Abstract
Background and purpose:
Fluoroquinolones are potent anti-microbial agents with multiple effects on host cells and tissues. Previous studies have highlighted their pro-apoptotic effect on human cancer cells and an immunoregulatory role in animal models of inflammatory bowel disease. We examined the effect of ciprofloxacin on proliferation, cell cycle and apoptosis of HT-29 cells, a human colonic epithelial cell line sensitive to transforming growth factor (TGF)-β1-mediated growth inhibition and its role in TGF-β1 production. We also examined the effect of ciprofloxacin on proliferation of HT-29 cells in combination with 5-fluorouracil (5-FU), a well-established pro-apoptotic agent.
Experimental approach:
Using subconfluent cultures of HT-29 and Caco-2 cells, we studied the effect of ciprofloxacin, TGF-β1 and 5-FU on proliferation, apoptosis, necrosis and cell cycle. The effect of ciprofloxacin on TGF-β1 mRNA expression and production was studied in RNA extracts and cell culture supernatants respectively, using confluent cultures.
Key results:
Ciprofloxacin decreased proliferation of HT-29 cells in a concentration- and time-dependent manner. This was mediated by accumulation of HT-29 cells into the S-phase but without any effect on apoptosis or necrosis. Additionally, ciprofloxacin enhanced the antiproliferative effect of 5-FU. Interestingly, ciprofloxacin was found to up-regulate TGF-β1 production by HT-29 cells and its anti-proliferative effect was abolished when TGF-β1 was blocked. Confirming this mechanism further, ciprofloxacin had no effect on Caco-2, a human colonic epithelial cell line that lacks functional TGF-β1 receptors.
Conclusions and implications:
We demonstrate a novel anti-proliferative and immunoregulatory effect of ciprofloxacin on human intestinal epithelial cells mediated via TGF-β1.
Keywords: ciprofloxacin, TGF-β1, colon cancer, HT-29 cells, inflammatory bowel disease, chemoprevention, immunomodulation
Introduction
Fluoroquinolones are broad spectrum antibiotics with few side effects that exert their antibacterial activity by inhibiting bacterial DNA-gyrase, which is responsible for supercoiling, transcription, replication and chromosomal separation of prokaryotic DNA (Shen, 1994). Ciprofloxacin is the traditional representative of the fluoroquinolones' family and it is used in various clinical settings like infectious enteritis and inflammatory bowel disease (Oldfield and Wallace, 2001; Greenberg, 2004). Recently, several immunomodulatory activities of quinolones have been reported including suppression of pro-inflammatory cytokines and induction of nitric oxide (Riesbeck, 2002; Kolios et al., 2006). Furthermore, there are reports that ciprofloxacin is able to induce apoptosis in various human cancer cell lines including the human colonic cancer cells (Herold et al., 2002). These non-antimicrobial effects of quinolones render them unique among other antibiotics and suggest a large spectrum of future clinical applications.
Transforming growth factor (TGF)-β1 is a multifunctional cytokine capable of regulating cell growth, differentiation and eventually tissue homeostasis. It participates in both innate and adaptive immune responses and acts as the key regulator of immune tolerance (Letterio and Roberts, 1998). On the other hand, defects in TGF-β1 regulation have been implicated in the pathogenesis of many diseases. Defective TGF-β1 production has been observed in Crohn's disease and ulcerative colitis potentially contributing to the perpetuation of inflammation (Becker et al., 2006). In oncogenesis, the TGF-β1 signalling pathway has been considered both suppressor and promoter; during early stages, it has been shown, in animal models, that epithelial cells lose their growth-inhibitory responsiveness to TGF-β which eventually promotes invasion and metastasis in the later stages of carcinoma development (Derynck et al., 2001; Siegel and Massague, 2003). In certain forms of cancer, transcriptional repression of TGF-β receptors (TGF-βRII or TGF-βRI) has been reported and may be primarily responsible for loss of sensitivity to TGF-β1 antiproliferative control contributing to tumour formation (Siegel and Massague, 2003).
We investigated the effect of ciprofloxacin on proliferation and survival of the colonic cancer cell lines HT-29 and Caco-2. We found that ciprofloxacin can suppress proliferation of HT-29 but has no effect on Caco-2 cells. The observed differential outcome parallels the expression of functional TGF-β receptors by the two cell lines with HT-29 being sensitive to TGF-β1, and Caco-2 being resistant to TGF-β1 (Matsushita et al., 1999; Chen et al., 2002; Zanetti et al., 2003). We further demonstrated that ciprofloxacin induces TGF-β1 expression and secretion by HT-29, resulting in suppression of proliferation through TGF-β1-dependent cell cycle arrest. We also demonstrated that ciprofloxacin enhances the antiproliferative effect of 5-fluorouracil (5-FU), a well-established treatment for colon cancer (Nita et al., 1998), on HT-29 colonic adenocarcinoma cells.
Methods
Cell culture
HT-29 and Caco-2 colonic epithelial cell lines were obtained from the European Collection of Animal Cell Cultures (ECACC, Porton Down, Wiltshire, UK). HT-29 cells are an epithelial cell line derived from a colon adenocarcinoma, which have characteristics of normal intestinal epithelium. HT-29 and Caco-2 were passaged weekly and grown at 37°C in McCoy's 5A and minimum essential medium alpha medium respectively, which were supplemented with 10% foetal bovine serum (FBS), penicillin/streptomycin (10 u·mL−1 and 10 µg·mL−1) and fungizone (0.5 µg·mL−1). Both cell lines were seeded at 2–3 × 104/cm2 in six-well plates and were maintained at 37°C in an atmosphere of 5% CO2 until confluent. In order to study TGF-β1 expression and production, 24 h before stimulation the confluent cell cultures were washed and cultured in fresh medium without FBS. Growth-arrested cultures were then stimulated with ciprofloxacin (100 µg·mL−1) for up to 48 h, as described in the results section. Cell culture supernatants were collected for protein determination and RNA extraction was performed as follows.
