Abstract
Background and purpose:
The atypical antipsychotic drug, zotepine, is effective in treatment of schizophrenia and acute mania, but the incidence of seizures during treatment is higher than with other antipsychotics. In addition, the mechanisms underlying the clinical actions of zotepine remain uncharacterized.
Experimental approach:
The effects of intraperitoneal administration of zotepine and haloperidol on the extracellular levels of noradrenaline, dopamine, 5-HT, GABA, and glutamate in the medial prefrontal cortex (mPFC) were compared. Neuronal activities induced by each drug in the ventral tegmental area (VTA), locus coeruleus (LC), dorsal raphe nucleus (DRN) and mediodorsal thalamic nucleus (MTN) were also analysed.
Key results:
Haloperidol did not affect extracellular neurotransmitter levels in the mPFC. In contrast, zotepine activated neuronal activities in all nuclei and increased the extracellular levels of noradrenaline, dopamine, GABA, and glutamate in the mPFC, but not 5-HT levels. The zotepine-stimulated neuronal activity in the VTA, LC, DRN and MTN enhanced the release of dopamine, noradrenaline, 5-HT, glutamate and GABA in the mPFC, although the enhanced GABAergic transmission possibly inhibited noradrenaline, dopamine and 5-HT release. The other afferent to mPFC, which releases dopamine and noradrenaline, was partially insensitive to GABAergic inhibition, but possibly received stimulatory AMPA/glutamatergic regulation from the MTN.
Conclusions and implications:
Our results indicated that the positive interaction between prefrontal catecholaminergic transmission and AMPA/glutamatergic transmission from MTN might explain the regulatory effects of zotepine on neurotransmitter release. A mechanism is suggested to account for the pharmacological profile of this atypical antipsychotic and for its pro-convulsive action.
British Journal of Pharmacology (2009) 157, 656–665; doi:10.1111/j.1476-5381.2009.00175.x; published online 9 April 2009
Keywords: antipsychotics, dopamine, GABA, glutamate, microdialysis, neurotransmitter release, noradrenaline, schizophrenia, 5-HT, zotepine
Introduction
Zotepine (2-[(8-chlorodibenzo[b,f]thiepine-10-yl)oxy]-N,N dimethylethylamine) was developed by Fujisawa Pharmaceutical Co. (Osaka, Japan: currently Astellas Pharmaceutical Inc.) in Japan as an antipsychotic drug. Zotepine has an atypical profile and is effective against negative (such as affective flattening, aboulia and social withdrawal) and positive (such as hallucination and delusion) symptoms, as well as cognitive deficits of schizophrenia. It is also less likely to induce extrapyramidal side effects compared with typical antipsychotics (Meyer-Lindenberg et al., 1997; DeSilva et al., 2006; Hashimoto et al., 2006). In addition, an open pilot study demonstrated the rapid and beneficial therapeutic effects of zotepine in patients with severe mania (Amann et al., 2005). Despite these clinical advantages of zotepine, the clinical incidence of seizures during treatment with zotepine is higher than with other antipsychotic drugs (Hori et al., 1992; Casey, 1997). The mechanism(s) underlying both the clinical actions of zotepine and the complicating seizures remain unclear.
The elevation of dopamine level in the medial prefrontal cortex (mPFC) is considered to be critical to the ability of atypical antipsychotics to improve cognitive dysfunction and negative symptoms of schizophrenia, since clozapine, risperidone, olanzapine, quetiapine and ziprasidone, but not halperidone, increased the extracellular dopamine level in mPFC (Meltzer et al., 2003). Haloperidol is a typical antipsychotic that acts mainly via dopamine D2 receptor antagonism (Bymaster et al., 1997). It does not significantly affect the extracellular levels of noradrenaline, dopamine or 5-HT in rat frontal cortex when administered by in vivo microdialysis (Rowley et al., 2000; Zhang et al., 2000; Adams and Moghaddam, 2001; Lopez-Gil et al., 2007). However, the atypical antipsychotics risperidone and clozapine significantly increased extracellular 5-HT levels in mPFC, although olanzapine had no such effect (Meltzer et al., 2003). Zotepine, which shows multiple antagonistic profiles with strong affinities to 5-HT2A receptors, 5-HT2C receptors, dopamine D2 receptors and transporters of noradrenaline and 5-HT (Tatsumi et al., 1999; Richelson and Souder, 2000), also increased the extracellular levels of noradrenaline and dopamine to varying degrees in the rat cortex, but not 5-HT levels (Rowley et al., 1998; Rowley et al., 2000; Nakamura et al., 2005). The contradictory effects of zotepine on extracellular monoamine levels in the mPFC are not explained by its binding profile.
The stimulating effects of the atypical antipsychotics, clozapine and olanzapine, on extracellular dopamine levels in the frontal cortex, do not correlate with neuronal activity in the ventral tegmental area (VTA). This region predominantly projects dopaminergic afferents to the mPFC, but depends on direct action on the frontal dopaminergic transmission system-associated monoamine receptors, as both typical and atypical antipsychotics enhance neuronal activity in the VTA (Gessa et al., 2000; Meltzer et al., 2003). The major dopaminergic, noradrenergic and 5-hydroxytryptaminergic afferents project to the mPFC from the VTA, locus coeruleus (LC) and dorsal raphe nucleus (DRN), respectively (Waterhouse et al., 1983; Van Eden et al., 1987; Kuroda et al., 1998; Lambe et al., 2000), but the major glutamatergic afferents project from other cortical regions and the mediodorsal thalamic nucleus (MTN) (Cotman and Monaghan, 1986; Jay et al., 1992; Kuroda et al., 1998). In the mPFC, the contacts between glutamatergic and monoaminergic neurons are possibly indirect and mediated by GABAergic interneurons, which are regulated by stimulatory NMDA receptors (Yonezawa et al., 1998; Zhu et al., 2004).
Based on these anatomical findings, the present study was designed to clarify the mechanisms of the clinical actions of zotepine. We also compared the effects of zotepine and haloperidol on neuronal activities in the VTA, DRN, LC and MTN. The extracellular levels of dopamine, noradrenaline, 5-HT, GABA and glutamate in mPFCs of freely moving rats were also analysed following administration of the two drugs, using telemetric monitoring for neuronal activity, high-speed and highly sensitive extreme liquid chromatography to determine GABA and glutamate levels, and ion-exchange high-performance liquid chromatography (HPLC) to determine levels of dopamine, noradrenaline and 5-HT in freely moving rats.
Methods
Experimental animals
All experiments described in this report were approved by the Ethics Review Committee for Animal Experimentation of Mie University. Male Sprague-Dawley rats (SLC, Shizuoka, Japan), weighing 250–300 g, were housed in air-conditioned rooms (temperature, 22 ± 2°C) set at 12-h light-dark cycle.
Preparation of microdialysis system
Each rat was placed in a stereotaxic frame and anaesthetized with 1.8% isoflurane. A concentric I-type dialysis probe (0.22-mm diameter; 3-mm exposed membrane; Eicom, Kyoto, Japan) was implanted in the mPFC (A = +3.2 mm, L = +0.8 mm, V = −5.5 mm, relative to bregma) according to the atlas of Paxinos and Watson (1998). The microdialysis probes placed into the mPFC covered the cortical layers II/III to VI. Perfusion experiments commenced 18 h after the rats had recovered from anaesthesia. The perfusion rate was always 1 µL·min−1, using modified Ringer's solution composed of (in mmol·L−1) 145 Na+, 2.7 K+, 1.2 Ca2+, 1.0 Mg2+ and 154.4 Cl−, buffered to pH 7.4 with 2 mmol·L−1 phosphate buffer and 1.1 mmol·L−1 Tris buffer (Okada et al., 2001; Okada et al., 2004). Each dialysate was injected into the liquid chromatography apparatus.
