Abstract
A microhematocrit concentration method (MH) for immediate diagnosis of Chagas' disease during the acute stage or in congenital cases was standardized. Parasitemia as low as 1,000 parasites per ml was detected, after centrifugation of six 50-microliters capillary tubes, by 10-min microscopic observation of each buffy coat spread between slide and cover glass. Operator's time was reduced by at least one-third when compared with a fresh blood observation (FB). In 12 of the 15 patients studied, diagnosis was performed in 4.9 +/- 3.08 min with MH, whereas 27.0 +/- 12.1 min were necessary when FB was used. In the three remaining patients whose FB results were negative, MH became positive after 13, 16, and 40 min. In our experience, FB proved to be more sensitive than previously reported. Suckling mouse inoculation also proved to be sensitive but, as in xenodiagnosis and in hemoculture, the delay in getting the final result was a limiting factor.
Full text
PDF
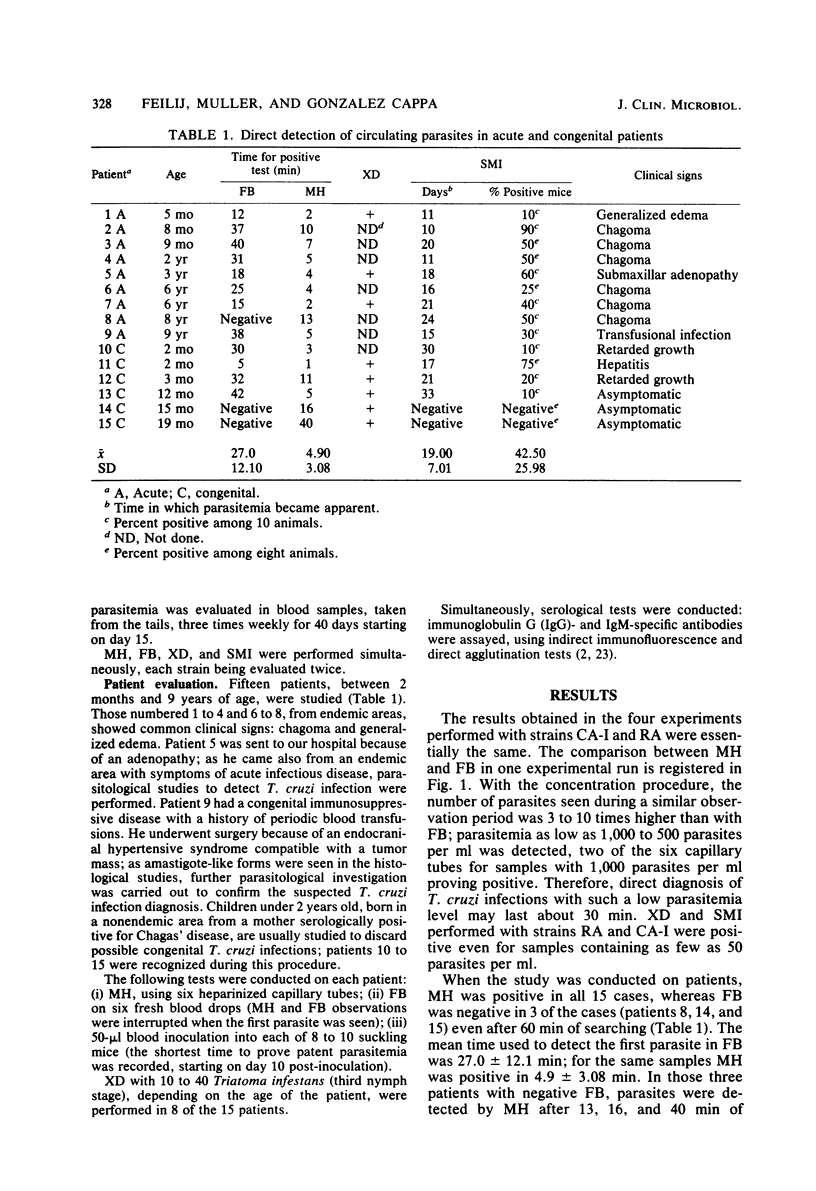

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramo Orrego L., Lansetti J. C., Bozzini J. P., Wynne de Martini G. J. Hemocultivo como método de diagnostico de la enfermedad de Chagas. Medicina (B Aires) 1980;40 (Suppl 1):56–62. [PubMed] [Google Scholar]
- Alvarez M., Cerisola J. A., Rohwedder R. W. Test de inmunofluorescencia para diagnóstico de la enfermedad de Chagas. Bol Chil Parasitol. 1968 Jan-Jun;23(1):4–8. [PubMed] [Google Scholar]
- BRENER Z., CHIARI E. VARIA C OES MORFOL'OGICAS OBSERVADAS EM DIFERENTES AMOSTRAS DE TRYPANOSOMA CRUZI. Rev Inst Med Trop Sao Paulo. 1963 Sep-Oct;5:220–224. [PubMed] [Google Scholar]