RT-PCR
Total RNA was extracted from HT-29 cells into TRIzol and treated with DNAse as described by the manufacturers. Multiplex RT-PCR was performed as previously described (Valatas et al., 2004). Briefly, 1 µg mRNA was denatured at 70°C for 10 min in the presence of 5 µmol·L−1 oligo (dT) 12–18 primer. It was then reverse transcribed in a 10 µL volume with Superscript II, 1X RT buffer, 1 mmol·L−1 deoxyribonucletide triphosphates (dNTPs), 5 mmol·L−1 DDT and 2.5 u·µL−1 RNAsin at 42°C for 60 min; 1 µL aliquots of cDNA were PCR amplified in a 25 µL reaction containing: 1 X PCR buffer and 2 mmol·L−1 MgCl2, 0.2 mmol·L−1 dNTPs, 0.5 mmol·L−1 sense and antisense primers, and 0.4 u polymerase. The oligonucleotide sequence and product size for specific primer pairs used are shown in Table 1. The conditions for amplification were: 5 min 94°C, 30 cycles of 30 s 94°C, 30 s annealing temperature, 30 s 72°C, followed by an extension for 7 min at 72°C. PCR products were resolved by electrophoresis on 2% agarose gels and visualized by ethidium bromide staining. In order to control for genomic contamination, an identical parallel PCR reaction (RT-negative) was performed for each sample containing starting material, which had not been reverse transcribed. The integrated density of the bands was used as quantitative parameter and was calculated by digital image analysis. The ratio of the integrated density of TGF-β1 divided by that of GAPDH, used as the housekeeping gene, was used to quantify the results.
Table 1.
Primer sequences used for the RT-PCR studies
| Gene | Primers | Product size |
|---|---|---|
| TGF-β1 | Sense: 5′-TGCTGAGGCTCAAGTTAAAA-3′ | 224 bp |
| Antisense: 5′-TGTGTTATCCCTGCTGTCAC-3′ | ||
| GAPDH | Sense: 5′-CAACGGATTTGGTCGTATTG-3′ | 184 bp |
| Antisense: 5′-GATGACAAGCTTCCCGTTCT-3′ |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; RT-PCR, reverse transcription polymerase chain reaction; TGF, transforming growth factor.
elisa for TGF-β1
HT-29 cells were seeded in six-well plates and cultured until confluence. Growth-arrested cultures were then stimulated with ciprofloxacin (100 µg·mL−1) for up to 48 h. Supernatants were collected and TGF-β1 production was assessed by elisa according to the manufacturer's instructions.
Cell viability and growth assay
HT-29 and Caco-2 cell lines were seeded in 24-well plates at an initial concentration of 1.5 × 104 cells per well and 2 × 104 cells per well respectively. This resulted in 60–70% confluence after 6 days of culture. The effect of ciprofloxacin, TGF-β1 and 5-FU was assessed 1 day after seeding. For incubations lasting 6 days, medium and compounds were renewed every 3 days. Each treatment was performed in quadruplicate. After 3 or 6 days of treatment, growth and viability of cells were measured by the tetrazolium salt assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenol tetrazolium bromide (MTT)] (Mosmann, 1983). In order to determine whether the antiproliferative effect of ciprofloxacin after 6 days of treatment is reversible, we removed media and plates were gently washed with Hank's buffered salt solution. Fresh media without ciprofloxacin was added and an MTT assay was performed 2 days later, which is after a total of 8 days in culture.
Apoptosis assays
Apoptosis in HT-29 cells was initially evaluated by 7-amino-actinomycin D (7-AAD) and annexin-V staining. HT-29 cells were seeded in six-well plates at a density of 7.5 × 104 per well. One day later, the medium was changed to medium containing 50 µg·mL−1 ciprofloxacin. After 6 days of incubation, cells were gently trypsinized and washed with ice-cold phosphate-buffered saline (PBS). Cells were resuspended in 100 µL of 1 X binding buffer, stained with 5 µL of FITC-annexin-V (50 µg·mL−1) and 10 µL of 7-AAD (1 mg·mL−1) and then incubated for 15 min on ice in the dark. Every sample was diluted with 385 µL of binding buffer and immediately analysed by flow cytometry (FACScalibur, Becton Dickinson). We identified three subsets of cells, based on the staining intensity: annexin−/7-AAD− (live cells), annexin+/7-AAD− (early apoptotic cells) and annexin+/7-AAD+ (late apoptotic and necrotic cells). Apoptosis was further examined by cell death detection elisa plus kit for analysis of DNA fragmentation according to manufacturer's instructions. HT-29 and Caco-2 cells were seeded in a 96-well plate at an initial concentration of 1.5 × 104 cells per well. After 24 h, the medium was replaced with ciprofloxacin-containing medium (0, 5 and 50 µg·mL−1). Cells were treated for 24 h and elisa was performed in supernatants and cell lysates in order to detect necrotic and apoptotic cells respectively.
Cell cycle analysis
HT-29 cells (1.5–2 × 105) were seeded in 25 cm2 flasks. After 24 h, medium was changed with fresh, supplemented or not (control) with ciprofloxacin (50 µg·mL−1). After 2 and 4 days of incubation (at a 50–60% cell confluence), cells were harvested with trypsin, washed in PBS without Ca2+/Mg2+, fixed in 70% ethanol and stored at −20°C until staining. Nuclear DNA staining was performed by propidium iodide (PI). Briefly, after the cells had been washed in PBS without Ca2+/Mg2+, 106 cells were stained in 2 mL of PI staining solution (50 µg·mL−1 PI, 1 mg·mL−1 RNase in PBS without Ca2+/Mg2+). DNA flow cytometry was performed in duplicate with a FACScalibur flow cytometer. Cell cycle analysis was performed by the WinMDI software (Windows Multiple Document Interface for Flow Cytometry).
Statistical analysis
Unless otherwise indicated, values represent mean ± s.e.mean of at least three independent experiments. Comparative statistical evaluation between groups was performed by anova. Bonferroni t post-hoc analysis and Student's t-test were used for statistical comparison between individual variables as appropriate. A probability value of P < 0.05 was taken as the criterion for statistical significance.
Materials
Recombinant human TGF-β1 and monoclonal anti-human LAP (TGF-β1) antibodies were purchased from R&D Systems (Abingdon, UK). TRIzol, Oligo (dT) 12–18 primer, Superscript II, RT buffers and dNTPs were purchased from Gibco BRL Life Technologies. RNAsin was from Promega (Southampton, UK). PCR buffers, DNAse and expand polymerase were purchased from Roche Molecular Biochemicals, (Lewes, Sussex, UK). Functional grade purified mouse IgG2a isotype control was purchased from E-bioscience (San Diego, CA, USA) and 5-FU from Teva Pharma (Milano, Italy). PI and FITC-annexin V were from BD Biosciences (San Diego, CA, USA), whereas 7-AAD was from Calbiochem-Novabiochem, (La Jolla, CA, USA). All other reagents, including ciprofloxacin, MTT and RNase, were from Sigma-Aldrich (Steinheim, Germany). All cell culture reagents and plastics were from Gibco BRL Life Technologies (Paisley, UK) and Nalge Nunc (Hereford, UK) respectively. The elisa kits were from R&D systems (Minneapolis, MN, USA). Our drug/molecular target nomenclature conforms to BJP's Guide to Receptors and Channels (Alexander et al., 2008).
