Abstract
Background and purpose:
Neuropeptides are involved in the regulation of food intake in the central nervous system, but they might also act on peripheral fat tissue via neuropeptide receptors.
Experimental approach:
We investigated the receptor expression and activity of pituitary adenylate cyclase-activating polypeptide (PACAP) and of neuropeptide Y at the mRNA and protein levels in the 3T3-L1 fibroblast line during differentiation into adipocytes. Intracellular calcium concentration was measured by calcium imaging.
Key results:
The PACAP receptors PAC1 and VPAC2 as well as the neuropeptide Y1 receptor were expressed at the mRNA level in fibroblasts, pre-adipocytes and adipocytes. The mRNA profile of the PAC1 receptor isoforms showed the HOP sequence, whereas the HIP-isoform was present in subconfluent 3T3-L1 fibroblasts only. At the protein level, the mature 3T3-L1 adipocytes produced the PAC1 and Y1 receptors; only the PAC1 receptor showed carbohydrate residues. Both neuropeptides induced an increase of intracellular calcium in mature adipocytes, which was absent in the precursor cells. These changes in calcium were mediated by Y1 and PAC1 receptors as demonstrated by the effects of specific receptor agonists and antagonists.
Conclusions and implications:
As the PAC1-HOP receptor variant seems to be responsible for PACAP-mediated calcium influx in many cell types, the HOP sequence might also mediate the increase in intracellular calcium in adipocytes. Because a high intracellular calcium level is associated with lipogenesis, peptidergic innervation of adipose tissue might be involved in stress-induced obesity.
British Journal of Pharmacology (2009) 157, 620–632; doi:10.1111/j.1476-5381.2009.00164.x; published online 27 April 2009
Keywords: NPY, PACAP, neuropeptide receptor, adipogenesis, adipose tissue, peptidergic innervation
Introduction
Adipocytes are the characteristic cells of adipose tissue. They undergo lipogenesis and lipolysis for the storage and release of energy to meet the needs of the body. Adipocytes are also endocrine cells due to the secretion of adipokines, which are highly influential on the immune system, blood vessels and insulin sensitivity (Waki and Tontonoz, 2007; Wang et al., 2008). Adipocyte-dependent physiological functions become pathophysiological effects when adipocytes develop excessively; it is a risk factor that may lead to diseases of the heart and circulatory system, such as diabetes and cancer (Visscher and Seidell, 2001). Studies on adipose tissue have given insight into key transcription factors [CCAAT/enhancer binding protein (C/EBP) family and peroxisome proliferator-activated receptor-γ (PPAR-γ)] involved in adipocyte differentiation (Gregoire et al., 1998)], the expression and function of new adipokines (Waki and Tontonoz, 2007; Wang et al., 2008) and cross-talk with other tissues, such as nerves (Turtzo et al., 2001; Kosacka et al., 2006). Signals affecting adipocyte differentiation and function are currently of considerable interest. According to a recent study, high extracellular Ca2+ levels impair adipogenesis by inhibiting the expression of key adipogenic genes in 3T3-L1 adipocytes (Jensen et al., 2004). Neuropeptide Y (NPY) and the pituitary adenylate cyclase-activating polypeptide (PACAP) act directly, although separately, on adipocytes; NPY has anti-lipolytic functions (Serradeil-Le Gal et al., 2000) and PACAP is lipogenic by potentiating insulin-dependent glucose uptake (Nakata et al., 1999) and lipolytic in the absence of insulin (Åkesson et al., 2003; 2005). Both neuropeptides might be partners in the regulation of energy utilization because NPY-positive neurones in the hypothalamic arcuate nucleus raise their intracellular calcium under the influence of PACAP (Nakata et al., 2004).
Neuropeptide Y is a 36-amino-acid neuropeptide that belongs to the pancreatic polypeptide family with a ubiquitous occurrence in the brain and peripheral organs. Hypothalamic NPY increases appetite signalling in a leptin-dependent manner. Peripheral NPY, which is preferentially found in sympathetic fibres, has pleiotropic functions on the vascular and endocrine systems (Zukowska et al., 2003; Kuo et al., 2007). It also acts as a vasoconstrictor and a vascular mitogen, effects that appear to be involved in the development of atherosclerosis via particular postsynaptic NPY receptors (Pons et al., 2004; Zukowska, 2005). Five NPY receptors have been classified: Y1, Y2, Y4, Y5 and Y6 (Ingenhoven and Beck-Sickinger, 1999; all receptor nomenclature follows Alexander et al., 2008). Their activation results in adenylate cyclase inhibition and a decrease in cAMP via Pertussis toxin-sensitive G-proteins. The Y1 receptor can also couple to other second messenger systems in control of direct Ca2+ influx (Prieto et al., 2000). In primary human adipocyte cultures, activation of the Y1 subtype inhibits lipolysis (Serradeil-Le Gal et al., 2000). The anti-lipolytic effect may additionally be mediated by a Y2 receptor-dependent process, as suggested by the finding that abdominal obesity increases in mice in the context of Y2 receptor up-regulation (Kuo et al., 2007). For 3T3-L1 pre-adipocytes, the NPY-activated proliferation and adipogenic differentiation were reported without further study of the NPY receptor profile (Kuo et al., 2007).
Similar to NPY, PACAP also occurs in the brain and peripheral nerve fibres. PACAP exists in two biologically active forms, the 38-amino-acid residue long PACAP and the N-terminally truncated PACAP-27 (Miyata et al., 1990). Both PACAP isoforms belong to the vasoactive intestinal polypeptide (VIP) family. Two PACAP receptors with similar affinity for VIP and PACAP are VPAC1 and VPAC2, whereas the PAC1 receptor prefers PACAP exclusively (Dautzenberg et al., 1999). By alternative splicing of the PAC1 gene, six receptor isoforms are generated with or without the inclusion of three 28-amino-acid sequences/cassettes (HIP, HOP-1 and/or HOP-2) in the third intracellular loop; the short form includes no cassettes (Spengler et al., 1993). The HOP splicing variant appears to mediate increased intracellular Ca2+ and neurotransmitter release in chromaffin cells by coupling to the phospholipase C pathway (Mustafa et al., 2007; Ushiyama et al., 2007). The PACAP molecule could be beneficial in the treatment of diabetes type 2, as this neuropeptide is one of the most effective insulinotropins known (Nakata and Yada, 2007). Intraperitoneal PACAP administration decreases blood glucose levels in rats and mice (Yada et al., 2000), probably by enhancing insulin secretion in pancreatic islets, which PACAP has been shown to accomplish in vitro as well as in vivo (Yamaguchi, 2001; Jamen et al., 2002). Furthermore, PACAP potentiates insulin-guided glucose uptake in 3T3-L1 adipocytes and primary adipocytes (Nakata et al., 1999; Åkesson et al., 2003). However, this lipogenic/anabolic effect turns into a lipolytic/catabolic process in the absence of insulin. It should be noted that the VPAC2 receptor is responsible for insulin-independent lipolysis resulting from PACAP application in primary adipocyte cultures (Åkesson et al., 2005). It is not known whether the VPAC2 receptor is also present in 3T3-L1 adipocytes.
We recently shown that neurones from postnatal dorsal root ganglia (DRG) considerably improve neurite outgrowth when co-cultured with 3T3-L1 adipocytes, and they even form synaptic contacts (Kosacka et al., 2005). Thus, the main aim of the current study was to clarify the mRNA profiles and activities of the PACAP and NPY receptors during 3T3-L1 adipogenesis, using a pharmacological approach, in order to obtain new insights into signal transduction elicited in these cells by the two neuropeptides.
Methods
Animals
All animal care and experimental procedures complied with and were approved by the local ethical guidelines of the ‘Regierungspräsidium Leipzig’, reg. no.: T07/07.
