Abstract
Background and purpose:
Elevated plasma total homocysteine (tHcy) level has been established as an independent risk factor for cardiovascular diseases. Danshensu, an active ingredient of Salvia miltiorrhiza, shows wide cardiovascular benefit. However, in terms of its own methylation, danshensu could elevate tHcy level, which would act against its cardiovascular benefit, thus posing a ‘therapeutic paradox’. As this paradox has not been fully assessed, we have evaluated the effects of danshensu on tHcy levels to uncover the underlying mechanisms.
Experiment approach:
We evaluated the influence of danshensu on homocysteine (Hcy) metabolism in rats with normal tHcy levels and in rat models of elevated tHcy (single intravenous methionine loading model and a hyperhomocysteinemic model after 3 weeks methionine dosing, with and without 3 weeks of danshensu treatment). We also quantified some metabolic intermediates (S-adenosyl methionine, S-adenosyl-l-homocysteine, cysteine and glutathione) relevant to Hcy metabolism in rat liver and kidney.
Key results:
Acute treatment with a single dose of danshensu in rats with normal tHcy did not change plasma tHcy. In contrast, danshensu significantly lowered tHcy in rats with elevated tHcy. The relatively higher cysteine and glutathione levels after treatment with danshensu indicated that its tHcy-lowering effect was via increased activity of the trans-sulphuration pathway.
Conclusions and implications:
Our results suggested that danshensu may act both acutely to increase trans-sulphuration and after chronic exposure to up-regulate the activity of the trans-sulphuration enzymes. The tHcy-lowering effect of danshensu is another cardiovascular benefit provided by S. miltiorrhiza and suggests a potential tHcy-lowering therapy.
Keywords: danshensu, homocysteine, polyphenols, Salvia miltiorrhiza, trans-sulphuration
Introduction
Elevated plasma total homocysteine (tHcy) level has been established as an independent risk factor for cardiovascular diseases. Several epidemiological studies had shown that moderately elevated plasma tHcy level was associated with an increased risk of fatal and non-fatal cardiovascular diseases (Nygard et al., 1997; Welch and Loscalzo, 1998; Wald et al., 2002). Homocysteine (Hcy) is a thiol containing amino acid formed by demethylation of methionine. Hcy can be further metabolized via two major pathways: (i) remethylated to methionine by methionine synthase; and (ii) trans-sulphurated to cysteine by cystathionine β-synthase and subsequently by cystathionine γ-synthase. Regulation of Hcy level is achieved by changing the activities of the two competitive pathways (Jacobs et al., 2001). Metabolism of Hcy via the trans-sulphuration pathway provides an endogenous route for cysteine synthesis, which is a significant source of glutathione (GSH) (Figure 1) (Martinov et al., 2000) and the importance of the Hcy-dependent trans-sulphuration pathway in the maintenance of the intracellular GSH pool and redox status has been shown (Vitvitsky et al., 2003).
Figure 1.
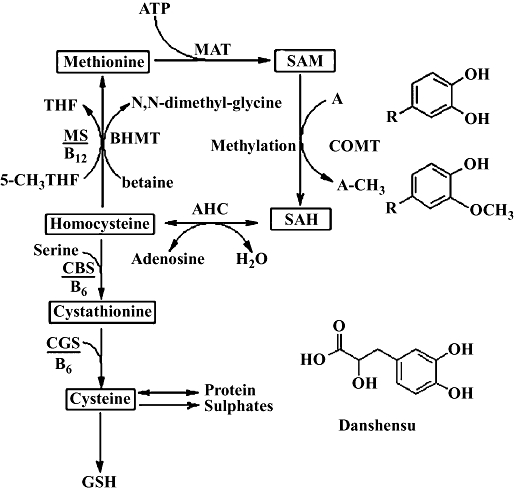
A simplified scheme of homocysteine metabolism and the relationship with polyphenol methylation. The methylation of a polyphenol by COMT is accompanied by conversion of SAM to SAH. The structure of danshensu (3,4-dihydroxyphenyl lactic acid) is shown at the bottom right hand corner; R represents -CH2CHOHCOOH. MAT, methionine adenosyltransferase; SAM, S-adenosylmethionine; A, methylation substrate; A-CH3, methylated substrate; COMT, catechol-O-methyltransferase; SAH, S-adenosylhomocysteine; AHC, S-adenosylhomocysteinase; MS, methionine synthase; BHMT, betaine homocysteine methyltransferase; CBS, cystathionine β-synthase; CGS, cystathionine γ-synthase.
Salvia miltiorrhiza has been widely used in China, Korea, Japan and other Asian countries, in the treatment of cardiovascular and cerebrovascular diseases. The beneficial effect of S. miltiorrhiza on cardiovascular system has been extensively documented (Cheng, 2007; Wu et al., 2007a; Han et al., 2008). Danshensu is a relatively simple polyphenol (3,4-dihydroxyphenyl lactic acid), found as an active component of extracts of S. miltiorrhiza, exhibiting cardiovascular protective effects (Wu et al., 2007b; Zhao et al., 2008). Moreover, danshensu was the most potent constituent of aqueous extracts of S. miltiorrhiza, protecting against Hcy-induced endothelial dysfunction (Chan et al., 2004). However, danshensu, like other polyphenols, is itself a substrate for endogenous methylating enzyme systems and thus consumes methionine and generates Hcy (see Figure 1). Treatment with danshensu could therefore elevate plasma tHcy level, which might cause cardiovascular toxicology. This possibility of raised plasma tHcy after danshensu might thus pose a ‘therapeutic paradox’, relative to its wide-ranging cardiovascular protection.
