Abstract
Background and purpose:
Bisphosphonates (BPs) are highly effective inhibitors of bone resorption. Nitrogen-containing bisphosphonates (N-BPs), such as zoledronic acid, induce the formation of a novel ATP analogue (1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester triphosphoric acid; ApppI), as a consequence of the inhibition of farnesyl pyrophosphate synthase and the accumulation of isopentenyl pyrophosphate (IPP). ApppI induces apoptosis, as do comparable metabolites of non-nitrogen-containing bisphosphonates (non-N-BPs). In order to further evaluate a pharmacological role for ApppI, we obtained more detailed data on IPP/ApppI formation in vivo and in vitro. Additionally, zoledronic acid-induced ApppI formation from IPP was compared with the metabolism of clodronate (a non-N-BP) to adenosine 5′(β,γ-dichloromethylene) triphosphate (AppCCl2p).
Experimental approach:
After giving zoledronic acid in vivo to rabbits, IPP/ApppI formation and accumulation was assessed in isolated osteoclasts. The formation of ApppI from IPP was compared with the metabolism of clodronate in MCF-7 cells in vitro. IPP/ApppI and AppCCl2p levels in cell extracts were analysed by mass spectrometry.
Key results:
Isopentenyl pyrophosphate/ApppI were formed in osteoclasts in vivo, after a single, clinically relevant dose of zoledronic acid. Furthermore, exposure of MCF-7 cells in vitro to zoledronic acid at varying times and concentrations induced time- and dose-dependent accumulation of IPP/ApppI. One hour pulse treatment was sufficient to cause IPP accumulation and subsequent ApppI formation, or the metabolism of clodronate into AppCCl2p.
Conclusions and implications:
This study provided the first conclusive evidence that pro-apoptotic ApppI is a biologically significant molecule, and demonstrated that IPP/ApppI analysis is a sensitive tool for investigating pathways involved in BP action.
Keywords: AppCCl2p, ApppI, bisphosphonate, cellular uptake, clodronate, IPP, osteoclast, zoledronic acid
Introduction
Bisphosphonates (BPs) are pyrophosphate analogues that concentrate specifically in bone and are highly effective inhibitors of bone resorption (Flanagan and Chambers, 1991; Rogers et al., 2000). Hence, these pharmacological compounds are widely used in the treatment of skeletal diseases associated with high osteoclast activity and accelerated bone turnover, such as osteoporosis (Delmas, 2002) and Paget's disease (Roux and Dougados, 1999). Furthermore, BPs are effective inhibitors of tumour-induced bone resorption and have been shown to modify the progression of skeletal metastasis in several forms of cancer, especially breast cancer and myeloma (Coleman, 2004).
Bisphosphonates can be divided into two pharmacological classes based on their chemical structure and their molecular mechanism of action: the non-nitrogen-containing bisphosphonates (non-N-BPs) are incorporated into cytotoxic ATP analogues in cells, and the more potent nitrogen-containing bisphosphonates (N-BPs) act through inhibition of the intracellular mevalonate pathway. Specifically, low potency non-N-BPs, such as clodronate, inhibit osteoclast function via intracellular metabolism into non-hydrolyzable analogues of ATP catalyzed by aminoacyl-tRNA synthetase enzymes (Rogers et al., 1996; Auriola et al., 1997; Frith et al., 1997). Consequently, accumulation of the ATP analogue within the cytosol triggers apoptosis by inhibiting the mitochondrial ADP/ATP translocase (Lehenkari et al., 2002). For N-BPs, the primary intracellular target is the inhibition of the enzyme farnesyl pyrophosphate synthase (FPPS) in the mevalonate pathway (Luckman et al., 1998; Benford et al., 1999). The activity of FPPS is required for the post-translational prenylation of small GTPases, such as Ras and Rap. As prenylation is required for the localization of these GTPases to subcellular membranes, N-BPs disrupt the function of small GTPases that are essential for osteoclast activity and survival. On the other hand, recent studies suggest that the anti-resorptive effect of N-BPs on osteoclasts may actually be due to accumulation of unprenylated small GTPases in their active state, that is, causing inappropriate activation of downstream signaling pathways, rather than loss of the prenylated proteins (Dunford et al., 2006; Russell et al., 2007).
Our recent research has revealed an additional effect of N-BPs, combining both of the above mentioned mechanisms of action (Mönkkönen et al., 2006). We discovered that potent N-BPs induce the intracellular formation of another ATP analogue, 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester triphosphoric acid (ApppI), as a result of the inhibition of FPPS in the mevalonate pathway and consequent accumulation of intracellular isopentenyl pyrophosphate (IPP). ApppI formation from IPP may be catalyzed by the same metabolic pathway (i.e. aminoacyl-tRNA-synthetases) as the ATP analogues of non-N-BPs (Mönkkönen et al., 2008), but ApppI does not contain a BP in its structure. As observed with the metabolite of a non-N-BP, clodronate, adenosine 5′(β,γ-dichloromethylene) triphosphate (AppCCl2p; Lehenkari et al., 2002), ApppI induced direct apoptosis through blockade of mitochondrial adenine nucleotide translocase (ANT) (Mönkkönen et al., 2006).
Previously, we have detected ApppI formation following N-BP treatment not only in macrophages, osteoclasts and various cancer cells in vitro, but also in vivo in peritoneal macrophages after local injection of high doses of zoledronic acid (Mönkkönen et al., 2006; 2007; 2008). However, in order to establish the biological significance of ApppI, the primary aim of this study was to elucidate whether it could be detected in osteoclasts, the pharmacological target cell for BPs, after in vivo administration of zoledronic acid at a clinically relevant dose. Another aim was to further evaluate the pharmacological role of ApppI by obtaining detailed data on its formation in vitro. MCF-7 cells, instead of osteoclasts, were selected for in vitro studies as a cell model, because earlier we have used this cell line to evaluate the role of pro-apoptotic ATP analogues in the anti-tumour properties of BPs, and observed abundant BP-induced IPP/ApppI or AppCCl2p accumulation in these cells (Mönkkönen et al., 2008). We studied the dependence of IPP/ApppI formation on zoledronic acid concentration and the time course of IPP/ApppI formation after pulse and continuous treatment with zoledronic acid. Furthermore, as pro-apoptotic effects of ApppI seem to be similar to those of other ANT-inhibiting ATP analogues, the formation of ApppI from IPP induced by zoledronic acid was compared with the metabolism of clodronate to its ATP analogue, AppCCl2p. The data provide further evidence that the pro-apoptotic ApppI is a biologically important molecule in the action of N-BPs.