Cultures of 3T3-L1 fibroblasts, pre-adipocytes and adipocytes
Mouse 3T3-L1 fibroblasts (American Type Culture Collection, Rockville, MD, USA) were maintained in Dulbecco's modified Eagle medium (DMEM) with 25 mmol·L−1 glucose and 10% foetal calf serum (all from Sigma, Deisenhofen, Germany). The cells were either grown as fibroblasts to subconfluence within 3 days or as pre-adipocytes to confluence within 5–6 days. On day 2 of confluence, pre-adipocytes were differentiated into adipocytes by DMEM supplemented with 1 µmol·L−1 insulin, 0.4 μg·mL−1 dexamethasone and 0.5 mmol·L−1 iso-butyl methylxanthine. Three days later, the medium was switched to DMEM containing 1 µmol·L−1 insulin for 3 days. Daily medium replacement turned 95% of the pre-adipocytes into mature adipocytes as evident from accumulated fat droplets. The three different cell types were either plated onto round glass coverslips mounted into 24-well culture plates or small-sized Petri dishes for morphological study and calcium imaging analysis, or plated into plastic flasks for Western blotting or PCR analysis.
Nile red staining
Cell cultures were rinsed with 0.1 mol·L−1 phosphate-buffered saline (PBS) and fixed with 2% PBS-buffered formaldehyde containing 0.2% Triton X-100 and 5% sucrose at 37°C for 5 min. After a thorough PBS rinse (three times, 5 min each), the cells were further permeabilized with 60% isopropanol solution in PBS (5 min) and incubated with 1:100 buffer diluted Nile red stock solution (20 µg·mL−1 in acetone; ICN Biomedicals Inc., Aurora, OH, USA) for 5 min. Cultures were washed in PBS three times and the coverslips inverted and finally mounted on object slides with Glycergel® (Dako, Hamburg, Germany).
RT-PCR analysis
Cultures developed in 250 mL flasks were scraped off in the stage of interest. The cells were homogenized in peqGold RNA-Pure™ (PEQLAB Biotechnology, Erlangen, Germany), and the RNA was deproteinized by phenol-chloroform and precipitated with 75% alcohol. The reverse transcription reaction was performed in a total volume of 20 µL and utilized 5 µg of RNA, 500 ng oligo (dT)15 primers (Promega, Mannheim, Germany), 4 µL first-strand buffer (Invitrogen, Karlsruhe, Germany), 2 µL 0.1 mol·L−1 dithiothreitol, 1 µL of a dNTP-mix (10 mmol·L−1 each), 1 µL RNaseOUT (Invitrogen) and 200 units of SuperScript™ II Reverse Transcriptase (Invitrogen). PCR reactions were performed in a total volume of 25 µL, using 1 µL of cDNA, 0.2 µmol·L−1 forward and reverse primers, 2.5 µL PCR-buffer (10×; Roche, Mannheim, Germany), 2 µL dNTP mix and 2.5 U Taq DNA-Polymerase (Roche). Initial denaturation at 95°C for 5 min was followed by 35 cycles [25 cycles for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] of denaturing at 95°C for 30 s, annealing at either 55°C (PAC1, HIP and HOP primers) or 60°C (Y1, Y5, VPAC1 and VPAC2) for 30 s, and extension at 72°C for 30 s. A final extension at 72°C for 10 min and subsequent cooling terminated the reaction. PCR products were analysed by electrophoresis on 1% agarose gel containing ethidium bromide and visualized by UV transillumination. To identify the PAC1 receptor, we used primers described by Jamen et al. (2002) that flank the HIP-HOP region, or the forward primer located in either the HIP or the HOP splicing variants for distinguishing isoforms. The VPAC1 and VPAC2 primers were taken from Rawlings et al. (1995). Other primer sets and PCR conditions were designed using Primer3 software (http://fokker.wi.mit.edu/primer3/input.htm). The primer pairs are shown in Table 1. Murine GAPDH cDNA fragments were amplified with specific primers and served as an internal standard. Amplified cDNA fragments were cloned into pGEM-T (Promega). Inserts of the expected size were sequenced at the local core unit for DNA technology and identified by comparisons with GenBank sequences (blast search).
Table 1.
Primers and details of the PCR analysis
| Receptor | mRNA Accession # at GenBank | Primers |
|---|---|---|
| PAC1 | NM_007407 | forward: 5′-CAT CCT TGT GCA GAA GCT GC-3′ |
| reverse: 5′-GGT GCT TGA AGT CCA TAG TG-3′ | ||
| HIP-cassette | NM_007407 | forward: 5′-ACA AAT TTA AGA CTG AGA GT-3′ |
| reverse: 5′-GGT GCT TGA AGT CCA TAG TG-3′ | ||
| HOP-cassette | NM_007407 | forward: 5′-TCC ACC ATT ACT CTA CGG CT-3′ |
| reverse: 5′-GGT GCT TGA AGT CCA TAG TG-3′ | ||
| VPAC1 | NM_011703 | forward: 5′-GGC CCC ATC CTC ATC TCC AT-3′ |
| reverse: 5′-CCG CCT GCA CCT CAC CAT TG-3′ | ||
| VPAC2 | NM_009511 | forward: 5′-ATG GAC AGC AAC TCG CCT CTC TTT AG-3′ |
| reverse: 5′-GAA GGA ACC AAC ACA TAA CTC AAA CAG-3′ | ||
| Y1 | NM_010934 | forward: 5′-TGA TTC GCT TGG TCT CAC TG-3′ |
| reverse: 5′-GTC CTT GCA GTG GCT TCT TC-3′ | ||
| Y2 | NM_008731 | forward: 5′-CCA TCT TCC GGG AAT AC-3′ |
| reverse: 5′-CTG AGG AAC CAC GTC A-3′ | ||
| Y5 | NM_016708 | forward: 5′-AGG CAG TGT TCC GAG CAG-3′ |
| reverse: 5′-AGA AGC GAC CGC ACT CAG-3′ | ||
| GAPDH | XM_983502 | forward: 5′-ATG GTG AAG GTC GGT GTG A-3′ |
| reverse: 5′-GGA AGC CCA TCA CCA TCT T-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blotting
For immunoblot analysis, cell cultures were lysed by ultrasonication in 60 mmol·L−1 Tris-HCl, pH 6.8, containing 2% sodium dodecyl sulphate (SDS) and 10% sucrose. The samples were then diluted 1:1 in sample buffer (250 mmol·L−1 TRIS-HCl at pH 6.8 and containing 4% SDS, 10% glycerin and 2% β-mercaptoethanol) and denatured at 70°C for 10 min. Protein concentration was assessed using the BCA™ protein assay (Pierbo Science, Bonn, Germany). Proteins (50 µg whole cell amount or 1 µg of purified glycoproteins) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose by electroblotting. Non-specific binding sites were blocked by pre-incubation with 5% non-fat milk for 60 min. The blots were incubated with polyclonal anti-PAC1 receptor antiserum (diluted 1:5000; obtained from Dr S. Schulz; described in Schulz et al., 2004) or polyclonal anti-Y1 receptor antiserum (1:2000; Alpha Diagnostics, San Antonio, TX, USA) at 4°C overnight. Immunoreactions were detected with the appropriate peroxidase-conjugated anti-rabbit IgG secondary antibody (1:10 000; Vector Laboratories, Peterborough, UK) at room temperature for 2 h. Peroxidase activity was visualized with an enhanced chemiluminescence kit (Amersham, Pharmacia, Freiburg, Germany). In addition, blots were stripped and incubated with an anti-GAPDH monoclonal antibody (diluted 1:3000, Research Diagnostics, Flanders, the Netherlands) followed by the anti-mouse IgG secondary antibody (1:4000; Vector Laboratories) to verify equal protein loading. To check specificity, antibodies were either pre-incubated with the corresponding blocking peptide (same source as the specific antibody) overnight at 4°C, or the protein extracts were deglycosylated with peptide N-glycosidase F (PNGase F; New England BioLabs, Beverly, MA, USA). We followed the manufacturer's protocol and additionally added 1 µL of protease inhibitor cocktail (Sigma) to prevent degradation by endogenous proteases.