Until now, the effect of danshensu on plasma tHcy level has never been reported. Although the effect of some polyphenols on plasma tHcy has been reported (de Bree et al., 2001; Hodgson et al., 2003; 2006; Hamelet et al., 2007), no clear conclusion has been reached. Recently, it was reported that catechin, a typical polyphenol, lowered plasma tHcy level, by via enhancing the rate of Hcy catabolism (Hamelet et al., 2007). The aim of the present paper is to assess the influence of danshensu on plasma tHcy level and provide a clear explanation for the possible ‘therapeutic paradox’ and previous inconsistent results with polyphenols.
A typical hyperhomocysteinemia (HHcy) model, in which Hcy remethylation is inhibited by the deficiency of folate, is commonly used for evaluating tHcy-lowering therapies (Huang et al., 2001). However, the general health of animals gets is considerably worse after more than 4 weeks, during the development of this HHcy model (Yagisawa et al., 2004). Thus, in order to comprehensively and accurately evaluate the influence of danshensu on Hcy metabolism, we conducted a series of experiments with other models. These included the single methionine loading model (Yagisawa et al., 2004), a 3-week methionine dosing model (Fukada et al., 2006), a hyperhomocysteinemic rat model (Zhou et al., 2001), with and without 3 weeks of danshensu treatment. Moreover, in order to further uncover the underlying mechanism, metabolic intermediates related to Hcy metabolism [S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), cysteine and GSH] were quantified in liver and kidney, two important tissues for Hcy metabolism. The results suggested that a single dose of danshensu did not acutely affect plasma tHcy level in rats with normal tHcy level, while acute or chronic treatment with danshensu significantly suppressed plasma tHcy in the rats with elevated tHcy. We therefore suggest that danshensu may be a potential tHcy-lowering drug, which has shown wide cardiovascular benefit and may avoid the nutritional problems accompanying present treatments for HHcy (Bonaa et al., 2006; Lonn et al., 2006).
Methods
Animals
All animal experiments were performed under a license granted by Jiangsu Science and Technology Office (China), with approval from the Animal Ethics Committee of China Pharmaceutical University. Every effort was made to minimize stress to rats. Male Sprague Dawley rats weighing 180–250 g were used throughout this study. They were housed at 22 ± 5°C with a 12-h dark/light cycle. All rats were provided with water and standard chow ad libitum for 1 week before experiments.
Experiments
All chemicals were dissolved in saline, except for tolcapone which was dissolved in saline containing 20% (v/v) PEG 200. During experiments, rats were fasted overnight and randomly assigned into different groups. About 200 µL blood was taken from orbital sinus alternatively after ethyl ether anaesthesia, and then the eye was quickly sterilized with alcohol and pressed with cotton. Blood samples were immediately collected into a polypropylene tube containing heparin-Na and centrifuged at 5000 g at 5°C for 3 min. Prepared plasma samples were kept at −20°C and analysed within 48 h.
Experiment 1: effect of i.v. danshensu on plasma tHcy in normal rats
Rats were assigned into three groups (n= 5): control treated with saline; intravenous (i.v.) danshensu (20 mg·kg−1); i.v. danshensu (20 mg·kg−1), 10 min after injection (i.p.) of tolcapone (10 mg·kg−1). Tolcapone is a known catechol-O-methyltransferase (COMT) inhibitor and can inhibit the methylation of danshensu. Blood was collected before and after 5, 15, 30, 60, 120 and 240 min of drug injection.
Experiment 2: effect of danshensu on plasma tHcy after a single i.v. methionine loading dose
Rats were assigned into four groups (n= 5): control treated with saline; i.v. methionine (0.8 mmol·kg−1); i.v. methionine (0.8 mmol·kg−1) plus danshensu (10 or 20 mg·kg−1). Blood was collected before and after 5, 15, 30, 60, 120 and 240 min of drug injection.
Experiment 3: effect of i.p. danshensu on plasma tHcy in rats during 3 weeks methionine dosing model
We conducted this experiment in order to assess the long-time effect of danshensu on plasma tHcy level with and without methionine dosing. Rats were assigned into four groups: control treated with saline (n= 5); i.p. danshensu (n= 10, 5 mg·kg−1·day−1); i.p. methionine (n= 10, 0.8 mmol·kg−1·day−1); combined methionine and danshensu, at the above dose (n= 6). During the experiment, blood was collected on the 5th, 7th, 9th, 11th, 19th and 21st day, before the daily drug injections.
Experiment 4: effect of single danshensu treatment on plasma tHcy in rats with hyperhomocysteinemia induced by methionine
The hyperhomocysteinemic (HHcy) rats (Durand et al., 1997) were the rats from the methionine group in experiment 3. After 3-week methionine (0.8 mmol·kg−1·day−1, i.p.) treatment and 24 h washout, 10 rats were fasted overnight and assigned into two groups: control with saline (n= 5); i.v. danshensu (n= 5, 20 mg·kg−1). The blood was collected before and after 5, 15, 30, 60, 120 and 240 min of drug (danshensu/saline) injection.
Experiment 5: effects of 3 weeks of danshensu treatment on Hcy metabolism
The rats were from the danshensu group in experiment 3. After 3-week danshensu treatment (5 mg·kg−1·day−1, i.p.) and 3-day washout, 10 rats were divided into two groups: control with saline (n= 5); i.v. methionine (n= 5, 0.8 mmol·kg−1). The blood was collected before and after 5, 15, 30, 60, 120 and 240 min of drug (methionine/saline) injection.
Experiment 6: analysis of metabolic intermediates related to Hcy in liver and kidney
In order to uncover the underlying mechanism of the effect of danshensu, we quantified the Hcy metabolism-related intermediates in liver and kidney. Rats were assigned into four groups: control treated with saline (n= 5); i.p. danshensu (n= 5, 5 mg·kg−1·day−1); i.p. methionine (n= 5, 0.8 mmol·kg−1·day−1); co-treatment with methionine and danshensu at the above dose (n= 5). The rats were treated for 3 weeks as above and fasted overnight on day 22. On day 23, rats were killed by cervical dislocation. The liver and kidney were immediately removed and stored at −20°C. Then the metabolic intermediates, SAM, SAH, cysteine and GSH were all quantified according to the methods described below.
