Abstract
Neuropeptides have been implicated in the psychopathology of stimulants of abuse. Neurotensin is a neuropeptide associated with the regulation of the nigrostriatal and mesolimbic dopamine pathways. In addition, the ventral tegmental area, a midbrain region implicated in the rewarding effects of most, if not all, addictive drugs, appears to be a particularly critical target for nicotine action. Because neurotensin has been linked with both mesolimbic and mesocortical dopamine function, we examined the impact of nicotine treatment on central nervous neurotensin systems by measuring changes in neurotensin tissue content because it has been shown such changes reflect alterations in release and activity of this peptide system. Male Sprague-Dawley rats received multiple administrations of (±) nicotine 4.0 mg/kg/day (0.8 mg/kg, i.p.; 5 × 2-h intervals) in the presence or absence of selective dopamine receptor antagonists (dopamine D1; SCH23390 or dopamine D2; eticlopride) or two doses of the non-selective nicotinic acetylcholine receptor antagonist (mecamylamine; 3.0 and 6.0 mg/kg, s.c.). The nicotine treatment significantly decreased neurotensin-like immunoreactivity content in the ventral tegmental area, as well as related regions such as prefrontal cortex, substantia nigra, and anterior striatal region 12–18 h after drug treatment, but not the nucleus accumbens. The nicotine-mediated decrease in the neurotensin-like immunoreactivity of the ventral tegmental area was selectively blocked by a specific dopamine D2, but not a dopamine D1, receptor antagonist, while mecamylamine attenuated at the low (3.0 mg/kg) and completely blocked at high (6.0 mg/kg) dose this nicotine effect. These findings with previous studies, suggest that nicotine-mediated dopamine release activates D2 receptors which in turn increases neurotensin release, turnover and acutely reduces tissue levels in the ventral tegmental area and other limbic and basal ganglia structures.
Keywords: neurotensin, substance P, metenkephalin, nicotine, mecamylamine, dopamine, ventral tegmental area, non-alpha 7 nicotinic receptor
1. Introduction
Activation of central nervous nicotinic system, such as that associated with the use of tobacco products, causes rewarding and cognitive effects that likely contribute to frequent abuse of these substances (Maskos et al., 2005; Singer et al., 2004). Thus, in order to develop more effective therapeutics for treating nicotine dependency it is important to elucidate the mechanisms responsible for the effects of this psychostimulant. Of relevance to this objective is an important site of action for nicotine, the ventral tegmental area. Support for the conclusion that nicotine mediates some of its most critical reward functions through this brain region (Ikemoto et al., 2006; Laviolette and van der Kooy, 2003; David et al., 2006) includes reports that: (i) it contains high concentrations of nicotinic acetylcholine receptors (Klink et al., 2001), such as alpha 4 and 7 and beta 2 subunits (Maskos et al., 2005; Klink et al., 2001; Wu et al., 2004), that have been linked to the addicting properties of nicotine-containing products (Maskos et al., 2005); (ii) systemically or directly administered nicotine in the ventral tegmental area results in the release of dopamine in this brain region (somatodendritic; Rahman et al., 2003; Chen et al., 2003), nucleus accumbens (Nisell et al., 1994; Sziraki et al., 2002; Ferrari et al., 2002; Rowell and Volk, 2004), and to some extent the prefrontal cortex (Nisell et al., 1996; Cao et al., 2005) due to the presence of nicotinic receptors on the corresponding dopamine projections; (iii) nicotinic receptors in the ventral tegmental area are responsible for both the reinforcing (Rowell and Volk, 2004) and cognitive-enhancing effects of nicotine (Maskos et al., 2005); and (iv) in striatal and ventral tegmental areas some dopaminergic neurotransmission is also under control of heteromeric autoreceptor complexes integrate by dopamine D2 autoreceptors and heteromeric non-alpha 7 nicotinic acetylcholine receptors (Quarta et al., 2007; Aghajanian and Bunney, 1977).
While considerable work has focused on the interaction between nicotinic and the mesolimbic and mesocortical dopaminergic systems, relatively little research has investigated the possible role of other ventral tegmental area neurotransmitter systems in mediating the pharmacological properties of nicotine (Naftchi at al., 1988; Singer et al., 2004). In this regard, a particularly interesting target for investigation are the neuropeptide neuromodulators. For example, there are significant ventral tegmental area levels of the neuropeptides neurotensin, substance P, and metenkephalin (Alburges et al., 2001a, b; Hanson et al., 2002), all of which have been linked to dopamine limbic and extrapyramidal dopamine pathway regulation and associated with the effects of potent psychostimulant drugs such as methamphetamine (Geisler and Zahm, 2006; Kelley et al., 1989; Kalivas, 1985) and cocaine (Hanson et al., 1992). However, previously none of these neuromodulators has been reported to be associated with nicotinic systems in the ventral tegmental area or other dopamine-rich areas. Of these neuropeptides, the role of neurotensin in the ventral tegmental area has been most studied. For example, activation of neurotensin receptors in the ventral tegmental area by local infusion of neurotensin has been demonstrated to: be excitatory on mesolimbic dopamine neurons (Cador et al., 1989); increase glucose utilization in the nucleus accumbens (Pontieri et al., 2000); cause locomotion (Kalivas and Taylor, 1985); be a positive reinforcer (Glimcher et al., 1987); contribute to dopamine-mediated sensitization (Kalivas and Taylor, 1985), such as that caused by administration of psychostimulants like amphetamine (Panayi et al., 2005); and contribute to neuroadaptations associated with psychostimulant drug administration (Reynolds et al., 2006). The exact origins of the endogenous neurotensin found in the ventral tegmental area relevant to regulating associated dopamine efferents have not been completely determined, although are thought to be associated with multiple sites such as the hypothalamus, dorsal raphe, lateral septum, accumbens shell and amygdala (Geisler and Zahm, 2006). Interestingly, findings suggesting that these neurotensin systems are influenced by psychostimulants demonstrate the neurotensin-producing capacity of some of these afferent pathways is enhanced after exposure to drugs such as methamphetamine (Geisler and Zahm, 2006). While neurotensin systems are clearly the most studied neuropeptide in the ventral tegmental area, it has been reported that injection of substance P or D-Ala-Met-enkephalin into the structure also cause dose-dependent increases in dopamine turnover in the nucleus accumben (Cador et al., 1989; Kalivas and Taylor, 1985) and influence operant responding (Kelley et al., 1989).
Because the neurotensin system has been the most researched of the neuropeptides associated with the ventral tegmental area, the present study primarily examined the possibility that neurotensin systems in ventral tegmental area are influenced by treatment with nicotine and consequently may contribute to the effects of this stimulant. The neurotensin response was assessed by evaluating levels of neurotensin-like immunoreactivity in the ventral tegmental area and associated structures after treatment with nicotine as we have previously shown that such changes can reflect alterations in neurotensin release and activation of its receptors (Wagstaff et al., 1994). Specifically, we observed that dopamine D2 agonists increase neurotensin release in both limbic and basal ganglia structures (Wagstaff et al., 1996) resulting in a rapid decrease in neurotensin tissue levels (Merchant et al., 1989) likely due to elevated turnover of the peptide.