DRG cells isolated from 3-day-old rat pups according to the protocol of Kosacka et al. (2005) were used as a positive control. Additionally, we performed a glycoprotein purification from the whole cell protein using wheat germ agglutinin-linked agarose beads (Sigma). Separation of the glycoprotein fraction was performed as previously described (Schulz et al., 2004).
Measurement of intracellular calcium levels by calcium imaging
3T3-L1 cells were loaded with 10 µmol·L−1 FURA2 acetoxymethyl ester (FURA2-AM; TEF Labs, Austin, TX, USA) and 0.0125% Pluronic® (TEF Labs) in 1 mL of standard Ringer solution (125 mmol·L−1 NaCl, 5 mmol·L−1 KCl, 2 mmol·L−1 CaCl2, 10 mmol·L−1 HEPES and 7.5 mmol·L−1 glucose; adjusted to pH 7.4 with NaOH) at 37°C for 30 min in the dark. The coverslips were placed in a superfusion chamber and continuously perfused at room temperature at a rate of 2 mL·min−1. Solutions were removed by a vacuum pump. After an initial 3 min rinsing, cells were superfused with Ringer solution containing either PACAP38, NPY (both AnaSpec Inc., San Jose, CA, USA) or specific agonists (Table 2) at a concentration of 100 nmol·L−1 for 2 min. Blocking experiments were performed in a 500 nmol·L−1 solution of the specific antagonist, including the specific Y1 receptor antagonist (R)-Nα-diphenylacetyl-N-(4-hydroxybenzyl) argininamide 3226 (BIBP 3226) (Sigma) or the specific PAC1 receptor antagonist PACAP(6-38) (American Peptide Company, Sunnyvale, CA, USA). After a 5 min pre-incubation with the antagonist, the cells were superfused with a solution containing 500 nmol·L−1 of the antagonist and 100 nmol·L−1 of the agonist for another 2 min. Prostaglandin F2α (PGF2α, 1 µmol·L−1) was used as a positive control.
Table 2.
Agonists and antagonists of NPY and PACAP receptors
| Reagent | Function | Reference | Source |
|---|---|---|---|
| NPY | Agonist for all NPY receptors | AnaSpec Inc., San Jose, CA, USA | |
| [Phe(7),Pro(34)] porcine NPY (pNPY) | Agonist for Y1 receptors | Söll et al., 2001,Höfliger et al., 2003,Lecklin et al., 2003 | Professor Beck-Sickinger, Leipzig, Germany |
| BIBP 3226 | Antagonist for Y1 receptors | Rudolf et al., 1994 | SIGMA |
| Ahx[5-24] pNPY | Agonist for Y2 receptors | El Bahh et al., 2002,Höfliger et al., 2003 | Professor Beck-Sickinger |
| [hPP1-17,A31, Aib32] pNPY | Agonist for Y5 receptors | Cabrele et al., 2000,Höfliger et al., 2003,Lecklin et al., 2003 | Professor Beck-Sickinger |
| PACAP | Agonist for all PACAP receptors | AnaSpec Inc., San Jose, CA, USA | |
| VIP | Agonist for VPAC1 & VPAC2 receptors | DeHaven and Cuevas, 2004 | BioTrend Chemicals AG, Zurich, Switzerland |
| PACAP(6-38) | Antagonist for PAC1 receptors | Bergström et al., 2003 | American Peptide Company Inc., Sunnyvale, CA, USA |
| [K15,R16,L27] VIP(1-7)/GRF(8-27) | Agonist for VPAC1 receptors | Gourlet et al., 1997 | Professor Gregoire, Brussel, Belgium |
| PGF2α | Positive control | Nakada et al., 1990,Miller et al., 1996 | SIGMA |
BIBP 3226, (R)-Nα-diphenylacetyl-N-(4-hydroxybenzyl) argininamide 3226; NPY, neuropeptide Y; PACAP, pituitary adenylate cyclase-activating polypeptide; PGF2α, prostaglandin F2α.
Experiments were performed on a Zeiss Axiovert 135 microscope (Carl Zeiss Jena GmbH, Jena, Germany) equipped with an Axiovert 135 UV transparent optic (Carl Zeiss). Dye-excitation illumination was provided by a dual-wavelength illuminator system (T.I.L.L. Photonics GmbH, Gräfelfing, Germany) consisting of a xenon arc lamp, variable speed reflective optic chopper and two monochromators, both under computer control. The excitation wavelengths were 340 and 380 nm. Emitted fluorescence was filtered at 510 nm by a photomultiplier tube and a photon-counting photometer. Changes in the intracellular calcium level were expressed as arbitrary units of the delta ratio (UΔR) of dye fluorescence at 340 and 380 nm. Fluorescence intensities for both excitation wavelengths were acquired in 2 s intervals. Calcium measurements were performed using objective magnification of 20× on areas with 10–20 fibroblasts, 30–50 pre-adipocytes or 20–30 mature adipocytes respectively.
Statistical analysis
Data are presented as mean ± SEM of at least three independent experiments. The data from calcium imaging were quantified using Sigma Plot software and evaluated by the multiple comparison Holm–Sidak test (SigmaStat Software, Jandel Scientific, San Rafael, CA, USA).
Results
Characterization of 3T3-L1 fibroblasts, pre-adipocytes and adipocytes
Using Nile red staining, 3T3-L1 fibroblasts exhibited typical fibroblast-like morphology during subconfluence (Figure 1A). Due to the contact inhibition, cell growth stopped at the confluence (Gregoire, 2001). At this stage, we termed these cells pre-adipocytes (Figure 1B). The terminology describing subconfluent 3T3-L1 cells is currently not precise. Also, the conversion of the 3T3-L1 fibroblast to the pre-adipocyte phenotype is insufficiently understood. Nevertheless, in the differentiation medium used, adipocytes developed over the course of the following 7 days. The cells converted from a fibroblast-like to a spherical shape and increased in size with an abundant lipid droplet accumulation (Figure 1C). All cell stages maintained their typical morphology during the loading with FURA2-AM for the calcium imaging experiments (Figure 1D–F).
Figure 1.

Characterization of 3T3-L1 fibroblasts, pre-adipocytes and adipocytes by staining. (A–C) Nile red staining of fibroblasts (A), pre-adipocytes (B) and adipocytes (C). (D–F) For comparison, the cell types are depicted by FURA2-AM loading. On culture day 3, 3T3 cells exhibited a fibroblast-like subconfluent morphology (A, D). On day 6, confluent pre-adipocytes display no lipid droplets (B), whereas adipocytes exhibit lipid droplets as signs of maturation (C). Scale bars represent 40 µm at a magnification of 40× (A–C) or 80 µm at a magnification of 20× (D–F).
Presence of the NPY and PACAP receptor mRNA during 3T3-L1 adipogenesis
In fibroblasts, pre-adipocytes and mature adipocytes, the mRNA encoding NPY and PACAP receptors was detected by RT-PCR analysis. Because 3T3-L1 cells were derived from mice, whole mouse brain RNA was used as a positive control. Amplification of whole mouse brain RNA with primer pairs specific for the Y1 receptor produced a band with the expected length of 295 bp (Figure 2A, left). Strong bands of the same length were found in fibroblasts, pre-adipocytes and mature adipocytes (Figure 2B). To verify the presence of Y2 receptor and Y5 receptor mRNA, primers were used that had generated clear amplification products of the expected length in the mouse brain mRNA extract: 201 bp for Y2 receptors and 217 bp for Y5 receptors (Figure 2A, left). Also in the mouse brain RNA, the Y5 receptor primers produced an additional band around 300 bp, which had been found previously in the hypothalamus as a long isoform of the Y5 receptor (Rodriguez et al., 2003). In contrast to the Y1 receptor, transcripts of the Y2 and Y5 receptors were absent from the three cell types (Figure 2B).
Figure 2.

