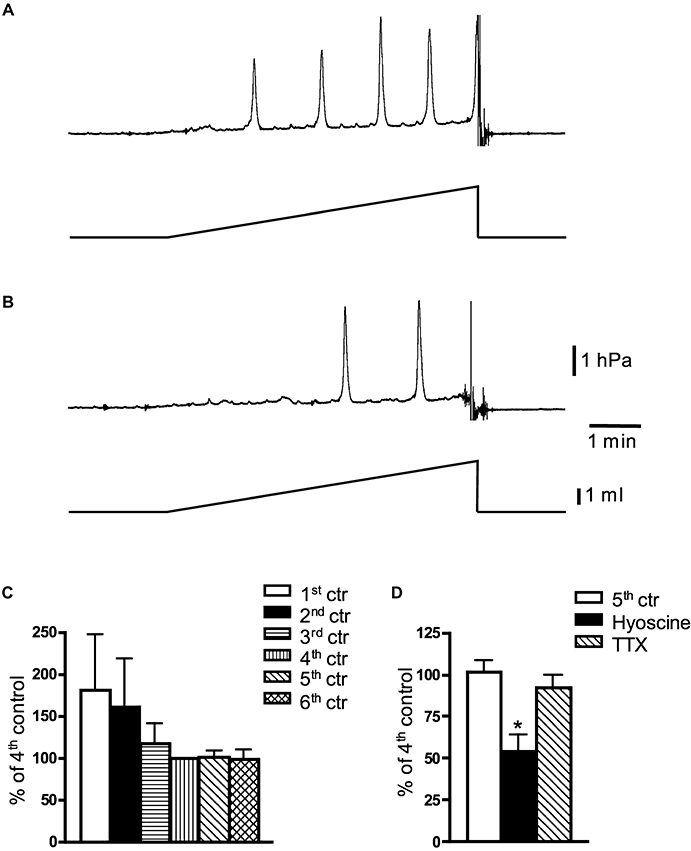
Effect of hyoscine and tetrodotoxin (TTX) on distension-induced contractile activity in the whole guinea pig bladder, in vitro. (A) Typical recording of 4th distension (0.5 mL·min−1 for 6 min)-evoked contractile activity and (B) 5th distension after 15 min of application of hyoscine (3 µmol·L−1). (C) Decay of distension-evoked contractile activity in control experiments (N= 5). Note that 4th–6th distensions evoked similar contractile activity of the bladder. (D) Averaged data of the effects of hyoscine (3 µmol·L−1, N= 5) and TTX (1 µmol·L−1, N= 5) on 3 mL distension-induced bladder contractions. *, significant difference from control, P < 0.01.
Isolated muscle strips
In most cases, three muscle strips (15–20 mm long × 2–3 mm wide), without urothelium, were excised from the guinea pig bladder [two lateral strips and one dorso-medial strip (Figure 5A)] and four strips from the rat bladder. Muscle strips were set up in 5 mL organ baths in oxygenated Krebs solution (at 36°C), under an initial load of 10 mN and allowed to equilibrate at least 1 h before beginning experiments. Contractile activity, measured as a change in length, was recorded by the isotonic transducer (Harvard Bioscience, model 52-9511, S. Natick, MA, USA) on a Maclab/8s data acquisition system with Chart 3.6 software (ADInstruments, Castle Hill, NSW, Australia) using a Macintosh G3 computer. Electrical field stimulation (EFS)-induced contractions were evoked by repetitive stimulation (10 Hz for 3 s, 0.3 ms duration, 90 V, at 3 min interval) delivered by a Grass S44 stimulator (Quincy, MA, USA). In order to study the effect of drugs on the contractile activity evoked by physostigmine, the area under the curve was calculated for 5 min in the presence of physostigmine, and after 25 min of physostigmine together with either hyoscine, TTX or ω-conotoxin GVIA (CTX) alone, or a combination of CTX, ω-agatoxin IVA (ATX) and SNX 482 together. In order to compare the effects of physostigmine between fresh and 5 day cultured tissues, the area under the curve for 30 min was normalized to the maximal contraction evoked by 100 µmol·L−1 carbachol and 80 mmol·L−1 KCl. In the guinea pig bladder, group data standardized to high KCl and 100 µmol·L−1 carbachol were nearly identical. However, in the rat tissue, contractions standardized to high KCl had much higher standard error of the mean than contractions standardized against carbachol. This effect appeared to be due to greater variability of responses to high KCl than to carbachol. For this reason, we used carbachol as the standard for comparison for both guinea pig and rat tissues.
Figure 5.
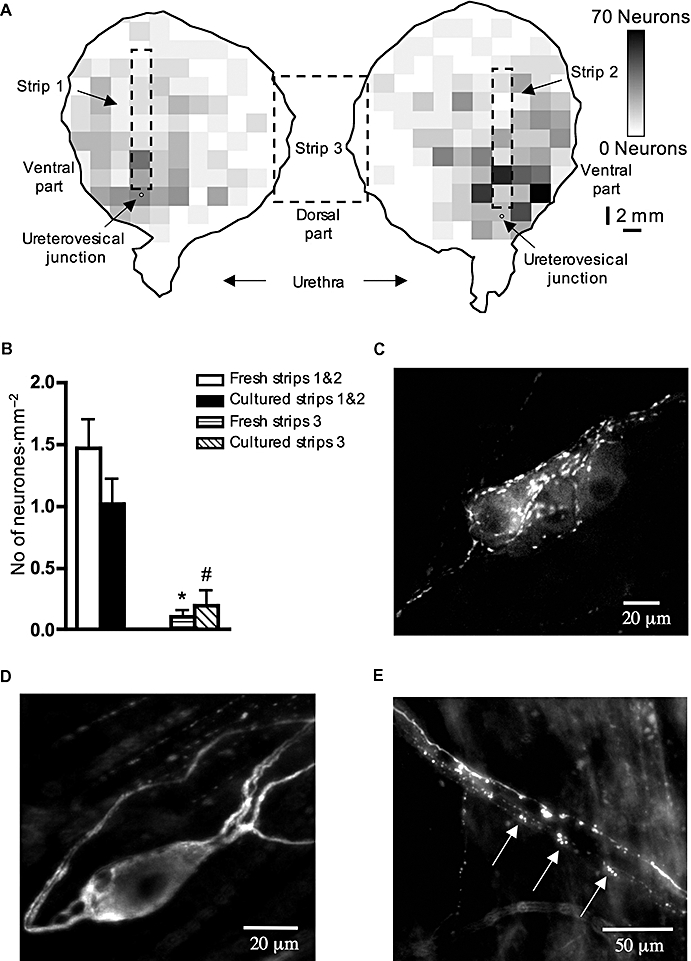
Intramural neurones in the whole guinea pig bladder and in isolated muscle strips. (A) Intramural neurones density map, indicating the number of intramural neurones in the whole bladder. Each square pixel on the map corresponds to 4 mm2; total number of neurones = 1805. (B) Averaged data of the density of intramural nerve cell bodies (number of neurones divided by area of the tissue) in fresh and 5 day cultured muscle strips from lateral (strips 1 & 2) and dorso-medial (strips 3) regions of the bladder. (C) Choline acetyltransferase-immunoreactive intramural neurones in a ganglion in the bladder wall. (D) Single intramural neurone located outside a ganglion shows multipolar morphology by tubulin immunoreactivity. (E) Clearly fragmented tubulin immunoreactive nerve fibres (marked by arrows) in the nerve trunk of the dorsal medial muscle strip after 5 days in culture. Such fragmented axons were not seen in freshly fixed preparations. Each value in (B) is the mean from 6–10 guinea pigs. * and #, significant difference from strips 1 & 2, P < 0.001.