The perfusion was started with modified Ringer's solution alone. Extracellular neurotransmitter levels in the mPFC were measured at 6 h after starting the perfusion. When the coefficients of variation of the level of each neurotransmitter reached less than 5% over 60 min (stabilization), control data were obtained over another 60-min period. The perfusion medium was then switched to modified Ringer's solution with or without the required agent: muscimol (50 µmol·L−1), 6,7-dinitroquinoxaline-2,3-dione (DNQX, 50 µmol·L−1) or MK-801 (50 µmol·L−1). After stabilization of the extracellular neurotransmitter levels, the rat was injected with either zotepine (1 and 3 mg·kg−1, i.p.) or haloperidol (0.1 and 1 mg·kg−1, i.p.).
Liquid chromatography
The extracellular levels of noradrenaline, dopamine and 5-HT were determined by ion-exchange HPLC with an electrochemical detector (HITEC-500, Eicom) and a graphite carbon electrode set at +450 mV (vs. an Ag/gCl reference electrode). The ion-exchange column (EicomPack CAX, 200 × 2.0 mm, Eicom) was maintained at 25°C and the flow rate of the mobile phase was set at 250 µL·min−1. The mobile phase comprised 0.1 mol·L−1 ammonium acetate buffer (pH 6.0) containing 0.05 mol·L−1 sodium sulphate, 0.3 mmol·L−1 EDTA-2Na, and 30% (v v−1) methanol.
The extracellular levels of glutamate and GABA were determined by extreme liquid chromatography (dual xLC 3185PU, Jasco, Tokyo) using ο-phthalaldehyde-derivatized fluorescence detection (xLC 3120FP, Jasco). The analytical column (X-PressPak V-C18, particle 2 µm, 50 × 2.0 mm, Jasco) was maintained at 40°C and the flow rate of the mobile phase was set at 500 µL·min−1. The mobile phase comprised two eluents: eluent A, 3.5 mmol·L−1 citrate buffer (pH 6.0); and eluent B, 3.5 mmol·L−1 citrate buffer (pH 3.5) containing 30% acetonitrile (v v−1), 30% ethanol (v v−1) and 40% water (v v−1). The following gradient elution was used: 0–0.2 min, 90% A–10% B; 2.2–2.5 min, 72% A–28% B; 4.6–5.0 min, 42% A–58% B; 6.1 min, 23% A–77% B; 6.2–7.0 min, 0% A–100% B.
Figure 1A and B, respectively, show typical chromatograms of monoamine analysis by ion-exchange HPLC equipped with an electrochemical detector, and of amino acid analysis using extreme high-pressure liquid chromatography equipped with a fluorescence detector.
Figure 1.
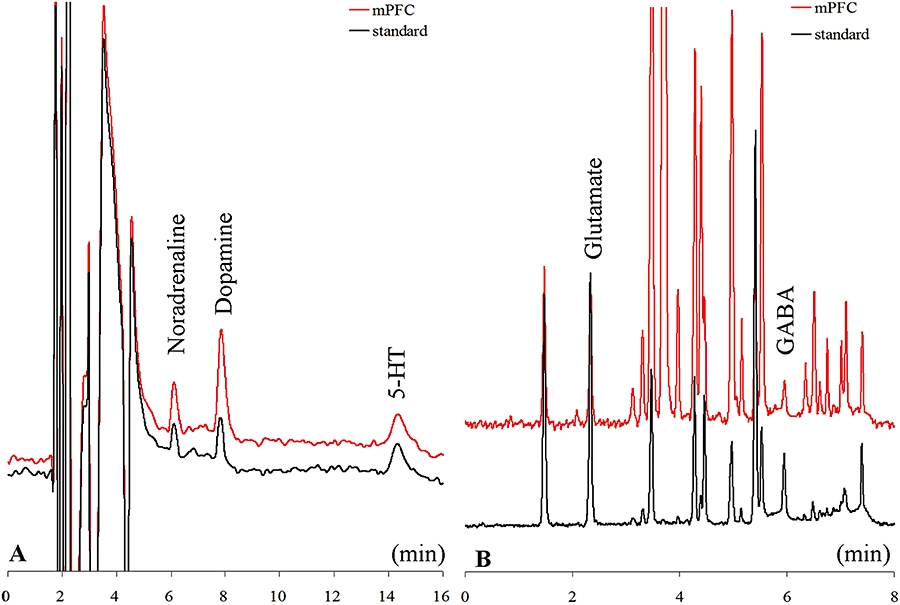
Typical chromatograms of monoamine analyses using ion-exchange high-performance liquid chromatography equipped with an electrochemical detector and amino acid analyses using extreme high-pressure liquid chromatography equipped with a fluorescence detector. The chromatograms in A were obtained from 20 µL of a standard solution containing 5 fmol (20 µL)−1 of noradrenaline, dopamine, and 5-HT (standard) or prefrontal perfusate (mPFC). The quantification limits for noradrenaline, dopamine and 5-HT were 0.1, 0.2 and 0.5 fmol (20 µL)−1 respectively. The chromatograms in B were obtained from 5 µL of a standard solution containing 1 pmol 5 µL−1 glutamate and 0.2 pmol 5 µL−1 GABA (standard), or prefrontal perfusate (mPFC). The quantification limits for glutamate and GABA were 10 and 20 fmol 5 µL−1 respectively.
Electrophysiological measurements
To determine the effects of zotepine and haloperidol on neuronal activity in freely moving rats, twisted bipolar teflon-insulated stainless steel microelectrodes (80-µm diameter) were chronically implanted under 1.8% isoflurane anaesthesia in the LC (A = −9.8 mm, L = +1.3 mm, V = −6.8 mm, relative to bregma), MTN (A = −1.8 mm, L = +0.8 mm, V = −4.6 mm, relative to bregma), VTA (A = −6.0 mm, L = +0.6 mm, V = −8.0 mm, relative to bregma) and DRN (A = −7.3 mm, L = +0.1 mm, V = −5.8 mm, relative to bregma) according to the atlas of Paxinos and Watson (1997). Another screw electrode was implanted in the bone 1 mm posterior to lambda as a reference electrode. Unit activities were converted to an integrated histogram by rate-averaging software and displayed as neuronal firing frequency (Hz).
Dopaminergic neurons in the VTA were identified using the following criteria: spike duration greater than 2 ms, firing rate of 0.5–9.0 Hz and biphasic/triphasic waveforms (Bunney et al., 1973). 5-Hydroxytryptaminergic neurons in the DRN were identified using the following criteria: spike duration greater than 0.8 ms and firing rate of 0.5–3.0 Hz (Aghajanian et al., 1970). Neurons in the LC were identified using the following criteria: spike duration greater than 2 ms and firing rate of 0.5–6.0 Hz (Aghajanian et al., 1977). Neurons in the MTN were identified using the following criteria: spike duration greater than 0.5 ms and firing rate of 0.5–10.0 Hz. The extracellular neuronal activity (firing frequency) recording commenced 24 h after recovery from anaesthesia, using a telemeter (Unimec, Tokyo) the low bandpass filter set at 100 Hz and high-bandpass at 3 kHz (Okada et al., 1998).