Methods
IPP/ApppI accumulation in osteoclasts in vivo
All animal care and experimental procedures complied with the NIH Guide for the Care and Use of Laboratory Animals. Rabbit osteoclasts were used as they have successfully been used to identify the formation of an methyleneadenosine 5′-triphosphate (AppCp) type metabolite of the non-N-BP clodronate (AppCCl2p) in vivo (Frith et al., 2001; Staal et al., 2003). Three-day-old rabbits were injected subcutaneously with 1 mg·kg−1 or 100 µg·kg−1 zoledronic acid in phosphate-buffered saline (PBS), or an equivalent volume of PBS alone. Each experiment involved one treated rabbit and one control rabbit. The osteoclasts were isolated 24 or 48 h after injection using a previously well-characterized method (Frith et al., 2001; Staal et al., 2003). Briefly, the rabbits were killed by overdose of halothane and long bones from each were removed and cleaned and then minced in 25 mL of serum-free α-MEM with L-glutamine (Life Technologies, Paisley, UK). The osteoclasts were released from the bone fragments by vortexing. The cell pellet was washed, resuspended in 1 mL of α-MEM containing 3.3 µg·mL−1 mouse anti-αvβ3 (23c6) antibody (Serotec AbD, Oxford, UK) and incubated at 37°C for 20 min. After incubation, the cells were washed twice in 1x PBS containing 0.1% (w/v) of bovine serum albumin (BSA). For separation of osteoclasts, the cell pellet was resuspended in 0.1% BSA in 1x PBS containing 2 × 107 anti-mouse IgG conjugated magnetic Dynal beads (Invitrogen, Paisley, UK), and incubated, with rotation, at 4°C for 30 min. After incubation, vitronectin receptor (VNR)-positive (osteoclast) and VNR-negative (non-osteoclast) cells were separated using a magnetic particle concentrator (Dynal). The erythrocytes within the non-osteoclast-fraction were then lysed in BD Pharm™ Lyse (BD Biosciences, Pharmingen, San Diego, CA, USA). As in previous studies (Frith et al., 2001), the purity of the osteoclast fraction was >90% multinucleated, VNR-positive cells. Finally, the osteoclast cells (with Dynal beads attached) and non-osteoclast cells were extracted with ice-cold acetonitrile (400 µL) and water (200 µL), and centrifuged at 14 000×g for 2 min at 4°C. The supernatants were transferred to fresh tubes and dried down in a SpeedVac concentrator (Stratech Scientific, London, UK) and stored at −70°C until mass spectrometric analysis. The methods for IPP/ApppI analysis and the protein content determinations are described below.
IPP/ApppI and AppCCl2p accumulation in MCF-7 cells in vitro
The experiments were performed using human oestrogen-dependent breast cancer cell line, MCF-7, obtained from the European Collection of Cell Cultures (Salisbury, UK). Cells were cultured at 37°C in RPMI-1640 medium supplemented with 10% fetal bovine serum and 100 IU·mL−1 penicillin-streptomycin in a 5% CO2 atmosphere. Cells were harvested using 0.25% trypsin. Following harvesting, cells were seeded in 6-well plates at a density of 1 × 106 cells per well and left to adhere overnight. Non-adherent cells were then removed and medium was replaced with treatment medium containing zoledronic acid or clodronate, or an equivalent volume of sterile PBS. For dose–response assay the cells were treated with 1, 10, 25, 50 or 100 µmol·L−1 of zoledronic acid for 24 h. For time-course experiments, two different treatment protocols were used: pulse and continuous treatment. For pulse treatment, the cells were exposed to BP for 1 h only, after which the drug was removed and replaced by fresh medium, and the samples were collected at 0, 1, 3, 6, 12, 18, 24 or 48 h. For continuous treatment, the cells were treated continuously with BP for 1, 3, 6, 12, 18, 24 or 48 h. Sample collection was performed by carefully scraping the cultured cells off from the wells and washing in ice-cold PBS. Cell extract preparations and protein content determinations were carried out as previously described (Mönkkönen et al., 2003). The viability of MCF-7 cells in dose–response assay and after pulse treatment with zoledronic acid was determined by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide (MTT) test (Mönkkönen et al., 2003).
For analysis, the evaporated cell extracts were redissolved in 150–200 µL of water containing 5 µmol·L−1 internal standard (AppCp) to compensate for the variability in ionization, and 0.25 mmol·L−1 phosphatase inhibitors (sodium fluoride and sodium orthovanadate) to prevent the degradation of IPP/ApppI. The molar amounts of IPP, ApppI, and AppCCl2p in cell extracts were determined by high pressure liquid chromatography (HPLC) negative ion electrospray ionization mass spectrometry (HPLC-ESI-MS) as previously described (Mönkkönen et al., 2007). Selected reaction monitoring (SRM) was used for analysis of the compounds in the sample and quantitation was based on the fragment ions characteristic to each molecule. The following transitions were monitored: m/z 245→159 for IPP, m/z 574→408 for ApppI, m/z 572→225 and m/z 574→227 for AppCCl2p (35Cl & 37Cl) and, m/z 504→406 for internal standard. The pattern of fragmentation for each sample was compared with that of the authentic standard. Standards were constructed by adding known amounts of synthetic IPP, ApppI or AppCCl2p to extracts from untreated cells. Quantitation was performed with LCquan 2.0 software (Thermo Finnigan), using the standard curve and the transitions mentioned above. Results shown are representative of at least three independent experiments (mean ± SEM).
Cellular uptake of BPs in vitro
Differences in cellular uptake of BPs could be a critical factor affecting formation of the ATP analogues. Therefore, in order to compare the kinetic profiles of drug uptake with that of ATP analogue formation in cells, the cellular uptake of [14C]-labelled BPs was assessed. The cells were treated with 25 µmol·L−1[14C] zoledronic acid or 500 µmol·L−1[14C] clodronate according to the two different time-course treatment protocols described above, except that the 18 h time point was excluded. After treatment, medium was recovered, the cells were rinsed five times with PBS solution and all wash-solutions were collected. Cell samples were harvested by carefully scraping the cells off the wells; two wells were pooled together, and washed in ice-cold PBS. Cell extract preparations and protein content determinations were carried out as previously described (Mönkkönen et al., 2003). For the radioactivity measurements, the soluble acetonitrile/water extracts were evaporated in a vacuum centrifuge and finally redissolved in 120 µl of Milli-Q water. Radioactivity of the cell medium, washes and cell extracts was determined by using liquid scintillation counting (Wallac Microbeta™ TriLux) after mixing with OptiPhase HiSase3 scintillation cocktail (Wallac). The level of drug uptake was quantified as molar amount of drug per mg protein. Results shown are representative of at least two independent experiments (mean ± SEM).