Results
Ciprofloxacin suppresses proliferation of HT-29 cells
Proliferation of HT-29 cells was assessed following culture of HT-29 for 6 days in the presence of various concentrations of ciprofloxacin (0–100 µg·mL−1). Proliferation rate was significantly (P < 0.001) suppressed by ciprofloxacin in a concentration (IC50= 9.46 µg·mL−1)- and time-dependent manner (Figure 1A and B). Maximum inhibition was 25% on day 3 and 36% on day 6 compared with untreated cells (Figure 1A). The effect of ciprofloxacin was reversible and cell proliferation could be reinstituted by removing ciprofloxacin from cultures (Figure 1B).
Figure 1.
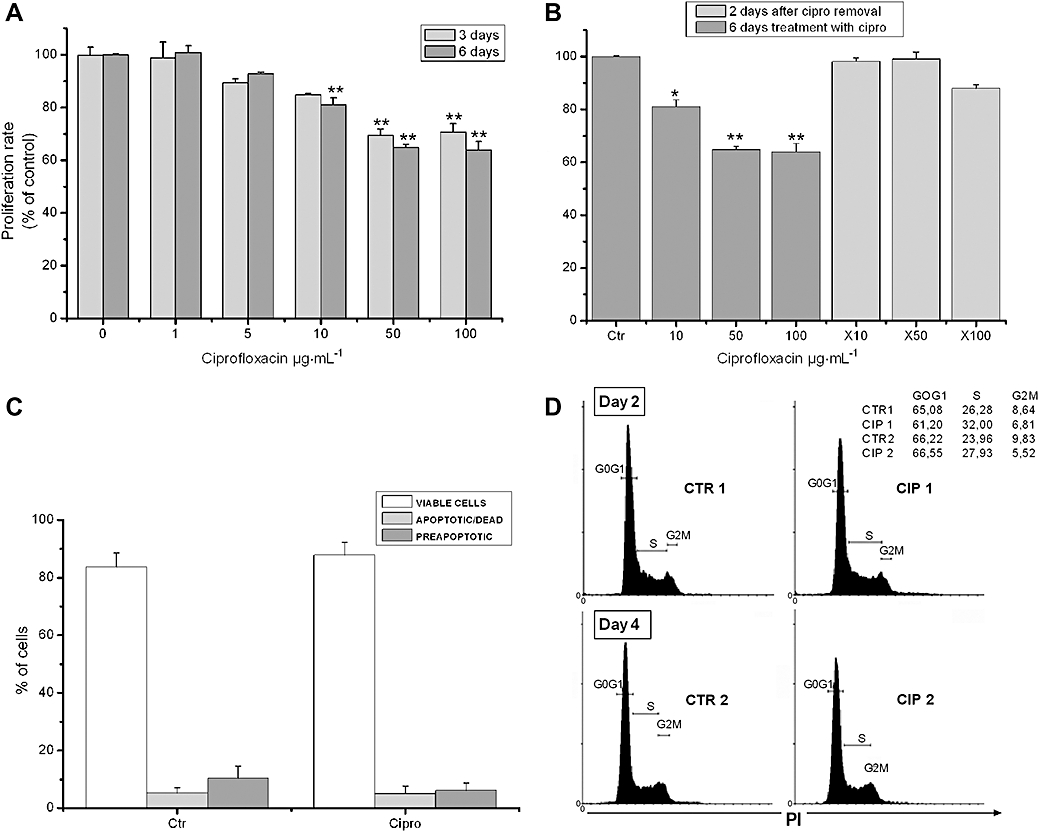
Ciprofloxacin suppresses HT-29 cell proliferation by inducing cell cycle arrest. (A) MTT assessed proliferation of HT-29 cells after treatment with 0–100 µg·mL−1 of ciprofloxacin for 3 and 6 days, as described in Methods. (B) MTT assessed proliferation of HT-29 cells treated with ciprofloxacin for 6 days, which was followed by removal of ciprofloxacin and subsequent 2 days treatment with vehicle; ×10, ×50 and ×100 indicate initial concentrations of ciprofloxacin (*P < 0.05, **P < 0.01 different from control values). (C) Apoptosis of HT-29 cells after treatment with vehicle (Ctr) or ciprofloxacin (50 µg·mL−1) evaluated by 7-AAD and annexin-V staining. (D) Effects of ciprofloxacin on cell cycle assessed by propidium iodide (PI) staining. HT-29 cells were treated for 2 days and 4 days with vehicle (CTR1, CTR2) or 50 µg·mL−1 of ciprofloxacin (CIP1, CIP2). Two representative graphs from six independent experiments are shown. 7-AAD, 7-amino-actinomycin D; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenol tetrazolium bromide.
In order to explain the observed anti-proliferative effect of ciprofloxacin, we further examined its ability to induce cell apoptosis and/or necrosis. Ciprofloxacin treatment did not induce apoptosis or necrosis of HT-29 cells as quantification of viable (84 ± 6%), pre-apoptotic (10 ± 3%) and apoptotic (6 ± 3%) cells by annexin-V and 7-AAD staining, revealed no difference from vehicle-treated cultures (Figure 1D). HT-29 cell necrosis and apoptosis following ciprofloxacin treatment were also measured in culture supernatants and cell lysates, respectively, by analysis of DNA fragmentation using anti-histone and anti-DNA antibodies and the above results were confirmed (Figure S1).
To evaluate whether ciprofloxacin could cause any specific perturbation of the cell cycle, an analysis of ciprofloxacin-treated HT-29 cells was performed at days 2 and 4 of exposure. Ciprofloxacin caused an accumulation of cells mostly in the S-phase in both time-points. In particular, the percentage of cells at the S-phase significantly increased from 25.94 ± 1.38 to 31.65 ± 0.98% (P= 0.028), and from 23.64 ± 0.66 to 27.72 ± 0.68% (P= 0.014) after treatment with 50 µg·mL−1 ciprofloxacin on days 2 and 4 respectively (Figure 1D). These results indicate that ciprofloxacin exerts its anti-proliferative effect on HT-29 cells by inducing cell cycle arrest.