The present study is the first report that nicotine treatment has a significant effect on the ventral tegmental area neurotensin systems and demonstrates that 5 administrations (i.p., 2-h intervals) of (±) nicotine significantly decreased neurotensin levels in the ventral tegmental area 12–18 hours after treatment, an effect that recovered by 24 hours. This nicotine-mediated reduction in the concentration of neurotensin was selectively blocked by a specific dopamine D2, but not a dopamine D1, receptor antagonist. The pretreatment with the non-selective nicotinic acetylcholine receptor antagonist, mecamylamine, prevented the effect of nicotine on neurotensin tissue content in ventral tegmental area. These findings suggest that nicotine enhances release and turnover of neurotensin in the ventral tegmental area, resulting in reduced tissue levels. This effect is mediated by activation of both nicotinic and dopaminic D2 receptors.
2. Materials and method
2.1. Animals
Male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC) weighing 250–330 g were maintained in a temperature-controlled environment and cared for according to NIH guidelines. Animals were kept on a 12-h light/dark cycle with food and water available ad libitum. Animals were allowed to acclimate for at least 2 weeks before their use. All the experiments were performed according to the guidelines of the University of Utah Institutional Animal Care and Use Committee.
2.2. Drug treatment and tissue dissection
Following the acclimatization period, two experimental protocols were used in this study: (a) animals were injected with 5 administrations of either saline (1.0 ml/kg, i.p.) or (±) nicotine freebase 4.0 mg/kg/day (0.8 mg/kg, i.p.; 5 injections at 2-h intervals) and were sacrificed 12, 18 or 24 h after treatment; (b) animals were pretreated with dopamine D1 (SCH 23390; 0.5 mg/kg/injection, i.p.), dopamine D2 (eticlopride; 0.5 mg/kg/injection, i.p.), or nicotine (mecamylamine; 3.0 or 6.0 mg/kg/injection, s.c.) receptor antagonists 15 min prior to the 5 administrations of (±) nicotine or saline and sacrificed 18 h after treatment. Brains were removed rapidly, frozen immediately on dry ice and stored at −80 °C. For regional studies, brain areas were dissected from consecutive 1-mm thick coronal slices as previously described (Alburges et al., 2001a, b). Based on the atlas of Paxinos and Watson (1986), the anterior striatum was dissected into very anterior striatum (1.70 mm anterior to bregma), medial anterior striatum (1.20 mm anterior to bregma), and lateral anterior striatum (1.20 mm anterior to bregma) regions. The nucleus accumbens was dissected into core and shell regions. The ventral tegmental area, prefrontal cortex, and substantia nigra regions were also removed. All tissue samples were subsequently stored at −80 °C until assayed for neuropeptides.
2.3. Drugs
Eticlopride hydrochloride (S(−)-3-Chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-hydroxy-2-methoxy-benzamide hydrochloride), SCH 23390 hydrochloride (R(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2, 3, 4, 5-tetrahydro-1H-3-benzazepine hydrochloride), mecamylamine hydrochloride, and (±) nicotine were acquired from Sigma-Aldrich (St. Louis, MO). All doses were calculated as freebase of the drug and were freshly prepared in physiological saline solution (0.9 % w/v NaCl, pH 7.4).
2.4. Radioimmunoassay
The solid-phase radioimmunoassay used to analyze the neuropeptide levels in this study was adapted from the methods previously described (Maidment et al., 1991; Alburges et al., 2001a, b). Tissue samples were homogenized in 0.01 M HCl. The resulting homogenate was then placed in boiling water for 10 min in order to inactivate peptidases. Homogenates were centrifuged (17,000 X g) for 30 min. Supernatant was then collected and an aliquot was used to determine the total protein for each tissue sample by the method of Bradford (1976). Remaining sample was lyophilized overnight and stored at −80°C until the radioimmunoassay was performed. The concentrations of neuropeptides were determined with a modified solid-phase radioimmunoassay technique described for neurotensin by Maidment et al., (1991). Lyophilized samples were reconstituted in 300 ml phosphate-buffered saline (pH 7.4) containing 0.1% (w/v/) gelatin and 0.1% (v/v) Triton X-100. Nunc-Immunoplates (ISC BioExpress, Kaysville, UT) were incubated overnight at 4°C with 50 ml of protein G solution (50 ng/100 ml in 0.1 M sodium bicarbonate; pH 9.0). After washing the wells three times with wash buffer [0.15 M K2HPO4, 0.02 M NaH2PO4, 0.2 mM ascorbic acid, 0.2% (v/v) Tween-20 and 0.1% (w/v) sodium azide; pH 7.5], 25 ml of a highly selective antiserum for one of the following were diluted in assay buffer [same as wash buffer containing 0.1% (w/v) gelatin]: metenkephalin diluted to 1:1,000; neurotensin diluted to 1:20,000; substance P diluted to 1:200,000. Following addition of metenkephalin antisera, wells were incubated overnight at room temperature. Wells for neurotensin and substance P assays were incubated with the respective antisera for 2 h at room temperature in order to allow the attachment of antibody to the protein G-coated surface. After incubation, wells were washed three times and 25 ml of sample or standard(s) were added to each well and incubated for 2 h at room temperature. After incubation, 25 ml of the labeled peptide ([125I]metenkephalin, [125I]neurotensin, or [125I]substance P) diluted with assay buffer to approximately 6500 dpm per 25 ml, were added to the wells and incubated for 2 h at room temperature. After incubation, wells were washed, separated and placed in 12X75-mm polypropylene tubes and counted in a five-channel Packard Cobra II Auto-Gamma counter (Packard Instrument Co., Meriden, CT). The total and nonspecific binding were defined by adding 25 ml of the labeled peptide to protein G-untreated and –treated wells, respectively. Quantities of neuropeptide immunoreactivity were determined by comparing bound to free [125I]metenkephalin in each sample to a standard curve (from 8 to 1000 pg/assay tube). Determination of the quantities of neurotensin and substance P, were made by comparing bound to free using a standard curve from 1 to 125 pg/assay tube. The reproducibility of the assay was evaluated using cerebellum tissue spiked with 62 and 250 pg of each peptide. This technique has been demonstrated to be very reproducible, resulting in less than 10% variability between assays and less than 5% between sample and standard duplicates (Hanson et al., 1997).
2.5. Antisera
The metenkephalin antiserum employed in this study was raised by Zymed Laboratories (South San Francisco, CA) using the single-point, site-directed peptide conjugation, antipeptide technique (Posnett et al., 1988; Tam, 1988). This highly selective metenkephalin antibody displayed less than 1% cross-reactivity with leu-enkephalin or dynorphin and did not significantly cross-react with 1000-fold excess concentration of neurotensin and has been used for other studies by this laboratory (Alburges et al., 2001a, b). The neurotensin and substance P anstisera were raised in New Zealand White rabbits as previously described (Letter et al., 1987; Ritter et al., 1984). These antisera recognize the neurotensin or the substance P carboxy terminus (respectively) and are highly selective, expressing no cross reactivity with 1000-fold excess concentrations of other endogenous neuropeptides such as dynorphin (for neurotensin or substance P antiserum), metenkephalin, cholecystockinin, substance P (for neurotensin antiserum) or substance K.
2.6. Statistical analysis
Results from these experiments are expressed as percentages of their respective controls in order to facilitate comparisons between groups (mean values ± S.E.M.). The control values (pg of neurotensin-, substance P- or metenkephalin-like immunoreactivity per mg of protein) for each experiment are indicated in the corresponding figure legend. Differences between means were analyzed using one-way analysis of variance followed by Fisher-Protected Least Significant Difference (PLSD). Differences were considered significant when the probability that they were zero was less than 5%.