Statistical analysis
For dose–response and pulse treatment studies, one-way anova with Tukey's multiple comparison tests was used to assess significant differences in the cell viability or IPP/ApppI formation.
Materials
Unlabelled and [14C]-labelled zoledronic acid [2-(imidazol-1-yl)-hydroxy-ethylidene-1,1-bisphosphonic acid, disodium salt, 4.75 hydrate] were kindly provided by Novartis Pharma AG (Basel, Switzerland), and clodronate (dichloromethylene-1,1-bisphosphonate) and [14C]clodronate by Schering Oy (Bayer Schering Pharma AG, Berlin, Germany). Stock solutions of BPs were prepared in PBS (pH 7.4; Gibco, UK) and solutions were filter-sterilized before use. The clodronate metabolite (AppCCl2p) and ApppI were synthesized as previously described (Lehenkari et al., 2002; Mönkkönen et al., 2006). IPP and AppCp were purchased from Sigma (St. Louis, MO, USA). Sodium fluoride was from Riedel-de-Haën (Germany) and sodium orthovanadate from Sigma (St. Louis, MO, USA). HPLC-grade methanol was from J.T. Baker (Deventer, the Netherlands) and dimethylhexylamine was obtained from Sigma Aldrich, (Milwaukee, WI, USA). Cell culture reagents were from Biowhittaker (Cambrex Bio Science, Verviers, Belgium). All the reagents used were of analytical grade or better.
Results
Zoledronic acid induces IPP/ApppI formation in osteoclasts in vivo
In this study, IPP and ApppI were monitored by MS/MS detection in osteoclasts following a clinically relevant dose of zoledronic acid in vivo. Analysis was carried out on lysates from osteoclasts that had been isolated and purified from neonatal rabbits by immunomagnetic bead separation ex vivo following subcutaneous injection with 1 mg·kg−1 zoledronic acid (for 24 and 48 h), or 100 µg·kg−1 zoledronic acid (for 24 h). For detection, we have previously developed a highly sensitive technique to identify ATP analogues in cell extracts, combining HPLC and tandem MS (Auriola et al., 1997). Using this procedure, the formation of IPP/ApppI was identified in extracts by monitoring the daughter fragment ions in the SRM mode. Typical SRM chromatograms of osteoclast extract isolated from untreated rabbit, osteoclast extract isolated from zoledronic acid-treated rabbit, and IPP and ApppI standards are shown in Figure 1. Major components were not detected in the chromatogram of osteoclast extracts generated from saline injected rabbits (Figure 1A and B). The peaks that eluted at 6.47 min (IPP) and 6.49 min (ApppI) were detected in SMR chromatograms of osteoclast extracts from zoledronic acid-treated rabbits (Figure 1C and D). Similar peaks were present in the chromatograms of osteoclast lysates from untreated rabbits, with added synthesized IPP and ApppI (Figure 1E and F).
Figure 1.
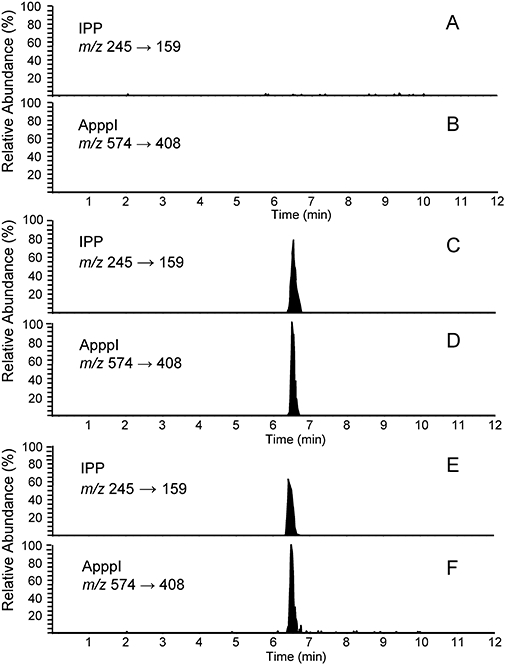
Identification of IPP and ApppI in rabbit osteoclasts in vivo. Vitronectin receptor-positive osteoclasts were isolated by immunomagnetic bead separation from rabbit bone marrow 24 h after single injection with 100 µg·kg−1 zoledronic acid (ZOL). Acetonitrile cell extracts were then analysed by HPLC-ESI-MS. Selective reaction monitoring chromatogram of osteoclast extract isolated from a saline injected rabbit (A, B), extract isolated from zoledronic acid-treated rabbit (C, D), untreated rabbit osteoclast extract with added 37 nmol·mg−1 protein IPP (E) and 11 nmol·mg−1 protein ApppI (F). The detection limits for IPP and ApppI are 2 pmol·mg−1 protein and 0.2 pmol·mg−1 protein respectively. No IPP or ApppI were detected in the chromatogram of osteoclast extracts generated from saline injected rabbits. The chromatograms are drawn on the same scale. ApppI, triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester; ESI, electrospray ionization; HPLC, high pressure liquid chromatography; IPP, isopentenyl pyrophosphate; MS, mass spectrometry.
Identical patterns of fragmentation obtained with authentic IPP and ApppI were identified from lysates of osteoclasts isolated from zoledronic acid-treated rabbits (Figure 2). The molecular ions of IPP (m/z 245) and ApppI (m/z 574) were fragmented, by CID, to form ions of m/z 79, 159, 177 and 227 (IPP) (Figure 2A and B), m/z 227 and 408 (ApppI) (Figure 2B and C). For internal standard, AppCp, two major fragment ions, m/z 486 and m/z 406 were observed (data not shown). The quantitation of IPP and ApppI was based on fragment ions, m/z 245→159 for IPP, m/z 574→408 for ApppI. The molar amounts of IPP and ApppI in each osteoclast fraction are presented in Figure 3. The maximum amount of IPP/ApppI that could be detected in osteoclast extracts isolated after 24 h of single injection with the higher dose (1 mg·kg−1) of zoledronic acid, corresponding to 360 pmol·mg−1 protein of IPP and 100 pmol·mg−1 protein of ApppI, followed by two and a half-fold decrease of IPP and fourfold decrease of ApppI at 48 h post-injection. After 24 h, 21.7% of the IPP was converted to ApppI. In addition, the same pattern was seen after treatment with a clinically relevant dose (100 µg·kg−1) of zoledronic acidedronic acid, corresponding to 21.5% conversion of IPP to ApppI. A minor amount of IPP/ApppI could also be detected in the non-osteoclast (VNR-negative) fractions (data not shown), probably due to contamination of this fraction with osteoclasts. These data confirm that zoledronic acid induces IPP/ApppI formation in osteoclasts in vivo after injection of a single clinical dose, 100 µg·kg−1, roughly equivalent to the 4 mg dose given to patients.