- Brener Z. Present status of chemotherapy and chemoprophylaxis of human trypanosomiasis in the Western Hemisphere. Pharmacol Ther. 1979;7(1):71–90. doi: 10.1016/0163-7258(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Chiari E., Brener Z. Contribuiço ao diagnóstico parasitológico da doença de Chagas na sua fase crônica. Rev Inst Med Trop Sao Paulo. 1966 May-Jun;8(3):134–138. [PubMed] [Google Scholar]
- D'Alessandro A., del Prado C. E. Search for Trypanosoma rangeli in endemic areas of Trypanosoma cruzi in Argentina and Brazil. Am J Trop Med Hyg. 1977 Jul;26(4):623–627. doi: 10.4269/ajtmh.1977.26.623. [DOI] [PubMed] [Google Scholar]
- Gonzalez S. M., Menes S., Schmunis G. A., Szarfman A., Vatuone N. H., Yanovsky J. F. La detección de aglutininas específicas en el diagnóstico de la enfermedad de Chagas (tripanosomiásis americana) Medicina (B Aires) 1976;36(4):364–375. [PubMed] [Google Scholar]
- González Cappa S. M., Bijovsky A. T., Freilij H., Muller L., Katzin A. M. Aislamiento de una cepa de trypanosoma cruzi a predominio de formas delgadas en la Argentina. Medicina (B Aires) 1981;41(1):119–120. [PubMed] [Google Scholar]
- González Cappa S. M., Chiale P., del Prado G. E., Katzin A. M., de Martini G. W., de Isola E. D., Abramo Orrego L., Segura E. L. Aislamiento de uan cepa de Trypanosoma cruzi de un Paciente con miocardiopatía chagásica cronica y su caracterización biológica. Medicina (B Aires) 1980;40 (Suppl 1):63–68. [PubMed] [Google Scholar]
- González Cappa S. M., Katzin A. M., Añasco N., Lajmanovich S. Comparative studies on infectivity and surface carbohydrates of several strains of Trypanosoma cruzi. Medicina (B Aires) 1981;41(5):549–555. [PubMed] [Google Scholar]
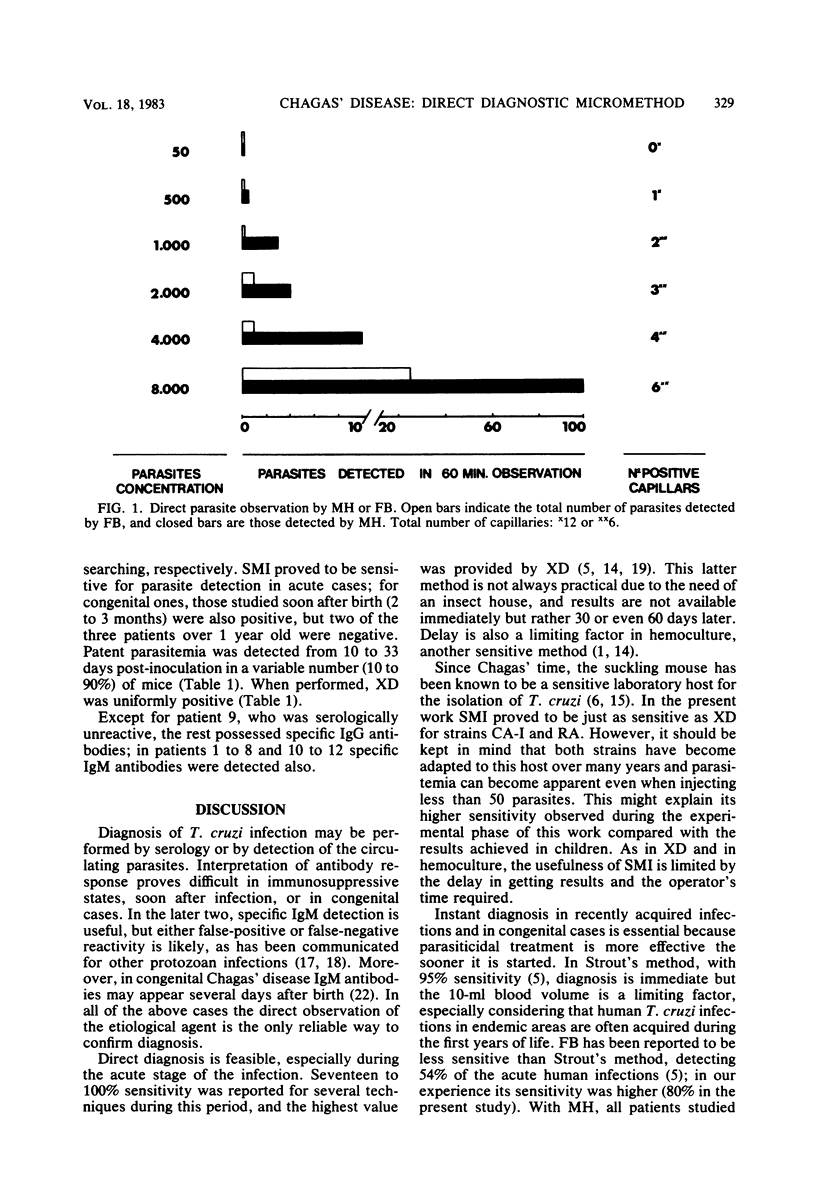
- PHILLIPS N. R. Experimental studies on the quantitative transmission of Trypanosoma cruzi: considerations regarding the standardization of materials. Ann Trop Med Parasitol. 1960 Apr;54:60–70. doi: 10.1080/00034983.1960.11685957. [DOI] [PubMed] [Google Scholar]