3. Results
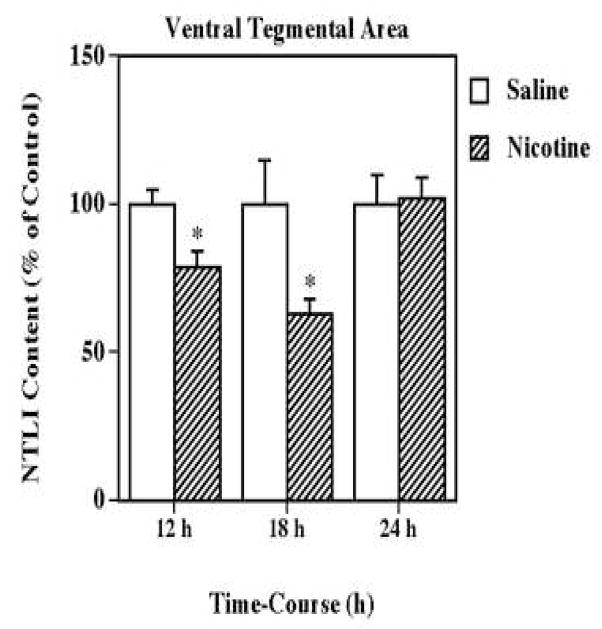
In order to determine if nicotine exposure influences the ventral tegmental area-associated neurotensin systems, the effect of multiple administrations (5 injections, 2-h intervals) of (±) nicotine (0.8 mg/kg/injection, i.p.) on neurotensin-like immunoreactivity levels in this structure were determined after 12, 18 and 24 h (Fig. 1). The tissue levels of neurotensin-like immunoreactivity were significantly decreased at both 12 (79 % of saline) and 18 (63 % of saline) h, but returned to control levels by 24 h after treatment. Due to this time-response pattern, all subsequent experiments were conducted within 24 h.
Figure 1.
Time-dependent effects of nicotine on neurotensin-like immunoreactivity (NTLI) content in the ventral tegmental area. Animals were administered 5 injections of (±) nicotine (0.8 mg/kg/injection, i.p., 2-h intervals) or saline and killed 12, 18 or 24 h following treatment. Results are expressed as percentages of control and represent mean values ± S.E.M. (n= 8 control and 9 drug-treated animals per group). The average control value of NTLI concentration was 490 ± 36 pg/mg protein. *P < 0.05 vs. control.
The mechanism responsible for this neurotensin response in the ventral tegmental area was determined by pretreating with selective dopamine D1 (SCH 23390) and dopamine D2 (eticlopride) receptor antagonists, and the non-selective nicotinic acetylcholine receptor antagonist, mecamylamine, prior to each nicotine administration (Fig. 2). None of the pretreatments with antagonist drugs alone significantly altered ventral tegmental area levels of neurotensin-like immunoreactivity. However, antagonism of dopamine D2, but not dopamine D1, receptors completely blocked the nicotine-induced neurotensin effect. In contrast, non-selective blockade of nicotinic receptors with a relatively low dose (3.0 mg/kg) of mecamylamine appeared to attenuate this nicotinic effect (80 % of saline); thus, the combination of nicotine and mecamylamine resulted in ventral tegmental area levels of neurotensin-like immunoreactivity that were not significantly different from saline, nicotine or mecamylamine groups alone. Administration of a higher dose (6.0 mg/kg) of mecamylamine completely blocked the nicotine-induced neurotensin effect (105 % of saline) in the ventral tegmental area (Fig. 3).
Figure 2.
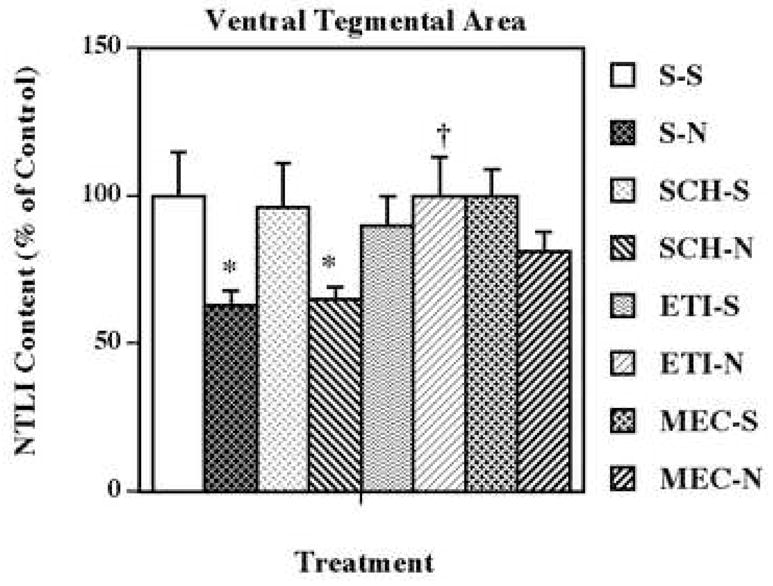
Effects of selective dopamine receptor and nicotinic acetylcholine receptor antagonists on nicotine-induced changes in neurotensin-like immunoreactivity (NTLI) content in the ventral tegmental area. Animals were given 5 administrations of (±) nicotine (N; 0.8 mg/kg/injection, i.p., 2-h intervals) or saline (S; control), alone or 15 min after administration of SCH 23390 (SCH; dopamine D1 receptor antagonist; 0.5 mg/kg/injection, i.p.), eticlopride (ETI; dopamine D2 receptor antagonist; 0.5 mg/kg/injection, i.p.), or mecamylamine (MEC; nicotinic acetylcholine receptor antagonist; 3.0 mg/kg/injection, s.c.). Animals were killed 18 h following the last treatment. Values represent the means ± S.E.M. expressed as percentages of control (n= 8 control and 9 drug-treated animals per group). The control value ± S.E.M. for NTLI concentrations (pg/mg protein) was 403 ± 65. *P < 0.05 vs. saline control; †P < 0.05 vs. corresponding nicotine group.
Figure 3.
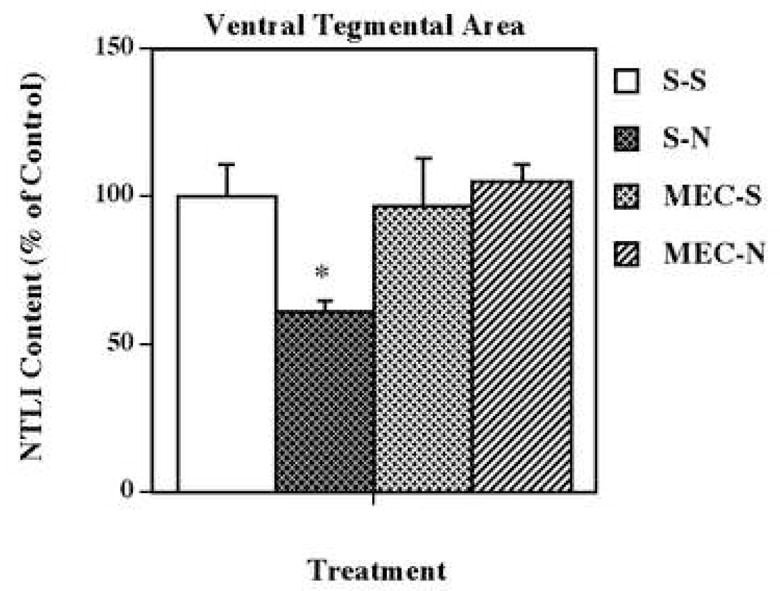
Effects of a high dose of mecamylamine on nicotine-induced changes in neurotensin-like immunoreactivity (NTLI) content in the ventral tegmental area. Animals were given 5 administrations of (±) nicotine (N; 0.8 mg/kg/injection, i.p., 2-h intervals) or saline (S; control), alone or 15 min after administration of the nicotinic antagonist mecamylamine (MEC; 6.0 mg/kg/injection, s.c.). Animals were killed 18 h following the last treatment. Values represent the means ± S.E.M. expressed as percentages of control (n= 8 or 9 in the control or drug-treated animals, respectively). The control value ± S.E.M. for NTLI concentrations (pg/mg protein) was 578 ± 62. *P < 0.05 vs. all other groups.