All these above experiments were summarized in Table 1.
Table 1.
Summary of the experimental conditions
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Experiment 1 (mg·kg−1) | Control saline | Danshensu (20) | Danshensu (20) | |
| Tolcapone (10) | ||||
| Experiment 2 (mg·kg−1) | Control saline | Methionine (0.8) | Methionine (0.8) | Methionine (0.8) |
| Danshensu (10) | Danshensu (20) | |||
| Experiment 3 (mg·kg−1·day−1) | Control saline | Danshensu (5) | Methionine (0.8) | Methionine (0.8) |
| n= 10 | n= 10 | Danshensu (5) | ||
| Experiment 4 (mg·kg−1) | Control saline | Danshensu (20) | ||
| Experiment 5 | Control saline | Methionine (0.8) | ||
| Experiment 6 (mg·kg−1·day−1) | Control saline | Danshensu (5) | Methionine (0.8) | Methionine (0.8) |
| Danshensu (5) |
Numbers in parentheses denote doses; for danshensu and tolcapone these are as mg·kg−1; for methionine, as mmol·kg−1. Experiments 1, 2, 4 and 5 involved a single i.v. dose of danshensu or methionine; experiments 3 and 6 involved chronic treatment with danshensu methionine or both given i.p., once daily for 3 weeks; n= 5 for all the groups except when otherwise indicated.
Analytical methods
The plasma tHcy concentration was determined according to previous method with minor modification (Katrusiak et al., 2001). Plasma samples (100 µL) were treated with 20 µL of dithiothreitol (DTT) (2 mmol·L−1, dissolved in 0.5 mol·L−1 phosphate buffer, pH 8.0) for 10 min at 25°C to reduce the disulphides and release protein-bound Hcy. Then it was mixed with 100 µL of 0.5 mmol·L−1 5,5'-dithio-bis-nitrobenzoic acid (DTNB) dissolved in the same buffer and approximately after 10 min deproteinization was achieved by the addition of 100 µL of 9% sulph osalicylic acid. Precipitated proteins were removed by centrifugation at 10 000×g for 10 min and the supernatant was used for high performance liquid chromatography (HPLC) analysis (Shimadzu 2010C). Chromatography of the sulphydryl-DTNB derivatives was accomplished using isocratic elution on Shimadzu C18 column (150 mm × 2.0 mm, 5 µm) at 40°C. Mobile phase A was methanol at 8% and B was water phase at 92% containing 0.1 mol·L−1 KH2PO4 (pH 3.8). The flow rate was 1.0 mL·min−1 and the sample injection volume was 10 µL.
For determination of SAM and SAH in tissues, we followed a previous method with minor modification (Kim et al., 2003). One gram of fresh liver (or kidney) was homogenized in 2 mL of 5% perchloric acid. The homogenate was centrifuged at 12 000×g for 10 min. The resulting supernatant was collected for HPLC analysis exactly as described by Kim et al. (2003).
Tissue cysteine was quantified by the acid-ninhydrin method (Gaitonde, 1967). To determine tissue tHcy, the tissues were homogenized in 0.5 mol·L−1 phosphate buffer (pH 8.0) at 1:2 ratios (v/v) and then treated as the plasma samples. GSH determination was carried out as described by Abdel-Zaher et al. (2008).
Data analysis
All results were expressed as the mean ± SD. Comparison of the means was made using a two-tailed Student's t-test. The AUC was tested after logarithmic transformation. The acceptable level of significance was established at P < 0.05 except when otherwise indicated.
Materials
Danshensu (purity 99%) was purchased from QingZe (Nanjing, China), DL-homocysteine, reduced GSH, DTNB, DTT, heptane sulphonic acid, sulphosalicylic acid, SAM and SAH were all obtained from Sigma-Aldrich (Shanghai, China). Methionine, cysteine, ninhydrin and polyethylene glycol 200 (PEG 200) were all purchased from WanQing (Nanjing, China).
Results
Experiment 1: effect of i.v. danshensu on plasma tHcy in normal rats
As shown in Figure 2, following the i.v. injection of danshensu, the mean values of plasma tHcy increased slightly but returned to baseline at about 2 h. However, both these mean values and the area under the plasma tHcy concentration curve over the 4 h post-danshensu observation period (ΔAUC0–4 h) showed great inter-individual variations (CV % of ΔAUC0–4 h was 102%). Thus, none of these data were significantly different from the initial and final values and no statistical difference for danshensu on plasma tHcy was recorded. As indicated in Figure 2, pretreatment with the COMT inhibitor, tolcapone, decreased the variability of plasma tHcy values after danshensu. The methylation of danshensu, measured directly in other experiments, also showed great inter-individual variation (data not shown). Thus, the highly variable effects of danshensu on plasma tHcy might be explained by large individual variations in methylation by COMT.
Figure 2.
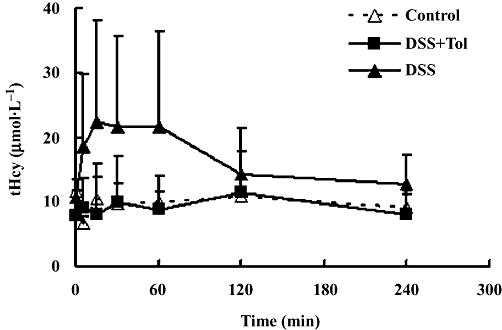
Time course of plasma levels of total homocysteine (tHcy) and the effects of danshensu (DSS). Control represents rats treated by i.v. saline; DSS, for rats given i.v. DSS 20 mg·kg−1, DSS + Tol for rats given i.v. DSS 20 mg·kg−1, 10 min after tolcapone i.p. 10 mg·kg−1 (dissolved in 20% PEG 200). Values are means ± SD, n= 5 for each groups.