In vivo studies
Male albino Wistar rats (Charles River, Calco, Italy), weighing 250–300 g, were used. The animals were anaesthetized with urethane (1.2 g·kg−1, s.c.) and a polyethylene catheter (PE 50, Clay Adams) was inserted into the right jugular vein for drug administration. A polyethylene cannula (PE 240, Clay Adams) was inserted into the trachea to facilitate breathing. Through a midline incision of the lower abdomen, the urinary bladder was exposed and prepared for intraluminal pressure recording via the trans-urethral route. A polyethylene catheter (PE 90, Clay Adams) was inserted into the bladder through the urethra and secured by a ligature. The urinary bladder was filled with saline (0.2–0.7 mL) and connected to a pressure transducer (Transpac IV, Abbott, Italy) for intraluminal pressure recording on a MacLab/8S ML 780 data acquisition system (ADInstruments, UK). Both ureters were ligated to maintain a constant bladder volume throughout the experiment. The animals were allowed to equilibrate, under anaesthesia, for 1 h before starting the experiment. TTX (5 nmol per animal) and physostigmine (5–500 nmol per animal) were applied topically onto the urinary bladder in a volume of 50 µL. Atropine (4.4 µmol·kg−1) was administered intravenously in a volume of 1 mL·kg−1.
Immunohystochemistry
All extrinsic and intrinsic nerves, including intramural neurones in the bladder, were immunohistochemically labelled using an antiserum raised against tubulin (Promega mouse anti-tubulin G712A, Madison, WI, USA, used at 1:500). Cholinergic neurones and their axons were identified using a polyclonal antiserum against choline acetyltransferase [goat anti-choline acetyltransferase (ChAT), AB144P, Chemicon, Temecula, CA, USA, used at 1:100]. Tissues were incubated with primary antibodies for 3 days and then overnight with secondary antibodies (donkey anti-goat Cy3 1:400; donkey anti-mouse Cy5 1:200, Jackson Immunoresearch Lab. Inc., West Grove, PA, USA) before mounting in bicarbonate-buffered glycerol (pH 8.6). Preparations were analysed on an Olympus AX70 epifluorescence microscope. Areas of half bladders and muscle strips were measured by mapping the outlines of the preparations using a modified version of NIH Image 1.62 software (NIH, Bethesda, MD, USA). Images of labelled neural structures were captured via a Hamamatsu digital camera (model C4742-95, Japan) and recorded on an Apple Macintosh G3 computer using IPLab software (Scanalytics Inc., Fairfax, VA, USA). Images were combined, cropped, scaled and adjusted for brightness and contrast in Adobe Photoshop CS.
Data analysis
Results are expressed throughout as mean ± standard error of the mean, with n referring to the number of strips and N to the number of animals. Statistical analysis was performed by Student's two-tailed t-test for paired or unpaired data or by repeated measures one-way anova with Tukey's comparison tests using Prism 4 software (GraphPad Software, Inc., San Diego, CA, USA). Differences were considered significant if P < 0.05.
Drugs
Atropine sulphate, hyoscine [(-) scopolamine] hydrobromide, physostigmine (eserine) hemisulphate and TTX were obtained from Sigma (St. Louis, MO, USA). CTX, ATX and SNX 482 were from Alomone Labs (Jerusalem, Israel).
Results
In the whole guinea pig bladder in vitro, containing only the residual volume of 0.75 mL, small spontaneous phasic bladder contractions occurred with a mean frequency of 2.1 ± 0.17 min−1 (N= 27) and mean peak amplitude of 0.24 ± 0.03 hPa (N= 27). As reported previously, these spontaneous contractions were resistant to TTX (Drake et al., 2003). In order to enhance the amplitude of these contractions, the bladder was infused with an additional 1.5 mL of Krebs solution allowed to equilibrate for a further 30 min in the presence of TTX (1 µmol·L−1). Application of hyoscine (3 µmol·L−1) in the continuing presence of TTX inhibited spontaneous contractions by 23 ± 4.2% (N= 4, P < 0.01, paired t-test, Figure 1A and C).
Figure 1.
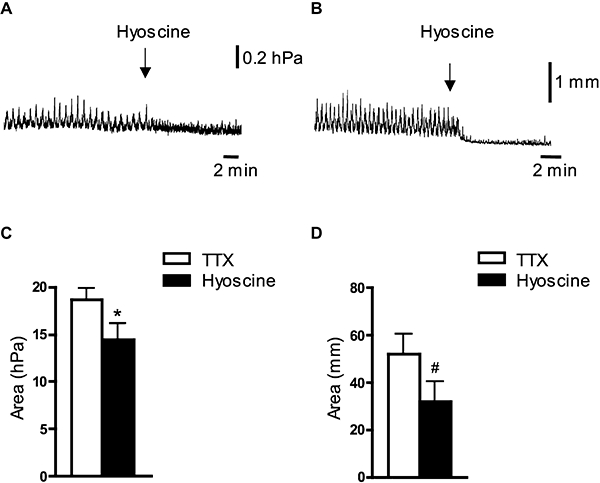
Effect of hyoscine on spontaneous contractile activity in the whole guinea pig bladder and isolated muscle strips in vitro. (A and B) Application of hyoscine (3 µmol·L−1) in the presence of tetrodotoxin (TTX) (1 µmol·L−1) inhibited spontaneous contractions of the whole bladder in vitro (A) and of muscle strips (B). (C and D) Averaged data of the effects of hyoscine (3 µmol·L−1) in the presence of TTX (1 µmol·L−1) on spontaneous contractions of the whole bladder (C, N= 4) and of isolated muscle strips (D, n= 8, N= 7). * and #, significant difference from TTX, P < 0.01 and P < 0.05 respectively.
Similarly, most isolated muscle strips from the guinea pig bladder (under an initial load of 10 mN) developed spontaneous contractions that were unaffected by TTX (1 µmol·L−1) (+8.2 ± 13% of control, N= 17, not significant, NS). Application of hyoscine (3 µmol·L−1), in the presence of TTX (1 µmol·L−1) caused a reduction of these spontaneous contractions by 40 ± 11% (n= 8, N= 7, P < 0.05, paired t-test) (Figure 1B and D). These data suggest that there is an ongoing, spontaneous release of acetylcholine, which affects local bladder contractility, and which is not due to action potential-dependent, TTX-sensitive activity of intramural neurones.
Effect of hyoscine and TTX on distension-induced contractility of the whole guinea pig bladder in vitro
Distension of the whole guinea pig bladder in vitro at 0.5 mL min−1 for 6 min evoked phasic contractions with mean amplitude of 1.82 ± 0.82 hPa and a frequency of 1 ± 0.25 min−1 (N= 25) (Figure 2A). The active contractile responses (measured as area under individual contraction peaks) evoked by distension declined over the next two to three distensions; however, the 4th, 5th and 6th distensions were not significantly different from each other (Figure 2C). TTX or hyoscine was therefore applied between 4th and 5th distensions and the contractility compared with 5th response to distension obtained in separate series of control experiments (N= 5) (Figure 2C). Application of TTX (1 µmol·L−1) did not significantly reduce phasic contractions evoked by bladder distension (−8 ± 8%, N= 5, NS, one-way anova) (Figure 2D). However, hyoscine (3 µmol·L−1) significantly reduced distension-evoked contractions by 46 ± 10% (N= 5, P < 0.01, one-way anova) (Figure 2A, B and D). The data indicate that release of acetylcholine, acting on muscarinic receptors, contributes to the contractile responses of the whole bladder in vitro evoked by ramp distension. However, TTX-sensitive neuronal activity does not appear to be required.