In order to assess the selectivity of the response of the neurotensin systems in the ventral tegmental area to nicotine treatment, the time-related effects of the nicotine treatment on neurotensin-like immunoreactivity levels in ventral tegmental area projection (Fig. 4) and parallel areas (Fig. 5) were examined. The multiple nicotine injections decreased neurotensin-like immunoreactivity levels in the prefrontal cortex (Fig. 4A) at 24 h (47 % of saline), but caused no significant changes in neurotensin content in either the nucleus accumbens core or shell (Fig. 4B) at either 12 or 24 h after treatment. In the parallel nigrostriatal system (Fig. 5), this same nicotine administration significantly decreased nigral neurotensin-like immunoreactivity levels (Fig. 5A) at both 12 (54 % of saline) and 24 (58 % of saline) h after treatment. There was also observed a nicotine-mediated decrease in neurotensin-like immunoreactivity levels in the very anterior striatum area (Fig. 5B) at 12 (59 % of saline), but not 24 h, while no significant changes in the tissue content of the neuropeptide were observed in any other striatal regions such as the medial and lateral anterior striatal areas (Figs. 5C and 5D).
Figure 4.
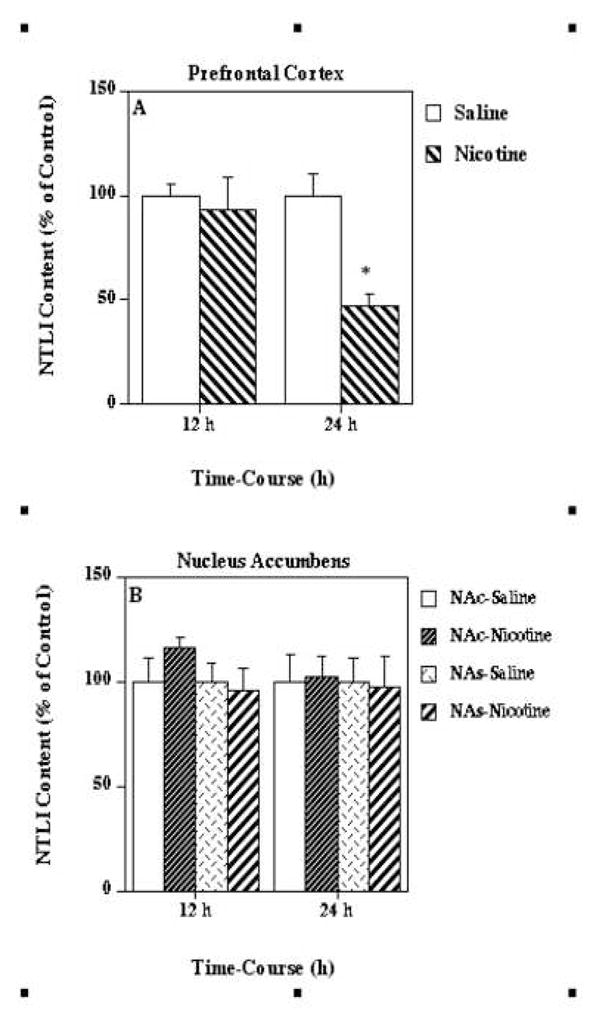
Effects of nicotine on neurotensin-like immunoreactivity (NTLI) levels in ventral tegmental area-related brain regions such as the prefrontal cortex (Fig. 4A) and the nucleus accumbens [core (NAc) and shell (NAs); Fig. 4B]. Animals were given injections of nicotine (as described for Fig. 1) or saline and killed 12 and 24 h following the treatment. Results are expressed as percentages of control and represent mean values ± S.E.M. (n= 8 control and 9 drug-treated animals per group). The average control values of NTLI concentrations for prefrontal cortex, NAs, and NAc were 37 ± 6 pg/mg protein, 270 ± 21 pg/mg protein, and 408 ± 26 pg/mg protein, respectively. *P < 0.05 vs. control.
Figure 5.
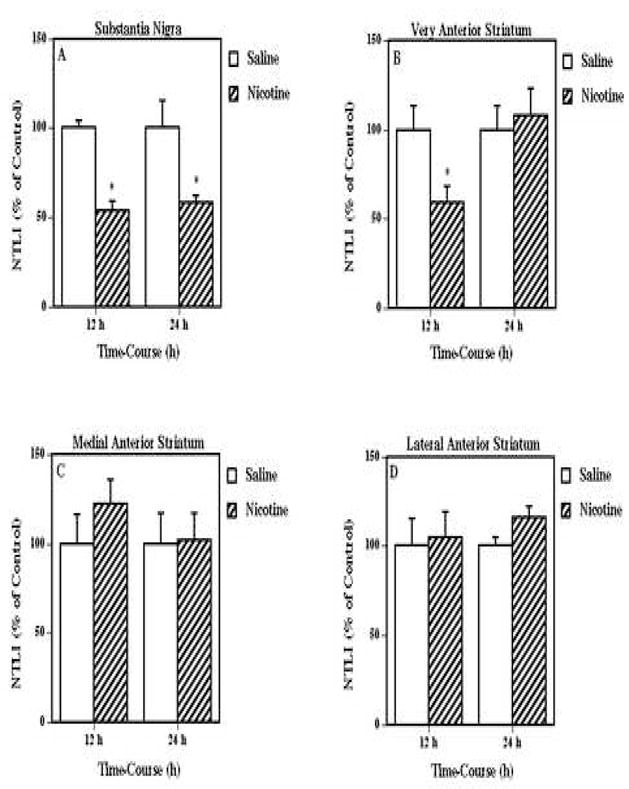
Effects of nicotine on neurotensin-like immunoreactivity (NTLI) content in other dopamine-related systems such as the substantia nigra (Fig. 5A), very anterior striatum (Fig. 5B), medial anterior striatum (Fig. 5C), and lateral anterior striatum (Fig. 5D). Animals were given injections of nicotine (as described for Fig. 1) or saline and killed 12 and 24 h following the treatment. Results are expressed as percentages of control and represent mean values ± S.E.M. (n= 8 control and 9 drug-treated animals per group). The average control values of NTLI concentrations for substantia nigra, very anterior striatum, medial anterior striatum, and lateral anterior striatum were 545 ± 60 pg/mg protein, 114 ± 12 pg/mg protein, 158 ± 17 pg/mg protein, and 80 ± 4 pg/mg protein, respectively. *P < 0.05 vs. control.
Finally, we determined if ventral tegmental area neuropeptide systems other than neurotensin were also altered by nicotine exposure. This was achieved by measuring ventral tegmental area levels of substance P- and metenkephalin-like immunoreactivity 12 and 24 h after multiple nicotine administrations (Fig. 6). This nicotine treatment significantly reduced substance P-like immunoreactivity in the ventral tegmental area (Fig. 6A) at 12 (56 % of saline), but not 24 h, while having no significant effect on metenkephalin-like immunoreactivity content in this structure at either time point (Fig. 6B).
Figure 6.