Experiment 2: effect of danshensu on plasma tHcy in single i.v. methionine loading
As shown previously (Yagisawa et al., 2004), plasma tHcy was elevated acutely and significantly after methionine loading. As summarized in Figure 3A, the elevation of plasma tHcy and the (ΔAUC0–4 h; Table 2) following methionine, was significantly attenuated by danshensu in a dose-dependent manner.
Figure 3.
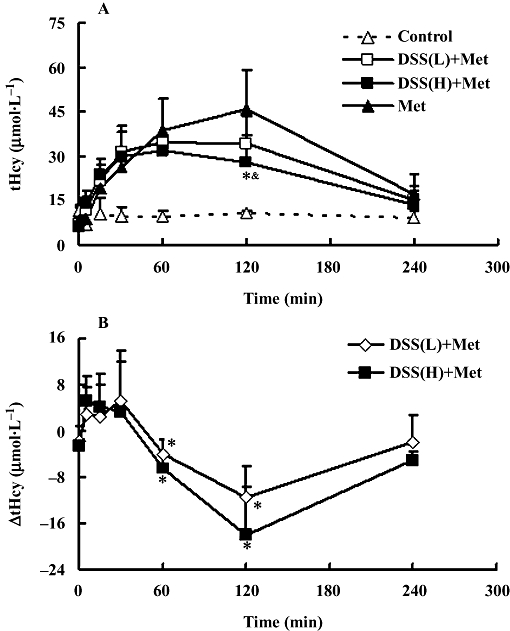
(A) Time course of plasma levels of total homocysteine (tHcy) in rats treated with i.v. methionine (Met) or methionine plus danshensu (DSS). Control represents rats i.v. saline, Met, rats were given i.v. 0.8 mmol·kg−1 methionine; DSS(L) + Met and DSS(H) + Met, rats were given Met i.v. 0.8 mmol·kg−1 plus danshensu 10 mg·kg−1 (L) or 20 mg·kg−1 (H) respectively. (B) The values on the Y axis (ΔtHcy) represent the difference in tHcy level between the Met group and the corresponding tHcy in DSS(L) + Met or DSS(H) + Met. Values are means ± SD, n= 5 for each groups. &P < 0.05, compared with Met group. *P < 0.05, compared with control (A) or zero (B).
Table 2.
Some pharmacokinetic parameters of plasma total homocysteine (tHcy) in rats treated with danshensu (DSS) and methionine (Met)
| Parameters | DSS | Met | DSS(L)+Met | DSS(H)+Met | pre-DSS+Met |
|---|---|---|---|---|---|
| Cmax (µmol·L−1) | 27 ± 16 | 45 ± 12 | 37 ± 4* (18%)a | 34 ± 3* (24%)a | 25 ± 10** (44%)a |
| Tmax (min) | 28 ± 19 | 96 ± 33 | 96 ± 33 | 78 ± 40 | 87 ± 48 |
| ΔAUC0–4 h (µmol·L−1·min) | 1744 ± 1791 | 5449 ± 1156 | 4277 ± 874* (22%)a | 3352 ± 893*▾(35%)a | 1129 ± 1142** (79%)a |
P < 0.05
P < 0.01 compared with group Met.
P < 0.05 compared with group DSS(L) + Met (Student's t-test). Values in parentheses for the AUV represent the % reduction, relative to the value with Met alone = 100%.
DSS, rats were given i.v. danshensu alone (20 mg·kg−1). Met, rats were given i.v. methionine alone (0.8 mmol·kg−1). DSS(H) + Met, rats were given i.v. methionine (0.8 mmol·kg−1) with high dose DSS (20 mg·kg−1). DSS(L) + Met, rats were given i.v. methionine (0.8 mmol·kg−1) with low dose DSS (10 mg·kg−1). pre-DSS + Met, rats were given i.p. danshensu 5 mg·kg−1·day−1 for 3 weeks and washed out (no treatment) for 3 days, then i.v. methionine (0.8 mmol·kg−1).
Each value is the mean ± SD for five rats except for pre-DSS + Met (n= 4 rats).
In Figure 3B, we have recalculated the data as differences between treated [DSS(L) + Met; DSS(H) + Met] and the control (Met) to expose a possible bidirectional effect on plasma tHcy in methionine loaded rats. Although only the value for the higher dose of danshensu at 120 min showed a statistically significant difference, there was a trend towards an initial rapid and short-lived rise in plasma tHcy followed by a longer lasting fall.
Experiment 3: effect of i.p. danshensu on plasma tHcy level in rats during 3 weeks methionine dosing
As indicated in Figure 4, 3 weeks of treatment with danshensu alone (i.p.; 5 mg·kg−1·day−1) did not affect plasma tHcy. However, daily dosing with methionine alone over the same time did increase plasma tHcy. Adding treatment with danshensu to the daily dose of methionine, largely suppressed the increase in plasma tHcy (Figure 4), which was consistent with our results after a single methionine load (see above).
Figure 4.