Effect of physostigmine on contractility of the whole guinea pig bladder and muscle strips in vitro
In order to study the mechanism of acetylcholine release, reflected by bladder contractility, we examined the effects of blocking acetylcholine esterase, the enzyme that degrades acetylcholine. A cholinesterase inhibitor, physostigmine (1 µmol·L−1), evoked powerful phasic contractions of the isolated guinea pig bladder in vitro, which were stable for more than 1 h (Figure 3A). Powerful phasic contractions were generated with mean frequency of 2.2 ± 0.17 min−1 (N= 12) and mean maximum amplitude of 5.6 ± 0.7 hPa (N= 12). These contractions were not affected by TTX (1 µmol·L−1) (+7 ± 15%, N= 4), but were inhibited by hyoscine (3 µmol·L−1) by 98 ± 1% (N= 4, P < 0.01, one-way anova) (Figure 3A and C). To test whether urothelium-released acetylcholine mediated this effect of physostigmine, the experiments were repeated on isolated smooth muscle strips of the guinea pig bladder corpus, in which the urothelium had been removed. Application of physostigmine (1 µmol·L−1) evoked phasic contractions with a slight increase in basal tone. The frequency (2.1 ± 0.1, N= 19) of these physostigmine-evoked contractions was similar to those of the whole bladder in vitro. TTX (1 µmol·L−1) did not change the excitatory effect of physostigmine in isolated muscle strips (−15 ± 8%, N= 6, NS), but hyoscine (3 µmol·L−1) significantly inhibited these contractions by 83 ± 6% (N= 6, P < 0.01, one-way anova) (Figure 3B and D). These observations suggest that the ongoing effect of TTX-resistant endogenous acetylcholine is not due to release from the urothelium.
Figure 3.
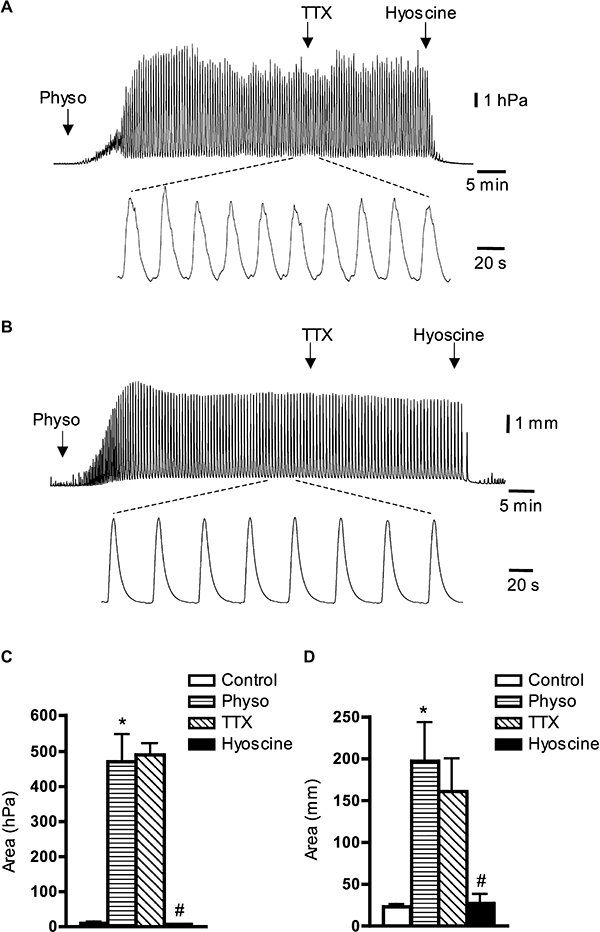
Typical tracings indicating the effects of tetrodotoxin (TTX) and hyoscine on the action of physostigmine (Physo) in the whole guinea pig bladder and isolated muscle strips in vitro. (A) Physostigmine (1 µmol·L−1) evoked powerful phasic contractions of the isolated whole guinea pig bladder in vitro; insert below shows physostigmine-induced contractions on faster time scale. TTX (1 µmol·L−1) did not affect, but hyoscine (3 µmol·L−1) nearly blocked phasic contractions evoked by physostigmine. (B) Physostigmine (1 µmol·L−1) evoked phasic contractions of isolated muscle strips; insert below shows physostigmine-induced contractions on faster time scale. TTX (1 µmol·L−1) did not affect contractions, but hyoscine (3 µmol·L−1) nearly abolished them. (C and D) Averaged data of the effects of TTX (1 µmol·L−1) and hyoscine (3 µmol·L−1) on physostigmine-induced contractions in the whole bladder in vitro (C) and in isolated muscle strips (D). Each value in (C) and (D) is the mean from four (C) and six (D) guinea pigs. *, significant difference from control, P < 0.001 in (C) and P < 0.01 in (D); #, significant difference from TTX, P < 0.001 in (C) and P < 0.05 in (D).
Action of selective Ca2+ channel blockers
In order to investigate which neuronal Ca2+ channel is responsible for spontaneous acetylcholine release, we studied the effects of selective Ca2+ channels blockers, such as CTX (for N type), ATX (for P/Q type) or by SNX 482 (for R-type). In the presence of TTX (1 µmol·L−1), the excitatory effect of physostigmine on isolated muscle strips was not affected by CTX (0.3 µmol·L−1, N= 6, NS). However, CTX (0.3 µmol·L−1) inhibited EFS (10 Hz)-induced contractions of the muscle strips by 33.6 ± 2.7% (n= 7, N= 7, P < 0.001, paired t-test) (Figure 4A and B). In the presence of TTX, physostigmine-evoked contractility was not affected by a cocktail of toxins, which included CTX (0.3 µmol·L−1), ATX (0.1 µmol·L−1) and SNX 482 (0.3 µmol·L−1) (n= 5, NS) (Figure 4C). The small reduction in the presence of cocktail of toxins was similar to the corresponding time control of the effect of physostigmine alone (N= 6) (Figure 4E). However, the cocktail of toxins [CTX (0.3 µmol·L−1), ATX (0.1 µmol·L−1) and SNX 482 (0.3 µmol·L−1)] significantly inhibited EFS (10 Hz)-induced contractions by 59 ± 4% (n= 5, N= 5, P < 0.001, paired t-test) (Figure 4D), which was significantly higher than the inhibitory effects of CTX alone (P < 0.01, one-way anova).
Figure 4.