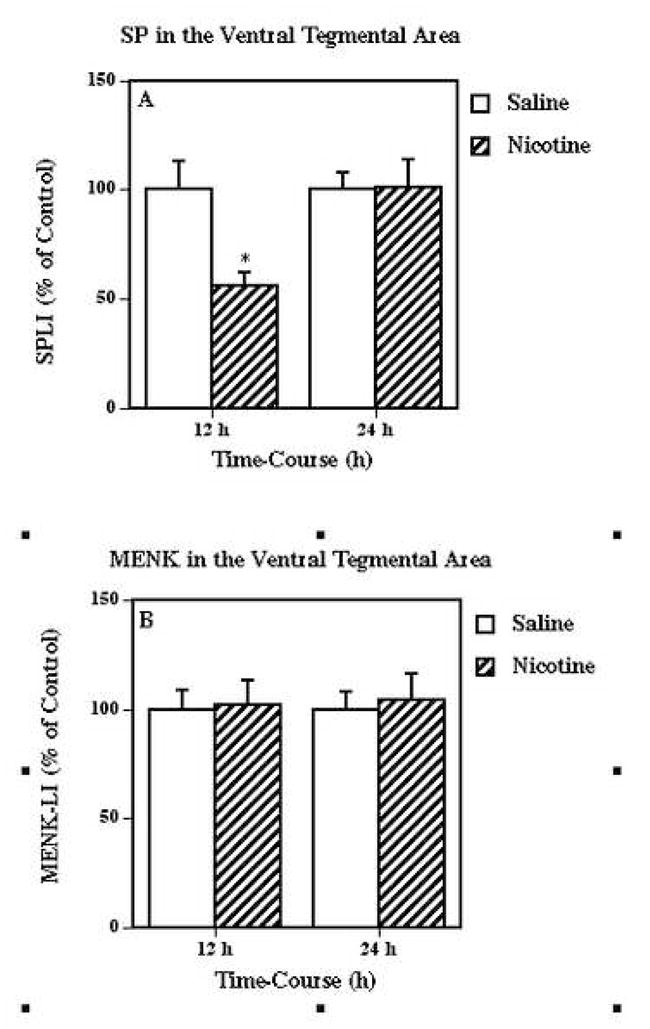
Effects of nicotine on substance P (SP; Fig. 6A) and metenkephalin (MENK; Fig. 5B) systems, in the ventral tegmental area. Animals received injections of nicotine (as described for Fig. 1) or saline and killed 12 and 24 h following the treatment. Results are expressed as percentages of control and represent mean values ± S.E.M. (n= 8 control and 9 drug-treated animals per group). The average control values of SPLI and MENK-LI concentrations for ventral tegmental area were 1036 ± 105 and 2446 ± 172 pg/mg protein, respectively. *P < 0.05 vs. control.
4. Discussion
It is well established that nicotine administration either through smoking of tobacco products or by administration of the pure drug has profound effects on the activity of ventral tegmental area-associated systems contributing to both the rewarding (and abuse potential) and cognitive properties of this substance (Maskos et al., 2005; Singer et al., 2004). These nicotine properties are at least partially associated with the high content of nicotinic receptors and likely their alpha 4 and 7, and beta 2 subunits (Maskos et al., 2005; Klink et al., 2001; Wu et al., 2004) in the ventral tegmental area and their regulation of dopamine efferent projections to particularly the nucleus accumbens (Nisell et al., 1994; Sziraki et al., 2002; Ferrari et al., 2002; Rowell and Volk, 2004), but also the prefrontal cortex (Nisell et al., 1996; Cao et al., 2005). In addition, previous studies have implicated ventral tegmental area alpha 7 nicotinic receptors in the nicotine withdrawal syndrome (Nomikos et al., 1999). Recently, studies by Quarta et al., (2007) revealed that striatal non-alpha 7 nicotinic acetylcholine receptor form part of a heteromeric dopamine autoreceptor complex that modulates dopamine release, and this nicotinic-dopaminergic network is also co-localized in the ventral tegmental area (Aghajanian and Bunney, 1977), suggesting a direct interaction between dopamine D2 and nicotinic receptor systems. However, other neuron-regulatory, such as neuropeptide (e.g. neurotensin), systems are also associated with the ventral tegmental area and also have been demonstrated to contribute to the regulation of dopamine efferent pathways and their consequent functional outcomes. In order to understand better the role of these other ventral tegmental systems, particularly as it relates to nicotine-mediated activities, we examined the response of neurotensin pathways to nicotine treatment. The selection of the nicotine dose was based on previous experiments where varying doses of (±) nicotine were used (from 0.4 to 3.2 mg/kg, i.p.; data not shown). Because higher doses of (±) nicotine (1.6 and 3.2 mg/kg, i.p.) severely impair motor response and induced seizures in the animals, the dose used in this study was 0.8 mg/kg, i.p. In addition, this dose and the multiple administrations-treatment paradigm we employed for nicotine has been previously used (Kane et al., 2000, 2001, 2005; Li et al., 2000, 2003; Matta et al., 2007) and is intended to mimic a drug exposure pattern expected from average human smokers. In response to this nicotine treatment, we observed a significant, but temporary reduction in ventral tegmental area levels of neurotensin-like immunoreactivity (Fig. 1). The relatively rapid recovery of the ventral tegmental area neurotensin system by 24 h after nicotine administration suggests this is a reversible effect. We confirmed that this neurotensin response to nicotine was linked to dopamine systems by the observation that this nicotine-mediated reduction in ventral tegmental area levels of neurotensin-like immunoreactivity was completely blocked by antagonism of the dopamine D2, but not dopamine D1 receptor (Fig. 2). This observation is consistent with other observations that selective activation of dopamine D2 receptors reduces neurotensin-like immunoreactivity content in limbic and extrapyramidal structures (Wagstaff et al., 1996; Merchant et al., 1989), while activation of dopamine D1 receptors has the opposite effect of increasing neurotensin-like immunoreactivity content in these tissues (Merchant et al., 1990). This finding suggests that exposure to nicotine causes release of dopamine in the ventral tegmental area, an effect previously reported (Rahman et al., 2003; Chen et al., 2003), which in turn activates dopamine D2 receptors leading to a reduction in the neurotensin-like immunoreactivity levels. Because activation of dopamine D2 receptors substantially increases the release of neurotensin in both limbic and extrapyramidal structures, it is likely nicotine treatment also causes release of neurotensin which enhances turnover and thereby temporarily reduces tissue levels of the peptide. This nicotine activation of dopamine D2 neurotransmission also agrees with recent studies by Scott et al., (2007) in human and Quarta et al., (2007) in animal models, revealing that a functional interactions exist between dopamine D2 receptors and nicotinic acetylcholine receptors in the basal ganglia. If stimulation of nicotinic receptors increases neurotensin release as suggested by the acute reduction in tissue we report herein, then studies examining the effects of ventral tegmental area administration of neurotensin may have relevance to the role of neurotensin in mediating nicotine functions. Thus, it has been found that application of neurotensin onto ventral tegmental area neurons: (i) is excitatory (Kalivas and Taylor, 1985; Jiang et al., 1994); (ii) stimulates dopamine release in ventral tegmental area and associated projection areas (Sotty et al., 2000; Legault et al., 2002); (iii) is rewarding (Glimcher et al., 1987); and (iv) can contribute to the process of sensitization to psychostimulants (Panayi et al., 2005) and dopamine itself (Kalivas and Taylor, 1985). Since nicotine alone can exert all of the properties listed above for neurotensin, perhaps activation of neurotensin systems contributes to these nicotine properties. Unfortunately, from this study it is not clear which neurotensin projections into the ventral tegmental area are associated with these nicotine-mediated changes; however, because the effects appear to be related to activation of dopamine D2 receptors by nicotine-stimulated dopamine release, the likely site for the nicotine action is the ventral tegmental area itself.