Statistical analysis
Data are expressed as mean ± SD. The dose-dependent effects of haloperidol and zotepine on the extracellular levels of the neurotransmitters were analysed by repeated measurements two-way analysis of variance (repeated two-way anova) using Tukey's multiple comparison. The effects of local perfusion with MK-801, muscimol and DNQX on the zotepine-induced elevation of extracellular neurotransmitter levels were analysed using two-way anova with Tukey's multiple comparison. The dose-dependent effects of antipsychotics on neuronal firing were analysed by one-way anova with Dunnett's multiple comparison. A P-value < 0.05 was considered statistically significant.
Materials
The following drugs were used in this study: zotepine (Astellas Pharma Inc, Tokyo), haloperidol (Sigma, St. Louis, MO), MK-801 (dizocilpine; Sigma), DNQX (Wako Chemicals, Osaka, Japan) and muscimol (Sigma). Haloperidol, zotepine, MK-801 and muscimol were dissolved in modified Ringer's solution containing less than 0.1% v v−1 acetate. The pH of the final solution was adjusted to 7.0 with phosphate buffer.
Results
Effects of antipsychotic drugs on neurotransmitter release in mPFC
The basal extracellular levels of noradrenaline, dopamine and 5-HT in mPFC were 6.8 ± 0.9, 13.2 ± 2.7 and 4.2 ± 0.6 fmol per sample (20 µL) respectively (Figures 1 and 2) (not corrected for in vitro dialysis probe recovery). The basal extracellular levels of glutamate and GABA were 2.4 ± 0.5 and 0.6 ± 0.1 pmol per sample (20 µL) respectively (Figures 1 and 3) (not corrected for in vitro dialysis probe recovery). The basal extracellular levels of noradrenaline, dopamine, 5-HT and GABA were tetrodotoxin-sensitive, Ca2+-dependent and K+-sensitive, but the basal extracellular glutamate level was tetrodotoxin-insensitive, Ca2+-independent and K+-sensitive (data not shown) (van Veldhuizen et al., 1990; Okada et al., 2001; Okada et al., 2004; Okada et al., 2005). In contrast, the K+-evoked release of all neurotransmitters was tetrodotoxin-sensitive and Ca2+-dependent (data not shown) (van Veldhuizen et al., 1990; Okada et al., 2001; Okada et al., 2004; Okada et al., 2005).
Figure 2.
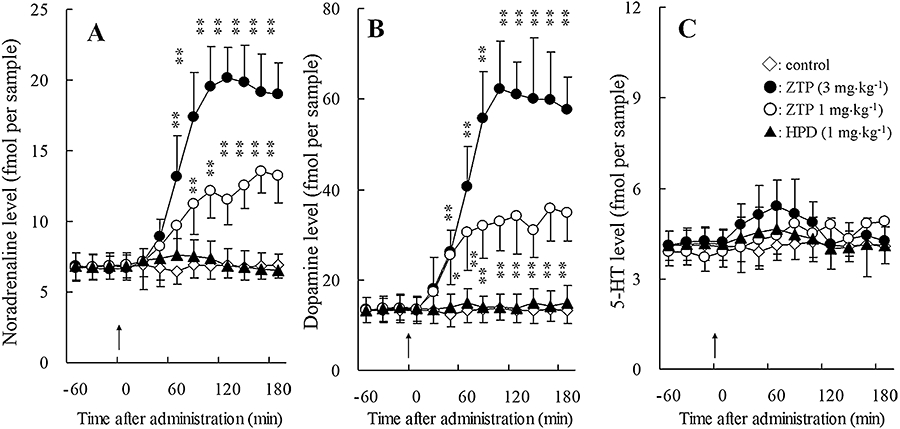
Effects of antipsychotic drugs on the extracellular levels of noradrenaline (A), dopamine (B), 5-HT and (C) GABA in the mPFC. Arrows indicate systemic administration of haloperidol (HPD: 1 mg·kg−1, i.p.) and zotepine (ZTP: 1 and 3 mg·kg−1, i.p.). The ordinates represent the mean ± SD (n = 8) neurotransmitter release (fmol per sample). Effects of the antipsychotic agents were compared using repeated two-way anova with Tukey's multiple comparison (*P < 0.05; **P < 0.01).
Figure 3.
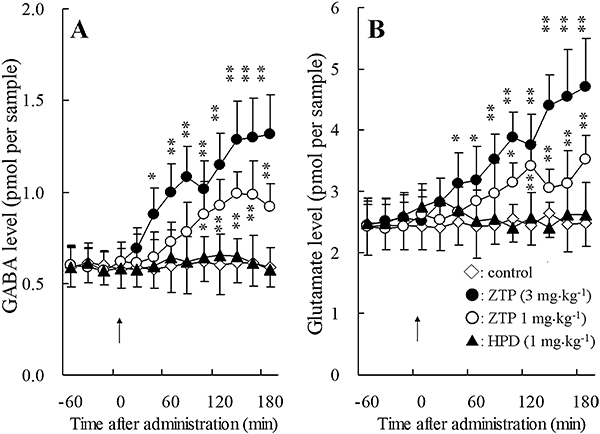
Effects of antipsychotic drugs on the extracellular levels of GABA (A) and glutamate (B) in the mPFC. Arrows indicate systemic administration of haloperidol (HPD: 1 mg·kg−1, i.p.) and zotepine (ZTP: 1 and 3 mg·kg−1, i.p.). The ordinates represent the mean ± SD (n = 8) neurotransmitter release (pmol per sample). Effects of the antipsychotic agents were compared using repeated two-way anova with Tukey's multiple comparison (*P < 0.05; **P < 0.01).
Systemic administration of 0.1 mg·kg−1 (data not shown) and 1 mg·kg−1 haloperidol (i.p.) did not affect the extracellular concentrations of noradrenaline, dopamine, 5-HT, GABA or glutamate (Figures 2 and 3). However, zotepine (1 and 3 mg·kg−1, i.p.) dose-dependently increased noradrenaline [repeated two-way anova: FDose(2, 21) = 50.9, P < 0.01; FTime(9, 189) = 149.5, P < 0.01; FDose*Time (18, 189) = 72.8, P < 0.01)], dopamine [repeated two-way anova: FDose(2, 21) = 59.1, P < 0.01; FTime(9, 189) = 188.7, P < 0.01; FDose*Time(18, 189) = 79.2, P < 0.01)], GABA [repeated two-way anova: FDose(2, 21) = 22.3, P < 0.01; FTime(9, 189) = 33.7, P < 0.01; FDose*Time(18, 189) = 12.1, P < 0.01)], and glutamate [repeated two-way anova: FDose(2, 21) = 17.3, P < 0.01; FTime(9, 189) = 32.3, P < 0.01; FDose*Time(18, 189) = 11.3, P < 0.01)] release without affecting 5-HT levels in the mPFC (Figures 2 and 3).