Figure 2.
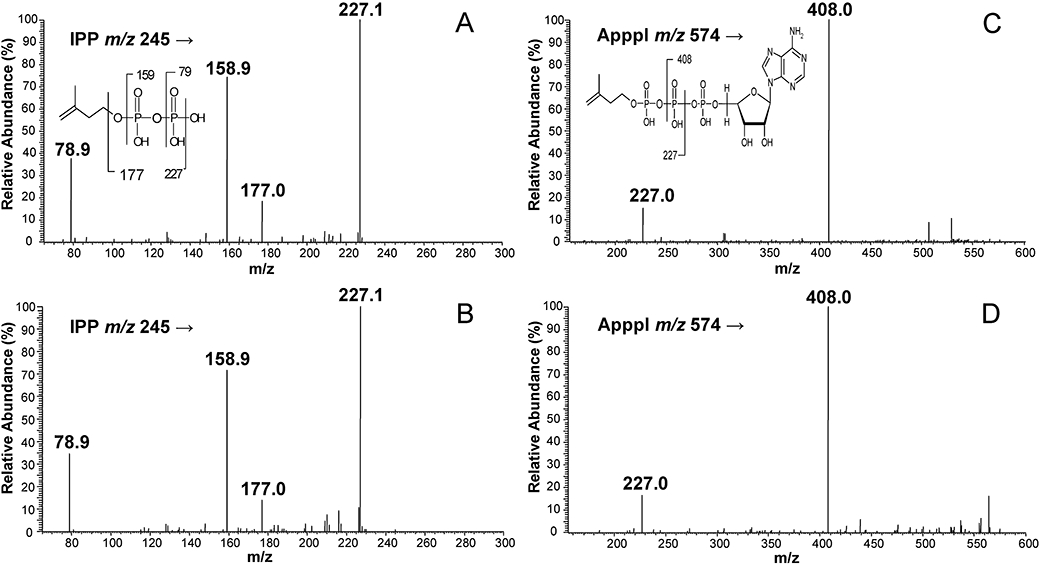
Mass spectrometric identification of IPP and ApppI. MS/MS spectra of IPP from the peak of Figure 1C (A) and Figure 1E (B), MS/MS spectra of ApppI from the peak of Figure 1D (C) and Figure 1F (D). m/z = mass-to-charge ratio. ApppI, triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester; IPP, isopentenyl pyrophosphate; MS, mass spectrometry.
Figure 3.
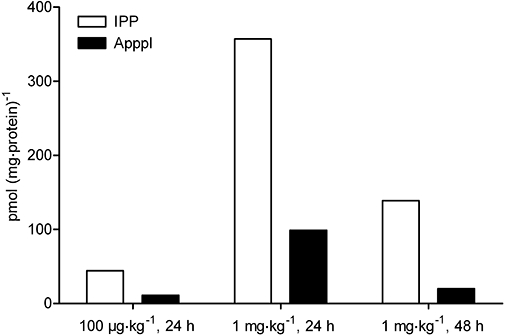
IPP/ApppI concentrations (pmol·mg−1 protein) in each osteoclast fraction from zoledronic acid (ZOL)-treated rabbits. Animals were injected subcutaneously with 100 µg·kg−1 or 1 mg·kg−1 zoledronic acid in PBS. The osteoclasts were isolated by immunomagnetic bead separation 24 or 48 h after injection. The molar amounts of IPP/ApppI in acetonitrile cell extracts were determined by HPLC-ESI-MS. ApppI, triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester; ESI, electrospray ionization; HPLC, high pressure liquid chromatography; IPP, isopentenyl pyrophosphate; MS, mass spectrometry; PBS, phosphate-buffered saline.
Zoledronic acid induces dose-dependent IPP/ApppI formation in MCF-7 cells in vitro
To investigate the dependence of IPP/ApppI formation on zoledronic acid concentration, MCF-7 cells were incubated with 1–100 µmol·L−1 zoledronic acid for 24 h. At 1–50 µmol·L−1 concentration, zoledronic acid induced dose-dependent IPP accumulation and ApppI formation in cells (Figure 4A). However, treatment of cells with 25 µmol·L−1 zoledronic acid for 24 h resulted in a statistically non-significant increase in IPP/ApppI formation compared with the value after incubation with 10 µmol·L−1 zoledronic acid. In addition, zoledronic acid at a high concentration of 100 µmol·L−1 did not induce more IPP/ApppI compared with 50 µmol·L−1 zoledronic acid, thus the maximum inhibition of FPPS and subsequent IPP/ApppI accumulation in cells was achieved with 50 µmol·L−1 of zoledronic acid. However, both concentrations, 50 and 100 µmol·L−1, significantly (P < 0.001) reduced the viability of MCF-7 cells by 17% and 28%, respectively, when assessed by the MTT test (Figure 4B).
Figure 4.
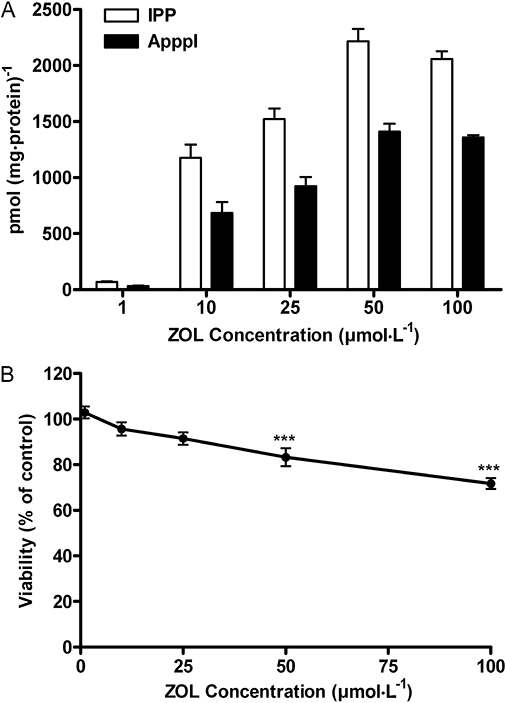
Effect of 1-100 µmol·L−1 zoledronic acid (ZOL) on IPP/ApppI formation (A), and the cell viability (B) of confluent MCF-7 cells after 24 h, assessed by mass spectrometry and MTT test respectively. (mean ± SEM, n= 9).***P < 0.001 compared with control. ApppI, triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester; IPP, isopentenyl pyrophosphate; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide.