In contrast to dopamine D2 receptor blockade, administration of the broad nicotine receptor antagonist, mecamylamine, did not clearly block the neurotensin response to nicotine, although attenuation of this effect was suggested (Fig. 2). The lack of a definitive effect of low dose of mecamylamine pretreatment on the neurotensin response to nicotine was not surprising, because the non-selective action of this antagonist, acting on both alpha 7 and non-alpha 7 nicotinic acetylcholine receptors (Quarta et al., 2007; George et al., 2000; Kempsill and Pratt, 2000; O’Dell and Christensen, 1988). However, a higher dose of mecamylamine, administered prior to the nicotine treatment, did completely block the nicotine-induced decrease in neurotensin-like immunoreactivity effect in the ventral tegmental area (Fig. 3) confirming that these neuropeptide effects are mediated by nicotinic receptors.
Because we observed that the nicotine-induced neurotensin changes in the ventral tegmental area were mediated by dopamine D2 receptors, we tested the possibility that nicotine treatment also influences neurotensin systems located in other brain regions where nicotine exposure causes dopamine release (Emmett and Greenfield, 2005). This was accomplished by assessing neurotensin-like immunoreactivity content in the ventral tegmental projection areas of the prefrontal cortex and nucleus accumbens. Nicotine treatment was found to decrease neurotensin-like immunoreactivity in the prefrontal cortex after 24 h, but did not alter this peptide in either the core or shell of the nucleus accumbens at either 12 or 24 h (Fig. 4). Nicotine treatment also altered neurotensin systems of the parallel nigrostriatal pathway as demonstrated by decreases in neurotensin-like immunoreactivity levels in the substantia nigra (at both 12 and 24 h) and very anterior striatum (12 h), but not in other anterior striatal regions (Fig. 5). These findings suggest that dopamine-linked neurotensin systems are broadly altered by exposure to nicotine. It is interesting that in those regions where nicotine-associated neurotensin-like immunoreactivity changes occurred, they were always decreases suggesting the involvement of a dopamine D2 receptor mechanism indirectly mediated by nicotine-related dopamine release and likely reflect increases in neurotensin release in these brain areas for the reasons discussed above.
While the functional relevance of these neurotensin changes caused by nicotine are not known, it has been demonstrated that neurotensin pathways influence the effects of others psychostimulants, thus, it contributes to cocaine sensitization (Rompré and Bauco, 2006), reinstatement of cocaine seeking (Lopak and Erb, 2005), and the hyperactivity caused by cocaine and amphetamine (Boules et al., 2007). Although the role for neurotensin systems in mediating the effects of cocaine and amphetamines has been extensively investigated, a role for neurotensin in nicotine effects is little studied. However, there is a report that stimulation of neurotensin receptors block nicotine-induced locomotor sensitization suggesting that neurotensin agonists should be explored for nicotine and psychostimulant abuse (Fredrickson et al., 2003).
Although the effect of nicotine treatment on neurotensin systems has not been previously reported, the effects of other potent psychostimulants on neurotensin pathways have been extensively studied. In general, this research has demonstrated that drugs such as the amphetamines and cocaine also alter neurotensin-like immunoreactivity levels in dopamine-rich areas. However, for the most part in contrast to the nicotine findings, high doses of these potent psychostimulants increase neurotensin-like immunoreactivity content in extrapyramidal and limbic structure and their effects are blocked by dopamine D1 receptors (Gygi et al., 1994; Hanson et al., 1989; Merchant et al., 1988). The differential role of dopamine D1 receptors in the potent psychostimulants and nicotine effects on neurotensin systems likely are due to differences in the response of dopamine pathways to these drugs. Thus, high doses of cocaine and the amphetamines are able to elevate extracellular dopamine levels considerably more than nicotine (Di Chiara and Imperato, 1988). Consequently, because the dopamine D2 receptor has a higher affinity than the dopamine D1 receptor (Seeman and Grigoriadis, 1985; Dearry et al., 1990), it would be expected that nicotine treatment would be more likely to cause dopamine-dependent effects mediated by dopamine D2, rather than dopamine D1, mechanisms. A suggested potential mechanism for the nicotine-mediated decrease in neurotensin-like immunoreactivity of the ventral tegmental area is that nicotine directly stimulated dopamine release by activation of nicotinic acetylcholine receptors localized in dopaminergic terminals which in turn increased neurotensin turnover (i.e. elevated release and metabolism) resulting in a temporary net loss of neurotensin-like immunoreactivity tissue content.
Finally, we examined the possibility that other dopamine-related neuropeptide systems in the ventral tegmental area (Cador et al., 1989) might also be altered by nicotine treatment (see Fig. 6). These studies revealed that nicotine administrations reduced substance P-like immunoreactivity levels in the ventral tegmental area at 12, but not at 24 h, while content of metenkephalin-like immunoreactivity was not altered at either time point examined in this area. These findings suggest that while the neurotensin responses were somewhat selective, other, but not all, neuropeptide systems associated with the ventral tegmental area may also contribute to the nicotine effects.
In summary, we observed that nicotine exposure similar to that which occurs in heavy tobacco smokers significantly alters neurotensin systems associated with the ventral tegmental area. These effects are expressed as reductions in neurotensin-like immunoreactivity tissue content, reversible and mediated by an interaction between nicotinic and dopaminergic systems through dopamine D2 receptor mechanisms and likely reflect an increase in neurotensin release. These responses might have implication in the psychopharmacological action of nicotine. In addition, neurotensin projections associated with dopamine pathways from the ventral tegmental area, as well as the substantia nigra, were similarly altered by nicotine treatment. These findings suggest that neurotensin systems may contribute to the effects of nicotine use on both limbic and extrapyramidal systems.