The mRNA profile of receptors for neuropeptide Y and pituitary adenylate cyclase-activating polypeptide (PACAP) at different stages of 3T3-L1 cell adipogenesis as determined by RT-PCR. (A) Whole mouse brain RNA was used to validate the expected sizes of the receptor products. (B) Neuropeptide Y1 receptor (Y1R) was present in each stage of adipogenesis, whereas the Y2 (Y2R) and Y5 receptor (Y5R) were absent in 3T3-L1 cell extracts. (C) The PACAP receptors, PAC1 and VPAC2 were detected in the three cell types of adipogenesis. The VPAC1 receptors appeared only in fibroblasts. A, mature adipocytes; FB, fibroblasts; PA, pre-adipocytes.
To detect the PAC1 receptor mRNA, primers able to differentiate between a possible insertion of either one HIP or HOP splicing variants (386 or 389 bp), both cassettes (473 bp) or no cassette for the short receptor variant (305 bp) were used. The primers revealed two amplification products in the mouse brain mRNA, 305 and 390 bp (Figure 2A, right). These two bands indicated the presence of the short form of the PAC1 receptor and a variant with an insertion of one 28-amino-acid cassette. The upper band was consistently seen throughout all adipogenic types, whereas the lower band was absent in pre-adipocytes (Figure 2C). The VPAC1 receptor primers produced transcripts of 298 bp only in 3T3 fibroblasts and no products in pre-adipocytes and adipocytes. Primers specific for the VPAC2 receptor generated strong bands corresponding to 325 bp that were consistently present throughout 3T3-L1 adipogenesis, which was similar to the expression of PAC1 receptors (Figure 2C). All products seen in mature adipocytes were cloned and successfully sequenced. They were at least 99% identical with the GenBank sequences.
The PAC1 receptor mRNA isoforms
To examine the predominant isoforms of the PAC1 receptor, we used mouse brain RNA to validate the success of our RT-PCR procedures. The transcript containing the HOP sequence resulted in the expected band with a length of 259 bp (Figure 3, upper row). Amplification products resulting from the primers containing the HOP sequence revealed a 259 bp band in fibroblasts, pre-adipocytes and adipocytes (Figure 3, second row). The HOP-amplicons were also confirmed by sequencing; they were 100% identical with the GenBank sequences. Amplification of the transcripts produced two bands for the HIP-isoform, one of the expected size of 329 bp and another of 413 bp. The latter represented a non-specific amplification according to the sequencing outcome. The 413 bp transcript is likely to have occurred as co-product during the partial reverse transcription and may not arise in the full-length transcription. The lower band of 329 bp was only found in fibroblasts and was identified successfully by sequencing (99% identity) as HIP-related. This indicated that the HIP-isoform of the PAC1 receptor was present only in 3T3-L1 fibroblasts (Figure 3, third row). GAPDH amplification revealed equal sampling (Figure 3, lower row).
Figure 3.
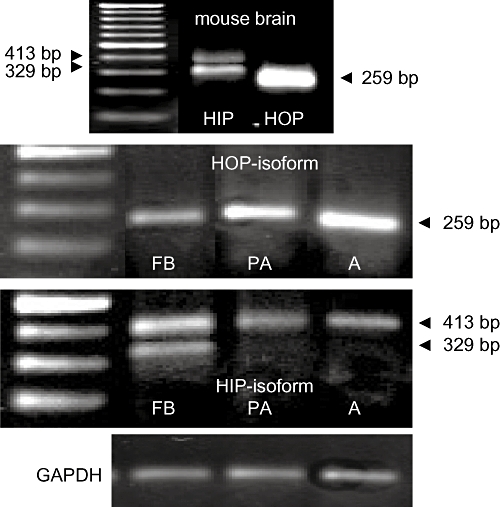
RT-PCR products of mouse brain mRNA for the PAC1 variants. Upper row: Amplicons of 329 and 413 bp corresponding to the HIP-isoform of PAC1 (left lane) and 259 bp band generated when using HOP-specific primers. Second row: Primers for HOP-cDNA produced strong amplicons with the expected size of 259 bp in fibroblasts (FB), pre-adipocytes (PA) and mature adipocytes (A). Third row: A 413 bp band appeared in each cell stage of adipogenesis. The negative outcome of sequencing determined them to be non-specific. The HIP-cassette of 329 bp, which was only verified in 3T3-L1 fibroblasts, was confirmed by sequencing. Lower row: The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) band indicates equal sample loading.
Presence of Y1 and of PAC1 receptor proteins in mature adipocytes
Investigating the lysates of mature adipocytes for the presence of the Y1 receptor protein by Western blotting, three bands of roughly 45, 60 and 70 kDa were found. Considering that both receptors as G-protein coupled receptors have putative N-glycosylation sides, deglycosylation with PNGase F treatment was performed up to 4 h. After enzymatic digestion, the immunodensity of the three bands did not change. The positive control with DRG cell cultures gave similar results. Specifity of two immunobands (70 and 45 kDa) was assumed because the application of the pre-absorbed antibody led to the disappearance of the 70 kDa band and the distinct decrease of the 45 kDa band. Further, the glycoprotein fraction showed no Y1 protein-related band (all data not shown).
Western blot analysis of cell lysates for PAC1 receptor protein revealed two bands with 55 and 50 kDa. The 55 kDa band disappeared, whereas the 50 kDa protein remained after PNGase F treatment for 2 h (Figure 4, left). Thus, the 55 kDa band represented the glycosylated form. In the DRG cell cultures this band convincingly increased in intensity (not shown). Blocking the PAC1 receptor-antibody with the specific immunopeptide caused a disappearance and decrease of the 55 kDa and the 50 kDa immunoband, respectively (Figure 4, middle). The glycoprotein fraction contained only one strong band at 55 kDa (Figure 4, right).
Figure 4.

By using Western blot analysis with the PAC1 receptor antibody (1:5000), two distinct bands with 50 and 55 kDa were detected. Enzymatic deglycosylation led to the disappearance of the 55 kDa band (left). After blocking the antibody with the specific immunopeptide, the blot lacked the 55 kDa band, and the 50 kDa band seemed to diminish (middle).The glycoprotein fraction by separation of glycoproteins with wheat germ agglutinin-linked beads showed only the 55 kDa band (right). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
NPY- and PACAP-mediated intracellular calcium increase in adipocytes
Pilot experiments for a positive control were performed with 30 or 60 mmol·L−1 KCl or 100 mmol·L−1 ATP. Neither solution affected intracellular calcium levels (data not shown). PGF2α, which is known to be responsible for calcium mobilization in 3T3-L1 pre-adipocytes but not in fibroblasts and mature adipocytes (Nakada et al., 1990; Miller et al., 1996), was then successfully used as a positive control; stimulation with 1 µmol·L−1 PGF2α resulted in a transient rise of intracellular calcium levels in all stages of 3T3-adipogenesis. In comparison, we observed a notably lower calcium peak after pre-incubation with PACAP.
The extent of the calcium response was also examined in the presence of 100 nmol·L−1 NPY, which was applied for 2 min. Fibroblasts exhibited a moderate calcium increase, whereas confluent pre-adipocytes did not respond (Figure 5A, left and middle graphs). In mature adipocytes, NPY stimulation led to a conspicuous calcium peak (Figure 5A, right graph). By measuring the peak extension for the intracellular calcium increase, a significantly higher response to NPY was noted for mature adipocytes compared with fibroblasts (P < 0.05) and with pre-adipocytes (P < 0.01; Figure 5B, left graph). The statistically significant difference was confirmed by the multiple comparison Holm–Sidak test. Comparing the number of reactive cells per area, a greater proportion of mature adipocytes were responsive than pre-adipocytes (P < 0.01). Fibroblast cultures with an intermediate proportion lacked a statistically significant difference from pre-adipocytes and adipocytes (Figure 5B, right panel).
Figure 5.

Calcium response of 3T3-L1 cells after stimulation with neuropeptide Y (NPY) or prostaglandin F2α (PGF2α). (A) Low calcium responses were seen in subconfluent cultures of fibroblasts (left) and pre-adipocytes (middle) and moderate calcium mobilization was seen in confluent cultures of mature adipocytes (right) after stimulation with 100 nmol·L−1 NPY for 2 min. Stimulation with 1 µmol·L−1 PGF2α was used to check the viability of cells after a 10 min buffer wash to remove neuropeptides. (B) Measuring the extension of the calcium peak (left) and the proportion of reactive cells per defined area (right) revealed statistically significant differences between adipocytes and pre-adipocytes and between fibroblasts and adipocytes. Data from three independent experiments are shown as mean ± SEM. *P≤ 0.05, **P≤ 0.01. A, mature adipocytes; FB, fibroblasts; PA, pre-adipocytes.