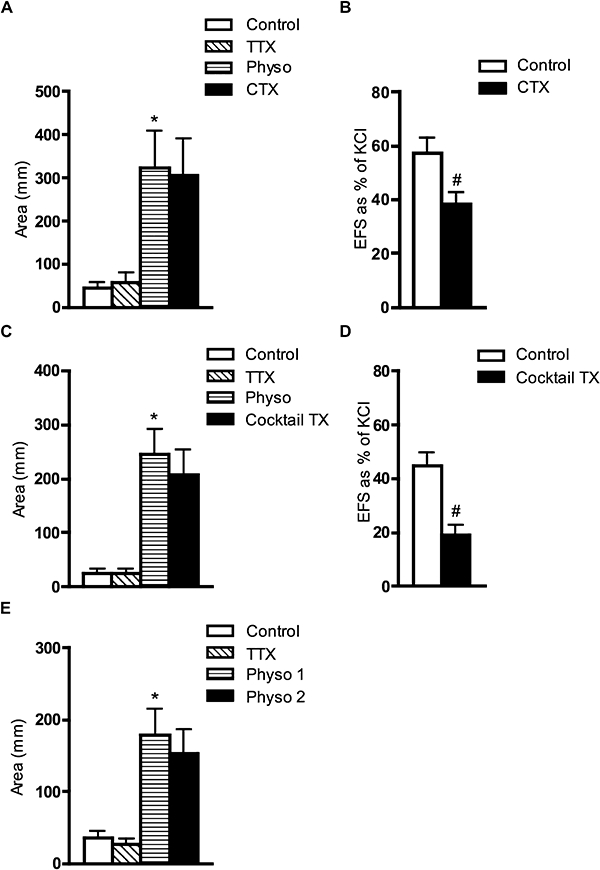
Effects of CTX alone and of a cocktail of toxins (CTX, ATX and SNX 482) on physostigmine (Physo)- and EFS (10 Hz)-induced contractions in isolated muscle strips of the guinea pig bladder. (A) Averaged data from six guinea pigs shows the lack of effect of CTX (0.3 µmol·L−1) on physostigmine (1 µmol·L−1)-induced contractions in the presence of TTX (1 µmol·L−1). (B) Averaged data (N= 7) of the effect of CTX (0.3 µmol·L−1) on EFS (10 Hz)-induced contractions. (C) Averaged data from five guinea pigs of the effects of the cocktail of toxins [CTX (0.3 µmol·L−1), ATX (0.1 µmol·L−1) and SNX 482 (0.3 µmol·L−1)] on physostigmine (1 µmol·L−1)-induced contractions, in the presence of TTX (1 µmol·L−1). (D) Averaged data (N= 5) of the effect of the same cocktail of toxins on EFS (10 Hz)-induced contractions revealing significant, but incomplete blockade. (E) Averaged data (N= 6) of the corresponding time control for the effect of 1 µmol·L−1 physostigmine (physo 1 = 25–30 min and physo 2 = 45–50 min) alone. *, significant difference from TTX, P < 0.01; #, significant difference from control, P < 0.001. ATX, ω-agatoxin IVA; CTX, ω-conotoxin GVIA; EFS, electrical field stimulation; TTX, tetrodotoxin.
Intramural neurones in the guinea pig bladder and effect of physostigmine
Neuronal Ca2+ channel blockers did not inhibit the contractile effects of physostigmine. This raises the question as to whether acetylcholine is spontaneously released from nerves or from non-neuronal cells, for example, ChAT-positive intramuscular interstitial cells (Gillespie et al., 2006a). The guinea pig bladder has a rich network of autonomic intramural ganglia in the muscular coat with the highest density of nerve cell bodies near the entry point of the ureters and urinary arteries (Gabella, 1990). The majority of the intramural neurones are located in intramural ganglia (Figure 5C), but about 10% of the neurones (10 ± 2%, n= 1297, N= 3) are single and located in the muscle coat of the bladder (Figure 5D). These single neurones are multipolar and have similar morphology to the Dogiel type II intrinsic afferent neurones in the gut (Brookes and Costa, 2002). Essentially, all (98 ± 0.01%; n= 1297, N= 3) of tubulin-stained intramural neurones were ChAT-imunnoreactive (Figure 5C), indicating that nearly all of these neurones are likely to be cholinergic.
By plotting the density of intramural neurones in the guinea pig bladder, we confirmed that the highest density of nerve cell bodies was in the area around ureterovesical junctions, while the apex and dorso-medial part of the bladder corpus largely lacked intramural neurones (Figure 5A). Physostigmine (1 µmol·L−1) produced similar contractile effects, measured as area under the curve, in isolated strips made from dorso-medial and lateral regions (218 ± 55 mm, N= 9 and 180 ± 21 mm, n= 17, N= 9, NS respectively), despite the lateral regions containing approximately 15 times more intramural nerve cell bodies than dorso-medial regions [1.47 ± 0.22 neurones·mm−2 (n= 20, N= 10)] for lateral regions (Figure 5B, strips 1 & 2) compared with 0.1 ± 0.05 neurones·mm−2 (n= 10, N= 10) for dorsal-medial region (Figure 5B, strip 3)]. However, one difference between the two types of strips was apparent. In lateral strips, physostigmine in most cases evoked phasic contractions with a little change in a basal tone, whereas in dorso-medial strips, phasic contractions were usually superimposed on the increased basal tone.
We also studied the effect of degeneration of extrinsic nerves by culturing isolated muscle strips in organ culture for 5 days. This treatment evoked a degeneration of a significant proportion of tubulin-positive nerve fibres in both lateral and dorso-medial muscle strips; with a substantial loss of nerve fibres overall and many nerve fibres becoming clearly fragmented (Figure 5E). This degeneration was never seen in fresh tissue. The physostigmine (1 µmol·L−1)-induced contractile effect (standardized against maximal contractions evoked by 100 µmol·L−1 carbachol) was significantly reduced by organ culture in the strips from dorso-medial region from 13 ± 4% (n= 7, N= 7) to 4 ± 1% (n= 6, N= 6, P < 0.05) (Figure 6A and C). However, in lateral strips, organ culture for 5 days did not significantly influence the contractile effect of physostigmine [1 µmol·L−1, 9 ± 3% (n= 4, N= 4) in fresh tissue and 8 ± 1% (n= 12, N= 6, NS)] in cultured tissue (Figure 6B and D). We also examined cultured tissue for nerve cell bodies. Similar to fresh tissue, cultured strips from the lateral region had significantly more nerve cell bodies than cultured strips from the dorso-medial region (1.02 ± 0.20 neurones·mm−2, n= 12, N= 6, compared with 0.19 ± 0.13 neurones·mm−2, n= 6, N= 6, P < 0.001, one-way anova, see Figure 5B). From these findings, it can be concluded that intrinsic cholinergic nerve cell bodies survive well for 5 days in organ culture. However, extrinsic axons show substantial degeneration. The proportionally greater reduction in physostigmine-induced contractility in dorso-medial strips, compared with lateral strips, can be explained by a proportionally greater contribution by extrinsic nerve fibres in the dorso-medial strips. EFS-induced contractions in dorso-medial strips (standardized as a percentage of the maximal contraction evoked by 80 mmol·L−1 KCl) were reduced by 5 days of organ culture from 53 ± 3% (n= 6, N= 6) in fresh tissue to 14 ± 3% (n= 5, N= 5, P < 0.001, one-way anova). In lateral strips, EFS-induced contractions were reduced by organ culture to a much lesser extent (from 53 ± 6%, n= 8, N= 5 to 34 ± 5%, n= 10, N= 5, P < 0.05, one-way anova). This suggests that a larger proportion of the cholinergic innervation of lateral strips arises from intrinsic neurones, which survive culture well. In dorso-medial strips, a greater proportion arises from extrinsic neurones, which degenerate during 5 days of organ culture. Taken together, the data indicate that there is spontaneous release of acetylcholine from both extrinsic autonomic and intrinsic intramural cholinergic fibres in the guinea pig bladder.
Figure 6.