IPP/ApppI and AppCCl2p formation, and cellular uptake of [14C] BPs in MCF-7 cells after pulse treatment in vitro
Zoledronic acid and clodronate were used to compare the kinetics of formation of IPP/ApppI and AppCCl2p respectively. Pulse exposure to BP for 1 h, either 25 µmol·L−1 zoledronic acid or 500 µmol·L−1 clodronate, was sufficient to induce IPP/ApppI or AppCCl2p accumulation, respectively, in MCF-7 breast cancer cells during the observation period of 0–48 h (Figure 5A and B). ApppI levels correlated well with the increase in the IPP concentration in cells, confirming that ApppI is a result of IPP accumulation in cells after FPPS inhibition. ApppI formation from IPP could be detected immediately after drug removal (0 h), although it was close to the detection limit (0.2 pmol·mg−1 protein). The amount of IPP and ApppI increased gradually up to 12 h, and reached the maximum level of IPP (49.2 pmol·mg−1 protein) and ApppI (8.7 pmol·mg−1 protein), then decreased until end of the experiment. The decrease in levels of IPP/ApppI after 12 h of drug removal was not a consequence of cell death as treatment did not affect the viability of confluent MCF-7 cells, assessed by MTT assay (data not shown). By contrast to the formation of IPP/ApppI, the metabolism of clodronate into AppCCl2p in cells reached the maximum level (21 pmol·mg−1 protein) immediately after drug removal, followed by a considerable decrease of metabolite at 1 h after drug exposure (Figure 5B). However, the amount of the metabolite increased again up to 12 h and then decreased gradually until the end of the experiment.
Figure 5.
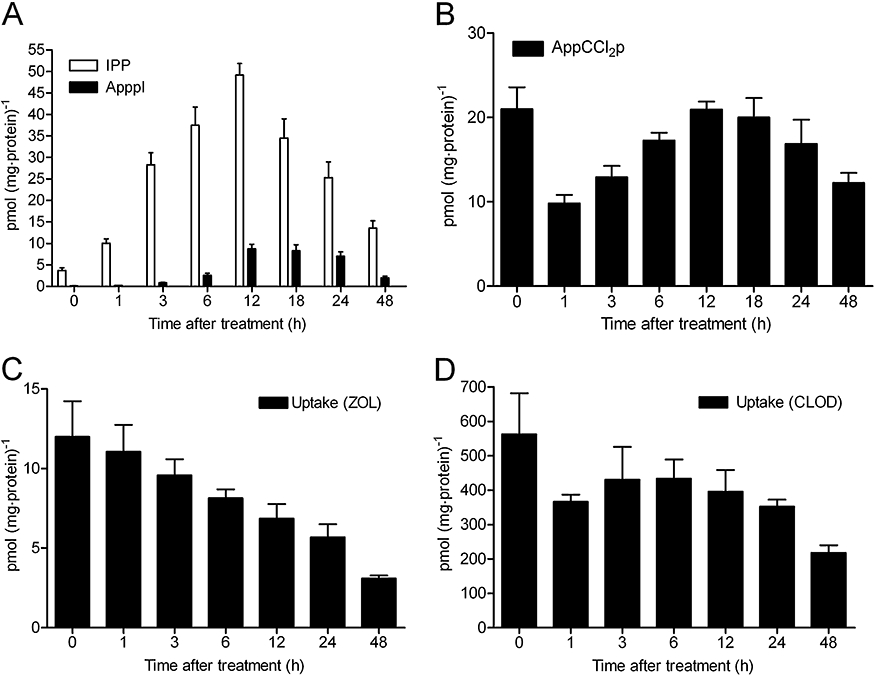
Zoledronic acid (ZOL)-induced IPP/ApppI formation (A), accumulation of AppCCl2p (B), the cellular uptake of zoledronic acid (C) and clodronate (CLOD; D), in confluent MCF-7 cells after a pulse exposure to BPs. The MCF-7 cells were treated with 25 µmol·L−1 ZOL or 500 µmol·L−1 CLOD for 1 h, and the samples were collected 0–48 h after drug removal. The intracellular concentration of BPs was determined by comparing the radioactivity of medium, washes and cell extracts relative to amount of protein in the cell extract. The molar amounts of IPP/ApppI or AppCCl2p were determined in acetonitrile cell extracts by using HPLC-ESI-MS (mean ± SEM, n= 4–8). ApppI, triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester; AppCCl2p, adenosine 5′(β,γ-dichloromethylene) triphosphate; BP, bisphosphonate; ESI, electrospray ionization; HPLC, high pressure liquid chromatography; IPP, isopentenyl pyrophosphate; MS, mass spectrometry.
The maximum amount of [14C]-labelled BPs (12 pmol·mg−1 protein of zoledronic acid and 562 pmol·mg−1 protein of clodronate) was detected in cell extracts immediately after drug removal (Figure 5C and D). After this, the amount of zoledronic acid in cells decreased constantly until the end of observation time at 48 h (Figure 5C). The cellular uptake of clodronate after pulse treatment differed substantially from the cellular uptake of zoledronic acid. This was illustrated by the kinetic profile of the clodronate uptake, which was closely similar to the profile seen in clodronate metabolism (Figure 5B and D). Although, the differences between the time points of 1–48 h were not significant. In both cases of BP uptake, zoledronic acid and clodronate were still found in cells after 48 h.
IPP/ApppI and AppCCl2p formation, and cellular uptake of [14C] BPs in MCF-7 cells after continuous treatment in vitro
For continuous drug treatment, the cells were treated with 25 µmol·L−1 zoledronic acid or 500 µmol·L−1 clodronate for 1–48 h. IPP and ApppI, and AppCCl2p were detectable after 1 h of exposure of the cells to BP, although the observed amount of ApppI was around detection limit and thus not visible in the figure (Figure 6A and B). The amount of IPP/ApppI and AppCCl2p increased gradually and time-dependently during the exposure. Therefore, the highest intracellular concentrations were achieved at 48 h of BP exposure, and corresponded to 1624 pmol·mg−1 protein of IPP, 472 pmol·mg−1 protein of ApppI and 758 pmol·mg−1 protein of AppCCl2p, in cells.