Stimulation with a 100 nmol·L−1 solution of PACAP led to a moderate calcium response in mature adipocytes, as illustrated in Figure 6A (right graph). This PACAP-mediated response was significantly greater than the pre-adipocyte response (mean values in Figure 6B, left graph; P < 0.01), but not significantly different from the response in fibroblasts (Figure 6B, left graph). This was supported by more responding cells in the adipocyte cultures compared with fibroblasts or pre-adipocytes (Figure 6B, right graph).
Figure 6.

Calcium response of 3T3-L1 cells after stimulation with PACAP or prostaglandin F2α (PGF2α). (A) Fibroblasts (left), pre-adipocytes (middle) and mature adipocytes (right) were stimulated with 100 nmol·L−1 pituitary adenylate cyclase-activating polypeptide (PACAP) for 2 min. The treatment resulted in an increase of cell calcium only in mature adipocytes. The cells were treated with 1 µmol·L−1 PGF2α as a positive control after a 10 min buffer wash. (B) Statistically significant differences between adipocytes and pre-adipocytes were detected by measuring the extent of the calcium elevation. In addition, significantly more adipocytes were responsive compared with subconfluent fibroblasts and pre-adipocytes. Data from three independent experiments are represented as mean ± SEM. *P≤ 0.05, **P≤ 0.01. A, mature adipocytes; FB, fibroblasts; PA, pre-adipocytes.
The Y1 receptor mediates the calcium peak triggered by NPY in adipocytes
To obtain pharmacological evidence of NPY-mediated calcium mobilization, we used specific agonists for the Y1, Y2 and Y5 NPY receptors, investigated at the mRNA level (see above). The value of calcium mobilization by 100 nmol·L−1 NPY (55.7 ± 9.9 UΔR) was defined as 100%. Equal molar doses of the Y1 receptor agonist [Phe(7),Pro(34)] pNPY imitated the NPY-induced calcium elevation and was not significantly different (Figure 7A). On the other hand, neither the Y2 receptor agonist Ahx[5-24] pNPY nor the Y5 receptor agonist [hPP1-17,A31,Aib32] pNPY led to comparable responses (Figure 7A). Differences between the NPY and Y1 receptor agonist responses and the Y2 receptor or Y5 receptor agonists were highly significant (P < 0.01 for all). For blocking experiments with the Y1 receptor antagonist, BIBP 3226, we removed the first NPY dose with a 15 min buffer wash. The second NPY application resulted in a less effective, but distinct, intracellular calcium elevation (Figure 7A). Pre-incubation of 500 nmol·L−1 BIBP 3226 for 5 min with an additional co-application of the Y1 receptor antagonist during the final 2 min completely blocked the NPY-induced calcium elevation in mature adipocytes (4.9%), compared with the unblocked second NPY-mediated calcium peak (P < 0.01; Figure 7A and B).
Figure 7.

The neuropeptide Y (NPY)-triggered calcium peak in mature adipocytes is mediated by its Y1 receptor. (A) An equal dose of the Y1 receptor agonist [Phe(7),Pro(34)] pNPY mimicked the effects of NPY, whereas the Y2 receptor agonist Ahx[5-24] pNPY and Y5 receptor agonist [hPP1-17,A31,Aib32] pNPY did not influence calcium levels, as revealed by a measurement of the calcium peak extension. Additionally, the NPY-triggered peak was reproducible 15 min after the first NPY application. (B) The second NPY-mediated calcium increase was blocked completely by the Y1 receptor antagonist BIBP 3226 in mature adipocytes. Treatment with PGF2α was used as a positive control. Data from three independent experiments are represented as mean ± SEM. *P≤ 0.05, **P≤ 0.01. BIBP 3226, (R)-Nα-diphenylacetyl-N-(4-hydroxybenzyl) argininamide 3226; PGF2α, prostaglandin F2α.
The PAC1 receptor is involved in the PACAP-induced calcium elevation in adipocytes
To define the receptors involved in PACAP-dependent intracellular calcium elevation in mature adipocytes, we used VIP, which binds to the VPAC1 and VPAC2 receptors with an affinity similar to PACAP. Stimulation with 100 nmol·L−1 VIP resulted in much smaller changes in intracellular calcium than with PACAP (100 nmol·L−1; 31.7 ± 3.3 UΔR: defined as 100%) (Figure 8A). Additionally, application of the VPAC1 receptor agonist [K15,R16,L27]VIP/GRF(8-27) did not affect intracellular calcium (20.6%), whereas the 100 nmol·L−1 PACAP application resulted in the expected calcium mobilization (Figure 8A). Differences between PACAP, VIP and the VPAC1 receptor agonists were significant (P < 0.01).
Figure 8.

Calcium mobilization induced by pituitary adenylate cyclase-activating polypeptide (PACAP) is mediated by the PAC1 receptor. (A, B) The PACAP-mediated calcium increase was not mimicked by VIP (binds to VPAC1 and VPAC2 receptors) or the VPAC1 receptor agonist [K15,R16,L27]VIP/GRF(8-27). Notably, the first PACAP-mediated intracellular calcium increase was not reproducible with a second PACAP application after a 60 min buffer wash to remove neuropeptides. (C) The PACAP-mediated calcium increase was completely abolished by pre-incubation with the PAC1 receptor-specific antagonist PACAP(6-38). Prostaglandin F2α (PGF2α) was used as a positive control. Data from three independent experiments are presented as mean ± SEM. *P≤ 0.05, **P≤ 0.01, ***P≤ 0.001.
A 15 min buffer wash was used to remove the first dose of PACAP before applying a second dose between 15 and 60 min. The calcium responses (21.7% and less) were far below that of the first PACAP dose (Figure 8A). Because of the lack of a second PACAP-triggered calcium increase, we had to inhibit the first PACAP-mediated calcium peak. After 5 min of pre-incubation with 500 nmol·L−1 PACAP(6-38), a specific PAC1 receptor antagonist, the standard dose of PACAP (100 nmol·L−1) was added. This treatment with the antagonist severely reduced the normal calcium response to the test dose of PACAP (1.7 ± 0.4 UΔR; P < 0.01; Figure 8A and C).
Discussion and conclusions
This study presents a comprehensive analysis of the NYP and PACAP receptor mRNA during the differentiation of 3T3-L1 fibroblasts into mature adipocytes. This new knowledge is helpful for future research on in vitro adipocyte function. In addition, the receptor activity was pharmacologically examined in the presence of 100 nmol·L−1 NPY/PACAP to observe the calcium profiles of FURA2-AM-loaded 3T3-L1 cell types. Although these concentrations were above circulating physiological levels, it might be close to the local concentration of NPY and PACAP at the postsynaptic site in vivo. Evidence is also provided by selective agonist and antagonist treatments that the Y1 receptor and PAC1 receptor are coupled to GQ-α-subunits in mature adipocytes and being engaged in phospholipase effectors. The G-proteins appear to be absent in their precursor cells, because fibroblasts and pre-adipocytes also expressed the receptor mRNA, but did not exhibit an intracellular Ca2+ increase upon exposure to ligand.