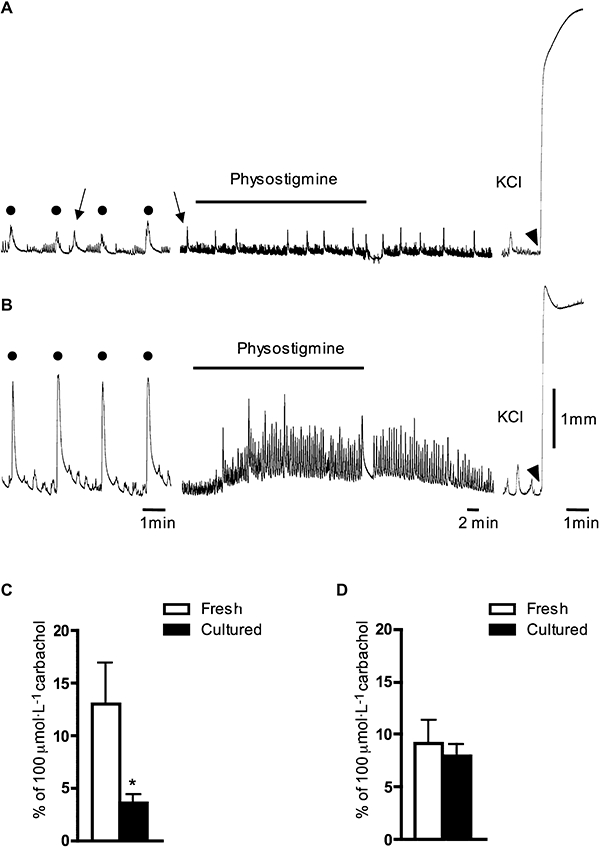
Effects of physostigmine and electrical field stimulation (EFS) (10 Hz) on 5 day cultured dorso-medial and lateral muscle strips in the guinea pig bladder. In contrast to high KCl, physostigmine (1 µmol·L−1) and EFS (10 Hz) evoked significantly smaller contractile responses in dorso-medial strips (A) than in lateral strips after 5 days in culture (B). An application of 80 mmol·L−1 KCl is shown by arrowhead in (A) and (B). In (A), arrows show spontaneous contractions while filled dots mark EFS-induced contractions (in A and B). (C and D) Averaged data of the effects of physostigmine (1 µmol·L−1) in fresh and 5 day cultured strips from dorso-medial (C) and lateral (D) regions of the bladder. Note that the effects of physostigmine in (A–D) are in the presence of tetrodotoxin (1 µmol·L−1). Each value in (C) and (D) is the mean from four to seven guinea pigs. *, significant difference from fresh control tissue, P < 0.05.
Effect of physostigmine in the rat bladder muscle strips
In muscle strips from the rat bladder, TTX (1 µmol·L−1) nearly blocked EFS (10 Hz)-induced contractions (which were 23 ± 4% of the peak contraction evoked by 100 µmol·L−1 carbachol, n= 11, N= 4) within 15–20 min. However, in most cases, a small residual contraction remained (3 ± 1% of 100 µmol·L−1 carbachol-induced contraction, n= 11, N= 4), which may have been the result of direct electrical activation of smooth muscle by EFS and/or direct release of transmitters from nerve terminals. In the presence of TTX (1 µmol·L−1), physostigmine (3 µmol·L−1) in most cases evoked significant increase in the muscle tone with superimposed small, phasic contractions (Figure 7A). In contrast to young animals, the bladders of adult rats have been reported not to contain intramural nerve cell bodies (Alian and Gabella, 1996). In muscle strips of mature adult (8–10 months old) rats, 24% of strips still had a few intramural nerve cell bodies detectable. An average density of intramural neurones was 0.005 ± 0.004 neurones·mm−2 (n= 19, N= 5) in fresh strips and 0.024 ± 0.01 neurones·mm−2 (n= 15, N= 4) in 5 day organ-cultured strips (Figure 7B). Physostigmine-induced contractions (standardized as a percentage of 100 µmol·L−1 carbachol-induced contraction) in the rat bladder strips were greatly reduced after 5 days in organ culture: from 12 ± 2.7% in fresh tissue (n= 14, N= 4) to 2.5 ± 0.7% in cultured tissues (n= 15, N= 4, P < 0.01, unpaired t-test, Figure 7C). In addition, EFS-induced contractions were abolished in cultured tissue (n= 15, N= 4). The data indicate that the major source of spontaneously released acetylcholine in the rat bladder arises from extrinsic cholinergic fibres.
Figure 7.
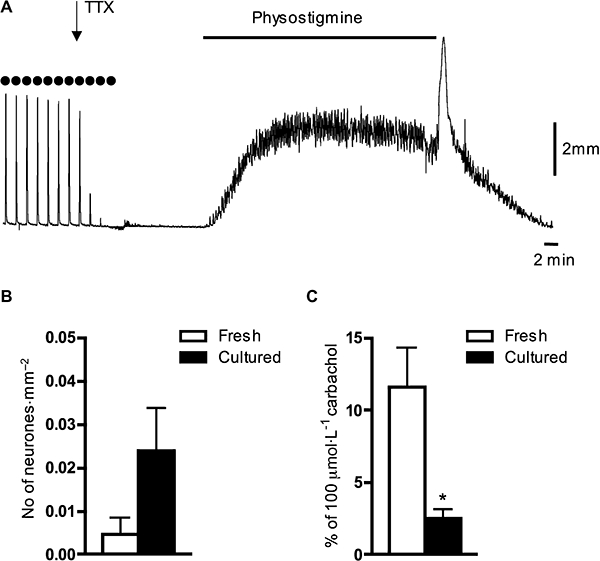
Effects of physostigmine and density of intramural neurones in the fresh and cultured muscle strips of the rat bladder. (A) Typical traces demonstrate the effects of physostigmine (3 µmol·L−1) on a fresh muscle strip of the rat bladder in the presence of tetrodotoxin (TTX) (1 µmol·L−1). Note that TTX blocked EFS-induced contractions (evoked by 10 Hz, marked by filled dots). (B) Averaged data on the density of intramural neurones (number of neurones divided by area of the tissue) in fresh and 5 day cultured muscle strips from the rat bladder. Note the very different vertical scale from Figure 5B. (C) Physostigmine (3 µmol·L−1)-induced contractile effect was significantly reduced after 5 days in organ culture compared with control fresh strips. Each value in (B) and (C) is the mean from four to five rats. *, significant difference from fresh tissue, P < 0.01.
Effect of physostigmine in the rat bladder in vivo
In vivo experiments were carried out on rats, as this species has been extensively used to study the bladder micturition reflex. In adult rats in vivo, isovolumetric distension (0.2–0.3 mL) of the urinary bladder activated a series of rhythmic contractions, with an amplitude of 30 ± 2 mmHg (N= 11). TXX (5 nmol, 50 µL) applied directly to the bladder wall produced a rapid and irreversible block of distension-induced reflex contractions (Figure 8). Topical application of physostigmine (5–50–500 nmol, 50 µL), 10 min later, induced a TTX-resistant and dose-dependent tonic contractile response of 4.5 ± 4.4, 6.7 ± 1.2 and 14.4 ± 5.1 mmHg respectively (N= 4–7). Atropine (4.4 µmol·kg−1, i.v.) rapidly abolished the tonic contraction, restoring basal tone. The maximal contraction evoked by 500 nmol of physostigmine applied topically was about 50% of the peak amplitude of distension-induced reflex contractions.
Figure 8.