The anti-proliferative effect of ciprofloxacin is mediated through TGF-β1
Recent studies have shown that TGF-β1 may be involved in the regulation of epithelial cell homeostasis (Letterio and Roberts, 1998). Data derived mostly from studies on epithelial cell lines suggest that TGF-β1 is produced by epithelial cells themselves, in an autocrine manner, and regulates epithelial cell proliferation and survival (Bierie and Moses, 2006). We wanted to see if changes in TGF-β1 production could account for the anti-proliferative effect of ciprofloxacin on HT-29 cells.
At first, we examined the effect of TGF-β1 (0–100 ng·mL−1) on the proliferation of HT-29 cells. TGF-β1 treatment significantly suppressed HT-29 cell proliferation in a concentration-dependent manner, with the highest concentration resulting in a 40% inhibition (P < 0.001, Figure 2A). We next investigated whether TGF-β1 is produced by HT-29, and whether this production could be modified by treatment with ciprofloxacin. TGF-β1 production was measured by elisa in confluent serum-free cell culture supernatants after 24 and 48 h of treatment with vehicle or ciprofloxacin (0 and 100 µg·mL−1). Vehicle-treated HT-29 cells were found to produce substantial amounts of TGF-β1 at 24 and 48 h (Figure 2B). Furthermore, treatment of HT-29 cells with ciprofloxacin resulted to a significant increase in TGF-β1 production at 24 and 48 h (Figure 2B).
Figure 2.
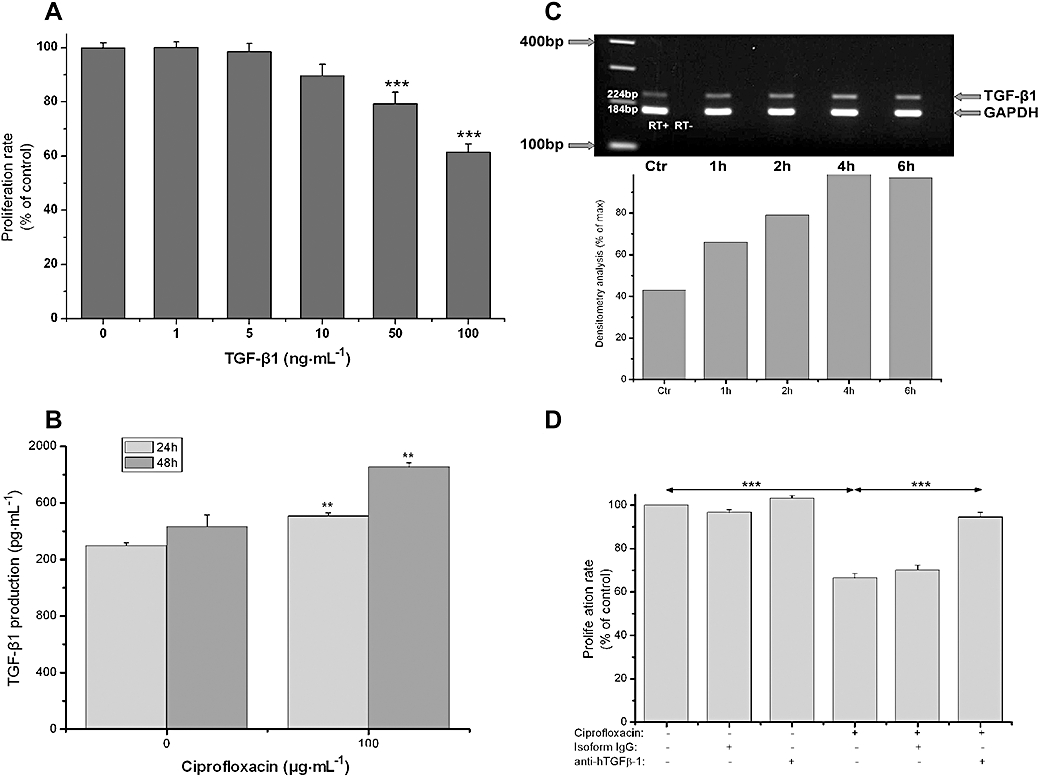
Ciprofloxacin-induced cell cycle arrest is mediated through TGF-β1. (A) MTT assessed proliferation of HT-29 cells after treatment with 0–100 ng·mL−1 of TGF-β1 for 3 days. (B) TGF-β1 production by HT-29 cells after 24 and 48 h treatment with vehicle (0) or ciprofloxacin (100 µg·mL−1) evaluated by elisa in cell culture supernatants. (C) Expression of TGF-β1 mRNA by HT-29 cells. Total RNA was extracted from HT-29 cells after treatment with ciprofloxacin (100 µg·mL−1) for 1 to 6 h and multiplex RT-PCR for TGF-β1 and GAPDH was performed. Upper panel: representative PCR blot of mRNA expression for TGF-β1 and GAPDH. Lower panel: the ratio of the integrated density of TGF-β1 divided by that of GAPDH, measured by densitometry analysis, is expressed as a percentage of the maximum TGF-β1 mRNA expression after stimulation with ciprofloxacin (100 µg·mL−1). One experiment representative of three independent experiments with similar results is shown. (D) MTT assessed proliferation of HT-29 cells after treatment with ciprofloxacin (100 µg·mL−1) for 6 days in the presence of 10 µg·mL−1 of neutralizing anti-human-TGF-β1 (LAP) antibody or 10 µg·mL−1 of purified mouse IgG2a isotype control (*P < 0.05, **P < 0.01, ***P < 0.001). GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenol tetrazolium bromide; RT-PCR, reverse transcription polymerase chain reaction; TGF, transforming growth factor.
Furthermore, we investigated whether the observed increase of TGF-β1 production occurs via induction of TGF-β1 mRNA expression or via ciprofloxacin-induced modifications at the post-translational level. Therefore, we examined the effect of ciprofloxacin on TGF-β1 mRNA expression by semi-quantitative RT-PCR. Untreated HT-29 cells showed a basal TGF-β1 expression, which was increased at least twofold within 4 h of ciprofloxacin treatment (Figure 2C).