Figure 6.
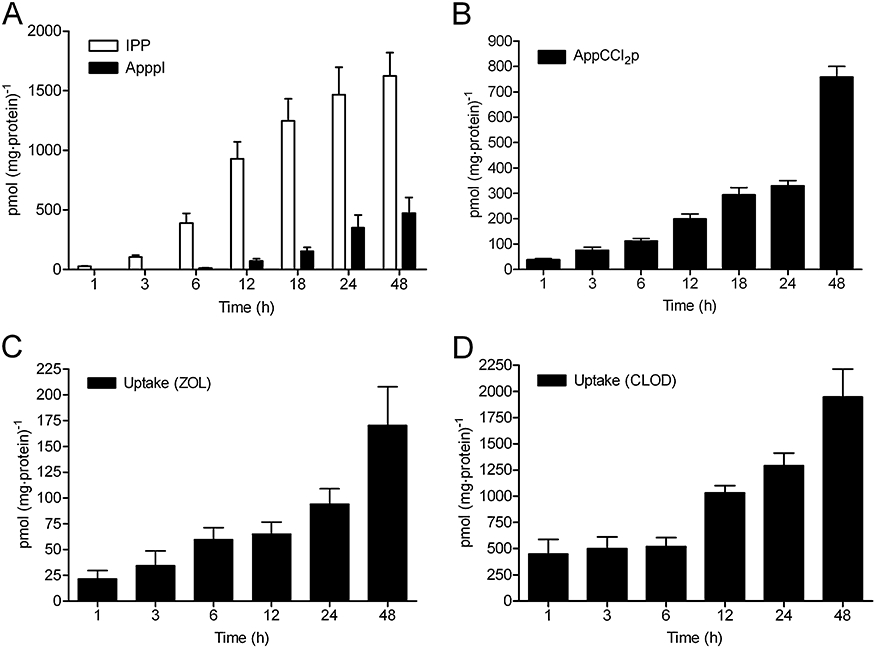
Zoledronic acid(ZOL)-induced IPP/ApppI formation (A), accumulation of AppCCl2p (B), the cellular uptake of zoledronic acid (C) and clodronate (CLOD; D), in confluent MCF-7 cells. The cells were treated with 25 µmol·L−1 ZOL or 500 µmol·L−1 CLOD continuously for 1–48 h. The intracellular concentration of BPs was determined by comparing the radioactivity of medium, washes and cell extracts relative to amount of protein in the cell extract. The molar amount of IPP/ApppI or AppCCl2p were determined in acetonitrile cell extracts by using HPLC-ESI-MS (mean ± SEM, n= 4–8). ApppI, triphosphoric acid 1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester; AppCCl2p, adenosine 5′(β,γ-dichloromethylene) triphosphate; BP, bisphosphonate; ESI, electrospray ionization; HPLC, high pressure liquid chromatography; IPP, isopentenyl pyrophosphate; MS, mass spectrometry.
The amount of [14C]-labelled BPs taken up by the cells increased gradually during 1–48 h of continuous exposure (Figure 6C and D). Therefore, the highest intracellular amounts of zoledronic acid (170 pmol·mg−1 protein) and clodronate (1946 pmol·mg−1 protein) were detected at 48 h, after treatment with 25 µmol·L−1 zoledronic acid and 500 µmol·L−1 clodronate respectively.
Discussion
Bisphosphonates are the most potent inhibitors of bone resorption and target selectively to bone mineral, where they are released and internalized specifically by osteoclasts (Sato et al., 1991; Frith et al., 2001). The non-N-BPs are incorporated into cytotoxic metabolites in cells (Auriola et al., 1997; Frith et al., 1997), while the more potent N-BPs inhibit FPPS in the intracellular mevalonate pathway (Amin et al., 1992), both mechanisms ultimately leading to osteoclast apoptosis. Recently, we discovered that N-BPs, such as zoledronic acid, induce the formation of a novel ATP analogue ApppI as a consequence of the inhibition of FPPS, and the subsequent accumulation of IPP (Mönkkönen et al., 2006). As observed for AppCCl2p, the comparable metabolite of clodronate, ApppI, is a pharmacologically active compound capable of inducing apoptosis in osteoclasts and therefore we sought more detailed data on its formation.
In this study, we show that ApppI was formed in rabbit osteoclasts following a single dose of zoledronic acid in vivo. Earlier, ApppI has been detected in peritoneal macrophages in vivo after local injection of a high dose of zoledronic acid (Mönkkönen et al., 2007), but this is the first published study to show the presence of pro-apoptotic ApppI in osteoclasts, the pharmacological target cell for BPs, in vivo. Importantly, ApppI formation was detected when zoledronic acid was given at the dose of 100 µg·kg−1, roughly equivalent to a 4 mg single dose given to patients (Daubine et al., 2007). Although bone turnover and metabolism are different in humans and rabbits, the result indicated that ApppI was formed in osteoclasts in vivo after a clinically relevant dose of zoledronic acid. IPP/ApppI accumulation in osteoclasts was lower at 48 h post-injection by comparison with that at 24 h post-injection, perhaps due to possible changes in the activity of the metabolizing enzymes in the mevalonate pathway, or to apoptosis of ApppI-containing osteoclasts. Additionally, minor amounts of IPP/ApppI were also detected in non-osteoclast cell fractions (data not shown), which could have been a contamination from osteoclasts and thus not a true effect of zoledronic acid on non-osteoclast cells. This hypothesis is also supported by earlier in vivo data demonstrating that N-BPs have a very low or no detectable effect on protein prenylation in non-osteoclast bone cells when given in vivo at doses that cause a robust inhibition of protein prenylation in osteoclasts (Frith et al., 2001; Coxon et al., 2005). However, on the basis of this study, the possibility that cell types other than osteoclasts in the bone microenvironment can internalize BPs and consequently accumulate IPP/ApppI cannot be excluded.