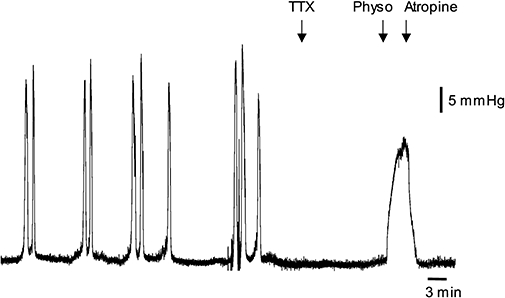
Effects of physostigmine on the rat bladder in vivo. Typical tracings showing the effects of physostigmine (Physo; 500 nmol, 50 µL applied topically, indicated by arrow) in the rat bladder, after topical application of tetrodotoxin (TTX) (5 nmol, 50 µL). Note that TTX blocked the distension-induced reflex contractions. Atropine (4.4 µmole·kg−1, i.v.) abolished the effect of physostigmine.
Discussion
Our data provide evidence that there is a functionally significant spontaneous release of acetylcholine from extrinsic and intrinsic cholinergic nerves in the guinea pig and rat bladders, which is resistant to N, P/Q and R type Ca2+ channel antagonists and hence may be due to Ca2+ entry through a novel type of neuronal Ca2+ channel.
Recently, it has been established that there are two main sources of acetylcholine release in the bladder: a neuronal source (which can be evoked by EFS and blocked by TTX) and a TTX-resistant, non-neuronal source, which mainly emanates from the urothelium (Yoshida et al., 2004; 2008). A subpopulation of intramuscular interstitial cells in the guinea pig bladder has been reported to contain ChAT and may therefore represent a third potential cellular source of acetylcholine (Gillespie et al., 2006a). It has been suggested that in the guinea pig bladder, spontaneous contractions (autonomous activity) are generated and coordinated by a specialized system of interstitial cells and intramural ganglia (Gillespie et al., 2004). TTX and atropine reportedly had no effect on spontaneous micro contractions of the isolated guinea pig bladder (Drake et al., 2003). In our study, however, hyoscine caused a small but significant reduction in spontaneous contractions of both the whole bladder and isolated muscle strips. Methodologically, the present study differed from the previous in vitro experiments in several ways: (i) the residual volume was about 2.25 mL in the present study, which is about twice that used previously (Drake et al., 2003); and (ii) effects of hyoscine were studied in the presence of TTX in the present study. When spontaneous contractions of the whole bladder or muscle strips were increased by physostigmine, hyoscine had a large inhibitory effect on contractility, indicating the importance of an ongoing cholinergic contribution for their generation. In addition, hyoscine, but not TTX, decreased distension-evoked contractile activity of the whole bladder. Thus, our data indicate that there is not only a spontaneous release of acetylcholine, but also that such release involves sufficient quantities to affect bladder muscle contractility.
These observations pose questions as to the source or sources of spontaneously released acetylcholine. Potential sources might include intrinsic neurones, axons of extrinsic neurones, interstitial cells or urothelial cells. When isolated muscle strips without the urothelium were studied, the contractile effects of physostigmine and inhibitory effects of hyoscine were similar to the whole bladder. This suggests that the urothelium is unlikely to be the major source of acetylcholine; this must reside within the outer muscle layers. The role of extrinsic nerves was evaluated by causing their degeneration by maintaining muscle strips in organ culture for 5 days. While extrinsic nerves degenerate under these conditions, intrinsic nerve cell bodies and their axons survive this procedure well (Song et al., 1995). One set of observations makes it unlikely that interstitial cells are the major source of spontaneously released acetylcholine. Rat muscle strips largely lack intrinsic neurones. After 5 days of organ culture, during which extrinsic nerves degenerate, these preparations therefore become largely denervated. Confirming this, EFS stimulation failed to evoke contractions in cultured muscle strips from rats and, importantly, physostigmine-induced contractions were nearly absent. This suggests that nerve fibres are the major source of spontaneously released acetylcholine. It should be noted that spontaneous contractile activity is well preserved in cultured muscle strips, suggesting that interstitial cells remain alive and functional. Studies of guinea pig bladder strips in organ culture were consistent with these findings. Dorso-medial strips of the guinea pig bladder contain relatively few intrinsic nerve cell bodies and thus probably receive most of their innervation from extrinsic neurones. Lateral muscle strips had about 15 times as many intramural neurones, which probably play a relatively larger role in their contractility. After 5 days in culture when extrinsic nerves showed significant degeneration, both EFS- and physostigmine-induced contractions were significantly inhibited in dorso-medial strips. However, physostigmine-induced contractions was not affected in lateral strips. This suggests that spontaneous release of acetylcholine occurs from both intrinsic (intramural) and extrinsic postganglionic parasympathetic nerves.
We cannot exclude the possibility that in a whole bladder, acetylcholine is released from the urothelium during distension (Kanai et al., 2007). It has been recently demonstrated that both stretch and focal application of carbachol to neonatal rat bladders evoked Ca2+ transients and membrane potential transients in smooth muscle near the urothelial–suburothelial interface. These then spread to the detrusor muscle. However, in adult bladders, while the same stimuli evoked Ca2+ and membrane potential waves in the urothelial–suburothelial regions, they did not propagate into the detrusor. Spontaneous activity in the detrusor appeared to be initiated near the serosal surface of the bladder wall (Kanai et al., 2007). It has been suggested that spontaneous release of acetylcholine from urothelium may regulate bladder tone (Yoshida et al., 2008). Under the conditions of the present study, non-neuronal acetylcholine (from the urothelium) appeared to contribute little to the TTX-resistant cholinergic contractile activity in the normal adult bladder. In adult rats, acetylcholine acting on muscarinic cholinergic receptors in the urothelium also evokes release of an unidentified diffusible factor that inhibits the underlying detrusor smooth muscle (Chess-Williams, 2002).
In most synapses in the central nervous system and autonomic nervous system, transmitter release depends on Ca2+ influx through N, P/Q and occasionally R type voltage-dependent Ca2+ channels, with much less involvement of L and T type Ca2+ channels (Takahashi and Momiyama, 1993; Wheeler et al., 1994; Wu et al., 1998; Waterman, 2000). Interestingly, a significant portion of acetylcholine release from preganglionic sympathetic nerve terminals in pelvic ganglia of guinea pigs and rats is resistant to blockade of L, T, N, P/Q and R type Ca2+ channels and is probably mediated by a novel, unidentified Ca2+ channel (Smith and Cunnane, 1999; Smith et al., 2000; Jobling et al., 2004). In the mammalian neuromuscular junction, acetylcholine release is mediated predominantly by P/Q type Ca2+ channels (Uchitel et al., 1992) while in amphibians and birds, N type Ca2+ channels predominate (De Luca et al., 1991). In the mouse bladder, neuromuscular transmission evoked by repetitive (10 Hz) EFS was inhibited by about 80% by mixture of selective N and P/Q type Ca2+ antagonists (Waterman, 1996). CTX alone reduces EFS-induced contractions of guinea pig bladder strips (Maggi et al., 1988), but a significant component of neuromuscular transmission (∼40%) evoked by EFS (10 Hz, 30 stimuli) was resistant to a cocktail of toxins, including antagonists for N type (CTX), P/Q type (ATX) and R type Ca2+ channels (SNX 482). In the present study, physostigmine-induced contractions of muscle strips from the guinea pig bladder, in the presence of TTX, were not affected by this cocktail of antagonists. It is likely that spontaneous release of acetylcholine from autonomic postganglionic and intramural neurones in the guinea pig bladder occurs through novel, unidentified types of Ca2+ channels.