In order to verify if ciprofloxacin action is strictly dependent on TGF-β1, we tried to prevent the anti-proliferative effect of ciprofloxacin by anti-TGF-β1 blockade. Proliferation of HT-29 cells was measured after 6 days of treatment with ciprofloxacin (50 µg·mL−1) added alone or in combination with 10 µg·mL−1 of monoclonal anti-human LAP (TGF-β1) neutralizing antibody (Figure 2D). As expected, ciprofloxacin suppressed HT-29 cell proliferation by 35% after 6 days of treatment. On the contrary, the addition of 10 µg·mL−1 of monoclonal anti-human LAP (TGF-β1) neutralizing antibody abolished the ciprofloxacin-induced suppression suggesting that the antiproliferative effect of ciprofloxacin is mediated by TGF-β1.
To further confirm this result, we examined the effect of ciprofloxacin on cell proliferation, apoptosis or necrosis of Caco-2, a human colonic epithelial cell line. In contrast to HT-29, Caco-2 lack functional TGF-β1 receptors (Biasi et al., 2002). Therefore, if the anti-proliferative effect of ciprofloxacin depends on TGF-β1, ciprofloxacin would not be expected to suppress Caco-2 proliferation. Initially, we confirmed the un-responsiveness to TGF-β1 by culturing Caco-2 cells in the presence of various concentrations of TGF-β1 (0–100 ng·mL−1). TGF-β1 had no effect on Caco-2 proliferation (Figure 3A). Moreover, treatment of Caco-2 cells with various concentrations of ciprofloxacin (0–100 µg·mL−1) confirmed that in the absence of TGF-β1 signalling ciprofloxacin has no effect on cell proliferation (Figure 3B). Similar to HT-29, as expected, analysis of DNA fragmentation showed no effect of ciprofloxacin on Caco-2 cell apoptosis/necrosis (data not shown).
Figure 3.
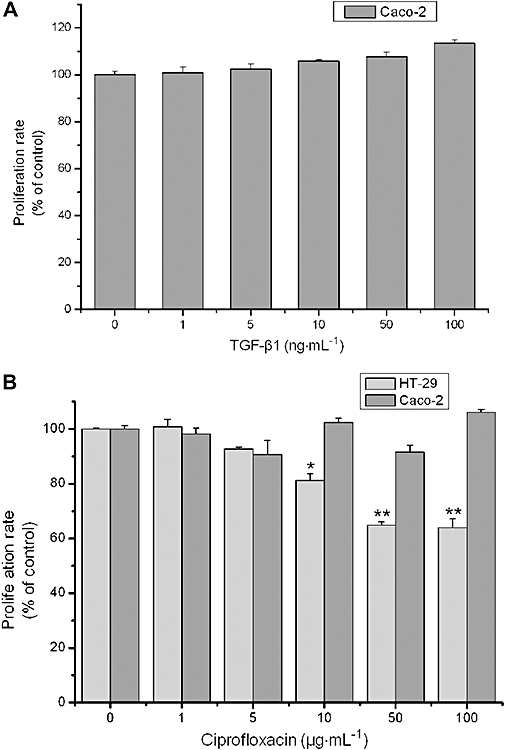
Ciprofloxacin has no effect on TGF-β1-resistant Caco2 cells. (A) MTT assessed proliferation of Caco-2 cells after treatment with TGF-β1 for 3 days. (B) MTT assessed proliferation of HT-29 and Caco-2 cells after treatment with ciprofloxacin for 6 days (*P < 0.05, **P < 0.01 different from control values). MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenol tetrazolium bromide; TGF, transforming growth factor.
Ciprofloxacin acts synergistically with 5-FU to suppress HT-29 cell proliferation
5-fluorouracil is a well-established chemotherapeutic agent in colonic cancer (Nita et al., 1998). We wanted to investigate if ciprofloxacin could enhance the anti-proliferative effect of 5-FU in colonic epithelial cells. In order to examine their possible synergistic effect, we cultured HT-29 with 5-FU or ciprofloxacin or a combination of them both (Figure 4). After 6 days of treatment with 1 mmol·L−1 5-FU, the proliferation rate of HT-29 cells was suppressed by 31.12 ± 1.75%. The addition of 50 µg·mL−1 ciprofloxacin further suppressed HT-29 proliferation by 59.37 ± 2.06% (Figure 4). Thus, the addition of ciprofloxacin in culture significantly enhanced the anti-proliferative effect of 5-FU on HT-29 cells (P < 0.001, Figure 4).
Figure 4.
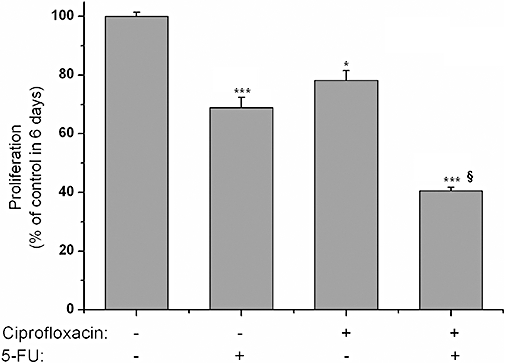
Ciprofloxacin augments the anti-proliferative effect of 5-FU. MTT assessed proliferation of HT-29 cells after treatment for 6 days with ciprofloxacin (100 µg·mL−1) added alone or in combination with 1 mmol·L−1 of 5-FU. (*P < 0.05, ***P < 0.001 different from control values, §P < 0.05 different from 5-FU treatment). 5-FU, 5-fluorouracil; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenol tetrazolium bromide.
Discussion
In the present study, we demonstrated that ciprofloxacin inhibits proliferation of the colonic epithelial adenocarcinoma cell line HT-29 in a concentration- and time-dependent manner. These results extend those found previously showing the antiproliferative effect of ciprofloxacin on human cancer cell lines. The antitumour efficacy of ciprofloxacin and other members of the fluoroquinolone family has been reported in several cancer cell types including leukaemic (Somekh et al., 1989), bladder (Seay et al., 1996; Aranha et al., 2000; Kamat and Lamm, 2004), prostate (El-Rayes et al., 2002), osteoblast (Holtom et al., 2000), osteosarcoma (Miclau et al., 1998), neuroblastoma (Norris et al., 2001) and colon cancer cell lines (Rabau et al., 1995; Herold et al., 2002). Data from the literature suggest that ciprofloxacin in high concentrations (>100 µg·mL−1) can induce apoptosis, possibly in part by blocking mitochondrial DNA synthesis (Lawrence et al., 1996; Aranha et al., 2002; Herold et al., 2002). However, lower concentrations of ciprofloxacin (20–100 µg·mL−1) suppress proliferation by arresting the cell cycle (Riesbeck et al., 1990; Holtom et al., 2000).