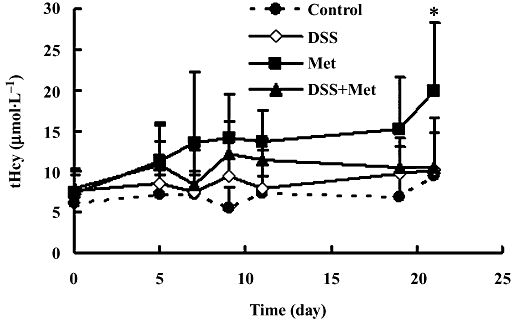
Time course of plasma levels of total homocysteine (tHcy) during chronic treatment. Rats were treated with i.p. saline (Control, n= 4), methionine (Met, 0.8 mmol·kg−1·day−1), danshensu (DSS, 5 mg·kg−1·day−1) or danshensu (5 mg·kg−1·day−1) plus methionine (0.8 mmol·kg−1·day−1) (DSS + Met), for 3 weeks. Values are means ± SD, n= 5 for each groups. *P < 0.05, compared with Control group.
Experiment 4: effect of danshensu on plasma tHcy in rats with hyperhomocysteinemia induced by methionine
Our model of HHcy was induced by 3-week treatment with daily methionine (see methods and above) and in these HHcy rats, we re-tested the effects of a single i.v. dose of danshensu. As shown in Figure 5, danshensu now caused a clear and marked fall in plasma tHcy, maintained for at least 4 h after the danshensu. As danshensu did not affect plasma Hcy in normal rats (Figure 2), its effects on plasma tHcy may only be detectable when plasma tHcy is raised.
Figure 5.
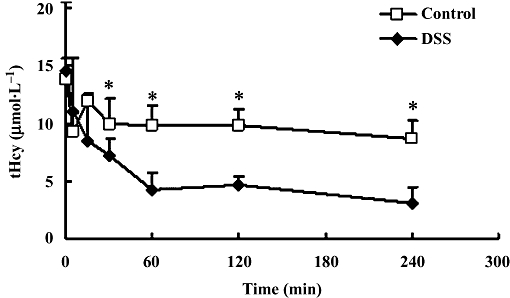
Time course of plasma levels of total homocysteine (tHcy) in rats with hyperhomocysteinemia (HHcy) after single injections of i.v. 20 mg·kg−1 danshensu (DSS) and saline (Control). Values are means ± SD, n= 5 for each groups. *P < 0.05.
Experiment 5: Hcy metabolism after 3-week chronic treatment with danshensu
Here we have re-tested the effects of a single methionine loading dose in rats chronically treated with danshensu (for 3 weeks; see Methods). The clear rise in plasma tHcy up to 2 h after methionine, as already shown in Figure 3A, was completely suppressed in rats chronically treated with danshensu (Figure 6). Also, the ΔAUC0–4 h was only 19% of that without danshensu pretreatment in experiment 1 (Table 2). It is relevant to note that these danshensu treated rats have normal plasma tHcy and that the methionine load was given 3 days after the end of danshensu treatment, that is 3 days of washout, in which time any circulating danshensu should be lost.
Figure 6.
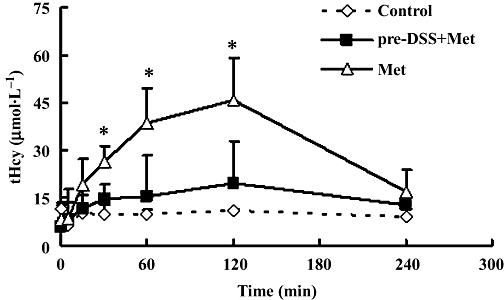
Time course of plasma levels of total homocysteine (tHcy) in rats given 21 days danshensu pretreatment (pre-DSS + Met). Plasma tHcy was raised by a single i.v. injection of 0.8 mmol·kg−1 methionine in pre-DSS rats or in rats with no treatment (Met). Control group were given i.v. saline. Values are means ± SD, n= 5 for each groups. *P < 0.05, compared with pre-DSS + Met group.
Experiment 6: analysis of metabolic intermediates related to Hcy in liver and kidney
The tissue levels of Hcy-related metabolic intermediates are governed by the relative activity of the remethylation or the trans-sulphuration pathways of Hcy metabolism, especially in liver, the central tissue for sulphur amino acid metabolism (Stead et al., 2000).
Compared with control (Table 3), chronic treatment with methionine (3 weeks daily i.p. injections) induced a considerable elevation of tissue tHcy which was markedly suppressed by danshensu (Table 3), in liver and kidney, consistent with the results in plasma.
Table 3.
Concentrations of metabolic intermediates related to homocysteine (tHcy) metabolism in liver and kidney of rats
| Groups | Weight gain |
Liver |
SAM/SAH |
Kidney |
SAM/SAH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tHcy | Cysteine | GSH | SAM | SAH | tHcy | Cysteine | GSH | SAM | SAH | ||||
| g | nmol·g−1 | mmol·g−1 | µg·g−1 | nmol·g−1 | mmol·g−1 | µg·g−1 | |||||||
| Control | 77.4 ± 6.7 | 9.0 ± 2.8 | 66.3 ± 19.1 | 7.0 ± 0.8 | 22.4 ± 2.9 | 5.4 ± 0.4 | 4.1 ± 0.7 | 0.7 ± 0.2 | 22.4 ± 5.0 | 2.8 ± 0.2 | 7.1 ± 2.4 | 4.3 ± 0.5 | 2.0 ± 0.6 |
| DSS | 73.0 ± 30.1 | 10.2 ± 4.2 | 96.5 ± 29.7* | 8.4 ± 0.5* | 18.4 ± 2.5 | 8.6 ± 5.2 | 2.2 ± 0.6* | 0.8 ± 0.2 | 37.4 ± 3.8* | 3.2 ± 0.2* | 5.4 ± 1.5* | 3.2 ± 1.5 | 1.9 ± 0.8 |
| Met | 64.8 ± 9.8 | 13.0 ± 1.2* | 135.7 ± 8.5* | 7.6 ± 0.9 | 55.5 ± 7.8* | 11.67 ± 3.0* | 7.6 ± 3.5* | 0.9 ± 0.2* | 25.9 ± 4.5 | 3.1 ± 0.4* | 7.9 ± 1.7 | 3.9 ± 0.3 | 2.8 ± 0.4* |
| Met + DSS | 89.5 ± 24.4 | 10.0 ± 1.6# | 163.9 ± 9.1*# | 8.9 ± 0.6# | 86.3 ± 16.4*# | 9.4 ± 3.6* | 5.9 ± 1.6* | 0.7 ± 0.1# | 26.9 ± 4.8 | 3.8 ± 0.2# | 6.5 ± 1.7 | 3.1 ± 0.9*# | 2.1 ± 0.1# |
P < 0.05 compared with control group.