In the guinea pig and human bladders, a well-developed network of intramural neurones exists, but its functions are not well defined (Gabella, 1990; Dixon et al., 2000). These neurones may comprise intramural neural circuits rather than just acting as simple relay neurones (Gillespie et al., 2006b). The highest density of nerve cell bodies in the guinea pig bladder was found to be around the entry points of the ureters and in the bladder neck, similar to a previous report (Gabella, 1990). From acetylcholinesterase staining, it was suggested that the majority of intramural neurones in the guinea pig bladder were likely to be cholinergic (Zhou and Ling, 1998); however, it is known that cholinesterase activity is not confined to cholinergic nerve cells (Costa et al., 1987). Previous experiments on the guinea pig bladder using more precise methods, visualizing ChAT or vesicular acetylcholine transporter (VAChT), were not successful. In the present study, 98% of tubulin-stained intramural neurones were found to be ChAT-immunoreactive, indicating that the great majority of these neurones are indeed cholinergic. In the human bladder, it was reported that 75% of intramural neurones were VAChT-immunoreactive (Dixon et al., 2000). The lack of effect of TTX on distension-induced bladder contractile activity, in vitro, suggests that intrinsic neural circuitry makes a very small contribution (if any) to such responses, as reported previously (Lagou et al., 2004).
The present study has established that spontaneous release of acetylcholine occurs in the whole bladder in vitro, and in isolated muscle strips, and that this is functionally significant. The relevance of this finding in vivo was also addressed. In vivo experiments in rats demonstrated that physostigmine, in the presence of an effective neural blockade by TTX, produced a tonic contraction of the bladder, similar to in vitro preparations. This contraction was blocked by a muscarinic antagonist and was therefore cholinergic. It appears that spontaneous release of acetylcholine may occur at a functionally significant level in vivo, where the innervation of the bladder is intact.
A number of studies have shown that urgency is poorly correlated with intravesical pressure in patients with painful bladder syndromes. One possible explanation is that enhanced localized contractile activity of the bladder (even without an overall increase in intravesical pressure) may lead to increased activation of sensory nerves, which causes excessive urgency (Coolsaet et al., 1993). In women with chronic pelvic pain syndromes, the bladder has more localized contractions than in healthy subjects (Van Os-Bossagh et al., 2001). Patients with ‘sensory urgency’ were reported to have a higher prevalence of localized contractile activity than a control, asymptomatic group. In most cases, episodes of urgency were accompanied by measurable local contractions but without detectable changes in intravesical pressure (Drake et al., 2005). Acetylcholine may play an important role in controlling localized bladder contractility in the overactive bladder. Edrophonium, a cholinesterase inhibitor, evoked or amplified involuntary detrusor contractions during filling in 80% of patients with overactive bladder, but did not affect detrusor function in those without frequency, urgency or urge incontinence (Yossepowitch et al., 2001). In overactive bladder due to outflow obstruction, there is an inhibition of acetylcholinesterase activity (Brading, 1997). Interestingly, anti-muscarinic drugs appear to act mainly during the storage phase by increasing bladder capacity and reducing urgency. However, parasympathetic cholinergic nerves are silent during storage phase, becoming active only during emptying (de Groat et al., 1993). It has recently been hypothesized that spontaneous release of acetylcholine from nerves in the bladder could play a role in the overactive bladder by increasing local contractile activity that leads to overstimulation of afferent fibres (Andersson, 2004). The present results provide evidence for spontaneous release of acetylcholine, even when neuronal firing activity is blocked by TTX. Whether this mechanism is present in the normal human bladder and whether it is enhanced in overactive bladder would be of great interest. Recently, it has been shown that a decrease of serum cholinesterase is related to occurrence of overactive bladder (Sugaya et al., 2007).
It has been proposed that in the elderly, increased spontaneous release of acetylcholine from non-neuronal sources (urothelium) may regulate bladder tone and possibly activate sub-urothelial afferent nerves via muscarinic receptors, leading to lower urinary tract symptoms including urgency and frequency (Yoshida et al., 2004; 2008; Kim et al., 2005). Anti-muscarinic drugs used for the treatment of overactive bladder, such as oxybutynin and darifenacin, reduced the activity of both A and C fibre bladder afferents (Iijima et al., 2007). Bladder afferent nerves may be positively modulated by excitatory mediators released from the urothelium, such as acetylcholine, ATP, prostaglandins and tachykinins (Birder, 2005; Kullmann et al., 2008). The present study suggests that these could be supplemented by spontaneous release of acetylcholine from nerve endings within the bladder wall.
In conclusion, the present study provides evidence that there is spontaneous, TTX-resistant, release of acetylcholine from autonomic cholinergic (extrinsic and intrinsic) nerves that significantly affect bladder contractility. This release is resistant to blockade by N, P/Q and R type Ca2+ channel antagonists. It is likely that some of the therapeutic actions of anti-muscarinic drugs in an overactive bladder are due to their effect on spontaneously released acetylcholine.
Acknowledgments
This study has been supported by the National Health and Medical Research Council of Australia Grant #375123.
Glossary
Abbreviations:
- ATX
ω-agatoxin IVA
- ChAT
choline acetyltransferase
- CTX
ω-conotoxin GVIA
- TTX
tetrodotoxin
Statement of conflicts of interest
None.
References
- Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alian M, Gabella G. Decrease and disappearance of intramural neurons in the rat bladder during post-natal development. Neurosci Lett. 1996;218:103–106. doi: 10.1016/s0304-3940(96)13129-3. [DOI] [PubMed] [Google Scholar]
- Andersson KE. Antimuscarinics for treatment of overactive bladder. Lancet Neurol. 2004;3:46–53. doi: 10.1016/s1474-4422(03)00622-7. [DOI] [PubMed] [Google Scholar]
- Birder LA. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol. 2005;289:F489–495. doi: 10.1152/ajprenal.00467.2004. [DOI] [PubMed] [Google Scholar]
- Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57–67. doi: 10.1016/s0090-4295(97)00591-8. [DOI] [PubMed] [Google Scholar]
- Brookes S, Costa M. Cellular Organisation of the Mammalian Enteric Nervous System. Vol. 14. London & New York: Taylor & Francis; 2002. [Google Scholar]
- Chess-Williams R. Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton Autac Pharmacol. 2002;22:133–145. doi: 10.1046/j.1474-8673.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- Coolsaet BL, Van Duyl WA, Van Os-Bossagh P, De Bakker HV. New concepts in relation to urge and detrusor activity. Neurourol Urodyn. 1993;12:463–471. doi: 10.1002/nau.1930120504. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB, Llewellyn-Smith IJ. Histochemistry of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1987. pp. 1–40. [Google Scholar]
- De Luca A, Rand MJ, Reid JJ, Story DF. Differential sensitivities of avian and mammalian neuromuscular junctions to inhibition of cholinergic transmission by omega-conotoxin GVIA. Toxicon. 1991;29:311–320. doi: 10.1016/0041-0101(91)90284-x. [DOI] [PubMed] [Google Scholar]