Acknowledgments
This work was supported by U.S. Public Health Service Grants DA09407 and DA00378. The authors would like to thank Dr. Paul S. Frankel and Mr. Lloyd Bush for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK, Bunney BS. Dopamine ‘autoreceptors’: pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedeberg’s Arch Pharmacol. 1977;297:1–7. doi: 10.1007/BF00508803. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Keefe KA, Hanson GR. Unique responses of limbic met-enkephalin systems to low and high doses of methamphetamine. Brain Res. 2001a;905:120–126. doi: 10.1016/s0006-8993(01)02514-8. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Keefe KA, Hanson GR. Contrasting response by basal ganglia metenkephalin systems to low and high doses of methamphetamine in a rat model. J Neurochem. 2001b;76:721–729. doi: 10.1046/j.1471-4159.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- Boules M, Iversen I, Oliveros A, Shaw A, Williams K, Robinson J, Fredrickson P, Richelson E. The neurotensin receptor agonist NT69L suppresses sucrose-reinforced operate behavior in the rat. Brain Res. 2007;1127:90–98. doi: 10.1016/j.brainres.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cador M, Rivet J, Kelley A, Le Moal M, Stinus L. Substance P, neurotensin and enkephalin injections into the ventral tegmental area: comparative study on dopamine turnover in several forebrain structures. Brain Res. 1989;486:357–363. doi: 10.1016/0006-8993(89)90523-4. [DOI] [PubMed] [Google Scholar]
- Cao YJ, Surowy CS, Puttfarcken PS. Different nicotinic acetylcholine receptor subtypes mediating striatal and prefrontal cortical [3H]dopamine release. Neuropharmacology. 2005;48:72–79. doi: 10.1016/j.neuropharm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sharples TJ, Phillips KG, Benedetti G, Broad LN, Zwart R, Shor E. The nicotine alpha 4 beta 2 receptor selective agonist, TC-2559, increases DA neuronal activity in VTA of rat midbrain slices. Neuropharmacology. 2003;45:334–344. doi: 10.1016/s0028-3908(03)00189-8. [DOI] [PubMed] [Google Scholar]
- David V, Besson M, Changeux J, Granon S, Cazala P. Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology. 2006;50:1030–1040. doi: 10.1016/j.neuropharm.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in mesolimbic system of freely moving rats. Proc Nat Acad Sci USA. 1988;85:5272–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearry A, Gingrich J, Falardeau R, Bates M, Caron M. Molecular cloning and expression of the genes for a human D1 dopamine receptor. Nature (London) 1990;347:72–76. doi: 10.1038/347072a0. [DOI] [PubMed] [Google Scholar]
- Emmett S, Greenfield S. Correlation between dopaminergic neurons, acetylcholinesterase and nicotinic acetylcholine receptors containing the alpha 3- or alpha 5-subunit in the rat substantia nigra. J Chem Neuroanat. 2005;30:34–44. doi: 10.1016/j.jchemneu.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Le Novera N, Picciotto MR, Changeux JP, Zoli M. Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci. 2002;15:1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x. [DOI] [PubMed] [Google Scholar]
- Fredrickson P, Boules M, Yerbury S, Richelson E. Blockade of nicotine-induced locomotor sensitization by a novel neurotensin analog in rats. Eur J Pharmacol. 2003;458:111–118. doi: 10.1016/s0014-2999(02)02689-4. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Neurotensin afferents of the ventral tegmental area in the rat: [1] reexamination of their origins and [2] responses to acute psychostimulant and antipsychotic drug administration. Eur J Neurosci. 2006;24:116–134. doi: 10.1111/j.1460-9568.2006.04928.x. [DOI] [PubMed] [Google Scholar]
- George TP, Verrico CD, Picciotto MR, Roth RH. Nicotinic modulation of mesoprefrontal dopamine neurons: pharmacology and neuroanatomic characterization. J Pharmacol Exp Ther. 2000;295:58–66. [PubMed] [Google Scholar]
- Glimcher P, Giovino A, Hoebel B. Neurotensin self injection in the ventral tegmental area. Brain Res. 1987;403:147–150. doi: 10.1016/0006-8993(87)90134-x. [DOI] [PubMed] [Google Scholar]
- Gygi S, Gibb JW, Hanson GR. Differential effects of antipsychotic and psychotomimetic drugs on neurotensin systems of discrete extrapyramidal and limbic regions. J Pharmacol Exp Ther. 1994;270:192–197. [PubMed] [Google Scholar]
- Hanson GR, Bush L, Keefe KA, Alburges ME. Distinct responses of basal ganglia substance P systems to low and high doses of methamphetamine. J Neurochem. 2002;82:1171–1178. doi: 10.1046/j.1471-4159.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Bush LG, Taylor VL, Gibb JW, Davis K, Schmidt CJ. Comparison of neurotensin responses to MDL 100,907, a selective 5-HT2A antagonist, with clozapine and haloperidol. Brain Res Bull. 1997;42:211–219. doi: 10.1016/s0361-9230(96)00258-4. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Smiley P, Johnson M, Letter A, Bush L, Gibb JW. Response of neurotensin systems of the basal ganglia to cocaine treatment. Eur J Pharmacol. 1989;160:23–30. doi: 10.1016/0014-2999(89)90650-x. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Singh N, Merchant K, Johnson M, Bush L, Gibb JW. Responses of limbic and extrapyramidal neurotensin systems to stimulants of abuse. Involvement of dopaminergic mechanism. Ann NY Acad Sci. 1992;668:165–172. doi: 10.1111/j.1749-6632.1992.tb27348.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Win M, Liu Z. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Pessia M, North R. Neurotensin excitation of rat ventral tegmental neurons. J Physiol. 1994;474:119–129. doi: 10.1113/jphysiol.1994.sp020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P. Sensitization to repeated enkephalin administration into the ventral tegmental area of the rat. II Involvement of the mesolimbic dopamine system. J Pharmacol Exp Ther. 1985;235:544–550. [PubMed] [Google Scholar]
- Kalivas P, Taylor S. Behavioral and neurochemical effect of daily injection with neurotensin into the ventral tegmental area. Brain Res. 1985;358:70–76. doi: 10.1016/0006-8993(85)90949-7. [DOI] [PubMed] [Google Scholar]
- Kane JK, Hwang Y, Konu O, Loughlin SE, Leslie FM, Li MD. Regulation of homer and group I metabotropic glutamate receptors by nicotine. Eur J Neurosci. 2005;21:1145–1154. doi: 10.1111/j.1460-9568.2005.03945.x. [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Li MD. Hypothalamic orexin-A binding are downregulated by chronic nicotine treatment in the rat. Neurosci Lett. 2001;298:1–4. doi: 10.1016/s0304-3940(00)01730-4. [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–3629. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- Kelley A, Cador M, Stinus L, Le Moal M. Neurotensin, substance P, neurokinin-alpha, and enkephalin: injection into ventral tegmental area in the rat produces differential effects on operant responding. Psychopharmacology (Berl) 1989;97:243–252. doi: 10.1007/BF00442258. [DOI] [PubMed] [Google Scholar]
- Kempsill FEJ, Pratt JA. Mecamylamine but not the alpha 7 receptor antagonist alpha-bungarotoxin blocks sensitization to the locomotor stimulant effects of nicotine. Br J Pharmacol. 2000;131:997–1003. doi: 10.1038/sj.bjp.0703560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux J. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette S, van der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry. 2003;8:50–59. doi: 10.1038/sj.mp.4001197. [DOI] [PubMed] [Google Scholar]
- Legault M, Congar P, Michel F, Trudeau L. Presynaptic action of neurotensin on cultural ventral tegmental area dopaminergic neurons. Neuroscience. 2002;111:179–189. doi: 10.1016/s0306-4522(01)00614-5. [DOI] [PubMed] [Google Scholar]
- Letter AA, Merchant KM, Gibb JW, Hanson GR. Effect of methamphetamine on neurotensin concentrations in rat brain regions. J Pharmacol Exp Ther. 1987;24:443–447. [PubMed] [Google Scholar]