Effects of antipsychotic drugs on neuronal firing frequencies in the VTA, DRN, LC and MTN
To clarify the mechanisms by which zotepine increased extracellular neurotransmitter concentrations in the mPFC, the effects of systemic administration of zotepine (1 and 3 mg·kg−1, i.p.) and haloperidol (0.1 and 1 mg·kg−1, i.p.) on neuronal activity in the VTA, DRN, LC and MTN were analysed.
Systemic administration of zotepine (1 and 3 mg·kg−1, i.p.) significantly increased VTA neuronal firing frequencies over the subsequent 3 h (Figure 4A), and this stimulation correlated with the increased extracellular dopamine levels in the mPFC. In contrast, systemic administration of haloperidol (1 mg·kg−1, i.p.) transiently increased neuronal firing frequencies in the VTA over the first 60 min, but after that time the frequencies returned to pretreatment values (Figure 4B). Consequently, we next investigated the neuronal firing frequencies in the VTA, DRN, LC and MTN during 60–180 min after the systemic administration (i.p.) of zotepine and haloperidol. Zotepine (1 and 3 mg·kg−1, i.p.) significantly increased neuronal firing frequencies in the VTA [one-way anova; F(2, 21) = 9.6, P < 0.01], DRN [one-way anova; F(2, 21) = 17.9, P < 0.01], LC [one-way anova; F(2, 21) = 57.8, P < 0.01] and MTN [one-way anova; F(2, 21) = 55.8, P < 0.01] in a dose-dependent manner, while (0.1 and 1 mg·kg−1, i.p.) had no effects on neuronal firing frequencies in the VTA [one-way anova; F(2, 21) = 3.1, P = 0.068], DRN [one-way anova; F(2, 21) = 1.8, P = 0.186], LC [one-way anova; F(2, 23) = 3.2, P = 0.061] and MTN [one-way anova; F(2, 23) = 2.45, P = 0.110] (Figure 4).
Figure 4.
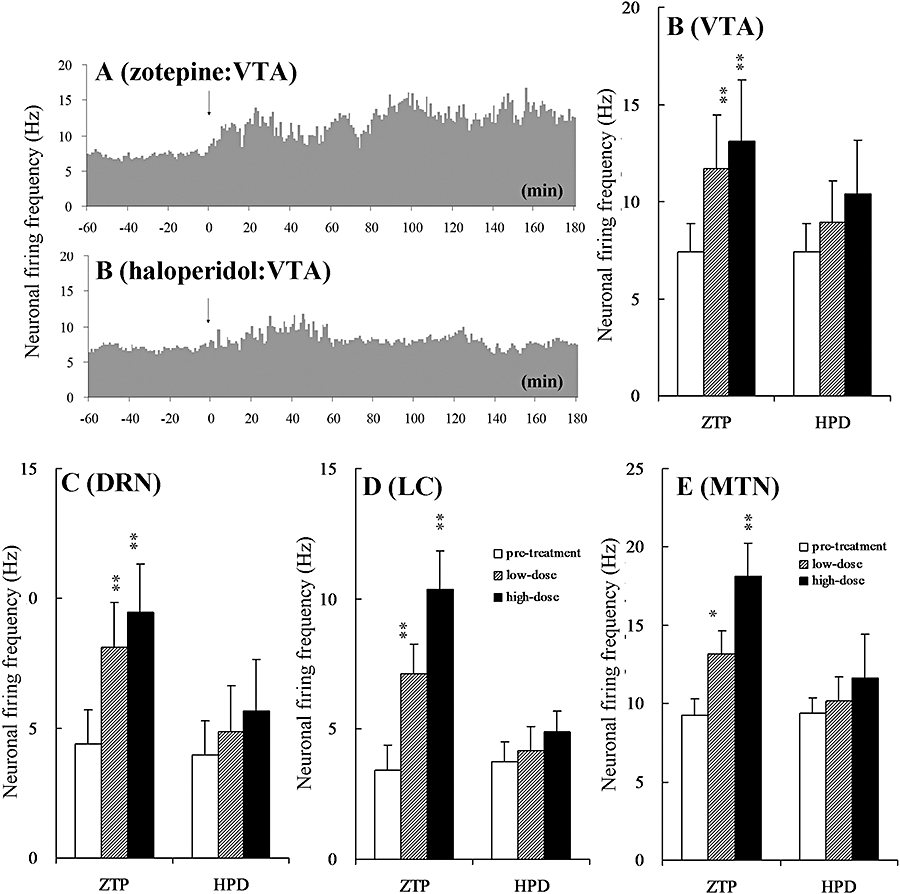
Typical histograms showing the effects of (A) zotepine (3 mg·kg−1 zotepine, i.p.) and (B) haloperidol (1 mg·kg−1 haloperidol, i.p.) on neuronal firing frequencies in the VTA. Arrows indicate systemic administration of zotepine (ZTP: 3 mg·kg−1, i.p.) and haloperidol (HPD: 1 mg·kg−1, i.p.). The ordinates represent the mean neuronal firing frequencies (spikes sec−1: Hz). Dose-dependent effects of the antipsychotic agents on neuronal firing frequencies in the (C) VTA, (D) DRN, (E) LC and (F) MTN. The ordinates represent the mean neuronal firing frequencies during 60–180 min after administration of haloperidol or zotepine. Low-dose: HPD at 0.1 mg·kg−1 and ZTP at 1 mg·kg−1; high-dose: HPD at 1 mg·kg−1 and ZTP at 3 mg·kg−1. The dose-dependent effects were compared using one-way anova with Dunnett's multiple comparison (*P < 0.05; **P < 0.01).
Interaction between local perfusion with MK-801, DNQX, or muscimol and systemically administered zotepine on neurotransmitter release in the mPFC
The effect of zotepine (i.p.) on the mean values of extracellular neurotransmitter level was analysed at 60–180 min after zotepine administration, since the significant zotepine-induced rise in extracellular neurotransmitter level was observed 1 h after administration of zotepine.
A two-way anova indicated the significant effects of perfusion with 50 µmol·L−1 MK-801 and systemic administration of zotepine (3 mg·kg−1, i.p.) on extracellular levels of noradrenaline [two-way anova: Fzotepine(1, 28) = 380.9, P < 0.01; FMK-801(1, 28) = 49.1, P < 0.01; Fzotepine*MK-801(1, 28) = 4.2, P < 0.05], dopamine [two-way anova: Fzotepine(1, 28) = 258.7, P < 0.01; FMK-801(1, 28) = 20.4, P < 0.01; Fzotepine*MK-801(1, 28) = 4.3, P < 0.05], 5-HT [two-way anova: Fzotepine(1, 28) = 46.4, P < 0.01; FMK-801(1, 28) = 197.6, P < 0.01; Fzotepine*MK-801(1, 28) = 43.8, P < 0.01], GABA [two-way anova: Fzotepine(1, 28) = 59.1, P < 0.01; FMK-801(1, 28) = 178.9, P < 0.01; Fzotepine*MK-801(1, 28) = 39.9, P < 0.01] and glutamate [two-way anova: Fzotepine(1, 28) = 78.0, P < 0.01; FMK-801(1, 28) = 15.9, P < 0.01; Fzotepine*MK-801(1, 28) = 5.4, P < 0.01] in the mPFC (Figures 5 and 6). Perfusion with 50 µmol·L−1 MK-801 increased the extracellular levels of noradrenaline (P < 0.01), dopamine (P < 0.01) and 5-HT (P < 0.01), but decreased that of GABA (P < 0.01) without affecting glutamate level in mPFC (Figures 5 and 6). Perfusion with 50 µmol·L−1 MK-801 significantly augmented the stimulatory effects of zotepine (3 mg·kg−1, i.p.) on extracellular levels of noradrenaline (P < 0.01), dopamine (P < 0.01) and glutamate (P < 0.01), but inhibited the stimulatory effects of zotepine on extracellular GABA level (P < 0.01). Surprisingly, under a functional NMDA receptor, zotepine did not affect 5-HT release in mPFC, whereas zotepine markedly increased 5-HT release (P < 0.01) in the same area under NMDA receptor blockade by perfusion with 50 µmol·L−1 MK-801 (Figure 5C).