- Dixon JS, Jen PY, Gosling JA. The distribution of vesicular acetylcholine transporter in the human male genitourinary organs and its co-localization with neuropeptide Y and nitric oxide synthase. Neurourol Urodyn. 2000;19:185–194. doi: 10.1002/(sici)1520-6777(2000)19:2<185::aid-nau9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Drake MJ, Harvey IJ, Gillespie JI. Autonomous activity in the isolated guinea pig bladder. Exp Physiol. 2003;88:19–30. doi: 10.1113/eph8802473. [DOI] [PubMed] [Google Scholar]
- Drake MJ, Harvey IJ, Gillespie JI, Van Duyl WA. Localized contractions in the normal human bladder and in urinary urgency. BJU Int. 2005;95:1002–1005. doi: 10.1111/j.1464-410X.2005.05455.x. [DOI] [PubMed] [Google Scholar]
- Furness JB. The Enteric Nervous System. Oxford: Blackwell; 2006. [Google Scholar]
- de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modifications in animal models of human disease. In: Maggi CA, editor. The Autonomic Nervous System: Nervous Control of the Urogenital System. Vol. 6. London: Harwood; 1993. pp. 227–289. [Google Scholar]
- Gabella G. Intramural neurons in the urinary bladder of the guinea-pig. Cell Tissue Res. 1990;261:231–237. doi: 10.1007/BF00318664. [DOI] [PubMed] [Google Scholar]
- Gillespie JI, Markerink-van Ittersum M, De Vente J. cGMP generating cells in the bladder wall: identification of distinct networks of interstitial cells. BJU Int. 2004;94:1114–1124. doi: 10.1111/j.1464-410X.2004.05186.x. [DOI] [PubMed] [Google Scholar]
- Gillespie JI, Markerink-van Ittersum M, De Vente J. Interstitial cells and cholinergic signalling in the outer muscle layers of the guinea-pig bladder. BJU Int. 2006a;97:379–385. doi: 10.1111/j.1464-410X.2006.05989.x. [DOI] [PubMed] [Google Scholar]
- Gillespie JI, Markerink-van Ittersum M, de Vente J. Sensory collaterals, intramural ganglia and motor nerves in the guinea-pig bladder: evidence for intramural neural circuits. Cell Tissue Res. 2006b;325:33–45. doi: 10.1007/s00441-006-0166-8. [DOI] [PubMed] [Google Scholar]
- Hanani M, Maudlej N. Intracellular recordings from intramural neurons in the guinea pig urinary bladder. J Neurophysiol. 1995;74:2358–2365. doi: 10.1152/jn.1995.74.6.2358. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559:567–581. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima K, De Wachter S, Wyndaele JJ. Effects of the M3 receptor selective muscarinic antagonist darifenacin on bladder afferent activity of the rat pelvic nerve. Eur Urology. 2007;52:842–847. doi: 10.1016/j.eururo.2007.02.057. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A. Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol. 2007;293:F1018–F1025. doi: 10.1152/ajprenal.00183.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling P, Gibbins IL, Lewis RJ, Morris JL. Differential expression of calcium channels in sympathetic and parasympathetic preganglionic inputs to neurons in paracervical ganglia of guinea-pigs. Neuroscience. 2004;127:455–466. doi: 10.1016/j.neuroscience.2004.05.005. [DOI] [PubMed] [Google Scholar]
- de Jongh R, van Koeveringe GA, van Kerrebroeck PE, Markerink-van Ittersum M, de Vente J, Gillespie JI. Damage to the bladder neck alters autonomous activity and its sensitivity to cholinergic agonists. BJU Int. 2007;100:919–929. doi: 10.1111/j.1464-410X.2007.07129.x. [DOI] [PubMed] [Google Scholar]
- Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, et al. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol. 2007;292:F1065–F1072. doi: 10.1152/ajprenal.00229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Yoshimura N, Masuda H, de Miguel F, Chancellor MB. Antimuscarinic agents exhibit local inhibitory effects on muscarinic receptors in bladder-afferent pathways. Urology. 2005;65:238–242. doi: 10.1016/j.urology.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Artim DE, Birder LA, de Groat WC. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci. 2008;28:1977–1987. doi: 10.1523/JNEUROSCI.4694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagou M, Drake MJ, Gillespie JI. Volume-induced effects on the isolated bladder: a possible local reflex. BJU Int. 2004;94:1356–1365. doi: 10.1111/j.1464-410X.2004.05113.x. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–836. [PubMed] [Google Scholar]
- Maggi CA, Santicioli P, Meli A. Postnatal development of myogenic contractile activity and excitatory innervation of rat urinary bladder. Am J Physiol Regul Integr Comp Physiol. 1984;247:R972–R978. doi: 10.1152/ajpregu.1984.247.6.R972. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Patacchini R, Santicioli P, ITh L, Giuliani S, Geppetti P, et al. The effect of omega conotoxin GVIA, a peptide modulator of the N-type voltage sensitive calcium channels, on motor responses produced by activation of efferent and sensory nerves in mammalian smooth muscle. Naunyn Scmiedeberg's Arch Pharmacol. 1988;338:107–113. doi: 10.1007/BF00174856. [DOI] [PubMed] [Google Scholar]
- Smith AB, Cunnane TC. Calcium channels controlling acetylcholine release in the guinea-pig isolated anterior pelvic ganglion: an electropharmacological study. Neuroscience. 1999;94:891–896. doi: 10.1016/s0306-4522(99)00286-9. [DOI] [PubMed] [Google Scholar]
- Smith AB, Motin L, Lavidis NA, Adams DJ. Calcium channels controlling acetylcholine release from preganglionic nerve terminals in rat autonomic ganglia. Neuroscience. 2000;95:1121–1127. doi: 10.1016/s0306-4522(99)00505-9. [DOI] [PubMed] [Google Scholar]
- Song ZM, Brookes SJ, Llewellyn-Smith IJ, Costa M. Ultrastructural studies of the myenteric plexus and smooth muscle in organotypic cultures of the guinea-pig small intestine. Cell Tissue Res. 1995;280:627–637. doi: 10.1007/BF00318365. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Onaga T, Nishjima S, Miyazato M, Oshiro Y, Hokama S, et al. Relationship between serum cholinesterase and urinary bladder activity in patients with or without overactive bladder and/or neurogenic bladder. Biomed Res. 2007;28:287–294. doi: 10.2220/biomedres.28.287. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Uchitel OD, Protti DA, Sanchez V, Cherksey BD, Sugimori M, Llinas R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Nat Acad Sci USA. 1992;89:3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Os-Bossagh P, Kosterman LM, Hop WC, Westerhof BE, de Bakker JV, Drogendijk AC, et al. Micromotions of bladder wall in chronic pelvic pain (CPP): a pilot study. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:89–96. doi: 10.1007/s001920170071. [DOI] [PubMed] [Google Scholar]
- Waterman SA. Multiple subtypes of voltage-gated calcium channel mediate transmitter release from parasympathetic neurons in the mouse bladder. J Neurosci. 1996;16:4155–4161. doi: 10.1523/JNEUROSCI.16-13-04155.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SA. Voltage-gated calcium channels in autonomic neuroeffector transmission. Pr Neurobiol. 2000;60:181–210. doi: 10.1016/s0301-0082(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Wu LG, Borst JG, Sakmann B. R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc Nat Acad Sci USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004;63:17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Masunaga K, Satoji Y, Maeda Y, Nagata T, Inadome A. Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in urothelium and its clinical significance. J Pharmacol Sci. 2008;106:193–198. doi: 10.1254/jphs.fm0070115. [DOI] [PubMed] [Google Scholar]
- Yossepowitch O, Gillon G, Baniel J, Engelstein D, Livne PM. The effect of cholinergic enhancement during filling cystometry: can edrophonium chloride be used as a provocative test for overactive bladder? J Urol. 2001;165:1441–1445. [PubMed] [Google Scholar]
- Zhou Y, Ling EA. Co-localization of nitric oxide synthase and some neurotransmitters in the intramural ganglia of the guinea pig urinary bladder. J Comp Neurol. 1998;394:496–505. [PubMed] [Google Scholar]