P < 0.05 compared with Met group; Student's t-test.
Each value is the mean ± SD for five rats. Weight gain in the increase in rat weight the experimental period of 21 days. Control group, rats were given i.p. saline. DSS, rats were given i.p. danshensu 5 mg·kg−1·day−1 for 3 weeks. Met, rats were given i.p. methionine 0.8 mmol·kg−1·day−1 for 3 weeks. Met + DSS, rats were given i.p. Met 0.8 mmol·kg−1·day−1 with DSS 5mgkg−1·day−1 for 3 weeks.
SAM, S-adenosylmethionine; SAH, S-adenosyl-l-homocysteine; tHcy, total homocysteine; GSH, reduced glutathione.
In liver, chronic treatment with danshensu (Table 3) markedly elevated cysteine but had no significant influence on SAM, SAH and the SAM/SAH ratio. After chronic treatment with methionine, liver cysteine, SAM and SAH but not GSH were all raised (Table 3) and combining chronic treatments raised cysteine and SAM still further with a clear increase in GSH.
In the same rats, kidney levels of cysteine and GSH were increased by chronic treatment with danshensu (Table 3). However, danshensu lowered SAM without affecting SAH and the SAM/SAH ratio, findings different from those in liver. Chronic methionine alone did not change SAM and SAH levels but raised the SAM/SAH ratio, which indicated that methionine may enhance the methylation potential of danshensu in kidney (Zhang et al., 2008). Adding danshensu to methionine for 3 weeks had two major effects in kidney; the SAM/SAH ratio was restored to normal and GSH was raised.
In summary, chronic treatment with danshensu, either alone or in combination with methionine, raised both cysteine and GSH levels in liver and kidney, which would be compatible with an increased activity of the trans-sulphuration pathway, induced by danshensu.
Discussion and conclusions
We undertook these experiments because ingested danshensu, like other polyphenols, consumes methionine by being methylated by COMT and this consumption of methionine increases levels of Hcy, which is a cardiovascular risk factor. At the same time, danshensu has been shown to provide cardiovascular benefits and these two opposing effects constitute the ‘therapeutic paradox’ that we have explored in our experiments. Our results clearly showed that danshensu did not affect plasma tHcy in normal rats after short- or longer-term treatments. By contrast, danshensu markedly attenuated the raised tHcy levels induced by methionine, suggesting that danshensu could provide a potential therapy for HHcy.
This effect of lowering tHcy was observed in a range of experimental conditions: after a single i.v. methionine load or after 3 weeks of chronic methionine dosing, using single or chronic dosing with danshensu. Overall, danshensu only lowered plasma tHcy when it had been raised either by an acute methionine load or by chronic methionine administration (HHcy rats). Further, danshensu altered metabolic intermediates of Hcy in liver and kidney, two major sites of Hcy metabolism. Based on the relative amounts of these intermediates (SAM, SAH, cysteine and GSH), we can evaluate, overall, the main pathway of Hcy metabolism and begin to uncover the biochemical mechanisms underlying the lowering of tHcy by danshensu. The relatively higher cysteine and GSH levels, both in liver and kidney, of rats given danshensu alone, suggested that danshensu might increase the activity of the trans-sulphuration pathway (see Figure 1), as a means of lowering Hcy levels. Although the particular step(s) in the trans-sulphuration pathway were not identified in our experiments, our results suggested that danshensu acted both acutely, after a single i.v. injection, and chronically, perhaps by up-regulating one or more of the enzymes in the trans-sulphuration pathway. It should be noted that whereas the effect of a single dose of danshensu on raised plasma tHcy was over by 4 h, suggesting a rapidly acting, short-lived mechanism, the effects of chronic treatment with danshensu were assessed days after ending chronic treatment, when plasma and tissue levels of danshensu should have been low, if not negligible. Thus, the more persistent mode of action suggested by the effects of chronic danshensu could be different from that involved in its acute effects.
Danshensu, a catechol-containing polyphenol, is an abundant and structurally representative compound of the water-soluble active components in S. miltiorrhiza. Polyphenols are abundant in tea, wine and many other foods (D'Archivio et al., 2007). The association between dietary polyphenols and plasma tHcy level has been investigated for many years but the results are still controversial (de Bree et al., 2001; Hodgson et al., 2003). Our results indicated that danshensu did not affect plasma tHcy in normal rats, while clearly lowering acutely raised plasma tHcy in normals or in a model of HHcy rats. This is the first report about the bidirectional effect of danshensu on Hcy metabolism and the distinct effects in different animal states gave us some understanding of the relationship between polyphenols and Hcy metabolism. It appears that the effect of polyphenols on plasma tHcy level was largely dependent on its initial value. For groups with normal tHcy, polyphenols may show minor, even slightly elevating effects, on tHcy levels. In contrast, for groups with elvated tHcy, polyphenols should exhibit a lowering effect. Previous reports had indicated that SAH always rises in parallel with Hcy, because the reaction catalysed by adenosyl-homocysteinase is a reversible process and is always at equilibrium due to the high activity of adenosylhomocysteinase (Martinov et al., 2000). The bidirectional effect probably represents accumulating SAH in relatively higher tHcy state, as shown in Table 3. SAH is an endogenous and potent inhibitor of COMT (Rivett and Roth, 1982), which is exclusively responsible for methylation of polyphenols. Thus, it is likely that methylation of these polyphenols would be largely inhibited by the accumulated SAH level in higher Hcy state, which was supported by the lower concentration of methylated metabolites of danshensu in the methionine group of rats (data not shown). If methylation were suppressed, the tHcy-raising effect of polyphenol methylation would be diminished and the potential tHcy-lowering effect would be dominant in controlling plasma tHcy levels. Although this explanation may not hold for all polyphenols (Stamler et al., 1993), for those polyphenols with a relatively lower extent of methylation and also exhibiting Hcy-lowering effect, such as danshensu, it provides a reasonable resolution of the paradox.