Figure 5.
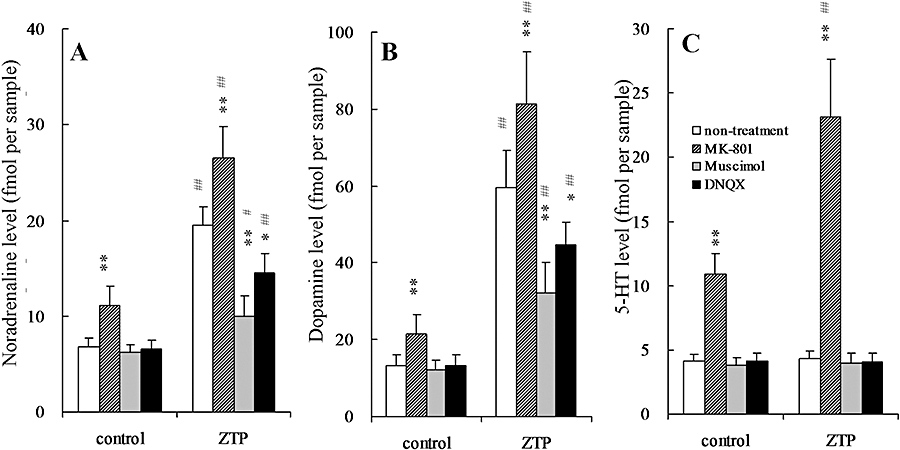
Effects of glutamate receptor antagonists (MK-801 and DNQX) and a GABAA receptor agonist (muscimol) on zotepine-induced elevation of extracellular levels of (A) noradrenaline, (B) dopamine and (C) 5-HT in the mPFC. The ordinates represent the mean ± SD (n = 8) extracellular neurotransmitter levels (fmol per sample) during 60–180 min after zotepine administration (ZTP: 3 mg·kg−1, i.p.). The perfusion medium was switched from MRS to MRS without (non-treatment) or with 50 µmol·L−1 MK-801, 50 µmol·L−1 muscimol or 50 µmol·L−1 DNQX. Following stabilization, the rats were injected with either the vehicle (control) or zotepine (ZTP: 3 mg·kg−1, i.p.). Data were compared using two-way anova with Tukey's multiple comparison (*P < 0.05; **P < 0.01 vs. non-treatment, #P < 0.05; ##P < 0.01 vs. control).
Figure 6.
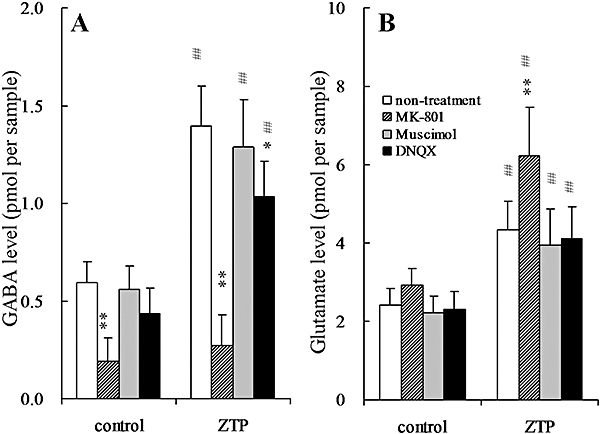
Effects of glutamate receptor antagonists (MK-801 and DNQX) and a GABAA receptor agonist (muscimol) on zotepine-induced elevation of extracellular levels of (A) GABA and (B) glutamate in the mPFC. The ordinates represent the mean ± SD (n = 8) extracellular neurotransmitter levels (pmol per sample) during 60–180 min after zotepine administration (ZTP: 3 mg·kg−1, i.p.). The perfusion medium was switched from MRS to MRS without (non-treatment) or with 50 µmol·L−1 MK-801, 50 µmol·L−1 muscimol or 50 µmol·L−1 DNQX. Following stabilization, the rats were injected with either the vehicle (control) or zotepine (ZTP: 3 mg·kg−1, i.p.). Data were compared using two-way anova with Tukey's multiple comparison (*P < 0.05; **P < 0.01 vs. non-treatment, ##P < 0.01 vs. control).
A two-way anova indicated the significant effects of perfusion with 50 µmol·L−1 muscimol and systemic administration of zotepine (3 mg·kg−1, i.p.) on extracellular levels of noradrenaline [two-way anova: Fzotepine(1, 28) = 202.2, P < 0.01; Fmusimol(1, 28) = 76.8, P < 0.01; Fzotepine*musimol(1, 28) = 60.8, P < 0.01], dopamine [two-way anova: Fzotepine(1, 28) = 301.5, P < 0.01; Fmusimol(1, 28) = 11.6, P < 0.01; Fzotepine*musimol(1, 28) = 11.3, P < 0.01], GABA [two-way anova: Fzotepine(1, 28) = 132.9, P < 0.01; Fmusimol(1, 28) = 1.2, P > 0.1; Fzotepine*musimol(1, 28) = 0.3, P > 0.1] and glutamate [two-way anova: Fzotepine(1, 28) = 54.9, P < 0.01; Fmusimol(1, 28) = 1.6, P > 0.1; Fzotepine*musimol(1, 28) = 0.1, P > 0.1] in the mPFC (Figures 5 and 6). Perfusion with 50 µmol·L−1 muscimol alone did not affect the release of noradrenaline, dopamine, 5-HT, GABA or glutamate in mPFC (Figures 5 and 6); however, perfusion with 50 µmol·L−1 muscimol significantly reduced the stimulatory effects of zotepine (3 mg·kg−1, i.p.) on extracellular levels of noradrenaline (P < 0.01) and dopamine (P < 0.01), but not 5-HT, GABA or glutamate levels in the mPFC (Figures 5 and 6).
A two-way anova indicated the significant effects of perfusion with 50 µmol·L−1 DNQX and systemic administration of zotepine (3 mg·kg−1, i.p.) on extracellular levels of noradrenaline [two-way anova: Fzotepine(1, 28) = 339.2, P < 0.01; FDNQX(1, 28) = 21.9, P < 0.01; Fzotepine*DNQX(1, 28) = 17.9, P < 0.01], dopamine [two-way anova: Fzotepine(1, 28) = 187.2, P < 0.01; FDNQX(1, 28) = 34.6, P < 0.01; Fzotepine*DNQX(1, 28) = 29.9, P < 0.01], GABA [two-way anova: Fzotepine(1, 28) = 237.2, P < 0.01; FDNQX(1, 28) = 18.9, P < 0.01; Fzotepine*DNQX(1, 28) = 4.3, P < 0.05] and glutamate [two-way anova: Fzotepine(1, 28) = 62.4, P < 0.01; FDNQX(1, 28) = 0.6, P > 0.1; Fzotepine*DNQX(1, 28) = 0.06, P > 0.1] in the mPFC (Figures 5 and 6). Perfusion with 50 µmol·L−1 DNQX alone did not affect the release of noradrenaline, dopamine, 5-HT, GABA or glutamate in mPFC (Figures 5 and 6); however, perfusion with 50 µmol·L−1 DNQX significantly reduced the stimulatory effects of zotepine (3 mg·kg−1, i.p.) on extracellular levels of noradrenaline (P < 0.01), dopamine (P < 0.01) and GABA (P < 0.05), but not 5-HT or glutamate levels in the mPFC (Figures 5 and 6).