As shown earlier (Mönkkönen et al., 2008), MCF-7 cells produced high amounts of both IPP and ApppI, and were thus an appropriate model to characterize the kinetics of ApppI formation reliably. Treatment of MCF-7 cells with increasing concentrations of zoledronic acid induced IPP/ApppI formation in a dose-dependent manner up to 50 µmol·L−1 concentration. However, as zoledronic acid at high concentrations of 50 and 100 µmol·L−1 reduced cell viability, 25 µmol·L−1 zoledronic acid was chosen for further time-course studies. Time-course studies on the continuous treatment with 25 µmol·L−1 zoledronic acid or 500 µmol·L−1 clodronate demonstrated that both BPs and IPP/ApppI or AppCCl2p accumulate in cells in a time-dependent manner. These results together with the data obtained after in vivo treatment (with 1 mg·kg−1 zoledronic acid) illustrate that after 24 h, approximately 20% of IPP was converted to ApppI in cells. Interestingly, at the same time point, a similar amount (26%) of internalized clodronate was metabolized to AppCCl2p, suggesting that the same metabolizing enzymes with same capacity might be responsible for the ApppI formation from IPP, and for clodronate metabolism. Additionally, time-course results revealed that 1 h pulse treatment with BP was sufficient to cause IPP accumulation and subsequent ApppI or AppCCl2p formation in cells during the entire observation time. However, the accumulation profile of AppCCl2p after a 1 h exposure with clodronate differed from that seen with zoledronic acid-induced IPP/ApppI accumulation. In contrast to IPP/ApppI, the formation of clodronate metabolite attained the maximum intracellular amount immediately after drug removal, but then unexpectedly decreased to the next observation time point. Differences between the profiles were to some extent expected, as formation of AppCCl2p from clodronate via aminoacyl-tRNA synthetases is more straightforward and more rapid than that of ApppI, which is formed after inhibition of FPPS in the mevalonate pathway, by zoledronic acid. As metabolism of clodronate depends on cellular uptake, drug uptake was thought to account for the abrupt reduction in accumulation profile of AppCCl2p. Although the clodronate uptake profile was found to be similar to that of the metabolite accumulation profile, it could not reliably account for the observation, and thus warrants further studies.
Similar formation profiles of IPP/ApppI were observed both in vivo, after a single injection of 1 mg·kg−1 zoledronic acid, and in vitro after a pulse exposure. In these studies, IPP levels were observed to decrease significantly after 24 h of treatment, although cellular uptake studies in vitro showed that there were still considerable amounts of zoledronic acid present in cells 48 h after the initial exposure. This was a rather surprising result, as IPP binds and further stabilizes the inhibited FPPS-N-BP complex in a closed conformation, leading to sustained inhibition of the enzyme (Rondeau et al., 2006; Dunford et al., 2008). However, the results in this study suggest that FPPS might be partly restored even in the presence of intracellular zoledronic acid. Previously, similar results have been obtained in a cholesterol biosynthesis study where 4 h treatment of PC-3 cells with 20 µmol·L−1 zoledronic acid completely inhibited cholesterol biosynthesis, but as early as 24 h later, about 10% of the biosynthesis was rescued, reaching 90% after 48 h (Goffinet et al., 2006). It is conceivable that the expression of FPPS is elevated through increased protein synthesis, or that feedback inhibition by IPP on upstream enzymes leads to decreased flux through the mevalonate pathway (and hence decreased IPP synthesis). There are no known alternative pathways for the production of FPP from IPP in mammalian cells (Edwards and Ericsson, 1999). However, some studies have postulated that IPP could be recycled back to HMG-CoA and eventually to acetyl-CoA by a sequence of reactions (Edmond and Popjak, 1974; Brown and Goldstein, 1980).
In this study, we obtained more detailed information of zoledronic acid-induced IPP/ApppI formation in vivo and in vitro and provide the first conclusive evidence that pro-apoptotic ApppI is formed in osteoclasts in vivo, even after a single clinically relevant dose of zoledronic acid. This result is of considerable importance, as it establishes the biological significance of this molecule. Both in vivo and in vitro data further support the proposition that ApppI formation results from the accumulation of IPP after FPPS inhibition. However, both studies reveal that some changes in the activity of the metabolizing enzymes in the mevalonate pathway might occur fairly shortly after drug exposure. This observation needs to be studied further, as it could indicate that in order to achieve more efficacious treatment, such as in cancer therapy, continuous or frequent pulse treatment with BPs is needed. This hypothesis is supported by previous in vivo findings demonstrating that treatment of animals with a single clinical dose did not inhibit tumour burden, whereas a low dose of zoledronic acid administered to animals on a daily or weekly intermittent schedule not only inhibits bone destruction, but also exhibits anti-tumour effects (Daubine et al., 2007; Stresing et al., 2007).
Isopentenyl pyrophosphate/ApppI analysis was shown to be a useful and sensitive tool for investigating pathways involved in BP action. In addition, identification of the molecular mechanism of action of ApppI may help in further understanding the differences and similarities between N-BPs and non-N-BPs in general. Furthermore, ApppI studies have revealed the potential of N-BPs to affect a wide range of cellular processes via the inhibition of FPPS, and may also help to explain the observed preclinical and clinical activity of these compounds. Further studies to clarify the relationship between IPP/ApppI, cancer cell apoptosis and γ,δ-T-cell activation are in progress.
Acknowledgments
This work was financially supported by the Academy of Finland, Finnish Cultural Foundation and Oskar Huttunen Foundation. The skilful technical assistance Mrs Lea Pirskanen is gratefully acknowledged. We thank Novartis Pharma AG (Switzerland) and Schering AG (Germany) for their kind gift of zoledronic acid and clodronate, respectively.
Glossary
Abbreviations:
- ApppI
1-adenosin-5′-yl ester 3-(3-methylbut-3-enyl) ester triphosphoric acid
- AppCCl2p
adenosine 5′(β,γ-dichloromethylene) triphosphate
- BP
bisphosphonate
- ESI
electrospray ionization
- FPP
farnesyl pyrophosphate
- IPP
isopentenyl pyrophosphate
- MS
mass spectrometry
Conflict of interest
The authors state no conflict of interest.