- Pyndiah N., Krech U., Price P., Wilhelm J. Simplified chromatographic separation of immunoglobulin M from G and its application to toxoplasma indirect immunofluorescence. J Clin Microbiol. 1979 Feb;9(2):170–174. doi: 10.1128/jcm.9.2.170-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Desmonts G. Congenital toxoplasmosis: variability in the IgM-fluorescent antibody response and some pitfalls in diagnosis. J Pediatr. 1973 Jul;83(1):27–30. doi: 10.1016/s0022-3476(73)80307-5. [DOI] [PubMed] [Google Scholar]
- STROUT R. G. A method for concentrating hemoflagellates. J Parasitol. 1962 Feb;48:100–100. [PubMed] [Google Scholar]
- Salgado A. de A. Consideraciones sobre metodología y sensibilidad del xenodiagnóstico. Bol Chil Parasitol. 1969 Jan-Mar;24(1):9–13. [PubMed] [Google Scholar]
- Schmuñis G. A., Szarfman A., Coarasa L., Guilleron C., Peralta J. M. Anti-Trypanosoma cruzi agglutinins in acute human Chagas' disease. Am J Trop Med Hyg. 1980 Mar;29(2):170–178. doi: 10.4269/ajtmh.1980.29.170. [DOI] [PubMed] [Google Scholar]
- Szarfman A., Urman J., Otalora A., Larguia A., Yanovsky J. F. Specific agglutinins and immunoglobulin levels in congenital Chagas infection. Medicina (B Aires) 1975 May-Jun;35(3):245–250. [PubMed] [Google Scholar]
- Woo P. T. Evaluation of the haematocrit centrifuge and other techniques for the field diagnosis of human trypanosomiasis and filariasis. Acta Trop. 1971;28(3):298–303. [PubMed] [Google Scholar]
- de Isola E. D., Sanchez D., Katzin V. Triatoma infestans: influencia de la alimentación artificial sobre su ciclo de vida. Medicina (B Aires) 1980;40 (Suppl 1):207–212. [PubMed] [Google Scholar]