Discussion
The present data on haloperidol, which is mainly and antagonist at dopamine D2 receptors (Bymaster et al., 1997), are in agreement in general with those reported by previous investigators (Rowley et al., 2000; Zhang et al., 2000; Adams and Moghaddam, 2001; Lopez-Gil et al., 2007). However, one study demonstrated that lower doses of haloperidol (0.004–0.3 mg·kg−1, s.c.) increased the extracellular levels of dopamine and noradrenaline in the mPFC dose-dependently (Westerink et al., 1998). The reason for these discrepant results regarding the effects of haloperidol on the extracellular monoamine levels in the frontal cortex is not clear at present. Several atypical antipsychotics that antagonize 5-HT2A and dopamine D2 receptors (olanzapine, risperidone and ziprasidone) also increased the mPFC extracellular dopamine levels (Ichikawa et al., 1998; Meltzer et al., 2003). Therefore, potent receptor antagonism for both 5-HT2A and dopamine D2 receptors seems to be important for the clinical actions of these atypical antipsychotic drugs (Meltzer et al., 2003). Risperidone and clozapine significantly increase extracellular 5-HT levels in the mPFC, unlike olanzapine. Thus, the ability of atypical antipsychotic drugs to increase extracellular 5-HT level might not be directly related to their affinity for 5-HT2A receptors (Ichikawa et al., 1998). Zotepine has multiple binding profiles with strong affinities to α1-adrenoceptors, 5-HT2A receptors, 5-HT2C receptors and dopamine D2 receptors (Tatsumi et al., 1999; Richelson and Souder, 2000). This profile therefore gives zotepine the pharmacological features of an atypical antipsychotic drug. The present study clearly demonstrated the different pharmacological effects of haloperidol and zotepine on neurotransmitter release in the mPFC, with haloperidol having no such effects and zotepine increasing the extracellular levels of noradrenaline, dopamine, GABA and glutamate in the mPFC without affecting 5-HT levels. In a previous study, a 5-HT2A receptor antagonist had no effect on the extracellular levels of noradrenaline, dopamine or 5-HT, while a 5-HT2C receptor antagonist increased that of noradrenaline and dopamine without affecting 5-HT (Gobert and Millan, 1999). Thus, it is possible that the zotepine-induced elevation of noradrenaline and dopamine levels noted in this study without affecting 5-HT levels is due to its 5-HT2C receptor antagonism. However, zotepine inhibits both noradrenaline and 5-HT transporters without exhibiting any affinity for dopamine transporters (Tatsumi et al., 1999). Inhibitors of noradrenaline, dopamine and 5-HT transporters increased the extracellular levels of monoamines (Okada et al., 1999; Rowley et al., 2000; Valentini et al., 2004). Therefore, the antagonistic profiling of zotepine against receptors and transporters does not explain its stimulatory effects on noradrenaline and dopamine levels without affecting 5-HT. To clarify the mechanisms underlying these contradictory results, we compared the effects of zotepine and haloperidol on neuronal activities associated with the monoaminergic and glutamatergic afferents to the mPFC.
The effects of clozapine, olanzapine and haloperidol on extracellular dopamine levels in the mPFC do not correlate with their effects on neuronal activity in the VTA under anaesthesia (Gessa et al., 2000). Intravenous administration of these three antipsychotic drugs transiently activated neuronal activity in the VTA, whereas extracellular dopamine levels in the mPFC were increased by clozapine and olanzapine, but not affected by haloperidol (Gessa et al., 2000). Thus, the atypical antipsychotic-induced increase in extracellular dopamine levels in the mPFC possibly depends on direct action on the mPFC dopaminergic system. In the present study, intraperitoneal administration of zotepine stimulated the VTA neuronal activity for 180 min, and this correlated with the increased extracellular dopamine level in the mPFC. In contrast, intraperitoneal administration of haloperidol also transiently stimulated VTA neuronal activity, but there was no correlation between this effect and extracellular dopamine level in the mPFC. Similarly, neuronal activities in the LC, DRN and MTN were not affected by haloperidol, whereas systemic injection of zotepine stimulated neuronal activity in all nuclei. The present study could not explain these different effects of haloperidol and zotepine on neuronal activity.
Whole-cell patch-clamp experiments using rat dorsal root ganglia demonstrated that zotepine, but not haloperidol, inhibited GABAergic inhibitory Cl− currents (Yokota et al., 2002), suggesting neuronal hyperexcitability in the brain. Alternatively, the stimulatory effects of zotepine on extracellular levels of dopamine, noradrenaline and glutamate depend on neuronal activities in the VTA, LC and MTN, respectively, although there was no correlation between zotepine-induced DRN neuronal stimulation and the effects on extracellular 5-HT level in the mPFC.
The major dopaminergic and 5-hydroxytryptaminergic afferents project to deeper layers in the mPFC from respective VTA and DRN nuclei (Waterhouse et al., 1983; Van Eden et al., 1987; Kuroda et al., 1998; Lambe et al., 2000). Afferents from the LC project to both deep and superficial layers of the mPFC (Waterhouse et al., 1983; Van Eden et al., 1987; Kuroda et al., 1998; Lambe et al., 2000), which comprise noradrenergic terminals and terminals co-releasing noradrenaline with dopamine (Devoto et al., 2005). The glutamatergic afferents project from other cortical regions and the MTN to superficial layers in the mPFC (Cotman and Monaghan, 1986; Jay et al., 1992; Kuroda et al., 1998). These previous findings established the NMDA-GABA hypothesis: that the contacts between glutamatergic and dopaminergic neurons in the mPFC are indirect and mediated by GABAergic interneurones, which are regulated by stimulatory NMDA receptors (Yonezawa et al., 1998; Zhu et al., 2004). The present results support this NMDA-GABA hypothesis, since the local perfusion of MK-801, an NMDA receptor antagonist, reduced and increased the extracellular levels of GABA and monoamine in the mPFC respectively. The stimulatory effect of MK-801 on the extracellular monoamines was inhibited by activation of GABAA receptors (Yonezawa et al., 1998; Zhu et al., 2004). Local perfusion with muscimol also inhibited the stimulatory effect of zotepine on the extracellular levels of noradrenaline and dopamine. Surprisingly, when NMDA receptor signalling was inhibited, zotepine increased the extracellular levels of noradrenaline, dopamine and 5-HT. Thus, the 5-hydroxytryptaminergic projection to the mPFC seems to be regulated predominantly by GABAergic inhibition. Contrary to 5-HT, the noradrenergic and dopaminergic projections probably consist of two types of terminals, sensitive and insensitive regulation by GABA. In addition, AMPA receptor inhibition prevented zotepine-induced elevation of noradrenaline and dopamine extracellular levels, with no effect on 5-HT levels. These results suggest that both afferents of noradrenaline and dopamine in mPFC are regulated by stimulatory AMPA/glutamatergic regulation, but 5-hydroxytryptaminergic afferents are not regulated by AMPA/glutamatergic effects. A major glutamatergic afferent to mPFC is projected from the MTN (Cotman and Monaghan, 1986; Jay et al., 1992; Kuroda et al., 1998). Therefore, the present results suggest that terminals of both noradrenergic and dopaminergic neurons are possibly stimulated by AMPA/glutamatergic regulation, via MTN projections.