In respect to the PAC1 receptor protein, an unglycosylated and glycosylated form was here found in mature adipocytes. Binding and activity of specific agonists are improved by glycosylation of receptors (Rengifo et al., 2007). Thus, the glycosylated PAC1 receptor might be more active in mature adipocytes than the unglycosylated one. The lack of response of intracellular calcium to re-stimulation with PACAP in our cells points to desensitization of the PACAP receptors, as reported earlier for chromaffin cells and for cortex slices (Taupenot et al., 1999; Niewiadomski et al., 2002). In contrast to PAC1 receptors, no glycosylation was detected for two bands of the Y1 receptor immunoreactivity. The 44 kDa receptor form is the native form, and the 70 kDa receptor protein the modified form by posttranslational processing as also suggested by others, who found the 70 kDa Y1 receptor protein only in the cytosolic fraction (Wolak et al., 2003). The ongoing debate on the potential existence of a glycosylated Y1 receptor has not yet been validated by enzymatic digestion of the protein extract under study (Sheikh and Williams, 1990; Migita et al., 2001). How the 70 kDa Y1 receptor is involved in the intracellular calcium rise in mature adipocytes stimulated by NPY, remains unclear.
In addition, intra- and extracellular calcium changes strongly affect the energy homeostasis and dysregulation may result in obesity. In pre-adipocytes, high extra- and intracellular calcium levels attenuate proliferation and differentiation (Miller et al., 1996; Ntambi and Takova, 1996; Shi et al., 2000). Our data support recent findings that NPY stimulates pre-adipocyte proliferation in the absence of a NPY-mediated calcium increase (Kuo et al., 2007). This finding is recently described as an Y1 receptor-mediated effect (Yang et al., 2008). On the other hand, in 3T3-L1 adipocytes, NPY application leads to lipid accumulation and cell differentiation. In the present study, we observed Ca2+-elevating effects through NPY stimulation more for mature adipocytes than for fibroblasts and pre-adipocytes. There is consensus that the intracellular calcium pools are modified via phospholipase C and that Y1 receptor-dependent signalling transduces phospholipase C activation in myocytes (Heredia Mdel et al., 2005). Notably, an intracellular calcium increase promotes triglyceride accumulation and lipid storage in mature adipocytes by up-regulating fatty acid synthase, a key enzyme of de novo lipogenesis (Kim et al., 1996), and by elevated glucose uptake via insulin-responsive glucose transporter translocation (Whitehead et al., 2001; Worrall and Olefsky, 2002). Considering the pathophysiological relevance for humans, long-term exposure of adipocytes to PACAP and NPY might result in obesity, which actually has been shown in mice under stress-induced NPY up-regulation (Kuo et al., 2007).
A discrepancy between mRNA expression and the function of NPY receptors seems to exist between in vitro and in vivo studies. According to the literature, primary human adipocytes derived from abdominal adipose tissue express three NPY receptors at the mRNA level: Y1, Y2 and Y5. However, the binding profile of selective radioactive ligands is only typical of the Y1 receptor, which has an anti-lipolytic effect (Serradeil-Le Gal et al., 2000). We demonstrated that the Y1 receptor gene is expressed in all stages of the adipogenesis of 3T3-L1 cells, but it was associated with an intracellular calcium increase only in NPY-stimulated mature adipocytes.
The PACAP-dependent signalling occurs via two principal routes (Alexander et al., 2008). In the Gsα-linked transduction, there is the activation of the receptor and of the adenylate cyclase system. With increase of cAMP and without significant influence on the intracellular calcium pools, protein kinase A is stimulated, which activates the hormone-sensitive lipase. This appears to be the pathway for PACAP-stimulated insulin-independent lipolysis in primary rat adipocytes derived from epididymal fat pads. It is exclusively mediated by the VPAC2 receptors (Åkesson et al., 2005). Interestingly, when rat pancreatic islets are stimulated by PACAP, insulin is released by adenylate cyclase signalling, which is coupled to the PAC1 and VPAC1 receptors (Jamen et al., 2002). The insulinotropic activity of PAC1 receptor has also been documented for PAC1 receptor gene-deficient mice with impaired glucose tolerance (Jamen et al., 2000). In the current study, no calcium mobilization was found for the HIP-isoform of PAC1 receptors as well as for VPAC2 receptors.
In the Gq/12-linked transduction, the HOP isoform of the PAC1 receptor appears to interact with the GQ-α-subunit, which induces a phospholipase C-dependent inositol trisphosphate (IP3) elevation with an intracellular calcium increase (Mustafa et al., 2007). Also produced is diacylglycerol, which triggers different isoforms of protein kinase C. This pathway is associated with a lipogenetic effect in 3T3-L1 adipocytes (Nakata et al., 1999; Åkesson et al., 2003). Yet in the present study, repeated stimulation with PACAP consistently failed to increase the accumulation of lipid droplets.
Although only the VPAC2 receptor is expressed in pancreas and skeletal muscle, all three PACAP receptor types are present in the human heart and adipose tissue (Wei and Mojsov, 1996). In the present study, 3T3-L1 pre-adipocytes and adipocytes expressed PAC1 receptors in addition to VPAC2 receptors. All three receptor types were expressed only in 3T3-L1 fibroblasts. This finding could indicate a low amount of the VPAC2 receptor mRNA not being functionally significant, or reflect a minor contribution of Gsα-linked cAMP-generating activity of the PACAP receptors. We assume that all three receptors are required to induce the transcription of specific genes via increased levels of cAMP, which is known to bind to the cAMP response element in specific promoters. The cAMP-dependent increase of C/EBP-β and C/EBP-δ in the early stages of adipocyte conversion induces an elevated expression of C/EBP-α, which is, together with the PPAR-γ, one of the most clearly identified transcription factors of adipogenesis (Wu et al., 1996; Gregoire et al., 1998). Notably, in astrocyte cultures, PACAP induces the C/EBP transcription factors, including C/EBP-β and C/EBP-δ (Cardinaux and Magistretti, 1996). In sympathetic neurones, which express the PAC1 receptor-HOP, the PACAP-dependent transcripts are related to peptide plasticity and nerve regeneration (Braas et al., 2007). Furthermore, pre-adipocytes from breast adipose tissue transiently increase prolactin expression and function via cAMP-activating ligands (McFarland-Mancini et al., 2006). We are presently studying the production and release of neurotropic and angiogenic factors by 3T3-L1 cells as related to the treatment with NPY and PACAP.
In conclusion, the 3T3-L1 adipocyte cell line expresses the Y1 receptors, the PAC1 receptor-HOP isoform and VPAC2 receptors in fibroblasts, pre-adipocytes and mature adipocytes. However, only mature adipocytes exhibit an intracellular Ca2+ increase, which could result from activation of both Y1 and PAC1-HOP receptors.
Acknowledgments
We would like to thank Dr S. Schulz as well as PD Dr R. Stumm (University of Magdeburg, Germany) for obtaining the PAC1 receptor antibodies and giving helpful advice on the glycoprotein purification. We also thank Professor F. Gregoire and Professor P. Robberecht (Université Libre de Bruxelles, Belgium) for sending us the VPAC1 receptor agonist [K15,R16,L27]VIP/GRF(8-27) and Professor A. Beck-Sickinger (University of Leipzig, Germany) for the kind gift of the Y1 receptor agonist [Phe(7),Pro(34)] pNPY, the Y2 receptor agonist Ahx[5-24] pNPY and the Y5 receptor agonist [hPP1-17,A31,Aib32] pNPY. For excellent technical support, we are indebted to Constanze Franke. We would also like to thank San Francisco Edit for their assistance in editing this manuscript. This study was supported by the Medical Faculty of the University of Leipzig and by the Translational Centre of Regenerative Medicine (TRM project nr. 1101SF).
Glossary
Abbreviations:
- BIBP 3226
(R)-Nα-diphenylacetyl-N-(4-hydroxybenzyl) argininamide 3226
- C/EBP
CCAAT/enhancer binding protein family
- DMEM
Dulbecco's modified Eagle medium
- DRG
dorsal root ganglia
- FURA2-AM
FURA2 acetoxymethyl ester
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- NPY
neuropeptide Y
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PBS
phosphate-buffered saline
- PGF2α
prostaglandin F2α
- PNGase F
peptide N-glycosidase F
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- SDS
sodium dodecyl sulphate
- UΔR
arbitrary units of the delta ratio
- Y1–6
NPY-(1–6) receptor
Conflict of interest
The authors state no conflict of interest.