- Li MD, Kane JK. Effect of nicotine on the expression of leptin and forebrain leptin receptors in the rat. Brain Res. 2003;991:222–231. doi: 10.1016/j.brainres.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Li MD, Kane JK, Parker SL, McAllen K, Matta SG, Sharp BM. Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Res. 2000;867:157–164. doi: 10.1016/s0006-8993(00)02283-6. [DOI] [PubMed] [Google Scholar]
- Lopak V, Erb S. Activation of central neurotensin receptors reinstates cocaine seeking in rat: modulation by a D1/D5, but not D2/D3, receptor antagonist. Psychopharmacology (Berl) 2005;182:297–304. doi: 10.1007/s00213-005-0089-1. [DOI] [PubMed] [Google Scholar]
- Maidment NT, Siddall BJ, Rudolph VR, Erdelyi E, Evans CJ. Dual determination of extracellular cholecystokinin and neurotensin fragments in rat forebrain: microdialysis combined with a sequential multiple antigen radioimmunoassay. Neuroscience. 1991;45:81–93. doi: 10.1016/0306-4522(91)90105-w. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles B, Pons S, Besson M, Guiard B, Guilloux J, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans A, Mallet J, Gardier A, David V, Faure P, Granon S, Changeux J. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;435:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Prameswaran N, Perkins KA, Picciotto MR, Ouik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Merchant KM, Bush L, Gibb JW, Hanson GR. Neurotensin-dopamine interactions in the substantia nigra. J Pharmacol Exp Ther. 1990;255:775–780. [PubMed] [Google Scholar]
- Merchant KM, Gibb JW, Hanson GR. Role of dopamine D-1 and D-2 receptors in the regulation of neurotensin systems of the neostriatum and nucleus accumbens. Eur J Pharmacol. 1989;160:409–412. doi: 10.1016/0014-2999(89)90098-8. [DOI] [PubMed] [Google Scholar]
- Merchant KM, Letter A, Gibb JW, Hanson GR. Changes in the limbic neurotensin system induced by doaminergic drugs. Eur J Pharmacol. 1988;153:1–9. doi: 10.1016/0014-2999(88)90581-x. [DOI] [PubMed] [Google Scholar]
- Naftchi NE, Maker H, Lapin E, Sleis J, Lajtha A, Leeman S. Acute reduction of brain substance P induced by nicotine. Neurochem Res. 1988;13:305–309. doi: 10.1007/BF00972478. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Hertel P, Panagis G, Svensson TH. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in rat. Synapse. 1996;22:369–381. doi: 10.1002/(SICI)1098-2396(199604)22:4<369::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Hildebrand BE, Panagis G, Svensson TH. Nicotine withdrawal in the rat: role of alpha 7 nicotinic receptors in the ventral tegmental area. Neuroreport. 1999;10:697–702. doi: 10.1097/00001756-199903170-00007. [DOI] [PubMed] [Google Scholar]
- O’Dell TJ, Christensen BN. Mecamylamine is a selective non-competitive antagonist of N-methyl-D-aspartate- and aspartate-induced currents in horizontal cells dissociated from catfish retina. Neurosci Lett. 1988;94:93–98. doi: 10.1016/0304-3940(88)90276-5. [DOI] [PubMed] [Google Scholar]
- Panayi F, Colussi-Mas J, Lambas-Senas L, Renaud B, Scarna H, Berod A. Endogenous neurotensin in the ventral termental area contributes to amphetamine behavioral sensitization. Neuropsychopharmacology. 2005;30:871–879. doi: 10.1038/sj.npp.1300638. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego, CA: 1986. [Google Scholar]
- Pontieri F, Rasura M, Scontrini A, Buttarelli F. Effects of intra-VTA injection of neurotensin on local cerebral glucose utilization in freely moving rats. Peptides. 2000;21:1751–1753. doi: 10.1016/s0196-9781(00)00326-0. [DOI] [PubMed] [Google Scholar]
- Posnett DN, McGrath H, Tam JP. A novel method for producing antipeptide antibodies. J Biol Chem. 1988;261:1719–1725. [PubMed] [Google Scholar]
- Quarta D, Ciruela F, Patkar K, Borycz J, Solinas M, Lluis C, Franco R, Wise RA, Goldberg SR, Hope BT, Woods AS, Ferré S. Heteromeric nicotinic acetylcholine-dopamine autoreceptor complexes modulate striatal dopamine release. Neuropsychopharmacology. 2007;32:35–42. doi: 10.1038/sj.npp.1301103. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Effects of acute and chronic nicotine on somatodendritic dopamine release of the VTA: in vivo microdialysis study. Neurosci Lett. 2003;348:61–64. doi: 10.1016/s0304-3940(03)00723-7. [DOI] [PubMed] [Google Scholar]
- Reynolds S, Geisler S, Berod A, Zahm D. Neurotensin antgonist acutely and robustly attenuates locomotion that accompanies stimulation of an neurotensin-containing pathway from rostrobasal forebrain to the ventral tegmental area. Eur J Neurosci. 2006;24:188–196. doi: 10.1111/j.1460-9568.2006.04791.x. [DOI] [PubMed] [Google Scholar]
- Ritter JK, Schmidt CJ, Gibb JW, Hanson GR. Increases of substance P-like immunoreactivity within striatal-nigral structures after subacute methamphetamine treatment. J Pharmacol Exp Ther. 1984;229:233–240. [PubMed] [Google Scholar]
- Rompré PP, Bauco P. Neurotensin receptor activation sensitizes to the locomotor stimulant effect of cocaine: a role for NMDA receptors. Brain Res. 2006;1085:77–86. doi: 10.1016/j.brainres.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Rowell P, Volk K. Nicotinic activation of mesolimbic neurons assessed by rubidium efflux in rat accumbens and ventral tegmentum. Neurosignals. 2004;13:114–121. doi: 10.1159/000076564. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Domino EF, Heitzeg MM, Koeppe RA, Ni L, Guthrie S, Zubieta JK. Smoking modulation of mu-opioid and dopamine D2 receptors-mediated neurotransmission in humans. Neuropsychopharmacology. 2007;32:450–457. doi: 10.1038/sj.npp.1301238. [DOI] [PubMed] [Google Scholar]
- Sotty F, Brun P, Leonetti M, Steinberg R, Soubrie P, Renaud B, Suaud-Chagny M. Comparative effects of neurotensin, neurotensin (8–13 and (D-Tyr[11] neurotensin applied into the ventral tegmental area on extracellular dopamine in the rat prefrontal cortex and nucleus accumbens. Neuroscience. 2000;98:485–492. doi: 10.1016/s0306-4522(00)90023-x. [DOI] [PubMed] [Google Scholar]
- Seeman P, Grigoriadis D. Dopamine D2 receptor dissociation constant for spiperone: identical values using 3H-labeled agonist or 3H-labeled antagonist. Biochem Pharmacol. 1985;34:4065–4066. doi: 10.1016/0006-2952(85)90388-0. [DOI] [PubMed] [Google Scholar]
- Singer S, Rossi S, Verzosa S, Hashim A, Lonow R, Cooper T, Sershen H, Lajtha A. Nicotine-induced changes in neurotransmitter levels in brain areas associated with cognitive function. Neurochem Res. 2004;29:1779–1792. doi: 10.1023/b:nere.0000035814.45494.15. [DOI] [PubMed] [Google Scholar]
- Sziraki I, Sershen H, Hashim A, Lajtha A. Receptors in the ventral tegmental area mediating nicotine-induced dopamine release in the nucleus accumbens. Neurochem Res. 2002;27:253–261. doi: 10.1023/a:1014844823534. [DOI] [PubMed] [Google Scholar]
- Tam JP. Synthetic peptide vaccine design: Synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff J, Gibb JW, Hanson GR. Dopamine D2-receptors regulate neurotensin release from nucleus accumbens and striatum as measured by in vivo microdialysis. Brain Res. 1996;721:196–203. doi: 10.1016/0006-8993(96)00132-1. [DOI] [PubMed] [Google Scholar]
- Wagstaff J, Bush L, Gibb JW, Hanson GR. Endogenous neurotensin antagonizes methamphetamine-enhanced dopaminergic activity. Brain Res. 1994;665:237–244. doi: 10.1016/0006-8993(94)91343-9. [DOI] [PubMed] [Google Scholar]
- Wu J, George AA, Schroeder KM, Xu L, Marxer-Miller S, Lucero L, Lukas RJ. Electrophysiological, pharmacological, and molecular evidence for alpha 7-nicotinic acethylcholine receptors in rat midbrain dopamine neurons. J Pharmacol Exp Ther. 2004;311:80–91. doi: 10.1124/jpet.104.070417. [DOI] [PubMed] [Google Scholar]