Glutathione is the most abundant low-molecular weight thiol and plays a key role in the cellular defense against oxidative stress. As our results indicated in Table 3, GSH was obviously elevated by danshensu both in liver and kidney, which would provide another explanation for the overall antioxidant activity of danshensu. Many papers have attributed the cardiovascular protection of danshensu to its antioxidant and radical scavenging properties, based on its catechol structure (Zhao et al., 2008), but the antioxidative potential of catechol-containing compounds has been challenged (Boots et al., 2002; 2003) on the grounds that this type of antioxidant shifted oxidative damage from lipid peroxidation to thiol arylation via semiquinone or quinone radical formation. Here we have provided a new mechanism for the antioxidant effects of danshensu, which is increased GSH biosynthesis, which would also be a novel cardiovascular protective action for S. miltiorrhiza.
It had been reported that many drugs exhibit a potential for raising tHcy (Desouza et al., 2002). Unlike these drugs, danshensu had no effect on plasma tHcy in rats with normal tHcy level. In contrast, danshensu can clearly lower elevated tHcy and stabilize tHcy levels via activation of the trans-sulphuration pathway. Such effects would be beneficial in the therapy of cardiovascular disease, especially for those patients who are simultaneously prescribed drugs exhibiting tHcy-raising potential, such as bezafibrate (Dierkes et al., 1999), fenofibrate (de Lorgeril et al., 1999) and nicotinic acid (Basu and Mann, 1997). For the patients at risk of plasma tHcy elevation from other causes, such as vitamin deficiency or kidney dysfunction, danshensu might also be beneficial by preventing elevation of tHcy, but further clinical assessment of this possible use is needed.
Acknowledgments
The authors gratefully acknowledge financial support from the National Natural Science Foundation of the People's Republic of China (No. 30772609).
Glossary
Abbreviations:
- DTNB
5,5'-dithio-bis-nitrobenzoic acid
- DTT
dithiothreitol
- GSH
glutathione
- Hcy
homocysteine
- SAH
S-adenosyl-l-homocysteine
- SAM
S-adenosyl methionine
- tHcy
total homocysteine
Conflict of interest
The authors state no conflict of interest.
References
- Abdel-Zaher AO, Abdel-Hady RH, Mahmoud MM, Farrag MM. The potential protective role of alpha-lipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2008;243(3):261–270. doi: 10.1016/j.tox.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Basu TK, Mann S. Vitamin B-6 normalizes the altered sulfur amino acid status of rats fed diets containing pharmacological levels of niacin without reducing niacin's hypolipidemic effects. J Nutr. 1997;127(1):117–121. doi: 10.1093/jn/127.1.117. [DOI] [PubMed] [Google Scholar]
- Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- Boots AW, Haenen GR, den Hartog GJ, Bast A. Oxidative damage shifts from lipid peroxidation to thiol arylation by catechol-containing antioxidants. Biochim Biophys Acta. 2002;1583(3):279–284. doi: 10.1016/s1388-1981(02)00247-0. [DOI] [PubMed] [Google Scholar]
- Boots AW, Kubben N, Haenen GR, Bast A. Oxidized quercetin reacts with thiols rather than with ascorbate: implication for quercetin supplementation. Biochem Biophys Res Commun. 2003;308(3):560–565. doi: 10.1016/s0006-291x(03)01438-4. [DOI] [PubMed] [Google Scholar]
- Chan K, Chui SH, Wong DY, Ha WY, Chan CL, Wong RN. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sci. 2004;75(26):3157–3171. doi: 10.1016/j.lfs.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121(1):9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- D'Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita. 2007;43(4):348–361. [PubMed] [Google Scholar]
- de Bree A, Verschuren WM, Blom HJ, Kromhout D. Lifestyle factors and plasma homocysteine concentrations in a general population sample. Am J Epidemiol. 2001;154(2):150–154. doi: 10.1093/aje/154.2.150. [DOI] [PubMed] [Google Scholar]
- de Lorgeril M, Salen P, Paillard F, Lacan P, Richard G. Lipid-lowering drugs and homocysteine. Lancet. 1999;353(9148):209–210. doi: 10.1016/S0140-6736(05)77220-2. [DOI] [PubMed] [Google Scholar]
- Desouza C, Keebler M, McNamara DB, Fonseca V. Drugs affecting homocysteine metabolism: impact on cardiovascular risk. Drugs. 2002;62(4):605–616. doi: 10.2165/00003495-200262040-00005. [DOI] [PubMed] [Google Scholar]
- Dierkes J, Westphal S, Luley C. Serum homocysteine increases after therapy with fenofibrate or bezafibrate. Lancet. 1999;354(9174):219–220. doi: 10.1016/S0140-6736(99)02153-4. [DOI] [PubMed] [Google Scholar]
- Durand P, Lussier-Cacan S, Blache D. Acute methionine load-induced hyperhomocysteinemia enhances platelet aggregation, thromboxane biosynthesis, and macrophage-derived tissue factor activity in rats. FASEB J. 1997;11(13):1157–1168. [PubMed] [Google Scholar]