The enhancement of noradrenergic transmission in the mPFC via antipsychotic-induced activation of the LC contributes to the observed improvements in cognitive dysfunction (Lim et al., 2007). Patients with schizophrenia showed no overall change in the number of neurons in the brain cortex, whereas fewer neurons were present in the MTN (Harrison and Weinberger, 2005). Both post-mortem and magnetic resonance imaging (MRI) volumetric studies identified functional interrelationship deficits between the frontotemporal cortex and MTN (Mitelman et al., 2005). Taken together with these clinical findings, the present results suggest that, if dysfunctional glutamatergic transmission is important for the pathophysiology of schizophrenia, then activation of glutamatergic transmission from the MTN to the mPFC possibly contributes mechanistically to the efficacy of zotepine against schizophrenia. Contrary to its clinical advantages, zotepine-induced overload of glutamatergic transmission associated with the MTN might contribute to the pro-convulsive action of zotepine (Hori et al., 1992; Fleischhacker et al., 1994), since MTN and other dorsal midline nuclei have a significant role in the primary seizures circuits of limbic seizures as well as in the spread of seizure activity to other regions (Bertram et al., 2008).
In conclusion, the present study demonstrated that systemic administration of zotepine increased the extracellular levels of noradrenaline, dopamine, glutamate and GABA, but not those of 5-HT, in the mPFC through interaction with the activated neuronal projection located outside the mPFC (or at least in the VTA, LC, DRN and MTN). Zotepine activated neuronal activity in the VTA, LC, DRN and MTN, thus increasing dopamine, noradrenaline, 5-HT, glutamate and GABA release in the mPFC. The enhanced releases of dopamine from the VTA, 5-HT from the DRN and noradrenaline from the LC were inhibited by enhanced GABAergic transmission. Contrary to 5-HT, the afferents of dopamine and noradrenaline are stimulated by enhanced AMPA/glutamatergic transmission and are partially insensitive to GABAergic inhibition in the mPFC. The particularly striking and positive interaction between the prefrontal catecholaminergic transmission and AMPA/glutamatergic transmission from the MTN emphasizes the relevance of mechanistic targets for the clinical actions and pro-convulsive propensity of zotepine.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science and Culture (18390316 and 18659330), a Grant from the Mitsubishi Pharma Research Foundation and a Grant from the Japan Epilepsy Research Foundation. We thank A/Prof. F.G. Issa (http://www.word-medex.com.au) for the careful reading and editing of the manuscript.
Glossary
Abbreviations:
- anova
analysis of variance
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- DRN
dorsal raphe nucleus
- HPLC
high-performance liquid chromatography
- LC
locus coeruleus
- MK-801
dizocilpine
- mPFC
medial prefrontal cortex
- MTN
mediodorsal thalamic nucleus
- VTA
ventral tegmental area
Conflict of interest
The authors state no conflict of interest.
References
- Adams BW, Moghaddam B. Effect of clozapine, haloperidol, or M100907 on phencyclidine-activated glutamate efflux in the prefrontal cortex. Biol Psychiatry. 2001;50:750–757. doi: 10.1016/s0006-3223(01)01195-7. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Foote WE, Sheard MH. Action of psychotogenic drugs on single midbrain raphe neurons. J Pharmacol Exp Ther. 1970;171:178–187. [PubMed] [Google Scholar]
- Aghajanian GK, Cedarbaum JM, Wang RY. Evidence for norepinephrine-mediated collateral inhibition of locus coeruleus neurons. Brain Res. 1977;136:570–577. doi: 10.1016/0006-8993(77)90083-x. [DOI] [PubMed] [Google Scholar]
- Amann B, Sterr A, Mergl R, Dittmann S, Seemuller F, Dobmeier M, et al. Zotepine loading in acute and severely manic patients: a pilot study. Bipolar Disord. 2005;7:471–476. doi: 10.1111/j.1399-5618.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Zhang D, Williamson JM. Multiple roles of midline dorsal thalamic nuclei in induction and spread of limbic seizures. Epilepsia. 2008;49:256–268. doi: 10.1111/j.1528-1167.2007.01408.x. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- Bymaster FP, Rasmussen K, Calligaro DO, Nelson DL, DeLapp NW, Wong DT, et al. In vitro and in vivo biochemistry of olanzapine: a novel, atypical antipsychotic drug. J Clin Psychiatry. 1997;58(Suppl. 10):28–36. [PubMed] [Google Scholar]
- Casey DE. The relationship of pharmacology to side effects. J Clin Psychiatry. 1997;58:55–62. [PubMed] [Google Scholar]
- Cotman CW, Monaghan DT. Anatomical organization of excitatory amino acid receptors and their properties. Adv Exp Med Biol. 1986;203:237–252. doi: 10.1007/978-1-4684-7971-3_18. [DOI] [PubMed] [Google Scholar]
- DeSilva P, Fenton M, Rathbone J. Zotepine for schizophrenia. Cochrane Database Syst Rev. 2006;4 doi: 10.1002/14651858.CD001948.pub2. CD001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Flore G, Saba P, Fa M, Gessa GL. Stimulation of the locus coeruleus elicits noradrenaline and dopamine release in the medial prefrontal and parietal cortex. J Neurochem. 2005;92:368–374. doi: 10.1111/j.1471-4159.2004.02866.x. [DOI] [PubMed] [Google Scholar]
- Fleischhacker WW, Hummer M, Kurz M, Kurzthaler I, Lieberman JA, Pollack S, et al. Clozapine dose in the United States and Europe: implications for therapeutic and adverse effects. J Clin Psychiatry. 1994;55(Suppl. B):78–81. [PubMed] [Google Scholar]
- Gessa GL, Devoto P, Diana M, Flore G, Melis M, Pistis M. Dissociation of haloperidol, clozapine, and olanzapine effects on electrical activity of mesocortical dopamine neurons and dopamine release in the prefrontal cortex. Neuropsychopharmacology. 2000;22:642–649. doi: 10.1016/S0893-133X(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Sudo T, Hirano M, Motomura H, Tagawa K, Nashiro S, et al. Efficacy and safety of zotepine for patients with treatment-resistant schizophrenia. Schizophr Res. 2006;87:332–333. doi: 10.1016/j.schres.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Hori M, Suzuki T, Sasaki M, Shiraishi H, Koizumi J. Convulsive seizures in schizophrenic patients induced by zotepine administration. Jpn J Psychiatry Neurol. 1992;46:161–167. doi: 10.1111/j.1440-1819.1992.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Kuroki T, Dai J, Meltzer HY. Effect of antipsychotic drugs on extracellular serotonin levels in rat medial prefrontal cortex and nucleus accumbens. Eur J Pharmacol. 1998;351:163–171. doi: 10.1016/s0014-2999(98)00308-2. [DOI] [PubMed] [Google Scholar]
- Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatory amino acid pathway from the hippocampus to the prefrontal cortex. Contribution of AMPA receptors in hippocampo-prefrontal cortex transmission. Eur J Neurosci. 1992;4:1285–1295. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Yokofujita J, Murakami K. An ultrastructural study of the neural circuit between the prefrontal cortex and the mediodorsal nucleus of the thalamus. Prog Neurobiol. 1998;54:417–458. doi: 10.1016/s0301-0082(97)00070-1. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Goldman-Rakic PS, Aghajanian GK. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb Cortex. 2000;10:974–980. doi: 10.1093/cercor/10.10.974. [DOI] [PubMed] [Google Scholar]
- Lim EP, Verma V, Nagarajah R, Dawe GS. Propranolol blocks chronic risperidone treatment-induced enhancement of spatial working memory performance of rats in a delayed matching-to-place water maze task. Psychopharmacology (Berl) 2007;191:297–310. doi: 10.1007/s00213-006-0664-0. [DOI] [PubMed] [Google Scholar]
- Lopez-Gil X, Babot Z, Amargos-Bosch M, Sunol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Gruppe H, Bauer U, Lis S, Krieger S, Gallhofer B. Improvement of cognitive function in schizophrenic patients receiving clozapine or zotepine: results from a double-blind study. Pharmacopsychiatry. 1997;30:35–42. doi: 10.1055/s-2007-979481. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Byne W, Kemether EM, Hazlett EA, Buchsbaum MS. Metabolic disconnection between the mediodorsal nucleus of the thalamus and cortical Brodmann's areas of the left hemisphere in schizophrenia. Am J Psychiatry. 2005;162:1733–1735. doi: 10.1176/appi.ajp.162.9.1733. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Ago Y, Itoh S, Koyama Y, Baba A, Matsuda T. Effect of zotepine on dopamine, serotonin and noradrenaline release in rat prefrontal cortex. Eur J Pharmacol. 2005;528:95–98. doi: 10.1016/j.ejphar.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Okada M, Kawata Y, Mizuno K, Wada K, Kondo T, Kaneko S. Interaction between Ca2+, K+, carbamazepine and zonisamide on hippocampal extracellular glutamate monitored with a microdialysis electrode. Br J Pharmacol. 1998;124:1277–1285. doi: 10.1038/sj.bjp.0701941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Kawata Y, Murakami T, Wada K, Mizuno K, Kondo T, et al. Differential effects of adenosine receptor subtypes on release and reuptake of hippocampal serotonin. Eur J Neurosci. 1999;11:1–9. doi: 10.1046/j.1460-9568.1999.00415.x. [DOI] [PubMed] [Google Scholar]
- Okada M, Nutt DJ, Murakami T, Zhu G, Kamata A, Kawata Y, et al. Adenosine receptor subtypes modulate two major functional pathways for hippocampal serotonin release. J Neurosci. 2001;21:628–640. doi: 10.1523/JNEUROSCI.21-02-00628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Zhu G, Yoshida S, Hirose S, Kaneko S. Protein kinase associated with gating and closing transmission mechanisms in temporoammonic pathway. Neuropharmacology. 2004;47:485–504. doi: 10.1016/j.neuropharm.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Okada M, Yoshida S, Zhu G, Hirose S, Kaneko S. Biphasic actions of topiramate on monoamine exocytosis associated with both soluble N-ethylmaleimide-sensitive factor attachment protein receptors and Ca(2+)-induced Ca(2+)-releasing systems. Neuroscience. 2005;134:233–246. doi: 10.1016/j.neuroscience.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotoxic coordinates. 4th Ed. San Diego: Academic Press; 1998. [Google Scholar]
- Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Kilpatrick IC, Needham PL, Heal DJ. Elevation of extracellular cortical noradrenaline may contribute to the antidepressant activity of zotepine: an in vivo microdialysis study in freely moving rats. Neuropharmacology. 1998;37:937–944. doi: 10.1016/s0028-3908(98)00094-x. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Needham PL, Kilpatrick IC, Heal DJ. A comparison of the acute effects of zotepine and other antipsychotics on rat cortical dopamine release, in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:187–192. doi: 10.1007/s002109900170. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Jansen K, Blakely RD, Richelson E. Pharmacological profile of neuroleptics at human monoamine transporters. Eur J Pharmacol. 1999;368:277–283. doi: 10.1016/s0014-2999(99)00005-9. [DOI] [PubMed] [Google Scholar]
- Valentini V, Frau R, Di Chiara G. Noradrenaline transporter blockers raise extracellular dopamine in medial prefrontal but not parietal and occipital cortex: differences with mianserin and clozapine. J Neurochem. 2004;88:917–927. doi: 10.1046/j.1471-4159.2003.02238.x. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Hoorneman EM, Buijs RM, Matthijssen MA, Geffard M, Uylings HB. Immunocytochemical localization of dopamine in the prefrontal cortex of the rat at the light and electron microscopical level. Neuroscience. 1987;22:849–862. doi: 10.1016/0306-4522(87)92964-2. [DOI] [PubMed] [Google Scholar]
- van Veldhuizen MJ, Feenstra MG, Boer GJ, Westerink BH. Microdialysis studies on cortical noradrenaline release: basic characteristics, significance of extracellular calcium and massive post-mortem increase. Neurosci Lett. 1990;119:233–236. doi: 10.1016/0304-3940(90)90841-v. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Lin CS, Burne RA, Woodward DJ. The distribution of neocortical projection neurons in the locus coeruleus. J Comp Neurol. 1983;217:418–431. doi: 10.1002/cne.902170406. [DOI] [PubMed] [Google Scholar]
- Westerink BH, de Boer P, de Vries JB, Kruse CG, Long SK. Antipsychotic drugs induce similar effects on the release of dopamine and noradrenaline in the medial prefrontal cortex of the rat brain. Eur J Pharmacol. 1998;361:27–33. doi: 10.1016/s0014-2999(98)00711-0. [DOI] [PubMed] [Google Scholar]
- Yokota K, Tatebayashi H, Matsuo T, Shoge T, Motomura H, Matsuno T, et al. The effects of neuroleptics on the GABA-induced Cl- current in rat dorsal root ganglion neurons: differences between some neuroleptics. Br J Pharmacol. 2002;135:1547–1555. doi: 10.1038/sj.bjp.0704608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H. Involvement of gamma-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol. 1998;341:45–56. doi: 10.1016/s0014-2999(97)01435-0. [DOI] [PubMed] [Google Scholar]
- Zhang W, Perry KW, Wong DT, Potts BD, Bao J, Tollefson GD, et al. Synergistic effects of olanzapine and other antipsychotic agents in combination with fluoxetine on norepinephrine and dopamine release in rat prefrontal cortex. Neuropsychopharmacology. 2000;23:250–262. doi: 10.1016/S0893-133X(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Zhu G, Okada M, Uchiyama D, Ohkubo T, Yoshida S, Kaneko S. Hyperactivity of endoplasmic reticulum associated exocytosis mechanism contributes to acute phencyclidine intoxication. J Pharmacol Sci. 2004;95:214–227. doi: 10.1254/jphs.fp0040044. [DOI] [PubMed] [Google Scholar]