References
- Åkesson L, Ahrén B, Manganiello VC, Holst LS, Edgren G, Degerman E. Dual effects of pituitary adenylate cyclase-activating polypeptide and isoproterenol on lipid metabolism and signaling in primary rat adipocytes. Endocrinology. 2003;144:5293–5299. doi: 10.1210/en.2003-0364. [DOI] [PubMed] [Google Scholar]
- Åkesson L, Ahrén B, Edgren G, Degerman E. VPAC2-R mediates the lipolytic effects of pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide in primary rat adipocytes. Endocrinology. 2005;146:744–750. doi: 10.1210/en.2004-0504. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels. BrJ Pharmacol. 2008;153(Suppl. 2):1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström AL, Hannibal J, Hindersson P, Fahrenkrug J. Light-induced phase shift in the Syrian hamster (Mesocricetus auratus) is attenuated by the PACAP receptor antagonist PACAP6-38 or PACAP immunoneutralization. Eur J Neurosci. 2003;18:2552–2562. doi: 10.1046/j.1460-9568.2003.03000.x. [DOI] [PubMed] [Google Scholar]
- Braas KM, Schutz KC, Bond JP, Vizzard MA, Girard BM, May V. Microarray analyses of pituitary adenylate cyclase activating polypeptide (PACAP)-regulated gene targets in sympathetic neurons. Peptides. 2007;28:1856–1870. doi: 10.1016/j.peptides.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrele C, Langer M, Bader R, Wieland HA, Doods HN, Zerbe O, et al. The first selective agonist for the neuropeptide YY5 receptor increases food intake in rats. J Biol Chem. 2000;275:36043–36048. doi: 10.1074/jbc.M000626200. [DOI] [PubMed] [Google Scholar]
- Cardinaux JR, Magistretti PJ. Vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, and noradrenaline induce the transcription factors CCAAT/enhancer binding protein (C/EBP)-beta and C/EBP delta in mouse cortical astrocytes: involvement in cAMP-regulated glycogen metabolism. J Neurosci. 1996;16:919–929. doi: 10.1523/JNEUROSCI.16-03-00919.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg FM, Mevenkamp G, Wille S, Hauger RL. N-terminal splicevariants of the type I PACAP receptor: isolation, characterization and ligand binding/selectivity determinants. J Neuroendocrinol. 1999;11:941–949. doi: 10.1046/j.1365-2826.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- DeHaven WI, Cuevas J. VPAC receptor modulation of neuroexcitability in intracardiac neurons: dependence on intracellular calcium mobilization and synergistic enhancement by PAC1 receptor activation. J Biol Chem. 2004;279:40609–40621. doi: 10.1074/jbc.M404743200. [DOI] [PubMed] [Google Scholar]
- El Bahh B, Cao JQ, Beck-Sickinger AG, Colmers WF. Blockade of neuropeptide Y(2) receptors and suppression of NPY's anti-epileptic actions in the rat hippocampal slice by BIIE0246. Br J Pharmacol. 2002;136:502–509. doi: 10.1038/sj.bjp.0704751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlet P, Vandermeers A, Vertongen P, Rathe J, De Neef P, Cnudde J, et al. Development of high affinity selective VIP1 receptor agonists. Peptides. 1997;18:1539–1545. doi: 10.1016/s0196-9781(97)00228-3. [DOI] [PubMed] [Google Scholar]
- Gregoire FM. Adipocyte differentiation: from fibroblast to endocrine cell. Exp Biol Med (Maywood) 2001;226:997–1002. doi: 10.1177/153537020122601106. [DOI] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- Heredia Mdel P, Delgado C, Pereira L, Perrier R, Richard S, Vassort G, et al. Neuropeptide Y rapidly enhances [Ca2+]i transients and Ca2+ sparks in adult rat ventricular myocytes through Y1 receptor and PLC activation. J Mol Cell Cardiol. 2005;38:205–212. doi: 10.1016/j.yjmcc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Höfliger MM, Castejón GL, Kiess W, Beck Sickinger AG. Novel cell line selectively expressing neuropeptide Y-Y2 receptors. J Recept Signal Transduct Res. 2003;23:351–360. doi: 10.1081/rrs-120026974. [DOI] [PubMed] [Google Scholar]
- Ingenhoven N, Beck-Sickinger AG. Molecular characterization of the ligand-receptor interaction of neuropeptide Y. Curr Med Chem. 1999;6:1055–1066. [PubMed] [Google Scholar]
- Jamen F, Persson K, Bertrand G, Rodriguez-Henche N, Puech R, Bockaert J, et al. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J Clin Invest. 2000;105:1307–1315. doi: 10.1172/JCI9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamen F, Puech R, Bockaert J, Brabet P, Bertrand G. Pituitary adenylate cyclase-activating polypeptide receptors mediating insulin secretion in rodent pancreatic islets are coupled to adenylate cyclase but not to PLC. Endocrinology. 2002;143:1253–1259. doi: 10.1210/endo.143.4.8739. [DOI] [PubMed] [Google Scholar]
- Jensen B, Farach-Carson MC, Kenaley E, Akanbi KA. High extracellular calcium attenuates adipogenesis in 3T3-L1 preadipocytes. Exp Cell Res. 2004;301:280–292. doi: 10.1016/j.yexcr.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Kim JH, Mynatt RL, Moore JW, Woychik RP, Moustaid N, Zemel MB. The effects of calcium channel blockade on agouti-induced obesity. FASEB J. 1996;10:1646–1652. [PubMed] [Google Scholar]
- Kosacka J, Figiel M, Engele J, Hilbig H, Majewski M, Spanel-Borowski K. Angiopoietin-1 promotes neurite outgrowth from dorsal root ganglion cells positive for Tie-2 receptor. Cell Tissue Res. 2005;320:11–19. doi: 10.1007/s00441-004-1068-2. [DOI] [PubMed] [Google Scholar]
- Kosacka J, Nowicki M, Kacza J, Borlak J, Engele J, Spanel-Borowski K. Adipocyte-derived angiopoietin-1 supports neurite outgrowth and synaptogenesis of sensory neurons. J Neurosci Res. 2006;83:1160–1169. doi: 10.1002/jnr.20811. [DOI] [PubMed] [Google Scholar]
- Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- Lecklin A, Lundell I, Salmela S, Männistö PT, Beck-Sickinger AG, Larhammar D. Agonists for neuropeptide Y receptors Y1 and Y5 stimulate different phases of feeding in guinea pigs. Br J Pharmacol. 2003;139:1433–1440. doi: 10.1038/sj.bjp.0705389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland-Mancini M, Hugo E, Loftus J, Ben-Jonathan N. Induction of prolactin expression and release in human preadipocytes by cAMP activating ligands. Biochem Biophys Res Commun. 2006;344:9–16. doi: 10.1016/j.bbrc.2006.03.168. [DOI] [PubMed] [Google Scholar]
- Migita K, Loewy AD, Ramabhadran TV, Krause JE, Waters SM. Immunohistochemical localization of the neuropeptide Y Y1 receptor in rat central nervous system. Brain Res. 2001;889:23–37. doi: 10.1016/s0006-8993(00)03092-4. [DOI] [PubMed] [Google Scholar]
- Miller CW, Casimir DA, Ntambi JM. The mechanism of inhibition of 3T3-L1 preadipocyte differentiation by prostaglandin F2alpha. Endocrinology. 1996;137:5641–5650. doi: 10.1210/endo.137.12.8940395. [DOI] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, et al. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) Biochem Biophys Res Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- Mustafa T, Grimaldi M, Eiden LE. The HOP cassette of the PAC1 receptor confers coupling to Ca2+ elevation required for pituitary adenylate cyclase-activating polypeptide-evoked neurosecretion. J Biol Chem. 2007;282:8079–8091. doi: 10.1074/jbc.M609638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada MT, Stadel JM, Crooke ST. Mobilization of extracellular Ca2+ by prostaglandin F2 alpha can be modulated by fluoride in 3T3-L1 fibroblasts. Biochem J. 1990;272:167–174. doi: 10.1042/bj2720167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata M, Yada T. PACAP in the glucose and energy homeostasis: physiological role and therapeutic potential. Curr Pharm Des. 2007;13:1105–1112. doi: 10.2174/138161207780618948. [DOI] [PubMed] [Google Scholar]