References
- Amin D, Cornell SA, Gustafson SK, Needle SJ, Ullrich JW, Bilder GE, et al. Bisphosphonates used for the treatment of bone disorders inhibit squalene synthase and cholesterol biosynthesis. J Lipid Res. 1992;33:1657–1663. [PubMed] [Google Scholar]
- Auriola S, Frith J, Rogers MJ, Koivuniemi A, Mönkkönen J. Identification of adenine nucleotide-containing metabolites of bisphosphonate drugs using ion-pair liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl. 1997;704:187–195. doi: 10.1016/s0378-4347(97)00490-8. [DOI] [PubMed] [Google Scholar]
- Benford HL, Frith JC, Auriola S, Mönkkönen J, Rogers MJ. Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol Pharmacol. 1999;56:131–140. doi: 10.1124/mol.56.1.131. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980;21:505–517. [PubMed] [Google Scholar]
- Coleman RE. The role of bisphosphonates in breast cancer. Breast. 2004;13(Suppl.)(1):S19–S28. doi: 10.1016/j.breast.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Coxon FP, Ebetino FH, Mules EH, Seabra MC, McKenna CE, Rogers MJ. Phosphonocarboxylate inhibitors of rab geranylgeranyl transferase disrupt the prenylation and membrane localization of rab proteins in osteoclasts in vitro and in vivo. Bone. 2005;37:349–358. doi: 10.1016/j.bone.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Daubine F, Le Gall C, Gasser J, Green J, Clezardin P. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst. 2007;99:322–330. doi: 10.1093/jnci/djk054. [DOI] [PubMed] [Google Scholar]
- Delmas PD. Treatment of postmenopausal osteoporosis. Lancet. 2002;359:2018–2026. doi: 10.1016/S0140-6736(02)08827-X. [DOI] [PubMed] [Google Scholar]
- Dunford JE, Rogers MJ, Ebetino FH, Phipps RJ, Coxon FP. Inhibition of protein prenylation by bisphosphonates causes sustained activation of rac, Cdc42, and rho GTPases. J Bone Miner Res. 2006;21:684–694. doi: 10.1359/jbmr.060118. [DOI] [PubMed] [Google Scholar]
- Dunford JE, Kwaasi AA, Rogers MJ, Barnett BL, Ebetino FH, Russell RGG, et al. Structure-activity relationships among the nitrogen-containing bisphosphonates in clinical use, and other analogues: time-dependent inhibition of farnesyl pyrophosphate synthase. J Med Chem. 2008;51:2187–2195. doi: 10.1021/jm7015733. [DOI] [PubMed] [Google Scholar]
- Edmond J, Popjak G. Transfer of carbon atoms from mevalonate to n-fatty acids. J Biol Chem. 1974;249:66–71. [PubMed] [Google Scholar]
- Edwards PA, Ericsson J. Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu Rev Biochem. 1999;68:157–185. doi: 10.1146/annurev.biochem.68.1.157. [DOI] [PubMed] [Google Scholar]
- Flanagan AM, Chambers TJ. Inhibition of bone resorption by bisphosphonates: interactions between bisphosphonates, osteoclasts, and bone. Calcif Tissue Int. 1991;49:407–415. doi: 10.1007/BF02555852. [DOI] [PubMed] [Google Scholar]
- Frith JC, Mönkkönen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res. 1997;12:1358–1367. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- Frith JC, Mönkkönen J, Auriola S, Mönkkönen H, Rogers MJ. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum. 2001;44:2201–2210. doi: 10.1002/1529-0131(200109)44:9<2201::aid-art374>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Goffinet M, Thoulouzan M, Pradines A, Lajoie-Mazenc I, Weinbaum C, Faye JC, et al. Zoledronic acid treatment impairs protein geranyl-geranylation for biological effects in prostatic cells. BMC Cancer. 2006;6:60. doi: 10.1186/1471-2407-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehenkari PP, Kellinsalmi M, Napankangas JP, Ylitalo KV, Mönkkönen J, Rogers MJ, et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002;61:1255–1262. doi: 10.1124/mol.61.5.1255. [DOI] [PubMed] [Google Scholar]
- Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- Mönkkönen H, Tormalehto S, Asunmaa K, Niemi R, Auriola S, Vepsäläinen J, et al. Cellular uptake and metabolism of clodronate and its derivatives in caco-2 cells: a possible correlation with bisphosphonate-induced gastrointestinal side-effects. Eur J Pharm Sci. 2003;19:23–29. doi: 10.1016/s0928-0987(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Mönkkönen H, Auriola S, Lehenkari P, Kellinsalmi M, Hassinen IE, Vepsäläinen J, et al. A new endogenous ATP analog (ApppI) inhibits the mitochondrial adenine nucleotide translocase (ANT) and is responsible for the apoptosis induced by nitrogen-containing bisphosphonates. Br J Pharmacol. 2006;147:437–445. doi: 10.1038/sj.bjp.0706628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mönkkönen H, Ottewell PD, Kuokkanen J, Mönkkönen J, Auriola S, Holen I. Zoledronic acid-induced IPP/ApppI production in vivo. Life Sci. 2007;81:1066–1070. doi: 10.1016/j.lfs.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Mönkkönen H, Kuokkanen J, Holen I, Evans A, Lefley D, Jauhiainen M, et al. Bisphosphonate–induced ATP analog formation and its effect on inhibition of cancer cell growth. Anticancer Drugs. 2008;19:391–399. doi: 10.1097/CAD.0b013e3282f632bf. [DOI] [PubMed] [Google Scholar]
- Rogers MJ, Brown RJ, Hodkin V, Blackburn GM, Russell RG, Watts DJ. Bisphosphonates are incorporated into adenine nucleotides by human aminoacyl-tRNA synthetase enzymes. Biochem Biophys Res Commun. 1996;224:863–869. doi: 10.1006/bbrc.1996.1113. [DOI] [PubMed] [Google Scholar]
- Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Mönkkönen J, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- Rondeau JM, Bitsch F, Bourgier E, Geiser M, Hemmig R, Kroemer M, et al. Structural basis for the exceptional in vivo efficacy of bisphosphonate drugs. Chem Med Chem. 2006;1:267–273. doi: 10.1002/cmdc.200500059. [DOI] [PubMed] [Google Scholar]
- Roux C, Dougados M. Treatment of patients with Paget's disease of bone. Drugs. 1999;58:823–830. doi: 10.2165/00003495-199958050-00005. [DOI] [PubMed] [Google Scholar]
- Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, et al. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88:2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal A, Frith JC, French MH, Swartz J, Harrity TW, Tamasi J, et al. The ability of statins to inhibit bone resorption is directly related to their inhibitory effect on HMG-CoA reductase activity. J Bone Miner Res. 2003;18:88–96. doi: 10.1359/jbmr.2003.18.1.88. [DOI] [PubMed] [Google Scholar]
- Stresing V, Daubine F, Benzaid I, Mönkkönen H, Clezardin P. Bisphosphonates in cancer therapy. Cancer Lett. 2007;257:16–35. doi: 10.1016/j.canlet.2007.07.007. [DOI] [PubMed] [Google Scholar]