The effect of ciprofloxacin in our model was not attributed to apoptosis but rather to cell cycle arrest that could be reversed after the removal of the quinolone. Previously published studies have demonstrated a 35% inhibition of mammalian cell growth by ciprofloxacin in concentrations higher than 20 µg·mL−1 (Forsgren et al., 1987). However, in our experiments ciprofloxacin suppressed proliferation in concentrations as low as 10 µg·mL−1, a concentration that can be achieved in human tissues using standard dosing regimens of ciprofloxacin. Serum concentrations of ciprofloxacin that can be achieved in patients after standard dosing regimens are 0.5–10 µg·mL−1 (Crump et al., 1983; Bergeron, 1989; Wolfson and Hooper, 1991; Shah et al., 1994), while the IC50 of the toxic effect of ciprofloxacin in cell lines is 40–80 µg·mL−1 (Riesbeck, 2002). In our experiments, the IC50 value for the antiproliferative effect of ciprofloxacin in HT-29 cells was 9.46 µg·mL−1, which is within achievable concentrations in human tissues.
In our study, TGF-β1 suppressed proliferation of HT-29 cells but had no effect on Caco-2 cells, acting similarly to ciprofloxacin. These results are in accordance with already published data showing that in contrast to HT-29 cells, Caco-2 cells show a decreased expression of the two main TGF-β1 receptors, RI and RII. In addition, these receptors are located at the basolateral cell surface in this cell line (Biasi et al., 2002). There is a discrepancy between our results and the results reported by Zanetti et al. (2003) that demonstrate an apoptotic effect in Caco-2 cells after treatment with 10 ng·mL−1 of TGF-β1. In our hands, TGF-β1 had no effect on the growth of Caco-2 cells. In fact, we observed a trend towards higher rates of proliferation when higher concentrations of TGF-β1 were used. Additionally, we observed no change in Caco-2 apoptosis or necrosis with TGF-β1 treatment (data not shown).
The TGF-β family of peptides, acting in an autocrine or paracrine manner, can effectively inhibit growth of intestinal epithelium. In particular, TGF-β1 has been shown to inhibit cell growth and to induce apoptosis of normal colonic epithelial cell types (Elliott and Blobe, 2005). Specifically, TGF-β1 has been shown to activate cytostatic gene responses at any point in the cell-cycle phases G1 (primarily), S or G2 (Siegel and Massague, 2003). With regard to apoptosis, TGF-β acts on multiple pathways producing a variety of responses depending on the cell type (Bierie and Moses, 2006). It is thought that TGF-β1 exerts an inhibitory effect in the early stages of cancer, while in the later stages of cancer progression cancer cells escape from the inhibitory control of TGF-β1, to some extent, due to mutations on the TGF-β1 signal transduction pathways. Multiple effects of TGF-β1 in tissue homeostasis, like its effect on angiogenesis, can also affect tumour progression (Seoane, 2006).
The observation that both ciprofloxacin and TGF-β1 suppress proliferation of HT-29 cells led us to further examine a possible effect of ciprofloxacin on the production of TGF-β1. TGF-β1 production by HT-29 cells has been described previously (Kucharzik et al., 1997). In the present study, we demonstrated for first time that stimulation of HT-29 cells with ciprofloxacin increases TGF-β1 production. The effective concentration of ciprofloxacin was considerably higher than the expected levels of ciprofloxacin in tissues of treated patients (Naber et al., 1993; Weidekamm and Portmann, 1993; Madu et al., 1996). The ciprofloxacin-induced concentration of TGF-β1 seems to be less than the concentration needed to observe an effect on HT-29 proliferation. However, in order to interpret this result we have to bear in mind that the maximal anti-proliferative effect of ciprofloxacin was observed after 6 days of treatment, whereas the ciprofloxacin-induced TGF-β1 was measured after 2 days of treatment. This experimental design was considered necessary as it is impossible to perform a 6 day treatment experiment in the serum-free conditions needed to measure true TGF-β1 production. Therefore, the relevant concentrations of ciprofloxacin-induced TGF-β1 in these experiments cannot be estimated. Furthermore, possible differences between autocrine and paracrine actions of self-produced versus exogenously added TGF-β1 may also play a role. In order to establish a causal relationship between the ciprofloxacin anti-proliferative effect and the ciprofloxacin-induced TGF-β1 production, we showed that treatment of HT-29 cells with neutralizing TGF-β1 antibody in the presence of ciprofloxacin reversed the antiproliferative effect of ciprofloxacin at almost all concentrations used. Our observations suggest that ciprofloxacin exerts its anti-proliferative effect on HT-29 cells by increasing TGF-β1 production. Epithelial cell-derived TGF-β1 can then suppress cell proliferation acting in an autocrine or/and a paracrine way.
Our results indicate a possible antineoplastic activity of ciprofloxacin in the premalignant or early stages of colonic adenocarcinoma, when TGF-β-1 transduction pathways remain intact (Manning et al., 1991; Matsushita et al., 1999). On the other hand, inducing TGF-β receptors expression in Caco-2 cells, as previously reported (Chen et al., 2002), can lead to their sensitization to the antiproliferative effect of TGF-β1. Such a sensitization procedure may possibly alter the effect of ciprofloxacin on Caco-2 cell proliferation and theoretically, it could be used in some cases of advanced colonic neoplasias in order to induce the TGF-β1-depedent anti-proliferative effect of ciprofloxacin.
It has already been reported that ciprofloxacin has an additive effect with well-known chemotherapeutic agents in several human cancer cell lines, including head and neck cancer (Norris et al., 2001), colon cancer (Herold et al., 2003), prostate cancer (El-Rayes et al., 2002). In these in vitro experiments, ciprofloxacin either sensitized multiresistant cancer cells to chemotherapy or increased efficacy of the chemotherapeutic agent (Norris et al., 2001). In our work, we showed for first time an additive effect of ciprofloxacin on the anti-proliferative effect of 5-FU in colonic cancer cells. Our results warrant further investigation of the in vivo relevance of these findings, as they highlight a possible adjuvant role of ciprofloxacin in 5-FU regiments for TGF-β1-sensitive colonic cancers.