- Nakata M, Shioda S, Oka Y, Maruyama I, Yada T. Insulinotropin PACAP potentiates insulin-stimulated glucose uptake in 3T3 L1 cells. Peptides. 1999;20:943–948. doi: 10.1016/s0196-9781(99)00085-6. [DOI] [PubMed] [Google Scholar]
- Nakata M, Kohno D, Shintani N, Nemoto Y, Hashimoto H, Baba A, et al. PACAP deficient mice display reduced carbohydrate intake and PACAP activates NPY-containing neurons in the rat hypothalamic arcuate nucleus. Neurosci Lett. 2004;370:252–256. doi: 10.1016/j.neulet.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Niewiadomski P, Nowak JZ, Sedkowska P, Zawilska JB. Rapid desensitization of receptors for pituitary adenylate cyclase-activating polypeptide (PACAP) in chick cerebral cortex. Pol J Pharmacol. 2002;54:717–721. [PubMed] [Google Scholar]
- Ntambi JM, Takova T. Role of Ca2+ in the early stages of murine adipocyte differentiation as evidenced by calcium mobilizing agents. Differentiation. 1996;60:151–158. doi: 10.1046/j.1432-0436.1996.6030151.x. [DOI] [PubMed] [Google Scholar]
- Pons J, Lee EW, Li L, Kitlinska J. Neuropeptide Y: multiple receptors and multiple roles in cardiovascular diseases. Curr Opin Investig Drugs. 2004;5:957–962. [PubMed] [Google Scholar]
- Prieto D, Buus CL, Mulvany MJ, Nilsson H. Neuropeptide Y regulates intracellular calcium through different signalling pathways linked to a Y(1)-receptor in rat mesenteric small arteries. Br J Pharmacol. 2000;129:1689–1699. doi: 10.1038/sj.bjp.0703256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings SR, Piuz I, Schlegel W, Bockaert J, Journot L. ). Differential expression of pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide receptor subtypes in clonal pituitary somatotrophs and gonadotrophs. Endocrinology. 1995;136:2088–2098. doi: 10.1210/endo.136.5.7720658. [DOI] [PubMed] [Google Scholar]
- Rengifo J, Gibson CJ, Winkler E, Collin T, Ehrlich BE. Regulation of the inositol 1,4,5-trisphosphate receptor type I by O-GlcNAc glycosylation. J Neurosci. 2007;27:13813–13821. doi: 10.1523/JNEUROSCI.2069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Audinot V, Dromaint S, Macia C, Lamamy V, Beauverger P, et al. Molecular identification of the long isoform of the human neuropeptide Y Y5 receptor and pharmacological comparison with the short Y5 receptor isoform. Biochem J. 2003;369:667–673. doi: 10.1042/BJ20020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf K, Eberlein W, Engel W, Wieland HA, Willim KD, Entzeroth M, et al. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur J Pharmacol. 1994;271:11–13. doi: 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- Schulz S, Röcken C, Mawrin C, Weise W, Höllt V, Schulz S. Immunocytochemical identification of VPAC1, VPAC2, and PAC1 receptors in normal and neoplastic human tissues with subtype-specific antibodies. Clin Cancer Res. 2004;10:8235–8242. doi: 10.1158/1078-0432.CCR-04-0939. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Lafontan M, Raufaste D, Marchand J, Pouzet B, Casellas P, et al. Characterization of NPY receptors controlling lipolysis and leptin secretion in human adipocytes. FEBS Lett. 2000;475:150–156. doi: 10.1016/s0014-5793(00)01649-5. [DOI] [PubMed] [Google Scholar]
- Sheikh SP, Williams JA. Structural characterization of Y1 and Y2 receptors for neuropeptide Y and peptide YY by affinity cross-linking. J Biol Chem. 1990;265:8304–8310. [PubMed] [Google Scholar]
- Shi H, Halvorsen YD, Ellis PN, Wilkison WO, Zemel MB. Role of intracellular calcium in human adipocyte differentiation. Physiol Genomics. 2000;3:75–82. doi: 10.1152/physiolgenomics.2000.3.2.75. [DOI] [PubMed] [Google Scholar]
- Söll RM, Dinger MC, Lundell I, Larhammer D, Beck-Sickinger AG. Novel analogues of neuropeptide Y with a preference for the Y1-receptor. Eur J Biochem. 2001;268:2828–2837. doi: 10.1046/j.1432-1327.2001.02161.x. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Taupenot L, Mahata M, Mahata SK, O'Connor DT. Time-dependent effects of the neuropeptide PACAP on catecholamine secretion: stimulation and desensitization. Hypertension. 1999;34:1152–1162. doi: 10.1161/01.hyp.34.5.1152. [DOI] [PubMed] [Google Scholar]
- Turtzo LC, Marx R, Lane MD. Cross-talk between sympathetic neurons and adipocytes in coculture. Proc Natl Acad Sci U S A. 2001;98:12385–12390. doi: 10.1073/pnas.231478898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiyama M, Ikeda R, Sugawara H, Yoshida M, Mori K, Kangawa K, et al. Differential intracellular signaling through PAC1 isoforms as a result of alternative splicing in the first extracellular domain and the third intracellular loop. Mol Pharmacol. 2007;72:103–111. doi: 10.1124/mol.107.035477. [DOI] [PubMed] [Google Scholar]
- Visscher TL, Seidell JC. The public health impact of obesity. Annu Rev Public Health. 2001;22:355–375. doi: 10.1146/annurev.publhealth.22.1.355. [DOI] [PubMed] [Google Scholar]
- Waki H, Tontonoz P. Endocrine functions of adipose tissue. Annu Rev Pathol. 2007;2:31–56. doi: 10.1146/annurev.pathol.2.010506.091859. [DOI] [PubMed] [Google Scholar]
- Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. J Cell Physiol. 2008;216:3–13. doi: 10.1002/jcp.21386. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mojsov S. Multiple human receptors for pituitary adenylyl cyclase activating polypeptide and vasoactive intestinal peptide are expressed in a tissue-specific manner. Ann N A Acad Sci. 1996;805:624–627. doi: 10.1111/j.1749-6632.1996.tb17531.x. [DOI] [PubMed] [Google Scholar]
- Whitehead JP, Molero JC, Clark S, Martin S, Meneilly G, James DE. The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J Biol Chem. 2001;276:27816–27824. doi: 10.1074/jbc.M011590200. [DOI] [PubMed] [Google Scholar]
- Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- Worrall DS, Olefsky JM. The effects of intracellular calcium depletion on insulin signaling in 3T3-L1 adipocytes. Mol Endocrinol. 2002;16:378–389. doi: 10.1210/mend.16.2.0776. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada T, Sakurada M, Filipsson K, Kikuchi M, Ahrén B. Intraperitoneal PACAP administration decreases blood glucose in GK rats, and in normal and high fat diet mice. Ann N A Acad Sci. 2000;921:259–263. doi: 10.1111/j.1749-6632.2000.tb06974.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N. Pituitary adenylate cyclase activating polypeptide enhances glucose-evoked insulin secretion in the canine pancreas in vivo. JOP. 2001;2:306–316. [PubMed] [Google Scholar]
- Yang K, Guan H, Arany E, Hill DJ, Cao X. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J. 2008;22:2452–2464. doi: 10.1096/fj.07-100735. [DOI] [PubMed] [Google Scholar]
- Zukowska Z. Atherosclerosis and angiogenesis: what do nerves have to do with it? Pharmacol Rep. 2005;57:229–234. [PubMed] [Google Scholar]
- Zukowska Z, Pons J, Lee EW, Li L. Neuropeptide Y: a new mediator linking sympathetic nerves, blood vessels and immune system? Can J Physiol Pharmacol. 2003;81:89–94. doi: 10.1139/y03-006. [DOI] [PubMed] [Google Scholar]