- Fukada S, Shimada Y, Morita T, Sugiyama K. Suppression of methionine-induced hyperhomocysteinemia by glycine and serine in rats. Biosci Biotechnol Biochem. 2006;70(10):2403–2409. doi: 10.1271/bbb.60130. [DOI] [PubMed] [Google Scholar]
- Gaitonde MK. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelet J, Demuth K, Dairou J, Ledru A, Paul JL, Dupret JM, et al. Effects of catechin on homocysteine metabolism in hyperhomocysteinemic mice. Biochem Biophys Res Commun. 2007;355(1):221–227. doi: 10.1016/j.bbrc.2007.01.142. [DOI] [PubMed] [Google Scholar]
- Han JY, Fan JY, Horie Y, Miura S, Cui DH, Ishii H, et al. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Ther. 2008;117(2):280–295. doi: 10.1016/j.pharmthera.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Hodgson JM, Burke V, Beilin LJ, Croft KD, Puddey IB. Can black tea influence plasma total homocysteine concentrations? Am J Clin Nutr. 2003;77(4):907–911. doi: 10.1093/ajcn/77.4.907. [DOI] [PubMed] [Google Scholar]
- Hodgson JM, Devine A, Puddey IB, Beilby J, Prince RL. Drinking tea is associated with lower plasma total homocysteine in older women. Asia Pac J Clin Nutr. 2006;15(2):253–258. [PubMed] [Google Scholar]
- Huang RF, Hsu YC, Lin HL, Yang FL. Folate depletion and elevated plasma homocysteine promote oxidative stress in rat livers. J Nutr. 2001;131(1):33–38. doi: 10.1093/jn/131.1.33. [DOI] [PubMed] [Google Scholar]
- Jacobs RL, Stead LM, Brosnan ME, Brosnan JT. Hyperglucagonemia in rats results in decreased plasma homocysteine and increased flux through the transsulfuration pathway in liver. J Biol Chem. 2001;276(47):43740–43747. doi: 10.1074/jbc.M107553200. [DOI] [PubMed] [Google Scholar]
- Katrusiak AE, Paterson PG, Kamencic H, Shoker A, Lyon AW. Pre-column derivatization high-performance liquid chromatographic method for determination of cysteine, cysteinyl-glycine, homocysteine and glutathione in plasma and cell extracts. J Chromatogr B Biomed Sci Appl. 2001;758(2):207–212. doi: 10.1016/s0378-4347(01)00182-7. [DOI] [PubMed] [Google Scholar]
- Kim SK, Choi KH, Kim YC. Effect of acute betaine administration on hepatic metabolism of S-amino acids in rats and mice. Biochem Pharmacol. 2003;65(9):1565–1574. doi: 10.1016/s0006-2952(03)00115-1. [DOI] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- Martinov MV, Vitvitsky VM, Mosharov EV, Banerjee R, Ataullakhanov FI. A substrate switch: a new mode of regulation in the methionine metabolic pathway. J Theor Biol. 2000;204(4):521–532. doi: 10.1006/jtbi.2000.2035. [DOI] [PubMed] [Google Scholar]
- Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337(4):230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- Rivett AJ, Roth JA. Kinetic studies on the O-methylation of dopamine by human brain membrane-bound catechol O-methyltransferase. Biochemistry. 1982;21(8):1740–1742. doi: 10.1021/bi00537a006. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Osborne JA, Jaraki O, Rabbani LE, Mullins M, Singel D, et al. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest. 1993;91(1):308–318. doi: 10.1172/JCI116187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead LM, Brosnan ME, Brosnan JT. Characterization of homocysteine metabolism in the rat liver. Biochem J. 2000;350(3):685–692. Pt. [PMC free article] [PubMed] [Google Scholar]
- Vitvitsky V, Mosharov E, Tritt M, Ataullakhanov F, Banerjee R. Redox regulation of homocysteine-dependent glutathione synthesis. Redox Rep. 2003;8(1):57–63. doi: 10.1179/135100003125001260. [DOI] [PubMed] [Google Scholar]
- Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- Wu B, Liu M, Liu H, Li W, Tan S, Zhang S, et al. Meta-analysis of traditional Chinese patent medicine for ischemic stroke. Stroke. 2007a;38(6):1973–1979. doi: 10.1161/STROKEAHA.106.473165. [DOI] [PubMed] [Google Scholar]
- Wu L, Qiao H, Li Y, Li L. Cardioprotective effects of the combined use of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytother Res. 2007b;21(8):751–756. doi: 10.1002/ptr.2157. [DOI] [PubMed] [Google Scholar]
- Yagisawa M, Okawa N, Shigematsu N, Nakata R. Effects of intravenous betaine on methionine-loading-induced plasma homocysteine elevation in rats. J Nutr Biochem. 2004;15(11):666–671. doi: 10.1016/j.jnutbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Zhang ZC, Xu M, Sun SF, Guo H, Qiao X, Han J, et al. Determination of danshensu in rat plasma and tissues by high-performance liquid chromatography. J Chromatogr Sci. 2008;46(2):184–190. doi: 10.1093/chromsci/46.2.184. [DOI] [PubMed] [Google Scholar]
- Zhao GR, Zhang HM, Ye TX, Xiang ZJ, Yuan YJ, Guo ZX, et al. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem Toxicol. 2008;46(1):73–81. doi: 10.1016/j.fct.2007.06.034. [DOI] [PubMed] [Google Scholar]
- Zhou J, Moller J, Danielsen CC, Bentzon J, Ravn HB, Austin RC, et al. Dietary supplementation with methionine and homocysteine promotes early atherosclerosis but not plaque rupture in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21(9):1470–1476. doi: 10.1161/hq0901.096582. [DOI] [PubMed] [Google Scholar]