Several immunomodulatory activities of quinolones have recently been elucidated including regulation of several cytokines and other inflammatory mediators (Riesbeck, 2002; Dalhoff and Shalit, 2003; Kolios et al., 2006). Our work suggests a novel immunomodulatory effect of quinolones on a well-differentiated colon epithelial cell line without the prerequisite presence of a triggering inflammatory cytokine. The recent report that ciprofloxacin suppresses experimental colitis in mice suggests a possible in vivo anti-inflammatory effect of ciprofloxacin in non-infectious colitis (Lahat et al., 2007). In addition to reducing and modifying colonic bacterial load, induction of TGF-β1 by ciprofloxacin might be associated with the observed end result.
In conclusion, we demonstrated a new mechanism of action of ciprofloxacin that is the induction of TGFβ-1 by colonic epithelial cells, which concurs with the previously reported immunomodulatory and growth inhibitory effects of ciprofloxacin. Whether this mechanism of action can operate in vivo during non-infectious gut inflammation or colonic cancer is a matter that needs further investigation.
Glossary
Abbreviations:
- 5-FU
5-fluorouracil
- 7-AAD
7-amino-actinomycin D
- FBS
foetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenol tetrazolium bromide
- PBS
phosphate-buffered saline
- PI
propidium iodide
- RT-PCR
reverse transcription polymerase chain reaction
Conflict of interest
The authors state no conflict of interest.
Supporting information
Additional Supporting information may be found in the online version of this article:
Figure S1 Ciprofloxacin treatment had no effect on apoptosis or necrosis of HT-29 cells. Apoptosis and necrosis of HT-29 cells after treatment with 0 (vehicle), 50 and 100 μg·mL−1 ciprofloxacin, evaluated by analysis of DNA fragmentaction as described in Methods.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn.) 2008;153(Suppl.)(2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranha O, Wood DP, Jr, Sarkar FH. Ciprofloxacin mediated cell growth inhibition, S/G2-M cell cycle arrest, and apoptosis in a human transitional cell carcinoma of the bladder cell line. Clin Cancer Res. 2000;6:891–900. [PubMed] [Google Scholar]
- Aranha O, Zhu L, Alhasan S, Wood DP, Jr, Kuo TH, Sarkar FH. Role of mitochondria in ciprofloxacin induced apoptosis in bladder cancer cells. J Urol. 2002;167:1288–1294. [PubMed] [Google Scholar]
- Becker C, Fantini MC, Neurath MF. TGF-beta as a T cell regulator in colitis and colon cancer. Cytokine Growth Factor Rev. 2006;17:97–106. doi: 10.1016/j.cytogfr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Bergeron MG. The pharmacokinetics and tissue penetration of the fluoroquinolones. Clin Invest Med. 1989;12:20–27. [PubMed] [Google Scholar]
- Biasi F, Tessitore L, Zanetti D, Cutrin JC, Zingaro B, Chiarpotto E, et al. Associated changes of lipid peroxidation and transforming growth factor beta1 levels in human colon cancer during tumour progression. Gut. 2002;50:361–367. doi: 10.1136/gut.50.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- Chen A, Davis BH, Sitrin MD, Brasitus TA, Bissonnette M. Transforming growth factor-beta 1 signaling contributes to Caco-2 cell growth inhibition induced by 1,25(OH)(2)D(3) Am J Physiol Gastrointest Liver Physiol. 2002;283:G864–G874. doi: 10.1152/ajpgi.00524.2001. [DOI] [PubMed] [Google Scholar]
- Crump B, Wise R, Dent J. Pharmacokinetics and tissue penetration of ciprofloxacin. Antimicrob Agents Chemother. 1983;24:784–786. doi: 10.1128/aac.24.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalhoff A, Shalit I. Immunomodulatory effects of quinolones. Lancet Infect Dis. 2003;3:359–371. doi: 10.1016/s1473-3099(03)00658-3. [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- El-Rayes BF, Grignon R, Aslam N, Aranha O, Sarkar FH. Ciprofloxacin inhibits cell growth and synergises the effect of etoposide in hormone resistant prostate cancer cells. Int J Oncol. 2002;21:207–211. [PubMed] [Google Scholar]
- Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- Forsgren A, Schlossman SF, Tedder TF. 4-Quinolone drugs affect cell cycle progression and function of human lymphocytes in vitro. Antimicrob Agents Chemother. 1987;31:768–773. doi: 10.1128/aac.31.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GR. Antibiotics should be used as first-line therapy for Crohn's disease. Inflamm Bowel Dis. 2004;10:318–320. doi: 10.1097/00054725-200405000-00021. [DOI] [PubMed] [Google Scholar]
- Herold C, Ocker M, Ganslmayer M, Gerauer H, Hahn EG, Schuppan D. Ciprofloxacin induces apoptosis and inhibits proliferation of human colorectal carcinoma cells. Br J Cancer. 2002;86:443–448. doi: 10.1038/sj.bjc.6600079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold C, Ganslmayer M, Ocker M, Blauberger S, Zopf S, Hahn EG, et al. Overadditive anti-proliferative and pro-apoptotic effects of a combination therapy on colorectal carcinoma cells. Int J Oncol. 2003;23:751–756. [PubMed] [Google Scholar]
- Holtom PD, Pavkovic SA, Bravos PD, Patzakis MJ, Shepherd LE, Frenkel B. Inhibitory effects of the quinolone antibiotics trovafloxacin, ciprofloxacin, and levofloxacin on osteoblastic cells in vitro. J Orthop Res. 2000;18:721–727. doi: 10.1002/jor.1100180507. [DOI] [PubMed] [Google Scholar]
- Kamat AM, Lamm DL. Antitumor activity of common antibiotics against superficial bladder cancer. Urology. 2004;63:457–460. doi: 10.1016/j.urology.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Kolios G, Manousou P, Bourikas L, Notas G, Tsagarakis N, Mouzas I, et al. Ciprofloxacin inhibits cytokine-induced nitric oxide production in human colonic epithelium. Eur J Clin Invest. 2006;36:720–729. doi: 10.1111/j.1365-2362.2006.01710.x. [DOI] [PubMed] [Google Scholar]
- Kucharzik T, Lugering N, Winde G, Domschke W, Stoll R. Colon carcinoma cell lines stimulate monocytes and lamina propria mononuclear cells to produce IL-10. Clin Exp Immunol. 1997;110:296–302. doi: 10.1111/j.1365-2249.1997.tb08331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat G, Halperin D, Barazovsky E, Shalit I, Rabau M, Klausner J, et al. Immunomodulatory effects of ciprofloxacin in TNBS-induced colitis in mice. Inflamm Bowel Dis. 2007;13:557–565. doi: 10.1002/ibd.20077. [DOI] [PubMed] [Google Scholar]
- Lawrence JW, Claire DC, Weissig V, Rowe TC. Delayed cytotoxicity and cleavage of mitochondrial DNA in ciprofloxacin-treated mammalian cells. Mol Pharmacol. 1996;50:1178–1188. [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Madu AA, Mayers M, Perkins R, Liu W, Drusano GL, Aswani R, et al. Aqueous and vitreous penetration of ciprofloxacin following different modes of systemic administration. Exp Eye Res. 1996;63:129–136. doi: 10.1006/exer.1996.0101. [DOI] [PubMed] [Google Scholar]
- Manning AM, Williams AC, Game SM, Paraskeva C. Differential sensitivity of human colonic adenoma and carcinoma cells to transforming growth factor beta (TGF-beta): conversion of an adenoma cell line to a tumorigenic phenotype is accompanied by a reduced response to the inhibitory effects of TGF-beta. Oncogene. 1991;6:1471–1476. [PubMed] [Google Scholar]
- Matsushita M, Matsuzaki K, Date M, Watanabe T, Shibano K, Nakagawa T, et al. Down-regulation of TGF-beta receptors in human colorectal cancer: implications for cancer development. Br J Cancer. 1999;80:194–205. doi: 10.1038/sj.bjc.6690339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miclau T, Edin ML, Lester GE, Lindsey RW, Dahners LE. Effect of ciprofloxacin on the proliferation of osteoblast-like MG-63 human osteosarcoma cells in vitro. J Orthop Res. 1998;16:509–512. doi: 10.1002/jor.1100160417. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Naber KG, Sorgel F, Kinzig M, Weigel DM. Penetration of ciprofloxacin into prostatic fluid, ejaculate and seminal fluid in volunteers after an oral dose of 750 mg. J Urol. 1993;150:1718–1721. doi: 10.1016/s0022-5347(17)35877-9. [DOI] [PubMed] [Google Scholar]
- Nita ME, Nagawa H, Tominaga O, Tsuno N, Fujii S, Sasaki S, et al. 5-Fluorouracil induces apoptosis in human colon cancer cell lines with modulation of Bcl-2 family proteins. Br J Cancer. 1998;78:986–992. doi: 10.1038/bjc.1998.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris MD, Madafiglio J, Gilbert J, Marshall GM, Haber M. Reversal of multidrug resistance-associated protein-mediated drug resistance in cultured human neuroblastoma cells by the quinolone antibiotic difloxacin. Med Pediatr Oncol. 2001;36:177–180. doi: 10.1002/1096-911X(20010101)36:1<177::AID-MPO1042>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Oldfield EC, 3rd, Wallace MR. The role of antibiotics in the treatment of infectious diarrhea. Gastroenterol Clin North Am. 2001;30:817–836. doi: 10.1016/s0889-8553(05)70212-0. [DOI] [PubMed] [Google Scholar]
- Rabau M, Nyska A, Dayan D. In vitro effect of ciprofloxacin on HT-29 human colon carcinoma cell line: assessment of cell proliferation by thymidine uptake and silver nucleolar organizer regions (AgNOR) histomorphometry. Arch Toxicol. 1995;70:124–126. doi: 10.1007/BF02733673. [DOI] [PubMed] [Google Scholar]
- Riesbeck K. Immunomodulating activity of quinolones: review. J Chemother. 2002;14:3–12. doi: 10.1179/joc.2002.14.1.3. [DOI] [PubMed] [Google Scholar]
- Riesbeck K, Bredberg A, Forsgren A. Ciprofloxacin does not inhibit mitochondrial functions but other antibiotics do. Antimicrob Agents Chemother. 1990;34:167–169. doi: 10.1128/aac.34.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seay TM, Peretsman SJ, Dixon PS. Inhibition of human transitional cell carcinoma in vitro proliferation by fluoroquinolone antibiotics. J Urol. 1996;155:757–762. [PubMed] [Google Scholar]
- Seoane J. Escaping from the TGFbeta anti-proliferative control. Carcinogenesis. 2006;27:2148–2156. doi: 10.1093/carcin/bgl068. [DOI] [PubMed] [Google Scholar]
- Shah A, Lettieri J, Kaiser L, Echols R, Heller AH. Comparative pharmacokinetics and safety of ciprofloxacin 400 mg i.v. thrice daily versus 750 mg po twice daily. J Antimicrob Chemother. 1994;33:795–801. doi: 10.1093/jac/33.4.795. [DOI] [PubMed] [Google Scholar]
- Shen LL. Molecular mechanisms of DNA gyrase inhibition by quinolone antibacterials. Adv Pharmacol. 1994;29A:285–304. doi: 10.1016/s1054-3589(08)60550-5. [DOI] [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- Somekh E, Douer D, Shaked N, Rubinstein E. In vitro effects of ciprofloxacin and pefloxacin on growth of normal human hematopoietic progenitor cells and on leukemic cell lines. J Pharmacol Exp Ther. 1989;248:415–418. [PubMed] [Google Scholar]
- Valatas V, Kolios G, Manousou P, Notas G, Xidakis C, Diamantis I, et al. Octreotide regulates CC but not CXC LPS-induced chemokine secretion in rat Kupffer cells. Br J Pharmacol. 2004;141:477–487. doi: 10.1038/sj.bjp.0705633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidekamm E, Portmann R. Penetration of fleroxacin into body tissues and fluids. Am J Med. 1993;94:75S–80S. [PubMed] [Google Scholar]
- Wolfson JS, Hooper DC. Pharmacokinetics of quinolones: newer aspects. Eur J Clin Microbiol Infect Dis. 1991;10:267–274. doi: 10.1007/BF01967000. [DOI] [PubMed] [Google Scholar]
- Zanetti D, Poli G, Vizio B, Zingaro B, Chiarpotto E, Biasi F. 4-hydroxynonenal and transforming growth factor-beta1 expression in colon cancer. Mol Aspects Med. 2003;24:273–280. doi: 10.1016/s0098-2997(03)00022-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


